Abstract
Objective
Most longitudinal studies of depressive symptoms reported mean symptom scores that tend to obscure interindividual heterogeneity in the symptom experience. The identification of subgroups of patients with distinct trajectories of depressive symptoms may help identify high risk individuals who require an intervention. This study aimed to identify subgroups of breast cancer patients (n=398) with distinct trajectories of depressive symptoms in the first six months after surgery, as well as predictors of these trajectories.
Methods
Growth mixture modeling was used to identify the latent classes based on Center for Epidemiological Studies-Depression scale scores completed prior to and monthly for six months after surgery.
Results
Four latent classes of patients with distinct depressive symptom trajectories were identified: Resilient (38.9%), Subsyndromal (45.2%), Delayed (11.3%), and Peak (4.5%). Patients in the Subsyndromal class were significantly younger than patients in the Resilient class. Compared to the Resilient class, Subsyndromal, Delayed, and Peak classes had higher mean trait and state anxiety scores prior to surgery. Except for axillary lymph node dissection (ALND), disease- and treatment-related characteristics did not differ across the classes. A greater proportion of women in the Subsyndromal class had an ALND compared to those in the Resilient class.
Conclusions
Breast cancer patients experience different trajectories of depressive symptoms after surgery. Of note, over 60% of these women were classified into one of three distinct subgroups with clinically significant levels of depressive symptoms. Identification of phenotypic and genotypic predictors of symptom trajectories after cancer treatment warrants additional investigation.
Keywords: breast cancer, depression, anxiety, growth mixture modeling, latent profiles, psychological distress
INTRODUCTION
Women with breast cancer are more likely than women without breast cancer to experience depressive symptoms (Den Oudsten, Van Heck, Van der Steeg, Roukema, & De Vries, 2009). The prevalence of depressive symptoms in women with breast cancer ranges from 1% to 50% (Bower, 2008; Burgess et al., 2005; Fann et al., 2008; Kissane et al., 2004; Pinder et al., 1993) and rates appear to be highest in the first six to twelve months after diagnosis (Helgeson, Snyder, & Seltman, 2004; Lam et al., 2010). These highly variable prevalence rates may be attributed to the use of heterogeneous depression measures, varied time points for assessments across the trajectory of breast cancer treatment, and reliance on mean scores to identify distinct patient subgroups. However, given the negative impact of depressive symptoms on quality of life (QOL) (Bower, 2008; Fann, et al., 2008) and treatment adherence (DiMatteo, Lepper, & Croghan, 2000), a need exists to identify women at highest risk for depression in order to institute appropriate interventions.
The use of cross-sectional versus longitudinal designs has contributed to the heterogeneity of findings regarding the prevalence and predictors of depressive symptoms in patients with breast cancer (Deshields, Tibbs, Fan, & Taylor, 2006; van't Spijker, Trijsburg, & Duivenvoorden, 1997). Most studies that attempted to identify predictors of depression were cross-sectional, which limits our understanding of how depressive symptoms change during and after treatment (Millar, Purushotham, McLatchie, George, & Murray, 2005; Stiegelis, Ranchor, & Sanderman, 2004). Among the limited number of longitudinal studies, most reported mean symptom scores for the entire sample, which obscures underlying heterogeneity in the symptom experience over time. In contrast, recent studies have demonstrated substantial heterogeneity in levels of distress and depression during and after treatment for breast cancer (Deshields, et al., 2006; Henselmans et al., 2010; Lam, Bonanno, et al., 2010; Lam, Shing, Bonanno, Mancini, & Fielding, 2010; Millar, et al., 2005)
For example, Deshields and colleagues (2006) evaluated patterns of depression over 6 months in 84 women with breast cancer, using the Center for Epidemiological Studies – Depression scale (CES-D). Five distinct patterns of depression were identified in this sample. At the end of radiation therapy (RT), 30% of women scored above the clinically significant cutpoint (≥16) on the CES-D. This proportion fell to 24% at 3 months and 21% at 6 months after RT. However, the composition of the depressed group was not the same at each timepoint, which underscores the heterogeneity of depressive symptoms over time. As noted by Henselmans and colleagues (2010), because a dichotomous, case-based definition of depression was used, Deshields et al. may have failed to identify subtle differences in depressive symptom profiles in these women.
Advances in longitudinal data analysis facilitate detection of underlying patterns of change in symptom severity over time (B. Muthen & Muthen, 2000). One such method, growth mixture modelling (GMM), enables the identification of subgroups of patients, referred to as latent growth classes, whose symptoms share a similar trajectory over time. Only four studies were found that identified distinct subgroups of patients with breast cancer based on longitudinal assessments of psychological distress (Helgeson, et al., 2004; Henselmans, et al., 2010; Lam, Bonanno, et al., 2010; Lam, Shing, et al., 2010). Each of these studies identified four, rather similar subgroups of patients with distinct distress trajectories over time periods that ranged from eight months to six years post-diagnosis. In terms of psychological predictors of subgroup membership, women with a lower sense of mastery, those who were less optimistic, those who had higher neuroticism scores, those will a lower sense of personal control, and those will less social support were more likely to be classified in the higher distress groups (Helgeson, et al., 2004; Henselmans, et al., 2010; Lam, Bonanno, et al., 2010). In most cases, the various subgroups of women did not differ on a variety of demographic and clinical characteristics (e.g., stage of disease, type of surgery). However, women who reported a higher number of physical complaints during treatment were more likely to be classified in the higher distress groups (Henselmans, et al., 2010; Lam, Bonanno, et al., 2010). Taken together, these findings suggest that subgroups of patients with breast cancer with distinct experiences of distress over time can be identified using more sophisticated approaches for longitudinal data analysis. In addition, these findings suggest that predictors associated with subgroup membership can be determined that would facilitate the identification of women who are at greater risk for the development of psychological distress. However, the conclusions that can be drawn about the trajectories of depressive symptoms in women with breast cancer are limited because previous studies used general measures of distress rather than a specific measure of depression.
Depression and anxiety have some overlapping features and frequently co-occur in the general population (Das-Munshi et al., 2008; Kessler et al., 2008) and in cancer patients (Brown, Kroenke, Theobald, Wu, & Tu, 2010). Their co-occurrence is associated with impaired functioning and QOL (Merikangas et al., 2003). Although these symptoms are not synonymous, in the psycho-oncology literature, they are often subsumed under the concept of “distress” (Jacobsen et al., 2005). Anxiety, common among breast cancer patients (Burgess, et al., 2005; Stark et al., 2002), should be distinguished from depression, because interventions differ depending on the nature and severity of symptoms. In addition, while anxiety can take many forms (e.g., uncertainty about the future, treatment-related anxiety, pre-existing generalized anxiety disorder), the relationships between anxiety and depressive symptoms have not been well studied in breast cancer patients following surgery (Burgess, et al., 2005). As in the general population, this relationship is likely to be complex. Trait anxiety (i.e., a relatively stable individual characteristic that precedes a cancer diagnosis) was associated with decreased QOL in women with breast cancer (van der Steeg, De Vries, & Roukema, 2010) and predicted depression (De Vries, Van der Steeg, & Roukema, 2009) and trajectories of depression (Deshields, et al., 2006). Therefore, anxiety is an important symptom to examine in relationship to depressive symptoms.
Given the paucity of longitudinal research on the heterogeneity of depressive symptoms in patients with breast cancer (Fann, et al., 2008; Helgeson, et al., 2004), the purposes of this study were: to identify distinct latent classes of breast cancer patients based on self-reported depressive symptoms from just prior to surgery through six months after surgery and to evaluate for differences in demographic and clinical characteristics and underlying trait anxiety among these latent classes. In addition, the levels and trajectories of anxiety symptoms among the different latent classes were evaluated. Based on the literature (Compas et al., 1999; Helgeson, et al., 2004; Kroenke, Kubzansky, Schernhammer, Holmes, & Kawachi, 2006), we hypothesized that younger age and higher levels of trait and state anxiety would be associated with the subgroups of patients with higher levels of depressive symptoms.
METHODS
Patients and settings
This longitudinal study is part of a larger study that evaluated neuropathic pain and lymphedema in a sample of women who underwent breast cancer surgery. Patients were recruited from Breast Care Centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices. Women were eligible if they were ≥18 years of age; would undergo breast cancer surgery on one breast; were able to read, write, and understand English; and provided written informed consent. Patients were excluded if they were: having bilateral breast cancer surgery and/or had distant metastasis at the time of diagnosis. A total of 516 patients were approached and 410 enrolled in the study (response rate 79.4%). The major reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to complete the baseline assessment prior to surgery.
Instruments
At enrollment, demographic information was obtained (age, gender, marital status, education, ethnicity, employment status, living situation, financial status). At each subsequent assessment, patients provided information on current treatments for breast cancer. Medical records were reviewed to obtain information on stage of disease, surgical procedure, neoadjuvant treatment, and reconstructive surgery.
The Karnofsky Performance Status (KPS) scale is widely used to evaluate functional status in patients with cancer and has well established validity and reliability (Karnofsky, Abelmann, Craver, & Burchenal, 1948). Patients rated their functional status using the KPS scale that ranged from 30 (“I feel severely disabled and need to be hospitalized”) to 100 (“I feel normal; I have no complaints or symptoms”).
The Self-Administered Comorbidity Questionnaire (SCQ), which was developed to assess comorbidity in clinical and health service research settings (Sangha, Stucki, Liang, Fossel, & Katz, 2003), consists of 13 common medical conditions, including depression, described in plain language (i.e., no prior medical knowledge needed). Patients were asked to indicate if they currently had the condition (“yes/no”), and if “yes,” to indicate whether they received treatment for it (“yes/no”) and whether it limited their activities (“yes/no”). Patients could add two additional conditions not listed on the instrument. Each condition yields a maximum of three points. Therefore, the maximum score totals 45 points if the open-ended items are used and 39 points if only the 13 closed-ended items are used. SCQ-13 scores are reported in this paper. The SCQ has well-established validity and reliability and has been used in studies of patients with a variety of chronic conditions (Brunner et al., 2008; Sangha, et al., 2003).
The Center for Epidemiologic Studies-Depression (CES-D) scale consists of 20 items representing the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of ≥ 16 indicating the need for clinical evaluation for major depression. The CES-D has well-established concurrent and construct validity (Carpenter et al., 1998; Radloff, 1977; Sheehan, Fifield, Reisine, & Tennen, 1995). In the current study, the Cronbach’s alpha was 0.90.
The Spielberger State-Trait Anxiety Inventories (STAI-T and STAI-S) consist of 20 items, each rated from 1 to 4. Total scores for each scale can range from 20 to 80, with higher scores indicating greater anxiety. The STAI-T measures a person’s predisposition to anxiety and estimates how a person generally feels. The STAI-S measures an individual’s transitory emotional response, with items assessing worry, nervousness, tension, and apprehension related to how a person feels “right now”. Scores ≥ 31.8 and ≥ 32.2 suggest high levels of trait and state anxiety, respectively (Fletcher et al., 2008; Spielberger, Gorsuch, Suchene, Vagg, & Jacobs, 1983). Both inventories have well-established criterion and construct validity and internal consistency (Bieling, Antony, & Swinson, 1998). In the current study, the Cronbach’s alphas for the STAI-T and STAI-S were 0.88 and 0.95, respectively.
Study Procedures
The Committee on Human Research at the University of California, San Francisco and at each study site approved the study. During the patient’s preoperative visit, a staff member explained the study to the patient. For those women willing to participate, the staff member introduced the patient to the research nurse, who met with the women, determined eligibility and obtained written informed consent prior to surgery. After providing consent, the patient completed the baseline study questionnaires (Assessment 0). Patients were contacted two weeks after surgery to schedule the first post-surgical appointment. The research nurse met with the patients in their home, the Clinical Research Center, or the clinic at 1, 2, 3, 4, 5, and 6 months after surgery. During each study visit, the women completed the study instruments.
Statistical Analyses
Descriptive statistics and frequency distributions were calculated for the sample characteristics and symptom severity scores.
Unconditional growth mixture modeling (GMM) with robust maximum likelihood estimation was carried out with Mplus Version 5.21 (L. K. Muthen & Muthen, 2009) to identify latent classes (i.e., subgroups of patients) with distinct depressive symptom trajectories over the six months of the study. In the basic latent growth curve analysis, a single growth curve representing the “average” change trajectory is estimated for the whole sample. However, it is usually true that substantial variation exists between the individual change trajectories and the estimated mean growth curve for the sample. This heterogeneity may be due to “error,” but it might also represent meaningful differences between unknown groups of individuals in the way they change across time. In the latter case, the possibility for examining the differences between these groups is lost when only a single growth curve is estimated (Byrne & Crombie, 2003) and (Duncan, Duncan, & Strycker, 2006).
GMM an extension of latent growth curve analysis, extends the estimation of a single growth curve – represented as latent variables (i.e., intercept and slope coefficients) and variance components for them – to the estimation of a new latent categorical variable that identifies latent growth curves for two or more previously unknown groups, called “classes” (Jung & Wickrama, 2008; Kreuter & Muthen, 2008; Mo & Bodner, 2007; B. Muthen & Muthen, 2000; B. O. Muthen, 2001a, 2001b, 2004). GMM can be employed in several ways. The basic GMM is the "unconditional model" in which separate growth classes are identified solely by examining differences in their growth trajectories (Mo & Bodner, 2007; B. O. Muthen, 2004). Classes can be distinguished due to differences in any combination of their intercepts, slopes (linear and nonlinear), and within-class variances in intercepts and slopes.
The simplest form of a latent class mixture model is the latent class growth model (LCGM; (Nagin, 1999; Roeder, Lynch, & Nagin, 1999)). In the LCGM, within-class variances and covariances for intercepts and slopes are assumed to be zero, because the class membership is presumed to account for all variation between individuals (within classes) in their growth trajectories (B. Muthen & Muthen, 2000). It is useful in GMM to begin estimation of the number of latent classes with a latent class growth analysis using LCGM (B. O. Muthen, 2004). GMM extends the estimation of multiple growth curves defined by the latent classes, such that covariances are not assumed to be zero within classes and may vary across classes.
The unconditional GMM may be extended through the incorporation of (1) covariates that characterize differences between observations; (2) concurrent events, or covariates or outcomes that are assessed and change across time; or (3) distal events, perhaps consequences of the differing change processes (Delucchi, Matzger, & Weisner, 2004; B. O. Muthen, 2004). Muthén (2001a, 2001b) refers to this extension as the general growth mixture model (GGMM).
The identification of the basic or unconditional growth model is the first step in such an analysis. It should be followed with an examination of how the latent classes differ on important covariates, and how class membership is associated with outcomes that depend on, or can be predicted by the differing change processes. The ideal approach to examine covariates of the change processes that lead to different latent classes, and to validate the meaning of the latent classes through the examination of their associations with concurrent events or their differences on distal events, is to incorporate these variables into increasingly complex structural models (Delucchi, et al., 2004; Kreuter & Muthen, 2008; B. O. Muthen, 2002, 2004; Petras et al., 2008). However, more complex models require larger samples, especially when the proportion of cases in some latent classes is small (e.g., perhaps only 10%). Tofighi and Enders (2008) evaluated several indices useful for choosing the number of classes for a GMM, including and not including covariates. In their Monte Carlo study, they found that including covariates in fact made estimation of the correct number of classes less reliable unless the sample size was 1000 or more.
In this study, the number of latent growth classes that best fit the data was identified using guidelines recommended by Jung and Wickrama (2008), Muthén (2004), Nylund, Asparaouhov, & Muthén (2007), and Tofighi and Enders (2008). First, a model with two latent growth classes was fit to the data, then a model with three latent growth classes was fit, and the procedure was repeated until the final iteration of the model was not supported. Model fit for the GMM was assessed statistically by identifying the model with the lowest Bayesian Information Criterion (BIC), and by testing the “K” versus “K-1” class models to determine whether a model with K classes fit the data better than a model with K-1 classes with the parametric bootstrapped likelihood ratio test (BLRT) (Jung & Wickrama, 2008; Nylund, et al., 2007; Tofighi & Enders, 2008). For example, after estimating the 3-class model (the K-class model), it was compared to the 2-class model (the K-1 class model) with the BLRT to determine whether the 3-class model fit the data better than the 2-class model. In addition to using the BLRT to compare models, we examined the Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (VLMR) for the “K” versus “K-1” class models. The VLMR test was shown in Monte Carlo studies to be anti-conservative in identifying the “correct” number of classes in some mixture models. That is, it sometimes identifies a K-class model as fitting the data better than the K-1 class model when in fact the K-1 class model is correct. However, when the VLMR test is non-significant, it does provide evidence that the K-class model is not better than the K-1-class model (Nylund, et al., 2007).
The fourth index used to evaluate model fit was entropy (i.e., the proportion of latent versus predicted class membership). It was estimated for each solution, with ≥ .80 being preferred. Better-fitting models should produce higher entropy values, indicating consistency between the latent and predicted class membership (Celeux & Soromenho, 1996; L. K. Muthen & Muthen, 1998–2010), perhaps analogous to classificiation indices for interrater agreement, or reliability coefficients. However, no fixed criterion exists for evaluating entropy, and it has been shown to be unreliable as a fit index with unbalanced class sizes (Henson, Reise, & Kim, 2007). In addition to evaluating the fit indices, the best fitting model was visually inspected by plotting observed against model-predicted values to determine whether the predicted trajectories followed the empiric trajectories for the classes, and to evaluate whether the predicted plots “made sense” theoretically and clinically (B. O. Muthen, 2004).
In summary, the criteria used for selecting the best fitting model in order of importance were the BIC followed by the BLRT. The VLMR was employed as a check against the extraction of too many classes by the BLRT, and the entropy measure was used descriptively to assess membership in the latent classes compared to the most likely class memberships predicted by the model, the model with higher entropy being preferred. Of course, none of these fit indices are relevant unless the smallest BIC can be reliably replicated with multiple random starts, or if the BLRT cannot reproduce the same log likelihood value in the majority of bootstrapped draws.
Intercepts and linear and quadratic slopes for each latent class were estimated for each model. Intercept and linear slope variances were estimated for each class and were allowed to differ across classes. Given the relatively small sample size, the within-class quadratic slope variance was fixed at zero, because estimation failed when quadratic slopes were free to vary even for the simplest (2-class) model. Even for the initial two-class model, it was necessary to fix the linear slope variance to zero for our largest class. Without setting the slope variance to zero, the model could not be estimated due to a non-positive definite covariance matrix for the class. The change trajectory for this large class was flat with minimal within-class variance, similar to what would be expected in a latent class growth model (LCGM) (Nagin & Tremblay, 2001; Roeder, et al., 1999). Mixture models are known to produce solutions at local maxima, so each model was fit with random starts to be sure that the solution for the model with the maximum log likelihood values was replicated (L. K. Muthen & Muthen, 1998–2010).
After identifying the latent class solution that best fit our data, differences among the predicted classes were examined for important covariates and concurrent outcomes outside our models. Although this approach is not the ideal strategy (Delucchi, et al., 2004; Kreuter & Muthen, 2008) (B. O. Muthen, 2002; Petras, et al., 2008), it was judged to be more prudent given our relatively small sample. Missing data for the depression scores were accommodated by Mplus Version 5.21 through the use of Full Information Maximum Likelihood and the use of the Expectation-Maximization algorithm. In this way, individuals are retained in the analysis even if their depression scores are missing at one or more occasions, unlike traditional analyses of longitudinal data such as repeated measures analysis of variance (ANOVA), in which a case is dropped even if only a single measure is missing. This method assumes that any missing data are ignorable [i.e., missing at random (B. O. Muthen, 2002; Schafer & Graham, 2002)].
Data were analyzed using SPSS Version 18.0 (SPSS, 2010) and Mplus Version 5.21 (L. K. Muthen & Muthen, 2009). ANOVA and Chi-square analyses were used to assess for differences in demographic and clinical characteristics and symptom severity scores among the GMM latent classes. Linear mixed effect model analyses were used to test for differences in State anxiety scores at baseline and over time among the latent classes, using the SPSS MIXED module. Post hoc contrasts were done to evaluate for differences among the GMM classes at baseline (i.e., intercept) as well as for GMM class×time interactions (i.e., Does the change over time in state anxiety vary across the different GMM classes?). Post hoc contrasts were done using the Bonferroni procedure to control the overall familywise alpha level of the six pairwise contrasts for the four GMM classes at 0.05. For any of the six pairwise contrasts, a p-value of < 0.008 (.05/6) was deemed statistically significant.
RESULTS
Patient Characteristics
As summarized in Table 1, most patients were Caucasian and well educated. Approximately 60% were married or partnered. Nearly one-quarter lived alone. Approximately 22% (n=87) of the women endorsed currently having depression on the SCQ.
Table 1.
Baseline Demographic and Clinical Characteristics of Total Sample (n=398) and of Four Latent Classes*
| Characteristic | Total Sample (n=398) |
Resilient Class (1) n=155 (38.9%) |
Subsyndromal Class (2) n=180 (45.2%) |
Delayed Class (3) n=45 (11.3%) |
Peak Class (4) n=18 (4.5%) |
Omnibus Statistics and Post hoc Comparisons** |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 54.9 (11.6) | 57.3 (11.0) | 53.0 (11.9) | 55.6 (11.0) | 52.4 (10.7) | F(3,394)=4.41; p=0.005 1>2 |
| Education (years) | 15.7 (2.7) | 15.8 (2.5) | 15.9 (2.8) | 14.5 (2.2) | 15.8 (3.4) | F(3,389)=3.51; p=0.015 |
| KPS Score | 93.2 (10.3) | 95.5 (8.7) | 91.1 (11.1) | 92.7 (11.5) | 95.6 (7.0) | F(3,387)=5.31; p=0.001 1>2 |
| SCQ-13 Score | 4.3 (2.8) | 4.0 (2.5) | 4.6 (3.1) | 4.4 (3.1) | 3.7 (2.4) | F(3,393)=1.42; p=0.237 |
| CES-D Total Score | 13.7 (9.8) | 6.8 (4.7) | 17.1 (8.6) | 24.1 (11.1) | 13.8 (8.0) | F(3,377)=78.66; p<0.001 1<2,3,4; 2<3; 3>4 |
| Trait Anxiety Score | 35.3 (9.0) | 30.6 (6.3) | 38.6 (9.2) | 38.8 (9.6) | 35.5 (8.0) | F(3,373)=28.12, p<0.001 1<2,3 |
| State Anxiety Score | 41.8 (13.5) | 35.0 (11.2) | 45.2 (12.6) | 50.4 (14.2) | 45.0 (13.5) | F(3,379)=26.91, p<0.001 1<2,3,4 |
| n (%) | n (%) | n (%) | n (%) | |||
| Ethnicity (% White) | 255 (64.4%) | 107 (69.5%) | 112 (62.6%) | 26 (57.8%) | 10 (55.6%) | χ2=21.98; p=0.23 |
| Marital status (% married/partnered) | 213 (54.1%) | 96 (62.3%) | 88 (49.2%) | 24 (54.5%) | 5 (29.4%) | χ2=21.53; p=0.12 |
| Works for pay (% yes) | 189 (47.8%) | 78 (50.3%) | 83 (46.6%) | 19 (42.2%) | 9 (52.9%) | χ2=1.23; p=0.75 |
| Lives alone (% yes) | 95 (24.2%) | 34 (22.1%) | 41 (23.0%) | 13 (29.5%) | 7 (41.2%) | χ2=3.87; p=0.28 |
| Stage of disease at diagnosis | ||||||
| 0 | 64 (16.9%) | 23 (15.5%) | 28 (16.3%) | 11 (25.6%) | 2 (12.5%) | χ2=25.86; p=0.21 |
| 1 | 143 (37.7%) | 66 (44.6%) | 51 (29.7%) | 19 (44.2%) | 7 (43.8%) | |
| IIA | 99 (26.1%) | 35 (23.6%) | 49 (28.5%) | 11 (25.6%) | 4 (25.0%) | |
| IIB | 39 (10.3%) | 12 (8.1%) | 23 (13.4%) | 1 (2.3%) | 3 (18.8%) | |
| IIIA | 18 (4.7%) | 5 (3.4%) | 13 (7.6%) | 0 (0%) | 0 (0%) | |
| IIIB | 6 (1.6%) | 2 (1.4%) | 4 (2.3%) | 0 (0%) | 0 (0%) | |
| IIIC | 8 (2.1%) | 3 (2.0%) | 4 (2.3%) | 1 (2.3%) | 0 (0%) | |
| IV | 2 (0.5%) | 2 (1.4%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Surgical treatment | ||||||
| Breast-conserving | 318 (79.9%) | 127 (81.9%) | 142 (78.9%) | 35 (77.8%) | 14 (77.8%) | χ2=0.69; p=0.88 |
| Mastectomy | 80 (20.1%) | 28 (18.1%) | 38 (21.1%) | 10 (22.2%) | 4 (22.2%) | |
| Sentinel node biopsy (% yes) | 328 (82.4%) | 133 (85.8%) | 144 (80.0%) | 36 (80.0%) | 15 (83.3%) | χ2=2.15; p=0.54 |
| Axillary lymph node dissection (% yes) | 149 (37.5%) | 52 (33.8%) | 82 (45.6%) | 10 (22.2%) | 5 (27.8%) | χ2=11.10; p=0.01 2>3 |
| Breast reconstruction at time of surgery (% yes) | 86 (21.7%) | 28 (18.2%) | 41 (22.8%) | 11 (24.4%) | 6 (33.3%) | χ2=2.88; p=0.41 |
| Neoadjuvant chemotherapy (% yes) | 79 (19.9%) | 26 (16.9%) | 44 (24.4%) | 6 (13.3%) | 3 (16.7%) | χ2=4.55; p=0.21 |
| Post-operative treatment*** | ||||||
| Adjuvant chemotherapy | 133 (33.4%) | 42 (27.1%) | 73 (40.6%) | 12 (26.7%) | 6 (33.3%) | χ2=7.83; p=0.05 |
| Radiation therapy | 224 (56.3%) | 93 (60.0%) | 95 (52.8%) | 26 (57.8%) | 10 (55.6%) | χ2=1.81; p=0.61 |
Abbreviations: KPS = Karnofsky Performance Status; SCQ = Self-Administered Comorbidity Questionnaire; CES-D = Center for Epidemiological Studies – Depression
All demographic, clinical, and symptom characteristics were assessed prior to breast cancer surgery.
Bonferroni post hoc pairwise comparisons with alpha = .05: Numbers refer to latent classes (e.g., for CES-D: 1<2,3,4 represents Resilient class had a lower mean CES-D score than the Subsyndromal, Delayed, or Peak classes). Only significant post hoc contrasts are shown.
Post-operative chemotherapy and radiation were coded dichotomously yes/no if the patient received these treatments at any point during the course of the study.
Results of GMM Analysis
Four distinct classes of depressive symptom trajectories were identified using GMM (see Figure 1). As shown in Table 2, a four-class solution provided the best model fit. The four-class model was selected because of its improvement over the three-class model (i.e., the 4-class BIC = 17010.84, which is smaller than the 3-class BIC = 17026.16; BLRT Χ2, p = .01, entropy = .71), with each class maintaining a reasonable size and interpretability (Jung & Wickrama, 2008). Although the entropy was weaker than .8 overall, the proportion of cases in each latent class compared to their predicted class membership was above .80 for every class. In addition, the VLMR test showed that the 5-class solution did not fit better than the 4-class solution. Visual inspection of the four-class solution confirmed that these trajectories appeared to make sense clinically (i.e., substantial subgroups with no symptoms (Resilient); with stable subsyndromal to mild depressive symptoms (Subsyndromal); and two smaller groups with changing symptom levels during and after treatment (see Discussion for further description of the clinical relevance of these trajectories)).
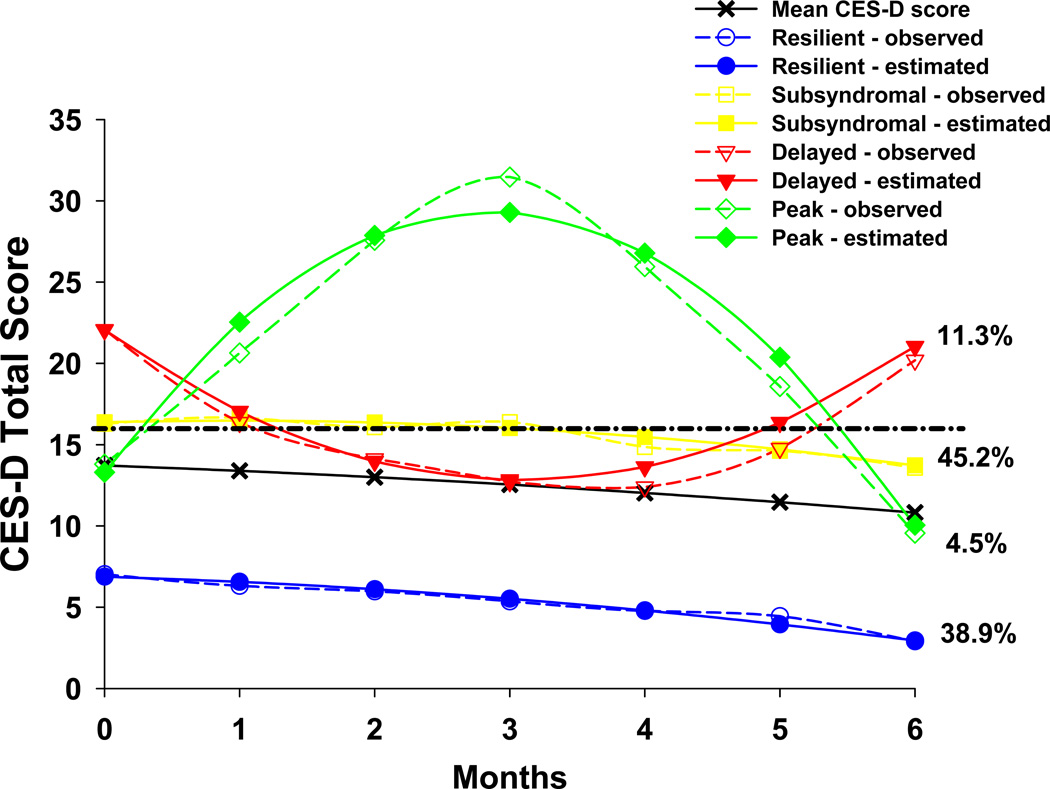
Figure 1.
Observed and estimated mean Center for Epidemiologic Studies-Depression (CES-D) trajectories for patients in each of the latent classes, as well as the mean CES-D scores for the total sample. Dashed and dotted black line indicates the cutoff score on the CES-D for clinically meaningful levels of depressive symptoms (i.e., ≥ 16)
Table 2.
Fit Indices for the GMM Class Solutions for 398 Breast Cancer Patients
| GMM | LL | AIC | BIC | Entropy | BLRT | VLMR (df) |
|---|---|---|---|---|---|---|
| 1-Classa | −8523.34 | 17171.67 | 17215.52 | n/a | n/a | n/a |
| 2-Class | −8495.90 | 17029.80 | 17105.54 | .64 | 112.16** | 112.16* (6) |
| 3-Class | −8441.24 | 16930.48 | 17026.16 | .69 | 90.82** | 90.82* (6) |
| 4-Classb | −8415.62 | 16891.24 | 17010.84 | .71 | 51.24* | 51.24* (6) |
| 5-Classc | −8395.19 | 16862.37 | 17005.88 | .70 | 40.87* | 40.87ns (6) |
= not significant;
p < .05;
p < .00005
Latent growth curve with linear and quadratic components; Chi2=77.186, 24 df, p < .00005, CFI = .94, RMSEA = .075
The 4-class model was selected as the best fitting model.
While a five-class solution had the smallest BIC, three other indicators led us to reject the 5-class solution in favor of the 4-class solution. Entropy decreased for the 5-class solution, indicating a worse fit; the best loglikelihood value was not replicated in 65 out of 77 bootstrap draws for the BLRT, making that test result unreliable; and the VLMR was not significant, indicating that too many classes had been extracted.
Abbreviations: GMM = Growth mixture model; LL = loglikelihood; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; BLRT = parametric bootstrapped likelihood ratio test for K-1 (H0) vs K classes; VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test for K-1 (H0) vs K classes.
A total of 38 missing data patterns were found across the seven assessments, with 96% of the sample providing data at baseline, declining to 86% at the last assessment. The covariance coverage (the percent of cases with data at each pair of assessments) ranged from a low of 77% for the 6th and 7th assessments, to a high of 91% for the 1st and 2nd assessments.
The parameter estimates for the four latent classes are listed in Table 3. The classes were named based on the overall shape of the trajectory. The largest percentage of patients was classified into the Subsyndromal class (n=180, 45.2%). This class had a mean total CES-D score prior to surgery that was just above the clinically significant CES-D cut-point of 16 (i.e., mean = 17.1). This mean score did not change significantly over the course of the study (Table 3). The second largest class was called the Resilient class (n=155, 38.9%). Prior to surgery, this subgroup’s mean CES-D score was 6.8. Their mean symptom scores remained stable over the course of the study. Two smaller classes contained 15.8% of the patients. The Delayed class (n=45, 11.3%) had a mean CES-D score that was elevated prior to surgery (i.e., mean = 24.1). This mean score decreased below the cutpoint of 16 at approximately one to two months after surgery, followed by an overall increase over the fifth and sixth months after surgery. The fourth class, called the Peak class’ (n=18, 4.5%), had a mean CES-D score (mean 13.8) that was lower than the mean CES-D score of the Subsyndromal group prior to surgery. However, the Peak group’s mean depressive symptom score increased steeply over the first three months after surgery, peaked at approximately three months, and then decreased to pre-surgical levels at six months post-surgery.
Table 3.
Parameter Estimates for Latent Classes from 7 Assessmentsa
| Parameter Estimatesb | Resilient Class (n = 155) |
Subsyndromal Class (n = 180) |
Delayed Class (n = 45) |
Peak Class (n = 18) |
|---|---|---|---|---|
| Means | Mean (S.E.) | Mean (S.E.) | Mean (S.E.) | Mean (S.E.) |
| Intercept | 6.88*** (0.76) | 16.38*** (1.20) | 22.06*** (2.37) | 13.30*** (1.96) |
| Linear Slope | −0.25 (0.35) | 0.21 (0.52) | −5.98*** (1.25) | 11.20*** (1.27) |
| Quadratic Slope | −0.07 (0.05) | −0.11 (0.08) | 0.97*** (0.20) | −1.96*** (0.18) |
| Variancesc | ||||
| Intercept | 3.67* (1.41) | 66.32*** (13.06) | 75.49** (22.10) | 27.94** (8.36) |
| Linear Slope | 0d | 1.55*** (0.34) | 4.41*** (0.99) | 2.12** (0.63) |
| 1 with S Covariance | 0d | −5.35*** (1.45) | −5.35*** (1.45) | −5.35*** (1.45) |
p < .01,
p < .001,
p < .0005
Trajectory group sizes are for classification of individuals based on their most likely latent class probabilities
Growth mixture model estimates were obtained with robust maximum likelihood estimation.
Variances for quadratic slopes were fixed at zero, and covariances between intercepts and slopes were held equal across classes, to aid in model convergence due to the relatively small sample sizes. Residual variances for the indicators at each assessment were held to be equal across classes, to allow comparisons of the growth parameters.
Fixed at zero
Abbreviations: S.E. = Standard Error
Differences in Demographic and Clinical Characteristics among the Four Latent Classes
As shown in Table 1, statistically significant differences were found among the four latent classes in age, education, and KPS Score. No significant differences were found among the four latent classes in comorbidities (SCQ score), ethnicity, marital status, working for pay, or living alone.
Post hoc contrasts for significant overall findings revealed that patients in the Subsyndromal class were significantly younger than those in the Resilient class. In terms of education, the post hoc contrasts were not significant at the predetermined threshold. In terms of KPS scores, those in the Subsyndromal class had lower scores than those in the Resilient class. The subgroups did not differ in terms of stage at diagnosis. The only treatment variables that distinguished among the groups, in omnibus tests, were having an axillary lymph node dissection (ALND) and having postoperative adjuvant chemotherapy. Post-hoc contrasts revealed that a higher percentage of women in the Subsyndromal class had an ALND (n=82, 45.6%) compared to those in the Delayed (n=10, 22.2%) class. Post hoc analyses for adjuvant chemotherapy did not reveal significant differences among the latent classes.
Statistically significant differences were found among the GMM latent classes in the percentage of women who endorsed depression on the SCQ (χ2=8.94, p=0.03). Across the four GMM classes, 16.8% of the Resilient class (i.e., 26 of 155 women), 23.9% of the Subsyndromal class (i.e., 43 of 180 women), 35.6% of the Delayed class (16 of 45 women), and 11.1% of the Peak class (2 of 18 women) reported depression. Post hoc contrasts revealed that a higher percentage of women in the Subsyndromal class than in the Resilient class reported a current problem with depression.
Differences in Trait and State Anxiety Scores Among the Four Latent Classes
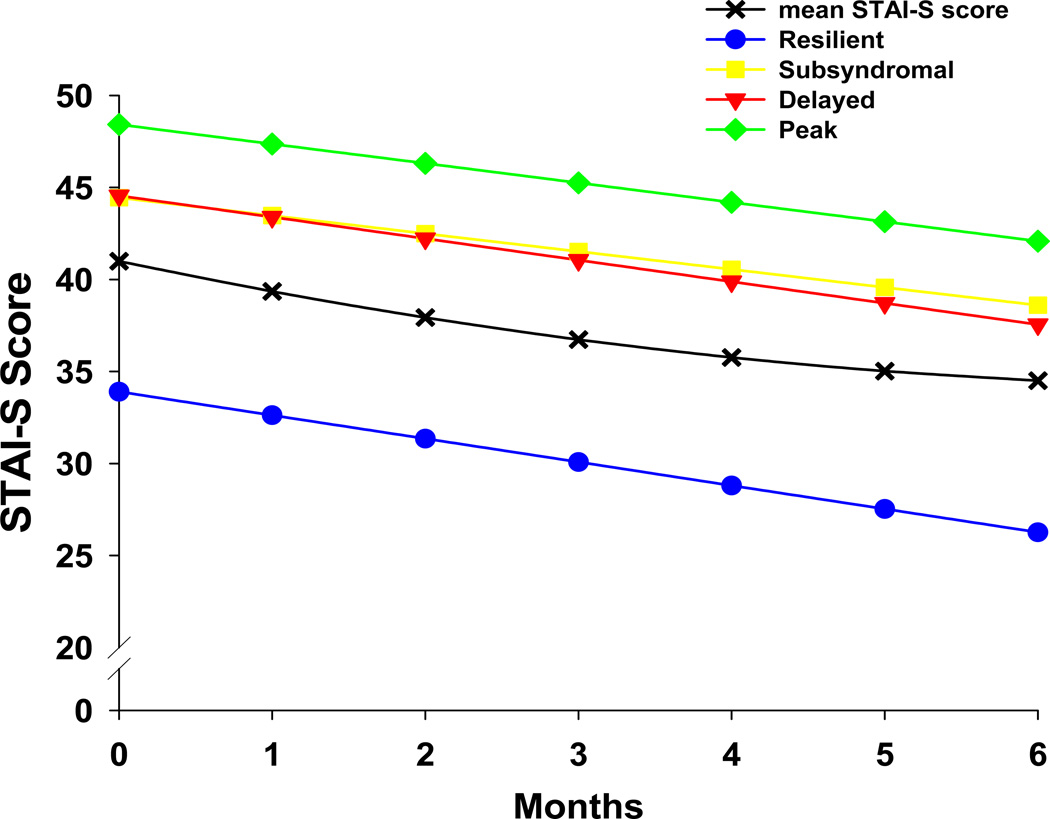
As Table 1 shows, the Subsyndromal and Delayed classes had higher mean trait anxiety (STAI-T) scores prior to surgery compared to the Resilient class (both p<0.001). Figure 2 illustrates the differences at baseline and over time in mean state anxiety (STAI-S) scores among the four classes. At baseline, the Resilient class had a significantly lower mean STAI-S score compared to the other three latent classes (all p<0.001). The post hoc contrasts for the class by time interactions for changes in STAI-S scores over time showed no differences among the four latent classes in the trajectory of anxiety symptoms.
Figure 2.
Changes over time in mean Speilberger State Anxiety Inventory (STAI-S) scores for patients in each of the latent classes. Post hoc contrasts for intercept 1 < 2, 3, and 4; all p < 0.0001. No differences among the CES-D groups on the slopes for state anxiety.
DISCUSSION
Findings from this study provide additional evidence of four distinct distress trajectories in oncology patients when newer methods of longitudinal analysis are employed(Helgeson, et al., 2004; Henselmans, et al., 2010; Hou, Law, Yin, & Fu, 2010; Lam, Bonanno, et al., 2010; Lam, Shing, et al., 2010). However, several important differences between this study and previous research warrant discussion.
In Deshields and colleagues’ longitudinal study (2006), five distinct subgroups of women were identified based on cutpoint analyses of CES-D scores (i.e., Never Depressed, Recover, Become Depressed, Stay Depressed, Vacillate) at three timepoints following the completion of RT. Compared to our findings (i.e., 38.9% in the Resilient class), a higher percentage of women were classified in the “Never Depressed” group. This difference may be attributed to the fact that variation within and between subgroups was minimized by the use of dichotomous categorizations. In addition, it is possible that a number of women in their “Never Depressed” group would be classified in our Subsyndromal class because the mean CES-D score in the Deshields study was 12.7 (at the end of treatment) which is slightly lower than the mean CES-D of 13.7 for the entire sample in our study. Finally, depressive symptoms were assessed at the completion of RT compared to our study that enrolled women prior to surgery for breast cancer. Consistent with our study findings, compared to the “Never Depressed” group, the other four depression groups had significantly higher state anxiety scores.
In a study that evaluated psychological adjustment, using the Mental Health Component Score of the SF-36, in women with breast cancer (n=287) over a four year period (Helgeson, et al., 2004), four distinct subgroups were identified. Consistent with our findings (i.e., 38.9% in the Resilient class), approximately 43% of their sample were classified as having low levels of distress across the four years. In addition, 12% of their sample had a deteriorating pattern of psychological distress which is similar to the 11.3% of patients classified in the Delayed class in our study. Personal and social resources distinguished among the four subgroups which suggest that these factors need to be evaluated as potential predictors of mental distress.
In another longitudinal study that followed breast cancer patients (n=171) over a period of twelve months (Henselmans, et al., 2010), four subgroups with distinct distress trajectories were identified using the General Health Questionnaire (GHQ; i.e., no distress (36%), distress during the active treatment phase only (33%), distress in the re-entry and survivorship phase (15%), and chronic distress (15%)). The five assessment points were selected to correspond with clinical events (i.e., soon after diagnosis (T1), after surgery (T2), after adjuvant therapy (T3), in the re-entry phase (T4), and in the short-term survivorship phase (T5)). Unlike our study, women who had received neoadjuvant chemotherapy were excluded from this study. Despite differences in measurement points and sample inclusion and exclusion criteria, the assessments in our study appear to provide a more in-depth view of the initial period prior to and following surgery for breast cancer. An examination of their trajectories from T1 to T3 suggests an intriguing yet speculative possibility—namely, correspondence of their No-distress (36%) group to our Resilient class (39%); their Recover group (33%), which experienced “distress only right after diagnosis and in the active treatment phase” to our Subsyndromal (45%) class; their “Late” group (15%) to our Delayed class (11%), and their Chronic group (15%) to our Peak class (5%). The latter two comparisons are the most speculative, given the small sample sizes of these groups in both studies. Further work is warranted in independent samples of patients with a variety of cancer diagnoses to confirm these subgroups with distinct depressive symptom or distress trajectories.
In the study by Henselmans and colleagues (2010), lower levels of neuroticism, higher levels of mastery and optimism, and fewer physical complaints from adjuvant treatment distinguished the no distress group from the other three groups. Consistent with their previous work, in the multivariate analyses, mastery was the only unique predictor of group membership (Helgeson, et al., 2004). Consistent with our findings discussed below, their findings support the notion that personality and trait-based variables play key roles in women’s experiences of distress after a breast cancer diagnosis.
Finally, in a recent study that reported on psychological distress (using the Chinese Health Questionnaire) over an 8 month period in a sample of Chinese women (n=285) (Lam, Bonanno, et al., 2010), four subgroups with distinct distress trajectories were identified (i.e., resilience (66%), chronic distress (15%), recovered (12%), and delayed-recovery (7%)). Although their assessment points (i.e., 5 days, 1 month, 4 months, and 8 months) are not directly comparable to those in the present study, it is interesting that two of the subgroups were similar to those identified in our study (i.e., minimal distress and delayed-recovery that had a parabolic trajectory). In addition, the largest changes in distress within the subgroups occurred over the first three assessments (4 months after surgery) which corresponds to the first five assessments in our study and suggests that our findings may provide more detailed information on the trajectories of psychological distress in the 6 months following breast cancer surgery. The large proportion of women classified as resilient (66%) in Lam et al. study is somewhat smaller than the Resilient class (39%) identified in our study. This difference may be due to the different measures used to assess psychological distress versus depressive symptoms. Optimism and less physical symptom distress at 1 month following surgery distinguished the resilient group from the other groups. In addition, Lam and colleagues reported that their chronic distress group had the poorest outcomes, in terms of distress and social adjustment, at six years follow up (Lam, Shing, et al., 2010).
Taken together, these findings suggest several conclusions and point to important directions for future research. First, subgroups of patients with breast cancer with distinct trajectories of distress or depression can be identified using newer methods of longitudinal data analysis. Second, personal characteristics (e.g., optimism, mastery) and higher levels of physical symptoms post-treatment, but not disease and treatment characteristics, appear to distinguish among these subgroups. Third, levels of trait and state anxiety, which differed significantly among the subgroups in our study and the study by Deshields and colleagues (2006), need to be evaluated as a risk factor for elevated depressive symptoms. Fourth, the specific measures chosen to assess psychological distress leads to different proportions of women classified as resilient.
This final point deserves particular mention. In our study, the largest latent class (Subsyndromal; 45%) had depressive symptom scores that on average were just above the clinically significant CES-D cutpoint of 16. Because a psychiatric evaluation was not done on these patients, definitive conclusions cannot be drawn about whether or not these patients met diagnostic criteria for specific clinical syndromes (e.g., major depression, dysthymic disorder, mixed anxiety and depression). However, it is possible that at least some portion of these patients experienced subsyndromal depression (i.e., symptoms of depression that do not meet the full threshold criteria for a major depressive episode). This hypothesis is supported by the finding that the Subsyndromal class exhibited significantly higher baseline trait and state anxiety scores and higher anxiety scores over time than the Resilient class. In addition, this class reported a lower functional status score than the Resilient class. Both the size and characteristics of this class are important because subsyndromal depression is associated with decreased functional status and QOL in the general population (Das-Munshi, et al., 2008; Forsell, 2007; Judd, Paulus, Wells, & Rapaport, 1996). Additional research is warranted, ideally using in-depth psychological and psychiatric assessments, to characterize women’s experience of depressive and anxiety symptoms during and after treatment for breast cancer.
The Delayed and Peak classes together accounted for 16% of the sample. Although no specific disease or patient characteristics differentiated between these two classes, the Peak class had mean depressive symptoms at baseline that were below those of the Subsydromal class and reached a peak at three months post-surgery. It is possible that this class may have experienced an increase in depressive symptoms related to variables not described here (e.g., higher levels of treatment-related physical symptoms). Both of these classes had higher state anxiety at baseline compared to the Resilient class, which underscores the role of anxiety in predisposing to higher levels of depressive symptoms after surgery. Moreover, the different longitudinal symptom patterns of these classes highlight the heterogeneity of the symptom experience and the need to evaluate patients at different time points rather than simply before or after surgery.
As predicted, the distinct latent classes differed from one another in terms of age, with the Resilient group being significantly older than the Subsyndromal group. This finding is consistent with previous reports that found that, on average, older cancer patients, including those with breast cancer, have lower levels of depressive symptoms and better overall QOL compared to younger patients (Helgeson, et al., 2004; Kroenke et al., 2004; Parker, Baile, de Moor, & Cohen, 2003). Various explanations are offered for why younger adults are more likely to have elevated depressive symptoms in the context of cancer (Compas, et al., 1999; Kroenke, et al., 2004). Proposed factors include differences in the types of treatments received, the severity of side effects (e.g., abrupt menopause, infertility, sexual dysfunction), and the use of more adaptive coping mechanisms (Compas, et al., 1999; Mosher & Danoff-Burg, 2005). However, in at least one study of older women with breast cancer (Ganz et al., 2003), the older age groups had higher study refusal rates. Therefore, it is possible that older patients who agree to participate in psychosocial or symptom-related research, particularly studies that require multiple assessments over a prolonged period of time, may be relatively healthier and less distressed than non-participating counterparts.
The majority of the patients in the study had relatively high functional status scores. The lower functional status score reported by patients in the Subsyndromal class compared to the Resilient class, while statistically significant and clinically meaningful (effect size, d=0.43), may be explained by the lower functional status noted in population-based studies between subsyndromal depression and functional status (Judd, et al., 1996; Judd, Schettler, & Akiskal, 2002).
The finding that state anxiety distinguished the Resilient class from the other three classes at baseline through six months of follow-up highlights the important relationship between these two symptoms. However, levels of state anxiety did not change in the same way as depression, demonstrating that the symptoms are not synchronous. In the general population, anxiety can occur independently or jointly with depressive symptoms (Stein, Kirk, Prabhu, Grott, & Terepa, 1995). Deshields and colleagues (2006) reported that scores on the STAI-S differed among the identified subgroups at the first study timepoint. Moreover, women in their sample who were categorized as having depression initially but then “recovered” had greater reductions in anxiety over time. Taken together, these findings underscore the need for longitudinal assessments of both anxiety and depression, as well as the need for further work to clarify distinct predictors of each symptom.
This study has several clinical implications. First, the finding that nearly half of the patients (Subsyndromal class, 45%) had slightly elevated or subsyndromal levels of depressive symptoms suggests that identification of these patients may be as important as identifying those patients with clearly elevated symptoms. Given that most screening instruments (e.g., Distress Thermometer (Jacobsen, et al., 2005)) use fewer items to screen for depression, it is unclear whether these patients would have been identified with such brief screeners. Thus, this substantial subgroup, may remain unidentified, yet may need referral or intervention. Little research exists on the effects of subsyndromal depressive symptoms on treatment outcomes, functional status, and QOL in cancer patients. Future research needs to clarify the prevalence, correlates, and outcomes of subsyndromal levels of depressive as well as anxiety symptoms.
Second, the consistent finding of a resilient class of patients across several studies suggest that resilience is common in adults who experience significant trauma, including a life-threatening illness (Bonanno, 2004). Additional research is needed to identify factors that protect individuals from the stressful effects associated with a cancer diagnosis and its treatment. From both clinical and research perspectives, distress interventions should be designed and tailored for individuals at highest risk. In contrast, resilient individuals may not need an intervention or may need a different type of intervention to maintain their resilience.
Third, these trajectories may represent underlying traits, which, at times of increased stress, predispose individuals to different trajectories of psychological symptoms. As mentioned above, previous studies reported on the relationship between a number of putative predisposing, personality-related factors (e.g., optimism, neuroticism, trait anxiety) and distress or depressive symptoms in women with breast cancer (Den Oudsten, et al., 2009; Henselmans, et al., 2010). Further work is needed to understand the degree to which traits influence psychological distress (both depression and anxiety) in cancer patients, and to develop and test interventions to improve coping skills in those predisposed to greater psychological distress by virtue of their underlying traits.
Finally, further work is needed to understand the longitudinal relationships among depressive symptoms and other prevalent symptoms in cancer patients, particularly fatigue, pain, and sleep disturbance. Research on the underlying neurobiology of depression in cancer suggests that these symptoms may share a common underlying basis (Raison & Miller, 2003).
Several limitations must be acknowledged. While information on depressive and anxiety symptoms were obtained through valid self-report measures, future studies need to include a clinical evaluation of previous and concurrent psychiatric comorbidities. The fact that the major reasons for refusal were being too overwhelmed with their cancer treatment or too busy may have led to an underestimation or overestimation of depressive symptoms in this sample. It is possible that the four latent classes may reflect some unique characteristics of this sample.
Finally, while other studies have used GMM to identify distinct latent classes of patients with and without cancer based on self-reports of depressive symptoms or distress (Carragher, Adamson, Bunting, & McCann, 2009; Colman, Ploubidis, Wadsworth, Jones, & Croudace, 2007; Henselmans, et al., 2010; Hunter, Muthen, Cook, & Leuchter, 2010), the findings from this study must be interpreted with caution until they are replicated in future studies. Ideally future studies should be done with sample sizes that are large enough to allow for confirmatory analyses of both the number and trajectories of the latent classes, as well as the phenotypic and genotypic characteristics that are unique to each class.
In addition to demographic and clinical variables, investigators should examine coping styles, personality traits, and other pre-existing individual characteristics as potential mediators and moderators of latent class membership. Research with other seriously or chronically ill populations would assist investigators and clinicians to understand how individual differences manifest in response to illness. If these latent classes with distinct symptom trajectories are reproduced, these findings would strengthen the notion that underlying traits are essential to understanding the incidence and course of distress or resilience in individuals affected by chronic and serious illnesses like cancer.
Acknowledgements
This study was funded by grants from the National Cancer Institute and the National Institute of Nursing Research (CA107091 and CA118658). Dr. Aouizerat is funded through the National Institutes of Health Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund. Dr. Miaskowski is an American Cancer Society Clinical Research Professor. This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties.
REFERENCES
- Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behavioral Research and Therapy. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. Journal of Clinical Oncology. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19:734–741. doi: 10.1002/pon.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BioMed Central Musculoskeletal Disorders. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. British Medical Journal. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BM, Crombie G. Modeling and testing change: An introduction to the latent growth curve model. Understanding Statistics. 2003;2:177–203. [Google Scholar]
- Carpenter JS, Andrykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, Cunningham LL. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues in Mental Health Nursing. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- Carragher N, Adamson G, Bunting B, McCann S. Subtypes of depression in a nationally representative sample. Journal of Affective Disorders. 2009;113:88–99. doi: 10.1016/j.jad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13(2):195–212. [Google Scholar]
- Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Biological Psychiatry. 2007;62(11):1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Compas BE, Stoll MF, Thomsen AH, Oppedisano G, Epping-Jordan JE, Krag DN. Adjustment to breast cancer: age-related differences in coping and emotional distress. Breast Cancer Research and Treatment. 1999;54:195–203. doi: 10.1023/a:1006164928474. [DOI] [PubMed] [Google Scholar]
- Das-Munshi J, Goldberg D, Bebbington PE, Bhugra DK, Brugha TS, Dewey ME, Prince M. Public health significance of mixed anxiety and depression: beyond current classification. British Journal of Psychiatry. 2008;192(3):171–177. doi: 10.1192/bjp.bp.107.036707. [DOI] [PubMed] [Google Scholar]
- De Vries J, Van der Steeg AF, Roukema JA. Trait anxiety determines depressive symptoms and fatigue in women with an abnormality in the breast. British Journal of Health Psychology. 2009;14:143–157. doi: 10.1348/135910708X310200. [DOI] [PubMed] [Google Scholar]
- Delucchi KL, Matzger H, Weisner C. Dependent and problem drinking over 5 years: a latent class growth analysis. Drug and Alcohol Dependence. 2004;74:235–244. doi: 10.1016/j.drugalcdep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Den Oudsten BL, Van Heck GL, Van der Steeg AF, Roukema JA, De Vries J. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psychooncology. 2009;18:1230–1237. doi: 10.1002/pon.1518. [DOI] [PubMed] [Google Scholar]
- Deshields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA. An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. [Google Scholar]
- Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, Gralow J. Major depression after breast cancer: a review of epidemiology and treatment. General Hospital Psychiatry. 2008;30(2):112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Miaskowski CA. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- Forsell Y. A three-year follow-up of major depression, dysthymia, minor depression and subsyndromal depression: results from a population-based study. Depression and Anxiety. 2007;24:62–65. doi: 10.1002/da.20231. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. Journal of Clinical Oncology. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychology. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychology. 2010;29:160–168. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- Henson JM, Reise SP, Kim KH. Detecting mixtures from structural model differences using latent variable modeling: A comparison of relative model fit statistics. Structural Equation Modeling. 2007;14:202–226. [Google Scholar]
- Hou WK, Law CC, Yin J, Fu YT. Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: a growth mixture modeling approach. Health Psychology. 2010;29:484–495. doi: 10.1037/a0020809. [DOI] [PubMed] [Google Scholar]
- Hunter AM, Muthen BO, Cook IA, Leuchter AF. Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. Journal of Psychiatric Research. 2010;44:90–98. doi: 10.1016/j.jpsychires.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PB, Donovan KA, Trask PC, Fleishman SB, Zabora J, Baker F, Holland JC. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–1502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MP, Wells KB, Rapaport MH. Socioeconomic burden of subsyndromal depressive symptoms and major depression in a sample of the general population. American Journal of Psychiatry. 1996;153:1411–1417. doi: 10.1176/ajp.153.11.1411. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatric Clinics of North America. 2002;25:685–698. doi: 10.1016/s0193-953x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychological Medicine. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Australian and New Zealand Journal of Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- Kreuter F, Muthen B. Analyzing criminal trajectory profiles: Briding multilevel and group-based approaches using growth mixture modeling. Journal of Quantitative Criminology. 2008;24:1–31. [Google Scholar]
- Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. Journal of Clinical Oncology. 2004;22:1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- Lam WW, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, Fielding R. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology. 2010;19:1044–1051. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- Lam WW, Shing YT, Bonanno GA, Mancini AD, Fielding R. Distress trajectories at the first year diagnosis of breast cancer in relation to 6 years survivorship. Psychooncology. 2010 doi: 10.1002/pon.1876. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, Angst J, Zurich Cohort S. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Archives of General Psychiatry. 2003;60:993–1000. doi: 10.1001/archpsyc.60.9.993. [DOI] [PubMed] [Google Scholar]
- Millar K, Purushotham AD, McLatchie E, George WD, Murray GD. A 1-year prospective study of individual variation in distress, and illness perceptions, after treatment for breast cancer. Journal of Psychosomatic Research. 2005;58:335–342. doi: 10.1016/j.jpsychores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Mo W, Bodner TE. Growth mixture modeling: Identifying and predicting unobserved subpopulations with longitudinal data. Organizational Research Methods. 2007;10:635–656. [Google Scholar]
- Mosher CE, Danoff-Burg S. A review of age differences in psychological adjustment to breast cancer. Journal of Psychosocial Oncology. 2005;23:101–114. doi: 10.1300/j077v23n02_07. [DOI] [PubMed] [Google Scholar]
- Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Muthen BO. Latent variable mixture modeling. In: Marcoulides GA, Schumacher RE, editors. New developments and techniques in structural equation modeling. Mahwah, NJ: Lawrence Erlbaum Associates; 2001a. [Google Scholar]
- Muthen BO. Second generation structural equation modeling with a combination of categorical and continuous latent variables: New opportunities for latent class-latent growth modeling. In: Collins LM, Sayer AG, editors. New Methods for Analysis of Change. 1st ed. Washington, DC: American Psychological Association; 2001b. pp. 291–332. [Google Scholar]
- Muthen BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29:81–117. [Google Scholar]
- Muthen BO. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan DW, editor. Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. 5th ed. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- Muthen LK, Muthen BO. MPlus (Version 5.21) Los Angeles: Muthen & Muthen; 2009. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Parker PA, Baile WF, de Moor C, Cohen L. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003;12:183–193. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- Petras H, Kellam SG, Brown CH, Muthen BO, Ialongo NS, Poduska JM. Developmental epidemiological courses leading to antisocial personality disorder and violent and criminal behavior: effects by young adulthood of a universal preventive intervention in first- and second-grade classrooms. Drug and Alcohol Dependence. 2008;95:S45–S59. doi: 10.1016/j.drugalcdep.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder KL, Ramirez AJ, Black ME, Richards MA, Gregory WM, Rubens RD. Psychiatric disorder in patients with advanced breast cancer: prevalence and associated factors. European Journal of Cancer. 1993;29A:524–527. doi: 10.1016/s0959-8049(05)80144-3. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biological Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- Roeder K, Lynch KG, Nagin DS. Modeling uncertainty in latent class membership: A case study in criminology. Journal of the American Statistical Association. 1999;94:766–776. [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. Journal of Personality Assessment. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- SPSS. SPSS for Windows (Version 18) Chicago, Illinois: SPSS, Inc.; 2010. [Google Scholar]
- Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. Journal of Clinical Oncology. 2002;20:3137–3148. doi: 10.1200/JCO.2002.08.549. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kirk P, Prabhu V, Grott M, Terepa M. Mixed anxiety-depression in a primary-care clinic. Journal of Affective Disorders. 1995;34:79–84. doi: 10.1016/0165-0327(95)00002-5. [DOI] [PubMed] [Google Scholar]
- Stiegelis HE, Ranchor AV, Sanderman R. Psychological functioning in cancer patients treated with radiotherapy. Patient Education and Counseling. 2004;52(2):131–141. doi: 10.1016/s0738-3991(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. Charlotte, NC: Information Age Publishing; 2008. [Google Scholar]
- van't Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosomatic Medine. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- van der Steeg AF, De Vries J, Roukema JA. Anxious personality and breast cancer: possible negative impact on quality of life after breast-conserving therapy. World Journal of Surgery. 2010;34:1453–1460. doi: 10.1007/s00268-010-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]