Abstract
Objective(s)
To examine the practice patterns and attitudes of obstetricians and gynecologists surrounding treatment of abnormal uterine bleeding (AUB).
Study Design
We conducted a cross-sectional study of members of the American College of Obstetricians and Gynecologists. Surveys, which were distributed using a sequential mixed method (both web-based and mail-based) approach, included questions about practice characteristics, practice patterns, and knowledge about treatment options for AUB.
Results
Four hundred seventeen out of 802 questionnaires were returned (52%). The most commonly selected first line choice for AUB treatment was combined oral contraceptives (97% anovulatory, 98% ovulatory). The levonorgestrel intrauterine system was the next most frequently selected option (63% anovulatory, 53% ovulatory). Respondents did not score high on questions about the effectiveness of treatments for AUB. Only 25% (n=86) answered at least two out of the three questions correctly.
Conclusions
Continued education is necessary to increase the utilization of the most effective treatment options for AUB.
Keywords: Abnormal uterine bleeding, evidence, attitudes, physician survey, treatment preferences
INTRODUCTION
Menstrual disorders are the most common gynecologic conditions in the general population. (1) Abnormal uterine bleeding (AUB) can mean both heavy and irregular menstrual bleeding, and many patients experience the combination of these symptoms. (2) The substantial impact of abnormal uterine bleeding (AUB) lies not only in its prevalence, but its affect on quality of life, associated loss of productivity, and major health care costs. (3–5)
AUB contributes to approximately 400,000 hospitalizations and several times that number of ambulatory visits in the United States each year, and physicians encounter many challenges when delivering medical care to these women. (6) The multiple etiologies of AUB, numerous available medical and surgical treatment options, and inconsistent measurement and reporting of treatment outcomes in studies contribute to the confusing nature of the body of literature on AUB. This has resulted in a body of literature which can be difficult to interpret and translate to clinical decision-making. (7) Adding to those challenges, recent studies have highlighted major variations in how clinicians and researchers define the commonly accepted terminologies used to describe the clinical signs and causes of menstrual disorders. (8) Clinical practice guidelines for the medical management of AUB have not been published in the United States, though guidelines from the United Kingdom and New Zealand are available. (9–11)
Despite the prevalence of AUB and its significant impact on the quality of life of women, in the United States there is no standardized way of evaluating or treating women with this problem. The clinical guidelines published in other countries, which suggest a standardized way of treating women with AUB, are based on a comprehensive systematic review of the evidence on the relative effectiveness of each treatment option. This study was performed to examine the practice patterns and attitudes of obstetricians and gynecologists in the U.S. about medical treatment of women with AUB. We aimed to evaluate whether physicians were choosing the most effective treatment options for patients with AUB and their attitudes about the effectiveness of commonly used treatment options.
MATERIALS AND METHODS
We conducted a cross-sectional survey of members of the American College of Obstetricians and Gynecologists (ACOG) from October 2008 through May 2009. The study was approved by the Women and Infants Hospital Institutional Review Board. (IRB # 08-0093)
Questionnaires were sent to 802 ACOG members. Six hundred two recipients were members of the Collaborative Ambulatory Research Network (CARN), which is a group of practicing obstetrician-gynecologists who volunteer to participate in survey research. (12) The other 200 recipients were randomly selected ACOG members who had not received a survey from ACOG in the previous 2 years. Other ACOG studies which surveyed both CARN members and a random sample of ACOG members have found that CARN members had been in practice longer than non-CARN members, but that there were no differences between groups in terms of distribution of responses to the survey questions.(13) ACOG surveys typically achieve a 30–50% response rate; With at least 350 eligible responses, we would have the ability to detect at least a 15% difference in physician demographic characteristics with alpha 0.05 and power of 80%.
We distributed the surveys using a sequential mixed method approach; all potential participants with email addresses received a web-based version and then all potential participants without an email address or who did not respond to the web-based version received a mailed version. This approach has been described as a way to reduce non-response error, especially among web-based surveys. (14) We showed in a previous analysis of this web-based survey that this sequential mixed method approach provided adequate representation and that web-based data collection was an appropriate approach for surveying obstetricians and gynecologists. (15)
For the web-based version of the survey, we used DatStat Illume (DatStat Illume is a trademark of DatStat, Incorporated, Seattle, Washington) and designed the web-based survey using standards suggested by Crawford et al. (16) DatStat Illume, a sophisticated computer software package with excellent data security, allows for complex skip patterns. The content of the survey was the same for the web and paper-based surveys and included multiple-choice questions about the physician and his/her practice, practice patterns for the evaluation and treatment of AUB, inquiries about the concepts that should be included in a comprehensive AUB questionnaire, and awareness of the evidence (as of the time of the survey) on medical treatment options for AUB. Practice patterns for the treatment of AUB were assessed using case scenarios. (Figure 1) Respondents were asked to choose the top three treatment options they preferred for each clinical scenario. Questions evaluating awareness of the evidence on treatment options for AUB were based on Cochrane Collaboration reviews. (Figure 1) (17, 18, 19, 20) Both the web-based and the paper-based survey were reviewed by experts and critiqued by colleagues of the Principal Investigator. Initially, we conducted a pilot survey with 25 physicians at Women & Infants Hospital (Providence, RI). The questionnaire and protocol for participant contact were revised based on feedback from the pilot participants.
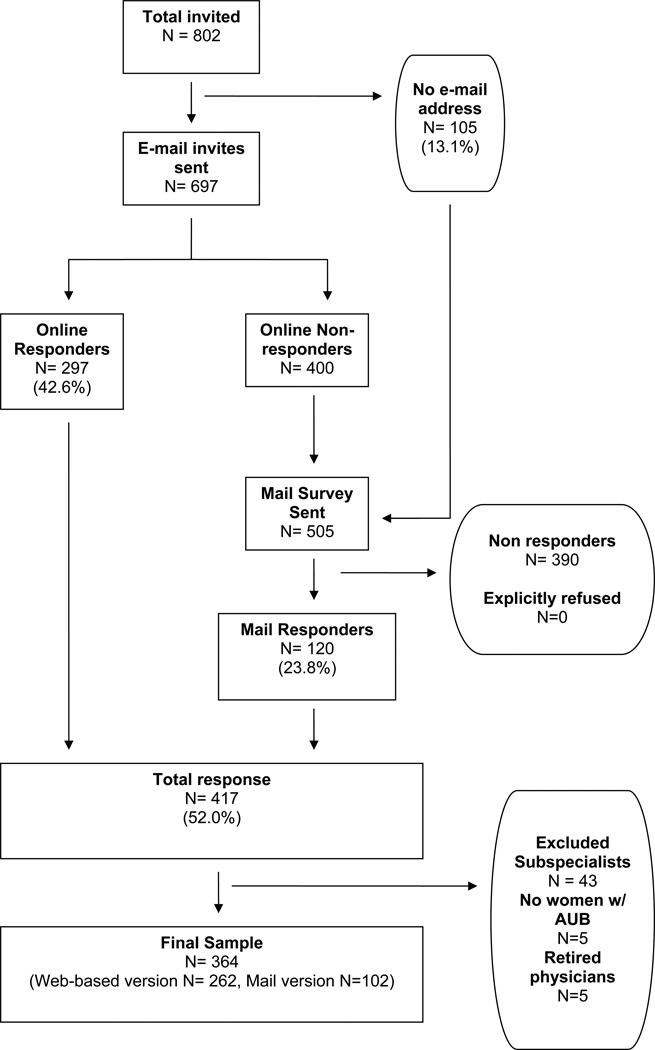
Figure 1.
Participant flow diagram
We followed principles of data collection recommended by Dillman and colleagues to maximize response rate (14, 21) First, pre-notification letters of the survey were mailed (n=802). (See figure 2) Ten days after the pre-notification letter, all study participants with a valid email address were sent an email invitation to participate in the web-based survey, which included an embedded link to a page that explained informed consent and included an embedded link to the secure and confidential website for survey completion. Four email reminders were sent to non-responders. For non-responders to the web-based survey as well as physicians with an unavailable e-mail address, a paper-based version of the survey was sent by mail six weeks after the pre-notification letters.
The informed consent page of the web-survey included information about the study, including the risks and benefits. Participants were asked to click a “button” at the end of page if they understood the study and consented to participate. For the paper survey, a letter containing information on informed consent was attached to the survey. The end of the letter addressed informed consent by the following: “By reading this letter, completing the paper survey, and submitting the paper survey, you are consenting to participate in this study”.
Web-based responses were automatically entered into the DatStat Illume Web database and surveys returned by mail were manually entered by a research assistant into the same DatStat software program that was used by study participants. Entered data were verified by the Principal Investigator and discrepancies were confirmed by a third reviewer.
Analyses were conducted using SAS 9.1 (Cary, NC). Categorical variables were compared using Chi square and Fisher’s exact tests, where appropriate, and two-sided p-values were calculated, with p<0.05 considered statistically significant.
RESULTS
Of the 802 physicians in the study sample, 697 (87%) had email addresses on file and were sent the web-based version of the survey. (Figure 2) Two-hundred ninety seven completed the web-based version (42.6%) and 120 physicians responded to the mail-based survey request (23.8%). In total, 417 questionnaires were returned, resulting in a response rate of 52.0%. Because the intent of the content of the survey was to examine obstetrician-gynecologists’ knowledge and practice patterns related to AUB, we excluded subspecialists, physicians who responded that they did not provide care for women with AUB, and physicians who returned the mail survey with a note that they were “retired” (n=53). A total of 364 surveys from obstetrician-gynecologists remained in our sample.
Overall, representation was obtained from all geographical districts and the sample was equally divided in terms of gender. (Table 1) Of respondents, the majority (69%) responded that they had completed residency 11 or more years ago, 53% reported that they evaluated 11 or more patients with heavy menstrual bleeding per month on average, and 53% of respondents indicated that they know of friends or family members who experience problems with heavy menstrual bleeding.
Table 1.
Respondent Demographics
| Demographics | N (column %)a N=359b |
|
|---|---|---|
| Type of sub-specialty | ||
| General Ob-Gyn | 337(94) | |
| Reproductive Endocrinology Subspecialty | 15(4) | |
| Urogynecology | 9(3) | |
| Minimally invasive gynecology/laparoscopy | 35(10) | |
| Clinical Research | 16(4) | |
| Membership status | ||
| Collaborative Ambulatory Research Network Member (CARN) | 293 (82) | |
| Non-CARN member | 66 (18) | |
| Gender | ||
| Male | 167(49) | |
| Female | 173(51) | |
| Years since completing residency | ||
| < 5 years | 25 (7) | |
| 5–10 years | 82 (24) | |
| 11–20 years | 94 (27) | |
| > 20 years | 143 (42) | |
| Type of Practice | ||
| Private practice | 283(82) | |
| Community hospital faculty | 16(5) | |
| University hospital faculty | 37(11) | |
| Geographical Districts | ||
| Midwest | 76(23) | |
| Northeast | 69(21) | |
| South | 116(36) | |
| West | 65(20) | |
| Proportion of time providing direct patient care | ||
| 0–50% | 24(7) | |
| 51–75% | 40(12) | |
| 76–100% | 276(81) | |
| Average number of patients evaluated per month with heavy Menstrual bleeding | ||
| 1–10 | 165 (47) | |
| 11 or more | 184 (53) | |
| Average number of ablations performed per month | ||
| None | 65 (19) | |
| 1–5 | 254 (73) | |
| 6 or more | 30 (9) | |
| Average number of hysterectomies performed per month | ||
| None | 51 (15) | |
| 1–5 | 272 (78) | |
| 6 or more | 27 (8) | |
| Has friends or family members with heavy menstrual bleeding | ||
| Yes | 182 (53) | |
| No | 104 (31) | |
| Did not know | 56 (16) | |
| Median time to complete questionnairec | Median, IQR 14 minutes (8) |
|
Could add up to > 100% because multiple choices could be selected
Column may not add up to total (n= 359) because of skip patterns and item nonresponse
Only for online surveys
For the case scenario on treatment of regular ovulatory heavy menstrual bleeding (Figure 1, Scenario A), the most commonly selected first line choices for treatment were COCs (97.7%) and the levonorgestrel intrauterine system (LNG-IUS) (62.7%), and nonsteroidal anti-inflammatory drugs (NSAIDs) (52.6%). (Table 2) We found that the prevalence of choosing COCs to treat ovulatory heavy menstrual bleeding did not differ by demographic and clinical practice factors. (Table 3) However, frequency of selection of LNG-IUS as a first line preference for treatment varied by type of practice. Ninety five percent of university hospital faculty chose it as a first line preference compared to 58% of private practice physicians (p=0.0001). Both physicians who completed residency greater than 20 years ago and men were less likely to choose NSAIDs as a first line agent than physicians who completed residency more recently and women. (p=0.002 and p=0.05, respectively) The most commonly selected first line preferences for treating anovulatory heavy menstrual bleeding (Figure 1, Scenario B) were COCs (96.5%), LNG-IUS (52.6%), and oral progestins (52.6%). (Table 2) The selection of oral progestins and LNG-IUS varied by type of practice; a higher proportion of private physicians than university hospital faculty chose oral progestins. (57% vs. 32%, p=0.01). (Table 3) Conversely, a higher proportion of university hospital faculty than private physicians chose the LNG-IUS. (78% vs. 50%, p=0.003)
Table 2.
Preferences for first line treatment for heavy menstrual bleedinga
| Treatments | N (%)b n=344 |
|
|---|---|---|
| Non-emergent OVULATORY/REGULAR BLEEDING | ||
| Combined estrogen and progesterone therapy | 336 (98%) | |
| Non-steroidal anti-inflammatory drugs | 181 (53%) | |
| Oral Progestin | 102 (30%) | |
| Depot medroxyprogesterone acetate | 84 (24%) | |
| Levonorgestrel Intrauterine system | 216 (63%) | |
| Other medical optionsc | 13 (4%) | |
| Surgical optionsd | 47 (14%) | |
| Non-emergent ANOVULATORY/IRREGULAR BLEEDING | ||
| Combined estrogen and progesterone therapy | 332 (96%) | |
| Non-steroidal an ti-inflammatory drugs | 77 (22%) | |
| Oral Progestin | 181 (53%) | |
| Depot medroxyprogesterone acetate | 93 (27%) | |
| Levonorgestrel Intrauterine system | 181 (53%) | |
| Other medical optionsc | 9 (3%) | |
| Surgical optionsd | 84 (24%) | |
| ACUTE UTERINE BLEEDING IN A 38 YEAR OLD SMOKER | ||
| Combined estrogen and progesterone therapy | 127 (37%) | |
| Non-steroidal anti-inflammatory drugs | 76 (22%) | |
| Oral Progestin | 208 (60%) | |
| Depot medroxyprogesterone acetate | 97 (28%) | |
| Levonorgestrel Intrauterine system | 98 (29%) | |
| Estrogen single agent | 96 (28%) | |
| Dilation and curettage | 246 (72%) | |
| Endometrial ablation | 95 (26%) | |
| Hysterectomy | 41 (12%) | |
Respondents were asked to select their top three choices for first line therapeutic options for three different case scenarios depicting ovulatory heavy menstrual bleeding, anovulatory heavy menstrual bleeding, and acute uterine bleeding. See Box 1 for a description of the case scenarios.
Add up to more than 100% - respondents could choose up to three options
Other medical options include Danazol, single agent estrogen, GnRH agonists
Surgical options include dilation and curettage, endometrial ablation, and hysterectomy
Table 3.
Comparisons of the top three first line preferences for treatment of non- emergent OVULATORY heavy menstrual bleeding (shaded) and ANOVULATORY heavy menstrual bleeding (unshaded). Number and row percent who indicated each therapy by each demographic characteristic.
| OVULATORY HEAVY MENSTRUAL BLEEDING | ANOVLUATORY HEAVY MENSTRUALBLEEDING | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COCsa | LNG IUSa | NSAIDSa | COCsa | oral progestin | LNG IUSa | ||||||||
| n=336b | P | n=216b | P | n=181b | P | n=332b | P | n=181b | P | n=181b | P | ||
| Years since residency | |||||||||||||
| < 5 years | 24 (96) | 0.20 | 18 (72) | 0.29 | 15 (60) | 0.002 | 23 (92) | 0.49 | 7 (28) | 0.05 | 12 (48) | 0.38 | |
| 5–10 years | 80 (98) | 57 (70) | 52 (63) | 79 (96) | 43 (52) | 49 (60) | |||||||
| 11–20 years | 94 (100) | 57 (61) | 56 (60) | 92 (98) | 48 (51) | 51 (54) | |||||||
| > 20 years | 138 (97) | 84 (59) | 58 (41) | 138 (97) | 83 (58) | 69 (48) | |||||||
| Type of Practice | |||||||||||||
| Privatec | 276 (98) | 1.0 | 163 (58) | 0.0001 | 144 (51) | 0.25 | 272 (96) | 0.55 | 160 (57) | 0.01 | 141 (50) | 0.003 | |
| Communityd | 16 (100) | 12 (75) | 11 (69) | 15 (94) | 7 (44) | 6 (38) | |||||||
| Universitye | 36 (97) | 35 (95) | 23 (62) | 37 (100) | 12 (32) | 29 (78) | |||||||
| Geographic Districts | |||||||||||||
| Midwest | 75 (99) | 0.14 | 52 (68) | 0.77 | 39 (51) | 0.98 | 74 (97) | 0.65 | 36 (47) | 0.67 | 46 (61) | 0.15 | |
| Northeast | 68 (99) | 42 (61) | 37 (54) | 67 (97) | 39 (57) | 29 (42) | |||||||
| South | 115 (99) | 73 (63) | 59 (51) | 114 (98) | 64 (55) | 61 (53) | |||||||
| West | 61 (94) | 40 (62) | 33 (51) | 62 (95) | 35 (54) | 36 (55) | |||||||
| Gender | |||||||||||||
| Male | 161 (96) | 0.17 | 98 (59) | 0.11 | 78 (47) | 0.05 | 164 (98) | 0.08 | 87 (52) | 0.92 | 85 (51) | 0.52 | |
| Female | 171 (99) | 116 (67) | 99 (57) | 164 (95) | 91 (53) | 94 (54) | |||||||
Abbreviations: COCs – combined oral contraceptives, LNG-IUS – levonorgestrel intrauterine system, NSAIDS – Nonsteroidal anti inflammatory drugs
Numbers in columns do not always add up to the column total given item nonresponse
Private practice
Community hospital faculty
University hospital faculty
Physicians selected surgical options more than any other option for the treatment of acute uterine bleeding in a 38 year old smoker (80%). (Figure 1, Scenario C) (Table 2) Preferences for treatment of this patient with acute uterine bleeding did not differ by years since completing residency, type of practice, geographic district or gender. (data not shown)
Data were analyzed for three questions which assessed awareness of the current evidence on treatment options for AUB. Correct answers were devised from Cochrane Reviews. (17–20) Only 25% (n=86) answered at least two out of the three questions correctly. We analyzed whether or not demographic factors or clinical volume were associated with the number of correct responses to the knowledge questions. We found only one difference; physicians in university hospital practice were more likely to answer at least 2 out of 3 questions correctly (36%) than private practice physicians (23%, p=0.04).
In terms of the individual questions, nearly all physicians responded correctly that LNG-IUS is an effective treatment for heavy menstrual bleeding (n=336, 98%). For the question about treating patients with ovulatory heavy menstrual bleeding with 14 days of oral progestins, only 23% responded correctly that it was ineffective. Forty seven percent of physicians who selected progestins as a first line treatment were unsure of the Cochrane Collaboration’s conclusion about their effectiveness and 23% of physicians incorrectly thought they were found to be effective. Regarding physicians’ knowledge of a systematic review of treating patients with ovulatory heavy menstrual bleeding with COCs, 22% of respondents were unsure of the conclusions of the review and 74% responded incorrectly that the systematic review concluded that COCs were effective.
COMMENTS
AUB, a prevalent symptom among women in the United States, affects quality of life, ability to work, and healthcare costs.(1,3,4) However, little is known about how American obstetrician-gynecologists treat this symptom in the clinical encounter and standard guidelines have not been implemented in the United States to guide evidence-based treatment of heavy or irregular menstrual bleeding Our study of obstetricians and gynecologists who were members of ACOG had two major findings (1) participants most frequently selected COCs for the treatment of both ovulatory and anovulatory heavy menstrual bleeding; and (2) participants overall lacked awareness of the current (as of October 2008–May 2009) evidence on effectiveness of frequently utilized treatment options for AUB.
Respondents to the survey most frequently chose COCs as a preference for first line treatment for both ovulatory (97.7%) and anovulatory (96.5%) heavy menstrual bleeding. The LNG-IUS was selected as a first line treatment less frequently (62.7% and 52.6%). This finding does not reflect the recommendation of the clinical guidelines for treatment of heavy menstrual bleeding published in the United Kingdom in 2007 or the previous guidelines published in 1999. (9, 22) Based on systematic review of the evidence, the 2007 guidelines from the National Institute of Clinical Excellence recommends that the LNG-IUS be chosen above all other treatment options because of its effectiveness in reducing bleeding (71–96%) and the quality of the evidence on its effectiveness.(9) Combined oral contraceptives, tranexamic acid, or NSAIDs were suggested as second line options. This was a change from the 1999 National Evidence Based Guidelines from the Royal College of Obstetricians and Gynecologists (RCOG) which suggested that nonhormonal treatments (tranexamic acid or NSAIDs) be chosen as the first treatment option for patients with heavy menstrual bleeding. (22) A 2006 survey of gynecologists on endometrial ablation conducted in the United Kingdom showed that only 0.4% chose COCs as a first line treatment for heavy menstrual bleeding, 78.7% chose nonhormonal treatment (tranexamic acid or NSAIDs), and 20.7% chose the LNG-IUS, which was consistent with 1999 United Kingdom guidelines. (23) In addition to these differences between U.S. and U.K. study populations, we saw differences between physicians within our own study on preferences for treatment.
Our study is not the first to show differences in diagnosis and management of common medical problems between the United States and United Kingdom. (24, 25) Countries with clinical practice guidelines and easily accessible evidence based reviews likely manage patients more similarly to one another and differently from countries without clinical practice guidelines.(24) Guidelines for the management of heavy menstrual bleeding were published in the United Kingdom in 1999 and in 2007. (9, 22) The United States does not currently have clinical practice guidelines for the medical management of heavy or irregular menstrual bleeding.
Deciding on the best medical therapy for AUB can be quite challenging because of the numerous treatment options available. (7) Physiologically it makes sense that COCs, the most frequently chosen treatment option in this study, should effectively reduce menstrual bleeding. Materials published by ACOG suggest that COCs can be effective for the treatment of heavy menstrual bleeding. (26) However, systematic reviews conducted by the Cochrane collaboration found insufficient evidence to support the effectiveness of COCs in treating ovulatory or anovulatory heavy menstrual bleeding.(19, 20) At the time the systematic review was published in 2009, only one small study showed that COCs reduced menstrual bleeding by 43%, and evidence supporting the effectiveness of COCs was limited. (27) However, since that publication (and after our study was completed), three randomized clinical trials on the effectiveness of COCs for women with heavy menstrual bleeding were published. Two of these studies found that although COCs decreased the amount of menstrual blood lost, they were less effective than the LNG-IUS. (28,29) The other study found that 44% of women with heavy menstrual bleeding who were treated with a novel COC preparation experienced completely normal menstrual periods after treatment. (30). Another commonly used medical treatment for AUB, oral progestin, has been shown to be ineffective for ovulatory heavy menstrual bleeding if used in just the luteal phase (for 10–14 days of the menstrual cycle) but may be effective if used for 21 days of the cycle.(17, 18) Confusion surrounding terminologies for describing AUB, the two slightly different regimens (10–14 days versus 21 days for oral progestins), and the fact that luteal phase progestins are approved by the Food and Drug Administration for the treatment of AUB may have contributed to why physicians may have answered the question on luteal phase progestins incorrectly. (8)
Though some treatments have been compared head-to-head, many treatments have not been compared to one another and clinicians may be interpreting the available literature differently. Systematic reviews and clinical guidelines can help clarify the literature when it is inconsistent. Although Cochrane reviews have been published on oral progestins, COCs, the LNG-IUS, NSAIDS, and antifibrinolytics, these reviews may be underutilized by physicians because they do not have access to them or may be intimidated by the sheer length of the reviews.(17–20, 31–33) The results of our study suggests that obstetricians and gynecologists in the United States may not be accessing these evidence based reviews given our findings that participants in our study were unaware of the conclusions of the reviews on COCs and luteal phase progestins. Although 98% of respondents correctly answered that the LNG-IUS is an effective treatment for heavy menstrual bleeding and the evidence on effectiveness of the LNG-IUS is high quality, the LNG-IUS was chosen less frequently than COCs as a first-line treatment. Access to the LNG-IUS could potentially affect physician choice of treatment options, though to our knowledge there is no evidence supporting access as a reason for less prescribing of the LNG-IUS for heavy menstrual bleeding. National published guidelines or emphasis of these already published international guidelines by professional organizations, such as the American College of Obstetricians and Gynecologists could potentially improve physician knowledge on AUB and increase their utilization of more effective treatments for patients with AUB.
This study had several strengths. First, the data represent responses from a national sample of gynecologists with good representation from all geographic districts. Second, adequate “coverage” of the intended study population was achieved given that email addresses were available for 87% of the study sample. However, while our response rate was relatively high for recent physician surveys, still 47% of physicians did not respond, resulting in some potential response bias. Also, we only assessed awareness of the current body of evidence pertaining to COCs, oral progestins, and the LNG-IUS, which we anticipated would be the most commonly selected treatment options based on our clinical experience. Therefore, we cannot evaluate level of awareness of the body of evidence on other treatment options, such as antifibrinolytics, NSAIDs, and endometrial ablation.
Physician surveys provide important information about knowledge, practice patterns, and attitudes. Our survey suggests that U.S. obstetricians and gynecologists may treat patients with AUB differently than obstetricians and gynecologists in other countries, choosing less effective treatment options over more effective options based on the current available evidence. Additionally, treatment preferences did not necessarily reflect knowledge about the effectiveness of the options. These findings highlight the need to develop or more effectively disseminate educational materials and practice guidelines on evidence based treatment of AUB.
Box 1. Case scenarios and questions on awareness of the evidence on treatment options for AUB.
| CASE SCENARIOSa |
The following two items refer to patients who DO NOT require emergency treatment of heavy menstrual bleeding. b Assume that the patient has no contraindications for any of the listed therapeutic options.c
|
The following item refers to patients who DO require emergency treatment of heavy menstrual bleeding.d
|
ASSESSMENT OF AWARENESS OF THE EVIDENCE ON AUB TREATMENT OPTIONS
|
For all case scenarios, respondents were asked to assume that the patient did not have endometrial hyperplasia or cancer
Respondents were instructed that emergency treatment of heavy menstrual bleeding is defined as a situation in which you feel a patient with acute heavy bleeding necessitates an emergency room evaluation, and/or hospital admission, and/or blood transfusion, and/or immediate medical or surgical intervention
Response options included combination estrogen-progestin therapy (pills, patch, ring) equivalent of once per day, danazol, estrogen (single agent), GnRH agonist, non-steroidal anti-inflammatory drugs, oral progestin once daily for 14 days/month, oral progestin once daily for 21 days/month, intramuscular progestin (Depo-medroxyprogesterone acetate), levonorgestrel intrauterine system, dilation and curettage, endometrial ablation, hysterectomy
Response options for “emergency treatment” included all of the options listed above in “c” plus combination estrogen-progestin therapy equivalent to more than one pill per day, intrauterine balloon, and oral progestin equivalent to multiple doses daily
Response options included effective, NOT effective, there is not enough evidence to make a conclusion, unsure
Response options included true, false, there is not enough evidence to make a conclusion, unsure
Acknowledgments
ACKNOWLEDGEMENT OF FINANCIAL SUPPORT
This research was supported by (1) National Institutes of Health-funded K23-HD057957 Career Development Award (Matteson) and (2) The Centers for Disease Control and Prevention and Grant #UA6 MC 19010 from the Maternal and Child Health Bureau, Health Resources and Services Administration, Department of Health and Human Services (Schulkin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STUDY LOCATION
This study was conducted at Women and Infants Hospital, Alpert Medical School of Brown University, Providence, RI and at The American College of Obstetricians and Gynecologists, Washington D.C.
DISCLOSURE: none of the authors have a conflict
Contributor Information
Kristen A. Matteson, Division of Research, Department of Obstetrics and Gynecology Women and Infants Hospital, Alpert Medical School of Brown University, Providence, RI.
Britta L. Anderson, The American College of Obstetricians and Gynecologists, Washington D.C..
Stephanie B. Pinto, Division of Research, Department of Obstetrics and Gynecology Women and Infants Hospital, Alpert Medical School of Brown University, Providence, RI.
Vrishali Lopes, Division of Research, Department of Obstetrics and Gynecology Women and Infants Hospital, Alpert Medical School of Brown University, Providence, RI.
Jay Schulkin, The American College of Obstetricians and Gynecologists, Washington D.C..
Melissa A. Clark, Department of Community Health, Alpert Medical School of Brown University, Providence, RI and Women and Infants Hospital, Alpert Medical School of Brown University, Providence.
REFERENCES
- 1.Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health. 1996;86:195–199. doi: 10.2105/ajph.86.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo RA. Abnormal Uterine Bleeding: Ovulatory and anovulatory dysfunctional uterine bleeding, management of acute and chronic excessive bleeding 2007. In: Katz VL, Lentz GM, Lobo RA, Gershenson DM, editors. Comprehensive Gynecology. Philadelphia, PA: Mosby Elselvier; 2007. pp. 915–931. [Google Scholar]
- 3.Cote I, Jacobs P, Cumming DC. Use of health services associated with increased menstrual loss in the United States. Am J Obstet Gynecol. 2003;188:343–348. doi: 10.1067/mob.2003.92. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health care costs and utilization in abnormal uterine bleeding. Value Health, Published on behalf of the International Society for Pharmacoeconomics and Outcomes Research. 2007;10:173–182. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 5.Frick KD, Clark MA, Steinwachs DM, Langenberg P, Stovall D, Munro MG, Dickersin K and the STOP-DUB Research Group. Economic burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatment. Women’s Health Iss. 2009;19(1):70–78. doi: 10.1016/j.whi.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Thompson BL, Ponce de Leon R, Kieke B, Velebil P, WIngo PA. Trends in hospitalization for abnormal uterine bleeding in the United States: 1980–1992. J Women’s Health. 1997 doi: 10.1089/jwh.1997.6.73. [DOI] [PubMed] [Google Scholar]
- 7.Rahn DD, Abed H, Sung VW, Matteson KA, Rogers RG, Morrill MY, Barber MD, Schaffer JI, Wheeler TL, 2nd, Balk EM, Uhlig K for the Society of Gynecologic Surgeons—Systematic Review Group. Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials. J Clin Epidemiol. 2010 Aug 11; doi: 10.1016/j.jclinepi.2010.03.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser IS, Critchley HOD, Munro MG, Broder M. A process designed to lead to international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding. Fert Steril. 2007;87:466–476. doi: 10.1016/j.fertnstert.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Health and Clinical Excellence (NICE) Clinical Guideline CG 44: Heavy Menstrual Bleeding. 2007 [Google Scholar]
- 10.Vilos GA, Lefebvre G, Graves GR. Guidelines for the management of Abnormal Uterine Bleeding. J Obstet Gynaecol Can. 2001;23(8):704–709. [Google Scholar]
- 11.National Health Committee, New Zealand. Guidelines for the Management of Heavy Menstrual Bleeding. Christchurch: 1998. [Google Scholar]
- 12.Hill LD, Erickson K, Holzman GB, Power ML, Schulkin J. Practice trends in outpatient obstetrics and gynecology: findings of the Collaborative Ambulatory Research Network, 1995–2000. Obstet Gynecol Surv. 2001;56(8):505–516. doi: 10.1097/00006254-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Morgan MA, Goldenberg RL, Schulkin J. Obstetricians-gynecologists’ practices regarding preterm birth at the limit of viability. J Matern-Fetal Neo M. 2008;21(2):115–121. doi: 10.1080/14767050701866971. [DOI] [PubMed] [Google Scholar]
- 14.Shaefer DR, Dillman DA. Development of a standard email methodology: Results of an experiment. Public Opin Quart. 1998;62:378–397. [Google Scholar]
- 15.Matteson KA, Anderson BL, Pinto SB, Lopes V, Schulkin J, Clark MA. Surveying Ourselves: Examining the Use of a Web-Based Approach for a Physician Survey. Eval Health Prof. 2010 Dec 29; doi: 10.1177/0163278710391086. [Epub ahead of print] PubMed PMID: 21190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford S, McCabe SE, Pope D. Applying web-based survey design standards. Journal of Prevention and Intervention in the Community. 2005;29(1/2):43–66. [Google Scholar]
- 17.Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 2005;(Issue 4) doi: 10.1002/14651858.CD002126.pub2. Art. No.: CD002126. [DOI] [PubMed] [Google Scholar]
- 18.Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 1998;(Issue 4) Art. No.: CD001016. [Google Scholar]
- 19.Hickey M, Higham JM, Fraser I. Progestogens versus oestrogens and progestogens for irregular uterine bleeding associated with anovulation. Cochrane Database of Systematic Reviews. 2007;(Issue 4) doi: 10.1002/14651858.CD001895.pub2. Art. No.: CD001895. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD000154.pub2. Art. No.: CD000154. [DOI] [PubMed] [Google Scholar]
- 21.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2nd ed. New York: Wiley; 2000. [Google Scholar]
- 22.Royal College of Obstetricians and Gynecologists. The Management of Menorrhagia in Secondary Care. vol. 101. London: RCOG Press; 1999. National Evidence Based Clinical Guidelines; pp. 470–473. (evidence based clinical guideline number 5) [Google Scholar]
- 23.Deb S, Flora K, Atiomo W. A survey of preferences and practices of endometrial ablation/resection for menorrhagia in the United Kingdom. Fertil Steril. 2008 Nov;90(5):1812–1817. doi: 10.1016/j.fertnstert.2007.08.052. Epub 2008 Feb 20. PubMed PMID: 18083167. [DOI] [PubMed] [Google Scholar]
- 24.von dem Knesebeck O, Bönte M, Siegrist J, Marceau L, Link C, Arber S, Adams A, McKinlay J. Country differences in the diagnosis and management of coronary heart disease - a comparison between the US, the UK and Germany. BMC Health Serv Res. 2008;8:198. doi: 10.1186/1472-6963-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams PC, Skinner JS, Cohen M, McBride R, Fuster V. Acute coronary syndromes in the United States and United Kingdom: a comparison of approaches. The Antithrombic Therapy is Acute Coronary Syndromes Research Group. Clin Cardiol. 1998;21(5):348–352. doi: 10.1002/clc.4960210510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American College of Obstetricians and Gynecologists Practice Bulletin. ACOG Practice Bulletin Number 110. Washington, DC: ACOG; 2010. Noncontraceptive uses of hormonal contraceptives. [Google Scholar]
- 27.Fraser IS, McCarron G. Randomized trial of 2 hormonal and 2 prostaglandin-inhibiting agents in women with a complaint of menorrhagia. Aust N Z J Obstet Gynaecol. 1991;31(1):66–70. doi: 10.1111/j.1479-828x.1991.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 28.Endrikat J, Shapiro H, Lukkari-Lax E, Kunz M, Schmidt W, Fortier M. A Canadian, multicentre study comparing the efficacy of a levonorgestrel-releasing intrauterine system to an oral contraceptive in women with idiopathic menorrhagia. J Obstet Gynaecol Can. 2009;31(4):340–347. doi: 10.1016/S1701-2163(16)34151-2. [DOI] [PubMed] [Google Scholar]
- 29.Shabaan MM, Zakherah MS, El-Nashar SA, Sayed GH. Levonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83(1):48–54. doi: 10.1016/j.contraception.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Jensen JT, Parke S, Mellinger U, Machlitt A, Fraser IS. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787. doi: 10.1097/AOG.0b013e3182118ac3. [DOI] [PubMed] [Google Scholar]; 31 Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 2000;(Issue 4) doi: 10.1002/14651858.CD000249. Art. No.: CD000249. [DOI] [PubMed] [Google Scholar]
- 31.Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 2000;(Issue 4) doi: 10.1002/14651858.CD000249. Art. No.: CD000249. [DOI] [PubMed] [Google Scholar]
- 32.Lethaby A, Augood C, Duckitt K. Nonsteroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 1998;(Issue 3) Art. No.: CD000400. [Google Scholar]
- 33.Beaumont H, Augood C, Duckitt K, Lethaby A. Danazol for heavy menstrual bleeding. Cochrane Database of Systematic Reviews. 2002;(Issue 2) doi: 10.1002/14651858.CD001017. Art. No.: CD001017. [DOI] [PubMed] [Google Scholar]