Abstract
Purpose
To evaluate the impact of low ER/PR expression and effect of endocrine therapy on survival outcomes in HER2-negative tumors with ER/PR < 10%, previously labeled as triple negative.
Methods
In a retrospective review, 1257 patients were categorized according their ER/PR percentages into three groups, ER/PR <1% (Group A), ER/PR 1–5% (Group B) and ER/PR 6–10% (Group C). Kaplan-Meier product limit method was used to estimate survival outcomes. Cox proportional hazards models was used to adjust for patient and tumor characteristics.
Results
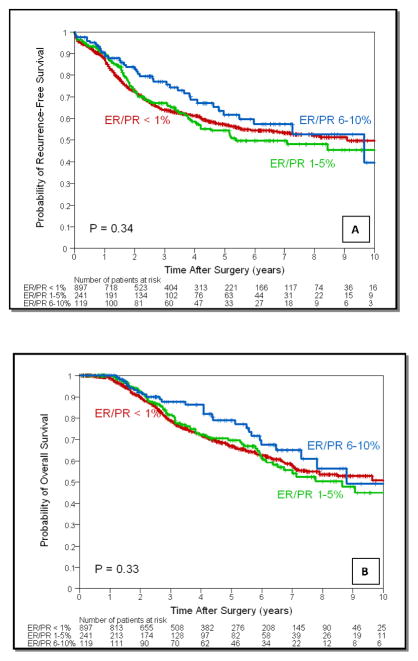
Group A, B and C had 897 (71.4%), 241 (19.2%) and 119 (9.4%) patients respectively. After a median follow up of 40 months there was no significant difference in 3-year recurrence free survival (RFS): 64%, 67% and 77% (P = 0.34) or overall survival (OS): 79%, 81% and 88% (P = 0.33) for groups A, B and C respectively. ER/PR expression was not an independent predictor for RFS (HR=1.10, 95% CI: 0.86–1.39, P=0.46 for group B and HR=0.96, 95% CI: 0.66–1.38, P=0.81 for group C, compared to group A), or OS (HR=1.11, 95% CI: 0.84–1.46, P=0.46 for group B and HR=0.94, 95% CI: 0.63–1.42, P=0.78 for group C, compared to group A). Endocrine therapy had no impact on survival outcomes (RFS: P=0.10; OS: P=0.45) among groups.
Conclusions
In this cohort, a low ER/PR level (1–5%) does not appear to have any significant impact on survival outcomes. There was a tendency for survival advantages in the ER/PR 6–10% is seen. Benefit of endocrine therapy in these patients is unclear.
Keywords: Estrogen Receptor, Progesterone Receptor, Breast cancer, Prognosis, Immunohistochemistry
INTRODUCTION
Breast cancer is a heterogeneous disease.1 Principally, it can be categorized as either receptor positive breast cancers (those that express estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2)) or receptor negative breast cancers.2 This classification is based on availability of targeted therapies (antiestrogens, aromatase inhibitors and trastuzumab) directed at these growth receptors.3,4,5 These targeted agents have resulted in considerable improvement in breast cancer survival and prognosis in receptor positive subtypes in the last few decades. However, therapeutic success in receptor negative subtypes has been disappointing with a median survival of about 1 year in metastatic disease.6 These receptor negative subtypes also known as triple-negative breast cancers (TNBC) represent about 15% of all breast cancers.7 They are characterized by aggressive clinical behavior and lack of effective targeted therapies.8
The triple-negative phenotype is associated with low or negative expression of ER, PR and HER2. Even so, lack of standardization of the detection method, assays and threshold for positivity of these markers has been a persistent issue.9,10 The issues regarding reproducibility and accuracy of reporting ER/PR and HER2 are problematical and nearly 20% of current testing is considered inaccurate.11,12 The recent American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) guidelines aspire to bring uniformity in recommendations regarding use of targeted therapies by improving accuracy of testing for these receptors and their utility as predictive markers.11,12
The joint panel of ASCO Clinical Practice Guidelines Committee (CPGC) and CAP Council on Scientific Affairs (CSA) recommended that ER and PR staining ≥ 1% by immunohistochemistry (IHC) be considered positive.11 Endocrine therapy should be considered in all these patients due to its sizeable impact on mortality and relatively low toxicity.3,13 However, the panel recognized that most prospective data showing correlation between the amount of ER and response to therapy was derived using ligand-binding assays (LBA) not IHC. The guidelines also stated that decision regarding endocrine therapy in patients with low levels of ER by IHC (1% to 10%) should be made after weighing risks and benefits.11
Prior to the current guidelines, the threshold for ER/PR positivity was ≥ 10 fmol/mg cytosol protein by LBA or ≥ 10% of tumor cells positive by IHC.14 Hence, HER2-negative breast cancers with ER/PR < 10% by IHC were considered as TNBC. In this retrospective study we identified a cohort of patients formerly defined as having TNBC and stratified them according to their level of ER/PR expression into one of three groups, ER/PR < 1%, ER/PR 1–5% and ER/PR 6–10%. We sought to evaluate the prognostic significance of low level of ER/PR (1% – 10%) by IHC. We also performed an exploratory analysis to assess the efficacy of endocrine therapy in patients with low ER/PR expression.
MATERIALS AND METHODS
Patients
We retrospectively identified all patients categorized as having TNBC (ER/PR < 10% and HER2/neu-negative) from the Breast Cancer Management System database at The University of Texas MD Anderson Cancer Center (MDACC). The analysis included all such patients diagnosed or treated at our institution between January 1990 and June 2009. Clinicopathological factors were assessed from the database and wherever data was missing medical records were reviewed to gather information. Patients with missing information regarding the percentage of estrogen and/or progesterone receptor expression by immunohistochemistry were excluded. A total of 1967 patients were identified. After excluding 710 patients with absent information regarding numerical ER/PR percentages a total of 1257 patients were included in the final analysis. All pathologic specimens were reviewed by dedicated breast pathologists including the IHC slides of cases from outside the institution. When the quality of the staining was unsatisfactory, staining was repeated at MDACC central IHC laboratory. The histologic type of all tumors was defined as per World Health Organization’s classification system.15 The histologic grade was defined according to the modified Black’s nuclear grading system.16 Immunohistochemical analysis to determine ER and PR status was performed using standard procedures on 4-μm sections of paraffin-embedded tissues stained with monoclonal antibodies: 6F11 (Novacastra Laboratories Ltd., Burlingame, CA) for ER and 1A6 (Novacastra Laboratories Ltd., Burlingame, CA) for PR. ER and PR levels were reported as the percentage of nuclear staining in tumor cells.
Statistical Analysis
Patients were categorized according to the percentage of ER/PR expression by immunohistochemistry into one of three groups Group A with ER/PR < 1%, Group B with ER/PR 1–5%, and Group C with ER/PR 6–10%. A concordant ER/PR pair was defined as both ER and PR less than 1% (or both ER/PR 1%–10%). Whenever ER/PR was discordant ER was taken as the defining value for classification.17 Patient and clinical characteristics included age, tumor size, nodal status, lymphovascular invasion (LVI), grade, histology, adjuvant radiation, surgery, post mastectomy radiation therapy (PMRT), and adjuvant chemotherapy. Distant metastasis-free survival (DMFS), recurrence-free survival (RFS), and overall survival (OS) were measured from the date of diagnosis to the date of first documented distant metastasis, local or distant recurrence and death respectively. Patients not experiencing the relevant end point were censored at last follow-up.
Patient and clinical characteristics were tabulated and compared between the three ER/PR groups with the chi-square test. Kaplan-Meier product limit method was used to estimate the survival outcomes of all patients according to the three ER/PR groups; groups were compared with the log-rank statistic. Cox proportional hazards models were fit to determine the association of ER/PR percentages with survival outcomes after adjustment for other patient and disease characteristics. Subset analyses were carried out within patients who did or did not receive adjuvant hormonal therapy. P-values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, NC) and S-Plus 7.0 (Insightful Corporation, Seattle, WA).
RESULTS
Group A (ER/PR < 1%) had a total of 897 (71.4%) patients. Among the remaining 360 (28.6%) patients, 241 (19.2%) patients were classified as Group B (ER/PR 1–5%) and 119 (9.4%) patients as Group C (ER/PR 6–10%). Patient and clinical characteristics by ER/PR groups are shown in Table 1.
Table 1.
Baseline characteristics by Estrogen/Progesterone Receptor levels.
| Group A ER/PR < 1% (N=897) |
Group B ER/PR 1 – 5% (N=241) |
Group C ER/PR 6 – 10% (N=119) |
|||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P-value | |
| Age (Median) | 51 | 51 | 49 | ||||
| Age | |||||||
| ≤ 50 | 439 | 48.9 | 117 | 48.5 | 63 | 52.9 | |
| > 50 | 458 | 51.1 | 124 | 51.5 | 56 | 47.1 | 0.69 |
| Tumor Size | |||||||
| T1 | 468 | 52.7 | 134 | 55.6 | 73 | 62.9 | |
| T2 | 358 | 40.3 | 86 | 35.7 | 40 | 34.5 | |
| T3–4 | 62 | 7.0 | 21 | 8.7 | 3 | 2.6 | 0.09 |
| Lymph Nodes | |||||||
| N0 | 541 | 62.0 | 156 | 67.2 | 71 | 60.7 | |
| N1 | 231 | 26.5 | 49 | 21.1 | 29 | 24.8 | |
| N2 | 53 | 6.1 | 10 | 4.3 | 10 | 8.5 | |
| N3 | 48 | 5.5 | 17 | 7.3 | 7 | 6.0 | 0.38 |
| Lymphovascular Invasion | |||||||
| Negative | 633 | 70.7 | 181 | 75.4 | 93 | 78.2 | |
| Positive | 262 | 29.3 | 59 | 24.6 | 26 | 21.8 | 0.12 |
| Grade | |||||||
| I or II | 76 | 8.7 | 36 | 15.3 | 24 | 20.5 | |
| III | 798 | 91.3 | 200 | 84.7 | 93 | 79.5 | 0.0001 |
| Histology | |||||||
| Ductal | 800 | 89.2 | 215 | 89.2 | 99 | 83.2 | |
| Other | 97 | 10.8 | 26 | 10.8 | 20 | 16.8 | 0.15 |
| Adjuvant Radiation | |||||||
| No | 386 | 43.0 | 105 | 43.6 | 49 | 41.2 | |
| Yes | 511 | 57.0 | 136 | 56.4 | 70 | 58.8 | 0.91 |
| Surgery | |||||||
| Breast consevation | 478 | 53.3 | 134 | 55.6 | 60 | 50.4 | |
| Mastectomy | 419 | 46.7 | 107 | 44.4 | 59 | 49.6 | 0.64 |
| Postmastectomy Radiotherapy | |||||||
| No | 317 | 75.7 | 82 | 76.6 | 40 | 67.8 | |
| Yes | 102 | 24.3 | 25 | 23.4 | 19 | 32.2 | 0.39 |
| Adjuvant Chemotherapy | |||||||
| Anthracycline-based | 189 | 28.5 | 54 | 32.1 | 25 | 29.1 | |
| Taxane-based | 38 | 5.7 | 12 | 7.1 | 3 | 3.5 | |
| Anthracycline and Taxane | 403 | 60.9 | 92 | 54.8 | 53 | 61.6 | |
| Other | 32 | 4.8 | 10 | 6.0 | 5 | 5.8 | 0.78 |
Patient & Clinical Characteristics
The median age was 51, 51 and 49 years in groups A, B and C respectively (range 21 to 98 years). Ninety-one (91%) percent of Group A (ER/PR < 1%) patients had grade III tumor compared to 85% in Group B (ER/PR 1–5%) and 80% in Group C (ER/PR 6–10%) (P = 0.0001). Four percent (4%) patients in Group A (ER/PR < 1%) had received hormonal therapy compared to 14% in Group B (ER/PR 1–5%) and 40% in Group C (ER/PR 6–10%) (P < 0.0001). Other patient and clinical characteristics including age, tumor size, nodal status, LVI, histology, adjuvant radiation, surgery, PMRT and adjuvant chemotherapy were similar among the three ER/PR groups.
Survival outcomes
Median follow-time for all patients was 40 months (range 0 to 233 months). There were 465 recurrences, 335 in Group A (ER/PR < 1%), 92 in Group B (ER/PR 1–5%) and 38 in Group C (ER/PR 6–10%) (P = 0.34). The RFS estimates with 95% CI by patient and tumor characteristics are listed in Table 2. The 3-year RFS rate for the entire cohort was 65% (95% CI: 62% – 68%). The 3-year RFS was 64% for Group A, 67% for Group B and 77% for Group C (P = 0.34) (Figure 1a). Cox proportional hazards model showed that younger age, larger tumor, positive nodal status, LVI, not receiving adjuvant radiation, and not receiving adjuvant chemotherapy were associated with increased risk of recurrence (Table 3). ER/PR levels did not have a significant impact on RFS in group B (ER/PR 1–5%) (HR=1.10, 95% CI: 0.86–1.39, P = 0.46) and group C (ER/PR 6–10%) (HR=0.96, 95% CI: 0.66–1.38, P = 0.81) compared to patients in group A (ER/PR < 1%). There were 367 deaths, 265 in Group A, 72 in Group B and 30 in Group C (P = 0.33). The OS estimates with 95% CI by patient and tumor characteristics are listed in Table 3. The 3-year OS rate for the entire cohort was 80% (95% CI: 78% – 83%). The 3-year OS estimate was 79% for Group A, 81% for Group B and 88% for Group C (P = .33) (Figure 1b). Cox proportional hazards model showed that younger age, larger tumor, positive nodal status, lymphovascular invasion, not receiving adjuvant radiation, and not receiving adjuvant chemotherapy were associated with increased risk of death (Table 3). After adjustment for other patient and tumor characteristics, ER/PR percentages did not have a significant impact on OS in group B (ER/PR 1–5%) (HR = 1.11, 95% CI 0.84 to 1.46, P = 0.46) and group C (ER/PR 6–10%) (HR = 0.94, 95% CI 0.63 to 1.42, P = 0.78) compared to patients in group A (ER/PR < 1%).
Table 2.
Three-year Recurrence-Free Survival and Overall Survival Estimates by Patient and Clinical characteristics.
| N Patients | Recurrence-Free Survival
|
Overall Survival
|
|||||
|---|---|---|---|---|---|---|---|
| N Events | 3-Year Estimate (95% CI) | P | N Events | 3-Year Estimate (95% CI) | P | ||
| Total | 1257 | 465 | 0.65(0.62, 0.68) | 367 | 0.80(0.78, 0.83) | ||
| Estrogen/Progesterone receptor | |||||||
| < 1% | 897 | 335 | 0.64(0.60, 0.67) | 265 | 0.79(0.76, 0.82) | ||
| 1 – 5% | 241 | 92 | 0.67(0.60, 0.73) | 72 | 0.81(0.75, 0.86) | ||
| 6 – 10% | 119 | 38 | 0.77(0.67, 0.84) | .34 | 30 | 0.88(0.79, 0.93) | .33 |
| Estrogen/Progesterone receptor | |||||||
| < 1% | 897 | 335 | 0.64(0.6, 0.67) | 265 | 0.79(0.76, 0.82) | ||
| 1 – 10% | 360 | 130 | 0.7(0.65, 0.75) | .61 | 102 | 0.84(0.79, 0.87) | .49 |
| Patients with hormonal therapy | |||||||
| ER/PR < 1% | 37 | 18 | 0.61(0.42, 0.75) | 12 | 0.93(0.76, 0.98) | ||
| ER/PR 1 – 5% | 33 | 15 | 0.73(0.53, 0.85) | 13 | 0.89(0.69, 0.96) | ||
| ER/PR 6 – 10% | 48 | 10 | 0.87(0.71, 0.94) | .10 | 9 | 0.95(0.8, 0.99) | .45 |
| Patients with hormonal therapy | |||||||
| ER/PR < 1% | 37 | 18 | 0.61(0.42, 0.75) | 12 | 0.93(0.76, 0.98) | ||
| ER/PR 1 –10% | 81 | 25 | 0.81(0.69, 0.89) | .10 | 22 | 0.92(0.82, 0.97) | .43 |
| Age | |||||||
| ≤ 50 | 619 | 277 | 0.59(0.54, 0.63) | 217 | 0.76(0.72, 0.79) | ||
| > 50 | 638 | 188 | 0.72(0.68, 0.75) | < .0001 | 150 | 0.84(0.81, 0.87) | < .0001 |
| Tumor size | |||||||
| T1 | 675 | 174 | 0.79(0.75, 0.82) | 136 | 0.88(0.85, 0.90) | ||
| T2 | 484 | 217 | 0.56(0.51, 0.61) | 167 | 0.76(0.71, 0.80) | ||
| T3–4 | 86 | 63 | 0.27(0.17, 0.37) | < .0001 | 54 | 0.56(0.44, 0.66) | < .0001 |
| Lymph nodes | |||||||
| N0 | 768 | 196 | 0.77(0.74, 0.80) | 137 | 0.90(0.87, 0.92) | ||
| N1–3 | 454 | 253 | 0.48(0.43, 0.53) | < .0001 | 216 | 0.67(0.62, 0.71) | < .0001 |
| Grade | |||||||
| I or II | 136 | 40 | 0.78(0.69, 0.84) | 41 | 0.86(0.78, 0.91) | ||
| III | 1091 | 410 | 0.64(0.61, 0.67) | .01 | 315 | 0.80(0.77, 0.82) | .17 |
| Histology | |||||||
| Ductal | 1114 | 411 | 0.66(0.63, 0.69) | 323 | 0.81(0.78, 0.84) | ||
| Other | 143 | 54 | 0.63(0.53, 0.7) | .61 | 44 | 0.73(0.64, 0.80) | .52 |
| Lymphovascular invasion | |||||||
| Negative | 907 | 253 | 0.74(0.71, 0.77) | 198 | 0.86(0.84, 0.89) | ||
| Positive | 347 | 209 | 0.43(0.37, 0.49) | < .0001 | 167 | 0.65(0.59, 0.70) | < .0001 |
| Adjuvant radiation | |||||||
| No | 540 | 229 | 0.57(0.52, 0.62) | 174 | 0.74(0.69, 0.78) | ||
| Yes | 717 | 236 | 0.71(0.68, 0.75) | < .0001 | 193 | 0.85(0.81, 0.87) | < .0001 |
| Adjuvant chemotherapy | |||||||
| Anthracycline- based | 268 | 104 | 0.70(0.64, 0.75) | 82 | 0.87(0.82, 0.90) | ||
| Taxane-based | 53 | 15 | 0.64(0.46, 0.77) | 11 | 0.75(0.56, 0.86) | ||
| Anthracycline- and Taxane-based | 548 | 193 | 0.64(0.59, 0.68) | 153 | 0.80(0.76, 0.84) | ||
| Non-Anthracycline/Taxane-based | 47 | 24 | 0.69(0.53, 0.80) | .75 | 18 | 0.86(0.72, 0.94) | .10 |
(ER = estrogen receptor; PR = progesterone receptor; CI = confidence interval
Figure 1.
Table 3.
Cox proportional hazards models for RFS and OS.
| RFS
|
OS
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| ER/PR 1–5% vs. ER/PR <1% | 1.11 | 0.88 to 1.41 | 0.37 | 1.10 | 0.84 to 1.44 | 0.47 |
| ER/PR 6–10% vs. ER/PR <1% | 0.92 | 0.65 to 1.30 | 0.64 | 0.88 | 0.60 to 1.31 | 0.54 |
| Age: ≥ 50 years vs. < 50 | 0.73 | 0.60 to 0.88 | 0.001 | 0.75 | 0.61 to 0.93 | 0.009 |
| Tumor Size: T2 vs. T1 | 1.69 | 1.37 to 2.08 | <.0001 | 1.58 | 1.24 to 2.00 | 0.0002 |
| Tumor Size: T3/4 vs. T1 | 3.40 | 2.48 to 4.66 | <.0001 | 2.59 | 1.84 to 3.65 | <.0001 |
| Lymph Nodes: N1–3 vs. N0 | 1.85 | 1.50 to 2.28 | <.0001 | 2.14 | 1.68 to 2.72 | <.0001 |
| LVI: yes vs. no | 2.05 | 1.66 to 2.53 | <.0001 | 1.79 | 1.42 to 2.27 | <.0001 |
| Adjuvant Radiation: yes vs. no | 0.48 | 0.40 to 0.58 | <.0001 | 0.61 | 0.49 to 0.75 | <.0001 |
LVI = lymphovascular invasion; HR = hazard ratio; CI = confidence interval
There were 394 distant recurrences. The overall 3-year distant metastasis free survival (DMFS) rate was 71% (95% CI: 68%–74%). The 3-year DMFS estimate was 69%, 73% and 80% for groups A, B and C respectively (P = 0.26). ER/PR 1–5% and ER/PR 6–10% did not have significant lower risk of distant metastasis compared to ER/PR < 1% in the multivariate model.
Effect of endocrine therapy
Subset analyses were performed among 118 patients who received endocrine therapy and 1139 patients who did not received endocrine therapy (Table 2). The group receiving endocrine therapy did not differ significantly from the group not receiving endocrine therapy with relation to patient and clinical characteristics including age, tumor size, nodal status, LVI, histology, adjuvant radiation, surgery, PMRT, and adjuvant chemotherapy. The only significant difference was seen in terms of histological grade of tumor. Seventy-five percent (75%) of the patients who received hormonal therapy had grade III tumor compared to 90% who did not (P < .0001).
Among patients who received endocrine therapy, we observed no significant difference in 3-year RFS estimates among the groups ER/PR < 1% (61%; 95% CI: 42–75%), ER/PR 1–5% (73%; 95% CI: 53–85%) and ER/PR 6–10% (87%; 95% CI: 71–94%) (P = 0.10). The 3-year OS estimates were also similar among the groups: ER/PR < 1% (93%; 95% CI: 76–98%), ER/PR 1–5% (89%; 95% CI: 69–96%) and ER/PR 6–10% (95%; 95% CI: 80–.99%) (P = 0.45). Similarly, among patients who did not received endocrine therapy, there was no significant difference in 3-year RFS estimates among the groups ER/PR < 1% (64%; 95% CI: 60–67%), ER/PR 1–5% (66%; 95% CI: 58–73%) and ER/PR 6–10% (70%; 95% CI: 56–79%) (P = 0.93). There was also no significant difference in 3-year OS estimates: ER/PR < 1% (78%; 95% CI: 75–81%), ER/PR 1–5% (80%; 95% CI: 73–86%) and ER/PR 6–10% (83%; 95% CI: 71–.90%) (P = 0.45). Receipt of hormonal therapy did not significantly impact RFS within Group A (P = 0.57) and Group B (P = 0.84) patients but it had a marginal impact among Group C (P = 0.05) patients. Hormonal therapy did not significantly impact OS within the three groups of patients (Group A: P = 0.79; Group B: P = 0.53; Group C: P = 0.23).
When ER/PR < 1% was compared to ER/PR 1–10%, the survival outcomes were not significantly different (Table 2), P=0.10 for RFS and 0.43 for OS.
DISCUSSION
In this retrospective review of patients previously labeled as triple-negative breast cancers (defined as HER2/neu-negative and ER/PR < 10% by IHC), we sought to evaluate the association of low level of ER/PR expression (ER/PR 1% – 10%) with survival outcomes when compared to those with ER/PR < 1%. We observed that low levels of ER/PR expression (1–5%) by IHC did not appear to have a significant impact on survival outcomes. There appears to be a trend to a survival advantage in favor of patients with ER/PR 6–10%. Analyzed outcomes such as 3-year RFS, OS, and DMFS appear similar with ER/PR < 1%, ER/PR 1–5% and ER/PR 6–10%. Further, we also observed that addition of endocrine therapy to patients with low ER/PR expression (ER/PR 1–10%) did not appear to have significant effect on survival outcomes when compared patients with ER/PR < 1%. Our findings suggest that overall prognosis of patients with low ER/PR expression (ER/PR 1–10%) by IHC is probably similar to patients with undetectable ER/PR expression (ER/PR < 1%).
The recent ASCO/CAP guidelines have decreased the threshold for positivity of ER/PR assays by IHC to ≥ 1%.11 This recommendation will definitely increase the proportion of patients receiving endocrine therapy. Although, the clinical significance of substantial benefit of endocrine therapy in ER-positive cases is indisputable, we believe its application to patients with low level of ER/PR expression, specifically those with ER/PR 1–10%, need further study.18
Most of the prospective data regarding efficacy of endocrine therapy and level of ER expression has been derived from LBA not IHC.19,20,21,22,23 All available data for IHC has thus far been retrospective comparisons with LBA.19,20,21,22,23 Apart from the shortcomings that exist with such retrospective comparisons, the sample size for the subset of patients with ER/PR 1–10% in these studies was too small to draw any realistic conclusion. The Breast International Group (BIG) 1–98 trial compared 8,010 postmenopausal women with ER positive breast cancer randomized to treatment with letrozole and/or tamoxifen.24 Viale et al. performed a subsequent retrospective analysis to evaluate the predictive and prognostic value of receptor status on 3,596 patients from BIG 1–98 and showed that DFS was statistically significantly different according to ER expression.25 They concluded that a cutoff of 1% indicated better prognosis and at least some degree of endocrine responsiveness. However the total number of patients with ER level of 1–9% was 44. Likewise, the study by Regan et al. had 33 patients and Thomson et al. had 6 patients with ER/PR 1–10%.22,23 Our study is the largest in terms of number of patients in the subgroup with ER/PR expression 1–10% by IHC (n = 360) and also in terms of patients who received endocrine therapy in this subgroup (n = 118). The College of American pathologists’ consensus statement 1999 acknowledged that although there was high level of correlation between IHC and LBA, there were few studies demonstrating predictive abilities of IHC assays.26 The previous studies, as seen above were underpowered to assess the impact such low levels of ER/PR expression and effect of endocrine therapy on survival outcomes in this particular subgroup (ER/PR 1–10%). We therefore feel that, further corroborative studies are needed prior to establishing a firm threshold for ER/PR positivity.
Also, there is significant heterogeneity in studies with regards to the scoring system used for IHC. Barnes et al. evaluated and compared LBA and various scoring systems for IHC, namely histo (H) score, proportion score, category score, quick score and Allred score.19 The analysis done by Harvey et al. which advocated a cutoff of ≥ 3 IHC score exemplifies the disparities among these semi-quantitative scoring systems.20 Harvey et al used the Allred score (Intensity score + Proportion Score) which is not the same as proportion score advocated by the recent recommendations. As is evident, these scoring systems are not interchangeable and thus it is difficult to make irrefutable conclusions regarding cutoff value from data derived from these older studies. Collins et al. demonstrated that ER expression is essentially bimodal in distribution.27 In a review of immunostains from 825 cases majority (99%) were either ER strongly positive (Allred score 7 or 8) or absolutely negative (Allred score 0). This corresponds to either staining in at least 70% of cells (80% cases) or a complete absence of staining (19%). The remaining 1% cases had staining in 20–60% of cells (Allred score 5 or 6). Weak ER staining (1–10%) was rare. This furthers the discussion that the distribution curves in a random sample will always be skewed towards improved survival in ER/PR > 1% group when compared to ER/PR < 1% due to the dominant prevalence of higher level of ER expression. This may explain why better outcomes for the group with ER/PR positive status in studies may not reflect the response of low ER/PR level tumors per se. Most of the studies mentioned above had a heterogeneous population with regards to HER2/neu status and did not make any discrimination between HER2 positive or negative disease. HER2/neu amplification is negatively correlated with estrogen receptor and progesterone receptor status.28 In an unselected population for HER2/neu status, there will be a greater prevalence of HER2/neu amplification in tumors with ER/PR level of <1% than ER/PR 1–10%. This could be a potential explanation of the poorer prognosis of ER/PR < 1% compared to ER/PR 1–10% seen in these studies. There is also significant crosstalk between estrogen receptor and HER2/neu leading to alteration in efficacy of endocrine therapy.29 We therefore looked at HER2/neu negative patients only to remove any confounding effects resulting from interactions of HER2/neu with ER/PR and to rule out HER2/neu both as a prognostic variable and a predictive factor for trastuzumab therapy.
Although, the benefit of endocrine therapy is convincing enough to recommend it in all patients, even those with minimal ER/PR expression, we must be keep in mind that tamoxifen and aromatase inhibitor therapy is not without its fair share of side effects. The vasomotor symptoms (night sweats and hot flashes), gynecologic symptoms (vaginal discharge and genital itching), sexual dysfunction, increased rate of endometrial cancer (risk ratio = 2.53), stroke, pulmonary embolism and deep-vein thrombosis symptoms are still worrisome in women using tamoxifen.30,31 Aromatase inhibitors (AIs) are better tolerated and have fewer aforementioned side effects, but are still associated with increased risk of osteopenia, osteoporosis and fractures.32 In light of this side effect profile, a realistic assessment of benefit in patients with ER/PR expression between 1 and 10% will be instrumental in helping patients and clinicians make an informed decision regarding endocrine therapy in this subgroup of patients.
The major drawback of our study is the limited sample size. Even though this is one of the largest reviews of TNBC and ER/PR 1–10% group, it is still modest as compared to breast cancer study standards. Although it doesn’t rule out clinically significant differences in outcomes or benefit from adjuvant endocrine therapy in this subgroup, we feel that the results are provocative enough to encourage larger analysis. Of note there was a 13% absolute difference in RFS and a 9% absolute difference in OS between groups A and C. Although the difference was statistically nonsignificant, the small number of patients in Group C results in insufficient power to rule out a clinically meaningful impact. We therefore feel that additional analyses to address this question are clearly indicated for ER/PR expression between 6%–10%.
Another source of error is that there was no central testing for IHC in our study. The pathologies were received from a variety of outside laboratories and although slides were reviewed by an MDACC pathologist there could still be inaccuracies due to lacking in assay standardization across laboratories.33 Quantitative estimation of receptor expression varies substantially between laboratories due to differences in methodology, tissue storage and type of antibody used, and this variation cannot be eliminated by a central review.9,34,35 However, this weakness was across groups and should not effect overall outcomes of the study.
We therefore conclude that the prognosis of tumors with low ER/PR (ER/PR 1–10%) expression, especially ER/PR 1–5% does not differ significantly from tumors with undetectable ER/PR levels (ER/PR <1%). The response of this subset (ER/PR 1–10%) of breast cancers to endocrine therapy has not been established as yet. Hence, a randomized prospective trial for tumors with such low levels of ER/PR expression by IHC (1% – 10%) is required to lift the shroud of uncertainty that envelops the treatment of this faction with endocrine therapy. The appropriate threshold for ER/PR positivity and endocrine therapy can then be established with certainty.
Acknowledgments
Funding: The work was supported in part by National Cancer Institute 1K23CA121994-01, National Cancer Institute Breast Specialized Program for Research Excellence (Developmental Grant) P50-CA116199 (to AMG). National Cancer Institute through The University of Texas M. D. Anderson’s Cancer Center Support Grant (P30 CA016672). The M. D. Anderson Breast Cancer Management System is supported in part by the Nelly B. Connally Breast Cancer Research Fund.
References
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Arimidex, Tamoxifen, Forbes JF, Cuzick J, et al. Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes A, Jasani B, Balaton AJ, et al. Study of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe. Documentation of poor reliability and identification of insufficient microwave antigen retrieval time as a major contributory element of unreliable assays. Am J Clin Pathol. 2001;115:44–58. doi: 10.1309/H905-HYC1-6UQQ-981P. [DOI] [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 11.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 13.Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 14.Giobbie-Hurder A, Price KN, Gelber RD International Breast Cancer Study Group, BIG 1–98 Collaborative Group. Design, conduct, and analyses of Breast International Group (BIG) 1–98: a randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials. 2009;6:272–287. doi: 10.1177/1740774509105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The World Health Organization histological typing of breast tumors second edition. The World Health Organization. Am J Clin Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 16.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 17.Bezwoda WR, Esser JD, Dansey R, Kessel I, Lange M. The value of estrogen and progesterone receptor determinations in advanced breast cancer. Estrogen receptor level but not progesterone receptor level correlates with response to tamoxifen. Cancer. 1991;68:867–872. doi: 10.1002/1097-0142(19910815)68:4<867::aid-cncr2820680432>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Redmond C, Brown A, et al. Treatment of primary breast cancer with chemotherapy and tamoxifen. N Engl J Med. 1981;305:1–6. doi: 10.1056/NEJM198107023050101. [DOI] [PubMed] [Google Scholar]
- 19.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of estrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74:1445–1455. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 21.Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. [PubMed] [Google Scholar]
- 22.Thomson CS, Twelves CJ, Mallon EA, Leake R, Scottish Cancer Trials Breast Group Scottish Cancer Therapy Network. Adjuvant ovarian ablation vs CMF chemotherapy in premenopausal breast cancer patients: trial update and impact of immunohistochemical assessment of ER status. Breast. 2002;11:419–429. doi: 10.1054/brst.2002.0451. [DOI] [PubMed] [Google Scholar]
- 23.Regan MM, Viale G, Mastropasqua MG, et al. International Breast Cancer Study Group. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 24.Thürlimann B, Keshaviah A, et al. Breast International Group (BIG) 1–98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 25.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol. 2007;25:3846–3852. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;24:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 27.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am J Clin Pathol. 2005;123:16–20. doi: 10.1309/hcf035n9wk40etj0. [DOI] [PubMed] [Google Scholar]
- 28.Berns EM, Klijn JG, Van Staveren IL, et al. Prevalence of amplification of the oncogenes c-myc, HER2/neu, and int-2 in one thousand human breast tumours: correlation with steroid receptors. Eur J Cancer. 1992;28:697–700. doi: 10.1016/s0959-8049(05)80129-7. [DOI] [PubMed] [Google Scholar]
- 29.Dowsett M, Harper-Wynne C, Boeddinghaus I, Salter J, Hills M, Dixon M. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res. 2001;61:8452–8458. [PubMed] [Google Scholar]
- 30.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 31.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 32.Arimidex, Tamoxifen, Buzdar A, Howell A, et al. Alone or in Combination Trialists’ Group. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 33.Layfield LJ, Gupta D, Mooney EE. Assessment of Tissue Estrogen and Progesterone Receptor Levels: A Survey of Current Practice, Techniques, and Quantitation Methods. Breast J. 2000;6:189–196. doi: 10.1046/j.1524-4741.2000.99097.x. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88:1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 35.Cheang MC, Treaba DO, Speers CHSK, et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24:5637–5644. doi: 10.1200/JCO.2005.05.4155. [DOI] [PubMed] [Google Scholar]