Abstract
Although there is increasing clinical acceptance of acupuncture and electroacupuncture (EA) as a treatment of substance abuse-related disorders, our understanding of this treatment remains incomplete. Previous clinical and pre-clinical studies have shown that acupuncture and EA are effective in reducing ethanol consumption. Recent studies have shown that Sprague–Dawley (SD) rats under an intermittent-access two-bottle choice drinking procedure (IE procedure) voluntarily drank high amounts of ethanol. However, an effect of EA on ethanol consumption of the SD rats under this drinking procedure has not been demonstrated.
In the present study, we demonstrated that SD rats escalated their ethanol intake and subsequently developed ethanol dependence under the IE procedure. A single low (2 Hz), but not high frequency (100 Hz) EA treatment applied at the bilateral acupoint Zusanli (ST36), but not at the tail reduced voluntary intake of, and preference for ethanol, but not sucrose. Furthermore, repeated EA treatments decreased the intake of and preference for ethanol, without resulting in a rebound increase in ethanol intake when the EA treatments were terminated. These observations indicate that EA may be a useful treatment for alcohol abuse.
Keywords: Acupuncture, Addiction, Alcohol dependence, Sprague-Dawley rats, treatment
1. Introduction
Acupuncture, consisting of stimulating certain points on the body by means of needles, has been regarded widely as an effective mean for some medical conditions, including nausea, pain (Jindal et al., 2008) and drug abuse (Kim et al., 2005b). It has been shown that manual acupuncture or acupuncture combined with electrical stimulation (electroacupuncture, EA) in specific frequencies applied to certain body sites can facilitate the release of specific neuropeptides in the central nervous system (CNS), eliciting profound physiological effects and even activating self-healing mechanisms (Han, 2003). However, many questions regarding the efficacy and basic mechanisms of acupuncture in general, and for alcohol abuse in particular, have not been well addressed. Previous clinical and preclinical studies have shown that acupuncture is an effective treatment for alcohol drinking behaviors and alcohol withdrawal syndrome (Yoshimoto et al., 2001; Karst et al., 2002; Trumpler et al., 2003; Kim et al., 2005a; Kunz et al., 2007; Overstreet et al., 2008). More recent animal studies have shown that acupuncture at the specific acupoint Shenmen (HT7) can normalize dopamine release induced by both the presence and withdrawal of ethanol (EtOH) in the nucleus accumbens (Yoon et al., 2004; Zhao et al., 2006) and can inhibit the activity of GABA neurons in the ventral tegmental area and reduce EtOH self-administration (Yang et al.., 2010). These results suggest that acupuncture may be an effective adjunct therapy for the treatment of alcoholism.
Zusanli (ST36) is one of the most effective acupuncture points with a wide range of effects such as: analgesic, spasmolytic, and homeostatic (Stux and Pomeranz, 1987). Many studies have verified the effectiveness of this point in experimental animals (Futaesaku et al., 1995; Han et al., 1999; Kim et al., 2002; Yun et al., 2002; Dong et al., 2009). Furthermore, it has been shown that EA at ST36 increased μ-receptor binding sites (Gao et al., 1997; Yun et al., 2002). Alternated frequency EA between 2 and 100 Hz or a single low frequency (2 Hz) at ST36 decreased alcohol intake in inbred alcohol-preferring P (iP) rats (Overstreet et al., 2008) and physically restricted Sprague-Dawley (SD) rats (Yoshimoto et al., 2001; Overstreet et al., 2008). However, in Overstreet’s study, it showed that EA blocked the increase of alcohol intake induced by alcohol deprivation, but not ongoing drinking. In Yoshimoto’s study, on the other hand, EA suppressed the increased time-access alcohol-drinking behavior induced by physical restraint. Information of EA effects on ongoing EtOH drinking in alcohol dependent subjects is lacking. Recent studies have demonstrated that SD rats under the IE procedure voluntarily consumed high amounts of EtOH (Li et al., 2010; Moorman and Aston-Jones, 2009). However, an effect of acupuncture on EtOH consumption in SD rats under this drinking procedure has not been demonstrated. In this study, we examined the effect of EA at ST36 on EtOH consumption of SD rats under the IE procedure.
2. Materials and Methods
Subjects used in this study were male Sprague-Dawley rats (n = 34, Taconic Farms, Hudson, NY) weighing between 180 and 200 g at the start of the experiment. Rats were housed individually in polycarbonate cages (Thoren Caging System Inc., Hazleton, PA, USA) in ventilation racks. Animals received food and water ad libitum. The room was illuminated on a 12 h light/dark schedule with lights off at 3:00 PM. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Medicine and Dentistry of New Jersey and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
After acclimating to the homecage environment for 1 week, animals (n = 26) were trained to voluntarily drink EtOH under the intermittent-access two-bottle choice drinking procedure as described previously (Simms et al., 2008). Briefly, animals were given 24-h concurrent access to one bottle of 20% (v/v) EtOH in water and one bottle of water, starting at 3:00 p.m. on Monday. After 24 h, the EtOH bottle was replaced with a second water bottle that was available for the next 24 h. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. In each EtOH drinking day, the placement of the EtOH bottle was alternated to control for side preferences. Animal body weight was determined once per week on Wednesdays. The amount of EtOH or water consumed was determined by weighing the bottles before access and after 2 h or 24 h of access. EtOH consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. The preference ratio of EtOH intake was calculated by the following formula: Preference ratio (%) = EtOH solution intake (ml/2 h or 24 h)/total fluid intake (ml/2 h or 24 h EtOH solution + ml/2 h or 24 h water). The daily volume of EtOH consumed and preference ratio of EtOH for three sessions in a given week were averaged. All EtOH solutions were prepared in tap water from 95% (v/v) EtOH (Pharmco, Brookfield, CT, USA). When SD rats had reached a consistent baseline level after 12 sessions of access to EtOH and water, measurements of blood EtOH concentrations (BECs) and physical withdrawal signs were conducted respectively at 13th and 14th drinking session. EA or sham EA procedures were performed always on Wednesday after 16th drinking session. Animals in the control group (EtOH naïve controls) were allowed access to water and food without limitation. There were no significant differences in body weight between the control and the EtOH-drinking rats at the end of the experiments.
To determine whether EA-induced reduction in drinking was selective to EtOH, a separate group (n = 8) of rats were trained to drink 5 % sucrose solution under intermittent-access two-bottle choice drinking procedure, similar to that for EtOH drinking. 5% sucrose was selected according to a recent rodent study on the effect of the opioid receptor antagonists, SoRI-9409 on alcohol intake (Nielsen et al., 2008). When SD rats had reached a consistent baseline level after 16 drinking sessions of access to sucrose and water, EA or sham procedures were performed always on Wednesday.
Blood samples were collected from the lateral tail vein following 1 h or 24 h of access to 20% EtOH and water from a subgroup of rats (n = 9). The samples were centrifuged at room temperature (approximately 21–22) for 15 min at 8000 rpm, and 10 μl plasma from each blood sample was analyzed using nicotinamide adenine dinucleotide-alcohol dehydrogenase (NAD-ADH) enzyme spectrophotometric method (Poklis and Mackell, 1982; Weiss et al., 1993).
Previous work using a chronic intermittent vapor exposure model found that EtOH-dependent animals showed mild but not severe (e.g. spontaneous tonic–clonic convulsions) (Majchrowicz, 1975) signs of physical withdrawal following removal from alcohol vapors (Richardson et al., 2008). To characterize withdrawal severity in the present model, withdrawal signs were quantified after removal from the 24-h access to EtOH or EtOH naïve controls using methodology similar to earlier reports (Macey et al., 1996; Richardson et al., 2008). Specifically, distal limb flexion response, tail stiffness and abnormal body posture were measured using a subjective 0–2 point scale to score withdrawal symptoms (0 = undetectable, 1 = moderate, 2 = severe). Ventromedial distal limb flexion responses were determined by lifting the rat up by the scruff of the neck and observing retraction of the lower legs toward the body. Tails were examined for rigidity and/or curvature upward toward the back. The presence of a broad-based stance and/or abnormal gait indicated abnormal body posture. Statistical analyses were conducted on overall withdrawal severity scores that were determined for each animal by summing the scores for all three signs, yielding a range from 0 to 6. Using this method, we quantified withdrawal severity after rats removed from the 24-h access to EtOH (n = 12) and in EtOH naïve controls (n = 12), in which rats were allowed access to water and food without limitation. The scores for each rat were done by a person who did not know the treatment condition. There was no significant difference in body weight between the control and the EtOH-drinking rats when performed measurements of physical withdrawal signs.
Twenty min of EA (or sham EA) was given to rats 30 min prior to the access to 20% EtOH or 5% sucrose. EA was applied at the acupoint of Zusanli (ST36), located near the knee joint of the hind limb, 2 mm lateral to the anterior tubercle of the tibia. Sham EA was applied at nonacupoints 0.2 1/5 tail length from the proximal region of the tail (Yang et al., 2002; Zhao et al., 2006). Seven rats under the intermittent-access two-bottle choice drinking procedure were dedicated to the single EA treatment (low frequency, 2 Hz) experiment, and twelve rats were dedicated to the repeated EA treatment (low frequency, 2 Hz) experiment. All rats were prehandled for 2 min/day for 3 consecutive days prior to acupuncture treatments to reduce stress and facilitate handling. On the test day, under light anesthetization with isoflurane, the rats of both EA and sham EA groups were lightly restrained, which involved being fixed on a rack with a towel covering their eyes. Under these conditions, the rats were calm and their limbs and tails could freely extrude. Two stainless-steel needles with diameter of 0.35 mm and length of 13 mm were inserted vertically to a depth of about 3 mm into ST36 of both legs (for EA group). After the animals woke up from anesthesia, EA stimulation or sham EA was conducted for 20 min. A constant current with square-wave stimulation produced by a programmed pulse generator (Han Actens WQ 1002F, Aeron Optoelectronic Technology Corp., Beijing, China) was given via the two needles for the EA group. The frequency of EA was 2 Hz, and the intensity of the stimulation was adjusted to provoke light trembling of muscles (about 0.2–0.3 mA). There is a considerable body of literature on the subject of EA frequency. A frequency of 100 Hz has proved effective in increasing preprodynorphin mRNA expression, as well as accelerating the release of dynorphin in the CNS (Guo et al., 1996; Yoshimoto et al., 2001; Han, 2003). Five animals after the single 2 Hz EA treatment were allowed to continue drinking EtOH under the intermittent-access drinking procedure and were administered high frequency (100 Hz) EA at ST36 after a two week recovery from the single 2 Hz EA administration. For the sham EA treatment, needles were placed into nonacupoints of the tail, and no current stimulation was applied. In the low-frequency and high-frequency experiments, all subjects received each treatment in a counterbalanced manner, with one acupuncture treatment per week. EtOH (or sucrose) and water intakes were then recorded at 2 h after the onset of drinking. Change in drinking due to EA administration was calculated by comparing to both the previous day’s consumption (“baseline”) and to the sham treatment.
Repeated EA treatment experiments were conducted on two groups of rats: one group received 2 Hz EA at ST36 and the other was given sham-EA at the tail for 3 consecutive drinking sessions (Wednesday through Monday). During the 6 treatment days, the rats had three EtOH-drinking sessions (day 1, Wednesday; day 3, Friday; and day 6, Monday). EtOH consumption during the drinking session immediately before EA administration and after the last EA administration was recorded respectively as the baseline or post-treatment baseline drinking level.
Results are presented as mean ± S.E.M. where appropriate. For baseline drinking behavioral, the data were analyzed using a one-way repeated measures analysis of variance (RM ANOVA) with Newman-Keuls post hoc analysis when a signi cant overall main effect was found (p < 0.05). Experiments for the single EA were conducted in a within-subjects design and analyzed by paired t-test. Data from repeated EA treatment experiments and physical withdrawal signs were subjected to a two-way RM ANOVA with Student Newman–Keuls post hoc comparisons to extract significant main effects and interactions. Statistical significance was declared at p < 0.05. The BEC data were analyzed by linear regression.
3. Results
An intermittent-access two-bottle choice drinking (IE) procedure led SD rats to consume high amounts of EtOH and depend on EtOH
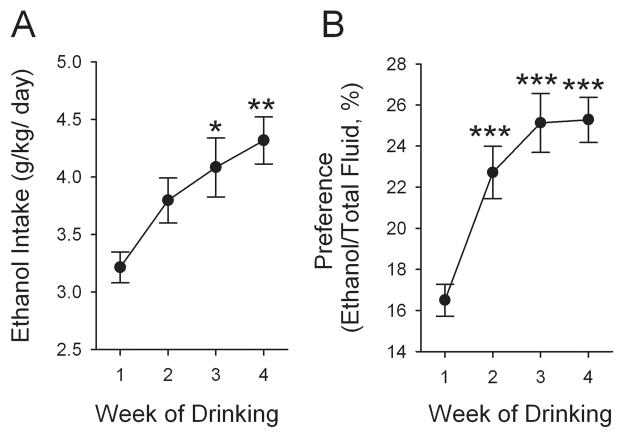
In this experiment, 26 rats were trained to self-administer alcohol under the IE procedure. This procedure led the majority of the SD rats to robustly increase EtOH intake (see below). Nevertheless, by week 4, 6 rats drank less than 1.5 g/kg/24 h. These rats were excluded from the statistical analysis, leaving 20 animals for further study. EtOH intake of these 20 rats increased (F3,222= 4.88, p < 0.01, one-way ANOVA, n = 20) across weeks of access to EtOH, from 3.2 ± 0.1 g/kg/24 h on week 1 to 4.3 ± 0.2 g/kg/24 h on week 4 (Fig. 1A). This was accompanied with a significant escalation in EtOH preference ratio over time (F3,222 = 11.39, p < 0.001, n = 20), from 16.7 ± 0.8 % on week 1 to 25.0 ± 1.1 % on week 4 (Fig. 1B).
Figure 1.
EtOH drinking habit develops and stabilizes over weeks under the intermittent-access two-bottle choice drinking (IE) procedure. (A) EtOH intake increased in a 24-h access period across weeks of access to EtOH (F3,222= 4.73, p = 0.004). (B) Preference for EtOH was also significantly elevated over time (F3,222 = 11.39, p < 0.001). The values are expressed as mean ± standard error of the mean of EtOH intake or preference. **p < 0.01 and ***p < 0.001 compared with the first drinking week (one-way RM ANOVA followed by Newman–Keuls post hoc test), n = 20.
To determine whether the rats were consuming physiologically relevant amounts of EtOH, we measured BEC of 9 rats from the above drinking group at the 13th drinking session. BEC was determined immediately after the 1 h of access to EtOH. The amount of EtOH consumed during the initial 1 h significantly correlated with the BECs (r² = 0.67, p < 0.01, n = 9, Figure 2). The BECs were 30.2 ± 6.4 mg/dl. We also measured BECs after 24 h of access to EtOH, which were 15.6 ± 2.5 mg/dl.
Figure 2.
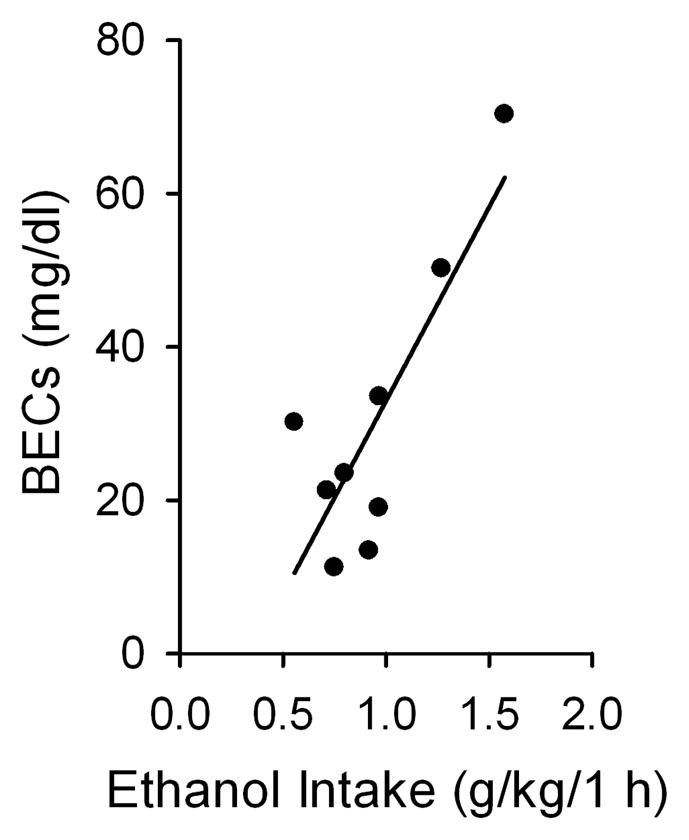
Blood EtOH concentrations (BECs; mg/dl) were obtained following 1 h of access to EtOH using the intermittent-access 20% EtOH drinking procedure. The blood samples for BEC analysis were collected approximately 75 min into the dark cycle. The amount of EtOH consumed in the initial 1 h of access to EtOH significantly correlated with the measured BECs (linear regression): r²= 0.67, p < 0.01, n = 9.
To determine whether the SD rats under the IE procedure develop physical dependence on EtOH, we measured the physical signs of withdrawal of 12 rats at the 14th drinking session. As indicated in Table 1, the physical signs were evident in rats withdrawn from EtOH, obtaining significance at 2 h, peaked from 4 to 8 h, and descended thereafter (main effect of Group, F1,154 = 7.4, p < 0.05; and Withdrawal time, F7,145 = 3.12, p < 0.001; interaction between both factors, F7,154 = 2.7, p < 0.05). Post-hoc analyses indicated significant differences between EtOH-naïve controls and EtOH intake rats at 2, 4, 6 and 8 h time-points (all p < 0.01), and significant increases in withdrawal signs at 2, 4, 6 and 8 h compared with 0 h in dependent animals (all p < 0.05).
Table 1.
Ratings of observational signs of withdrawal from EtOH drinking under the intermittent access two-bottle choice drinking paradigm.
| Withdrwal score (0–6) | Postwithdrawal Time (hr)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 24 | |
| Ethanol | 0.50 ± 0.19 | 1.33 ± 0.25#* | 1.58 ± 0.34#*** | 1.66 ± 0.37#*** | 1.41 ± 0.34#** | 1.16 ± 0.27 | 0.75 ± 0.25 | 0.67 ± |
| Control | 0.42 ± 0.23 | 0.42 ± 0.19 | 0.50 ± 0.15 | 0.50 ± 0.15 | 0.50 ± 0.15 | 0.67 ± 0.19 | 0.75 ± 0.25 | 0.67 ± |
The values are expressed as mean ± SEM.
p < 0.05,
p < 0.001compared with 0 h;
p < 0.01 compared with EtOH-naïve control.
Low but not high frequency EA reduces the intake of EtOH but not the intake of sucrose
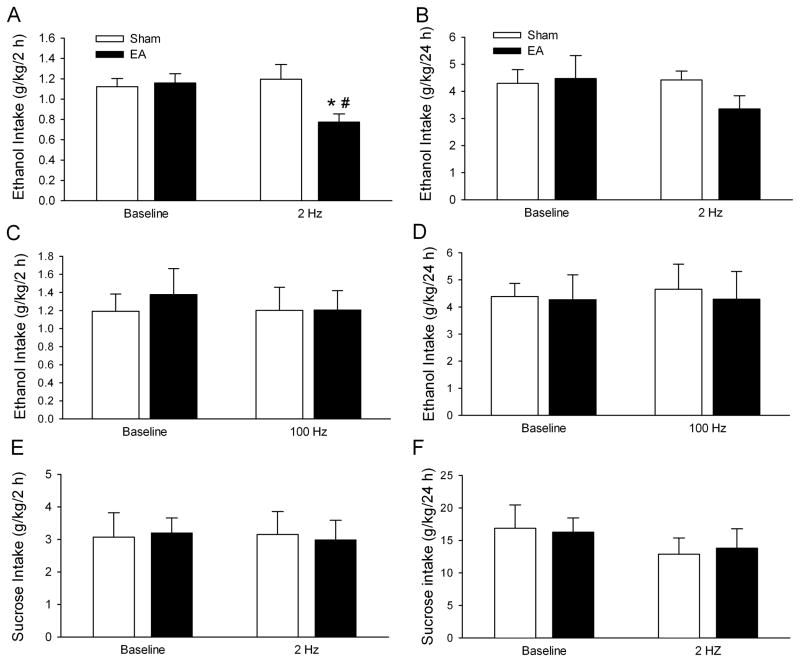
To assess the effect of EA on voluntary EtOH intake, 20 min of low frequency (2 Hz) EA was applied at ST36 before access to EtOH on an EtOH drinking day. As shown in Fig. 3A, EA treatment significantly decreased EtOH intake over the 2-h access period compared with sham EA treatment (t(12) = 2.53, p < 0.05) and baseline drinking levels (t(12) = 2.76, p < 0.05). EA treatment also significantly reduced the preference ratio for EtOH compared with sham EA treatment (t(12) = 3.34, p < 0.01; Table 2) and baseline EtOH preference ratio (t(12) = 3.36, p < 0.01; Table 2). Interestingly, the decrease in EtOH intake was accompanied by an increase in water intake compared with sham EA treatment (t(12) = 3.3, p < 0.01; Table 2). No significant difference was found between sham EA treatment and baseline levels for either water intake or preference ratio for EtOH. In addition, low frequency (2 Hz) EA also produced a tendency to decrease EtOH intake over the 24-h access period compared with sham EA treatment (t(12) = 1.84, p = 0.09, Fig. 3B).
Figure 3.
Low frequency (2 Hz), but not high frequency (100 Hz) EA, applied at ST36 selectively reduced EtOH intake in rats under the intermittent-access two-bottle choice drinking procedure. EA or sham EA was administered 30 min before the start of drinking session. A. Low frequency (2 Hz) EA applied at ST36 significantly decreased EtOH consumption 2 h after the onset of drinking (n = 7). B. High frequency (100 Hz) EA applied at ST36 did not significantly alter EtOH intake 2 h after the onset of drinking (n = 5). C. Low frequency (2 Hz) EA applied at ST36 did not affect sucrose intake 2 h after the onset of drinking, using the two-bottle choice 5% sucrose drinking procedure (n = 8). The values are expressed as mean ± SEM. *p < 0.05 compared with baseline; #p < 0.05 compared with sham (paired t-test).
Table 2.
The effects of EA applied at ST36 on water intake and preference for EtOH (or sucrose) after 2 hours of access to EtOH (or sucrose) and water
| Group | Baseline | EA Test | ||
|---|---|---|---|---|
| Water Intake (mL//kg/2 h) | Preference for EtOH or Sucrose (%) | Water Intake (mL//kg/2 h) | Preference for EtOH or Sucrose (%) | |
| EtOH Reinforcement | ||||
| Low Frequency Group | ||||
| Sham | 12.1 ± 2.1 | 41.0 ± 6.9 | 11.6 ± 1.0 | 38.7 ± 3.9 |
| EA 2 HZ | 12.7 ± 2.2 | 42.5 ± 5.5 | 18.2 ± 1.6# | 22.7 ± 2.1*# |
| High Frequency Group | ||||
| Sham | 12.2 ± 2.4 | 38.4 ± 4.5 | 12.5 ± 2.3 | 42.2 ± 7.0 |
| EA 100 HZ | 11.4 ± 1.5 | 41.8 ± 0.5 | 14.4 ± 2.4 | 35.5 ± 5.7 |
| Sucrose Reinforcement | ||||
| Sham | 0.8 ± 0.5 | 98.5 ± 0.8 | 2.1 ± 0.8 | 94.8 ± 2.7 |
| EA 2 HZ | 0.7 ± 0.3 | 98.9 ± 0.5 | 3.4 ± 1.2* | 94.1 ± 1.9* |
Values are expressed as mean ± SEM. Paired t-test.
p < 0.05 compared with baseline;
p < 0.01 compared with sham.
To determine whether the effect of EA is specific to EtOH intake, we measured the effects of EA on sucrose intake using an intermittent access of 5% sucrose in a two-bottle choice dinking procedure. We chose 5% instead of other concentrations of sucrose because in a previous study we found that there was not significant difference in sucrose preference between 0.01–5% of sucrose, (all >90%), and 5% sucrose provides the calories needed for the rats. Two Hz EA applied at ST36 did not significantly alter sucrose intake over the 2-h access period compared either with sham EA treatment or baseline drinking levels (p > 0.05, Fig. 3C). However, 2 Hz EA at ST36 caused an increase in water intake compared with baseline levels (t(14) = 2.20, p < 0.05, Table 2). The increase in water intake was accompanied by a significant decrease in preference for sucrose in relation to baseline sucrose preference (t(14) = 2.16, p < 0.05, Table 2). No significant difference was found between sham EA and baseline levels for either sucrose or water consumption (p > 0.05, Table 2). Low frequency (2 Hz) EA also did not alter sucrose intake over the 24-h access period (p > 0.05, Fig. 3D).
To determine whether the effect of EA depends on its frequency, high frequency (100 Hz) EA at ST36 for 20 min was administered to rats before access to EtOH. Under these experimental conditions, both the intake of and the preference ratio for EtOH were not significantly altered (all p > 0.05, Fig. 3E, Table 2). Furthermore, 100 Hz EA did not alter EtOH intake at the 24-h time point compared either with sham EA treatment or baseline drinking levels (all p > 0.05, Fig. 3F).
Repeated EA treatment decreased the intake of and the preference for EtOH
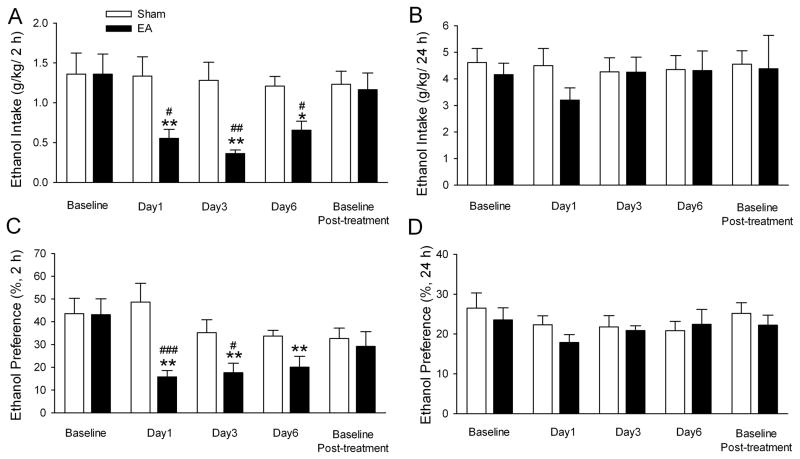
To determine the effect of repeated administrations of EA on EtOH intake, EA or sham EA was administered to another two groups of rats under the IE paradigm. When these rats had reached stable baseline levels of EtOH consumption after 4 weeks in the paradigm (see Fig. 1), repeated low frequency (2 Hz) EA at ST36 was administered. As shown in Fig. 4A, low frequency EA but not the sham treatment reduced EtOH intake on all test days at 2-h time point examined. There was no difference in EtOH baseline drinking during the EA-free days. In the treatment period, EtOH intake was significantly reduced among EA-treated animals (group effect [F1,40= 6.40, p = 0.02], time effect [F4,40 = 3.26, p = 0.02] and interaction term [F4,40 = 3.44, p = 0.017]). Post hoc analysis revealed that EtOH intake on day 1, 3 and 6 was decreased during the EA treatment period compared with baseline EtOH consumption (all p < 0.05, Fig. 4A). When EA treatments were terminated on day 7, the baseline EtOH-drinking levels returned to the pretreatment levels (Fig. 4A). No rebound increase in EtOH intake was observed. There was no overall main effect of sham EA on EtOH intake on all test days compared with baseline drinking levels.
Figure 4.
Repeated administration of low frequency (2 Hz) EA significantly decreased EtOH intake and preference. EA at low frequency (2 Hz) or sham EA were administered to two different groups of rats, on 6 consecutive days, 10 min before the start of EtOH- or water-drinking session. The effect was measured on day 1, 3, and 6. EtOH intake and preference was compared with the baseline drinking levels. There was no significant difference regarding the baseline drinking levels between the EA and the sham group. Significant reduction in EtOH intake (g/kg) and preference for EtOH during the 2 h EtOH access after the onset of drinking was found in EA (filled bars) but not in sham EA group (blank bars). The values are expressed as mean ± SEM. *p < 0.05, **p < 0.01 compared with baseline drinking levels, #p < 0.05, ##p < 0.01, ###p < 0.001compared with sham (two-way RM ANOVA followed by Newman-Keuls post hoc test), n = 6 animals in each group.
Although the preference for EtOH at 2-h time point examined was not significantly different among groups during the EA-free baseline period, this ratio was significantly reduced in the low frequency EA treated group (group effect [F1,40= 5.50, p = 0.04], the time effect [F4,40 = 4.42, p = 0.004], and the interaction term [F4,40 = 4.25, p = 0.006]). Post hoc analysis revealed that low frequency (2 Hz) EA decreased the preference for EtOH in all test days (all p < 0.01, Fig. 4C). There was no difference between the pretreatment and post-EA treatment baseline for the preference for EtOH (Fig. 4C). There was no overall main effect of sham EA on the preference for EtOH on test days compared with baseline preference for EtOH (Fig. 4C).
Furthermore, EA-induced reduction in the intake of and preference for EtOH on the all test days at the 24-h time point examined was not signi cant (EtOH intake: F1,40 = 0.65, p > 0.05; Preference for EtOH: F1,40 = 1.03, p > 0.05; Fig.4C and D).
4. Discussion
The present study demonstrates that an intermittent-access two-bottle choice drinking (IE) procedure led SD rats to consume high amounts of EtOH and exhibit some signs of physical withdrawal. A single low (2 Hz) but not high frequency (100 Hz) EA treatment applied at the acupoint ST36 selectively decreased voluntarily EtOH intake of and preference for EtOH in SD rats during the first 2-h of EtOH access under the IE procedure. Furthermore, repeated EA treatment decreased EtOH intake and preference for EtOH, without resulting in a rebound increase in EtOH intake when EA treatments were terminated.
A recent study suggested that EA is more effective in reducing alcohol intake in alcohol-dependent rats (Overstreet et al., 2008), but this experiment was conducted in selectively inbred alcohol-preferring (iP) rats, which are not always easy to access. This may limit further investigation on the efficacy and basic mechanisms of acupuncture. Recent evidence showed that the IE procedure led standard laboratory rats (such as Long-Evans and Wistar rats) to voluntarily consume high amounts of EtOH, which can be maintained over a long period (Steensland et al., 2007; Nielsen et al., 2008; Simms et al., 2008; Stuber et al., 2008; Carnicella et al., 2009). A recent study showed also that the IE procedure led SD rats to increase significantly their preference for EtOH when compared to daily access with sucrose fading (Moorman and Aston-Jones, 2009). In line with these data, in the current study, SD rats under the IE program significantly escalated their EtOH intake and preference for EtOH. Furthermore, BECs in these SD rats following 1 h of access to EtOH were similar to that of most (70%) of Long-Evans rats, which were under 60 mg/dl under the same drinking procedure (Simms et al., 2008). However, no SD rats in the current study reached the BECs seen in rat strains selectively bred for alcohol preference (Myers et al., 1998; Bell et al., 2006).
Unlike the continuous-access procedure, the IE procedure consists of repeated cycles of EtOH drinking and deprivation, which mimics the clinical course of alcoholism and induces dependence, as measured by physical and motivational signs upon withdrawal (Schulteis et al., 1995; Richardson et al., 2008). In the current study, mild withdrawal signs were observed from 2–8 h post EtOH withdrawal. To our knowledge, these are the first data showing withdrawal signs with this drinking procedure. It is unclear why the animas exhibit signs of withdrawal while their BEC indicates that they are not intoxicated.
Previous studies indicate that withdrawal signs require that the animals are intoxicated, which requires the BEC is above 80mg% (Brick and Erickson, 2009). One possibility is that the animals within the long-term alcohol-drinking with repeated alcohol deprivation exhibited a period of significant drinking or “loading up” during the initial period of EtOH access (Spanagel and Holter, 1999; (Bell et al., 2006). It has been reported that greater than 95% of the EtOH was consumed in the first 12 min of 1 h access session, leading to BECs that peaked early (Murphy et al., 2002; Bell et al., 2006). This alcohol preference shift is taken as a behavioral mechanism resulting from a strong motivation to drink alcohol following a deprivation period and lead to build up intoxicating levels of blood alcohol. This is supported by a recent study by Carnicella et al. (2009), indicating that the BECs of 50% Long-Evans rats under IE procedure were above 80 mg% following 30 min of drinking. Amount of EtOH consumed and BECs could have declined somewhat at a later time (Vetter-O’Hagen et al., 2009). Indeed, as demonstrated in the current study that BECs (15.6 ± 2.5 mg%) were lower when they were tested again after 24-h of access to EtOH. Furthermore, in the current study, the BEC levels may be underestimated since we centrifuged the samples at room temperature at which EtOH may evaporate.
Nevertheless, our data indicates that voluntarily EtOH intake under the IE procedure is sufficient to make SD rats develop mild physical dependence, which is comparable to that long-term voluntary alcohol consumption of only 3–4 g/kg/day in rats can induce mild withdrawal symptoms (Spanagel and Holter, 1999) (Holter et al., 1998) (Holter et al., 2000). Importantly, low frequency (2 Hz) EA at ST36 significantly and selectively decreased voluntarily EtOH intake in the EtOH dependent rats during the first 2-h of EtOH access. Furthermore, EA efficacy can be repeated when repeated EA treatments were administered. One caveat of current study is that we did not study the effect of EA on the physical signs of withdrawal. Nevertheless, we did previously show that a single low frequency EA at ST36, but not sham-EA, significantly attenuated the physical signs of withdrawal in SD rats chronically exposed to EtOH (Ye and Yu, 2003).
The endogenous opioid peptides in CNS have been linked to the positive reinforcing properties of EtOH (Herz, 1997). One possible mechanism is that the opiate, via the activation of the μ opioid receptors, reduce the GABAergic inhibitory transmission to the dopaminergic (DA) neurons in the ventral tegmental area (VTA) and thus increase the activity of VTA DA neurons by a mechanism of disinhibition. This possibility is supported by many previous preclinical and clinical studies showing that the opioid antagonists decrease EtOH self-administration. Conversely, during acute withdrawal from chronic EtOH exposure, the release of endogenous opioid peptides may be reduced. This may contribute to the reduced excitability of VTA DA neurons. This possibility is supported by a previous study showing the hyperactivity of GABA neurons in the VTA during acute withdrawal from chronic EtOH exposure (Gallegos et al., 1999), and by a very recent study (Barak et al., 2011) showing that the intermittent access procedure leads to a decrease of dopamine levels in the nucleus accumbens during acute withdrawal. Thus, animals may drink more alcohol to compromise the negative effect induced by hypofunction of DA system during withdrawal.
Previous studies have shown that analgesia effect induced low- or high frequency EA are both mediated by opioid peptides. The difference was that the former was mediated by μ and/or δ opioid receptors, whereas the latter was mediated by κ opioid receptors. These results suggest that different kinds of opioid peptides are released under different conditions (Han, 2003). Thus, 2 Hz EA could stimulate selectively the release of endogenous opioid peptide enkephalins and endorphins, which interact respectively with δ and μ opioid receptors (Guo et al., 1996; Han, 2003). By activation of μ opioid receptors, low frequency EA may reduce VTA GABAergic inhibitory transmission and increase the activity of VTA DA neurons and the release of dopamine and thus reduce the need for drinking EtOH. This possibility is supported by previous studies showing that EA decreased EtOH drinking behaviors in iP and Wistar rats by activating the endogenous opiate system (Yang et al., 2010; Overstreet et al., 2008). Previous studies have demonstrated that electrical stimulation itself may increase release of opioid peptides. Thus, the effect of low frequency EA may due to a non-specific effect of electrical stimulation. However, this is possibility is not supported by our data which show that only low but not high frequency EA reduced alcohol drinking.
The mechanisms underlying the lack of effect of high frequency (100 Hz) EA observed in the current study are not completely clear. One possible reason is that, as mentioned, high frequency EA may activate κ opioid receptors, and activation of κ opioid receptors can directly inhibit VTA DA neurons (Margolis et al., 2003).
In the current study, 100 Hz EA did not affect ongoing intake of and preference for EtOH, which was inconsistent with a previous study which showed that both low frequency (1 Hz) and high frequency (100 Hz) at ST36 reduced alcohol-drinking behavior of rats (Yoshimoto et al, 2001). While the mechanism underlying the difference requires further investigation, there are several apparent differences in the experimental conditions between our and Yoshimoto’s studies: (1) EtOH drinking procedures: Yoshimoto et al, stress induced alcohol-drinking and in the current study, the IE procedure; (2) Duration of EA: Yoshimoto et al., 10 min, versus 20 min in the current study; (3) Times of EA: Yoshimoto et al, twice a week for 1 or 3 consecutive weeks; current study, one time; and (4) index of drinking: Yoshimoto et al., EtOH drinking behavior versus ongoing drinking of EtOH in the current study.
Since both tail vein blood sampling and withdrawal score rating are stressful procedures, which could have interfered with the assessment of the acupuncture treatments. However, since these procedures were conducted on all groups of rats, the effect of acupuncture observed in the current study is unlikely caused by these interferences.
In summary, the current study demonstrated that EA at low (2 Hz) but not high frequency (100 Hz) at acupoint ST36 is effective in reducing voluntarily EtOH intake of and preference for EtOH in SD rats which consume high amount of EtOH in an intermittent-access 20% EtOH two-bottle choice drinking procedure. These findings may be useful for improving the efficacy of acupuncture treatment in human alcoholics and alcohol dependence. Further investigation into the potential mechanisms which mediate acupuncture effect in reducing alcohol drinking is necessary for a more comprehensive understanding of acupuncture treatments.
Highlights.
SD rats under an intermittent drinking paradigm increased ethanol intake.
2 Hz but not 100 Hz electroacupuncture (EA) at ST36 reduced ethanol intake of rats.
2 Hz but not 100 Hz EA at ST36 reduced ethanol preference of SD rats.
Multiple EA treatments decreased the intake of and preference for ethanol of SD rats
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barak S, Carnicella S, Yowell QV. Ron D Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Brick J, Erickson CK. Intoxication is not always visible: an unrecognized prevention challenge. Alcohol Clin Exp Res. 2009;33:1489–1507. doi: 10.1111/j.1530-0277.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Shun-Yue L, Shu-You W, Hui-Min M. Evaluation of influence of Acupuncture and Electro-acupuncture for Blood Perfusion of Stomach by Laser Doppler Blood Perfusion Imaging. Evid Based Complement Alternat Med. 2009;16:16. doi: 10.1093/ecam/nep050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaesaku Y, Zhai N, Ono M, Watanabe M, Zhao J, Zhang C, Li L, Shi X. Brain activity of a rat reflects apparently the stimulation of acupuncture. A radioautography using 2-deoxyglucose. Cell Mol Biol (Noisy-le-grand) 1995;41:161–170. [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- Gao M, Wang M, Li K, He L. Changes of mu opioid receptor binding sites in rat brain following electroacupuncture. Acupunct Electrother Res. 1997;22:161–166. doi: 10.3727/036012997816356662. [DOI] [PubMed] [Google Scholar]
- Guo HF, Tian J, Wang X, Fang Y, Hou Y, Han J. Brain substrates activated by electroacupuncture of different frequencies (I): Comparative study on the expression of oncogene c-fos and genes coding for three opioid peptides. Brain Res Mol Brain Res. 1996;43:157–166. doi: 10.1016/s0169-328x(96)00170-2. [DOI] [PubMed] [Google Scholar]
- Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Han Z, Jiang YH, Wan Y, Wang Y, Chang JK, Han JS. Endomorphin-1 mediates 2 Hz but not 100 Hz electroacupuncture analgesia in the rat. Neurosci Lett. 1999;274:75–78. doi: 10.1016/s0304-3940(99)00670-9. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Holter SM, Linthorst AC, Reul JM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacol Biochem Behav. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Jindal V, Ge A, Mansky PJ. Safety and efficacy of acupuncture in children: a review of the evidence. J Pediatr Hematol Oncol. 2008;30:431–442. doi: 10.1097/MPH.0b013e318165b2cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Passie T, Friedrich S, Wiese B, Schneider U. Acupuncture in the treatment of alcohol withdrawal symptoms: a randomized, placebo-controlled inpatient study. Addict Biol. 2002;7:415–419. doi: 10.1080/1355621021000006017. [DOI] [PubMed] [Google Scholar]
- Kim EH, Jang MH, Shin MC, Lim BV, Kim HB, Kim YJ, Chung JH, Kim CJ. Acupuncture increases cell proliferation and neuropeptide Y expression in dentate gyrus of streptozotocin-induced diabetic rats. Neurosci Lett. 2002;327:33–36. doi: 10.1016/s0304-3940(02)00372-5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chung JY, Kwon YK, Kim KJ, Yang CH, Hahm DH, Lee HJ, Pyun KH, Shim I. Acupuncture reduces alcohol withdrawal syndrome and c-Fos expression in rat brain. Am J Chin Med. 2005a;33:887–896. doi: 10.1142/S0192415X0500348X. [DOI] [PubMed] [Google Scholar]
- Kim YH, Schiff E, Waalen J, Hovell M. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005b;24:115–132. doi: 10.1300/j069v24n04_09. [DOI] [PubMed] [Google Scholar]
- Kunz S, Schulz M, Lewitzky M, Driessen M, Rau H. Ear acupuncture for alcohol withdrawal in comparison with aromatherapy: a randomized-controlled trial. Alcohol Clin Exp Res. 2007;31:436–442. doi: 10.1111/j.1530-0277.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myers RD, Robinson DE, West MW, Biggs TA, McMillen BA. Genetics of alcoholism: rapid development of a new high-ethanol-preferring (HEP) strain of female and male rats. Alcohol. 1998;16:343–357. doi: 10.1016/s0741-8329(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Cui CL, Ma YY, Guo CY, Han JS, Lukas SE, Lee DY. Electroacupuncture reduces voluntary alcohol intake in alcohol-preferring rats via an opiate-sensitive mechanism. Neurochem Res. 2008;33:2166–2170. doi: 10.1007/s11064-008-9791-9. [DOI] [PubMed] [Google Scholar]
- Poklis A, Mackell MA. Evaluation of a modified alcohol dehydrogenase assay for the determination of ethanol in blood. Clin Chem. 1982;28:2125–2127. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stux G, Pomeranz B. Acupuncture textbook and atlas. Berlin: Springer-Verlag; 1987. [Google Scholar]
- Trumpler F, Oez S, Stahli P, Brenner HD, Juni P. Acupuncture for alcohol withdrawal: a randomized controlled trial. Alcohol Alcohol. 2003;38:369–375. doi: 10.1093/alcalc/agg091. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Yang CH, Lee BB, Jung HS, Shim I, Roh PU, Golden GT. Effect of electroacupuncture on response to immobilization stress. Pharmacol Biochem Behav. 2002;72:847–855. doi: 10.1016/s0091-3057(02)00769-4. [DOI] [PubMed] [Google Scholar]
- Yang CH, Yoon SS, Hansen DM, Wilcox JD, Blumell BR, Park JJ, Steffensen SC. Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin Exp Res. 34:2137–2146. doi: 10.1111/j.1530-0277.2010.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Yu XC. Acupuncture antagonized observational signs of withdrawal from chronic alcohol administration in a rat model. Abstr 560, Research Society on Alcoholism. 2003 [Google Scholar]
- Yoon SS, Kwon YK, Kim MR, Shim I, Kim KJ, Lee MH, Lee YS, Golden GT, Yang CH. Acupuncture-mediated inhibition of ethanol-induced dopamine release in the rat nucleus accumbens through the GABAB receptor. Neurosci Lett. 2004;369:234–238. doi: 10.1016/j.neulet.2004.07.095. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Kato B, Sakai K, Shibata M, Yano, Yasuhara M. Electroacupuncture stimulation suppresses the increase in alcohol-drinking behavior in restricted rats. Alcohol Clin Exp Res. 2001;25:63S–68S. doi: 10.1097/00000374-200106001-00015. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Yoon SS, Lee BH, Kwon YK, Kim KJ, Shim I, Choi KH, Kim MR, Golden GT, Yang CH. Acupuncture normalizes the release of accumbal dopamine during the withdrawal period and after the ethanol challenge in chronic ethanol-treated rats. Neurosci Lett. 2006;395:28–32. doi: 10.1016/j.neulet.2005.10.043. [DOI] [PubMed] [Google Scholar]