Abstract
Our objectives for this report were to identify trajectories of youth gambling behavior, and to examine their relation to executive cognitive function (ECF) and associated problem behaviors. Philadelphia school children, enrolled at ages 10–12 years (n = 387; 49% male), completed three annual assessments of risk behaviors, ECF, impulsivity, problem behaviors and demographics. Across ages 10–15 years, using methods from Nagin et al., two groups were identified: Early Gamblers (n = 111) initiated early and continued in later assessments, and Later Gamblers (n = 276) initiated at later ages and gambled less. Betting money on cards and sports were the most frequently reported gambling behaviors. Using gambling group as outcome, final backward selection logistic regression model showed Early Gamblers are more likely male (P = 0.001), report more active coping (P = 0.042), impulsive behaviors (P ≤ 0.008), and have friends who gamble (P = 0.001). Groups were similar in ECF, parental monitoring, marital status, SES, and race. Early Gamblers had higher incidence of problem behaviors and drug use (all P ≤ 0.006). Two gambling groups were identified in early adolescence with Early Gamblers showing higher levels of impulsivity and comorbid problems but similar levels of ECF compared to Late Gamblers. As more gambling groups are identified through later adolescence, ECF may emerge as a relevant precursor of problem gambling at this later time.
Keywords: Youth gambling, Trajectories, Executive cognitive function, Impulsivity, Adolescence
Introduction
In the past decade, gambling has become one of the most frequently reported addictive behaviors among youth (Gupta and Derevensky 1998; Hurt et al. 2008). Data from studies of youth gambling suggest that 24–40% of adolescents gamble weekly, 10–14% are at risk for gambling problems, and 2–9% meet diagnostic criteria for pathological gambling (Nower et al. 2004; Welte et al. 2008). Of importance, while many young gamblers do not become problem gamblers, early experiences with gambling at ages 8–10 years are associated with greater involvement in gambling as well as problem and pathologic gambling in adulthood (Gupta and Derevensky 1998). Other factors are associated with risk for the development of problem gambling such as gender, SES, parental variables and impulsive tendencies. Pathological gambling in adults has been linked concurrently to poor cognitive function; however, few longitudinal studies of pathological gambling have been completed that assess cognitive function before onset of problem gambling.
In a national survey of adolescents (Barnes et al. 2005), gambling involvement increased with age, and was more likely in participants who were male, not African American and of higher SES. However, African Americans who did gamble did so frequently, and low SES participants who gambled were more likely to be problem gamblers. Youth gamblers frequently engage in other problem behaviors such as drug use, have high levels of comorbid DSM-IV disorders including ADHD and conduct disorder (Hardoon et al. 2004; Kaminer et al. 2002), disruptive family relationships, and problems functioning in academic settings (Derevensky and Gupta 2000; Gupta and Derevensky 1998; Hardoon et al. 2004). Gambling in youth has been suggested to be a means of coping with stress, avoiding or escaping from problems, and/or decreasing boredom, with problem gamblers found to possess poor or maladaptive coping skills and more avoidant coping styles (Bergevin et al. 2006; Derevensky and Gupta 2000; Gupta and Derevensky 2000; Nower et al. 2004). Higher levels of parental monitoring are associated with lower levels of adolescent gambling behaviors but only for youth whose parents do not gamble (McComb and Sabiston 2010; Vachon et al. 2004). Youth who gamble excessively typically begin gambling at home with parents or other relatives, and also report having friends who gamble (Derevensky and Gupta 2000; Gupta and Derevensky 2000).
Youth gamblers who report a tendency toward impulsive behavior, specifically acting without thinking and the inability to delay gratification, may be at risk for problem or pathological gambling in adulthood (Nower et al. 2004). As noted by Nower et al., gambling often involves a high degree of sensory and mental stimulation, raising the likelihood that youth who seek intense and novel forms of sensation may be at risk for developing gambling problems (Nower et al. 2004). In a longitudinal study of predictors of early gambling behaviors, higher levels of teacher-rated impulsive behavior in children at age 5 years predicted a higher propensity toward self-reported gambling behavior in the sixth grade (Pagani et al. 2009). These observations suggest a developmentally continuous effect of impulsivity that places individuals on a trajectory toward gambling involvement in adolescence and adulthood (Pagani et al. 2009; Vitaro et al. 1999; Winters et al. 2002).
Studies in adult pathological gamblers show deficits in executive cognitive functions, such as working memory, cognitive control, and reward processing (Goudriaan et al. 2006). These functions, which encompass a cluster of abilities localized in the prefrontal cortex (PFC), are essential for cognitive and emotional self-regulation. The PFC undergoes a prolonged period of postnatal maturation, continuing through childhood, into adolescence, and beyond (De Luca et al. 2003; Huizinga et al. 2006; Luciana et al. 2005). Specifically, skills associated with cognitive control and reward processing reach adult levels by early adolescence whereas skills associated with working memory continue to develop through adolescence. Coordination of each of these skills become increasingly refined and well coordinated through later adolescence as demonstrated by studies showing improvements in task performance through at least age 20. These refinements in executive functions are part of a critical stage of development when levels of impulsive behaviors increase at a rapid rate, leading to an increased tendency for engagement in risk behaviors (Romer et al. 2011). The role of the PFC in impulse control and its late maturation suggest that the relatively high levels of risky behavior seen in adolescence may reflect immature prefrontal executive function (Romer et al. 2009, 2011; Steinberg 2008).
Adult gambling studies have investigated whether executive cognitive functions are impaired in pathological gamblers compared to control participants. For example, Goudriaan et al. reported that pathological gamblers showed diminished performance on inhibition, time estimation, cognitive flexibility, and planning tasks compared to control participants (Goudriaan, et al. 2006), with Roca et al. reporting impairment in decision making, inhibitory control, memory, and verbal fluency (Roca et al. 2008). Cavedini et al. found that pathological gamblers exhibited decision-making impairments similar to those of frontal lobe damaged patients (Brand et al. 2005; Cavedini et al. 2002). The executive cognitive function deficits observed in pathological gamblers are thought to reflect dysfunction in PFC circuitry (Brand et al. 2005; Goudriaan et al. 2006). Directionality of this relationship, however, has not been determined as few studies have compared executive cognitive functions in gamblers before pathological gambling is diagnosed.
For this report, we examined executive cognitive functions in relation to gambling in a community sample of youth first assessed at ages 10–12 years. Our objectives were: 1) to identify trajectories of gambling behavior; 2) to examine executive cognitive functions (in particular: working memory, cognitive control, and reward processing) in relation to gambling trajectories; and 3) to identify associated problem behaviors. We hypothesized that: 1) early gamblers, identified in our community sample of youth, would have lower scores on measures of working memory, cognitive control, and reward processing, independent of other previously identified risk factors for gambling; and 2) early gamblers would have higher rates of other problem behaviors.
Methods
Participants
Participants were 387 youth, enrolled at ages 10–12 years in a longitudinal, multi-cohort study of risk behavior, impulsivity and executive cognitive functions. Seventy percent of the subjects attended seven Philadelphia schools where onsite enrollment occurred. The remaining 30% attended other Philadelphia area schools and were recruited through flyers distributed at schools and posted in local venues such as libraries. Parental consent and youth assent were obtained in accordance with the protocol approved by the IRB of The Children’s Hospital of Philadelphia. Youth were reimbursed for their time and travel.
Three hundred and eighty seven children were enrolled. The resultant cohort is 49% male. Sixty-four percent of subjects are White, 24% are African American, 9% are Asian, and 3% are other (including 10 who self-identify as both African American and White). Nine percent are Hispanic. Subject households consist of a median of 4 individuals, and 64% of subjects have caregivers who are married. The SES of our cohort is 40.6 ± 15.4 (the inverted mean) which corresponds to the lower range of middle-class (Hollingshead and Redlich 2007). Median years of parental education is 14. Three hundred and seventy three participants (96%) completed the second assessment and 367 (95%) completed the third assessment. At Assessment 1, participant mean age was 11.4 ± 0.9, Assessment 2, 12.6 ± 0.9, and at Assessment 3, 13.5 ± 0.9 years. At enrollment, 10% were in grade 4, 49% in grade 5, 26% in grade 6, and the rest (15%) in grade 7.
Procedure/Measures
At each annual assessment, participants were tested one-on-one by examiners trained by psychologists to administer all questionnaires and tasks in a standardized manner. Demographic information was collected using semi-structured interview at each assessment. Tasks were administered using pencil and paper as well as touch-screen laptops using e-Prime (Schneider et al. 2002) and MediaLab (Jarvis 2004) and audio-computer assisted self-interviewing (ACASI) method of both visual and aural presentation to ensure maximum comprehension and confidentiality (Metzger et al. 2000).
Gambling
Frequency and diversity of gambling for money were assessed through self-report questionnaires inquiring about seven types of gambling behaviors, such as playing cards, placing bets on sports, and purchasing lottery tickets. Lifetime experience with gambling was assessed with yes/no questions such as “Have you ever bet on cards for money?”. Subjects responding “yes” to lifetime questions completed a more detailed assessment of frequency of gambling including questions such as, “How often do you place bets on sports?”, rated on a 7-point scale with responses ranging from “tried only once” to “daily”. For each annual assessment, responses to the gambling questions were collapsed to form one variable with three possible conditions: no gambling, no recent gambling, and recent gambling in the last 30 days.
Executive Cognitive Function
Three frontal systems associated with executive cognitive functions were evaluated at each of the assessments: working memory, which is the ability to hold the goals of a complex task in mind; cognitive control, which is responsible for inhibitory control and conflict monitoring; and reward processing, which is the use of positive and negative feedback to guide responses. For Assessment 1, nine tasks were administered, four for working memory (Letter Two-Back, Corsi Block Tapping, Digit Span from Wechsler Intelligence Scale for Children-Fourth Edition [WISC-IV], and Spatial Working Memory), three for cognitive control (Counting Stroop, Eriksen Flanker, and Stop Signal), and two for reward processing (Reversal Learning and Balloon Analogue Risk Task [BART]) (Romer et al. 2009). All tasks were administered using e-Prime, except for two: 1) the Digit Span task from the WISC-IV (Wechsler 2003), which was administered in standard Wechsler aural format using paper and pencil; and 2) the BART, which was administered using software available from Lejuez (Hunt et al. 2005; Lejuez et al. 2002, 2003). At Assessment 2, because of concern for ceiling effects on the Letter Two-Back task, we added the Object Two-Back, an alternative and more difficult form of the Two-Back. For statistical analyses, a Working Memory composite score was created using the mean of the z-scores for each task which were computed using winsorized raw scores from all assessments. We did not compute composite scores for the Cognitive Control or Reward Processing tasks because the component tasks were not correlated.
Impulsivity
As described previously in Romer et al. (2009), two dimensions of impulsivity were measured: Acting without Thinking and Sensation Seeking. Acting without Thinking, which is characterized by quick cognitive decisions and lack of planning, was assessed using 13 yes/no questions from the Eysenck I7 Junior Impulsivity Subscale. Sensation Seeking, which is the desire for novel, complex, and intense experiences and willingness to take risks for the sake of such experiences, was measured on a 4-point scale with items ranging from “strongly agree” to “strongly disagree” using the 4 questions of the Reduced Brief Sensation Seeking Scale.
Parental Monitoring/Mentoring
Parental monitoring and mentoring were assessed using a twenty-item questionnaire tapping behaviors such as: 1) “Do your parents or the adults who take care of you expect you to call them if you are going to be late?” (rated on a 4-point scale with items responses ranging from “all of the time” to “never”); and 2) “How often do you talk with your parents or the adults who take care of you about the use of drugs?” (rated on a 3-point scale, “never”, “sometimes”, or “often”) (Griffiths 2003). Items were coded so that higher scores indicate more parental monitoring/mentoring.
Peer Influences
Questions derived from the Annenberg National Survey of Youth (Griffiths 2003) inquired about peer involvement in and approval of risk behaviors, such as: 1) “Of your friends and people your age that you spend time with, how many gamble for money such as playing the lottery, or betting on sports, or a card game?”, with a 5-point response ranging from “none” to “all”; and 2) “How do your friends feel about gambling for money such as playing the lottery or betting on sports or a card game?”, with a 4-point response ranging from “mostly disapprove” to “mostly approve”. For each annual assessment, ratings for the peer involvement and approval questions were summed, and the mean of these sums over the 3 assessments was used as a covariate in the main analysis. These peer influence scores were categorized as Low (bottom 25%), Moderate (middle 50%), or High (top 75%).
Coping
Eight questions adapted from Compas et al. assessed participant coping strategies (Compas et al. 2001). Frequency of five active coping strategies (e.g., “When people make you upset, how often do you try to get support from a friend or a family member?”) and three non-active coping strategies (e.g., “When people make you upset, how often do you just take things as they are and go with the flow?”) were assessed. Participants responded using a 4-point scale with response ranging from “all of the time” to “never”. Sums for active and non-active coping were computed and used as covariates in analysis.
Drug Use
Drug use behaviors were assessed using questions derived from the CDC’s State and Local Youth Risk Behavior Survey (YRBS) (Centers for Disease Control and Prevention 2003) and the Monitoring the Future Study (MTF) (Johnston et al. 2006). Drug use items included assessment of last 30 days and lifetime use of alcohol, marijuana, cigarettes, inhalants, chewing tobacco, cigars, methamphetamine, Ritalin, steroids, ecstasy, LSD, heroin, cocaine, and any IV drug use. For each substance, use in the last 30 days was coded as “1” and non-use was coded as “0”. These 14 individual scores were added together to create an overall recent drug use total score. Drug use trajectories used in analyses were based on this total score.
Problem Behaviors
Problem behaviors other than drug use were assessed with the Youth Self-Report (YSR) of the Achenbach System of Empirically Based Assessment (Achenbach and Rescorla 2001), which provides standardized ratings of Internalizing and Externalizing behavior problems. The Internalizing scale reflects problems with the self such as anxiety, depression, somatic complaints and social withdrawal. The Externalizing scale reflects problems with others such as aggressive and rule breaking behaviors. The YSR has very good reliability (Cronbach’s Alpha = 0.90) and is used extensively to identify clinically significant problem behaviors in youth. For analysis, scores collected at the last assessment were used and classified dichotomously as Normal (T score <60) or in the Borderline or Clinical range (T scores ≥60) based on YSR norms (Achenbach and Rescorla 2001).
Statistical Analyses
Group-based-trajectory modeling methods developed by Nagin et al. were used to group participants following similar developmental patterns for gambling (Jones et al. 1998; Nagin 1999; Nagin and Tremblay 2001). Trajectory modeling was also used to group participants following similar developmental patterns for executive cognitive functions, impulsivity, and drug use. Preliminary bivariate analyses, including Chi-square, t-tests, and logistic regression, were conducted to examine the relation between gambling group membership and important covariates. Finally, in our main analysis, a multivariable logistic regression model was fitted with gambling trajectory group as outcome and variables including all executive cognitive function and impulsivity trajectory groupings and other measured covariates that were significant at P ≤ 0.10 in preliminary bivariate analyses. A backward selection algorithm was applied.
Results
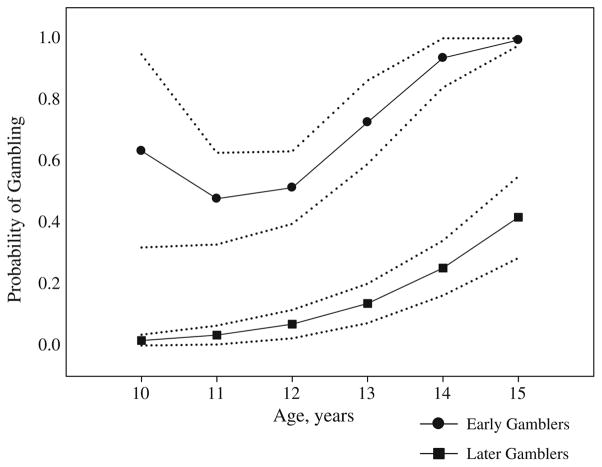
Using the collapsed variable for self-reported gambling for money (no gambling, no recent gambling, and recent gambling in last 30 days) for each of the three assessments, two gambling trajectories were identified as shown in Fig. 1. The first trajectory group (n = 111), termed Early Gamblers, constituted 29% of the sample. By definition, 100% of Early Gamblers reported some gambling behavior. A second trajectory group (n = 276), termed Later Gamblers, constituted 71% of the sample. Of this group, 92% reported no gambling at the first two of the three assessments, and 65% reported no gambling behavior at any assessment. Early Gamblers had a higher probability of gambling at age 10 and by age 15 their probability of gambling was 1.0, with Later Gamblers’ probability of gambling being 0.4 by age 15. The most frequently reported gambling behaviors were betting money on cards (27%) and sports (24%).
Fig. 1.
Trajectories of gambling for money in 387 adolescents calculated using methods of Nagin et al. (Jones et al. 1998; Nagin 1999; Nagin and Tremblay 2001). Shown are model estimates and upper and lower 95% confidence intervals (dotted lines) for Early Gamblers (closed circle) and later gamblers (closed square)
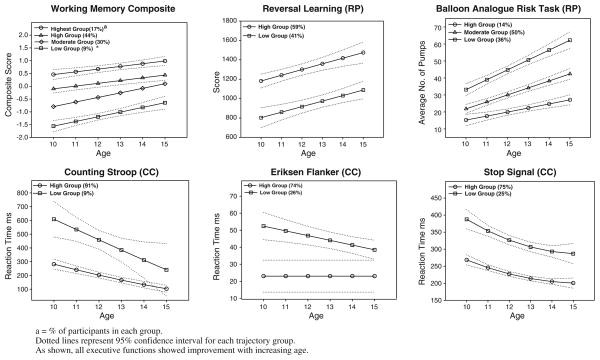
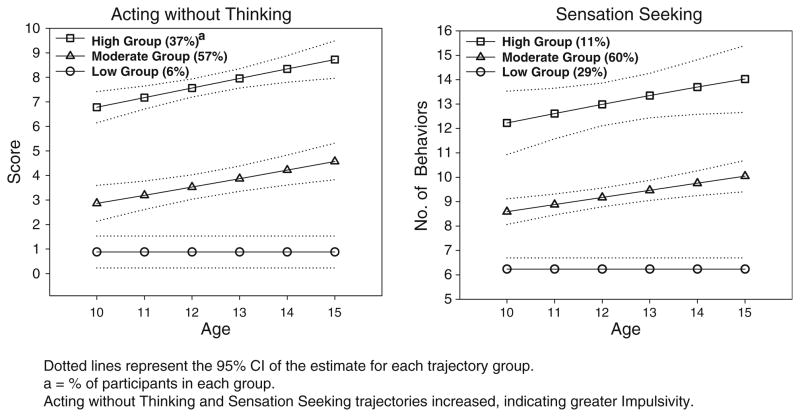
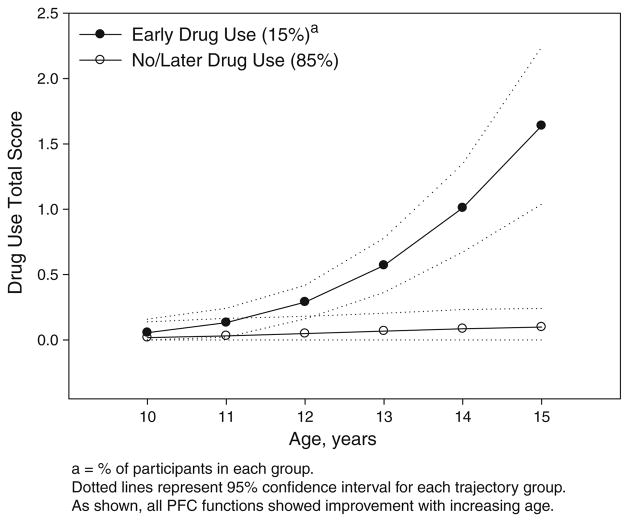
Trajectories for executive cognitive function are shown in Fig. 2. As shown, four trajectory groups were identified for the Working Memory composite and three groups for the Balloon Analogue Risk Task. For each of the other four executive cognitive function tasks, two trajectory groups were identified. All groups showed improvement with increasing age. Figure 3 shows trajectories for Acting without Thinking and Sensation Seeking. Three groups were identified for each of these two impulsivity measures. Figure 4 shows the two identified trajectories of Drug Use in our cohort. For analysis, trajectory groupings for the Working Memory composite, Balloon Analogue Risk Task, Acting without Thinking and Sensation Seeking were dichotomized, with groups representing the highest level compared to the remaining groups combined.
Fig. 2.
Trajectories of executive cognitive function
Fig. 3.
Trajectories of impulsivity
Fig. 4.
Trajectories of drug use
For descriptive purposes the results of our preliminary analyses examining the bivariate relations between gambling trajectories and important covariates are shown in Tables 1 and 2. Variables found to be related to gambling group status were gender, parental monitoring, peer influence, coping style, drug use and impulsivity. Race, SES, parental marital status and executive function were not related to gambling group status.
Table 1.
Bivariate relation between gambling trajectories and covariates
| Early gamblers | Later gamblers | P-value | |
|---|---|---|---|
| n | 111 | 276 | |
| Gender, Male | 75 [67]a | 114 [41] | <0.001 |
| Race, White | 75 [68] | 172 [62] | 0.35 |
| SESb | 39.5 ± 14.1c | 41.0 ± 15.9 | 0.37 |
| Parental marital status, married | 67 [64] | 180 [67] | 0.54 |
| Parental monitoring/mentoring | 2.6 ± 0.4 | 2.7 ± 0.3 | 0.023 |
| Peer influences | 4.9 ± 1.4 | 3.4 ± 1.2 | <0.001 |
| Coping | |||
| Active | 12.0 ± 3.1 | 12.7 ± 3.2 | 0.039 |
| Non-active | 5.3 ± 1.2 | 5.4 ± 1.2 | 0.22 |
n [%]
Hollingshead 2-factor index
Mean ± SD
Table 2.
Bivariate relation between gambling trajectories and trajectories of drug use, impulsivity, and executive function measures
| ORa | 95% CIb | P-value | |
|---|---|---|---|
| Drug usec | 2.02 | 1.14, 2.90 | <0.001 |
| Acting without thinkingc | 3.76 | 2.44, 5.81 | <0.001 |
| Sensation seekingc | 3.66 | 2.39, 5.59 | <0.001 |
| Executive cognitive functionc | |||
| Working memory | |||
| Composite score | 0.78 | 0.42, 1.44 | 0.43 |
| Cognitive control | |||
| Counting stroop | 1.04 | 0.48, 2.25 | 0.92 |
| Eriksen Flanker | 1.12 | 0.67, 1.86 | 0.67 |
| Stop signal | 1.32 | 0.80, 2.16 | 0.28 |
| Reward processing | |||
| Reversal learning | 0.77 | 0.50, 1.21 | 0.26 |
| BART | 1.13 | 0.81, 1.57 | 0.46 |
Early gamblers coded 1, later gamblers coded 0
Odds ratio
Confidence intervals
High trajectory coded as 1, low and moderate coded as 0
Variables found to be associated with Gambling trajectory group with a P-value less than or equal to 0.10 were included in the final regression model (Table 3). After backward selection, male gender, higher active coping, higher levels of peers influence, and higher levels of impulsivity, both Acting without Thinking and Sensation Seeking, increased odds of being in Early Gambling group. Of note, the relationship between active coping and gambling group was inverted from that seen in bivariate analyses. Further modeling showed that there was an interaction among peer influence, gambling trajectory group, and active coping. Subjects with lower levels of peer influence had high active coping, regardless of gambling trajectory group. Those with higher levels of peer influence had lower active coping overall, however, within that group, Early Gamblers had higher active coping than Late Gamblers (data not shown).
Table 3.
Final step of multivariate model predicting gambling group membership
| ORa | 95% CIb | P-value | |
|---|---|---|---|
| Genderc | 2.96 | 1.67–5.26 | <0.001 |
| Active coping | 1.10 | 1.00–1.21 | 0.042 |
| Peer influences | 1.92 | 1.52–2.42 | <0.001 |
| Acting without thinkingd | 2.07 | 1.02–3.56 | 0.008 |
| Sensation seekingd | 2.10 | 1.29–3.44 | 0.003 |
Backward logistic regression
Early gamblers coded 1, later gamblers coded 0
Odds ratio
95% confidence intervals
Males coded 1, females coded 0
High trajectory coded as 1, low and moderate coded as 0
The relationship between gambling trajectory group and problem behaviors measured in the YSR are shown in Table 4. Using this instrument, Early Gamblers were approximately twice as likely as Late Gamblers to be in the YSR Borderline or Clinical range for both Internalizing and Externalizing problems (P ≤ 0.003).
Table 4.
Prevalence of problem behaviors in youth gamblers
| Early gamblers (n = 111) | Later gamblers (n = 276) | P-value | |
|---|---|---|---|
| Internalizing Scale—Borderline or Clinical Range | 28 [26]a | 35 [14] | 0.003 |
| Externalizing Scale—Borderline or Clinical Range | 35 [33] | 40 [15] | <0.001 |
Achenbach System of Empirically Based Assessment—Youth Self-Report
n [%]
Discussion
Many studies examine the risk factors associated with youth gambling such as impulsivity, delinquency, and other DSM-IV disorders including substance use disorders. Fewer studies examine the association between executive cognitive function and youth gambling. The objectives of this report were to identify early trajectories of youth gambling in our cohort, to examine their relationships to executive cognitive function, and to identify associated problem behaviors.
In our community sample of youth assessed longitudinally, trajectory modeling yielded two gambling groups: Early Gamblers and Late Gamblers. While two distinct groups were identified after three assessments, data collection is ongoing and as shown in studies of other problem behaviors, it may be that modeling that includes older ages will yield more groups, including those youth who never engage in gambling behaviors, others who show early gambling experimentation but declining engagement at older ages and others who develop gambling behaviors that are pathological (Nagin 1999; Romer 2003). Future assessments will include the South Oaks Gambling Screen (SOGS), an instrument used for identification of pathological gambling behaviors (Stinchfield 2002). Using results of the SOGS in trajectory analyses will allow for valid identification of a problem gambler group in older adolescents.
The second objective in this study was to assess executive cognitive function in relation to gambling behaviors. We measured executive cognitive function using a number of tasks designed to tap areas shown by others to be compromised in adult pathological gamblers. We were surprised that Early Gamblers and Late Gamblers did not differ in working memory, cognitive control, or reward processing at the ages studied. There are at least two possible explanations for this finding. First, our measure of youth gambling behaviors is not an indicator of pathology, thus our Early Gamblers group likely includes a subset of youth who gamble but will not develop gambling behaviors that are problematic. As stated above, it is likely that modeling data from older adolescents will yield more distinct groups, including problem gamblers with a distinct profile of executive cognitive function that is consistent with those reported for adult gamblers. Second, executive cognitive function is not fully mature at ages examined in this study. Cognitive control and reward processing skills have likely reached adult levels for children up to age 13–15 with refinements that extend into later adolescence and working memory skills continue to develop into young adulthood (De Luca et al. 2003; Huizinga et al. 2006; Luciana et al. 2005). We speculate that executive cognitive function may emerge as a relevant factor in problem gambling as more distinct groups of gamblers emerge and executive function reaches peak developmental levels during later adolescence.
Our assessments of executive cognitive function to date will allow for later determination of the cognitive correlates of problem gambling behaviors and will inform regarding directionality of the relations between cognitive function and problem gambling observed in studies of older gamblers. It may be that problem gambling is a result of impairments in cognitive function related to impulsivity and self-regulation. On the other hand, it may be that patterns of cognitive stimulation associated with problem gambling induce changes in developmental pathways leading to compromised functioning of executive cognitive function closely associated with decision making and impulse control (Crockford et al. 2005). As the children in our study reach later adolescence, these relationships will be able to be more fully delineated.
Pathological gambling is associated with poor impulse control and the changes in the brain’s system of reward and aggression. Longitudinal tracking of cohorts such as ours, that are evaluated prior to the onset of problem levels of gambling, allows for determination of whether differences in impulse control and reward processing precede or follow more serious problem gambling behaviors. We assessed two dimensions of impulsivity, Acting without Thinking and Sensation Seeking, finding that Early Gamblers showed higher levels of both dimensions. This is consistent with studies of older subjects that measure impulsivity in relation to gambling behavior (Nower et al. 2004) but few studies measure impulsivity in relation to gambling at ages as young as 10–12 years old. Vitaro et al. examined a large low SES community sample of male adolescents across ages 12–15 using teacher and self-report ratings of impulsivity (Vitaro et al. 1998). They found that adolescents with the highest levels of impulsivity at earlier ages were more likely to have comorbid gambling and drug-use problems at age 17. Generalizability of these findings is limited to low SES males, and because early gambling behavior was not assessed, it is unclear how early gambling influenced emergence of later problem gambling. While gambling behaviors exhibited by the young adolescents in our study cannot be characterized as pathological, the early relations between gambling behaviors and impulsivity are similar to those found in older adolescents.
Similar to others (Hardoon et al. 2004; Vitaro et al. 1998), Early Gamblers in our young cohort were more likely to exhibit higher levels of internalizing and externalizing behaviors, placing the youth at increased risk for other problems. As noted above, Vitaro et al. found that the most impulsive adolescents were those reporting comorbid problems of gambling and drug use (Vitaro et al. 1998). Further analysis is needed to confirm this in our cohort, as we did not compare rates of drug use and other risk behaviors in our Early Gamblers and Late Gamblers groups. In addition, while our evaluation of gambling behaviors included frequency and diversity of gambling, we were not able to determine the level of pathology because we did not assess consequences of gambling. Despite this limitation, our findings support others showing increased levels of impulsivity and higher rates of other problem behaviors in youth who gamble.
A finding that we consider noteworthy is the interaction among gambling trajectory group membership, coping behaviors, and peer influences. In bivariate analyses, we found that Early Gamblers were more likely than Late Gamblers to have higher levels of peer influences and lower active coping strategies. These findings are consistent with the research suggesting that youth problem gamblers use gambling as a means of coping with stress, avoiding problems, and alleviating boredom (Gupta and Derevensky 2000; Nower et al. 2004). A review by Gupta and Derevensky states that 44% of adolescents participated in gambling activities because their friends were in engaged in similar practices. These findings suggest that a strong social learning and peer-modeling component is involved in the acquisition of gambling behaviors where quality friendships are often lost and replaced by newly acquired friends or gambling associates (Derevensky and Gupta 2000; Romer 2003). Our multivariate analysis revealed an interaction among these three variables, but the effect of active coping was statistically unstable, thus no strong conclusions can be drawn. Our findings suggest the need for careful examination of the possible interactions that exist between gambling and these two important variables that have been found by others to be closely related to adolescent gambling behaviors.
Our study examined executive cognitive function in youth at an age when gambling behavior may first occur. Although we found no difference in executive cognitive function between youth with early and later initiation of gambling, we speculate that differences may emerge in our cohort as they progress through adolescence. Continued follow-up will allow assessment of the directionality of the relation between executive cognitive function and pathologic gambling.
Acknowledgments
The project described was supported by Grant Numbers R01DA018913 from the National Institute on Drug Abuse and UL1-RR-024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Laura M. Betancourt, Email: betancourtl@email.chop.edu, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA
Nancy L. Brodsky, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA
Caitlin A. Brown, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA
Kathleen A. McKenna, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA
Joan M. Giannetta, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA
Wei Yang, Department of Biostatistics and Epidemiology, The University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Daniel Romer, Institute for Adolescent Risk/Annenberg Public Policy Center, The University of Pennsylvania, Philadelphia, PA, USA.
Hallam Hurt, Division of Neonatology, The Children’s Hospital of Philadelphia, 3535 Market St., Room 1433, Philadelphia, PA 19104, USA.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Barnes GM, Welte JW, Hoffman JH, Dintcheff BA. Shared predictors of youthful gambling, substance use, and delinquency. Psychology of Addictive Behaviors. 2005;19(2):165–174. doi: 10.1037/0893-164X.19.2.165. [DOI] [PubMed] [Google Scholar]
- Bergevin T, Gupta R, Derevensky J, Kaufman F. Adolescent gambling: Understanding the role of stress and coping. Journal of Gambling Studies. 2006;22:195–208. doi: 10.1007/s10899-006-9010-z. [DOI] [PubMed] [Google Scholar]
- Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision-making impairments in patients with pathological gambling. Psychiatry Research. 2005;133(1):91–99. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biological Psychiatry. 2002;51(4):334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2003 state and local youth behavior survey. 2003 http://www.cdc.gov.
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127(1):87–127. [PubMed] [Google Scholar]
- Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biological Psychiatry. 2005;58(10):787–795. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, et al. Normative data from the CANTAB. I: development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Derevensky JL, Gupta J. Youth gambling: A clinical and research perspective. e-Gambling: The Electronic Journal of Gambling Issues. 2000;2:1–10. [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101(4):534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Griffiths M. Adolescent gambling: Risk factors and implications for prevention, intervention, and treatment. In: Romer D, editor. Reducing adolescent risk-toward an integrated approach. Thousand Oaks, California: Sage Publications, Inc; 2003. pp. 223–238. [Google Scholar]
- Gupta R, Derevensky JL. Adolescent gambling behavior: A prevalence study and examination of the correlates associated with problem gambling. Journal of Gambling Studies. 1998;14(4):319–345. doi: 10.1023/a:1023068925328. [DOI] [PubMed] [Google Scholar]
- Gupta R, Derevensky JL. Adolescents with gambling problems: From research to treatment. Journal of Gambling Studies. 2000;16(2–3):315–342. doi: 10.1023/a:1009493200768. [DOI] [PubMed] [Google Scholar]
- Hardoon KK, Gupta R, Derevensky JL. Psychosocial variables associated with adolescent gambling. Psychology of Addictive Behaviors. 2004;18(2):170–179. doi: 10.1037/0893-164X.18.2.170. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: a community study. 1958. American Journal of Public Health. 2007;97(10):1756–1757. doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV. Construct validity of the Balloon Analog Risk Task (BART): Associations with psychopathy and impulsivity. Assessment. 2005;12(4):416–428. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta JM, Brodsky NL, Shera D, Romer D. Gambling initiation in preadolescents. Journal of Adolescent Health. 2008;43(1):91–93. doi: 10.1016/j.jadohealth.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Jarvis B. MediaLab (Version 2004.2.87) New York: Empirisoft; 2004. [Google Scholar]
- Johnston LD, Bachman JG, O’Malley PM. Monitoring the future: Questionnaire responses from the nation’s high school seniors. Ann Arbor: Institute for Social Research; 2006. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. 1998 available at www.stat.cmu.edu/~bjones/traj.html.
- Kaminer Y, Burleson JA, Jadamec A. Gambling behavior in adolescent substance abuse. Substance Abuse. 2002;23(3):191–198. doi: 10.1080/08897070209511489. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- McComb JL, Sabiston CM. Family influences on adolescent gambling behavior: A review of the literature. Journal of Gambling Studies. 2010;26(4):503–520. doi: 10.1007/s10899-010-9181-5. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Koblin B, Turner C, Navaline H, Valenti F, Holte S, et al. Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. American Journal of Epidemiology. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological Methods. 1999;4(2):139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nower L, Derevensky JL, Gupta R. The relationship of impulsivity, sensation seeking, coping, and substance use in youth gamblers. Psychology of Addictive Behaviors. 2004;18(1):49–55. doi: 10.1037/0893-164X.18.1.49. [DOI] [PubMed] [Google Scholar]
- Pagani LS, Derevensky JL, Japel C. Predicting gambling behavior in sixth grade from Kindergarten impulsivity a tale of developmental continuity. Archives of Pediatrics and Adolescent Medicine. 2009;163(3):238–243. doi: 10.1001/archpediatrics.2009.7. [DOI] [PubMed] [Google Scholar]
- Roca M, Torralva T, Lopez P, Cetkovich M, Clark L, Manes F. Executive functions in pathologic gamblers selected in an ecologic setting. Cognitive and Behavioral Neurology. 2008;21(1):1–4. doi: 10.1097/WNN.0b013e3181684358. [DOI] [PubMed] [Google Scholar]
- Romer D. Reducing adolescent risk toward an integrated approach. Thousand Oaks, CA: Sage; 2003. [Google Scholar]
- Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Yang W, Hurt H. Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Developmental Science. 2011 doi: 10.1111/j.1467-7687.2011.01061.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47(13):2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Steinberg L. A neurobehavioral perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchfield R. Reliability, validity, and classification accuracy of the South Oaks Gambling Screen (SOGS) Addictive Behaviors. 2002;27(1):1–19. doi: 10.1016/s0306-4603(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Vachon J, Vitaro F, Wanner B, Tremblay RE. Adolescent gambling: relationships with parent gambling and parenting practices. Psychology of Addictive Behaviors. 2004;18(4):398–401. doi: 10.1037/0893-164X.18.4.398. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Arseneault L, Tremblay RE. Impulsivity predicts problem gambling in low SES adolescent males. Addiction. 1999;94(4):565–575. doi: 10.1046/j.1360-0443.1999.94456511.x. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Ferland F, Jacques C, Ladouceur R. Gambling, substance use, and impulsivity during adolescence. Psychology of Addictive Behaviors. 1998;12(3):185–194. [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Welte JW, Barnes GM, Tidwell MC, Hoffman JH. The prevalence of problem gambling among U.S. adolescents and young adults: results from a national survey. Journal of Gambling Studies. 2008;24(2):119–133. doi: 10.1007/s10899-007-9086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Stinchfield RD, Botzet A, Anderson N. A prospective study of youth gambling behaviors. Psychology of Addictive Behaviors. 2002;16(1):3–9. doi: 10.1037//0893-164x.16.1.3. [DOI] [PubMed] [Google Scholar]