Abstract
Objective
The value of neoadjuvant chemotherapy (NAC) for the treatment of advanced ovarian cancer has yet to be determined. While NAC may facilitate and simplify complete cytoreduction and reduce the risk of surgery, the delay of surgery related to NAC needs to be balanced against any potential benefit.
Methods
Surveillance, Epidemiology and End-Results (SEER) data linked to Medicare claims were used to identify 6844 women with treated stage III/IV epithelial ovarian cancer (1995–2005). Patients were classified by primary treatment (surgery (PDS) or chemotherapy), and the primary chemotherapy group was characterized as having NAC or palliative chemotherapy (PC) based on whether there was documentation that surgery was recommended. We compared surgical complications and survival between the groups.
Results
4827 (71%) of women were treated with PDS, 958 received NAC (14%) and 1059 (15%) had PC. Only 577 (60%) of women with NAC underwent surgery and they had fewer ostomies (8.5% vs. 19.2%, p<0.001) and fewer infections, gastrointestinal and pulmonary complications than PDS (all p<0.01). Comparing NAC to PDS there was a 16% increase in the risk of death at 2 years (RR 1.16, 95%CI 1.01–1.34) for women with stage III disease and a 15% reduction in the risk for women with stage IV disease (RR 0.85, 95%CI 0.73–0.99).
Conclusions
NAC followed by surgery was associated with fewer surgical complications than PDS. The direction and magnitude of the difference in survival between women receiving NAC and those receiving PDS differed according to the stage of disease and follow up time.
Introduction
Ovarian cancer is an often lethal gynecologic malignancy, resulting in over 13,000 deaths in the United States 2010[1]. Most women with ovarian cancer are diagnosed at an advanced stage of disease (III/IV), and for most, primary debulking surgery (PDS) and platinum-based chemotherapy is considered the standard of care [2, 3]. “Optimal” debulking, is a form of cytoreduction that has been variably defined as the reduction tumor burden to less than 1–2cm of gross residual disease following PDS. Multiple series have demonstrated an inverse relationship between volume of residual disease following surgery and survival [4, 5]. It is unknown to what extent this is related to the biologic characteristics of the tumor or the influence of the operative technique being utilized[6].
The use of neoadjuvant chemotherapy (NAC) to support operative cytoreduction has been proposed as a strategy to improve the likelihood of optimal debulking and to reduce the need for more extensive and higher risk components of operative debulking (e.g. multiple bowel resections, upper abdominal procedures). Critics question whether the necessary delay to surgery that comes with NAC might decrease survival[7]. As a result of this concern, NAC has generally been reserved for patients felt to have high surgical risk profiles or those thought to be at high risk for suboptimal resection[6, 8]. Information about the utility of NAC in the US comes primarily from case series with small numbers of selected patients and these series have yielded conflicting results, depending on the methodology employed [9, 10]. A recent, multinational trial (EORTC-GCG/NCIC-CTG) randomized women with bulky stage IIIC/IV ovarian cancer to either PDS or 3 cycles of NAC followed by interval debulking surgery (IDS) using a non-inferiority design [11]. Postoperative mortality and short term complications were all less common among women in the NAC arm, with no differences in overall survival supporting that NAC was not inferior to PDS. The application of these data to a contemporary US population has been called into question[12] because the reported median survival of 29–30 months for both groups was only comparable to that seen in US trials for patients with suboptimal debulking at the time of primary surgery. Furthermore the frequency of optimal debulking (to <1cm of residual tumor diameter) following PDS in the trial was only 42%, significantly lower than that reported in contemporary US series[13, 14].
To address the use, safety, and utility of NAC in older patients in the community at large we employed the SEER-Medicare database. We examined the use of NAC, the proportion of patients who had IDS following NAC, rates of surgical complications and all-cause survival for women treated with PDS and NAC (with or without IDS).
Methods
Data Source
Data for this analysis came from a linkage between the Surveillance Epidemiology, End Results (SEER) database provided by the National Cancer Institute (NCI) and Medicare healthcare claims records provided by the Center for Medical Services (CMS) [15]. Internal Review Board approval was obtained from the Human Subjects Division of the University of Washington (IRB 37473). Details of the cohort have been previously described in detail[16]. In summary, cases were limited to women over 65 diagnosed from 1995–2005 with pathologically confirmed Stage III/IV invasive epithelial cancer diagnosed prior to autopsy and continuously covered by Medicare parts A+B and not enrolled in an HMO from the 12 months prior to diagnosis and at least 9 months following diagnosis. This analysis was limited to women with evidence of the receipt of either surgery or chemotherapy in their Medicare claims in the year following diagnosis as defined below.
Patient Characteristics and Treatment Identification
Sociodemographic variables were collected from SEER data. SEER registries were categorized according to geographic region. Median household income from zip code of residence was used as a proxy for socioeconomic status (quartiles). Tumor characteristics were determined from SEER. Comorbidity score was determined using claims for the 12 months prior to ovarian cancer diagnosis to calculate the Deyo adaptation [17] of the Charlson comorbidity index [18, 19].
Receipt of ovarian cancer directed surgery and chemotherapy was identified by searching Medicare claims for billing codes as previously reported[16]. The patients who received initial chemotherapy were further stratified by surgical intent at the time of diagnosis as reported in the SEER data. Women who had surgery recommended were labeled as having received neoadjuvant chemotherapy (NAC) and those not recommended surgery were labeled as having received palliative chemotherapy (PC), as previously described[16].
Outcomes
The receipt of IDS following NAC was defined using the same codes as PDS. Identification of additional procedures at the time of the primary surgery was performed by searching for International Classification of Disease-9 codes (ICD-9) in the inpatient billing records (appendix 1). Post operative complications and ostomies were defined as described by Earle et al and included both medical and surgical complications occurring within 30 days of the surgical episode in either outpatient or inpatient claims [20]. Overall survival was defined as the time from the first treatment until death from any cause or until censoring occurred.
Statistical Analysis
A univariate analysis was performed using the chi-square test for the frequency distribution of categorical variables and the t-test for means of continuous variables. We restricted our analysis of outcomes to women in whom at the onset of therapy, surgery was thought to be possible (i.e. excluding those in the palliative chemotherapy group). This was done to mimic the design of the EORTC trial which restricted enrollment to women who were thought to be surgical candidates[11].
Unadjusted Kaplan-Meier survival analysis method was used to compare the stage specific overall survival of women by treatment group [21]. The log-rank test was used to compare Kaplan Meier survival curves. Previous publications have identified women at high risk of surgical mortality based on age, stage and comorbidity score[22]. We grouped women into three risk categories into: Low risk= age <75, any stage with Charleston co-morbidity score<2; Intermediate risk= age <75, any stage with Charleston co-morbidity score ≥2; High risk = age 75+, stage III with Charleston co-morbidity score ≥1 or age 75+, stage IV with any Charleston co-morbidity score. A stratified Kaplan Meier survival curve was then made for women in each of the three risk categories comparing NAC to PDS.
A multivariate Cox proportional hazard regression model was constructed to evaluate the association between overall survival and treatment group, adjusting for confounding variables that were clinically relevant as well as those significant in the univariate analysis. The proportional hazards assumption was tested by examination of Schoenfeld residuals and this analysis revealed a violation of the proportional hazards assumption. A Poisson regression model was used to model the incident rate ratios for the fixed endpoints of survival at 1 and 2 years as the outcomes of interest. Poisson models were adjusted for age, race, histology, year of diagnosis, comorbidity score, socioeconomic status, marital status and geographic region and size. Robust standard errors were used for regressions all tests were two sided with an alpha of 0.05. STATA SE version 11.0 (College Station, TX) was used for all calculations.
Results
Of the 6844 women, 1059 (15.5%) were classified as having palliative chemotherapy (surgery not recommended) and were not included in the analysis. Of the remaining 5785 women, 4827 (83.4%) had PDS and 958 received NAC (16.6%). Demographic, clinical and pathologic characteristics of the women undergoing PDS and NAC varied considerably (Table 1). Women treated with NAC were more likely to have stage IV disease than those receiving PDS (47% vs. 33%) and were more often from regions with higher median incomes and from metropolitan areas than those treated with PDS. There was no difference in the age distribution or comorbidity scores between the groups.
Table 1.
Demographic, Clinical and Pathologic Characteristics by Treatment Group*
| Primary Debulking Surgery (PDS) n (%) |
Neoadjuvant Chemotherapy (NAC) n(%) |
|
|---|---|---|
| No. of patients | 4,827 | 958 |
|
| ||
| Age (years) | ||
| 65–69 | 1066 (22.08) | 188 (19.62) |
| 70–74 | 1383 (28.65) | 268 (27.97) |
| 75–79 | 1282 (26.56) | 270 (28.18) |
| 80–84 | 731 (15.14) | 171 (17.85) |
| 85+ | 365 (7.56) | 61 (6.37) |
|
| ||
| Race | ||
| White | 4352 (90.16) | 843 (88.00) |
| Black | 230 (4.76) | 60 (6.26) |
| Other | 174 (3.60) | 39 (4.07) |
|
| ||
| Median Household Income | ||
| First Quartile | 1140 (23.62) | 176 (18.37) |
| Second Quartile | 1158 (23.99) | 224 (23.38) |
| Third Quartile | 1136 (23.53) | 260 (27.14) |
| Fourth Quartile | 1194 (24.74) | 246 (25.68) |
|
| ||
| Marital Status | ||
| Married | 2190 (45.37) | 502 (52.40) |
| Not Married | 2499 (51.77) | 431 (44.99) |
|
| ||
| Region | ||
| Northeast | 933 (19.33) | 196 (20.46) |
| Midwest | 1016 (21.05) | 193 (20.15) |
| South | 677 (14.03) | 121 (12.63) |
| West | 2201 (45.60) | 448 (46.76) |
|
| ||
| Area of Residence | ||
| Large Metropolitan | 2762 (57.22) | 579 (60.44) |
| Metropolitan | 1290 (26.72) | 256 (26.72) |
| Urban | 293 (6.07) | 67 (6.99) |
| Less Urban | 381 (7.89) | 13 (1.36) |
| Rural | 101 (2.09) | 13 (1.36) |
|
| ||
| Stage | ||
| III | 3401 (65.05) | 482 (50.31) |
| IV | 1599 (33.13) | 454 (47.39) |
|
| ||
| Grade¥ | ||
| Low | 871 (18.04) | 98 (10.22) |
| High | 2983 (61.80) | 426 (44.47) |
| Unknown | 970 (20.10) | 434 (45.30) |
|
| ||
| Histology | ||
| Serous/Adenocarcinoma | 3674 (76.11) | 797 (83.19) |
| Mucinous | 197 (4.08) | 25 (2.61) |
| Endometroid | 304 (6.30) | 21 (2.19) |
| Clear Cell | 78 (1.62) | 13 (1.36) |
| Other Epithelial | 574 (11.89) | 102 (10.65) |
|
| ||
| Comorbidity Score | ||
| 0 | 3271 (67.76) | 615 (64.20) |
| 1 | 1023 (21.19) | 217 (22.65) |
| 2 | 326 (6.75) | 76 (7.93) |
| 3+ | 207 (4.29) | 50 (5.22) |
Not all totals add up to 100% because of rounding and missing data,
low grade= grade 1 or 2 according to the SEER data; high grade = grade 3 or 4 as listed in SEER
Of the 958 women treated with NAC, 577 (60.2%) had IDS in the year following diagnosis. The median time to surgery was 17 weeks and patients had a median of 4 cycles of chemotherapy prior to surgery. 89% of these women received additional chemotherapy following surgery with a median of 6 additional cycles. Among the 4827 women treated with PDS, only 75.8% were given chemotherapy following surgery with a median of 6 cycles received.
Characteristics and complications of surgery are listed in Table 2. Women having IDS after NAC were less likely to undergo a small bowel resection (3.8% vs. 6.4%, p<0.001) and almost half as likely to have a large bowel resection (11.1% vs. 20.6%, p<0.001) than those having PDS. Ostomies were performed in 19.2% of women having PDS compared to only 7.8% of those having IDS (p<0.001). There was no difference in the receipt of upper abdominal procedures between the groups. Women having IDS following NAC had an average length of hospital stay that was over 3 days shorter than women having PDS (7.89 days vs. 11.53 days, p<0.001) and they had fewer ICU admissions (28.2% vs. 42.7%, p<0.001). Surgical complications occurred more commonly following PDS than IDS. Specifically, women having PDS had more infections (17.7% vs. 11.4%, p<0.001), gastrointestinal complications (35.3% vs. 29.1%, p=0.003), pulmonary complications (11.2% vs. 3.8%, p<0.001) and more wound infections and disruptions (20.7% vs. 14.2%, p=0.001) than those treated with NAC prior to IDS. When women who were admitted emergently were excluded from this analysis, these results were not significantly changed (data not shown).
Table 2.
Additional Surgical Procedures and Surgical Complications by Treatment Group*
| Primary Debulking Surgery (PDS) n (%) |
Interval Debulking Surgery (IDS)# n(%) |
p value | |
|---|---|---|---|
| No. of patients | 4,827 | 577 | |
|
| |||
| Any Bowel Resection/Ostomy | 1442 (29.88) | 95 (16.46) | <0.001 |
| Small Bowel Resection | 309 (6.40) | 22 (3.81) | 0.014 |
| Large Bowel Resection | 992 (20.56) | 64 (11.09) | <0.001 |
| Ostomy | 928 (19.23) | 45 (7.80) | <0.001 |
| Rectal Resection | 250 (5.18) | 22 (3.81) | 0.156 |
| Any Upper Abdominal Procedure | 194 (4.02) | 17 (2.95) | 0.208 |
| Length of Stay Mean Days (SD) | 11.53 (10.27) | 7.89 (7.17) | <0.001 |
| ICU Stay | 2049 (42.71) | 160 (28.22) | <0.001 |
| Transfusion during surgical stay | 329 (6.86) | 31 (5.47) | 0.211 |
| Surgical Injury | 316 (6.55) | 31 (6.42) | 0.276 |
|
| |||
| 30-Day Complications | |||
| Cardiac | 556 (11.52) | 53 (9.19) | 0.094 |
| Thromboembolic Event | 229 (4.75) | 28 (4.85) | 0.909 |
| General Infections | 854 (17.70) | 66 (11.44) | <0.001 |
| Gastrointestinal | 1701 (35.25) | 168 (29.12) | 0.003 |
| Neurological or Renal¥ | 208 (4.25) | 13 (2.25) | 0.021 |
| Reoperation | 479 (9.93) | 49 (8.49) | 0.273 |
| Pulmonary | 542 (11.23) | 22 (3.81) | <0.001 |
| Wound Infection/Breakdown | 997 (20.66) | 82 (14.21) | 0.001 |
Surgical stay refers to the hospitalization at the time of either primary debulking surgery or interval debulking surgery following neoadjuvant chemotherapy, inpatient claims data for this hospitalization was missing for 41 patients. p values are using Chi2 or t-test comparing PDS to IDS
includes only women who had surgery following neoadjuvant chemotherapy.
combined categories for patient confidentiality due to low numbers.
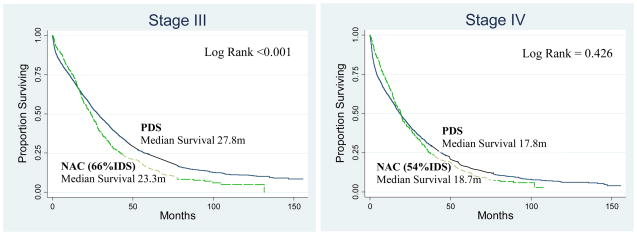
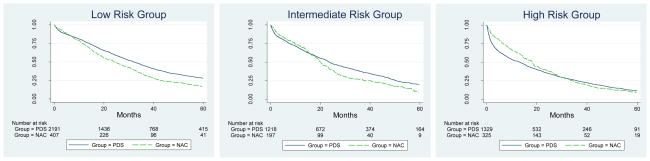
Overall survival by stage comparing women treated with PDS and NAC is illustrated in Figure 1. All women treated with NAC, regardless if they received IDS were included in this survival analysis (“intention to treat” analysis). The unadjusted median survival for patients with stage III disease was slightly longer at 27.8 months for PDS compared to 23.3 months for NAC. The unadjusted median survival for women with stage IV disease was not appreciably different between groups, 17.8 months with PDS compared with 18.7 months for NAC. As seen in Figure 1 both stage III and IV patients have crossover of the survival curves (at 15 and 19 months respectively) which initially favors NAC and subsequently favors PDS. Further stratification the survival curves by risk category (Figure 2) suggest that the timing of this crossover is related to the risk of surgical mortality. The initial benefit of NAC appears to be markedly extended in older patients with stage IV disease, and not present in the youngest women with stage III disease.
Figure 1. Kalpan Meier Survival Curves By Stage and Treatment Group.
PDS: Primary Debulking Surgery, NAC: Neoadjuvant Chemotherapy and % IDS is the proportion of women having Interval Debulking Surgery following NAC.
Figure 2. Kalpan Meier Survival Curves By Risk Group and Treatment.
PDS: Primary Debulking Surgery, NAC: Neoadjuvant Chemotherapy. Risk groups are based on the risk of 30-day surgical mortality: Low risk= age <75, any stage with Charleston co-morbidity score<2; Intermediate risk= age <75, any stage with Charleston co-morbidity score ≥2; High risk = age 75+, stage III with Charleston co-morbidity score ≥1 or age 75+, stage IV with any Charleston co-morbidity score.
Among women with stage III disease, the adjusted risk of death at one year was no different between NAC and PDS (RR 0.9, 95%CI 0.74–1.10). The risk of death at 2 years was increased for women with stage III disease for NAC by 16% compared to PDS (2yr RR 1.16, 95%CI 1.01–1.34). For women with stage IV disease NAC is associated with a reduction of the risk of death at 1 year by 31% (RR 0.69, 0.57–0.83) and 15% at 2 years compared to PDS (RR 0.85, 95%CI 0.73–0.99).
Discussion
The role of NAC in the treatment of advanced ovarian cancer has yet to be determined [8, 23]. This study demonstrates that among older American women for whom surgery was intended, NAC was used 17% of the time during 1995–2005, with only 60% of those treated with NAC going on to receive surgical debulking. For women having interval surgery following NAC, there was a relatively lower incidence of 30-day infections, wound complications, pulmonary and gastrointestinal complications compared with PDS. The impact of NAC on overall survival is a mixed effect and depends on the stage of the patient and the follow up time. Among women with stage III disease, receipt of NAC was associated with no difference in survival at one year, but a 16% decrease in survival at 2 years. NAC was associated with improved survival for stage IV patients of 31% at 1 and 15% at 2 years.
NAC for advanced ovarian cancer in the US has generally been reserved for patients felt to be high risk surgical candidates [6, 23]. In comparative evaluations of NAC and PDS these biases might make patients with NAC appear to have worse outcomes, as not all important differences (e.g frailty and social support) may be adequately measured and adjusted for. Despite this, several observational studies have suggested no difference in survival for women treated with NAC [24, 25]. Our study observed that among women with stage IV disease, there was an improvement in survival at 1 and 2 years between those treated with NAC compared with PDS. Women with stage III disease in this study however, had a 4 month shorter median survival when treated with NAC compared to those receiving PDS, and after adjustment for risk parameters a higher risk of death by 2 and 5 years. This may be related to unmeasured (and therefore unadjusted) higher risk patient characteristics among the group having NAC and/or a direct negative impact of the NAC on patients, perhaps due to the delay of surgery.
Our results indicate that the risk of death following either NAC or PDS is not constant and patients having NAC have an initially improved survival which then crosses over at 15–19 months to favor PDS. The time at which this crossover occurs is notably later than that reported in the randomized trial (2 months). This may be in part due to a difference in the frequency of early post-operative deaths (fewer following NAC), but may also be reflective of other differences between the study populations.
Some series identify patients for inclusion following a hospitalization that typically includes surgery and have found the proportion of women having surgery after NAC to exceed 94%[21, 25, 26]. This identification technique limits our understanding of the role of NAC because women who had only outpatient chemotherapy and were never able to proceed with surgery may not be included. The EORTC trial limited enrolment to women who were felt to be surgical candidates and did not perform IDS if women had disease progression while on NAC, and 88% of women randomized to NAC went on to have IDS[11]. In our series, only 60% of women had evidence of a debulking surgery following NAC. This may have been due to the inadvertent inclusion of some patients in the NAC group who may never have been surgical candidates or relate to the difficulties of assessing who is a surgical candidate before initiating NAC. We employed a technique for identifying patients using the “recommended for surgery” variable in the SEER dataset that was based on whether “surgery was offered”, but it is not known how often this recommendation was made by a clinician with experience in the management of ovarian cancer. The accuracy of the “surgery recommended” variable in SEER has not been assessed, even though this variable has been used by other investigators [27, 28] to aid in the delineation of surgical intent. The inclusion of women in the NAC group for whom surgery was not really a possibility may inappropriately decrease the survival benefit associated with NAC and may also explain why we found worse survival among Stage III patients having NAC. This may also be considered a conservative bias among patients with Stage IV disease where a survival benefit of NAC was observed.
Similarly, the ability to receive at least one cycle of chemotherapy following PDS is over 90% in most series [11, 29]. This is much higher than what was observed in this analysis (76%) and may be due to the older age of this cohort, a greater likelihood of refusal of chemotherapy or being considered too ill to receive chemotherapy, or a difference between care received in trials compared to care in the community. This inability to complete chemotherapy following surgery may account for a lower observed median survival in this analysis for women having PDS (24.1months) compared to that reported in other reports (30–65.6 months)[12]. We also observed a difference in the apparent amount of chemotherapy received between the PDS and NAC groups. While our ability to precisely quantify the number of cycles is limited but the use of billing data to estimate weeks of chemo the data suggests that women treated with NAC have a median number of cycles that is almost twice that of PDS (NAC=10, PDS=6). This difference in the amount of chemotherapy received may contribute to observed outcomes and the inability to explore this further with this observational data set is a limitation of this analysis.
Several studies have reported lower post-operative morbidity for women having IDS following NAC compared with women having PDS[11, 21, 30]. Our study results are consistent with these reports, documenting a lower incidence of many 30-day complications following surgery. The use of less extensive surgical procedures to achieve optimal debulking has also been reported following NAC[31, 32]. Our findings were somewhat consistent with these results, demonstrating women had fewer bowel resections following NAC. However we did not identify a reduction in the use of upper-abdominal procedures, likely due to the limited performance of these procedures in both groups. With these data it is not possible to assess the role that distribution of the tumor burden had (either before or after NAC) on surgical procedures. It is possible that the NAC patients had higher upper abdominal tumor burdens than the PDS group which may have required more upper abdominal procedures and they been treated with PDS. Thus no difference in the rate of these procedures between the groups may actually reflect an improvement due to NAC. The magnitude of the reduction in the need for bowel resections was similar to that observed in the ETORC trial, supporting the notion that this finding is a result of the NAC and not variation in provider behavior among clinicians favoring NAC[11].
There are several important limitations of this study including the restriction of our population to those over the age of 65. From 2003–2007 the median age at diagnosis for ovarian cancer in the US was 63 years, approximately 54.3% of women were under 65 when diagnosed and the findings of this study may not be reflective of their experience [33]. The use of claims data to identify treatment and comorbidity status also has the potential to introduce misclassification. While previous studies have determined a high level of agreement between Medicare data and chart review in the identification of surgery and chemotherapy[34, 35], the accuracy of diagnostic codes is lower for co-morbid conditions and treatment complications[19, 36]. Lastly some important clinical information was unavailable or inconsistently unavailable in a non-random fashion that may account for observed outcomes (e.g. performance status, histology) and we cannot account for the impact of such variables.
In summary our results indicate that NAC is an occasionally employed strategy (17%) for American women over the age of 65 in whom surgery is planned and that many (40%) who start a course of NAC never go on to have ovarian cancer-directed surgery. Our findings support the effectiveness of NAC in reducing surgical complications, an alternative that reduces peri-operative morbidity. The association between NAC and survival differed somewhat according to the stage of disease and follow up time, but the potential for confounding demands caution in the interpretation of these findings. Additional randomized trials are needed to study the role of NAC in the treatment of women with advanced ovarian cancer.
Supplementary Material
7. Highlights.
Among older women neoadjuvant chemotherapy for advanced ovarian cancer was associated with a fewer bowel resections and surgical complications.
Neoadjuvant chemotherapy’s impact on overall survival is a mixed and depends on the stage of the patient and time.
Acknowledgments
This work was supported by the Marsha Rivkin Center for Ovarian Cancer Research. Dr Thrall is the recipient of the Scientific Scholar Award from the Rivkin Center. This work is also supported by the National Cancer Institute (NCI) at the National Institutes of Health, Dr. Thrall is the recipient of an NCI-funded postdoctoral fellowship (T32-CA009515-26).
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
This study used the linked Surveillance Epidemiology, and End Results (SEER)-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Presented as a poster at the Forty Second Annual Meeting of the Society of Gynecologic Oncologists, Orlando, Florida, March 6–9, 2011.
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute NC. Cancer Facts and Figures - 2010. In: Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review 1975–2007. 2010. [Google Scholar]
- 2.National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: screening, treatment, and follow-up. Gynecol Oncol. 1994;55:S4–14. doi: 10.1006/gyno.1994.1333. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. Practice guidelines in Oncology v.2.2010 Ovarian Fallopian Tube and Primary Peritoneal Carcinomas. 2010. [Google Scholar]
- 4.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg LE, Rodriguez G, Hurteau JA. The role of neoadjuvant chemotherapy in treating advanced epithelial ovarian cancer. J Surg Oncol. 2010;101:334–43. doi: 10.1002/jso.21482. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Eisenhauer EL, Santillan A, Chi DS. Delaying the primary surgical effort for advanced ovarian cancer: a systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol Oncol. 2007;104:480–90. doi: 10.1016/j.ygyno.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Dewdney SB, Rimel BJ, Reinhart AJ, Kizer NT, Brooks RA, Massad LS, Zighelboim I. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119:18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–20. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 11.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Brady MF. EORTC 55971: does it apply to all patients with advanced state ovarian cancer? Gynecol Oncol. 2011;120:300–1. doi: 10.1016/j.ygyno.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, Mutch DG, Burger RA, Swart AM, Trimble EL, Accario-Winslow C, Roth LM. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C, Barakat RR. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Thrall MMGH, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in Treatment of Advanced Epithelial Ovarian Cancer in the Medicare Population. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2011.03.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:IV-26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 20.Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, Trimble EL, Bodurka DC, Bristow RE, Carney M, Warren JL. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 21.Hou JY, Kelly MG, Yu H, McAlpine JN, Azodi M, Rutherford TJ, Schwartz PE. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105:211–7. doi: 10.1016/j.ygyno.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, Chi DS, Bristow RE, Cliby WA. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011;120:23–8. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz PE. Contemporary considerations for neoadjuvant chemotherapy in primary ovarian cancer. Curr Oncol Rep. 2009;11:457–65. doi: 10.1007/s11912-009-0062-y. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz PE, Rutherford TJ, Chambers JT, Kohorn EI, Thiel RP. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol. 1999;72:93–9. doi: 10.1006/gyno.1998.5236. [DOI] [PubMed] [Google Scholar]
- 25.McLean KA, Shah CA, Thompson SA, Gray HJ, Swensen RE, Goff BA. Ovarian cancer in the elderly: outcomes with neoadjuvant chemotherapy or primary cytoreduction. Gynecol Oncol. 2010;118:43–6. doi: 10.1016/j.ygyno.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Everett EN, French AE, Stone RL, Pastore LM, Jazaeri AA, Andersen WA, Taylor PT., Jr Initial chemotherapy followed by surgical cytoreduction for the treatment of stage III/IV epithelial ovarian cancer. Am J Obstet Gynecol. 2006;195:568–74. doi: 10.1016/j.ajog.2006.03.075. discussion 574–6. [DOI] [PubMed] [Google Scholar]
- 27.Farjah F, Wood DE, Yanez ND, 3rd, Vaughan TL, Symons RG, Krishnadasan B, Flum DR. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–8. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairfield KM, Lucas FL, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the medicare population. Cancer. 2010 doi: 10.1002/cncr.25242. in press. [DOI] [PubMed] [Google Scholar]
- 29.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, Bristow RE, Chi DS, Cliby WA. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007;107:99–106. doi: 10.1016/j.ygyno.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Morice P, Brehier-Ollive D, Rey A, Atallah D, Lhomme C, Pautier P, Pomel C, Camatte S, Duvillard P, Castaigne D. Results of interval debulking surgery in advanced stage ovarian cancer: an exposed-non-exposed study. Ann Oncol. 2003;14:74–7. doi: 10.1093/annonc/mdg003. [DOI] [PubMed] [Google Scholar]
- 31.Kayikcioglu F, Kose MF, Boran N, Caliskan E, Tulunay G. Neoadjuvant chemotherapy or primary surgery in advanced epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11:466–70. doi: 10.1046/j.1525-1438.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, Bae DS. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. J Obstet Gynaecol Res. 2006;32:99–106. doi: 10.1111/j.1447-0756.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 33.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. Institute NC. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: 2010. [Google Scholar]
- 34.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40:IV-43–8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 35.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 36.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40:IV-62–8. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.