Summary
1. Seasonality of rainfall can exert a strong influence on animal condition and on host-parasite interactions. The body condition of ruminants fluctuates seasonally in response to changes in energy requirements, foraging patterns and resource availability, and seasonal variation in parasite infections may further alter ruminant body condition.
2. This study disentangles effects of rainfall and gastrointestinal parasite infections on springbok (Antidorcas marsupialis) body condition and determines how these factors vary among demographic groups.
3. Using data from four years and three study areas, we investigated i) the influence of rainfall variation, demographic factors and parasite interactions on parasite prevalence or infection intensity, ii) whether parasitism or rainfall is a more important predictor of springbok body condition and iii) how parasitism and condition vary among study areas along a rainfall gradient.
4. We found that increased parasite intensity is associated with reduced body condition only for adult females. For all other demographic groups, body condition was significantly related to prior rainfall and not to parasitism. Rainfall lagged by two months had a positive effect on body condition.
5. Adult females showed evidence of a “periparturient rise” in parasite intensity, and had higher parasite intensity and lower body condition than adult males after parturition and during early lactation. After juveniles were weaned, adult females had lower parasite intensity than adult males. Sex differences in parasitism and condition may be due to differences between adult females and males in the seasonal timing of reproductive effort and its effects on host immunity, as well as documented sex differences in vulnerability to predation.
6. Our results highlight that parasites and the environment can synergistically affect host populations, but that these interactions might be masked by their interwoven relationships, their differential impacts on demographic groups, and the different time scales at which they operate.
Keywords: Bovidae; Eimeria; endoparasites; Etosha National Park, Namibia; Strongylida; Strongyloides
Introduction
Estimates of animal body condition are used to determine the influence of factors such as environmental degradation, life history parameters and ecological interactions on animal health (Stevenson & Woods 2006). For ruminants, individual body condition varies cyclically with seasonal changes in energy and protein requirements (e.g., towards maintenance, growth, reproduction and lactation) and resource quality and availability (Parker, Barboza & Gillingham 2009). In balancing the body’s metabolic requirements against seasonal changes in the environment and the resource base, ruminants seasonally adjust their voluntary intake rate (Weber & Thompson 1998), digestive capacity, diet composition and foraging time (Owen-Smith 1994), all factors which contribute to the seasonal fluctuations in body condition.
Animal body condition can also be influenced by parasite infections; in wildlife populations numerous examples exist of associations between gastrointestinal parasite infections and decreased host condition (e.g. Holmstad, Hudson & Skorping 2005; Lello, Boag & Hudson 2005; Newey et al. 2005; Hakkarainen et al. 2007; Craig et al. 2008). In hosts gastrointestinal parasite infections can cause reduced voluntary intake rates, altered digestive function, altered protein metabolism (Fox 1997) and losses of endogenous protein (Van Houtert & Sykes 1996), all symptoms that would reduce the ability of infected individuals to maintain or increase body condition. Individuals may lose condition as a direct result of disease symptoms and in addition, animals in poorer condition may have a reduced ability to control parasite infections (Beldomenico et al. 2008) or may alter their foraging behavior in ways that increases exposure to parasites (Hutchings et al. 1999). Whatever the ultimate cause, a parasite-associated reduction in host condition may result in decreased survival of parasitized individuals (Gulland 1992; Hudson, Newborn & Dobson 1992; Murray, Cary & Keith 1997).
For wildlife species with many natural predators, reductions in body condition as a result of parasite infection may increase vulnerability to predation (Murray, Cary & Keith 1997). Our study species, springbok (Antidorcas marsupialis) is a medium-sized antelope with many natural predators including lion (Panthera leo) (Stander 1992), spotted hyena (Crocuta crocuta) (Trinkel 2010), black-backed jackal (Canis mesomelas) (Klare et al. 2010), cheetah (Acinonyx jubatus), leopard (P. pardus) (Hayward & Kerley 2008) and numerous other smaller mammalian and avian predators (Skinner & Louw 1996). Across springbok populations the adult sex ratio is skewed towards females, a pattern which is more pronounced in populations with larger predators (Bednekoff & Ritter 1997). In our study area of Etosha National Park, Namibia, a full suite of medium and large bodied carnivores is extant, and estimates of the springbok adult sex ratio vary from 1.5:1 to 2.0:1 females to males, implying that predators are more likely to kill male than female springbok (Bednekoff & Ritter 1997).
The goal of this study is to determine whether gastrointestinal parasites or rainfall has a larger influence on springbok body condition. However, distinguishing between environmental and parasite factors driving variation in body condition is complicated because there are many inter-related parasite and environmental factors that can affect body condition in opposing directions. Rainfall seasonality directly influences resource availability and parasitism by altering the development, survival and transmission of parasite life stages in the environment (Fayer 1980; Banks et al. 1990; O’Connor, Walkden-Brown & Kahn 2006). How individuals apportion their nutrient intake among bodily functions (e.g. maintenance, growth, reproduction, immunity) depends on resource availability and their age and reproductive status (Coop & Kyriazakis 1999). Furthermore, for a prey species, the condition observed in the population may be altered by predation, if predators selectively remove individuals in poorer condition or those of particular demographic groups.
In a prior study we showed that gastrointestinal parasitism of springbok is strongly seasonal and that the prevalence or intensity of parasite infections can relate to host age (Turner & Getz 2010). Here we build upon these patterns to disentangle the inter-related rainfall, parasite and demographic factors associated with changes in springbok physical condition. We test a series of models to investigate 1) the influence of rainfall, demographic factors and parasite interactions on parasite prevalence and infection intensity, 2) whether parasitism or rainfall is a more important predictor of springbok body condition and how this varies among demographic groups, and 3) how measures of parasitism and host condition vary across study areas situated along a rainfall gradient. We discuss how predation effects may or may not contribute to the patterns observed in this study.
Materials and Methods
STUDY AREA
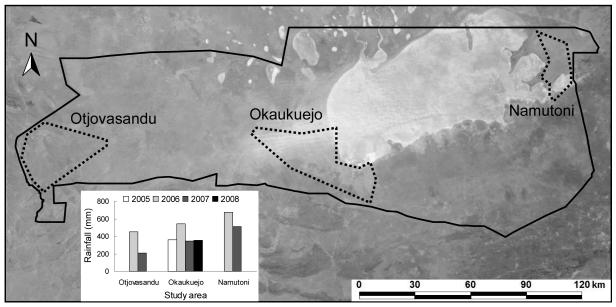
This research was conducted in Etosha National Park, a 22,915km2 reserve in northern Namibia, located between 18°30′-19°30′S and 14°15′-17°10′E. Etosha is a semiarid savanna system with a seasonal rainfall pattern, mainly falling November-April, with the greatest monthly rainfall occurring January-February (Engert 1997). The main study area is located at Okaukuejo in the center of Etosha (Fig. 1). There is a gradient of increasing rainfall from west to east across Etosha and two additional study areas (Otjovasandu and Namutoni) were selected at either end of this gradient (Fig. 1). These three areas differ in annual rainfall, soil type (Beugler-Bell & Buch 1997) and vegetation communities (le Roux et al. 1988). The mean annual rainfall from 1971-2008 was 301mm at Otjovasandu, 349mm at Okaukuejo, and 444mm at Namutoni. During 2006-2007 when all three areas were sampled, the rainfall differences between areas were larger than apparent in the annual means (Fig. 1).
Figure 1.
Etosha National Park in northern Namibia. The three study areas are indicated with dashed lines. Okuakuejo was the main study area with intermediate rainfall values; Otjovasandu was the drier area and Namutoni the wetter area. The inset figure shows annual rainfall in the years each area was sampled. The satellite image from Terra MODIS shows the large Etosha salt pan (available from http://visibleearth.nasa.gov/view_rec.php?id=4009).
STUDY SPECIES
Springbok are an arid-adapted antelope (tribe Antilopini) occurring in drier regions of southern Africa (Nagy & Knight 1994) and a commercially important game species. Springbok segregate sexually, have a territorial mating system and a six month gestation period. They are categorized as selective, mixed-feeding herbivores (Hofmann, Knight & Skinner 1995). Springbok are the most abundant larger herbivore in Etosha, with a population estimate of 15,600 (13,200-17,900 95% CI) in 2005 (cf. Ministry of the Environment and Tourism aerial survey records).
The parasites assessed in this study include nematodes in the superfamily Trichostrongyloidea (order Strongylida, hereafter strongyles) and the genus Strongyloides (order Rhabditida) and two coccidian morphotypes in the genus Eimeria (hereafter Eimeria A and Eimeria B; Fig. 2). A morphotype may represent an undescribed species or a complex of multiple species; further research is underway to determine species units. We did not attempt to differentiate the strongyles based on egg morphology, and many strongyle genera parasitize springbok: Agriostomum, Cooperia, Cooperioides, Dictyocaulus, Haemonchus, Impalaia, Longistrongylus, Nematodirus, Oesophagostomum, Ostertagia, Paracooperia and Trichostrongylus (Round 1968; Horak, Meltzer & de Vos 1982; De Villiers, Liversidge & Reinecke 1985).
Figure 2.
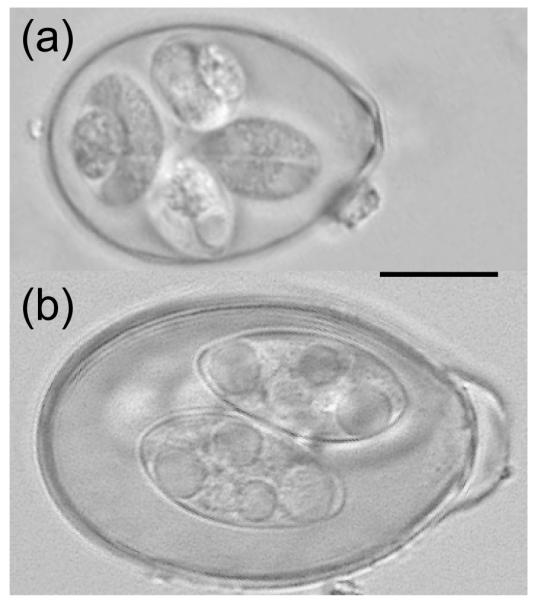
Eimeria oocyst morphotypes from springbok. These undescribed oocysts were considered generically for this study as A) Eimeria A, a tear-shaped oocyst and B) Eimeria B, an elliptical oocyst with micropyle cap (additional research is underway to determine if each morphotype represents a single species). The bar represents 10μm.
SAMPLE COLLECTION AND PARASITOLOGICAL ANALYSIS
Gastrointestinal parasitism was evaluated non-invasively using quantitative estimates of egg and oocyst shedding in springbok feces from opportunistically selected individuals. Fecal specimens were collected from our main study area in Okaukuejo between July 2005 and April 2008, for a total of 718 samples. Collecting periods were July-August 2005, February-October 2006, February-June 2007 and January-April 2008. Monthly sample sizes ranged from 27-48 individuals, with a mean of 37 samples per month. Samples were collected from the other two study areas in April-May 2006 (wet season), July-August 2006 (dry season) and March-April 2007 (wet season). Thirty fecal samples were collected per area per month of sampling, collecting a total of 160 samples from each area.
In all study areas, samples were collected between 7:00-13:00 and areas were sampled in rotation: Okaukuejo was always sampled in the first week of the month, Namutoni in the second week, and Otjovasandu in the third week. The laboratory for parasite analysis was based in Okaukuejo, so when working in other study areas, samples were collected over 2.5 days, stored in a refrigerator on the vehicle and then processed immediately on return to the laboratory. Samples were processed at most three days after collection and often at shorter time intervals.
In each sampling period (per month and area), we sampled at different waterholes and roads each day, to reduce the potential to resample individuals (elaborated in Turner and Getz 2010). On observing an individual defecate, its age, sex and body condition were recorded and the feces collected within 10 minutes of deposition. Age and sex were determined via horn growth and morphology and genitalia (Rautenbach 1971). Age was assessed in three categories: juveniles <1 year, yearlings 1-2 years, and adults 2+ years old. We adapted the condition scoring system developed by Berry and Louw (1982) for wildebeest to springbok. The condition categories (1=very poor, 2=poor, 3=fair, 4=good, 5=excellent) were based on the shape of the hindquarters and the visibility of the ribs and pelvis.
Fecal samples were evaluated for gastrointestinal parasite eggs or oocysts using a modification of the McMaster method for fecal egg counts (Gibbons et al. 2005; detailed in Turner and Getz 2010). In rare cases where the number of oocysts on the slide was too high to count accurately, the sample was diluted and the counts adjusted in proportion to the dilution factor. The McMaster technique is generally used to quantify an estimate of the number of eggs per gram of feces (the egg count seen in the two slide chambers multiplied by 50). We were interested in the relative differences in intensity, not estimates of parasites/gram of feces, and multiplying the count data by 50 creates large discontinuities in the dataset. Therefore, all intensity results were evaluated and reported based on slide counts rather than fecal egg counts.
DATA ANALYSIS
Two datasets were used to assess the objectives, the larger dataset from the main study area with monthly-level resolution (N=718 samples collected during 20 months over a 34 month period) and a seasonal-level dataset with samples collected from the three study areas (in two wet and one dry seasons). For the analyses among areas (N=524 samples), only the subset of data from the main study area that matched the sampling period for the other study areas was used. The main dataset was used to assess how parasitism and body condition were affected by host demography, rainfall and infection with other parasites. The study area dataset was then used to determine if parasitism and body condition differed in areas with higher and lower annual rainfall than the main study area. Statistical analyses were conducted in R 2.11.0 (R Development Core Team 2010).
Parasite prevalence and intensity
Statistical analyses of factors affecting parasite presence were conducted using logistic regressions, and analyses of factors affecting parasite intensity were performed using generalized linear models (GLMs) with a negative binomial error distribution and log link function. Parasite intensity is an estimate of parasite quantity shed by infected individuals, therefore when parasite intensity was the dependent variable in an analysis, only samples for which that parasite was present (i.e. count>0) were included. However, when intensity was an independent variable, all data for that parasite type, including zero values, were used in the analysis.
The prevalence or intensity of each parasite type was evaluated for relationships to host age and sex, monthly rainfall and associations with other parasites (as prevalence or intensity, to match the parasite measure of the dependent variable). The rainfall variables examined were monthly rainfall one and two months prior to sample collection; rainfall in the month of collection was not included because samples were collected in the first week of each month. Only proximate rainfall effects were examined as these are most relevant to the timescale required to complete parasite life cycles.
Springbok body condition
Although condition scores were recorded using a five-category scale, 94% of the individuals sampled had condition scores of good or fair. As a result, the condition scores were collapsed into two classes, lower condition (poor or fair condition scores) and higher condition (good or excellent condition scores) and analyzed using logistic regressions.
Since some parasite infections are more prevalent or intense in younger animals and others in adult animals (Turner & Getz 2010) and animals have different energetic requirements depending on their age and sex, the factors exerting a strong influence over an individual’s body condition will change over time. As a first level of analysis, we tested if there were significant differences in body condition among age and sex classes. As a result of this analysis, the data were partitioned into juvenile, yearling, adult female and adult male datasets. This allowed for an assessment of the rainfall and parasite factors affecting springbok condition independent from demographic factors and also a comparison of how the factors affecting body condition differ for different ages or by sex. Each of these analyses contrasted the effects of rainfall (one and two months prior) and the intensity of each parasite type on body condition.
Study area comparisons
The study area dataset was used to compare how environmental factors, including annual rainfall variation (study area), rainfall years (wet or dry sampling year), and season (wet or dry) affected measures of parasitism and body condition. Age was included as a factor in these analyses to control for age-related variation in parasitism or condition. Statistical tests for parasite prevalence, intensity and body condition were the same as used for the main dataset.
Springbok movement patterns provide evidence that animals can migrate seasonally along the edge of the Etosha pan (Panagi & Stander 1989; W.C. Turner unpublished data); therefore, springbok populations in the intermediate-rainfall and wetter study areas may not be fully independent. There is no evidence supporting springbok movement corridors between the drier area and the other two study areas. Given the potential non-independence of the wetter two study areas, we focus on the comparison between the drier and wetter area, which are separated by approximately 250kms.
Results
PARASITE PREVALENCE AND INTENSITY
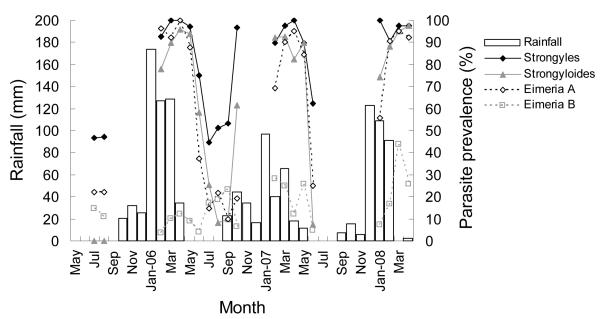
Strongyles, Strongyloides and Eimeria A all had positive associations between monthly rainfall and egg or oocyst shedding (Table 1; Fig. 3). Strongyle prevalence significantly increased with increased rainfall one and two months prior; however, strongyle intensity was only significantly related to rainfall one month prior. The prevalence and intensity of Eimeria A and Strongyloides were significantly related to rainfall one and two months prior. The prevalence of Eimeria B was not significantly related to either rainfall variable (Table 1).
Table 1.
Prevalence and intensity of springbok parasites. Relationships are shown among parasite prevalence or intensity and monthly rainfall, host demographics and parasite interactions. Prevalence was tested with logistic regression; infection intensity with GLM. The variables rain1 and rain2 are respectively the rainfall one and two months prior to sample collection; samples were collected in the first week of each month. A positive coefficient for sex indicates the parasite measure was higher in males than females. Associations in Eimeria B intensity were excluded due to low prevalence (16.7%; 120 positives per 718 samples).
| Independent variables |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age | sex | rain1 | rain2 | strongyles | Strongyloides | Eimeria A | Eimeria B | |||||||||||
| parasite type | parasite data types |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P | N |
| strongyles | prevalence | 0.338 (0.135) |
* | 0.503 (0.227) |
* | 0.013 (0.004) |
** | 0.015 (0.005) |
** | 1.092 (0.296) |
*** | 0.591 (0.281) |
* | 0.295 (0.332) |
ns | 718 | ||
| strongyles | intensity | 0.287 (0.059) |
*** | −0.345 (0.086) |
*** | 0.006 (0.0009) |
*** | 0.001 (0.001) |
ns | 0.007 (0.002) |
*** | 0.000005 (0.00009) |
ns | 0.00006 (0.0003) |
ns | 584 | ||
| Strongyloides | prevalence | −0.066 (0.137) |
ns | 0.036 (0.214) |
ns | 0.014 (0.003) |
*** | 0.019 (0.003) |
*** | 1.167 (0.285) |
*** | 1.220 (0.235) |
*** | −0.259 (0.306) |
ns | 718 | ||
| Strongyloides | intensity | −0.105 (0.069) |
ns | 0.057 (0.101) |
ns | 0.008 (0.001) |
*** | 0.003 (0.001) |
*** | 0.006 (0.002) |
** | −0.0002 (0.0001) |
^ | −0.0007 (0.0003) |
* | 450 | ||
| Eimeria A | prevalence | 0.071 (0.133) |
ns | 0.063 (0.210) |
ns | 0.018 (0.003) |
*** | 0.015 (0.003) |
*** | 0.581 (0.280) |
* | 1.203 (0.239) |
*** | −0.463 (0.3) |
ns | 718 | ||
| Eimeria A | intensity | −0.508 (0.097) |
*** | −0.099 (0.145) |
ns | 0.007 (0.001) |
*** | 0.010 (0.001) |
*** | 0.002 (0.003) |
ns | −0.002 (0.003) |
ns | −0.0006 (0.0005) |
ns | 438 | ||
| Eimeria B | prevalence | −0.745 (0.122) |
*** | −0.064 (0.210) |
ns | 0.002 (0.003) |
ns | 0.003 (0.002) |
ns | 0.403 (0.324) |
ns | −0.090 (0.289) |
ns | −0.286 (0.283) |
ns | 718 | ||
Significance levels are =p≤0.001,
=p≤0.01,
=p≤0.05,
=p≤0.1; ns=non-significant.
Figure 3.
Monthly variation in rainfall and parasite prevalence in springbok.
There were significant positive associations in prevalence among strongyles, Strongyloides and Eimeria A and between the intensities of strongyles and Strongyloides (Table 1). There were no significant relationships between Eimeria B and the prevalence of other parasites.
The prevalence and intensity of strongyles significantly increased as host age increased (Table 1). Strongyles were also more prevalent in males than females but strongyle intensity in infected individuals was higher in females than males (Table 1). The prevalence estimates of strongyle nematodes were: juvenile females 74%, juvenile males 66%, yearling females 83%, yearling males 91%, adult females 79% and adult males 87%. In contrast to strongyles, Eimeria spp. parasites had negative relationships with age. Eimeria B prevalence significantly decreased as age increased and although Eimeria A prevalence was not significantly related to host age, the intensity Eimeria A infections significantly decreased as age increased (Table 1). There were no statistically significant relationships between parasite prevalence or intensity and host sex or age for Strongyloides.
SPRINGBOK BODY CONDITION
Springbok were significantly more likely to be in the lower body condition class as age increased and there was a near-significant interaction of age×sex on springbok condition (logistic regression: age, z=−6.64, p<0.0001; sex, z=−1.49, p=0.137; age×sex, z=1.88, p=0.061; Fig. 4). The percentage by age of animals in the lower condition class was 14% juveniles, 42% yearlings and 56% adults in the lower condition class (51% of adult males and 62% of adult females were in the lower condition class). As a result of these demographic patterns in springbok body condition, the effect of rainfall versus parasites factors on condition were assessed separately for juveniles, yearlings, adult males and adult females.
Figure 4.
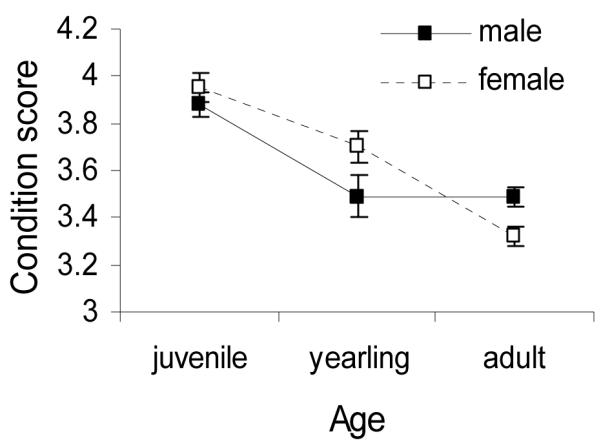
Springbok body condition by age and sex. Condition score is the average (1-5 scale) condition score recorded; means are presented ± standard errors.
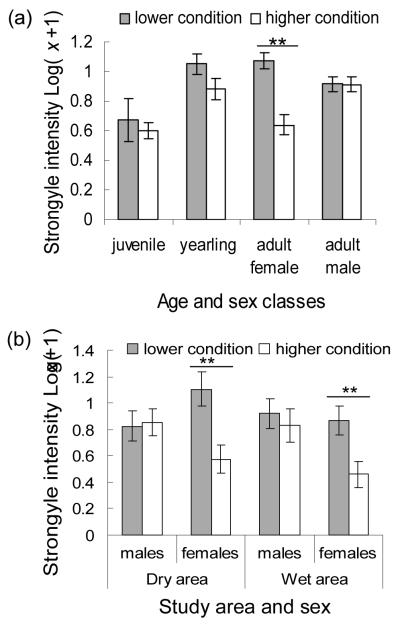
The relative importance of rainfall versus parasite factors on springbok body condition differed for the age and sex classes examined. The body condition of juveniles, yearlings and adult males was significantly related to rainfall whereas the condition of adult females was significantly related to strongyle intensity (Table 2). Although not always statistically significant, the directionality of relationships between condition and the rainfall variables was consistent among all groups examined: rainfall one month prior had a negative relationship with condition and rainfall two months prior had a positive relationship (Table 2). Juvenile springbok had a significant relationship between rainfall one month prior and body condition and a near-significant relationship between condition and rainfall two months prior. Yearlings and adult males had a significant relationship between condition and rainfall two months prior but not one month prior. Adult female condition was significantly lower for individuals with higher strongyle intensities (Fig. 5A; Table 2). For comparison, we repeated the model of adult female body condition excluding parasites from the analysis. When considering only rainfall variables, adult female condition was significantly and negatively related to rainfall one month prior (β=−0.009 SE=0.003, z=2.713, p=0.007). None of the other parasites examined had statistically significant relationships with springbok body condition.
Table 2.
Rainfall versus parasite effects on springbok body condition. Analyses were partitioned into demographic groups because of age and sex variation in body condition (Fig. 4).
| Independent variables |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rain one month prior |
rain two months prior |
strongyles | Strongyloides | Eimeria A | Eimeria B | ||||||||
| Age/sex categories |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P | N |
| juveniles | −0.018 (0.008) |
* | 0.015 (0.008) |
^ | 0.003 (0.024) |
ns | −0.018 (0.016) |
ns | 0.0001 (0.0005) |
ns | 0.010 (0.010) |
ns | 128 |
| yearlings | −0.004 (0.004) |
ns | 0.011 (0.004) |
** | 0.003 (0.011) |
ns | −0.018 (0.014) |
ns | −0.0007 (0.0005) |
ns | −0.018 (0.013) |
ns | 132 |
| adult males | −0.0001 (0.0032) |
ns | 0.007 (0.003) |
* | −0.007 (0.010) |
ns | −0.0007 (0.0080) |
ns | −0.0005 (0.0007) |
ns | 0.003 (0.007) |
ns | 217 |
| adult females | −0.007 (0.004) |
ns | 0.004 (0.003) |
ns | −0.024 (0.008) |
** | −0.002 (0.012) |
ns | 0.001 (0.0009) |
ns | −0.062 (0.048) |
ns | 220 |
Figure 5.
Springbok body condition and strongyle infection intensity. A. Strongyle intensity and body condition by demographic groups from the main study area. B. Strongyle intensity and body condition for adult males and adult females in the drier and wetter study areas. Means are presented ± standard errors; condition score is the average (1-5 scale) condition score recorded; strongyle intensity is presented as log(x+1) where x is the parasite count including zero values; significant differences are indicated with **.
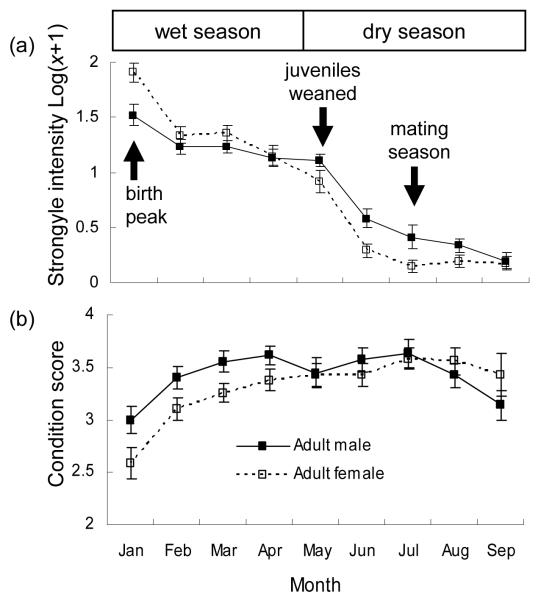
The differences between adult males and females in strongyle parasitism and body condition are presented in Fig. 6, to explore why adult males and females differed in the factors affecting body condition. Data are presented monthly to show how parasitism and condition change seasonally and how the differences between the sexes change in relation to the generalized timing of important life history events (i.e. parturition, lactation, breeding). Adult females have higher strongyle intensity and lower condition than adult males during the wet season, when females give birth and are lactating. In the dry season, after juveniles have been weaned, adult females have lower strongyle intensity than adult males and there are no detectable differences between the sexes in body condition. After the mating season, adult males experience a slight decrease in body condition compared to adult females.
Figure 6.
Sex differences in (a) strongyle parasitism and (b) condition in adult springbok. Data are presented monthly in relation to the average timing of reproductive events (Gasaway, Gasaway & Berry 1996; Skinner & Chimimba 2005; W.D. Versfeld pers. obs.). Means are presented ± standard errors; condition score is the mean (1-5 scale) condition scores recorded; strongyle intensity is presented as log(x+1) where x is the parasite count including zero values.
PARASITISM AND HOST CONDITION AMONG STUDY AREAS
Parasitism increased from one study area to the next along the rainfall gradient only for Strongyloides prevalence and intensity (Table 3). For the other parasites, there was either no significant relationship between parasitism and the study areas (e.g. the prevalence of strongyles, Eimeria A and Eimeria B) or the significant relationships in parasitism among areas did not directly follow the rainfall gradient. Strongyle intensity was significantly lower in the intermediate-rainfall area than the wetter area but did not differ significantly between the wetter and drier areas. Eimeria A intensity was significantly higher in the wetter than the drier area, but the intermediate-rainfall area had higher Eimeria A intensity than the wetter area.
Table 3.
Environmental factors affecting springbok parasitism and body condition across study areas. Models included factors for the year (drier or wetter) and the season (dry or wet) and area (driest vs wettest; intermediate (main) vs wettest). A positive coefficient (β) for the categorical variables indicates the parasite or condition measure was higher in the wetter of the two categories considered.
| Independent variables |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Season (dry vs wet) |
Year (drier vs wetter) |
Area (drier vs wetter) |
Area (main vs wetter) |
Age |
|||||||
| Data type |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P |
β (SE) |
P | N |
| strongyle prevalence | 3.320 (0.481) |
*** | −0.822 (0.739) |
ns | 0.298 (0.356) |
ns | 0.003 (0.336) |
ns | 0.020 (0.175) |
ns | 524 |
| strongyle intensity | 2.201 (0.132) |
*** | 0.149 (0.095) |
ns | 0.167 (0.111) |
ns | 0.267 (0.104) |
* | −0.026 (0.056) |
ns | 429 |
| Strongyloides prevalence | 4.075 (0.365) |
*** | 0.628 (0.313) |
* | 2.050 (0.352) |
*** | 0.729 (0.327) |
* | 524 | ||
| Strongyloides intensity | 2.882 (0.255) |
*** | 0.035 (0.115) |
ns | 1.754 (0.154) |
*** | 0.672 (0.129) |
*** | 316 | ||
| Eimeria A prevalence | 4.142 (0.364) |
*** | 0.232 (0.360) |
ns | 0.558 (0.349) |
ns | −0.199 (0.336) |
ns | 522 | ||
| Eimeria A intensity | 3.171 (0.326) |
*** | 0.609 (0.168) |
*** | 0.552 (0.209) |
** | −0.379 (0.192) |
* | −0.210 (0.108) |
^ | 334 |
| Eimeria B prevalence | −0.398 (0.365) |
ns | −0.609 (0.364) |
^ | −0.036 (0.379) |
ns | −0.468 (0.331) |
ns | −1.03 (0.156) |
*** | 522 |
| body condition | 0.610 (0.251) |
* | 1.449 (0.248) |
*** | −0.617 (0.247) |
* | 0.016 (0.231) |
ns | 0.823 (0.134) |
*** | 516 |
Significance levels are =p≤0.001,
=p≤0.01,
=p≤0.05,
=p≤0.1; ns=non-significant.
Age is added when warranted (see Table 1) to account for age-related variation in parasitism or condition.
Despite the large inter-annual variation in rainfall between the two years of study, only Eimeria A (intensity) and Strongyloides (prevalence) showed significant differences between the two years, with parasitism higher in the wetter than drier year (Table 3). All parasite estimates were significantly higher in wet than dry seasons, with the exception of Eimeria B. Eimeria B was not statistically related to any of the environmental variables examined, although it had a near significant negative relationship to year.
Springbok were in better condition in the wetter than drier years and in the wet than dry seasons (Table 3). Contrary to expectation, however, springbok in the drier area were significantly more often in the higher body condition class than in the other two areas (there was no significant difference in body condition between the intermediate and wetter areas). The sex differences for adults in the relationship between strongyle intensity and body condition (Fig. 5A for the main study area) were consistent in each of the additional study areas (Fig. 5B).
Discussion
The goal of this study was to disentangle the effects of rainfall and parasitism on seasonal fluctuations in springbok body condition. Springbok condition, and its relationship to rainfall or parasite factors, varied among demographic groups. The effects of rainfall on springbok body condition show both positive and negative relationships and direct and indirect influences on body condition, depending on the demographic group. Increased parasite intensity was associated with reduced body condition only for adult females. In all other demographic groups examined, body condition was related to rainfall and not parasite variables. Host resilience (i.e. productivity despite parasite infection) and resistance (i.e. ability to limit parasite establishment, growth, fecundity or persistence) to parasitism are affected by the balance between nutrition and the particular energy demands of the individual (Coop & Kyriazakis 1999)—factors that vary with age, sex and seasonality. This study demonstrates the importance of environmental variation and demographic influences on rates of parasitism and ultimately on animal body condition.
RAINFALL VS PARASITE EFFECTS ON BODY CONDITION
The relative importance of rainfall or parasite factors in describing patterns of springbok body condition varied among demographic groups (Table 2). For yearlings and adult males, rainfall positively influenced body condition, and condition improved significantly with rainfall at a two month lag. A lag between peak rainfall and improvement in body condition is expected and ruminants are generally in the best condition towards the end of the vegetation growing season (e.g. Parker et al. 1993; Gasaway, Gasaway & Berry 1996).
Adult female condition was lower when strongyle intensity was higher, and this was the only demographic group for which condition related to parasitism. Although rainfall was not a significant factor in the female condition model (Table 2), if parasite variables were excluded from the model then the condition of adult females was negatively related to rainfall one month prior. Strongyle intensity was strongly correlated with rainfall at a one month lag and intensity was higher in females than males (Table 1). The negative relationship between adult female condition and rainfall when parasite variables were excluded demonstrates an indirect, negative effect of rainfall on adult female condition. Juvenile condition was also negatively related to rainfall at a one month lag but there was no significant relationship to any of the parasite variables. Variation in juvenile condition is likely cuing to a factor correlated with rainfall other than the parasites measured in this study.
Strongyles were the only parasites that had a significant relationship with body condition and only for adult females (sex differences in condition and strongyle infections are discussed below). Of the parasites examined in this study, strongyle and Eimeria spp. infections are considered generally pathogenic in livestock, whereas Strongyloides spp. infection is more often asymptomatic (Bowman 2003). For Strongyloides spp. in springbok, this generalization is supported; there were no significant relationships between Strongyloides spp. and body condition, despite the significant associations between this parasite and infection with both strongyles and Eimeria A. Infections with the Eimeria morphotypes were more prevalent or intense in juvenile springbok, while infection with strongyles was more prevalent and intense in adults. Given the age-related patterns in parasite infections, the Eimeria spp. are the parasites most likely to affect juvenile springbok condition. The lack of a negative relationship between the Eimeria morphotypes and juvenile condition indicates that these parasites are not particularly pathogenic in free-ranging springbok.
PARASITISM AND CONDITION ALONG THE RAINFALL GRADIENT
Against expectations, springbok were in significantly better body condition in the drier than the wetter study area (Table 3). There was a decreased presence and intensity of Strongyloides and a decreased intensity of Eimeria A in the drier area, but no significant differences were detected between the two areas in the prevalence or intensity of strongyles, the only parasite which affected body condition in the main study area (for adult females).
Beyond rainfall, these areas also differ in vegetation structure and composition and in the diversity and density of herbivorous mammals, all factors that could influence body condition and parasitism. Springbok are an arid-adapted, mixed-feeding species and individuals graze when grasses are green and highly digestible (Bigalke & van Hensbergen 1990). The drier area has numerous short grass species preferred by grazing herbivores and also a high diversity of trees and shrubs (le Roux et al. 1988), perhaps providing a greater range of forage options for springbok throughout the year than seen in the higher rainfall areas. Springbok in the drier area are also at lower density than in the other study areas (cf. Ministry of the Environment and Tourism aerial survey records), and a reduction in intraspecific (and likely interspecific) competition can lead to improvements in body condition (Gaidet & Gaillard 2008). Differences in predation pressure are unlikely to drive the differences in condition among the study areas, as the most recent estimates of lion densities were similar between the drier and main study areas (drier area, 2.5-2.9 lions/100km2 [Stander 1991]; main area, 2.8 lions/100km2 [Berry 1981]; estimates for the wetter area are not available).
Rainfall seasonality was an important factor affecting parasitism, whether evaluated in terms of a lagged monthly rainfall effect (Table 1; Fig. 3) or as a categorical season variable in the study area comparison (Table 3). Of the environmental variables considered in the study area comparison, season had a larger effect on parasitism than did year or area effects. Despite the large inter-annual variation in rainfall between the two years of study, only Eimeria A (intensity) and Strongyloides (prevalence) showed significant differences between the two years, with parasitism higher in the wetter than drier year. Eimeria B showed no relationship with any of the environmental variables considered. This parasite was relatively rare, with a prevalence of 13.4-16.7% in the two datasets.
SEX DIFFERENCES IN PARASITISM AND CONDITION
Sex differences in parasitism are commonly observed and are attributed to ecological differences between males and females in parasite exposure or physiological differences in susceptibility (Zuk & McKean 1996). In this study, strongyle nematodes were more prevalent in males than females, and for infected springbok, strongyle intensity was higher in females than males (Table 1). The sex difference in prevalence implies a difference between males and females in exposure to strongyle larvae in the environment, a result that could be due to springbok social organization or mating system. Ruminants that sexually segregate often have differences in habitat selection, diet selection or activity patterns between the sexes (Bowyer 2004; Ruckstuhl 2007) all factors that could lead to sex differences in parasitism.
Springbok males segregate from females, and springbok social groups include mixed-sex groups of females and young, male bachelor groups and lone territorial males (Skinner & Chimimba 2005). A territorial mating system may influence the exposure of males to parasites; Ezenwa (2004) found strongyle infection intensity was higher in territorial males than bachelor males or females for Grant’s gazelles (Gazella granti) but not for Thomson’s gazelles (G. thomsoni). Adult males were not apportioned into group types in this study and it is possible that there are differences in parasite exposure for males on territories versus in bachelor herds. However, only adult males may become territorial (Skinner & Chimimba 2005) and strongyle prevalence was higher for males than females in the yearling and adult age classes. Male springbok segregate from females into bachelor herds before adulthood (Skinner & Chimimba 2005), therefore the observed sex differences in strongyle prevalence are more likely related to ecological differences resulting from sexual segregation than from territoriality.
The sex difference in strongyle intensity, where infected females had higher intensity than infected males (Table 1), may result from differences in immune responses to strongyle infection. Parasite egg intensity in feces provides an estimate of a host’s ability to resist an infection given that the parasite is present and reproducing. Host immune responses against adult nematodes include constraints to worm body size and fecundity (Rowe et al. 2008) or worm expulsion (Balic, Bowles & Meeusen 2000). In many species, females show signs of a rise in fecal egg counts following parturition, a phenomenon called the “periparturient rise” or “periparturient relaxation of immunity”. This increase in parasitism for reproductive females is linked to a decrease in immune function and is rapidly corrected when lactation ceases (Beasley, Kahn & Windon 2010). Late pregnancy and lactation are energetically costly for females: energy requirements for gestating females are 17-32% higher than non-gestating females and lactation requires an additional 2-3 times more energy than gestation (Robbins 1993). The periparturient rise is thought to result from tradeoffs in partitioning a finite nutrient pool between maintenance, reproductive activities and immune function (Coop & Kyriazakis 1999). Experimental reductions in energetic demands (i.e., removing nursing young; Houdijk et al. 2006) or increases in the forage nutrient supply (i.e., dietary crude protein; Jones et al. 2011) can lead to improvements in the expression of immunity in females.
Adult female springbok had higher strongyle intensity and were on average in poorer condition than adult males from the peak in parturition and for the first two months of lactation (Fig. 6). After juveniles are weaned, adult females had lower strongyle intensity compared to adult males. We did not distinguish females by reproductive status and the actual differences between lactating females and adult males in parasitism and condition may be more dramatic than seen in our results. In addition, the seasonal timing of reproductive effort for males and females is different. The most energetically costly time for female ungulates often occurs just prior to peak rainfall, allowing females access to high quality or abundant forage when nutrient requirements are at a maximum (Sinclair, Mduma & Arcese 2000). However, this means females likely have increased reproductive stress and a dip in immunity in the season when potential contact with parasite larvae is high. These patterns may explain why changes in body condition were significantly related to parasites for adult females but not for the other demographic groups (Table 2). The springbok mating season occurs in the dry season, and any reproductive-related increases in stress or decreases in immunity for males therefore occurs when there should be little to no contact with parasite larvae in the environment. Our results support that adult females are more negatively influenced by strongyles than are other demographic groups, which may be due to increased energetic demands of gestation and lactation for females and the seasonal differences in reproductive effort between the sexes.
Adult males were more often in better condition than were adult females (Fig. 4). Adult females may be more negatively influenced by strongyles than are adult males, as discussed above, causing females on average to be in poorer condition than males. However, since springbok are an important prey species for many predators, the potential role of selective predation on the observed sex differences in condition and strongyle parasitism cannot be ignored. Springbok are the primary food source for lions in Etosha and adult males are killed significantly more often than adult females compared to the population sex ratio (Stander 1992). Springbok are also the primary food source for spotted hyena (Trinkel 2010) and hyenas may hunt adult males more than adult females (Martina Trinkel, personal communication). Sex differences in springbok vulnerability to predation may be due to their social organization, which is very similar to that of Thomson’s gazelles. Adult male Thomson’s gazelles are hunted more by cheetahs than are adult females because males are more likely to be alone, in smaller groups or on the periphery of groups and are less vigilant than females (Fitzgibbon 1990).
A low overall hunting success rate implies that predators may be more successful hunting individuals in poorer condition (Temple 1987; Wirsing, Steury & Murray 2002; Packer et al. 2003). The success rate of lion hunts on springbok was low in comparison to other prey species, at 13% for springbok vs 11-52% for other species (Stander 1992). Given that adult males are more vulnerable to predation and that hunting success on springbok is low, this may indicate that lions selectively remove males in poorer condition. If lions selectively remove low condition-high strongyle intensity adult males from the population, this could create the infection/condition patterns observed in this study (Fig. 5). Females in poorer condition may have some degree of protection from predation by living in groups and if so, low condition-high parasite intensity females may be less likely to be removed from the population than similarly infected adult males. However, as territorial males likely have a higher predation risk than individuals in female or bachelor groups and must be in excellent condition to defend a territory, predator selection of more vulnerable adult males may not necessarily remove highly parasitized-low condition males from the population.
In reality, the two possible causes of the sex differences in strongyle intensity and body condition presented here are not mutually exclusive, and it is very likely that both sex differences in immunity and vulnerability to predation play a role in shaping patterns of parasitism and body condition observed in the springbok population. The sex differences in strongyle intensity and body condition were observed consistently across all of the study areas (Fig. 5) providing evidence that these patterns are general to the ecology of springbok and are not caused by the peculiarities of any one study area. Additional research is required to distinguish the relative importance of immunity versus predation on sex differences in parasitism and body condition, and how these factors may vary temporally or spatially.
CONCLUSION
Ecological systems are complex because there are typically many interacting factors affecting organisms at different scales. Environmental variability has been recognized as an important factor affecting host stress and immunocompetence (Altizer et al. 2006) and the behavior and ecology of organisms (Owen-Smith 2002; Hopcraft, Olff & Sinclair 2010). Parasites can have important effects on the life history parameters of their hosts, reducing survival (Gulland 1992; Hudson, Newborn & Dobson 1992; Murray, Cary & Keith 1997), fecundity (Stien et al. 2002; Lello, Boag & Hudson 2005) or offspring condition (Hakkarainen et al. 2007). Parasites and the environment can synergistically affect host populations, but the effects of parasites are often masked by the interwoven relationships among parasites, environmental variability and their differential effects on host demographic classes. This complexity is typified by the seasonal fluctuations in ruminant body condition, where animals must acquire the nutrients necessary for short-term survival but also for future reproductive events in dynamic systems alternating between resource abundance and scarcity. Host resilience and resistance to parasitism depends on the balance between nutritional quality of the resource base, the body’s energetic requirements and the magnitude of the detrimental parasite effects on the host. To understand the ecology of any single species, we must understand how environmental variation influences physiology, life history and the strength of species interactions in the web of consumers that surround the target organism, including both its predators and parasites (Getz 2011). The time scales at which species interactions can affect a target population vary substantially from multi-year cycles in the abundance of predators to seasonal cycles in resource abundance to weekly-monthly cycles in the abundance of parasites. Our results highlight the importance of considering the inertial effects of past influences on the current structure and health of a given population.
Acknowledgements
This manuscript benefited from comments from Steve Bellan, Carrie Cizauskas and Holly Ganz. We thank the Namibian Ministry of Environment and Tourism for permission to conduct this research and the staff in the Directorate of Scientific Services and Shayne Kötting at the Etosha Ecological Institute for logistical support and assistance. We thank Martina Küsters, Mathias Bosseau, Aimee Boursaw, Emily Kalenius, Birgit Kötting, Gabriel Shatumbu, Nigel Berriman, Johannes Kapner and Seth Guim for assistance with sample collection. This research was supported by a Fulbright fellowship, Andrew and Mary Thompson Rocca Scholarships, Professor Earl Storie Memorial Scholarship, G. Fitzgarrald Martin Scholarship, and a grant from the Department of Environmental Science, Policy and Management to WCT, and NIH Grant GM083863 to WMG.
References
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecology Letters. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Balic A, Bowles VM, Meeusen ENT. The immunobiology of gastrointestinal nematode infections in ruminants. Advances in Parasitology. 2000;45:181–241. doi: 10.1016/s0065-308x(00)45005-0. [DOI] [PubMed] [Google Scholar]
- Banks DJD, Singh R, Barger IA, Pratap B, le Jambre LF. Development and survival of infective larvae of Haemonchus contortus and Trichostrongylus colubriformis on pasture in a tropical environment. International Journal for Parasitology. 1990;20:155–160. doi: 10.1016/0020-7519(90)90095-5. [DOI] [PubMed] [Google Scholar]
- Beasley AM, Kahn LP, Windon RG. The periparturient relaxation of immunity in Merino ewes infected with Trichostrongylus colubriformis: Parasitological and immunological responses. Veterinary Parasitology. 2010;168:60–70. doi: 10.1016/j.vetpar.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Bednekoff PA, Ritter RC. Adult sex ratio of a wild population of springbok (Antidorcas marsupialis) at Nxai Pan, Botswana. South African Journal of Wildlife Research. 1997;27:22–24. [Google Scholar]
- Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M. Poor condition and infection: a vicious circle in natural populations. Proceedings of the Royal Society B-Biological Sciences. 2008;275:1753–1759. doi: 10.1098/rspb.2008.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry HH. Abnormal levels of disease and predation as limiting factors for wildebeest in the Etosha National Park. Madoqua. 1981;12:242–253. [Google Scholar]
- Berry HH, Louw GN. Seasonal nutritive status of wildebeest in the Etosha National Park. Madoqua. 1982;13:127–139. [Google Scholar]
- Beugler-Bell H, Buch MW. Soils and soil erosion in the Etosha National Park, northern Namibia. Madoqua. 1997;20:91–104. [Google Scholar]
- Bigalke RC, van Hensbergen HJ. Some behavioral considerations in springbok management. In: Skinner JD, Dott HM, editors. Proceedings of a Workshop on Springbok. The Zoological Society of Southern Africa and The Eastern Cape Game Management Association; Graaff Reinet: 1990. pp. 12–16. [Google Scholar]
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th edn W. B. Saunders; Philadelphia: 2003. [Google Scholar]
- Bowyer RT. Sexual segregation in ruminants: definitions, hypotheses, and implications for conservation and management. Journal of Mammalogy. 2004;85:1039–1052. [Google Scholar]
- Coop RL, Kyriazakis I. Nutrition-parasite interaction. Veterinary Parasitology. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Craig BH, Tempest LJ, Pilkington JG, Pemberton JM. Metazoan-protozoan parasite co-infections and host body weight in St Kilda Soay sheep. Parasitology. 2008;135:433–441. doi: 10.1017/S0031182008004137. [DOI] [PubMed] [Google Scholar]
- De Villiers IL, Liversidge R, Reinecke RK. Arthropods and helminths in springbok (Antidorcas marsupialis) at Benfontein, Kimberley. Onderstepoort Journal of Veterinary Research. 1985;52:1–11. [PubMed] [Google Scholar]
- Engert S. Spatial variability and temporal periodicity of rainfall in the Etosha National Park and surrounding areas in northern Namibia. Madoqua. 1997;20:115–120. [Google Scholar]
- Ezenwa VO. Host social behavior and parasitic infection: a multifactorial approach. Behavioral Ecology. 2004;15:446–454. [Google Scholar]
- Fayer R. Epidemiology of protozoan infections - coccidia. Veterinary Parasitology. 1980;6:75–103. [Google Scholar]
- Fitzgibbon CD. Why do hunting cheetahs prefer male gazelles? Animal Behaviour. 1990;40:837–845. [Google Scholar]
- Fox MT. Pathophysiology of infection with gastrointestinal nematodes in domestic ruminants: recent developments. Veterinary Parasitology. 1997;72:285–308. doi: 10.1016/s0304-4017(97)00102-7. [DOI] [PubMed] [Google Scholar]
- Gaidet N, Gaillard J-M. Density-dependent body condition and recruitment in a tropical ungulate. Canadian Journal of Zoology. 2008;86:24–32. [Google Scholar]
- Gasaway WC, Gasaway KT, Berry HH. Persistent low densities of plains ungulates in Etosha National Park, Namibia: testing the food-regulating hypothesis. Canadian Journal of Zoology. 1996;74:1556–1572. [Google Scholar]
- Getz WM. Biomass transformation webs provide a unified approach to consumer-resource modeling. Ecology Letters. 2011;14:113–124. doi: 10.1111/j.1461-0248.2010.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons LM, Jacobs DE, Fox MT, Hansen J. [Accessed June 2005];The RVC/FAO Guide to Veterinary Diagnostic Parasitology. Faecal Examination of Farm Animals for Helminth Parasites. 2005 http://www.rvc.ac.uk/review/Parasitology/Index/Index.htm.
- Gulland FMD. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Hakkarainen H, Huhta E, Koskela E, Mappes T, Soveri T, Suorsa P. Eimeria-parasites are associated with a lowered mother’s and offspring’s body condition in island and mainland populations of the bank vole. Parasitology. 2007;134:23–31. doi: 10.1017/S0031182006001120. [DOI] [PubMed] [Google Scholar]
- Hayward MW, Kerley GIH. Prey preferences and dietary overlap amongst Africa’s large predators. South African Journal of Wildlife Research. 2008;38:93–108. [Google Scholar]
- Hofmann RR, Knight MH, Skinner JD. On structural characteristics and morphophysiological adaptation of the springbok (Antidorcas marsupialis) digestive system. Transactions of the Royal Society of South Africa. 1995;50:125–142. [Google Scholar]
- Holmstad PR, Hudson PJ, Skorping A. The influence of a parasite community on the dynamics of a host population: a longitudinal study on willow ptarmigan and their parasites. Oikos. 2005;111:377–391. [Google Scholar]
- Hopcraft JGC, Olff H, Sinclair ARE. Herbivores, resources and risks: alternating regulation along primary environmental gradients in savannas. Trends in Ecology & Evolution. 2010;25:119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Horak IG, Meltzer DGA, de Vos V. Helminth and arthropod parasites of springbok, Antidorcas marsupialis, in the Transvaal and Western Cape Province. Onderstepoort Journal of Veterinary Research. 1982;49:7–10. [PubMed] [Google Scholar]
- Houdijk JGM, Jackson F, Coop RL, Kyriazakis I. Rapid improvement of immunity to Teladorsagia circumcincta is achieved through a reduction in the demand for protein in lactating ewes. International Journal for Parasitology. 2006;36:219–227. doi: 10.1016/j.ijpara.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Newborn D, Dobson AP. Regulation and stability of a free-living host-parasite system: Trichostongylus tenuis in red grouse. I. Monitoring and parasite reduction experiments. Journal of Animal Ecology. 1992;61:477–486. [Google Scholar]
- Hutchings MR, Kyriazakis I, Gordon IJ, Jackson F. Trade-offs between nutrient intake and faecal avoidance in herbivore foraging decisions: the effect of animal parasitic status, level of feeding motivation and sward nitrogen content. Journal of Animal Ecology. 1999;68:310–323. [Google Scholar]
- Jones LA, Houdijk JGM, Sakkas P, Bruce AD, Mitchell M, Knox DP, Kyriazakis I. Dissecting the impact of protein versus energy host nutrition on the expression of immunity to gastrointestinal parasites during lactation. International Journal for Parasitology. 2011;41:711–719. doi: 10.1016/j.ijpara.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Klare U, Kamler JF, Stenkewitz U, Macdonald DW. Diet, prey selection, and predation impact of black-backed jackals in South Africa. Journal of Wildlife Management. 2010;74:1030–1042. [Google Scholar]
- le Roux CJG, Grunow JO, Morris JW, Bredenkamp GJ, Scheepers JC. A classification of the vegetation of the Etosha National Park. South African Journal of Botany. 1988;54:1–10. [Google Scholar]
- Lello J, Boag B, Hudson PJ. The effect of single and concomitant pathogen infections on condition and fecundity of the wild rabbit (Oryctolagus cuniculus) International Journal for Parasitology. 2005;35:1509–1515. doi: 10.1016/j.ijpara.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Murray DL, Cary JR, Keith LB. Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. Journal of Animal Ecology. 1997;66:250–264. [Google Scholar]
- Nagy KA, Knight MH. Energy, water, and food use by springbok antelope (Antidorcas marsupialis) in the Kalahari Desert. Journal of Mammalogy. 1994;75:860–872. [Google Scholar]
- Newey S, Shaw DJ, Kirby A, Montieth P, Hudson PJ, Thirgood SJ. Prevalence, intensity and aggregation of intestinal parasites in mountain hares and their potential impact on population dynamics. International Journal for Parasitology. 2005;35:367–373. doi: 10.1016/j.ijpara.2004.12.003. [DOI] [PubMed] [Google Scholar]
- O’Connor LJ, Walkden-Brown SW, Kahn LP. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Veterinary Parasitology. 2006;142:1–15. doi: 10.1016/j.vetpar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Owen-Smith N. Foraging responses of kudus to seasonal changes in food resources: elasticity in constraints. Ecology. 1994;75:1050–1062. [Google Scholar]
- Owen-Smith N. Adaptive herbivore ecology: from resources to populations in variable environments. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecology Letters. 2003;6:797–802. [Google Scholar]
- Panagis K, Stander PE. Marking and subsequent movement patterns of springbok lambs in the Etosha National Park, South West Africa-Namibia. Madoqua. 1989;16:71–74. [Google Scholar]
- Parker KL, Barboza PS, Gillingham MP. Nutrition integrates environmental responses of ungulates. Functional Ecology. 2009;23:57–69. [Google Scholar]
- Parker KL, Gillingham MP, Hanley TA, Robbins CT. Seasonal patterns in body mass, body composition, and water transfer rates of free-ranging and captive black-tailed deer (Odocoileus hemionus sitkensis) in Alaska. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1993;71:1397–1404. [Google Scholar]
- R Development Core Team R: A language and environment for statistical computing. 2010 http://www.R-project-org.
- Rautenbach IL. M.Sc. thesis. University of Pretoria; Pretoria: 1971. Ageing criteria in the springbok, Antidorcas marsupialis (Zimmermann, 1780) [Google Scholar]
- Robbins CT. Wildlife feeding and nutrition. 2nd Edition Academic Press; New York: 1993. [Google Scholar]
- Round MC. Checklist of the Helminth Parasites of African Mammals of the Orders Carnivora, Tubulidentata, Proboscidea, Hyracoidea, Artiodactyla and Perrisodactyla. Commonwealth Agricultural Bureaux; Bucks, England: 1968. [Google Scholar]
- Rowe AK, McMaster K, Emery D, Sangster N. Haemonchus contortus infection in sheep: parasite fecundity correlates with worm size and host lymphocyte counts. Veterinary Parasitology. 2008;153:285–293. doi: 10.1016/j.vetpar.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Ruckstuhl KE. Sexual segregation in vertebrates: proximate and ultimate causes. Integrative and Comparative Biology. 2007;47:245–257. doi: 10.1093/icb/icm030. [DOI] [PubMed] [Google Scholar]
- Sinclair ARE, Mduma SAR, Arcese P. What determines phenology and synchrony of ungulate breeding in Serengeti? Ecology. 2000;81:2100–2111. [Google Scholar]
- Skinner JD, Chimimba CT. The Mammals of the Southern African Subregion. 3rd edn Cambridge University Press; 2005. [Google Scholar]
- Skinner JD, Louw GN. The springbok Antidorcas marsupialis (Zimmerman, 1780) Transvaal Museum Monograph. 1996;10:1–50. [Google Scholar]
- Stander PE. Demography of lions in the Etosha National Park, Namibia. Madoqua. 1991;18:1–9. [Google Scholar]
- Stander PE. Foraging dynamics of lions in a semiarid environment. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1992;70:8–21. [Google Scholar]
- Stevenson RD, Woods WA. Condition indices for conservation: new uses for evolving tools. Integrative and Comparative Biology. 2006;46:1169–1190. doi: 10.1093/icb/icl052. [DOI] [PubMed] [Google Scholar]
- Stien A, Irvine RJ, Ropstad E, Halvorsen O, Langvatn R, Albon SD. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. Journal of Animal Ecology. 2002;71:937–945. [Google Scholar]
- Temple SA. Do predators always capture substandard individuals disproportionately from prey populations? Ecology. 1987;68:669–674. [Google Scholar]
- Trinkel M. Prey selection and prey preferences of spotted hyenas Crocuta crocuta in the Etosha National Park, Namibia. Ecological Research. 2010;25:413–417. [Google Scholar]
- Turner WC, Getz WM. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. Journal of Wildlife Diseases. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtert MFJ, Sykes AR. Implications of nutrition for the ability of ruminants to withstand gastrointestinal nematode infections. International Journal for Parasitology. 1996;26:1151–1168. doi: 10.1016/s0020-7519(96)00120-8. [DOI] [PubMed] [Google Scholar]
- Weber ML, Thompson JM. Seasonal patterns in food intake, live mass, and body composition of mature female fallow deer (Dama dama) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1998;76:1141–1152. [Google Scholar]
- Wirsing AJ, Steury TD, Murray DL. Relationship between body condition and vulnerability to predation in red squirrels and snowshoe hares. Journal of Mammalogy. 2002;83:707–715. [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: Patterns and processes. International Journal for Parasitology. 1996;26:1009–1023. [PubMed] [Google Scholar]