Abstract
Objective
We evaluated the influence of physician-diagnosed migraine on blood pressure levels and the risk of hypertensive disorders of pregnancy in a clinic-based prospective cohort study of 3,373 healthy pregnant women.
Background
The relationship between migraine and blood pressure is controversial with results from several studies suggesting positive associations, while others suggest null or inverse associations. To our knowledge, no previous study has investigated blood pressure profiles among pregnant migraineurs.
Methods
We abstracted blood pressure values and delivery information from medical records of women presenting to prenatal clinics in Washington State. Mean blood pressure differences for pregnant migraineurs and non-migraineurs were estimated in regression models, using generalized estimating equations. We calculated, odds ratios (OR) and 95% confidence intervals (95%CIs) for gestational hypertension and preeclampsia in relation migraine status.
Results
Mean first, second and third trimester systolic blood pressure (SBP) were elevated among pregnant migraineurs as compared with non-migraineurs. Migraineurs had higher mean third trimester SBP (4.08 mm Hg) than non-migraineurs. Trimester-specific diastolic blood pressure (DBP) values were variably related with migraine status. Mean first (0.82 mm Hg) and third(2.39 mm Hg) trimester DBP were higher, and second trimester DBP values were lower (−0.24) among migraineurs as compared with non-migraineurs. Migraineurs had a 1.53-fold increased odds of preeclampsia (95%CI 1.09 to 2.16). Additionally, migraineurs who were overweight or obese had a 6.10-fold increased odds of preeclampsia (95%CI 3.83 to 9.75) as compared with lean non-migraineurs.
Conclusions
Pregnant migraineurs had elevated blood pressures, particularly SBP measured in the third trimester, and a higher risk of preeclampsia than pregnant women without migraine. Observed associations were more pronounced among overweight or obese migraineurs. Our findings add to the accumulating evidence of adverse pregnancy outcomes among migraineurs.
Keywords: Migraine, Blood Pressure, Hypertension, Preeclampsia, Pregnancy
INTRODUCTION
Migraine, a recurrent neurovascular headache disorder, is characterized by episodes of severe throbbing, pulsatile headache associated with nausea, vomiting, photophobia, phonophobia, and aversion to physical activity1, 2. The prevalence of migraine varies considerably across the life-course and is most prevalent among women during their childbearing years3–5. An expanding literature links migraine with a broad range of ischemic vascular disorders including ischemic stroke, angina, myocardial infarction, claudication, and cardiovascular mortality6–9. The risk of stroke has been reported to be greater among migraineurs with aura, those who smoke, those women taking oral contraceptives, and those with migraine occurring during the peripartum period10, 11. Although positive associations of migraine with elevated blood pressure have been documented by some12–15, but not all16–20 investigators, abnormalities in sympathetic function favoring the development and progression of hypertension are thought to account for increased risks of vascular ischemic disorders among migraineurs21.
Adverse perinatal outcomes, characterized by ischemic placental disorders, are noted to be increased among pregnant migraineurs4, 22. Pregnancies complicated by preterm delivery23, low birth weight23, placental abruption24, and hypertensive disorders of pregnancy including preeclampsia are now recognized to be more common among pregnant migraineurs4,23,25–29. Although the mechanisms for these observed associations are incompletely understood, available evidence suggest that these adverse perinatal outcomes may be attributable to lower cardiac output and relatively higher total peripheral vascular resistance, characteristics rarely detected in uncomplicated pregnancies30, 31. At present, it is unknown whether pregnant migraineurs have lower cardiac output or relatively higher total peripheral vascular resistance than pregnant non-migraineurs. To our knowledge, no previous study investigated blood pressure profiles among pregnant migraineurs. In order to better understand the influence of migraine on: (1) blood pressure patterns across gestation, and (2) the development of gestational hypertension and preeclampsia, we conducted a prospective analysis in a large cohort of pregnant women. We sought to investigate the extent to which, if at all, physician-diagnosed migraine is associated with (1) trimester-specific systolic, diastolic and mean arterial blood pressures; and (2) risk of gestational hypertension and preeclampsia. Because prior studies suggested that migraine-preeclampsia associations may be modified by pre-pregnancy overweight status26, we completed exploratory analyses to examine this possibility.
METHODS
Study Population and Setting
This analysis is based on data collected from a cohort of healthy women attending prenatal care clinics affiliated with Swedish Medical Center in Seattle, Washington and Tacoma General Hospital in Tacoma, Washington. The inclusion criteria for the study were initiation of prenatal care before 20 weeks gestation, participant age 18 years of age or older, participant able to speak and read English, and participant who planned to carry the pregnancy to term and to deliver at either hospital. At 14 weeks gestation, on average, participants reported socio-demographic, behavioral, and health characteristics in a structured interview. After delivery, study personnel abstracted data from participants’ hospital labor and delivery medical records and clinic records. Medical record abstractors recorded information on blood pressures and weight measured at routine prenatal care visits. Between December 1996 and February 2008, 5,063 eligible women were approached and 4,000 (approximately 79%) agreed to participate. The exclusion criteria for these analyses were as follows: early pregnancy loss (n=42), missing data on migraine diagnosis (n=267), pre-gestational chronic hypertension (n=166). We also excluded 152 women who enrolled in the study but who were lost to follow-up. A total of 3,373 women remained for analysis. All study procedures were approved by the Institutional Review Board of Swedish Medical Center. All participants provided written informed consent. Interviewers, medical records abstractors and data managers were blinded to specific study hypotheses. Diagnoses of hypertensive disorders were made without knowledge of migraine status.
Description of Covariates and Trimester-Specific Blood Pressure Assessment
At the time of enrollment in the study, a 45 to 60 minute structured questionnaire was administered by a trained interviewer. As reported previously, information on medical and reproductive histories and socio-demographic and lifestyle characteristics including age at physician diagnosis of migraine was collected32. History of migraine was ascertained by asking women the following questions: (1) “Has a physician ever told you that you have migraine?” and (2) “How old were you when a physician told you that you had migraine?” Pre-pregnancy weight and height were also based on self-reports made during the interview. Pre-pregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Our measure of weight gain during pregnancy was the weight gain between pre-pregnancy and 18 to 22 weeks gestation (henceforth referred to as early pregnancy). This measure of early pregnancy weight gain is not likely to be influenced by preeclampsia-associated edema, dietary modifications motivated by diagnosis of pregnancy complications, or decreased energy expenditure prompted by prescribed bed rest. We calculated early pregnancy weight gain by subtracting pre-pregnancy weight from weight at 18 to 22 weeks gestation. Early pregnancy weight gain values are expressed as kilograms. Gestational age of pregnancy was determined using self-reported last normal menstrual period and this date was confirmed by ultrasound exams completed prior to 20 weeks gestation. Gestational hypertension and preeclampsia were defined according to published diagnostic criteria33. Gestational hypertension was defined as sustained blood pressure of ≥140/90 mm Hg with readings performed ≥6 hours apart. Preeclampsia was defined as gestational hypertension further complicated by proteinuria which was based on urine protein concentration of ≥30 mg/dl or 1+ on a urine dipstick on ≥2 urine specimens collected ≥4 hours apart. We used the definition in the literature34 to define first, second, and third trimesters as follows: first trimester <15 weeks; second trimester 15–28 weeks; and third trimester ≥29 weeks gestation.
All pregnancy-associated blood pressure measurements (i.e., systolic and diastolic blood pressures) along with the date and gestational age when the blood pressure was taken were abstracted from participants’ clinical and hospital medical records. For the purposes of this study we used antepartum clinical blood pressures taken and recorded during routine visits. Blood pressures taken upon admission for inpatient observation, or admission to the emergency room were considered only when blood pressure from an expected antepartum visit was unavailable. Blood pressures taken during active labor or during the postpartum period were not considered in these analyses.
As would be expected in any antepartum population, a subgroup of women, particularly those with a complicated pregnancy, had more than the expected numbers of antepartum visits and recorded blood pressures. To prevent overrepresentation in the sample of such women, we randomly selected blood pressure readings from among appropriate gestational age categories if/when there was a larger than expected number of blood pressure recordings. In instances where blood pressures were taken twice (on the same day) to confirm an initial reading, we randomly selected one of the associated readings. Detailed descriptions of methods used to develop the blood pressure database have been described elsewhere35. An average of 12.2 (median: 13; interquartile range: 11–14) blood pressure values were recorded for each study participant. Details of the constitution of this database have been previously described35,36.
During the study period many different health care providers made blood pressure readings as part of routine clinical practice. Although the measures are not strictly standardized as would be in a clinical trial, blood pressures were taken using standard mercury sphygmomanometers (scaled to even numbers), and patients were rested and seated during examinations. Mean arterial pressure (MAP), considered an integrated parameter of blood pressure, is known to be more reproducible than individual systolic (SBP) and diastolic (DBP) blood pressures37. We therefore computed mean arterial pressures for each subject according to the following formula: MAP = DBP + ⅓ (SBP − DBP).
Statistical Analyses
The blood pressure values (SBP, DBP, MAP) were the primary dependent variables and physician diagnosed migraine (yes, no) was the primary covariate. Linear regression models were fitted using generalized estimating equations to adjust for repeated BP measurements on the same woman38. Based on exploratory investigations of the correlation between repeated measurements, an exchangeable correlation structure was assumed for all analyses. Robust estimates of standard errors (SE) were used throughout. All models were fitted with trimester as an effect modifier. Test statistics were constructed as the ratio of the relevant point estimate to its robust SE and associated P-values calculated from normal tables. Logistic regression procedures were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for gestational hypertension and preeclampsia in relation to migraine. These analyses were completed to address our secondary aim of evaluating the extent to which history of migraine is a risk factor for hypertensive disorders of pregnancy (e.g., gestational hypertension and preeclampsia combined; and each condition studied separately). Statistical significance was defined at P-value <0.05. Analyses were carried out using Stata Software, Version 9.239.
RESULTS
The socio-demographic characteristics of participants according to migraine are presented in Table 1. Approximately 17.4% of women (n=586) in this cohort reported a history of physician diagnosed migraine. Migraineurs and non-migraineurs were similar with regards to age, race/ethnicity, educational attainment, leisure time physical activity and smoking status. Migraineurs were more likely to be obese than non-migraineurs. First trimester SBP, DBP, and MAP values for the entire cohort were as follows (mean±SE): 112.4±0.2 mm Hg, 68.5±0.1 mm Hg, and 83.1±0.1 mm Hg.
Table 1.
Characteristics of the study cohort (N = 3,373) by categories of history of migraine diagnosis
| Characteristics | No Migraine | Yes Migraine | P-value |
|---|---|---|---|
| N = 2787 % |
N = 586 % |
||
| Participant age (y) | |||
| < 25 | 3.9 | 3.6 | 0.609 |
| 25–34 | 62.5 | 64.7 | |
| ≥ 35 | 33.6 | 31.7 | |
| Participant race/ethnicity | |||
| Non-Hispanic White | 86.1 | 88.6 | 0.068 |
| African American | 1.7 | 1.4 | |
| Asian | 7.8 | 4.8 | |
| Other | 4.5 | 5.3 | |
| Multiparous | 35.8 | 39.1 | 0.135 |
| < 12 years education | 3.7 | 4.4 | 0.371 |
| Smoked during pregnancy | 5.9 | 6.2 | 0.829 |
| Alcohol use during pregnancy | 10.2 | 9.2 | 0.475 |
| No exercise during pregnancy | 13.0 | 12.0 | 0.492 |
| No prenatal vitamin | 2.5 | 2.9 | 0.553 |
| Pre-pregnancy body mass index (kg/m2) | |||
| < 18.5 | 4.4 | 4.1 | 0.021 |
| 18.5–24.99 | 72.6 | 70.7 | |
| 25–29.99 | 16.0 | 14.5 | |
| ≥ 30 | 7.0 | 10.8 | |
| Early Pregnancy weight gain (kg)1 | 6.9±3.7 | 6.8±4.4 | 0.443 |
| Gestational age at delivery | |||
| < 28 | 0.5 | 0.2 | 0.364 |
| 28–36 | 9.7 | 8.2 | |
| 37–40 | 75.9 | 78.7 | |
| > 40 | 13.9 | 13.0 | |
| Gestational hypertension | 5.3 | 8.2% | 0.006 |
| Preeclampsia | 2.3 | 2.7 | 0.145 |
Early pregnancy weight gain is weight gained during first 20 weeks of gestation
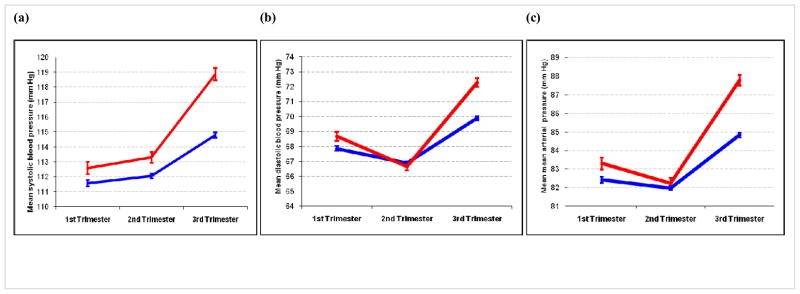
Mean second trimester SBP (112.9±0.1 mm Hg), DBP (67.6±0.1 mm Hg), and MAP (82.7±0.1 mm Hg) values were all slightly lower than mean values for the first trimester (p<0.0001). Mean third trimester SBP (115.6±0.2 mm Hg), DBP (70.4±0.1 mm Hg), and MAP (84.4±0.1 mm Hg) values were statistically significantly higher than first and second trimester values, respectively (P-values <0.0001). These somewhat J-shaped patterns of mean diastolic blood pressure across trimesters are consistent with reports in the literature40.
The associations of trimester-specific mean SBP and history of migraine are summarized in Table 2. Mean first trimester SBP was slightly elevated among migraineurs compared with non-migraineurs. Using non-migraineurs as the reference group, we noted that mean first trimester SBP values were 1.20 mm Hg higher among migraineurs. This difference in first trimester SBP was statistically significant. The differences in first trimester mean SBP for migraineurs vs. non-migraineurs remained essentially unchanged (Δ=1.13 mm Hg; 95% CI 0.3 to 2.0) after we adjusted for confounding by age, race/ethnicity, parity, smoking status, and prenatal vitamin use. Further adjustment for pre-pregnancy BMI slightly attenuated the difference (Δ=1.01 mm Hg; 95% CI 0.2 to 1.8). Second trimester mean SBP values were positively associated with migraine history. After adjusting for age, race/ethnicity, parity, smoking status, prenatal vitamin use and pre-pregnancy BMI, we noted that migraineurs had higher SBP values (1.25 mm Hg; 95% CI 0.5 to 2.0) than non-migraineurs. There was strong evidence that migraine was positively associated with mean SBP in the third trimester. After adjusting for age, race/ethnicity, parity, smoking status, prenatal vitamin use, and pre-pregnancy BMI, migraineurs had considerably higher mean third trimester SBP (4.08 mm Hg; 95% CI 3.3 to 4.9) than non-migraineurs.
Table 2.
Unadjusted and adjusted mean (SE) systolic blood pressure (SBP), by trimester and history of migraine
| Migraine | First Trimester | Second Trimester | Third Trimester | |||
|---|---|---|---|---|---|---|
| Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | |
| NO | 112.30 [0.18] | 112.77 [0.16] | 115.52 [0.18] | |||
| YES | 113.50 [0.43] | 1.20 (0.3 to 2.1) | 114.23 [0.38] | 1.46 (0.7 to 2.2) | 119.78 [0.43] | 4.26 (3.4 to 5.1) |
|
| ||||||
|
MODEL 1
| ||||||
| Migraine | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 112.88 [0.22] | 113.34 [0.16] | 116.09 [0.18] | |||
| YES | 114.01 [0.44] | 1.13 (0.3 to 2.0) | 114.72 [0.39] | 1.38 (0.6 to 2.1) | 120.29 [0.43] | 4.20 (3.4 to 5.0) |
|
| ||||||
|
MODEL 2
| ||||||
| Migraine | 2 Adjusted Mean [SE] | Δ (95% CI) | 2 Adjusted Mean [SE] | Δ (95% CI) | 2 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 111.58 [0.22] | 112.05 [0.16] | 114.79 [0.18] | |||
| YES | 112.59 [0.41] | 1.01 (0.2 to 1.8) | 113.30 [0.36] | 1.25 (0.5 to 2.0) | 118.87 [0.40] | 4.08 (3.3 to 4.9) |
Adjusted for age, race/ethnicity, parity, smoking and prenatal vitamin use. Adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age.
Adjusted for age, race/ethnicity, parity, smoking, prenatal vitamin use, and pre-pregnancy body mass index. Adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Associations of migraine with trimester specific mean DBP and MAP are summarized in Tables 3 and 4, respectively. Mean first trimester DBP, before adjusting for confounders, was higher for migraineurs (mean=69.21, SE=0.32, mm Hg) than non-migraineurs (mean=68.38, SE=0.12 mm Hg). As can been seen in the third part of Table 3, mean first-trimester DBP values continued to be elevated for migraineurs compared with non-migraineurs after we controlled for potential confounders, including pre-pregnancy BMI (Δ=0.82 mm Hg; 95% CI 0.2 to 1.4). Mean second trimester DBP values were not statistically significantly different between migraineurs and non-migraineurs (Δ=−0.24 mm Hg; 95% CI −0.8 to 0.3) after controlling for confounders including pre-pregnancy BMI. Conversely, migraine status was strongly and positively associated with higher mean third trimester DBP values (Δ=2.39 mm Hg; 95% 1.8 to 3.0) after adjustments for confounders.
Table 3.
Unadjusted and adjusted mean (SE) diastolic blood pressure (DBP), by trimester and history of migraine
| Migraine | First Trimester | Second Trimester | Third Trimester | |||
|---|---|---|---|---|---|---|
| Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | |
| NO | 68.38 [0.12] | 67.45 [0.11] | 70.41 [0.13] | |||
| YES | 69.21 [0.32] | 0.93 (0.3 to 1.5) | 67.32 [0.28] | −0.13 (−0.7 to 4.2) | 72.91 [0.30] | 2.50 (1.9 to 3.1) |
|
| ||||||
|
MODEL 1
| ||||||
| Migraine | *Adjusted Mean [SE] | Δ (95% CI) | *Adjusted Mean [SE] | Δ (95% CI) | *Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 68.75 [0.16] | 67.81 [0.11] | 70.77 [0.13] | |||
| YES | 69.65 [0.32] | 0.90 (0.3 to 1.5) | 67.66 [0.28] | −0.15 (−0.7 to 4.0) | 72.25 [0.30] | 2.48 (1.9 to 3.1) |
|
| ||||||
|
MODEL 2
| ||||||
| Migraine | **Adjusted Mean [SE] | Δ (95% CI) | **Adjusted Mean [SE] | Δ (95% CI) | **Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 67.86 [0.15] | 66.90 [0.11] | 69.89 [0.13] | |||
| YES | 68.68 [0.30] | 0.82 (0.2 to 1.4) | 66.66 [0.26] | −0.24 (−0.8 to 0.3) | 72.28 [0.29] | 2.39 (1.8 to 3.0) |
Adjusted for age, race/ethnicity, parity, smoking and prenatal vitamin use. Adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age.
Adjusted for age, race/ethnicity, parity, smoking, prenatal vitamin use, and pre-pregnancy body mass index. Adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
Table 4.
Unadjusted and adjusted mean (SE) mean arterial pressure (MAP), by trimester and history of migraine
| Migraine | First Trimester | Second Trimester | Third Trimester | |||
|---|---|---|---|---|---|---|
| Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | Unadjusted Mean [SE] | Δ (95% CI) | |
| NO | 83.02 [0.13] | 82.55 [0.11] | 85.43 [0.13] | |||
| YES | 84.04 [0.32] | 1.02 (0.4 to 1.6) | 82.96 [0.29] | 0.42 (−0.1 to 1.0) | 88.51 [0.31] | 3.08 (2.5 to 3.7) |
|
| ||||||
|
MODEL 1
| ||||||
| Migraine | *Adjusted Mean [SE] | Δ (95% CI) | *Adjusted Mean [SE] | Δ (95% CI) | *Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 83.45 [0.16] | 82.98 [0.10] | 85.86 [0.13] | |||
| YES | 84.43 [0.32] | 0.98 (0.4 to 1.6) | 83.36 [0.29] | 0.38 (−0.2 to 0.9) | 88.91 [0.31] | 3.05 (2.4 to 3.7) |
|
| ||||||
|
MODEL 2
| ||||||
| Migraine | **Adjusted Mean [SE] | Δ (95% CI) | **Adjusted Mean [SE] | Δ (95% CI) | **Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 82.42 [0.16] | 81.97 [0.11] | 84.83 [0.13] | |||
| YES | 83.30 [0.33] | 0.88 (0.3 to 1.5) | 82.24 [0.27] | 0.27 (−0.2 to 0.8) | 87.78 [0.29] | 2.95 (2.4 to 3.5) |
Adjusted for age, race/ethnicity, parity, smoking and prenatal vitamin use. Adjusted mean values reported for MODEL 1 are from a regression model based on the entire study cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age.
Adjusted for age, race/ethnicity, parity, smoking, prenatal vitamin use, and pre-pregnancy body mass index. Adjusted mean values reported for MODEL 2 are from a regression model based on the entire cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women from 25–34 years of age, with a pre-pregnancy BMI < 25 kg/m2.
A similar, though not as pronounced J-shaped pattern of MAP was observed across the three trimesters (Table 4). After adjusting for confounders, evidence of positive associations of mean first trimester MAP values with migraine status (Δ=0.88 mm Hg; 95% 0.3 to 1.5). There was no evidence of an association of migraine with second trimester MAP values (Δ=0.27 mm Hg; 95% −0.2 to 0.8). Third trimester MAP values, however, were statistically significantly higher among migraineurs as compared with non-migraineurs (Δ=2.95 mm Hg; 95% 2.4 to 3.5) after adjustment for confounders including pre-pregnancy BMI. Summaries of the associations of trimester-specific MAP with migraine status after stratification by parity (Table 5) and pre-pregnancy overweight status (Table 6) are provided. There was no evidence of effect modification (P-values of interaction terms all >0.05) by either covariate. Migraine appeared to be similarly associated with increased third trimester MAP values among nulliparous (Δ=3.31 mm Hg; 95% 2.6 to 4.0) women and among multiparous (Δ=3.26 mm Hg; 95% 1.5 to 3.3) women (Table 5). Migraine appeared to be more strongly associated with increased third trimester MAP values among overweight (Δ=3.43 mm Hg; 95% 2.3 to 4.6) women than among lean (Δ=2.80 mm Hg; 95% 2.1 to 3.5) women (Table 6). Lastly, observed associations between migraine and elevated blood pressures, particularly third trimester values, were evident even among women who remained normotensive throughout pregnancy. After excluding women who developed gestational hypertension or preeclampsia, migraineurs had increased third trimester MAP values (Δ=2.27 mm Hg; 95% 1.7 to 2.8) compared to non-migraineurs (Supplementary Table).
Table 5.
Adjusted mean (SE) mean arterial pressure (MAP), by trimester and history of migraine, after stratification by parity
| First Trimester | Second Trimester | Third Trimester | ||||
|---|---|---|---|---|---|---|
|
All Women (N = 3,373)
| ||||||
| Migraine | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 82.42 [0.16] | 81.97 [0.11] | 84.83 [0.13] | |||
| YES | 83.30 [0.33] | 0.88 (0.3 to 1.5) | 82.24 [0.27] | 0.27 (−0.2 to 0.8) | 87.78 [0.29] | 2.95 (2.4 to 3.5) |
|
| ||||||
|
Nulliparous Women (N = 2,146)
| ||||||
| Migraine | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 82.21 [0.18] | 81.90 [0.13] | 84.98 [0.16] | |||
| YES | 82.93 [0.38] | 0.72 (−0.3 to 1.5) | 82.16 [0.35] | 0.26 (−0.4 to 0.9) | 88.29 [0.38] | 3.31 (2.6 to 4.0) |
|
| ||||||
|
Multiparous Women (N = 1,227)
| ||||||
| Migraine | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 81.86 [0.27] | 81.12 [0.18] | 83.61 [0.21] | |||
| YES | 82.98 [0.49] | 1.12 (0.2 to 2.1) | 81.39 [0.41] | 0.27 (−0.5 to 1.1) | 85.97 [0.46] | 3.26 (1.5 to 3.3) |
Adjusted for age, race/ethnicity, smoking, and pre-pregnancy body mass index. Adjusted mean values reported are from a regression model based on the entire study cohort (N = 3,373) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women 25–34 years of age, with a pre-pregnancy BMI <25 kg/m2.
Adjusted for age, race/ethnicity, parity, smoking and pre-pregnancy body mass index. Adjusted mean values reported are from a regression model based on nulliparous women only (N = 2,146) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women 25–34 years of age, with a pre-pregnancy BMI <25 kg/m2.
Adjusted for age, race/ethnicity, parity, smoking and pre-pregnancy body mass index. Adjusted mean values reported are from a regression model based on multiparous women only (N = 1,227 with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women 25–34 years of age, with a pre-pregnancy BMI <25 kg/m2.
Table 6.
Adjusted mean (SE) mean arterial pressure (MAP), according to trimester and history of migraine, after stratification by overweight status
| First Trimester | Second Trimester | Third Trimester | ||||
|---|---|---|---|---|---|---|
|
All Women (N = 3,733)
| ||||||
| Migraine | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) | 1 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 82.42 [0.16] | 81.97 [0.11] | 84.83 [0.13] | |||
| YES | 83.30 [0.33] | 0.88 (0.3 to 1.5) | 82.24 [0.27] | 0.27 (−0.2 to 0.8) | 87.78 [0.29] | 2.95 (2.4 to 3.5) |
|
| ||||||
|
Lean Women (BMI < 25 kg/m2) (N = 2,584)
| ||||||
| Migraine | 2 Adjusted Mean [SE] | Δ (95% CI) | 2 Adjusted Mean [SE] | Δ (95% CI) | 2 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 82.41 [0.17] | 81.92 [0.12] | 84.80 [0.14] | |||
| YES | 83.08 [0.39] | 0.67 (−0.1 to 1.4) | 82.05 [0.30] | 0.13 (−0.5 to 0.7) | 87.60 [0.33] | 2.80 (2.1 to 3.5) |
|
| ||||||
|
Overweight Women (BMI ≥ 25 kg/m2) (N = 789)
| ||||||
| Migraine | 3 Adjusted Mean [SE] | Δ (95% CI) | 3 Adjusted Mean [SE] | Δ (95% CI) | 3 Adjusted Mean [SE] | Δ (95% CI) |
|
| ||||||
| NO | 87.69 [0.35] | 87.30 [0.22] | 90.18 [0.27] | |||
| YES | 89.24 [0.69] | 1.55 (0.4 to 2.7) | 88.03 [0.58] | 0.73 (−0.4 to 1.9) | 93.61[0.60] | 3.43 (2.3 to 4.6) |
Adjusted for age, race/ethnicity, parity, and smoking. Adjusted mean values reported are from a regression model based on the entire study cohort (N = 3,733) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women 25–34 years of age.
Adjusted for age, race/ethnicity, parity, and smoking. Adjusted mean values reported are from a regression model based on lean women only (N = 2,584) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking women 25–34 years of age, with a pre-pregnancy BMI <25 kg/m2
Adjusted for age, race/ethnicity, parity, and smoking. Adjusted mean values reported are from a regression model based on overweight women only (N= 789) with indicators variables specified to represent Non-Hispanic white, nulliparous, non-smoking, women 25–34 years of age, with a pre-pregnancy BMI ≥ 25 kg/m2
The secondary aim of this analysis was to assess the risk of incident gestational hypertension and preeclampsia among women in the cohort (Table 7). Approximately 7.4% of the women in the study cohort were diagnosed with either gestational hypertension (n=196) or preeclampsia (n=80). After adjustment for age, parity, smoking status, and pre-pregnancy BMI history of migraine was not statistically significantly associated with odds of gestational hypertension (OR=1.22; 95% CI 0.70 to 2.14). A history of migraine was associated with a 1.53-fold increased odds of preeclampsia (95% CI 1.09 to 2.16) after adjustment for confounders. Finally, in Table 8 we summarize results from analyses designed to assess independent and joint effects of migraine and pre-pregnancy overweight or obesity status on risk of incident hypertensive disorders of pregnancy. For these analyses, lean women without a history of migraine served as the reference group. Compared with the reference group, lean (<25 kg/m2) migraineurs did not have an increased risk of preeclampsia (adjusted OR=1.16; 95% CI 0.71 to 1.91). The OR for overweight or obese (≥25 kg/m2) non-migraineurs was 2.72 (95% CI 1.74 to 3.83). Migraineurs who were overweight or obese, had a 6.10-fold increased odds of preeclampsia (95% CI 3.83 to 9.75) (P-value for multiplicative interaction=0.183). Odds ratios of similar magnitudes were observed for gestational hypertension.
Table 7.
Multivariable adjusted odds ratios (OR) and 95% confidence interval (CI) of gestational hypertension and preeclampsia according to physician diagnosed migraine
| Migraine
|
||
|---|---|---|
| NO N = 2787 |
YES N = 586 |
|
| Normotensives n (%) | 2575 (92.4%) | 522 (89.1%) |
| Any Hypertensive Disorder in Pregnancy n (%) | 212 (7.6%) | 64 (10.9%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 1.49 (1.11 to 2.00) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 1.44 (1.07 to 1.94) |
| Incident Gestational Hypertension n (%) | 148 (5.3%) | 48 (8.2%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 1.23 (0.71 to 2.15) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 1.22 (0.70 to 2.14) |
| Incident Preeclampsia n (%) | 64 (2.3%) | 16 (2.7%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 1.60 (1.14 to 2.25) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 1.53 (1.09 to 2.16) |
Adjusted for age, parity, smoking during pregnancy, and pre-pregnancy body mass index,
Table 8.
Multivariable adjusted odds ratios (OR) and 95% confidence interval (CI) of gestational hypertension and preeclampsia according to physician diagnosed migraine and BMI categories
| No Migraine & Lean | Migraine & Lean | No Migraine & Overweight | Migraine & Overweight | |
|---|---|---|---|---|
| N = 2146 | N = 438 | N = 641 | N = 148 | |
| Normotensive women n (%) | 2024 (94.3%) | 412(94.1%) | 551 (86.0%) | 110 (74.3%) |
| Any Hypertensive Disorder of Pregnancy | 122 (5.7%) | 26 (5.9%) | 90 (14.0%) | 38 (25.7%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 1.05 (0.68 to 1.62) | 2.71 (2.03 to 3.62) | 5.73 (3.80 to 8.64) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 1.17 (0.69 to 1.66) | 2.77 (2.07 to 3.71) | 5.84 (3.87 to 8.83) |
| P-value for interaction | 0.025 | |||
| Incident Gestational Hypertension n (%) | 36 (1.7%) | 6 (1.4%) | 28 (4.4%) | 10 (6.8%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 0.82 (0.34 to 1.96) | 2.86 (1.73 to 4.72) | 5.11 (2.47 to 10.57) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 0.85 (0.36 to 2.04) | 2.89 (1.74 to 4.80) | 5.21 (2.51 to 10.84) |
| P-value for interaction | 0.056 | |||
| Incident Preeclampsia n (%) | 86(4.0%) | 20 (4.6%) | 62 (9.7%) | 28 (18.9%) |
| Unadjusted OR (95% CI) | 1.00 (REF) | 1.14 (0.69 to 1.88) | 2.65 (1.89 to 3.72) | 5.99 (3.75 to 9.56) |
| 1 Adjusted OR (95% CI) | 1.00 (REF) | 1.16 (0.71 to 1.91) | 2.72 (1.94 to 3.83) | 6.10 (3.82 to 9.75) |
| P-value for interaction | 0.183 | |||
Adjusted for age, parity, and smoking during pregnancy
CONCLUSIONS
Self-reported history of physician diagnosed migraine is associated with elevated blood pressures; particularly mean third trimester blood pressures. Adjusted mean third trimester SBP, DBP and MAP were 4.08, 2.39 and 2.95 mmHg, respectively, higher for migraineurs as compared with non-migraineurs. Migraineurs had a 1.53-fold increased odds of preeclampsia (95% CI 1.09 to 2.16). The migraine-preeclampsia association appeared to be modified by pre-pregnancy overweight status, though the p values for interaction did not reach statistical significance (p=0.183). Overweight or obese migraineurs, compared with lean non-migraineurs women, had a 6.10-fold increased odds of preeclampsia (95% CI 3.82 to 9.75).
To our knowledge there have been no published studies on migraine diagnosis status and trimester-specific blood pressures. Our study extends the literature by documenting elevated SBP across the three trimester of pregnancy, with differences getting more pronounced in the third trimester for migraineurs as compared with non-migraineurs. Mean first and third trimester DBP and MAP values were elevated, though second trimesters were either the same or lower among migraineurs as compared with non-migraineurs. Observed blood pressure elevations, particularly third trimester values, were independent of known preeclampsia risk factors including age, race/ethnicity. Moreover, differences were evident even after we stratified participants according to parity and pre-pregnancy overweight status, two of the most potent risk factors for hypertensive disorders of pregnancy.
Several studies have explored a possible association between migraine and hypertension in populations other than pregnant women, with contradictory results. Some studies have shown a positive association between migraine and blood pressure12–15, others have shown no association16. Four studies have also shown negative associations between migraine, blood pressure or hypertension17–20. In a population-based study that included 11,171 women Gudmundsson et al17 reported that migraineurs had lower pulse pressure, lower systolic and higher diastolic blood pressure values compared with non-migraineurs. Tzourio et al18 reported that migraine was associated with lower blood pressures and with smaller values of carotid wall thickness. Data from the Women’s Health Study indicated that older women with migraine had higher systolic and diastolic blood pressure than controls15. Additionally, results from the Genetic Epidemiology of Migraine Study14, indicated that migraineurs had about a 63% increased odds of high blood pressure (95% CI 1.2 to 2.1) as compared with non-migraineurs. Differences in operational research definitions of migraine, hypertension, as well as difference in blood pressure measurements, sample size and population characteristics are thought to account for these contradictory reports. It is noteworthy that associations between migraine and pregnancy-related hypertension, first hypothesized in 195941, have been documented and confirmed with far greater consistency4, 29
Our findings of increased risks of preeclampsia among migraineurs, however, are generally consistent with most4,23,25–29, though not all42,43, prior reports. Marcoux and colleagues, in their case-control study of Canadian women25, noted that migraineurs had a 2.4-fold increased risk of preeclampsia as compared with controls (95% CI 1.4 to 4.2). Adeney et al26, in their case-control study of American women residing in the Pacific Northwest region of the US, reported a positive association between migraine history and preeclampsia risk. The authors reported that women with a history of physician-diagnosed migraine had a 1.8-fold (95% CI, 1.1 to 2.7) increased risk of preeclampsia compared with women not having such a medical history. Similarly, Sanchez et al28, in their case-control study of Peruvian women, reported that migraineurs had a 4.0-fold increased risk of preeclampsia (95% CI 1.9 to 8.2) compared with non-migraineurs. Additionally, Facchinetti et al27, in their prospective cohort study 702 normotensive Italian women with singleton pregnancies, reported that migraine, classified using the International Headache Society diagnostic criteria, was associated with incident hypertensive disorders in pregnancy (OR=2.9; 95% CI 1.4 to 5.8). Although it is not clear why similar associations were not demonstrated in two studies42,43, differences in study population characteristic (including distribution of the severity of migraine), errors in classification of primary covariates, low statistical power and uncontrolled confounding may, at least in part, account for the differences across studies.
Observed associations of migraine with elevated blood pressures and pregnancy hypertensive disorders are biologically plausible. Abnormal vascular reactivity, increased platelet aggregation, and a high underlying ischemic stroke or cardiovascular risk profile may account for observed associations. Profound hemodynamic changes, representing adaptive responses necessary for meeting the circulatory needs of the mother and the developing feto-placental unit44 such as increased blood volume, stroke volume, and heart rate, and the haemostatic changes in favor of thrombosis may all compound the interactions between migraine and vascular complications observed among pregnant migraineurs. This hypothesis is supported by findings from Vanmolkoft et al45 who reported that migraineurs (with or without aura) have increased peripheral and central blood pressure, decreased diameter and compliance of superficial muscular arteries, and decreased endothelia dilatation in response to hyperemia as compared with controls45.
Our data suggest that there might be a different effect of second trimester diastolic and systolic blood pressure values, with the former having a positive and the latter a null or slightly negative association with migraine. This observation is in general agreement with results from a recent population based Icelandic study. Gudmundsson et al17 reported that migraine was negatively correlated with systolic blood pressure and positively correlated with diastolic pressure levels. Taken together, available data suggest that migraine may have effects on systolic and diastolic blood pressures that are either trimester-specific and/or differ in pregnant and non-pregnant individuals. Collectively, these data reinforce the notion46 that migraine-blood pressure associations are complex and likely to be missed in analyses that rely solely on generic diagnoses of hypertension.
Several limitations of our study merit discussion and consideration. First, migraine status was based on self-reports made during interviews. However, similar questions to the ones we used in the current study to ascertain migraine status have been widely used in epidemiological studies such the Women’s Health Study47 and the National Health and Nutrition Examination Surveys (NHANES)48–50; and investigators have documented good agreement between migraine classification based on self-reports with information derived from medical records review47,51–52. Nevertheless, we cannot exclude the possibility of that migraine status was reported with error in our study. We were also unable to differentiate migraineurs on the basis of features such as the frequency and severity of attacks and efficacy and type of therapies used to manage symptoms. Second, we relied on recorded clinical blood pressure measurements that are influenced by circadian rhythms, stress, diet, and other sources of within-person variation53. Errors in these measurements may also arise from misreading, digit preference, and use of an incorrect cuff size in non-research, clinical settings. These sources of error are likely unrelated to reports of migraine diagnosis, thus we speculate that our reported measures of association are likely biased towards the null. Third, we relied on self-reported pre-pregnancy weight and cannot exclude the potential for misclassification of participants BMI values.
Fourth, although we adjusted for several potential confounders, we cannot exclude the possibility of residual confounding due to misclassification of adjusted variables (e.g., pre-pregnancy BMI) or confounding by other unmeasured variables (e.g., medication use that may impact blood pressure). Specifically, we do not have information on pharmacological and non-pharmacological (e.g., bed rest) approaches used to manage incident preeclampsia and pregnancy induced hypertension in this cohort. In consideration of evidence suggesting that adiposity may be a mediating factor along the causal pathway of migraine status and blood pressure, we report results from multivariable models with and without adjustment for pre-pregnancy BMI. We also report results stratified by lean and overweight or obese status. Finally, the generalizability of our study may also be limited as our cohort was primarily comprised of Non-Hispanic White and well-educated women. On balance, the fact that results from our study were largely similar to those provided by other studies which included racially, ethnically and geographically diverse populations23,25,26,28,29, suggests that reported associations of migraine with trimester-specific blood pressure and hypertensive disorders are robust. Our results also suggest that the magnitudes of observed differences in trimester-specific blood pressures values, particularly third trimester values, for migraineurs versus non-migraineurs are of clinical significance.
Noted study limitations are offset by several strengths. First, data used in the present analyses are derived from a large well characterized pregnancy cohort with excellent follow-up (>95%) which minimized possible selection bias. Second, the prospective study design allows for the appropriate delineations of the temporal sequence of migraine diagnosis and alterations in antepartum blood pressure values. This strengthens inferences drawn from the study. Further, to our knowledge this is the first study to assess the influence of migraine status on systolic, diastolic and mean arterial blood pressure patterns across gestation.
In summary, our results, combined with those reported by others, indicate that risk for hypertensive disorders are increased among pregnant migraineurs; and that differences in blood pressure profiles, particularly for systolic blood pressure, exist across gestation. Additional prospective cohort studies are needed to more rigorously evaluate the extent to which migraines and/or its treatments are associated with the occurrence of preeclampsia. Results from studies that allow for characterizing migraine history according to age of onset, frequency, and triggers of migraine episodes will likely yield new information useful for developing strategies for the prevention and control of migraine among reproductive aged and pregnant women.
Supplementary Material
Figure 1.
Association between adjusted trimester-specific mean (a) systolic, (b) diastolic and (c) mean arterial blood pressure, according to migraine (red) and non-migraine (blue) status. The vertical lines represent 95% confidence intervals. The adjusted mean values are for Non-Hispanic white, nulliparous, college educated women who are 25–34 years of age with a pre-pregnancy body mass index <25 kg/m2.
Acknowledgments
Study funding: This research was supported by awards from the National Institutes of Health (R01HD-032562, and R01HD-055566).
This research was supported by awards from the National Institutes of Health(R01HD-055566 and R01HD-32562). The authors are indebted to the staff of the Center for Perinatal Studies for their expert technical assistance.
Footnotes
Conflict of interest statement: No conflict of interest
AUTHOR CONTRIBUTIONS
MAW had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication. MAW conceived, designed and obtained funding for the study. MAW, and RSM analyzed the data. MAW, BLP, BG, and DAE and SKA drafted the manuscript. All authors interpreted the data, critically revised the draft for important intellectual content, and gave final approval of the manuscript to be published.
References
- 1.Menon R, Bushnell CD. Headache and pregnancy. Neurologist. 2008;14:108–119. doi: 10.1097/NRL.0b013e3181663555. [DOI] [PubMed] [Google Scholar]
- 2.Machado RB, Pereira AP, Coelho GP, Neri L, Martins L, Luminoso D. Epidemiological and clinical aspects of migraine in users of combined oral contraceptives. Contraception. 2010;81:202–208. doi: 10.1016/j.contraception.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 4.Adeney KL, Williams MA. Migraine headaches and preeclampsia: an epidemiologic review. Headache. 2006;46:794–803. doi: 10.1111/j.1526-4610.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 6.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123:612–624. doi: 10.1016/j.amjmed.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864–1871. doi: 10.1212/WNL.0b013e3181a71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosamond W. Are migraine and coronary heart disease associated? An epidemiologic review. Headache. 2004;44 (Suppl 1):S5–12. doi: 10.1111/j.1526-4610.2004.04103.x. [DOI] [PubMed] [Google Scholar]
- 9.Tzourio C, Kittner SJ, Bousser MG, Alperovitch A. Migraine and stroke in young women. Cephalalgia. 2000;20:190–199. doi: 10.1046/j.1468-2982.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 10.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushnell CD, Jamison M, James AH. Migraines during pregnancy linked to stroke and vascular diseases: US population based case-control study. Br Med J (Clin Res Ed) 2009;338:b664. doi: 10.1136/bmj.b664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper WD, Glover DR, Hormbrey JM, Kimber GR. Headache and blood pressure: evidence of a close relationship. J Hum Hypertens. 1989;3:41–44. [PubMed] [Google Scholar]
- 13.Cirillo M, Stellato D, Lombardi C, De Santo NG, Covelli V. Headache and cardiovascular risk factors: positive association with hypertension. Headache. 1999;39:409–416. doi: 10.1046/j.1526-4610.1999.3906409.x. [DOI] [PubMed] [Google Scholar]
- 14.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64:614–620. doi: 10.1212/01.WNL.0000151857.43225.49. [DOI] [PubMed] [Google Scholar]
- 15.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. Erratum: JAMA 296(283):292, JAMA 296(286):654. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen BK, Olesen J. Symptomatic and nonsymptomatic headaches in a general population. Neurology. 1992;42:1225–1231. doi: 10.1212/wnl.42.6.1225. [DOI] [PubMed] [Google Scholar]
- 17.Gudmundsson LS, Thorgeirsson G, Sigfusson N, Sigvaldason H, Johannsson M. Migraine patients have lower systolic but higher diastolic blood pressure compared with controls in a population-based study of 21,537 subjects. The Reykjavik Study. Cephalalgia. 2006;26:436–444. doi: 10.1111/j.1468-2982.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio C, Gagniere B, El Amrani M, Alperovitch A, Bousser MG. Relationship between migraine, blood pressure and carotid thickness. A population-based study in the elderly. Cephalalgia. 2003;23:914–920. doi: 10.1046/j.1468-2982.2003.00613.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagen K, Stovner LJ, Vatten L, Holmen J, Zwart JA, Bovim G. Blood pressure and risk of headache: a prospective study of 22 685 adults in Norway. J Neurol Neurosurg Psychiatry. 2002;72:463–466. doi: 10.1136/jnnp.72.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiehe M, Fuchs SC, Moreira LB, Moraes RS, Fuchs FD. Migraine is more frequent in individuals with optimal and normal blood pressure: a population-based study. J Hypertens. 2002;20:1303–1306. doi: 10.1097/00004872-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Quarti-Trevano F, Dell’oro R, Mancia G. Essential hypertension and the sympathetic nervous system. Neurol Sci. 2008;29 (Suppl 1):S33–36. doi: 10.1007/s10072-008-0882-9. [DOI] [PubMed] [Google Scholar]
- 22.Contag SA, Mertz HL, Bushnell CD. Migraine during pregnancy: is it more than a headache? Nat Rev Neurol. 2009;5:449–56. doi: 10.1038/nrneurol.2009.100. [DOI] [PubMed] [Google Scholar]
- 23.Chen HM, Chen SF, Chen YH, Lin HC. Increased risk of adverse pregnancy outcomes for women with migraines: a nationwide population-based study. Cephalalgia. 2010;30:433–438. doi: 10.1111/j.1468-2982.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez SE, Williams MA, Pacora PN, et al. Risk of placental abruption in relation to migraines and headaches. BMC Womens Health. 2010;10:30. doi: 10.1186/1472-6874-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcoux S, Berube S, Brisson J, Fabia J. History of migraine and risk of pregnancy-induced hypertension. Epidemiology. 1992;3:53–56. doi: 10.1097/00001648-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Adeney KL, Williams MA, Miller RS, Frederick IO, Sorensen TK, Luthy DA. Risk of preeclampsia in relation to maternal history of migraine headaches. J Matern Fetal Neonatal Med. 2005;18:167–172. doi: 10.1080/14767050500260566. [DOI] [PubMed] [Google Scholar]
- 27.Facchinetti F, Allais G, Nappi RE, et al. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia. 2009;29:286–292. doi: 10.1111/j.1468-2982.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez SE, Qiu C, Williams MA, Lam N, Sorensen TK. Headaches and migraines are associated with an increased risk of preeclampsia in Peruvian women. Am J Hypertens. 2008;21:360–364. doi: 10.1038/ajh.2007.46. [DOI] [PubMed] [Google Scholar]
- 29.Allais G, Gabellari IC, Borgogno P, De Lorenzo C, Benedetto C. The risks of women with migraine during pregnancy. Neurol Sci. 2011;31 (Suppl 1):S59–61. doi: 10.1007/s10072-010-0274-9. [DOI] [PubMed] [Google Scholar]
- 30.Wallenburg H. Hemodynamics in hypertensive pregnancy. In: Rubin P, editor. Hypertension in pregnancy (Handbook of Hypertension) Vol. 21. Amsterdam: Elsevier Science Publishers; 2000. pp. 66–101. [Google Scholar]
- 31.Belfort M, Uys P, Dommisse J, Davey DA. Haemodynamic changes in gestational proteinuric hypertension: the effects of rapid volume expansion and vasodilator therapy. Br J Obstet Gynaecol. 1989;96:634–641. doi: 10.1111/j.1471-0528.1989.tb03276.x. [DOI] [PubMed] [Google Scholar]
- 32.Vo M, Ainalem A, Qiu C, Lee Peterlin B, Aurora SK, Williams MA. Body mass index and adult weight gain among reproductive age women with migraine. Headache. 2011 doi: 10.1111/j.1526-4610.2010.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 34.American College of Obstetricians and Gynecologists. Hypertension in pregnancy. ACOG Technical Bulletin. 1996;219:1–8. [Google Scholar]
- 35.Thompson ML, Miller RS, Williams MA. Construction and characterisation of a longitudinal clinical blood pressure database for epidemiological studies of hypertension in pregnancy. Paediatr Perinat Epidemiol. 2007;21:477–486. doi: 10.1111/j.1365-3016.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific bloodpressures and hypertensive disorders in pregnancy. Sleep. 2010;33:1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darovic G. Hemodynamic monitoring. Invasive and noninvasive clinical application. Philadelphia: WB Saunders Co; 2002. [Google Scholar]
- 38.Diggle P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford; New York: Clarendon Press; Oxford University Press; 1994. [Google Scholar]
- 39.Stata Corp. Stata Statistical Software: Release 8.2. Stata Corporation; College Station, TX: 2004. [Google Scholar]
- 40.MacGillivray I, Rose GA, Rowe B. Blood pressure survey in pregnancy. Clin Sci. 1969;37:395–407. [PubMed] [Google Scholar]
- 41.Rotton WN, Sachtleben MR, Friedman EA. Migraine and eclampsia. Obstet Gynecol. 1959;14:322–330. [PubMed] [Google Scholar]
- 42.Wainscott G, Sullivan FM, Volans GN, Wilkinson M. The outcome of pregnancy in women suffering from migraine. Postgrad Med J. 1978;54:98–102. doi: 10.1136/pgmj.54.628.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattsson P. Migraine headache and obesity in women aged 40–74 years: a population-based study. Cephalalgia. 2007;27:877–880. doi: 10.1111/j.1468-2982.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 44.Danzell JD. Pregnancy and pre-existing heart disease. J La State Med Soc. 1998;150:97–102. [PubMed] [Google Scholar]
- 45.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563–1570. doi: 10.1212/01.wnl.0000260964.28393.ed. [DOI] [PubMed] [Google Scholar]
- 46.Agostoni E, Aliprandi A. Migraine and hypertension. Neurol Sci. 2008;29 (Suppl 1):S37–39. doi: 10.1007/s10072-008-0883-8. [DOI] [PubMed] [Google Scholar]
- 47.Schurks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women’s Health Study. Cephalalgia. 2009;29:1086–1090. doi: 10.1111/j.1468-2982.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Von Behren J, Kreutzer R, Hernandez A. Self-reported asthma prevalence in adults in California. J Asthma. 2002;39:429–440. doi: 10.1081/jas-120004036. [DOI] [PubMed] [Google Scholar]
- 49.Lateef TM, Merikangas KR, He J, et al. Headache in a national sample of American children: prevalence and comorbidity. J Child Neurol. 2009;24:536–543. doi: 10.1177/0883073808327831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterlin BL. Post-traumatic stress disorder in migraine: further comments. Headache. 2009;49:787. doi: 10.1111/j.1526-4610.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- 51.Evaluation of national health interview survey diagnostic reporting. Vital Health Stat. 1994;120:1–116. [PubMed] [Google Scholar]
- 52.Edwards WS, Winn DM, Collins JG. Evaluation of 2-week doctor visit reporting in the national health interview survey. Vital Health Stat. 1996;2:1–46. [PubMed] [Google Scholar]
- 53.Ayala DE, Hermida RC, Mojon A, Fernandez JR, Iglesias M. Circadian blood pressure variability in healthy and complicated pregnancies. Hypertension. 1997;30:603–610. doi: 10.1161/01.hyp.30.3.603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.