Abstract
A dipstick assay, based on Leishmania infantum antigen, for the rapid detection of Leishmania-specific antibodies in canine serum samples was developed and evaluated. After determination of optimal dipstick test conditions, test performance was compared with two existing serological tests, i.e., the direct agglutination test (DAT) and the fast agglutination screening test (FAST). In the present study the dipstick test had a sensitivity of 99.2% and a specificity of 87.9%. The DAT had a sensitivity of 97.7% and a specificity of 95.2%, whereas the FAST had also a sensitivity of 97.7% and a specificity of 93.0%. High degrees of agreement were observed between the dipstick test and DAT (93.7%; κ value, 0.86), between the dipstick test and FAST (91.8%; κ value, 0.82), and between the DAT and FAST (95.2%; κ value, 0.90). The high sensitivity and ease of performance make the dipstick test very suitable for surveillance surveys.
Canine infections with Leishmania infantum or Leishmania chagasi are important as a cause of disease in dogs and as a reservoir for human leishmaniasis (26). Canine leishmaniasis (CanL) is a severe systemic disease with hair loss, skin lesions, epistaxis, anemia, wasting, swollen limbs and joints, lameness, renal failure, lymphadenopathy, ocular lesions, and diarrhea (25). CanL is endemic in countries around the Mediterranean Sea (caused by L. infantum) and Latin America (caused by L. chagasi). Studies in European foci have shown that the prevalence of CanL varies from 1 to 37% (1, 4). In addition, infections are now frequently reported as import cases in northern Europe (25). Since 2000, CanL is also found in foxhound populations in the United States and Canada (6, 11, 16), and it is suggested that the disease may become endemic in this region as well (6, 10). As the dog is considered to be the reservoir of Leishmania and thus for subsequent transmission of disease to humans (4, 7, 13), in some countries seropositive dogs are killed as a control measure. Elimination of dogs in areas of endemicity in China and Brazil has been correlated with a decreased incidence of human disease (8, 12, 18). However, this practice is resisted by dog owners, and its overall effectiveness, unless the whole dog population is removed, is questionable (9, 19).
Accurate and rapid diagnosis of CanL is of great importance in order to start early treatment and to prevent transmission, but this remains problematic. Clinical diagnosis is difficult due to variable symptomatology. Parasitological diagnosis relies on microscopic demonstration or culture of Leishmania parasites from aspirates, but sample retrieval is painful to the dog, microscopic identification in smears and biopsy sections requires experienced personnel, and the isolation of parasites by culturing is time-consuming, difficult, and expensive. Furthermore, more than half of all infected dogs lack clinical signs of leishmaniasis (1), but these asymptomatic dogs are just as infective to the vector as symptomatic dogs (3). Serology is used for indirect diagnosis of CanL, and several techniques have been developed to detect anti-Leishmania antibodies in clinical samples. However, several available serodiagnostic tests have inadequate sensitivity and/or specificity (18-20), which may result in misdiagnosis and thus in subsequent wrong treatment or unnecessary sacrifice of dogs. Furthermore, many tests are not practical due to the requirement of advanced equipment, making them not suitable for surveillance programs or for use in simple veterinary practice (22). There is thus a definite need for a rapid, sensitive, and specific diagnostic tool for CanL (19, 20).
Here we describe the development of a simple dipstick test for CanL based on L. infantum crude antigen. As L. chagasi and L. infantum, both of which belong to the Leishmania donovani complex, are most likely the same species (14), we have selected an L. infantum MHOM/CN/54 Peking strain as the antigen source. This parasite grows very well under laboratory conditions, and relatively large amounts of crude antigen can be obtained from this strain. Furthermore, preliminary research (24) suggested that antigen produced from a homologous parasite (L. infantum) slightly increased the sensitivity of serodiagnostic tests for canine infection with Leishmania parasites. The dipstick test was evaluated by using canine serum samples from several different regions where the disease is endemic or not endemic, and test performance was compared with those of our direct agglutination test (DAT) and the fast agglutination screening test (FAST), both traditionally based on L. donovani antigen, which are in use in our laboratory for the serodiagnosis of CanL and human visceral leishmaniasis (15, 17, 21, 22, 23).
MATERIALS AND METHODS
Preparation of dipstick test.
L. infantum MHOM/CN/54/Peking promastigotes were cultured under the same conditions as previously described for L. donovani (15). Parasites were harvested and washed three times with phosphate-buffered saline (PBS) (pH 7.2). The pellet was resuspended in water, sonicated, and centrifuged for 30 min at 10,000 × g and 20°C. The supernatant was collected, and its protein concentration was determined. This antigen preparation was than bound as a distinct line to a nitrocellulose strip. To obtain an internal control, goat anti-canine immunoglobulin G (IgG) (heavy plus light chains) (Nordic Immunological Laboratories, Tilburg, The Netherlands) was also applied to the nitrocellulose as a separate band. Unbound antigen and unbound internal control IgG were removed by brief washing with PBS (pH 7.2). Next, the coated strips were blocked with 3% egg white solution-0.01% NaN3 in PBS for 30 min. After four washes for 5 min each with PBS, the nitrocellulose was allowed to dry at room temperature. The antigen-containing membrane was subsequently attached to a plastic support with double-sided tape and cut into 2.5-mm-wide sticks that were stored at room temperature.
Execution of dipstick test.
Appropriate serum dilutions were made in 0.5% egg white in Tris-NaCl solution (0.24% Tris, 2.92% NaCl [pH 7.5]) plus 0.2% Tween 20. The dipsticks were prewetted with Tris-NaCl solution plus 0.2% Tween 20 prior to use and subsequently were incubated with diluted serum for 15 min at room temperature. Next, the dipsticks were washed three times for 2 min each with Tris-NaCl solution plus 0.2% Tween, followed by a 15-min incubation with diluted peroxidase conjugate (goat anti-dog IgG [Fc]-peroxidase) in 0.5% egg white in Tris-NaCl plus 0.2% Tween 20. After three washes for 5 min each with Tris-NaCl solution plus 0.2% Tween 20 and one wash with PBS, the dipsticks were incubated for 2 min in substrate solution (1 mg of 3,3-diaminobenzidine-4-hydrochloride in 2 ml of PBS and 1.3 μl of 30% H2O2). Finally, the dipsticks were washed thoroughly in water for 1 min and dried.
Determination of optimum dipstick test conditions.
In order to determine optimal dipstick test conditions, various antigen concentrations (500 μg/ml, 750 μg/ml, and 1 mg/ml), dilutions of reference sera (1:25, 1:50, and 1:100), and conjugate dilutions (1:100, 1:200, 1:400, 1:800, and 1:1,500) were tested. The reference sera used were from a healthy control dog from a region where the disease is not endemic with a DAT titer of <1:100 (negative control), from a healthy dog from an region of endemicity with a DAT titer of 1:200, from a dog with a suspected infection from an region of endemicity with a DAT titer of 1:800, from a symptomatic dog with a titer of 1:1,600, and from a dog with a parasitologically confirmed L. infantum infection and a DAT titer of 1:6,400.
DAT and FAST.
DAT and FAST based on freeze-dried L. donovani antigen were performed as described previously (21). The DAT results in an antibody titer (quantitative test), with a cutoff value of 1:400 (17, 21). The qualitative FAST results in a positive or negative result (21).
Serum samples.
All serum samples were heat inactivated (56°C, 30 min) prior to use to inactivate the complement system. Heat inactivation does not affect the test results.
The following sets of serum samples were used to determine the sensitivity and specificity of the final dipstick test and were also tested in the standard dog DAT and FAST for comparison.
(i) Healthy dogs (negative control serum samples; n = 44).
The samples from healthy controls, with no history and no clinical signs of CanL, included those from dogs from three areas of endemicity, i.e., the Kusadasi region in Turkey (n = 1), the state of Rio de Janeiro in Brazil (n = 13), and the Alto Douro region in Portugal (n = 9), and from dogs not from an area of endemicity (i.e., dogs that have never been outside The Netherlands), which were obtained from the Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands (n = 19) or stored at the serum bank at KIT Biomedical Research, Amsterdam, The Netherlands (n = 2).
(ii) Confirmed cases of CanL (positive control serum samples; n = 126).
The positive control samples included serum samples from parasitologically confirmed CanL cases from the Kusadasi region in Turkey (n = 2); serum samples from dogs with leishmaniasis which were presented at the Clinic for Companion Animals, Faculty of Veterinary Medicine, Utrecht University (all of these dogs had clinical signs of visceral leishmaniasis, and parasites were found in bone marrow or lymph node aspirates) (n = 25); serum samples from dogs from Minas Gerais state in Brazil, for which the suspicion of canine visceral leishmaniasis was confirmed by PCR (n = 31); serum samples from parasitologically positive CanL cases from the Alto Douro region in Portugal (n = 58); serum samples from dogs with clinical signs of CanL and high immunofluorescence assay titers from the state of Rio de Janeiro in Brazil (n = 2); and serum samples from dogs with confirmed CanL stored at the serum bank at KIT Biomedical Research (n = 8).
(iii) Dogs with other diseases (n = 36).
Serum samples from dogs with other confirmed diseases, which were presented at the Clinic for Companion Animals, Faculty of Veterinary Medicine, Utrecht University, (n = 21) were tested. This group comprised 10 dogs with cancer, 3 with autoimmune hemolytic anemia, 4 with gastroenteritis and severe wasting, 1 with hernia, 1 with pyometra and kidney failure, 1 with systemic histiocytosis, and 1 suffering from heart failure and lung cancer. All of these dogs have never been outside The Netherlands. In addition, serum samples from dogs (n = 15) from the Alto Douro region in Portugal that were suffering from Hepatozoon canis infection (n = 2); Babesia infection and seropositive for Leptospira spp. (n = 3); Demodex canis infection (n = 3); mixed infection with Toxocara canis, Taenia spp., Dipylidium caninum, or Sarcoptes scabiei var. canis (n = 2); Trichuris vulpis infection (n = 1); Ehrlichia canis infection (n = 1); cancer (n = 1); lymphoma (n = 1); and an unspecified immunological disorder (n = 1) were included in the evaluation.
Reproducibility.
Two observers independently read the results of the DAT, FAST, and dipstick assays, and their results were compared. If the interpretation of the dipstick or FAST results was different for the two observers or the reading of the DAT differed by more than one serum dilution, the sample was retested in the appropriate test. If the final results (i.e., positive or negative) of the dipstick test, FAST, and DAT differed from each other, the sample was reanalyzed in all tests to confirm the test results.
Data analysis.
The sensitivity (i.e., the probability that the assay will be positive when the infection is present) and the specificity (i.e., the probability that the assay will be negative when the infection is absent) of each diagnostic test (dipstick test, FAST, and DAT) in the present study were calculated by using the formulas (2) sensitivity = TP/(TP + FN) × 100% and specificity = TN/(TN+FP) × 100%, where TN is the number of true negatives, TP is the number of true positives, FN is the number of false negatives, and FP is the number of false positives. The sensitivities of the tests were assessed with sera from dogs with confirmed CanL (n = 126). Sera from healthy controls from areas of endemicity (n = 23) and from areas where the disease is not endemic (n = 21) and sera from dogs with confirmed cases of other diseases (n = 36) were used to determine test specificity.
In addition, the degree of agreement between the evaluated tests was determined. The agreement between the tests was determined by calculating κ values with 95% confidence intervals by using Epi-Info version 6. κ values express the agreement beyond chance; a κ value of 0.60 to 0.80 represents substantial agreement beyond chance, whereas a κ value of >0.80 represents almost perfect agreement beyond chance (2).
RESULTS
Optimization of the dipstick test.
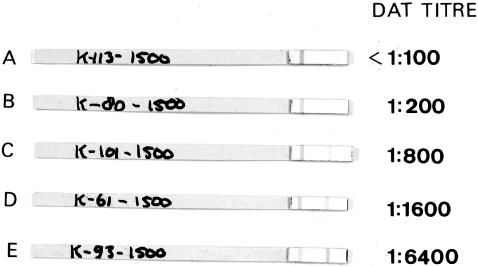
Dipstick optimization with reference sera showed that the best test result was obtained by using an antigen concentration of 750 μg/ml, a serum dilution of 1:50, and a conjugate dilution of 1:1,500. Under these conditions, the negative controls were negative and the sera from dogs with suspected cases and from those of dogs with symptomatic and confirmed cases tested positive with the dipstick test (Fig. 1). These conditions were used for further evaluation of the dipstick test with the large serum panel and in comparison with the DAT and FAST.
FIG. 1.
Optimization of dipstick test conditions. The best result was obtained by using an antigen concentration of 750 μg/ml, a serum dilution of 1:50, and a conjugate dilution of 1:1,500. Under these conditions, samples from a negative control from an area where CanL is not endemic (A) and a negative control from an area of endemicity (B) are negative with the dipstick test, whereas samples from dogs with suspected (C), symptomatic (D), and confirmed (E) cases of CanL are positive with the dipstick test.
Test evaluation.
None of the tests had to be repeated due to inconsistencies between the readings of the observers. A 100% agreement in the reading of the dipstick test, FAST, and DAT (a one-step difference in titer was allowed) was found between the two observers. The analyses performed with the dipstick test, FAST, and DAT were all valid, as the internal control of the dipstick test was always positive and the titers of appropriate control samples determined with the DAT corresponded to the predetermined titer of the control (a one-step difference in DAT titer was allowed). Ten samples for which different results was observed in the DAT, FAST, and dipstick test were retested with all three tests, and in all cases retesting resulted in the same result as initially obtained.
Results of the dipstick test, DAT, and FAST with sera from confirmed cases of CanL, healthy negative controls, and dogs with other diseases are summarized in Table 1. Calculation of the sensitivities of the diagnostic tests revealed that the dipstick test had a sensitivity of 99.2% and both the DAT and FAST had a sensitivity of 97.7%.
TABLE 1.
Comparison of dipstick test, DAT, and FAST with serum samples from dogs with confirmed CanL, healthy controls from regions where CanL is or is not endemic, and dogs with other diseases
| Category and region | n | No. positive/total
|
||
|---|---|---|---|---|
| Dipstick test | DAT | FAST | ||
| Confirmed CanL cases | ||||
| Alto Douro region, Portugal | 58 | 58/58 | 58/58 | 58/58 |
| Minas Gerais state, Brazil | 31 | 31/31 | 30/31 | 30/31 |
| Rio de Janeiro state, Brazil | 2 | 2/2 | 2/2 | 2/2 |
| Kusadasi region, Turkey | 2 | 2/2 | 2/2 | 2/2 |
| Import cases, The Netherlands | 25 | 24/25 | 23/25 | 23/25 |
| Serum bank KIT, The Netherlands | 8 | 8/8 | 8/8 | 8/8 |
| Total | 126 | 125/126 | 123/126 | 123/126 |
| Healthy controls | ||||
| Alto Douro region, Portugal | 9 | 1/9 | 0/9 | 0/9 |
| Rio de Janeiro state, Brazil | 13 | 2/13 | 1/13 | 1/13 |
| Kusadasi region, Turkey | 1 | 0/1 | 0/1 | 0/1 |
| The Netherlands | 19 | 4/19 | 0/19 | 0/19 |
| Serum bank KIT, The Netherlands | 2 | 0/2 | 0/2 | 1/2 |
| Total | 44 | 7/44 | 1/44 | 2/44 |
| Other diseases | ||||
| Alto Douro region, Portugal | 15 | 3/15 | 3/15 | 3/15 |
| The Netherlands | 21 | 1/21 | 0/21 | 1/21 |
| Total | 36 | 4/36 | 3/36 | 4/36 |
In total, seven of the negative control samples (n = 44) and four serum samples from dogs with other confirmed diseases (n = 36) tested positive with the dipstick test, resulting in an 87.9% specificity of this test. One negative control sample and three samples from dogs with other confirmed diseases tested positive with the DAT (titer of ≥1:400), resulting in a 95.2% specificity. The FAST had two false-positive results with the negative controls and four false-positive results with other diseases, resulting in a specificity of 93.0%.
A high degree of agreement (93.7%) was observed between the dipstick test and the DAT (Tables 2 to 4). The agreement beyond chance (κ value) was 0.86. High degrees of agreement were also observed between the dipstick test and FAST (91.8%; κ value, 0.82) and between the DAT and FAST (95.2%; κ value, 0.90).
TABLE 2.
Comparison between dipstick test DAT and for detection of Leishmania antibodies in canine serum samples
| DAT result | No. with the following dipstick test resulta:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 128 | 1 | 129 |
| Negative | 12 | 65 | 77 |
| Total | 140 | 66 | 206 |
Agreement, 93.7%; κ value, 0.86.
TABLE 4.
Comparison between DAT and FAST
| FAST result | No. with the following DAT resulta:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 123 | 7 | 130 |
| Negative | 3 | 73 | 76 |
| Total | 126 | 80 | 206 |
Agreement, 95.2%; κ value = 0.90.
DISCUSSION
A sensitive, specific, and simple diagnostic tool for CanL is essential to the veterinary community and for surveillance programs (20). Such a test should ideally have the format of a lateral flow test, because this can be used by general veterinarians and may ultimately prove to be more cost-effective than currently used diagnostics, in particular when used in mass screening surveys (20). As a first step towards the development of such a test, we have developed a simple dipstick test for the serodiagnosis of CanL. After optimum assay conditions were set, the dipstick test was used to screen a large panel of serum samples from healthy controls and from dogs with confirmed cases of CanL and other diseases. The observed sensitivities (97.7 and 97.7%) and specificities (95.2 and 93.0%) of the DAT and FAST, respectively, found in the present study were comparable to those observed in previous studies (15, 17, 21). The experimental dipstick test had a very good sensitivity of 99.2%. In fact, the dipstick test detected two more case of CanL than the other two tests did. In contrast, the specificity of the dipstick test was lower (87.9%). This lower specificity is attributed to the fact that the dipstick test showed four samples from healthy controls from The Netherlands, an area where the disease is not endemic, to be positive. These dogs have never been outside The Netherlands, nor did they show signs of any disease. The false-positive reaction of the dipstick test in these cases remains unexplained. In addition, the dipstick test found three healthy controls from areas of endemicity to be positive, one of which was also found to be positive with the DAT and FAST. As these samples were from dogs originating from a region of endemicity and a full parasitological examination of these dogs was not carried out, an asymptomatic Leishmania infection in these dogs cannot be ruled out. Furthermore, the DAT, FAST, and dipstick test all found the same cases of other diseases from Portugal positive. These dogs were suffering from either arthritis, E. canis, or distemper combined with demodex. One dog, from The Netherlands and suffering from cancer, was found positive with the dipstick test and FAST but not with the DAT.
The high sensitivity and ease of performance make the dipstick test very suitable for surveillance surveys. However, the lower specificity of the test compared to the DAT may result in some dogs being misdiagnosed as false positives. Therefore, the diagnosis of CanL must be made on the basis of the outcome of the diagnostic test in combination with clinical and epidemiological information.
Currently, there is only one dipstick test commercially available for leishmaniasis diagnosis, and this test is based on a recombinant antigen of a 39-amino-acid repeat that is part of a 230-kDa protein encoded by a kinesin-like gene of L. chagasi (5). To our knowledge, so far only one study has used this dipstick test to detect Leishmania antibodies in canine samples, and the test was shown to have 61 to 75% specificity and 72 to 77% sensitivity (20), which is lower than those observed for the test evaluated in the present study.
Although the dipstick test is based on an antigen of L. infantum (the causative agent of CanL in the Mediterranean region), it is also able to detect antibodies in serum samples of dogs infected with L. chagasi (Brazil). This enables the use of the dipstick in all major regions of CanL endemicity. Furthermore, it also detected anti-Leishmania antibodies in imported cases of CanL, allowing the use of the dipstick test in veterinary clinics in countries where CanL is not endemic.
TABLE 3.
Comparison between dipstick test and FAST
| FAST result | No. with the following dipstick test resulta:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 128 | 5 | 133 |
| Negative | 12 | 61 | 73 |
| Total | 140 | 66 | 206 |
Agreement, 91.8%; κ value, 0.82.
Acknowledgments
We thank E. G. M. Beijer (Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands), Y. Özbel and S. Özensoy (Department of Parasitology, Ege University, Izmir, Turkey), E. S. Da Silva (Laboratório de Leishmanioses, Centro de Pesquisas René Rachou-Fio-Cruz, Belo Horizonte, Minas Gerais, Brazil), and E. D. Da Silva (Laboratório de Reativos para Diagnostica, Bio-Manguinhos/Fiocruz, Rio de Janeiro, Brazil) for providing us with valuable canine serum samples.
S. J. Semião-Santos was supported by scholarship BDP/SFRH/5731 (FCT, Portugal).
REFERENCES
- 1.Abranches, P., M. C. Silva-Pereira, F. M. Conceiçao, G. M. Santos-Gomes, and J. G. Janz. 1991. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J. Parasitol. 77:557-561. [PubMed] [Google Scholar]
- 2.Altman, D. G. 1991. Practical statistics for medical research. Chapman & Hall, London, United Kingdom.
- 3.Alvar, J., R. Molina, M. San Andres, M. Tesouro, J. Nieto, M. Vitutia, F. Gonzalez, M. D. San Andres, J. Boggio, and F. Rodriguez. 1994. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol. 88:371-378. [DOI] [PubMed] [Google Scholar]
- 4.Bettini, S., and L. Gradoni. 1986. Canine leishmaniasis in the Mediterranean area and its implications for human leishmaniasis. Insect Sci. Appl. 7:241-245. [Google Scholar]
- 5.Burns, J. M., W. G. Shreffer, D. R. Benson, H. W. Ghalib, R. Badaro, and S. G. Reed. 1993. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2000. Visceral leishmaniasis outbreaks in dogs. Focus 9:4-5. [Google Scholar]
- 7.Cunha, S., M. Freire, C. Eulalio, J. Critosvao, E. Netto, W. D. Johnson, Jr., S. G. Reed, and R. Badaro. 1995. Visceral leishmaniasis in a new ecological nice near a metropolitan area of Brazil. Trans. R. Soc. Trop. Med. Hyg. 89:155-158. [DOI] [PubMed] [Google Scholar]
- 8.de Alencar, J. E. 1961. Profilaxia do calazar no Ceará, Brasil. Rev. Inst. Med. Trop. São Paulo 3:175-180. [PubMed] [Google Scholar]
- 9.Dietze, R., G. B. Barros, L. Teixeira, J. Harris, K. Michelson, A. Falqueto, and R. Corey. 1997. Effect of eliminating seropositve canines on the transmission of visceral leishmaniasis in Brazil. Clin. Infect. Dis. 25:1240-1242. [DOI] [PubMed] [Google Scholar]
- 10.Enserink, M. 2000. Has leishmaniasis become endemic in the U. S.? Science 290:1881-1883. [DOI] [PubMed] [Google Scholar]
- 11.Gaskin, A. A., P. Schantz, J. Jackson, A. Birkenheuer, L. Tomlinson, M. Gramiccia, M. Levy, F. Steurer, E. Kollmar, B. C. Hegarty, A. Ahn, and E. B. Breitschwerdt. 2002. Visceral leishmaniasis in a New York foxhound kennel. J. Vet. Intern. Med. 16:34-44. [DOI] [PubMed] [Google Scholar]
- 12.Leng, Y. J. 1982. A review of kala-azar in China from 1949-1959. Trans. R. Soc. Trop. Med. Hyg. 19:363-384. [DOI] [PubMed] [Google Scholar]
- 13.Marty, P., Y. Le Fichoux, D. Giordana, and A. Brugnetti. 1992. Leishmanin reaction in the population of a highly endemic focus of canine leishmaniasis in Alpes Maritimes, France. Trans. R. Soc. Trop. Med. Hyg. 86:249-250. [DOI] [PubMed] [Google Scholar]
- 14.Mauricio, I. L., J. R. Stothard, and M. A. Miles. 2000. The strange case of Leishmania chagasi Parasitol. Today 16:188-189. [DOI] [PubMed] [Google Scholar]
- 15.Oskam, L., R. J. Slappendel, E. G. Beijer, N. C. C. Kroon, C. W. van Ingen, S. Ozensoy, Y. Özbel, and W. J. Terpstra. 1996. Dog-DAT: a direct agglutination test using stabilized, freeze-dried antigen for the serodiagnosis of canine visceral leishmaniasis. FEMS Immunol. Med. Microbiol. 16:235-239. [DOI] [PubMed] [Google Scholar]
- 16.Owens, S. D., D. A. Oakley, K. Marryot, W. Hatchett, R. Walton, T. Nolan, A. Newton, F. Steurer, P. Schantz, and U. Giger. 2001. Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J. Am. Vet. Med. Assoc. 219:1076-1083. [DOI] [PubMed] [Google Scholar]
- 17.Özbel, Y., L. Oskam, S. Ozensoy, N. Turgay, M. Z. Alkan, C. L. Jaffe, and M. A. Ozce. 2000. A survey on canine leishmaniasis in western Turkey by parasite, DNA and antibody detection assays. Acta Trop. 74:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Palatnik de Sousa, C. B., W. R. dos Santos, J. C. Franca-Silva, R. da Costa, A. B. Reis, M. Palatnik, W. Mayrink, and O. Genaro. 2001. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am. J. Trop. Med. Hyg. 65:510-517. [DOI] [PubMed] [Google Scholar]
- 19.Reithinger, R., and C. R. Davies. 1999. Is the domestic dog (Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am. J. Trop. Med. Hyg. 61:530-541. [DOI] [PubMed] [Google Scholar]
- 20.Reithinger, R., R. J. Quinnell, B. Alexander, and C. R. Davies. 2002. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J. Clin. Microbiol. 40:2352-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schallig, H. D. F. H., G. J. Schoone, E. G. Beijer, C. C. M. Kroon, M. Hommers, Y. Özbel, S. Ozensoy, E. S. da Silva, L. M. Cardoso, and E. D. da Silva. 2002. Development of a fast agglutination screening test (FAST) for the detection of anti-Leishmania antibodies in dogs. Vet. Parasitol. 109:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Schallig, H. D. F. H., G. J. Schoone, C. C. M. Kroon, A. Hailu, F. Chappuis, and H. Veeken. 2001. Development and application of ‘simple’ diagnostic tools for visceral leishmaniasis. Med. Microbiol. Immunol. 190:69-71. [DOI] [PubMed] [Google Scholar]
- 23.Schallig, H. D. F. H., M., Canto-Cavelheiro, and E. S. da Silva. 2002. Evaluation of the direct agglutination test (DAT) and the rK39 dipstick test for the serodiagnosis of visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 97:1015-1018. [DOI] [PubMed] [Google Scholar]
- 24.Semiao-Santos, S. J. 1996. Ph. D. thesis. University of Amsterdam, Amsterdam, The Netherlands.
- 25.Slappendel, R. J., and E. Teske. 1999. A review of canine leishmaniasis presenting outside endemic areas, p. 54-59. In R. Killick-Kendrick (ed.), Canine leishmaniasis: an update. Hoechst Roussel Vet, Wiesbaden, Germany.
- 26.World Health Organization. 1990. Control of the leishmaniases—-report of a W. H. O. Expert Committee. WHO Tech. Rep. Ser. 793. [PubMed]