Abstract
Inflammation is a key factor in a number of neurodegenerative diseases including systemic lupus erythematosus (SLE). The complement system is an important mechanism in initiating and amplifying inflammation. Our recent studies demonstrate that C5a, a protein fragment generated during complement activation could alter the blood-brain barrier (BBB) integrity, and thereby disturb the brain microenvironment. To understand the mechanism by which this occurs, we examined the effects of C5a on apoptosis, translocation of nuclear factor-κB (NFκb) and the expression of Iκbα, MAPK, CREB and TJ protein, zona occludens (ZO-1) in mouse brain endothelial cells. Apoptosis was examined by DNA laddering and caspase-3 activity and the distribution of the ZO-1 and the p65 subunit of NFκB were determined by immunofluorescence. Inhibition of CD88 reduced translocation of NFκb into the nucleus, altered ZO-1 at the interfaces of neighboring cells, decreased caspase-3 activity and prevented apoptosis in these cells. Our results indicate that signaling through CD88 regulates the BBB in a NFκb dependent manner. These studies suggest that the C5a receptor, CD88 is a promising therapeutic target that will reduce NFκb signaling cascades in inflammatory settings.
Keywords: blood-brain barrier, complement, C5a, inflammation
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multifactorial neurodegenerative disease associated with a high degree of morbidity and mortality (Carroll 2004; Carroll 1998), with no optimum therapy. The internal milieu of the brain is maintained constant by the blood-brain barrier (BBB), which is disrupted in lupus patients during flares and in experimental models (Abbott et al. 2003; Jacob et al. 2010f; Nishimura et al. 2008; Huerta et al. 2006). The BBB is a complex organization of cerebral endothelial cells (EC) and pericytes surrounded and supported by astrocytes and astrocytic endfeet (Abbott et al. 2006; Brown et al. 2007; Davson H et al. 1993; Davson H and Segal MB 1995). Alteration of the BBB forming cells and/or disruption of the tight junctions can cause increased permeability, leading to “leakiness” resulting in the influx of proinflammatory molecules and cells that upset normal brain function and lead to vascular/neural injury. Mechanism/s for breakdown of the BBB is incompletely understood, but appears to involve direct effects of inflammatory molecules on endothelial regulation of BBB components, as well as indirect effects through cell-mediated injury. Unique endothelial structural features of the BBB include highly organized endothelial tight junctions, the absence of class II major histocompatibility complex, and a highly developed transport system. Exposure to proinflammatory molecules leads to endothelial dysfunction (Ducruet et al. 2009; Jacob et al. 2010c; Flierl et al. 2009). The changes that occur during this process are cytoskeletal remodelling, decreases the brain solute barrier, enhances leukocyte endothelial adhesion and migration and promotes shedding of endothelial ‘microparticles’ (EMP). This process plays an important role in the onset and progression of vascular disease.
The complement (C) cascade normally functions as a protective mechanism. However, when the complement system is excessively activated, the beneficial effects can become detrimental to the host (Carroll and Fischer 1997; Tenner and Fonseca 2006; Barnum 2002; Muller-Eberhard and Schreiber 1980; Muller-Eberhard 1988; Muller-Eberhard 1986). The anaphylatoxin, C5a, is a 74-aa fragment of C5 and binds to the G-coupled receptor, CD88 and the receptor, C5L2. C5L2 can act as a positive modulator of both anaphylatoxins, C3a and C5a (Chen et al. 2007; Flierl et al. 2008), while CD88 is a specific C5a receptor (deVries B. et al. 2003). CD88 is present on the blood cells such as neutrophils (Ibrahim et al. 2004) and platelets that infiltrate the brain in different inflammatory settings, and is also constitutively expressed on several cell types in the brain including endothelial cells (Farkas et al. 1998; Gasque et al. 1997; Gasque et al. 1995; Laudes et al. 2002; Monsinjon et al. 2003). It mediates a number of biological processes, including chemotaxis and degranulation of mast cells and basophils, vascular permeability, an increased generation of reactive oxygen species, and production of cytokines (Guo and Ward 2005). CD88 is down-regulated in the hippocampus of Alzheimer’s patients, but up-regulated in the caudate of Huntington’s patients and in animal models of neurological trauma (Fonseca et al. 2009; Singhrao et al. 1999).
C5a can be either neurotoxic (Ward 2008a; Fonseca et al. 2009; Jacob et al. 2010c) or neuroprotective (Mukherjee and Pasinetti 2001; Niculescu et al. 2003) depending on the setting (Woodruff et al. 2009). There is increasing evidence that C5a promotes pro-inflammatory conditions contributing to cellular apoptosis (Ward 2008b; Jacob et al. 2010b; Nauta et al. 2002; Niculescu et al. 2004). Recent studies have shown that C5a also has an anti-apoptotic effect on neutrophils, during sepsis. Ligation of CD88 activates PI-3K and MAPK survival signaling pathways in neutrophils, thus suppressing the apoptotic response (Guo et al. 2004). Gene expression altered by C5a in endothelial cells includes genes associated with apoptosis (caspase 3, caspase 8, cFLIP). It also causes activation of NFκB directly (Albrecht et al. 2004) or through EGFR activation (Schraufstatter et al. 2002).
In SLE patients, altered levels of the complement activation products, including anaphylatoxins have correlated with a poor outcome (Buyon et al. 1992; Hopkins et al. 1988). In experimental models, we have demonstrated that the inhibition of the complement cascade at different locations, including C5a/CD88 signaling attenuated the disease symptoms in brain (Alexander et al. 2005; Alexander et al. 2007; Alexander et al. 2003; Alexander et al. 2008). More recently, we and others have demonstrated that inhibition of CD88 protected the blood-brain barrier (BBB) integrity. The mechanism by which this occurs remains an enigma and was the goal of this study. We studied the effect of CD88 inhibition in cultured brain endothelial cell line, bEnd3 which emulate the primary endothelial cells. The results of this study demonstrate that signaling through CD88 regulates the translocation of NFκB and the expression of ZO-1 and could potentially lead to apoptosis and altered BBB permeability. Further, manipulation of CD88 would leave the complement cascade intact to perform the normal protective functions; therefore it is a more viable therapeutic candidate compared to a pan complement inhibitor.
MATERIALS AND METHODS
Cells in culture
bEnd3 cells
bEnd3 (American Type Tissue Culture Collection, Rockville, MD), an immortalized mouse brain endothelial cell line was used for these studies. Cells were seeded at a density of 0.5–1.0 × 104 cells/cm2 onto tissue culture-treated plastic ware and grown in DMEM with 4.5 g/l glucose, 3.7 g/l sodium bicarbonate, 4 mM glutamine, 10% FCS and 100 U/ml of penicillin and streptomycin. All cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells stained positively with Alexa 488 labeled PECAM and agglutinin indicating their endothelial characteristics. 2–3 pooled cultures were used, for each mRNA and protein isolation. The values given are the Mean ± S.D. of three independent experiments.
Primary culture
Cerebral microvascular endothelial cells were isolated from two week old C57BL6 mice as described earlier (Jacob et al. 2010c). Briefly, brains were collected in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1% penicillin and streptomycin (Invitrogen). The cortex was cut into small pieces and homogenized using 10 strokes with a dounce homogenizer (0.5 mm clearance). Homogenates were mixed 1:1 with 30% dextran and centrifuged at 3000g for 25 min. The pellet was digested in 3 ml of DMEM/F12 (Invitrogen) containing DNAse (10 μg/ml), and collagenase (2 mg/ml) at 37°C in a shaking water bath for 30 min. The pellet was washed in medium and plated onto collagen IV plated dishes (BD Biosciences, Bedford, MA, USA) and grown in DMED/F12 medium supplemented with 20% plasma derived serum (Animal Tech, Inc.), 2 mM glutamine, 0.5 U/ml heparin, 1 ng/ml basic FGF, penn/strep (100 U/ml) and gentamycin (50 μg/ml). 24 h after plating, red blood cells, cell debris and nonadherent cells were removed by washing with medium. Passage 2 endothelial cells were employed in the present study. Cells stained positively with anti-VE cadherin (Fig 2A) and anti-CD31 (not shown).
Fig 2. Endothelial cells (bEnd3 cells and primary endothelial cells) express CD88.
bEnd3 cells (A) and primary endothelial cells (results not shown) express CD88 (red) by immunofluorescence. Nuclei are stained blue (DAPI). CD88 expression was also assessed by western blotting in both bEnd3 and primary endothelial cells (B). The samples were normalized to actin expression.
Treatment of cells
Cells were treated with lupus serum (collected from 20 week old MRL/lpr mice), CD88 antagonist (1μM of cyclic hexapeptide AcF[OPdChaWR], CD88a; obtained from John Lambris, Pennsylvania (Thurman et al. 2007)) or 50μM pyrrolidine dithiocarbamate, (PDTC) (He et al. 2006), purchased from R & D Systems, MN). CD88a is a small cyclic peptide shown to block CD88 (Short et al. 1999; Finch et al. 1999), while PDTC is a potent NF-κB inhibitor. Once the bEnd3 cells reached confluency, medium was replaced with DMEM (Gibco-BRL, Chagrin Falls, OH) with 2% FBS for synchronization. Cells were then treated for 1 h with NF-κB inhibitor or CD88a. Subsequently, all plates were treated with lupus or control mouse serum and assessed 3 h later.
SDS-PAGE and Western Blot
Confluent cells were subjected to western blotting as described earlier (Jacob et al. 2010b). Cells were washed in PBS and homogenized homogenized in RIPA buffer and protein estimated using BCA reagent (Hill and Straka 1988). Equal amounts of samples were separated by SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore). Membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with chicken anti mouse CD88 (Dr Scott Barnum, Birmingham; 1:1000). Membranes were then incubated with peroxidase-conjugated anti-chicken antibody (Pierce). Chemiluminescent substrate (Pierce) was used to develop signals. Membranes were then stripped followed by probing with anti-actin (Sigma-Aldrich). Controls in which the primary antibody was omitted were negative.
Imaging of cells
Confluent cells were subjected to the different treatments (lupus serum, CD88a + lupus serum, and PTDC + lupus serum) for 3 h as described above. Cells were then fixed with 4% paraformaldehyde for 20 min, washed with PBS and permeabilized with 0.02% Tween-20 in PBS for 10 min. Non-specific binding was blocked by incubating the slides for 20 min with blocking buffer (5% normal goat serum and 3% bovine serum albumin in PBS). Slides were then incubated with Phycoerythrin (PE)-labelled anti-mouse CD88 antibody, clone 20/70 (Biolegend, 1:500), anti-NF-κB p65 (Santa Cruz Biotechnology, 1:500), or anti-ZO-1 (Chemicon, Temecula, CA, USA; 1: 2000) overnight and visualized using alexa-conjugated second antibody (Molecular Probes, Eugene, OR). To ensure specificity, staining was carried out without the primary antibodies. Cells were mounted with ProLong Gold Antifade (Molecular Probes, Eugene, OR) and observed using a Zeiss microscope. For each experiment, confocal microscope settings for image acquisition and processing were identical between control and treated monolayers, and 3 separate, random images were acquired and analyzed for each experimental condition. Images were processed using PhotoShop Imaging software.
Cytokine analysis
Cell homogenates were analyzed for multiple cytokines using the Luminex multianalyte technology (Luminex Corporation, Austin, TX, USA). Levels of MAPK, CREB and IκB, were measured as per the manufacturer’s instructions. Treated and control monolayers were homogenized and supernatants used. Minimum detection limit for the cytokines were 0.2 pg/ml for MAPK and CREB, 0.3 pg/ml for IκB. Levels detected below this limit were considered and reported as undetectable.
Ligase-mediated (LM) PCR
DNA was purified from 2–3 pooled cultures using the DNeasy kit (Qiagen) according to the manufacturer’s instructions. The extent of DNA laddering was amplified and detected by LM-PCR, using a commercially available kit (BD Clontech, Palo Alto, CA). To ensure that an equivalent amount of genomic DNA template was used for sample, standard PCR for the gene En-2 was performed, using primers contained in the above kit. DNA isolated from each culture was ligated with the supplied primer targets for 18 h at 16°C. The ligated DNA was then used as the substrate for PCR, using supplied primers and Advantage DNA polymerase (BD Clontech) for 23 cycles of 94°C for 1 min and 72°C for 3 min. The reaction product for each sample was electrophoresed through a 1.2% agarose gel, and ethidium bromide-stained bands were detected with UV light illumination.
Assay of caspase-3 activity
bEnd3 cells were homogenized in cell lysis buffer [25 mM HEPES (pH 7.4) buffer containing 2 mM DTT, 5 mM EDTA, and 10 mM digitonin] (Goswami et al. 1999). Lysates were then incubated on ice for 15 min and centrifuged at 10,000 × g for 10 min at 4°C, and protein concentrations were determined using the bicinchoninic acid assay. Soluble supernatants were either used immediately or were stored at −80°C. Aliquots of protein (50 μg) were incubated at 37°C with assay buffer [50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, and 10% glycerol] and 200 mM Ac-DEVD-pNA (BIOMOL). Hydrolysis of the DEVD-AFC substrate was followed for 15 min by fluorometry of the released AFC (excitation, 400 nm; emission, 505 nm), and activity was calculated from the slope. Addition of the caspase-3 inhibitor, Ac-DEVD-CHO(0.1 mM; BIOMOL), to the reaction mixture was used to confirm the specificity of the assay.
Quantitative real-time reverse transcriptase polymerase chain reaction
Quantitation of ZO-1 gene expression in bEnd3 cells was done by real time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) with Syber Green. Lupus and control serum treated cells were lysed and total RNA was extracted by Trizol reagent (Life Technologies). Isolated RNA was incubated with RNase-free DNase I (1–3 U/mg RNA; Roche, Mannheim, Germany) for 40 minutes at 37°C to remove DNA contaminants. The cDNA was synthesized by qScript™ cDNA supermix (Quanta Bioscience Inc., Gaithersburg, MD, US) according to manufacturer’s protocol. Real-time PCR was performed for ZO-1 mRNA. Products of each reaction yielded a single band when run on agarose gel, confirming specific amplification. Primers targeting tight junction genes were synthesized by Integrated DNA Technologies (Coralville, IA) and the sequences of the primers, taken from published studies, are as follows: 5′-TTCAAAGTCTGCAGAGACAATAGC-3′ (forward) and 5′-TCACATTGCTTAGTCCAGT TCC-3′ (reverse) for ZO-1 (Wang et al. 2008). PCR was performed in AB7700 SDS V1.7 (Applied Biosystems, Foster City, CA) with the program: 50°C 2 min, 94°C for 10 minutes and 40 cycles at 94°C for 15 s and 60°C for 1 minute. To validate the quantitative real-time RT-PCR protocol, melting curve analysis was performed to check for the absence of primer dimers. Using a standard curve generated from serial dilutions of brain cDNA, the ratio of ZO-1 expression relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was calculated for each experimental animal, and normalized relative to an average of ratios from the control group.
Statistical analyses
Data are expressed as Mean ± SEM and were analyzed using Minitab (version 12; Minitab) software. Numeric data from all experiments were first analyzed using the “graphical summary” function in Minitab 15 (State College, PA) to determine data normality (Anderson-Darling test) and 95% confidence intervals for mean, median, and SD. Parametric and nonparametric data were analyzed by one-way ANOVA and Kruskal-Wallis tests, respectively. The data were considered significant when p<0.05.
RESULTS
Brain endothelial cells express VE-cadherin and CD88
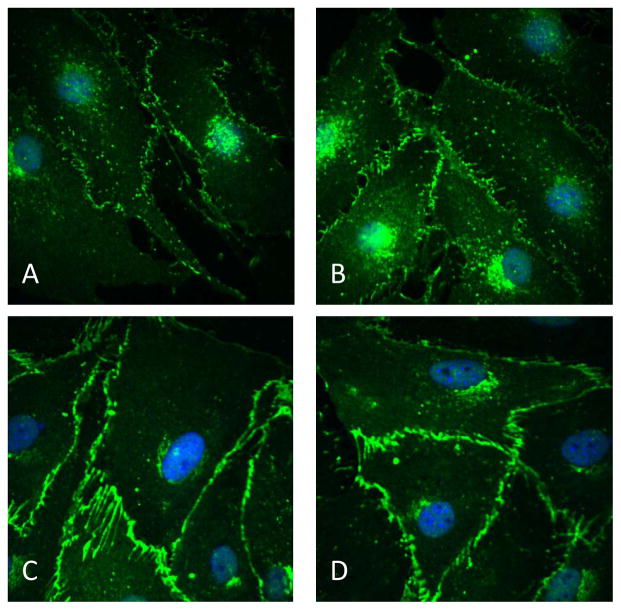
VE-cadherin present in endothelial cells is associated with actin, and plays an important role in maintaining vascular permeability. It is expressed by the bEnd3 cells and primary culture of endothelial cells from mouse brain. In cells treated with control serum, VE-cadherin had a punctuate appearance, and was attached to the cell membrane (Fig 1A and B). On the other hand, in cells treated with lupus serum, there was a substantial increase in expression with lines of staining running in from the cell surface (Fig 1C and D) similar to that described by Millan et al (Millan et al. 2010).
Fig 1. Expression of VE-cadherin is altered in endothelial cells by lupus serum.
Monolayer of bEnd3 cell and primary endothelial cells expressed VE-cadherin (green) on the cell surface (A and B). Treatment of cells with lupus serum increased the expression of VE-cadherin in endothelial cells and altered the staining pattern in a similar manner in both, primary cells and bEnd3 cells (C and D). Nuclei are stained blue (DAPI).
The endothelial cells express CD88 on their membranes (Fig 2A). This was further substantiated by western blotting (Fig 2B). As observed in our recent studies (Jacob et al. 2010d) and in the present study (VE-cadherin), since both bEnd3 cells and the primary cells demonstrate a similar response to lupus serum treatment, subsequent studies to understand the role of C5a/CD88 signaling in endothelial cells were performed using the bEnd3 cell line.
Aggregation and translocation of NF-κB in brain endothelial cells is significantly reduced by CD88 inhibition
NF-κB is a pleiotropic regulator of many genes involved in immune and inflammatory responses. Therefore, we investigated the translocation of NF-κB after cell stimulation with lupus serum for 3 h. Controls included cells treated with normal serum. On treatment with lupus serum, the NF-κB staining changed from a diffuse cytoplasmic to a prominent aggregated pattern. In addition, there was nuclear staining, suggesting that a redistribution of NFκB from the cytoplasm to the nucleus had occurred (Fig 3) compared to controls. Treatment of cells with CD88a prevented the aggregation and translocation of NF-κB induced by lupus serum in these cells.
Fig 3. Signaling through CD88 induces translocation of NFkb into the nucleus.
Monolayer of bEnd3 cells treated with control serum, lupus serum and CD88a+lupus serum were stained for NF-κB p65 (red) and CD31 (blue). Cells treated with control serum had NF-κB in the cytoplasm (arrowhead) and none in the nucleus compared to the lupus serum where the NF-κB was found in the nucleus (arrow). In cells pretreated with CD88a the NF-κB remained in the cytoplasm, indicating the important role of C5a in the process. Panel A, B, C and D show enlarged pictures of the cells demonstrating the C5a induced translocation of NF-κB into the nucleus (arrow).
Inhibition of CD88 prevents lupus serum induced alteration of signal transduction pathways in endothelial cells
We then further explored the signal transduction pathways involved in the activation of bEnd3 cells by C5a/CD88 signaling. Both p42 and p44 subunits of MAPK were upregulated in bEnd3 cells treated with lupus serum (Fig 4). In addition, the expression of CREB, the transcription factor that competes with NFκB for CBP binding was decreased. Further, the expression of Iκb, the cytoplasmic inhibitor of NFκB translocation into the nucleus, was reduced in the treated cells. Pretreatment of cells with CD88a prevented the change in the expression of these signaling molecules and the translocation of NFκB into the nucleus, suggesting that C5a/CD88 signaling promotes NFκB activation events.
Fig 4. Inhibition of CD88 reduces changes in signaling molecules in lupus serum-treated bEnd3 cells.
Monolayer of bEnd3 cells was treated with control serum, lupus serum and CD88a+lupus serum, and the homogenate subjected to luminex beads, as given in Methods. The three signaling molecules that were significantly altered were– MAPK, CREB and Iκbα. These molecules function in close correlation with NFκb. Pretreatment with CD88a prevented this change. P<0.02.
CD88 inhibition prevents cell death in bEnd3 cells treated with lupus serum
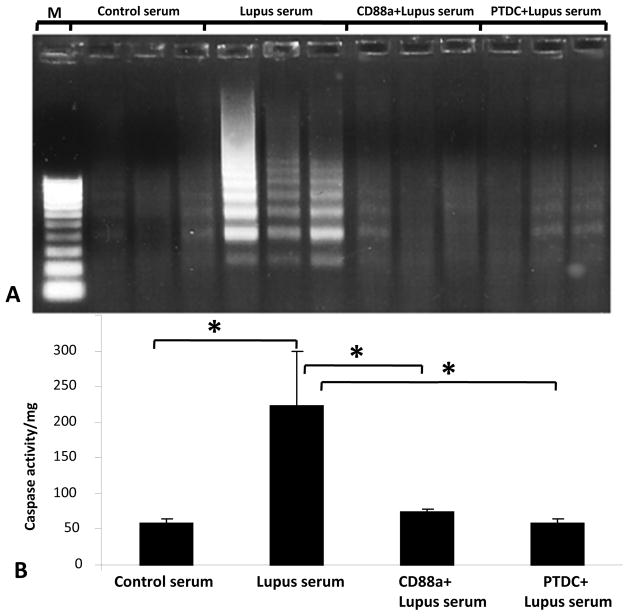
Activation of NF-κB plays a central role in the regulation of diverse cellular processes including inflammation and apoptosis. Apoptosis is an important event in lupus brains. In this study we examined the apoptotic response of mouse endothelial cells in a lupus setting. We treated the cells with lupus serum for 3 hours, based on data from our previous study (Jacob et al. 2010c). To investigate whether apoptosis occurred in these cells, we assessed the extent of DNA laddering. A substantial amount of DNA laddering was present in lupus-serum stimulated cells, which was reduced by pretreatment of cells with CD88a (Fig 5A), indicating that the apoptosis occurred in a C5a/CD88 dependent manner.
Fig 5. Signaling through CD88 induces apoptosis in bEnd3 cells.
Monolayer of bEnd3 cells treated with control serum, lupus serum, CD88a +lupus serum, and PTDC + lupus serum. A. DNA was isolated and subjected to LM-PCR. As seen in the Fig, significant DNA laddering occurred in cells treated with lupus serum compared to cells treated with control serum. This apoptotic laddering was prevented by both CD88a and NFκb inhibitor treatments. B. In bEnd3 cells treated as described in A, caspase activity was determined as described in Material and Methods. Lupus serum significantly increased caspase activity in bEnd3 cells (p<0.02), which was prevented by pretreatment with CD88a or PTDC.
Induction of caspase-3 activity in lupus serum treated endothelial cells is complement dependent
Key effectors of apoptosis are the proteolytic enzymes of the caspase family. Caspase-3, a critical mediator of apoptotic pathways in mammalian cells was increased in lupus serum treated cells compared to controls, as assessed by hydrolysis of the DEVD-AFC substrate (Fig. 5B). This increase in caspase-3 activity was significantly reduced in cells treated with CD88a and PTDC. These results suggest that apoptosis of the cells treated with lupus serum and induced by C5a/CD88 signaling occurred in an NFκB dependent manner.
CD88 inhibition prevents actin reorganization in lupus-treated endothelial cells through the NFkB pathway
Actin cytoskeleton maintains the structural integrity of the cell, and its reorganization could lead to increased vascular permeability. Rhodamine-phalloidin staining of paraformaldehyde fixed and permeabilized bEnd3 monolayer was performed to permit visualization of the actin filaments (Fig. 6). F-actin stress fiber formation induced by lupus serum in the endothelial cell monolayer was significantly reduced by pretreatment of cells with CD88a. In addition, the stress fiber formation was prevented by PTDC. These results indicate that signaling through CD88 signaling regulated the F-actin reorganization and thereby the vascular permeability through the NFκB pathway.
Fig 6. Inhibition of CD88 or NFκb reduces stress fiber formation in lupus serum treated endothelial cells.
Monolayers of bEnd3 cells were treated with 5% control serum, lupus serum, or CD88a or PTDC followed by lupus serum for 3 h. Typical patterns of Texas Red-phalloidin staining indicating actin rearrangement are shown in cells treated with lupus serum compared to cells treated with control serum. Pretreatment of cells with CD88a or PTDC significantly reduced actin rearrangement. Representative pictures from three independent experiments are shown. Nuclei were stained with DAPI.
Effect of signaling through CD88 on tight junction protein, ZO-1 in endothelial cells
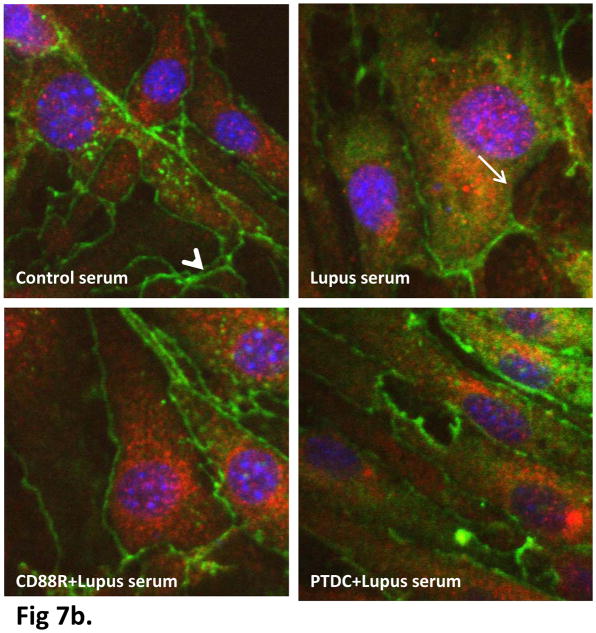
ZO-1 proteins function as a scaffolding protein critical in maintaining the integrity of the tight junctions and is known to be regulated by NF-κB activation (Ma et al. 2004). Therefore, we next examined the effect of lupus serum on mRNA and protein expression of tight junction protein ZO-1 in bEnd3 monolayer. Confluent monolayer of bEnd3 cells were treated with lupus serum for 3 h. Total mRNA was extracted and subjected to quantitative real time RT-PCR using primers specific to ZO-1. The mRNA expression of ZO-1 was down-regulated (P<0.02) in lupus serum exposed monolayers (1.98 ± 0.85) when compared to control serum-treated cells (3.73 ± 0.76) (Fig 7a). The decrease in ZO-1 mRNA expression was prevented by CD88 (3.17 ± 0.42) and NFκB (2.94 ± 0.24) inhibition. To correlate the decreased mRNA levels with tight junction protein expression, treated and control monolayers were stained for ZO-1 and compared by confocal microscopy. The protein expression correlated well with real-time quantitative RT-PCR results, showing distinct decrease in localization of ZO-1. The staining of ZO-1 became discontinued (arrow) along the cell contour of bEnd3 following incubation with lupus serum, while intact and continuous (arrowhead) and orderly ZO-1 staining was observed in bEnd3 cells treated with control serum (Fig 7b). ZO-1 maintains the intercellular connections between cells, and its reorganization could lead to increased vascular permeability. Pretreatment of cells with CD88a or PTDC was protective and maintained ZO-1 expression, indicating a complement-dependent disruption of the tight junction.
Fig 7.
Fig 7a. Signaling through CD88 alters ZO-1 expression in bEnd3 cells. Expression of ZO-1 was quantified by real-time PCR and normalized to expression of glyceraldehyde-3-phosphate dehydrogenase. Values are expressed as Mean ± S.D. Treatment with lupus serum caused a substantial decrease in expression of ZO-1. *P < 0.02 versus baseline.
Fig 7b. Inhibition of CD88 or NFκb prevents reduced ZO-1 expression in lupus serum treated endothelial cells. Monolayers of bEnd3 cells were treated with 5% control serum, lupus serum, or CD88a or PTDC followed by lupus serum for 3 h. ZO-1 expression was reduced and the continuity of the ZO-1 layer was lost in lupus serum treated cells. Pretreatment of cells with CD88a or PTDC significantly prevented these alterations. Representative pictures from three independent experiments are shown. ZO-1 was stained in green, CD31 in red and nuclei in blue.
DISCUSSION
BBB is a critical unit of the brain that maintains the internal milieu constant and protects the brain from circulating toxins and inflammatory cells. BBB damage occurs in a number of neuroinflammatory settings including SLE (Davies 2002a; Davies 2002b; Diamond et al. 2006) in which the concentration of several complement proteins are found to be altered. Different models are used to study the BBB in vitro. Although they have limitations, comparison of four different models including monolayer of bEnd3 cells demonstrate that the monolayer did not differ from cocultures in their hydraulic conductivity and diffusive permeability. Further, the permeability of these models were comparable with in vivo data obtained from rat pial microvessels (Li et al. 2010). In addition, since the immortalized cell line bEnd3 was shown to maintain characteristics of the BBB over repeated passages, and primary cultures had a number of disadvantages (Yuan et al. 2010), the studies described were conducted in these cells.
Our recent results suggest that C5a generated during complement activation causes structural alterations which increases the permeability of cultured endothelial cell monolayers, as estimated by actin filaments and the transendothelial electrical resistance (TEER), and thereby the BBB integrity. However, the mechanism by which C5a cause these changes still remains unknown. Our results in the present study suggest that C5a/CD88 signaling alters the BBB integrity in an NF-κB dependent manner, leading to apoptosis, altered tight junction proteins, and thereby BBB dysfunction. Pretreatment with the peptide antagonist, CD88a or NF-κB inhibitor significantly reduced these alterations, indicating the important role of C5a/CD88 signaling and NF-κB in causing the pathology.
The endothelial cell damage induced by C5a was evaluated using cultured endothelial cell monolayers. VE-cadherin was used as an endothelial cell marker. However, interestingly, the expression of VE-cadherin was altered in lupus setting. This is in line with actin reorganization that occurs when the cells are treated with lupus serum, since the endothelial adherens junctions seem to regulate actin polymerization and actomyosin contraction (Lampugnani 2010).
One significant effect of complement activation can be apoptosis, which can occur directly through cellular events stimulated by C5b-9, C5a, and Bb (Uwai et al. 2000), culminating in activation of caspase-3, the effector protein that performs the downstream function of apoptosis. Apoptosis was increased in lupus serum treated bEnd3 cells, as observed by LM-PCR. In addition, caspase-3 was up-regulated in these cells, indicating that apoptosis most likely occurred in a caspase-dependent fashion. Treatment of the cells with lupus serum decreased I-κB expression and increased translocation of NF-κB into the nucleus. NF-κB is known to play a pivotal role in endothelial activation, including endothelial apoptosis in a caspase-3 and bcl2 dependent manner. Inhibition of NF-κB prevented lupus serum induced apoptosis, in bEnd3 cells suggesting that this process occurred in a NF-κB dependent manner.
The activity of the transcription factor NF-κB is regulated through its association with cellular coactivators. Interaction with the coactivator cAMP response element binding protein (CREB)-binding protein (CBP) appears to be necessary to optimize the transcriptional activity of NF-κB. In vitro studies have directly shown competition between NF-κB p65 and CREB for limiting amounts of CBP (Parry and Mackman 1997). Our results show reduced expression of CREB in lupus serum treated bEnd3 cells, which could potentially result in enhanced NF-κB-dependent transcription. The prevention of these alterations by CD88a adds to the growing evidence that the changes induced by signaling through CD88 in these cells are NF-κB dependent.
Our data suggest that inhibition of CD88 reduced the cascade of events resulting in compromise of the BBB, which occurred in a NFκB dependent fashion resulting in apoptosis and altered ZO-1 expression. Since increased complement activation leads to increased circulating C5a in lupus, our results suggest that C5a maybe altering the downstream signaling cascade through CD88 binding and thereby altering the BBB integrity. However, a second ligand for CD88 has been described, the ribosomal protein S19 dimer released from apoptotic cells (Nishiura et al. 2009). The binding of RPS19 dimer is also inhibited by CD88a. Therefore our next sets of experiments are ongoing to delineate the effects of C5a from RPS19 in lupus setting. In conclusion, these results suggest that signaling through CD88 plays a key role in disrupting the BBB integrity through NFκb-dependent pathways, and is a potentially important therapeutic target for neuroinflammatory settings with altered complement cascade.
Acknowledgments
This work was supported by National Institutes of Health Grant R01DK055357 (to RJQ).
Abbreviations used
- C5a
Complement factor 5a
- CD88
C5a receptor
- SLE
Systemic lupus erythematosus
- CREB
cAMP response element binding protein
- ZO-1
zona occludens
- NFκB
nuclear factor-κB
- BBB
blood-brain barrier
- TNF-α
tumor necrosis factor-α
- PDTC
pyrrolidine dithiocarbamate
Footnotes
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: JJA and AJ. Performed the experiments: BH, PC, AJ and JJA. Analyzed the data: JJA and AJ. Contributed reagents/materials/analysis tools: RJQ. Wrote the paper: JJA, AJ and RJQ.
CONFLICT OF INTEREST:
Authors have no conflict of interest.
REFERENCE LIST
- Abbott NJ, Mendonca LL, Dolman DE. The blood-brain barrier in systemic lupus erythematosus. Lupus. 2003;12:908–915. doi: 10.1191/0961203303lu501oa. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Bao L, Jacob A, Kraus DM, Holers VM, Quigg RJ. Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim Biophys Acta. 2003;1639:169–176. doi: 10.1016/j.bbadis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Bao L, MacDonald RL, Quigg RJ. Complement-Dependent Apoptosis and Inflammatory Gene Changes in Murine Lupus Cerebritis. J Immunol. 2005;175:8312–8319. doi: 10.4049/jimmunol.175.12.8312. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Vezina P, Sekine H, Gilkeson GS, Quigg RJ. Absence of functional alternative complement pathway alleviates lupus cerebritis. Eur J Immunol. 2007;37:1691–1701. doi: 10.1002/eji.200636638. [DOI] [PubMed] [Google Scholar]
- Barnum SR. Complement in central nervous system inflammation. Immunol Res. 2002;26:7–13. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon JP, Tamerius J, Belmont HM, Abramson SB. Assessment of disease activity and impending flare in patients with systemic lupus erythematosus. Comparison of the use of complement split products and conventional measurements of complement. Arthritis Rheum. 1992;35:1028–1037. doi: 10.1002/art.1780350907. [DOI] [PubMed] [Google Scholar]
- Carroll MC. The lupus paradox. Nat Genet. 1998;19:3–4. doi: 10.1038/ng0598-3. [DOI] [PubMed] [Google Scholar]
- Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat Rev Immunol. 2004;4:825–831. doi: 10.1038/nri1456. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Fischer MB. Complement and the immune response. Curr Opin Immunol. 1997;9:64–69. doi: 10.1016/s0952-7915(97)80160-4. [DOI] [PubMed] [Google Scholar]
- Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- Davies DC. Blood-brain barrier breakdown and oedema formation in systemic sepsis and human brain tumours. J Anat. 2002a;200:528–529. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002b;200:639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H, Segal MB. Physiology of the CSF and the blood-brain barrier. CRC; New York: 1995. [Google Scholar]
- Davson H, Zlokovic BV, Rakic L, Segal MB. An introduction to the blood-brain barrier. Macmillan; London: 1993. [Google Scholar]
- deVries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–3889. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, DeGiorgio LA, Lee J, Triantafyllopoulou A, Cohen-Solal J, Volpe BT. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- Ducruet AF, Zacharia BE, Hickman ZL, Grobelny BT, Yeh ML, Sosunov SA, Connolly ES., Jr The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp Neurol. 2009;219:398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Baranyi L, Takahashi M, Fukuda A, Liposits Z, Yamamoto T, Okada H. A neuronal C5a receptor and an associated apoptotic signal transduction pathway. J Physiol. 1998;507(Pt 3):679–687. doi: 10.1111/j.1469-7793.1998.679bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Chen AJ, Nadeau BA, Day DE, Sarma JV, Huber-Lang MS, Ward PA. The complement anaphylatoxin C5a induces apoptosis in adrenomedullary cells during experimental sepsis. PLoS One. 2008;3:e2560. doi: 10.1371/journal.pone.0002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Rittirsch D, Huber-Lang M, Niederbichler AD, Hoesel LM, Touban BM, Morgan SJ, Smith WR, Ward PA, Ipaktchi K. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit Care. 2009;13:R12. doi: 10.1186/cc7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J Immunol. 2009a;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Gotze O, Morgan BP. Identification and characterization of the complement C5a anaphylatoxin receptor on human astrocytes. J Immunol. 1995;155:4882–4889. [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Gotze O, Morgan BP. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol. 1997;150:31–41. [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Kilkus J, Dawson SA, Dawson G. Overexpression of Akt (protein kinase B) confers protection against apoptosis and prevents formation of ceramide in response to pro-apoptotic stimuli. J Neurosci Res. 1999;57:884–893. [PubMed] [Google Scholar]
- Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- He HJ, Zhu TN, Xie Y, Fan J, Kole S, Saxena S, Bernier M. Pyrrolidine dithiocarbamate inhibits interleukin-6 signaling through impaired STAT3 activation and association with transcriptional coactivators in hepatocytes. J Biol Chem. 2006;281:31369–31379. doi: 10.1074/jbc.M603762200. [DOI] [PubMed] [Google Scholar]
- Hill HD, Straka JG. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988;170:203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Hopkins P, Belmont HM, Buyon J, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum. 1988;31:632–641. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J Biol Chem. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]
- Jacob A, Bao L, Brorson J, Quigg RJ, Alexander JJ. C3aR inhibition reduces neurodegeneration in experimental lupus. Lupus. 2010a;19:73–82. doi: 10.1177/0961203309348978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Hack B, Bai T, Brorson JR, Quigg RJ, Alexander JJ. Inhibition of C5a receptor alleviates experimental CNS lupus. J Neuroimmunol. 2010b;221:46–52. doi: 10.1016/j.jneuroim.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Hack B, Chiang E, Garcia JG, Quigg RJ, Alexander JJ. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010c;24(6):1682–8. doi: 10.1096/fj.09-138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG. Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol. 2010;9:16. doi: 10.1186/jbiol232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- Li G, Simon MJ, Cancel LM, Shi ZD, Ji X, Tarbell JM, Morrison B, III, Fu BM. Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann Biomed Eng. 2010;38:2499–2511. doi: 10.1007/s10439-010-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated protein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77:43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ, Schreiber RD. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Daha MR, Tijsma O, van de WB, Tedesco F, Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–792. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J Immunol. 2004;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Soane L, Niculescu F, Rus V, Raine CS, Shin ML, Rus H. Effects of membrane attack complex of complement on apoptosis in experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2003;1010:530–533. doi: 10.1196/annals.1299.098. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Harigai M, Omori M, Sato E, Hara M. Blood-brain barrier damage as a risk factor for corticosteroid-induced psychiatric disorders in systemic lupus erythematosus. Psychoneuroendocrinology. 2008;33:395–403. doi: 10.1016/j.psyneuen.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Nonaka H, Revollo IS, Semba U, Li Y, Ota Y, Irie A, Harada K, Kehrl JH, Yamamoto T. Pro- and anti-apoptotic dual functions of the C5a receptor: involvement of regulator of G protein signaling 3 and extracellular signal-regulated kinase. Lab Invest. 2009;89(6):676–94. doi: 10.1038/labinvest.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102–2110. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- Short A, Wong AK, Finch AM, Haaima G, Shiels IA, Fairlie DP, Taylor SM. Effects of a new C5a receptor antagonist on C5a- and endotoxin-induced neutropenia in the rat. Br J Pharmacol. 1999;126:551–554. doi: 10.1038/sj.bjp.0702338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhrao SK, Neal JW, Morgan BP, Gasque P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp Neurol. 1999;159:362–376. doi: 10.1006/exnr.1999.7170. [DOI] [PubMed] [Google Scholar]
- Tenner AJ, Fonseca MI. The double-edged flower: roles of complement protein C1q in neurodegenerative diseases. Adv Exp Med Biol. 2006;586:153–176. doi: 10.1007/0-387-34134-X_11. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- Uwai M, Terui Y, Mishima Y, Tomizuka H, Ikeda M, Itoh T, Mori M, Ueda M, Inoue R, Yamada M, Hayasawa H, Horiuchi T, Niho Y, Matsumoto M, Ishizaka Y, Ikeda K, Ozawa K, Hatake K. A new apoptotic pathway for the complement factor B-derived fragment Bb. J Cell Physiol. 2000;185:280–292. doi: 10.1002/1097-4652(200011)185:2<280::AID-JCP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wang H, Ding T, Brown N, Yamamoto Y, Prince LS, Reese J, Paria BC. Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse. Dev Biol. 2008;318:112–125. doi: 10.1016/j.ydbio.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PA. Role of the complement in experimental sepsis. J Leukoc Biol. 2008a;83:467–470. doi: 10.1189/jlb.0607376. [DOI] [PubMed] [Google Scholar]
- Ward PA. Sepsis, apoptosis and complement. Biochem Pharmacol. 2008b;76:1383–1388. doi: 10.1016/j.bcp.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TM, Ager RR, Tenner AJ, Noakes PG, Taylor SM. The Role of the Complement System and the Activation Fragment C5a in the Central Nervous System. Neuromolecular Med. 2009 doi: 10.1007/s12017-009-8085-y. [DOI] [PubMed] [Google Scholar]
- Yuan W, Li G, Gil ES, Lowe TL, Fu BM. Effect of surface charge of immortalized mouse cerebral endothelial cell monolayer on transport of charged solutes. Ann Biomed Eng. 2010;38:1463–1472. doi: 10.1007/s10439-010-9920-x. [DOI] [PubMed] [Google Scholar]