Abstract
Carbon fullerenes possess unique properties and their interactions with biomolecules have widespread applications. Functionalization of fullerenes with hydroxyl groups (fullerenols) can increase the solubility and potential for cellular interaction, but the health and safety effects of varying degrees of fullerene hydroxylation in biological systems is poorly understood. Existing reports regarding the toxicity and inflammatory potential of fullerenols give conflicting conclusions. To further elucidate the potential for toxicity of fullerenols, human epidermal keratinocytes (HEK) were exposed to fullerenols (low (C60(OH)20), medium (C60(OH)24), and high (C60(OH)32)) at concentrations ranging from 0.000544–42.5μg/ml for 24 and 48h. A statistically significant (p < 0.05) decrease in viability with alamar Blue (aB) was noted only with C60(OH)32 at 42.5μg/ml after 24h. Nanoparticle (NP) controls showed minimal NP/assay interference of the three fullerenols with the aB viability assay. Normalized IL-8 concentration for C60(OH)20 was not significantly different from control, while C60(OH)24 and C60(OH)32 showed a significant decrease at 24 and 48h. These results suggest that different hydroxylation of fullerenes caused no cytotoxicity or inflammation up to 8.55μg/ml. These findings suggest that extrapolation across similar NP will be dependent upon surface chemistry and concentration which may affect the degree of agglomeration and thus biological effects.
Keywords: Hydroxylated fullerenes, fullerenes, cytotoxicity, skin cells, keratinocytes, nanoparticles
1. INTRODUCTION
Fullerenes or buckyballs are molecular structures containing 60 carbon atoms (C60). These materials possess unique chemical, mechanical, electrical, optical, magnetic, and biological properties that make them useful for a variety of novel commercial and medical applications. Fullerenes are used in materials science, superconductivity applications, electronic circuits, nonlinear optics, pharmaceuticals, and in everyday items such as clothing, tennis rackets, bowling balls and numerous other applications. Information regarding the biodistribution and metabolism of C60 is limited, probably secondary to C60 being insoluble in aqueous solutions coupled with the lack of sensitive analytical techniques for their detection. There are conflicting reports as to the potential toxicity of fullerenes such as C60. While C60 itself has essentially no solubility in water, it has been shown to aggregate with either organic solvent inclusion or after partial hydrolysis to create the water-soluble species nC60. These aggregates have exceptionally low mobility in aqueous solutions but have been proposed to have high cellular toxicity.
Several methods have been employed that attempt to decrease the hydrophobicity of C60. One method uses stirring or sonication of C60 in water to form nC60, a stable, aqueous suspension of C60 (Oberdörster, 2004). A variety of solvents, such as tetrahydrofuran (THF), have also been added to the process in an effort to reduce the production time of nC60 (Deguchi et al., 2001). However, THF has since been implicated as the cause of reported nC60 toxicity (Fortner et al, 2005; Inman et al., 2006; Isakovic et al., 2006; Kovochich et al., 2009; Lyon and Alvarez, 2008; Spohn et al., 2009; Zhang et al., 2009). Recently, we reported on a novel method to prepare nC60 NP at high concentrations without the use of THF (Xia et al., 2006; 2010).
Functionalization of C60 can be achieved through the chemical addition of hydrophilic functional groups (e.g., -NH2, -OH, -COOH, etc.) bonded to the fullerene cage to increase solubility, and subsequently the potential for increased cellular interaction in an in vivo or in vitro setting. Functionalized fullerenes retain unique physicochemical characteristics and their biomolecular interactions are widely studied given their prospective utility in biomedical applications such as targeted drug delivery, reactive oxygen species (ROS) scavenging, MRI contrast agents, radiation protection, and gene therapy (Partha and Conyers, 2009).
There are several studies that have reported on the benefits of pristine and functionalized fullerenes illustrating their usefulness in biomedical applications. Pure C60 has been found to have strong antioxidant properties with greater antioxidant activity than vitamin E (α-tocopherol) (Wang et al., 1999), and prevent hepatocyte injury induced by carbon tetrachloride oxidation (Gharbi et al., 2005). C60 has also been shown to inhibit osteoclast differentiation and bone destruction in arthritis (Yudoh et al., 2009). Numerous studies have shown hydroxylated fullerenes to play a cytoprotective role in biological systems via ROS scavenging with a decrease in oxidative stress to cells or tissues (Andrievsky et al., 2005; Bogdanovic et al., 2004; Saitoh et al., 2011; Sayes et al., 2004; Torres et al., 2010; Tsai et al., 1997). Tsai demonstrated the free-radical scavenging activity of hydroxylated fullerenes which prevented hydrogen peroxide-elicited oxidative damage of brain tissue in rats (Tsai et al., 1997). Fullerenols have also been shown to reduce cardiotoxicity and modulate the cytotoxic effects associated with doxorubicin chemotherapy (Bogdanovic et al., 2004; Torres et al., 2010), and to mediate the decrease in the frequency of micronuclei and chromosome aberrations in hamster ovarian cells (Mrdanović et al., 2009). Additionally, fullerenols showed an increase in the cytoprotection of UV-induced oxidative damage proportional to increasing hydroxylation levels (Saitoh et al., 2011).
Many fullerenes have been investigated for use as carrier molecules for chemotherapeutic agents due to ease of surface modification. Studies in tumor-bearing mice have shown that multihydroxylated metallofullerenol NP had strong antitumor activity without toxic effects (Chen et al., 1998; 2005). A doxorubicin-fullerenol conjugate had comparable in vivo therapeutic results to free doxorubicin without the cardiotoxicity or splenic pathology typically seen with this chemotherapeutic agent (Chaudhuri et al., 2009). Functionalization of C60 with amino acid complexes via the incorporation of a substituted phenylalanine provided a route for intracellular drug delivery through cell membranes (Yang et al., 2007), and C60(OH)24 has been noted to accumulate in the tumor tissue of mouse tumor-bearing models (Qiang et al., 2006).
However, there is an increasing amount of literature demonstrating the cytotoxic effects of hydroxylated fullerenes (Isakovic et al., 2006; Johnson-Lyles et al., 2010; Jovanović et al., 2010; Mrdanović et al., 2009; Su et al., 2010; Usenko et al., 2007; Weilgus et al., 2010; Yamada et al., 2010). Hydroxylated fullerenes have been shown to significantly decrease cell viability and cause G1 phase cell cycle arrest in Chinese hamster ovarian and lung cells, but only a slight decrease in viability with no cell cycle effects in mouse fibrosarcoma cells (Su et al., 2010). Fullerenol toxicity was demonstrated in Chinese hamster ovarian cells with a lethal concentration (LC)50 found at 250μg/ml (Mrdanović et al., 2009), and also shown in rat astrocytes with an inhibitory concentration (IC)50 of fullerenol found at 1.74μg/ml coupled with decreased in vivo locomotor activity (Yamada et al., 2010). Hydroxylated fullerenes have been shown to induce cytoskeletal and mitochondrial aberrations in porcine renal tubule cells exposed to 5.8mg/ml (Johnson-Lyles et al., 2010). Weilgus et al. (2010) reported induced phototoxic damage due to lipid peroxidation in human retinal epithelial cells. Hydroxylated fullerenes have been reported to cause embryonic developmental abnormalities and increased mortality in zebrafish and bighead minnows at low concentrations (2.5μg/ml and 10ng/ml, respectively) (Jovanović et al., 2010; Usenko et al., 2007). Additionally, HEK exposed to fullerene-based amino acids depict a significant decrease in viability at 0.004, 0.04 and 0.4mg/ml compared to controls, with a significant increase in Interleukin (IL)-8 from 4h through 48h (Rouse et al., 2006). It has been proposed that the cytotoxicity of fullerenes is largely dependent on the functionalization of the compound (Sayes et al., 2004; 2005).
There continues to be much discussion and controversy regarding the toxicity of solubilized fullerenes within biological systems. The expected increase in usage of these NP in consumer products, industrial and biomedical applications raises a legitimate concern that these NP may be hazardous to human health. The objective of this study was to assess the response of HEK to three fullerenes with different degrees of hydroxylation (low (C60(OH)20, medium (C60(OH)24, and high (C60(OH)32) at various concentrations to determine whether the degree of hydroxylation influences toxicity and/or inflammatory potential in an established human cell line.
2. MATERIALS AND METHODS
2.1. Preparation of hydroxylated fullerenes
Hydroxylated fullerenes were prepared following the phase transfer method of Li et al. (1993). Sodium hydroxide (12.5M NaOH solution) was mixed with a solution of C60 (SES Research, Houston, TX) (in toluene) with the phase transfer agent (40% tetrabutylammonium hydroxide (TBAH) in water). After shaking for 30 min, the toluene solution, originally a deep violet, turned colorless forming a brown precipitate. The toluene phase was decanted and residual toluene removed via evaporation under vacuum. Deionized water was added and the solution stirred for 24h with air flow to dissolve the precipitate and allow the hydroxylation reaction to continue. Carbon dioxide was removed from the air flow by bubbling through a 1M NaOH column and the product washed with methanol to remove excess NaOH and TBAH. The product, C60(OH)24, was recovered by decanting the supernatant after centrifugation. With each consecutive washing, deionized water was added to dissolve the product and methanol was added to precipitate the product for centrifugation. Finally, the product was dissolved in water and filtered through a 0.2μm nylon filter for subsequent dose preparations.
For the fullerenols C60(OH)20 and C60(OH)32, the above procedure was modified, with 3% hydrogen peroxide (H2O2) added after methanol washing. The product was dissolved in 3% H2O2 and stirred for 24h in a sealed bottle, with the same washing and drying procedures performed for the H2O2 treated product. The color of this product was light yellow after H2O2 treatment compared to the reddish-brown color of the first product, indicative of further hydroxylation. The lower hydroxylation lot was prepared using 6M NaOH under the flow of Argon gas rather than air flow.
2.2. Characterization of hydroxylated fullerenes
X-ray photoelectron spectroscopy (XPS) (Kratos Axis ULTRA, Shimadzu) was employed to estimate the degree of fullerene hydroxylation. Survey and high-resolution scans of each fullerene powder deposited on double-stick copper tape to a thickness greater than 500μm (scan area: 300μm × 700μm) were collected to obtain elemental composition and functionality. The survey spectra were acquired using a pass energy of 160eV (step size 1eV) and high-resolution scans were collected at a pass energy of 20eV (step size 0.1eV). The binding energies were calibrated using C 1s peak (284.6eV). The peak areas of high-resolution scans of C 1s and O 1s were used to estimate the number of hydroxyl groups on the C60, using the average of three spectra for each sample. The XPS results were confirmed using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR). The spectra of a powder sample deposited on a germanium crystal were collected using a Thermo Electron Nicolet 8700 ATR-FTIR spectrometer at a resolution 4cm−1 with 256 scans. Background spectra were collected from a clean Ge crystal prior to depositing the sampling. A fullerol (C60(OH)24) obtained from a commercial source (MER Corp, AZ) was used as the standard reference material to estimate the number of hydroxyl groups.
A 1mg/ml dilution of each fullerenol was prepared in deionized water and size measured by dynamic light scattering (DLS) at 22°C using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). In addition, each fullerenol was diluted in cell culture medium (KGM-2) at 8.5μg/ml and 42.5μg/ml and the size determined by DLS following incubation at 37°C for 0, 7, 24, and 48h. At 24h and 48h, 10μl of each fullerenol in KGM-2 was mixed 1:20 in deionized water, placed onto a formvar-coated grid, and air-dried overnight. Samples were examined on a FEI/Philips EM208S TEM operating at an accelerating voltage of 80KV.
2.3. Cell culture with HEK
Cryopreserved primary neonatal human epidermal keratinocytes (HEK) (Lonza, Walkersville, MD) were seeded into T-75 cell culture flasks (75cm2; 500,000 cells/flask) and grown in 15ml of keratinocyte growth medium (KGM-2) at 37°C in a humidified 5% CO2 environment. Once the cells reached 70% confluency, they were harvested and passed into 96-well black microplates at 12,500 cells in 200μl/well and grown in the incubator. Peripheral wells remained unseeded, containing Hanks’ Balanced Salt Solution (HBSS) to provide a barrier to minimize evaporation of the culture medium.
2.4. C60(OH)x treatment of HEK
Upon reaching 70% confluency in the 96-well plates, HEK were exposed to the fullerenols in KGM-2 from stock solutions of (C60(OH)20, C60(OH)24, and C60(OH)32) in serial dilutions from 42.5 to 0.000544μg/ml for 24 and 48h. Control wells consisted of HEK in KGM-2 alone. Following incubation, aliquots of the medium from each well were saved, pooled by treatment group, and stored at −80°C for IL-8 analysis.
2.5. Alamar Blue (aB) viability assay
Standard cytotoxicity assays that assess chemical toxicity may generate conflicting results with carbon nanomaterials since dye-based assays may often provide false viability and cytokine data (Monteiro-Riviere and Inman, 2006; Monteiro-Riviere et al., 2009). Previous studies in our laboratory have demonstrated that the interference of carbon nanomaterials with the CellTiter 96® AQueous One (96AQ) and aB viability assays is minimal, so these assays are recommended for carbon NP toxicity assessment (Monteiro-Riviere et al., 2009). Preliminary fluorescence measurements found little interaction of the aB dye with the fullerenols, therefore the aB cell viability assay (Molecular Probes, Invitrogen, Eugene, OR) was chosen to assess toxicity in this study. The aB reagent was added to the medium at a 10% concentration and incubated with the cells for 3h. The aB reagent was converted from the non-fluorescent indicator dye resazurin to the highly red fluorescent metabolite resorufin via reduction reactions by metabolically active cells. Fluorescence of each well was quantitated on a Gemini EM spectrophotometer with an excitation wavelength of 545nm and an emission wavelength of 590nm. The resulting fluorescence was proportional to the number of viable cells per well. Fluorescence values were normalized to media controls and expressed as percent viability. Experimental controls were conducted with the aB assay to assess potential NP/assay interactions. A NP (no-cell) control (hydroxylated fullerenes in collagen-coated wells without cells) and a cell control (hydroxylated fullerenes exposed to HEK-reduced assay dye) were performed to mimic the experimental conditions of the studies.
2.6. Fluorescence spectrum
The fluorescence spectrum was measured to determine the interactions of the NP with the aB assay. NP were suspended in centrifuge tubes with aB medium (10μl/100μl KGM-2) at 0.000544, 0.0027, 0.0136, 0.068, 0.34, 1.7, 8.5, and 42.5μg/ml and incubated for 3h. The aB medium was transferred to a black-well plate and fluorescence read at intervals between 565nm and 640nm on a Gemini EM spectrophotometer with the excitation/emission wavelength 545/590nm.
2.7. Cytokine release
Human (IL)-8 was quantitated with the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA), using xMAP Technology® analysis software. Treatment and control media from the aB viability experiments were incubated with capture antibody (conjugated to magnetic beads) in a 96-well filter plate, fluorescently labeled, and analyzed in the Bio-Plex array reader. The resulting IL-8 data (detection limit 1.0 pg/ml) was quantitated relative to a standard curve and normalized to the aB viability data. To determine potential interactions of NP with the cytokine assay, fullerenols in KGM-2 at the highest concentration (42.5μg/ml) were spiked with a known concentration of IL-8, incubated for 24 and 48h, and assayed for IL-8. Any change in the cytokine concentration relative to the spiked sample control indicated particle interaction with the cytokine assay.
2.8. Cell uptake of fullerenols and transmission electron microscopy (TEM)
To evaluate NP cellular uptake in HEK, the cells were grown in 25cm2 flasks and exposed to 42.5μg/ml of C60(OH)20, C60(OH)24 and C60(OH)32 for 24 and 48h. Following exposure, HEK were harvested, rinsed in HBSS and fixed in Trump’s fixative. Cells were then rinsed in buffer, embedded as a pellet in 3% agar, dehydrated through graded ethanols, cleared in acetone, infiltrated and embedded in Spurr’s resin. Thin sections (~800Å) were mounted on copper grids and examined unstained on a FEI/Philips EM208S TEM.
2.9. Statistical analysis
The mean values for each treatment were calculated and the significant differences (p < 0.05) determined using the PROC GLM Procedure (SAS 9.1 for Windows; SAS Institute, Cary, NC). When significant differences were present, comparisons between each treatment and the control were conducted using the Dunnett’s test at the p < 0.05 level of significance.
3. RESULTS
3.1. Characterization of hydroxylated fullerenes
XPS analysis determined the number of hydroxyl groups on the fullerenes were 20, 24, and 32, which was confirmed by ATR-Fourier Transform Infrared Spectroscopy as 19, 23, and 33 (Table 1). Utilizing DLS, to measure the C60(OH)x stock solutions in water (1mg/ml) at 22°C, the mean sizes were C60(OH)20, 37.0nm; C60(OH)24, 97.0nm; and C60(OH)32, 274.5nm. Fullerenol agglomeration in the culture medium was determined via DLS by incubating the NP at 37°C for up to 48h. At 8.5μg/ml, fullerenes with 20 and 24 hydroxyl groups increased in size slightly and fullerenes with 32 hydroxyl groups decreased in size slightly, with the medium control (containing protein agglomerates) remaining relatively unchanged (Table 2). When the concentration was increased to 42.5μg/ml, size measurements increased dramatically (Table 3). Initially an increase in the hydroxylation level of the fullerenols resulted in less agglomeration but an increase in agglomeration was shown over time. While agglomeration generally increased over time, C60(OH)20 and C60(OH)24 leveled off after 24h with C60(OH)32 showing a size increase through 48h. The C60(OH)20 increased in size from 792.0nm at time 0 to peak at 26,716.7nm at 24h, while C60(OH)24 and C60(OH)32 increased by a magnitude of 1–2 after 48h (Table 3).
Table 1.
Characterization native hydroxylated fullerenes using XPS and confirmed by ATR-FTIR.
| Native Fullerenol | X-Ray Photoelectron Spectroscopya | Fourier Transform Infrared Spectroscopy a |
|---|---|---|
| C60(OH)20 | 20 | 19 |
| C60(OH)24 | 24 | 23 |
| C60(OH)32 | 32 | 33 |
Numbers represent measured hydroxyl group content of each fullerenol.
Table 2.
Size (nm) of 8.5μg/ml C60(OH)x in cell culture medium at 37°C by DLS.
| Fullerenol | 0h | 7h | 24h | 48h |
|---|---|---|---|---|
| Cell Culture Medium* | 158.0 | 136.5 | 139.0 | 155.3 |
| C60(OH)20 | 165.5 | 208.4 | 230.5 | 237.7 |
| C60(OH)24 | 127.8 | 149.1 | 151.1 | 160.7 |
| C60(OH)32 | 141.7 | 103.3 | 100.1 | 119.8 |
Culture medium contains protein agglomerates
Table 3.
Size (nm) of 42.5μg/ml C60(OH)x in cell culture medium at 37°C by DLS.
| Fullerenol | 0h | 7h | 24h | 48h |
|---|---|---|---|---|
| Cell Culture Medium* | 158.0 | 136.5 | 139.0 | 155.3 |
| C60(OH)20 | 792.0 | 7,848.0 | 26,716.7 | 23,354.0 |
| C60(OH)24 | 320.7 | 1,498.5 | 2,454.3 | 2,354.7 |
| C60(OH)32 | 186.4 | 703.0 | 2,520.3 | 3,645.3 |
Culture medium contains protein agglomerates
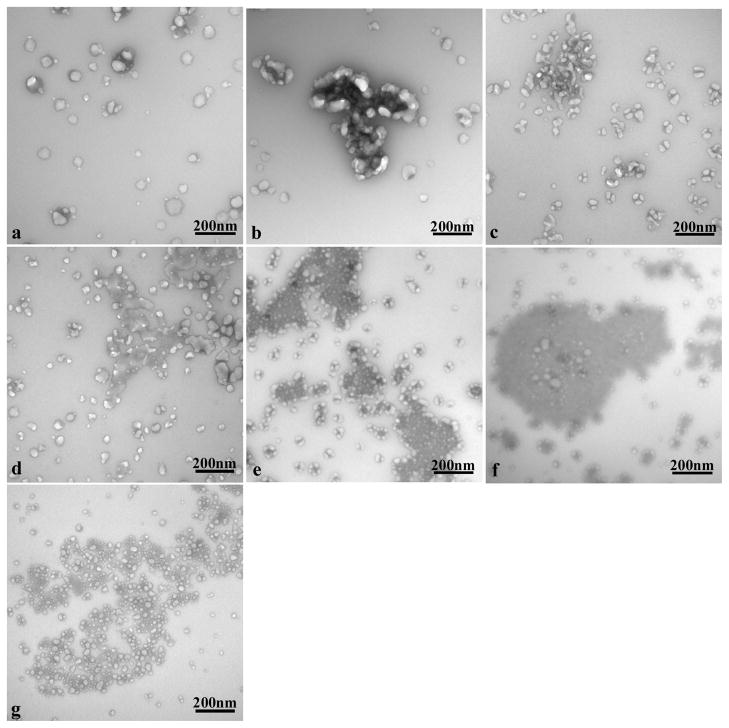
TEM of the fullerenols in the stock solution was performed to visualize the NP dispersion in the culture medium, and cannot be correlated to the DLS size measurements. Electron micrographs depicted agglomeration with a heterogeneous distribution of the fullerenols in the culture medium. Images of the media alone showed individual and agglomerated proteins at 48h (Figure 1a, unstained). At a concentration of 8.5μg/ml, the C60(OH)20 agglomerates were tightly surrounded by large proteins (Figure 1b, unstained), the C60(OH)24 agglomerates were fragmented and interspersed with the proteins (Figure 1c, unstained) and the C60(OH)32 agglomerates were interspersed with proteins in a more disperse pattern (Figure 1d, unstained). The 42.5μg/ml concentration of C60(OH)20 showed a pattern of localization of the protein media around the periphery of large nanoparticle agglomerates (Figure 1e, unstained ), the C60(OH)24 agglomerates showed clusters of proteins within large nanoparticle agglomerates (Figure 1f, unstained) and the C60(OH)32 agglomerates showed most of the protein to be evenly dispersed with the nanoparticles (Figure 1g, unstained).
Figure 1.
TEM of unstained C60(OH)x incubated in KGM-2 medium for 48h at 37°C. (a) KGM-2 medium control. (b) 8.5μg/ml C60(OH)20 in KGM-2. (c) 8.5μg/ml C60(OH)24 in KGM-2. (d) 8.5μg/ml C60(OH)32 in KGM-2. (e) 42.5μg/ml C60(OH)20 in KGM-2. (f) 42.5μg/ml C60(OH)24 in KGM-2. (g) 42.5μg/ml C60(OH)32 in KGM-2.
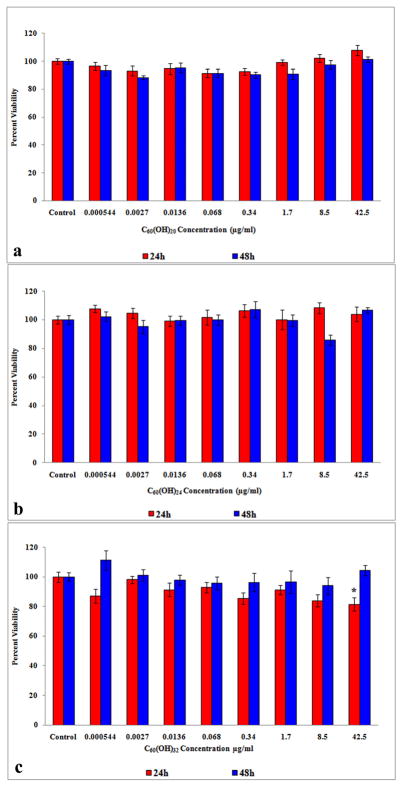
3.2. aB viability assay
The C60(OH)20 (Figure 2a) and C60(OH)24 (Figure 2b) did not cause a statistically significant (p < 0.05) change in viability relative to the controls at 24 or 48h but C60(OH)32 did cause a significant (p < 0.05) decrease in viability at 42.5μg/ml after 24h (Figure 2c).
Figure 2.
aB viability of HEK exposed to fullerenols at 24 and 48h. (a) Viability of HEK exposed to C60(OH)20. (b) Viability of HEK exposed to C60(OH)24. (c) Viability of HEK exposed to C60(OH)32. *denotes statistically significant at p < 0.05.
3.3. Experimental controls
NP controls (fullerenols exposed to aB assay medium, no cells) revealed no interactions between the NPs and the aB medium at 24 and 48h, while the cell controls (fullerenols exposed to HEK-reduced assay medium) showed little interaction between the NP and the reduced aB medium (data not shown). The lack of interaction of the assay dye with these NP suggests this assay is appropriate to assess cytotoxicity of these NP.
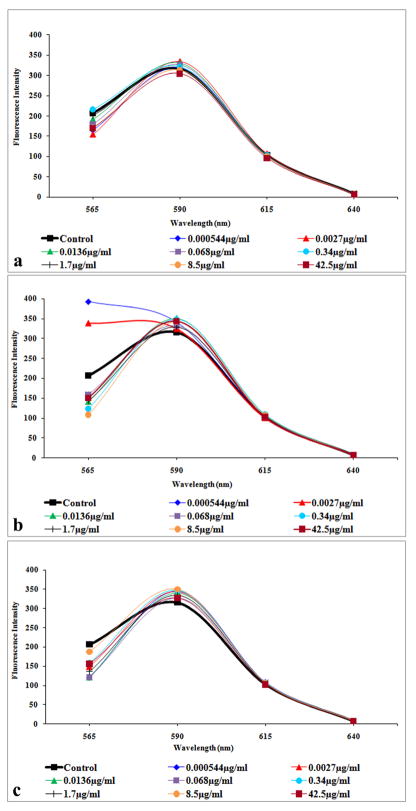
3.4. Fluorescence spectrum
The fluorescence spectrum for the aB medium peaked at 590nm for C60(OH)20 (Figure 3a), C60(OH)24 (Figure 3b) and C60(OH)32 (Figure 3c). Minimal difference was noted between the fluorescent intensity of each NP concentration and the control of each hydroxylated fullerene, indicating minimal NP interference with the aB medium.
Figure 3.
Fluorescent spectrum of C60(OH)x with aB medium. (a) Fluorescent spectrum of C60(OH)20. (b) Fluorescent spectrum of C60(OH)24. (c) Fluorescent spectrum of C60(OH)32. Spectra show minimal interaction of fullerenols with the aB medium.
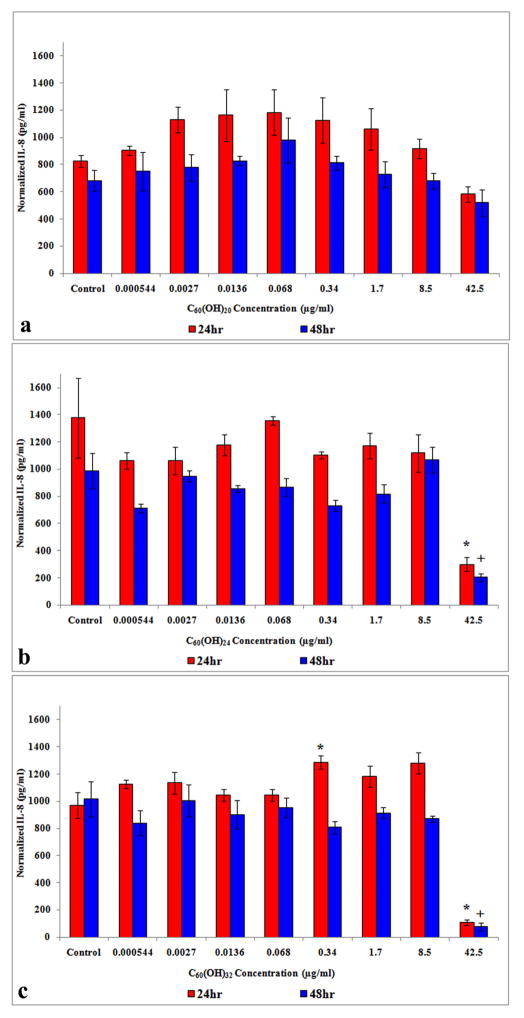
3.5. Cytokine release
With HEK treated with C60(OH)20, normalized IL-8 showed no statistically significant differences relative to the controls at 24 and 48h (Figure 4a), whereas HEK treated with C60(OH)24 (Figure 4b) and C60(OH)32 (Figure 4c) showed a statistically significant decrease at 42.5μg/ml after 24 and 48h. A significant increase in IL-8 release was also observed with C60(OH)32 treatment at 0.34μg/ml after 24h (Figure 4c).
Figure 4.
IL-8 release from HEK exposed to C60(OH)x for 24 and 48h. (a) IL-8 release with C60(OH)20. (b) IL-8 release with C60(OH)24 (c) IL-8 release with C60(OH)32. *denotes statistically significant at p < 0.05.
During cytokine analysis, spiked samples of fullerenols (spiked controls) with a known concentration of IL-8 were run concurrently and showed an interaction with the cytokine assay at 42.5μg/ml resulting in a suppression of the spiked IL-8. The IL-8 levels of suppression for the fullerenols compared to the controls (without fullerenols) at 24h was 29.9% with C60(OH)20, 69.7% with C60(OH)24 and 85.3% with C60(OH)32. Likewise at 48h, the observed suppression was 32.8, 78.3 and 87.3 for each, respectively.
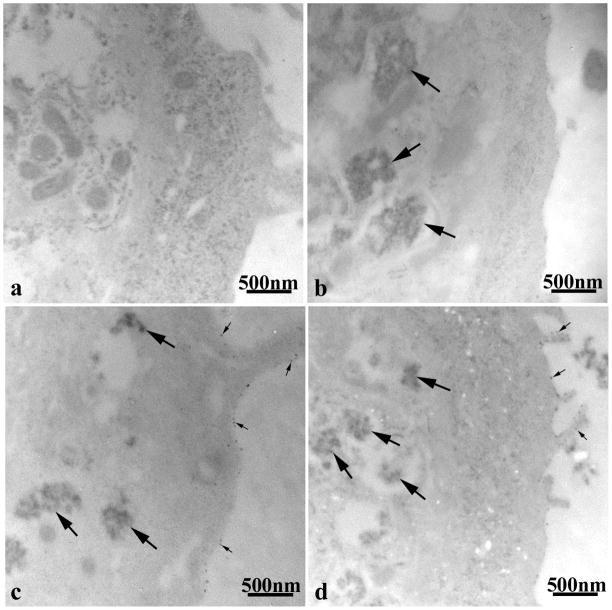
3.6. TEM of the cellular uptake of fullerenols
TEM of unstained HEK exposed to the three different fullerenols showed dramatic differences in the cellular uptake and localization of the NP. HEK controls exhibited normal cellular morphology with no NP aggregates (Figure 5a). HEK treated with C60(OH)20 for 24h depicted agglomerates within the cytoplasmic vacuoles of the cell (Figure 5b). Agglomerates of C60(OH)24 were also found within the cell and focally attached to the cell membrane (Figure 5c), while C60(OH)32 exposure resulted in smaller aggregates within the cells and fewer focal attachments along the periphery of the cell membrane (Figure 5d).
Figure 5.
TEM of unstained HEK treated with 42.5μg/ml of C60(OH)x for 24h. (a) Untreated control. (b) C60(OH)20. (c) C60(OH)24. (d) C60(OH)32. Large arrows denote fullerenol agglomerates in cytoplasmic vacuoles and small arrows show fullerenols attached to the cell membrane.
4. DISCUSSION
This study showed that C60(OH)20 and C60(OH)24, ranging from concentrations of 0.000544 – 42.5μg/ml, had no effect on HEK viability, suggesting they are not toxic to HEK at these concentrations. However, a significant decrease in cell viability was noted in HEK exposed to the highest concentration of 42.5μg/ml of C60(OH)32 at 24h (Figure 2c). By 48h, however, the cells appear to recover from the treatment. Since the NP controls, cell controls, and fluorescence spectra data all showed minimal interaction of the fullerenols with the aB assay, the viability data may indicate a negative response of HEK exposed to the most hydroxylated fullerenol at the highest tested concentration. Given that hydrophilicity is expected to increase as a result of functionalization, we anticipate an increase in the level of cellular interaction with the NP with greater functionalization, which follows that any decrease in viability would occur with the highest degree of hydroxylation.
The other marker of cellular response tested was the cytokine and pro-inflammatory mediator IL-8. It is expected that any potential response would be greatest at the highest concentrations. However, normalized IL-8 exposed to C60(OH)24 and C60(OH)32 showed a significant decrease at the highest tested concentration of 42.5μg/ml relative to controls at both 24 and 48h, while no significant cytokine release was noted with C60(OH)20. It is probable that most IL-8 released into the media dosed with the 42.5μg/ml was adsorbed by the large fullerenol agglomerates formed at this concentration, making some IL-8 unavailable for assay. Since cytokine release is expected to increase relative to the controls if the fullerenols caused an irritation effect, the decrease in IL-8 levels at these highest concentrations is most likely due to interactions between the fullerenols and the cytokine or assay, as confirmed by the spiked controls. Fullerenols at 42.5μg/ml spiked with a known concentration of IL-8 and run concurrently with the IL-8 assay showed a NP suppression effect. The level of IL-8 suppression with the hydroxylated fullerenes as compared to the control (without NP) at 24h was 29.9%, 69.7%, and 85.3% for C60(OH)20, -24, -32, respectively. Likewise at 48h, the observed suppression was 32.8%, 78.3%, and 87.3%. Due to these NP effects, the IL-8 concentrations may be greater than reported, and thus underestimated. Previously, this adsorption effect was reported with single-walled carbon nanotubes (Zhang et al., 2007). These data are important and again stress that inaccurate results may be the consequence at high concentrations of hydroxylated fullerenols due to their tendency to agglomerate in suspension. Finally, characterization studies suggest that fullerenol agglomeration increased with concentration and decreased with hydroxyl groups at 8.5 and 42.5μg/ml (Tables 2 and 3). Agglomerates are larger by 1–2 magnitudes at fullerenol concentrations of 42.5μg/ml (Table 3) compared to concentrations of 8.5μg/ml (Table 2) after 48h. It is interesting that the pattern of increasing IL-8 suppression is proportional to the increase in hydroxylation level, suggesting that the hydroxylation state may also affect the cytokine suppression of fullerenols. However, the mechanism behind this result is beyond the scope of the present study.
Conflicting toxicology results within the literature report the combined interaction of cell type, NP, functional groups and concentrations and seem to illustrate a cell-type dependent response that is specific to the particular constellation of factors present in any given study. Present data strengthens this observation and shows that many effects are only seen at high concentrations, which may exist outside of any potentially achievable biological exposure. Such studies must be interpreted with caution since the agglomeration seen at high concentrations in cell culture media may not reflect conditions in vivo. Recent publications have used different viability assays, some lacking NP controls, which have different particle/dye-based interactions as reported by our group (Monteiro-Riviere et al., 2009; Monteiro-Riviere et al., 2010; Samberg et al., 2010). Care should always be taken to run relevant controls in order to rule out any NP/assay interactions to provide the most accurate results. This assumes, of course, that any potential therapeutic value of hydroxylated fullerenols may not exist in the concentration range represented in the literature where a toxic range has been established. As with other substances, a cell-type dependent toxicity level will always exist for a given chemical, and it is well known that this level will depend upon the dose and duration of exposure and the physicochemical properties for each NP (Monteiro-Riviere and Tran, 2007).
In conclusion, given the lack of significance trends, the reported cell viability and (IL-8) release data indicate a lack of toxicity to HEK at concentrations of 0.000544 – 8.5μg/ml utilizing the aB assay after exposure to three hydroxylated fullerenes, C60(OH)20, C60(OH)24 and C60(OH)32. At the highest concentration tested (42.5μg/ml), HEK toxicity to C60(OH)32 was noted, with an 18.54% decrease in viability relative to the control at 24h. The degree of hydroxylation may also affect NP agglomeration in cell culture media which may ultimately affect the cytotoxic potential of C60(OH)x, but this was evident only at the highest levels of exposure used in these studies. Similarly, suppression of IL-8 release was seen to increase with hydroxylation and concentration, a finding impacted by particle interaction with either the assay or the cytokine. These findings suggest that extrapolation across even very similar NP will be dependent upon surface chemistry and concentration which may affect the degree of agglomeration and thus biological effects. Further work examining the toxic potential of hydroxylation levels of fullerenols on biological systems will be necessary before any therapeutic value of these particles can be exploited.
Degrees of hydroxylation of fullerenes and the concentration can influence toxicity
Degree of hydroxylation affects nanoparticle agglomeraion in media which affects toxicity
Inaccurate results at high concentrations of fullerenes may be due to agglomeration
Acknowledgments
The authors would like to thank Drs. A.R. Badireddy and M.R. Wiesner of the Department of Civil and Environmental Engineering Center for the Environmental Implications of Nano Technology, Duke University, Durham, NC for the XPS and ATR-FTIR characterization. This research was presented at the 50th Anniversary Annual Meeting of the Society of Toxicology in Washington DC in March 8, 2011. This research was supported by the National Institutes of Health (NIH-RO1 ES016138).
Footnotes
Conflict of interest- The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrievsky G, Klochkov V, Derevyanchenko L. Is the C60 fullerene molecule toxic? Fullerenes, Nanotubes, and Carbon Nanostructures. 2005;13:363–376. [Google Scholar]
- Bogdanović G, Kojić V, Dordević A, Canadanović-Brunet J, Vojinović-Miloradov M, Baltić VV. Modulating activity of fullerol C60(OH)22 on doxorubicin-induced cytotoxicity. Toxicol In Vitro. 2004;18:629–637. doi: 10.1016/j.tiv.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Paraskar A, Soni S, Mashelkar RA, Sengupta S. Fullerenol-cytoxic conjugates for cancer chemotherapy. ACSNano. 2009;3(9):2505–2514. doi: 10.1021/nn900318y. [DOI] [PubMed] [Google Scholar]
- Chen C, Xing G, Wang J, Zhao Y, Li B, Tang J, Jia G, Wang T, Sun J, Xing L, Yuan H, Gao Y, Meng H, Chen F, Chai Z, Fang X. Multihdroxylated [Gd@C82 (OH)22]n nanoparticles: antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5:2050–2057. doi: 10.1021/nl051624b. [DOI] [PubMed] [Google Scholar]
- Chen BX, Wilson SR, Das M, Coughlin DJ, Erlangerm BF. Antigenicity of fullerenes: Antibodies specific for fullerenes and their characteristics. Proc Natl Acad Sci. 1998;95:10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi S, Alargova RG, Tsujii K. Stable dispersions of fullerenes C60 and C70, in water, preparation and characterization. Langmuir. 2001;17:6013–6017. [Google Scholar]
- Fortner JD, Lyon DY, Sayes CM, Boyd AM, Falkner JC, Hotze EM, Alemany LB, Tao YJ, Guo W, Ausman KD, Colvin VL, Hughes JB. C-60 in water: nanocrystal formation and microbial response. Environ Sci Technol. 2005;39:4307–4316. doi: 10.1021/es048099n. [DOI] [PubMed] [Google Scholar]
- Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;12:2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- Inman AO, Sayes CM, Colvin VL, Monteiro-Riviere NA. Nano-C60 and derivatized C60 toxicity in human epidermal keratinocytes. Toxicol Sci. 2006;90(S–1):167. [Google Scholar]
- Isakovic A, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Mirkovic M, Dramicanin M, Harhaji L, Raicevic N, Nikolic Z, Trajkovic V. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol Sci. 2006;91:173–183. doi: 10.1093/toxsci/kfj127. [DOI] [PubMed] [Google Scholar]
- Johnson-Lyles DN, Peifley K, Lockett S, Neun BW, Hansen M, Clogston J, Stern ST, McNeil SE. Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2010;248:249–258. doi: 10.1016/j.taap.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović B, Anastasova L, Rowe EW, Palić D. Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820) Aquat Toxicol. 2010;101(2):474–482. doi: 10.1016/j.aquatox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Kovochich M, Espinasse B, Auffan M, Hotze EM, Wessel L, Xia T, Nel AE, Wiesner MR. Comparative toxicity of C60 aggregates toward mammalian cells: role of tetrahydrofuran (THF) decomposition. Environ Sci Technol. 2009;43:6378–6384. doi: 10.1021/es900990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Takeuchi A, Ozawa M, Xinhai Li XH, Saigob K, Kitazawaa K. C60 fullerol formation catalysed by quaternary ammonium hydroxides. J Chem Soc Chem Commun. 1993;23:1784–1785. [Google Scholar]
- Lyon DY, Alvarez PJJ. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ Sci Technol. 2008;42:8127–8132. doi: 10.1021/es801869m. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon. 2006;44:1070–1078. [Google Scholar]
- Monteiro-Riviere NA, Tran CL. Nanotoxicology: Characterization, Dosing, and Health Effects. Informa Healthcare; New York: 2007. [Google Scholar]
- Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol. 2009;234:222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Oldenburg SJ, Inman AO. Interactions of aluminum nanoparticles with human epidermal keratinocytes. J Appl Toxicol. 2010;30:276–285. doi: 10.1002/jat.1494. [DOI] [PubMed] [Google Scholar]
- Mrdanović J, Solajić S, Bogdanović V, Stankov K, Bogdanović G, Djordjevic A. Effects of fullerenol C60(OH)24 on the frequency of micronuclei and chromosome aberrations in CHO-K1 cells. Mutat Res. 2009;680:25–30. doi: 10.1016/j.mrgentox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Oberdörster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partha R, Conyers JL. Biomedical applications of functionalized fullerene-based nanomaterials. Int J Nanomed. 2009;4:261–275. [PMC free article] [PubMed] [Google Scholar]
- Qiang Ji Z, Sun H, Wang H, Xie Q, Liu Y, Wang Z. Biodistribution and tumor uptake of C60(OH)x in mice. J Nanopart Res. 2006;8:53–63. [Google Scholar]
- Rouse JG, Yang J, Barron AR, Monteiro-Riviere NA. Fullerene-based amino acid nanoparticle interactions with human epidermal keratinocytes. Toxicol In Vitro. 2006;20:1313–1320. doi: 10.1016/j.tiv.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Miyanishi A, Mizuno H, Kato S, Aoshima H, Kokubo K, Miwa N. Super-highly hydroxylated fullerene derivative protects human keratinocytes from UV-induced cell injuries together with the decreases in intracellular ROS generation and DNA damages. J Photochem Photobiol B. 2011;102:69–76. doi: 10.1016/j.jphotobiol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004;4:1881–1887. [Google Scholar]
- Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Spohn P, Hirsch C, Hasler F, Bruinink A, Krug HF, Wick P. C60 fullerene: a powerful antioxidant or a damaging agent? The importance of an in-depth material characterization prior to toxicity assays. Environ Pollut. 2009;157:1134–1139. doi: 10.1016/j.envpol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Su Y, Xu JY, Shen P, Li J, Wang L, Li Q, Li W, Xu GT, Fan C, Huang Q. Cellular uptake and cytotoxic evaluation of fullerenol in different cell lines. Toxicol. 2010;269:155–159. doi: 10.1016/j.tox.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Torres VM, Srdjenovic B, Jacevic V, Simic VD, Djordjevic A, Simplício AL. Fullerenol C60(OH)24 prevents doxorubicin-induced acute cardiotoxicity in rats. Pharmacol Rep. 2010;62:707–718. doi: 10.1016/s1734-1140(10)70328-5. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chen YH, Chiang LY. Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in-vitro. J Pharm Pharmacol. 1997;49:438–445. doi: 10.1111/j.2042-7158.1997.tb06821.x. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I, Tai L, Lee D, Kanakamma P, Shen C, Luh T, Cheng C, Hwang K. C60 and water-soluble fullerene derivatives as antioxidants against radical-initiated lipid peroxidation. J Med Chem. 1999;42:4614–4620. doi: 10.1021/jm990144s. [DOI] [PubMed] [Google Scholar]
- Wielgus AR, Zhao B, Chignell CF, Hu DN, Roberts JE. Phototoxicity and cytotoxicity of fullerol in human retinal pigment epithelial cells. Toxicol Appl Pharmacol. 2010;242:79–90. doi: 10.1016/j.taap.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Xia XR, Monteiro-Riviere NA, Riviere JE. Trace analysis of fullerenes in biological samples by simplified liquid-liquid extraction and high-performance liquid chromatography. J Chromat A. 2006;1129:216–222. doi: 10.1016/j.chroma.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Xia XR, Monteiro-Riviere NA, Riviere JE. Intrinsic biological property of colloidal fullerene nanoparticles (nC60): lack of lethality after high dose exposure to human epidermal and bacterial cells. Toxicol Lett. 2010;197:128–134. doi: 10.1016/j.toxlet.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Yamada T, Nakaoka R, Sawada R, Matsuoka A, Tsuchiy T. Effects of intracerebral microinjection of hydroxylated-[C60] fullerene on brain monoamine concentrations and locomotor behavior in rats. J Nanosci Nanotechnol. 2010;10:604–611. doi: 10.1166/jnn.2010.1720. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang K, Driver J, Yang J, Barron AR. The use of fullerene substituted phenylalanine amino acid as a passport for peptides through cell membranes. Org Biomol Chem. 2007;5:260–266. doi: 10.1039/b614298b. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Karasawa R, Masuko K, Kato T. Water-soluble fullerene (C60) inhibits the osteoclast differentiation and bone destruction in arthritis. Int J Nanomedicine. 2009;4:233–239. doi: 10.2147/ijn.s7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Cho M, Fortner JD, Lee J, Huang CH, Hughes JB, Kim JH. Delineating oxidative processes of aqueous C60 preparations: role of THF peroxide. Environ Sci Technol. 2009;43:108–113. doi: 10.1021/es8019066. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Zeng L, Barron AR, Monteiro-Riviere NA. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int J Toxicol. 2007;26:103–113. doi: 10.1080/10915810701225133. [DOI] [PubMed] [Google Scholar]