Abstract
Chronic alcohol consumption results in hepatotoxicity, steatosis, hypoxia, increased expression of inducible nitric oxide synthase (iNOS) and decreased activities of mitochondrial respiratory enzymes. The impact of these changes on cellular respiration and their interaction in a cellular setting is not well understood. In the present study we tested the hypothesis that nitric oxide (˙NO)-dependent modulation of cellular respiration and the sensitivity to hypoxic stress is increased following chronic alcohol consumption. This is important since ˙NO has been shown to regulate mitochondrial function through its interaction with cytochrome c oxidase, although at higher concentrations, and in combination with reactive oxygen species, can result in mitochondrial dysfunction. We found that hepatocytes isolated from alcohol-fed rats had decreased mitochondrial bioenergetic reserve capacity and were more sensitive to ˙NO-dependent inhibition of respiration under room air and hypoxic conditions. We reasoned that this would result in greater hypoxic stress in vivo, and to test this, wild-type and iNOS−/− mice were administered alcohol-containing diets. Chronic alcohol consumption resulted in liver hypoxia in the wild-type mice and increased levels of hypoxia-inducible factor 1α in the peri-venular region of the liver lobule. These effects were attenuated in the alcohol-fed iNOS−/− mice suggesting that increased mitochondrial sensitivity to ˙NO and reactive nitrogen species in hepatocytes and iNOS plays a critical role in determining the response to hypoxic stress in vivo. These data support the concept that the combined effects of ˙NO and ethanol contribute to an increased susceptibility to hypoxia and the deleterious effects of alcohol consumption on liver.
Keywords: ethanol, inducible nitric oxide synthase, bioenergetics, reserve capacity
1. Introduction
It has been shown that nitric oxide (˙NO) regulates several mitochondrial functions, including respiration and biogenesis. These new insights have led to a deeper understanding of the cross talk between ˙NO signaling pathways and major regulatory and metabolic pathways in the cell [1, 2]. For example, mitochondrial biogenesis can be regulated by the soluble guanylate cyclase pathway and ˙NO can modulate the response to hypoxia, depending on its concentration, through both mitochondrial-dependent and independent pathways [3–5]. However, under conditions associated with inflammation, increased reactive oxygen species (ROS) will decrease the concentration of ˙NO available to interact with cytochrome c oxidase and participate in reactions with other reactive species to generate secondary products that impair mitochondrial function through oxidation, nitration, and inactivation of mitochondrial proteins[6–8]. Chronic exposure to alcohol is particularly interesting in this respect since hepatotoxicity is associated with hypoxia, increased reactive nitrogen species (RNS) through induction of iNOS, protein nitration and lipid oxidation [9–12]. These oxidants are associated with oxidative damage to the mitochondrial respiratory chain and mtDNA particularly complex I and II [7, 10–15].
A role for the ˙NO-cytochrome c oxidase pathway in regulating oxygen (O2) gradients has also been proposed based upon both theoretical modeling and the observation that ˙NO is a more effective inhibitor of the most actively respiring (state 3) mitochondria [16, 17]. This suggests that under normal conditions, the binding of ˙NO to cytochrome c oxidase limits O2 consumption in the most actively respiring tissues, extending O2 gradients in organs such as the heart or liver [7, 16, 17]. Since alcohol-dependent hepatotoxicity is associated with increased superoxide and induction of iNOS it is likely that peroxynitrite is formed [10–12, 18]. Indeed, we have recently shown that amelioration of mitochondrial oxidant stress with a mitochondrial antioxidant, probably through scavenging peroxynitrite, can inhibit HIF-1α activation in response to chronic alcohol (EtOH) consumption[19]. Moreover, in response to ethanol (EtOH)-dependent hepatotoxicity, isolated liver mitochondria become more sensitive to respiratory inhibition by ˙NO [12, 15]. This will further contribute to tissue hypoxia and oxidative stress through increasing production of superoxide within the respiratory chain[20]. In addition, it is now clear that as the tissue becomes hypoxic the respiratory chain is capable of generating superoxide at complex III which is also modified in ethanol-dependent hepatotoxicity [21, 22].
Previous studies have shown that chronic EtOH consumption causes marked bioenergetic defects in both peri-venous and peri-portal hepatocytes. Upon exposure to hypoxia, which occurs in EtOH-induced hepatotoxicity, these defects become more pronounced and are associated with decreased aerobic and anaerobic ATP production [23–26]. Interestingly, it is now clear that mitochondria do not generally function close to their maximal respiratory function (State 3 in isolated mitochondria) in cells but a more intermediate respiratory state we have termed “state apparent” [27–29]. We have proposed that decreases in the specific activity of mitochondrial respiratory chain proteins decreases the bioenergetic reserve or spare capacity but this has not been shown in vivo. This is important given that it makes the cell potentially more susceptible to stress since we and others have shown reserve capacity is used at times of oxidative stress or increased work load [28–30]. Previous studies have shown with isolated mitochondria that the specific activities and respiratory control ratio of mitochondria isolated from alcohol treated animals are decreased [15, 31]. Based on this, we hypothesized that hepatocytes from chronic ethanol-fed animals will have a decreased reserve capacity. Furthermore, since the reserve capacity is decreased then mitochondria will be metabolically more active and closer to state 3 to maintain bioenergetic homeostasis under which conditions mitochondria are more sensitive to NO[29]. We hypothesized that the sensitivity to NO-dependent inhibition would therefore increase and this would be exacerbated by hypoxia (which is a feature of alcohol-dependent hepatotoxicity). In support of this it has been shown that inducible nitric oxide synthase (iNOS) is known to be increased in response to EtOH consumption, and mitochondria isolated from EtOH-treated animals are more susceptible to ˙NO-dependent inhibition of respiration[12, 15]. Given that induction of iNOS is also associated with protein nitration, we reasoned that ˙NO would exacerbate the effects of hypoxia and pathological effects of alcohol in the hepatocytes from EtOH-exposed animals[6, 9, 10, 32].
In support of this concept, we and others have demonstrated that EtOH-dependent hepatotoxicity is suppressed in iNOS−/− animals[10, 12]. These data suggest an important link between increased ˙NO formation from iNOS during chronic EtOH intoxication, enhanced sensitivity of mitochondrial respiration to ˙NO, and hypoxia. The adaptive response to hypoxia is orchestrated through hypoxia-inducible factor-1 (HIF-1), [33]. The effects of NO and iNOS on hypoxia and cellular bioenergetics were tested in a model of chronic EtOH-induced hepatotoxicity using hepatocytes isolated from Sprague-Dawley rats and studies examining effects in liver tissue from C57BL/6 and iNOS−/− mice.
2. Materials and Methods
2.1. Reagents and antibodies
All chemicals were purchased from Sigma-Aldrich (St.-Louis, MO) unless stated otherwise and were of the highest grade available.
2.2. Alcohol Feeding
Male Sprague-Dawley rats or wild type (C57BL/6) and iNOS−/− (B6.129P2-NOS2 tm/lau) mice were pair-fed an isocaloric liquid diet, with and without ethanol (4% for mice and 5% for rats), for 5–6 weeks as described previously[12, 22]. EtOH consumption was uniform throughout the study between wild type and iNOS−/− mice (data not shown). Animals were handled in accordance to “The Guide for the Care and Use of Laboratory Animals” approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
2.3. Hepatocyte preparations
Primary rat hepatocytes were isolated as previously described [23]. The viability of hepatocytes at isolation was 93±1% and was not different between control and EtOH-fed rats. In initial experiments we found that the efficiency of adherence for the ethanol treated cells was variable and compensated for this difference by seeding with a higher number of cells than the control. However, this resulted in experimental variation in the basal O2 consumption between hepatocyte preparations. Where feasible, this was corrected for by measurement of protein levels in the wells. To allow comparison of the data between conditions, changes are therefore expressed as a % of the basal OCR in each case and the range of OCR consumption in the individual experiments reported in the text.
2.4. Immunoblot analysis
Briefly, hepatocyte homogenates (20 µg) were separated using 12.5% SDS-PAGE followed by immunoblotting onto nitrocellulose. Antibody dilutions were 1/3000 for CYP2E1 (Millipore, Billerica, MA), 1/5000 for porin/VDAC (Invitrogen Carlsbad, CA), and 1/2000 for cytochrome c oxidase subunit IV (CcOX-IV) (Invitrogen Carlsbad, CA) in Tris-buffered saline containing 0.05% Tween-20 followed by HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (GE Healthcare, Piscataway, NJ). The efficiency of protein loading and protein transfer was monitored using Ponceau S staining which was then used for normalization. The intensities of protein bands were quantified prior to image saturation using AlphaEaseFC software (Alpha Innotech, Santa Clara, CA).
2.5. Mitochondrial Bioenergetics and enzyme activities
Citrate synthase and CcOX activities were measured as previously described [13, 22]. Citrate synthase activities are expressed in units of enzyme activity, where 1 unit = 1 µmol thionitrobenzoate generated/min. CcOX activities are expressed in k/sec, where k is the first order rate constant for the oxidation of cytochrome c. An XF24 analyzer (Seahorse Bioscience, Billerica, MA) was used to measure hepatocyte O2 consumption[29]. Hepatocytes from control and EtOH-fed rats were adhered to collagen-coated V7 plates (Seahorse Bioscience) for 24 hr. The seeding density was optimized for both control and EtOH-fed rat hepatocytes (Supplemental Figure 1), with 20,000 and 40,000 cells/well chosen for control and EtOH groups, respectively. XF24 assays were performed in unbuffered DMEM (pH 7.4) supplemented with 5.5 mM d-glucose, 1 mM sodium pyruvate, and 4 mM l-glutamine. Cellular mitochondrial function was measured as described previously using sequential injections of oligomycin (1 µg/mL), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP, 0.3 µM), and antimycin A (10 µM) plus rotenone (1 µM)[29]. The concentrations used were determined by titrating to yield their optimal effects (data not shown).
2.6. Hypoxia exposure
To measure the effect of changing O2 tension on hepatocyte respiration, an XF24 analyzer was placed in a sealed glove box (Plas-Labs, Lansing, MI) equilibrated to 1% O2 (11.5 µM O2) via repeated cycles of vacuuming the chamber and refilling it with argon. The OCR of hepatocytes was then measured over time as the O2 tension of the media decreased during equilibration from room air to 1% O2.
2.7. Immunohistochemistry
After EtOH treatment, mice were administered pimonidazole (120 mg/kg) in saline (1 mL/kg) via tail vein injection and then anesthetized after 60 min with ketamine:xylazine (60:10 mg/kg i.p)[34]. Livers were harvested, fixed in 10% buffered formalin and paraffin-embedded. Five micron sections were deparaffinized with xylene and rehydrated with a graded series of EtOH washes. After briefly treating with 0.01% protease (pronase E) and blocking with serum-free protein block, sections were incubated with mouse anti-pimonidazole antibody (1:50) for 40 min at room temperature. Sections were then blocked again for 10 min with 5% BSA in PBS, washed, and incubated with Alexa Fluor® 350-conjugated anti-mouse secondary (Invitrogen). Nuclei were counterstained with Oregon Green® 488-conjugated anti-rabbit antibody (Invitrogen). Images were acquired using a Leica fluorescent microscope with IPLAB Spectrum (Scanylytics, CA). The intensity of fluorescence was quantified by using SIMPLEPCI software (Compix, Cranberry Township, PA). HIF-1α levels were also assessed using formalin-fixed sections. Antigen unmasking was performed by incubating with 0.1 M sodium citrate (pH 6.0). Sections were washed, incubated for 1 hr with 10% goat serum, followed by overnight incubation at 4°C with anti-HIF-1α antibody (1:50, Novus Biologicals, Littleton, CO). Sections were then blocked and developed with secondary antibody as described above.
2.8. Nitric Oxide Measurement
The initial rates of NO production from DetaNONOate were measured polarographically after addition to the media used for the bioenergetics experiments as described in [35] and shown in Supplemental Figure 3. Since NO reacts with oxygen, the initial rate was selected to estimate the levels of NO being produced since this is essentially insensitive to oxygen in the media.
2.9. Statistics
Data are expressed as mean ± SEM. Experiments were performed using primary hepatocytes or liver tissue isolated from 6 pairs of control and EtOH-fed rats or wild type and iNOS−/− mice, respectively. Statistical significance was determined using Student’s t-test, with statistical significance set at p<0.05.
3. Results
3.1. Indices of chronic EtOH consumption in isolated hepatocytes
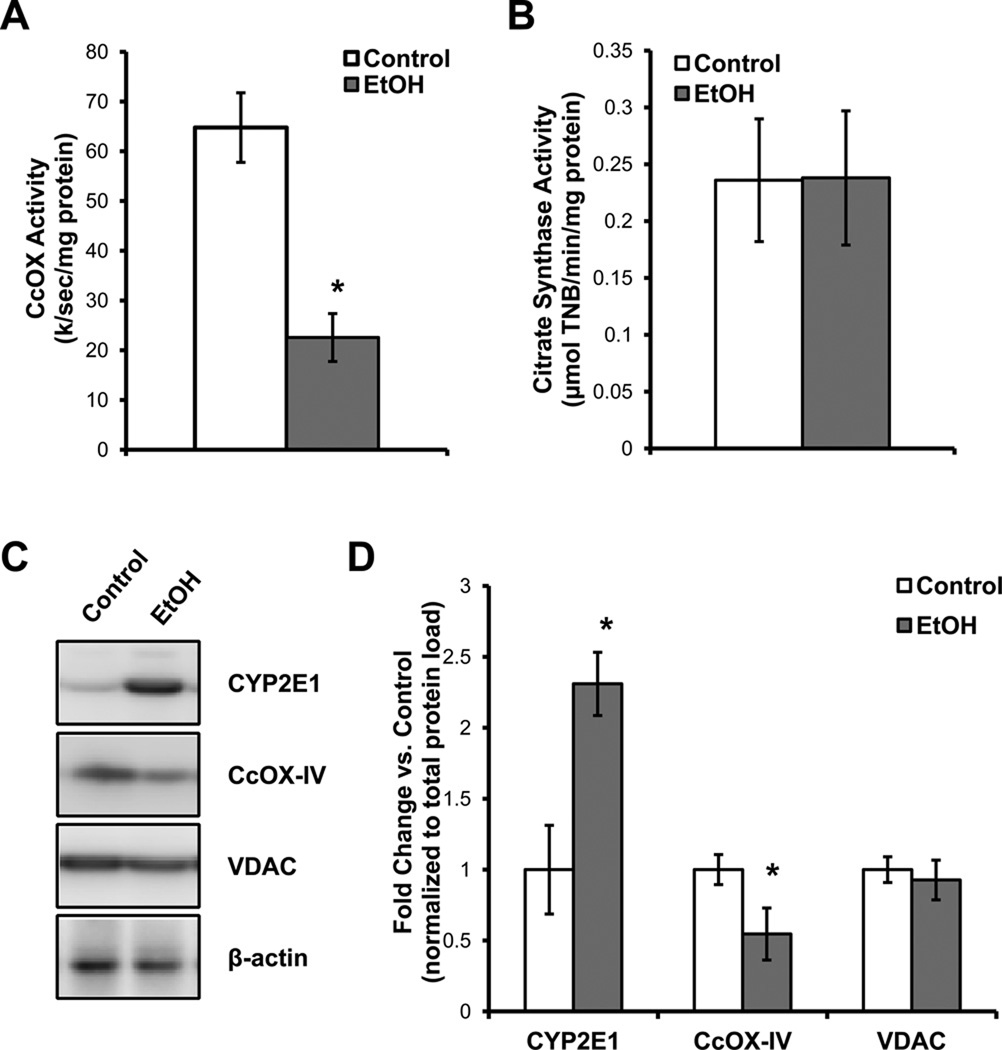
The activity and levels of key enzymes were determined from hepatocytes isolated from control and EtOH-fed rats. As reported previously for total liver homogenates and isolated mitochondria, EtOH consumption causes a decrease in the activity of cytochrome c oxidase (CcOX) and protein subunits whereas the levels of citrate synthase are unchanged (Figure 1A,B,C). Protein levels of the outer mitochondrial membrane protein VDAC were not changed by EtOH consumption (Figure 1C,D). Liver cytochrome P450 2E1 (CYP2E1) protein levels increased in response to chronic EtOH consumption, as expected (Figure 1C,D).
Figure 1. Effect of alcohol (EtOH) consumption on mitochondrial protein levels and activity.
(A) Cytochrome c oxidase (CcOX) activity in isolated hepatocytes from control and EtOH-fed rats. The activity is expressed as the first order rate constant for the oxidation of reduced cytochrome c (B) Citrate synthase activity in isolated hepatocytes from control and EtOH-fed rats and the activity is expressed as the formation of thionitrobenzoate (TNB). (C) Protein levels of cytochrome P450 2E1 (CYP2E1), cytochrome c oxidase subunit IV (CcOX-IV), voltage-dependent anion channel (VDAC), and β-actin from primary hepatocytes isolated from control and EtOH-fed rats, along with the quantification of the densitometry for the different proteins normalized to total protein and expressed as the fold change vs. control (D). Data are mean ± SEM. n=6 for each group. *p≤0.05 compared to control.
3.2. Primary hepatocytes show altered cellular bioenergetics in response to chronic EtOH exposure
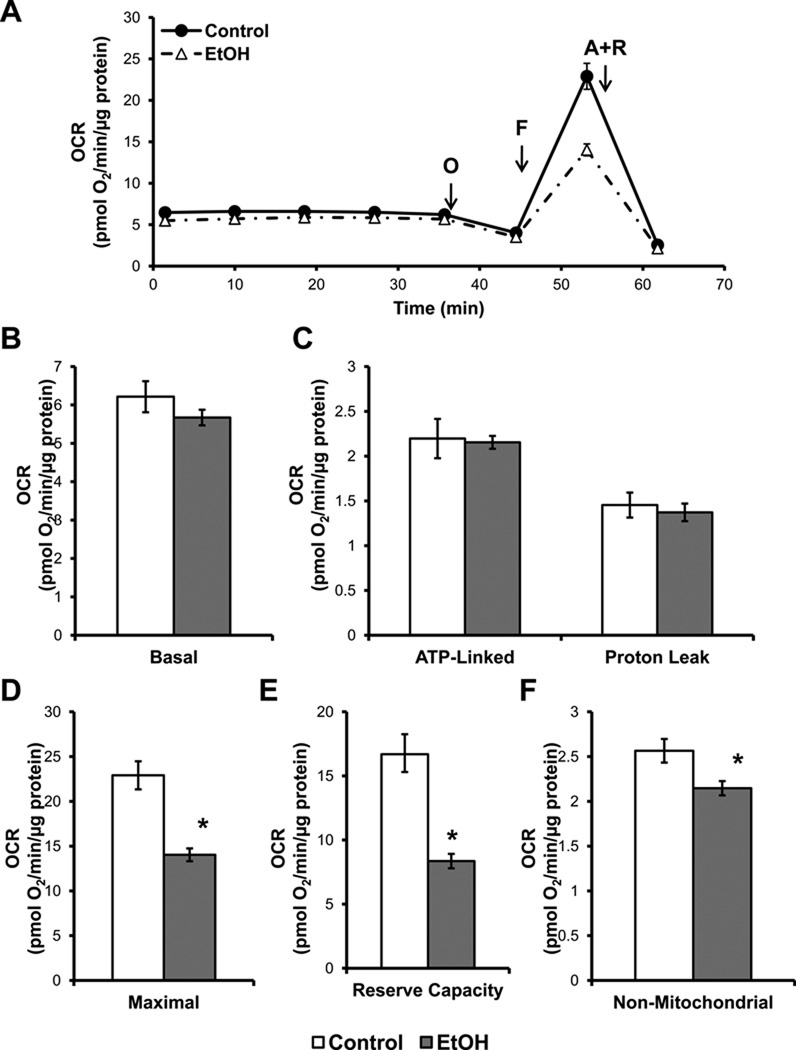
In order to determine if chronic EtOH consumption causes alterations in hepatocyte bioenergetics, mitochondrial function was measured using the XF24 analyzer. Chronic EtOH consumption had no effect on the basal OCR, ATP-linked OCR, or proton leak of hepatocytes (Figure 2A,B,C). The proton ionophore FCCP (0.3 µM) was then injected to stimulate maximal OCR in the cells. Interestingly, hepatocytes isolated from EtOH-fed rats exhibited a significantly diminished maximal OCR (Figure 2D). The difference between the maximal and basal OCR represents the amount of mitochondrial function available to hepatocytes for use under conditions of increased energy demand and/or stress and is termed the cellular bioenergetic reserve capacity[29]. Hepatocytes from EtOH-fed rats exhibited a 50% decrease in their available reserve capacity as compared to the controls (Figure 2E). Hepatocytes were then exposed to antimycin A (10 µM) and rotenone (1 µM) to fully inhibit the mitochondrial electron transport chain. This caused a substantial decrease in the OCR of hepatocytes, with all remaining O2 consumption attributed to non-mitochondrial sources. Hepatocytes isolated from EtOH-fed rats displayed a small, but statistically significant, decrease in non-mitochondrial OCR (Figure 2F). From these data we can estimate the relative turnover of the mitochondrial respiratory chain by assuming that the OCR in the presence of oligomycin represents State 4 respiration (limited by availability of ADP) and the rate after FCCP an estimate of State 3 respiration (unconstrained by ADP). This value we have termed state apparent [29] and is 3.8 ± 0.02 (mean ± S.E.M, n=5) in control hepatocytes, which is very close to 4 (equivalent to State 4 in isolated mitochondria), and decreases to 3.5 ± 0.01 (mean ± S.E.M., n= 3, p≤0.001) in hepatocytes isolated from rats after chronic ethanol consumption.
Figure 2. Chronic alcohol (EtOH) consumption decreases mitochondrial respiratory function in hepatocytes.
Primary hepatocytes were isolated from rats fed control and EtOH-containing diets for 6 wk and were plated at 20,000 cells/well (20.1 ± 0.4 µg protein) for control and 40,000 cells/well for EtOH-exposed hepatocytes (21.0 ± 0.3 µg protein). Hepatocytes were allowed to attach overnight prior to oxygen consumption rate (OCR) measurements. (A) OCR traces from control and EtOH-fed hepatocytes with serial injections of oligomycin (O, 1 µg/mL), FCCP (F, 0.3 µM), and antimycin A plus rotenone (A+R, 10 µM and 1 µM, respectively) to determine parameters of mitochondrial function. (B) Basal OCR of hepatocytes is measured prior to injection. (C) ATP-linked respiration was calculated from the decrease in OCR following oligomycin injection, with the remainder being attributed to proton leak (D). (E) Maximal OCR was measured following FCCP injection. (F) The reserve capacity was calculated from the difference between the maximal and basal OCR and represents the spare respiratory capacity available to the hepatocytes for use under increased energy demand or stress. (G) The non-mitochondrial OCR of hepatocytes was determined by injecting antimycin A and rotenone simultaneously to fully inhibit the mitochondrial electron transport chain, thereby all remaining O2 consumption is due to extra-CcOX sources. Results are mean ± SEM. n=5 per group. *p<0.05 compared to control.
3.3. Chronic EtOH exposure increases the sensitivity of hepatocytes to ˙NO-mediated inhibition of respiration
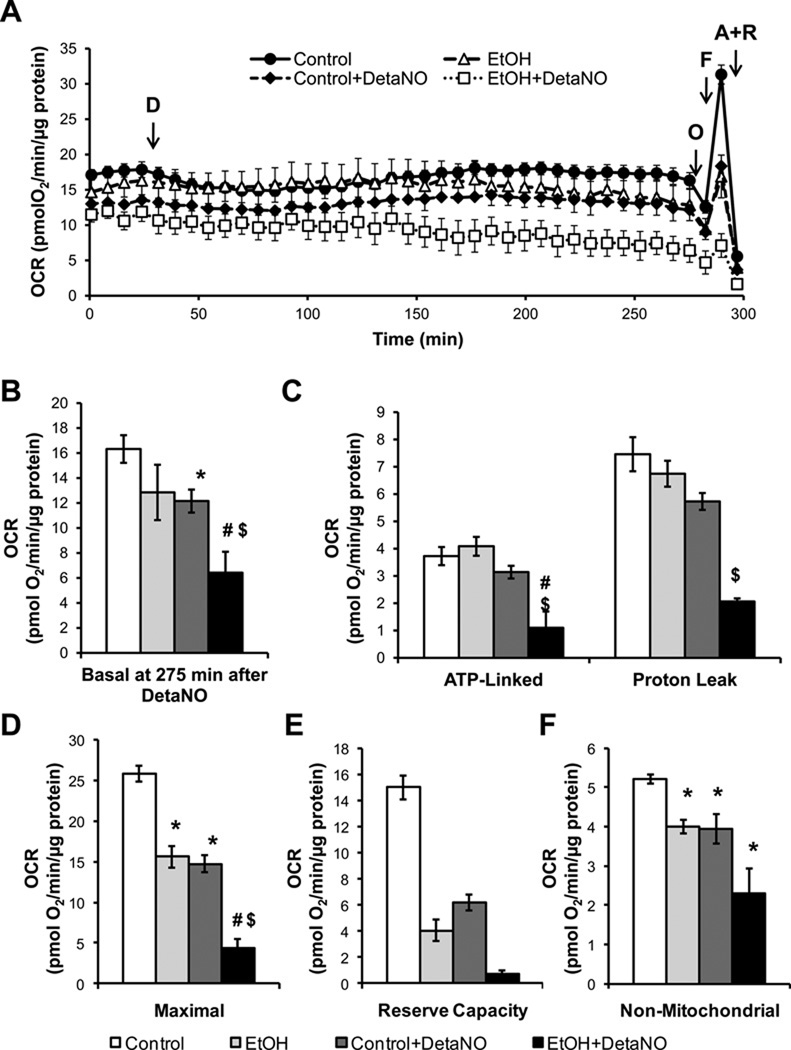
We and others have previously shown that isolated mitochondria in State 3 respiration are substantially more sensitive to inhibition by ˙NO than those in State 4 and that liver mitochondria isolated after chronic ethanol consumption are more sensitive to inhibition of respiration by ˙NO [16, 36, 37]. The effect of ˙NO on mitochondrial respiratory function of primary hepatocytes isolated from control and EtOH-fed rats was then assessed using the ˙NO donor DetaNONOate (DetaNO). Under these conditions the rate of ˙NO release was determined using an ˙NO electrode and found to be dependent on the concentration of DetaNONOate and ranged from 1.8 ± 0.4- 6.1 ± 0.06 nM/sec (supplementary Figure 3). This is similar to the values (approximately 3.5 nM/sec) reported for iNOS-dependent ˙NO release in Kupffer cells which are a major source of NO in the liver [38–40]. Concentrations of ˙NO metabolites in liver and plasma are elevated in the µM range after alcohol consumption and have been reported to be as high as 2.5 µM [11, 41]. As shown in Figure 3A, hepatocytes from control and EtOH-fed rats were exposed to 500 µM DetaNO (4.3 ± 0.6 ˙NO nM/sec) for 4 hr while monitoring the OCR, followed by the evaluation of mitochondrial function. As shown in Figure 3, EtOH consumption did not change basal OCR (Figure 3B) but decreased the maximal respiration (Figure 3D). However, hepatocytes from EtOH-fed rats treated with DetaNO exhibited a progressive decrease in the basal OCR after approximately 2 hr exposure to the ˙NO donor, resulting in a 40% inhibition after 4 hr (Figure 3A,B). Following oligomycin injection, the EtOH-exposed hepatocytes treated with DetaNO also showed a significant decrease in ATP-linked OCR and proton leak (Figure 3C), while EtOH consumption alone had no effect. The maximal OCR and reserve capacity of primary hepatocytes isolated from control and EtOH-fed rats was assessed using FCCP, and was severely suppressed by ˙NO in the EtOH treated animals (Figure 3D,E). Chronic EtOH consumption again resulted in a slight decrease in non-mitochondrial OCR (Figure 3F) which was insensitive to the addition of DetaNO. Viability of the hepatocytes was assessed following the experiments and was found to be unaltered by DetaNO treatment (data not shown).
Figure 3. Chronic alcohol (EtOH) consumption sensitizes hepatocytes to nitric oxide (˙NO)-induced mitochondrial dysfunction.
(A) The effect of ˙NO on respiration in hepatocytes isolated from control and alcohol (EtOH)-fed rats was determined by treating cells with DetaNONOate (D, 500 µM) for 4 hr followed by serial injections of oligomycin (O), FCCP (F), and antimycin A plus rotenone (A+R) to measure parameters of mitochondrial function. (B) Basal OCR (275 min post DetaNO) of hepatocytes is measured prior to oligomycin injection. (C) ATP-linked respiration is ascribed to the oligomycin-induced decrease in OCR and the remaining OCR following oligomycin injection is ascribed to proton leak. (D) Maximal OCR was measured following FCCP injection. (E) The reserve capacity was calculated from the difference between the maximal and basal OCR. (F) The non-mitochondrial OCR was determined by injecting antimycin A and rotenone simultaneously to fully inhibit the electron transport chain. Results expressed as percent of baseline (measurement prior to DetaNONOate or vehicle addition) and are mean ± SEM. Basal OCR values are 148 ± 3 pmol O2/min for control group and 118 ± 5pmol O2/min for the EtOH group. n=5 per group. *p<0.05 compared to Control. #p<0.05 compared to EtOH. $p<0.05 compared to Control + DetaNO.
3.4. Alterations in hepatocyte respiration in response to hypoxia and ˙NO are exacerbated by chronic EtOH consumption
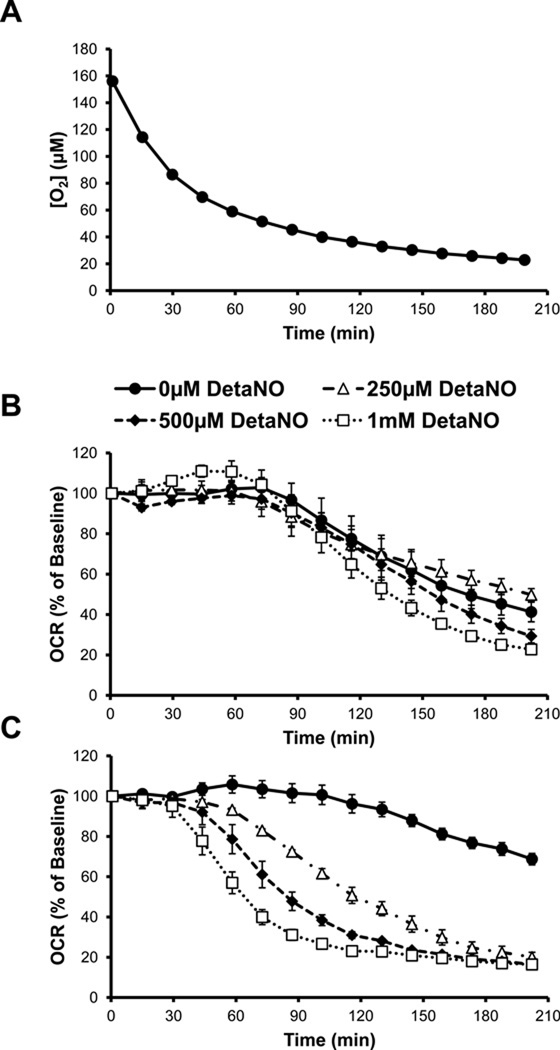
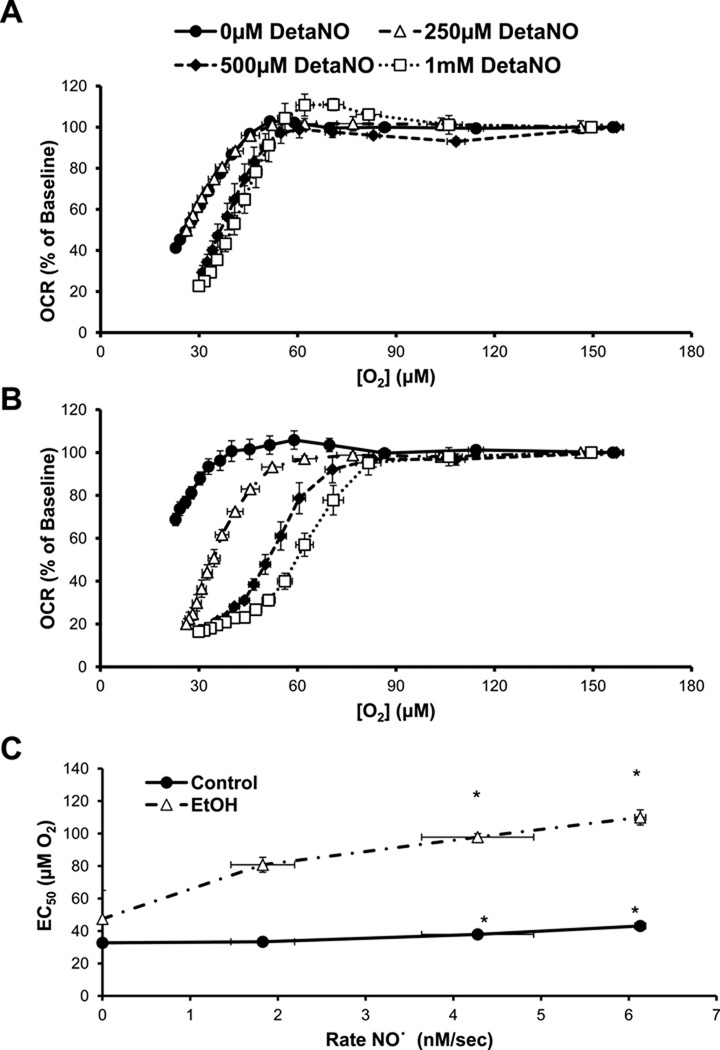
The effect of hypoxia on the mitochondrial bioenergetics of primary hepatocytes was also determined using the XF24 analyzer. To achieve this, cells were plated with media equilibrated to room air and then placed into an XF24 analyzer positioned in a sealed chamber with an atmosphere of 1% O2. The O2 levels in the cell culture plate were then allowed to reach equilibrium with the atmosphere in the hypoxia chamber. The media O2 concentration was measured every 8 min in the individual wells containing cells and decreased exponentially over 2 – 3 hrs as the media reached equilibrium with the atmosphere in the hypoxia chamber (Figure 4A). After approximately 160 min, the rate of change in O2 concentration was slower resulting in minimal level of approximately 20 µM for the course of the experiment. Over the same time course, the basal OCR of control hepatocytes was measured and remained unchanged for the first 60 min corresponding to an O2 concentration of approximately 60 µM, after which it began to decrease progressively (Figure 4B). In contrast, hepatocytes from EtOH-fed animals showed less dependence on changing O2 concentrations and a higher rate of oxygen consumption compared to controls at all oxygen tensions below 40 µM (Figure 4C). To determine the effect of ˙NO on the OCR of control hepatocytes in hypoxia, 250 µM – 1 mM DetaNO was added to the cells immediately prior to beginning the XF assay. Control hepatocytes were essentially resistant to exposure to ˙NO under hypoxic conditions, with only the highest levels of DetaNO (500 µM – 1 mM) modestly decreasing OCR below untreated control hepatocyte levels after 2 hr. In contrast, hepatocytes isolated from EtOH-fed rats were much more sensitive to ˙NO-induced inhibition of OCR at lower O2 tensions than the control hepatocytes.
Figure 4. Alcohol (EtOH) increases hepatocyte susceptibility to nitric oxide (˙NO)-induced inhibition of respiration during hypoxia.
(A) Changes in O2 tension in the room air-equilibrated media above attached hepatocytes over time following exposure to 1% O2 atmosphere. The effect of 0–1000 µM DetaNONOate (DetaNO) added immediately prior to the start of the assay on oxygen consumption rate (OCR) of hepatocytes from control rats (B) and EtOH-fed rats (C) over time as O2 decreases as seen in (A). Results are mean ± SEM. n=5 per group. In panel B, all points in the 250 µM, 500 µM, and 1 mM DetaNO curves were significantly different from vehicle-treated from 188 min, 173 min, and 130 min, respectively. In panel C, all points in the 250 µM and 500 µM DetaNO curves were significantly different from vehicle-treated from 58 min and all points in the 1 mM DetaNO curve were significantly different from vehicle treated from 44 min. Results expressed as percent of baseline (measurement prior to DetaNO or vehicle addition) and are mean ± SEM. Basal OCR values are 153 ± 9 pmol O2/min for controls and 109 ± 9 pmol O2/min for the EtOH group. n=5 per group.
Changes in OCR in control and EtOH hepatocytes were then plotted as a function of the O2 measured in the media at the indicated time points. As shown in Figure 5A, ˙NO treatment caused a right shift of the O2 dependency curve in control cells and was independent of the rate of NO release. As shown in Figure 5B hepatocytes isolated from EtOH-fed animals showed a dose-dependent shift of OCR inhibition by DetaNO under low O2 tensions. While increasing concentrations of DetaNO caused a slight increase in the EC50 for the dependence of OCR on O2 in control hepatocytes, the EC50 of EtOH-fed hepatocytes was significantly higher in response to increasing concentrations of ˙NO released from DetaNO (Figure 5C).
Figure 5. Chronic alcohol (EtOH) consumption alters hepatocyte response in OCR to decreasing O2 tension and nitric oxide (˙NO).
(A) The change in oxygen consumption rate (OCR) of control hepatocytes treated with DetaNONOate (DetaNO, 0–1000µM) was plotted as a function of the decreasing O2 tension of the media, as seen in Figure 4B. (B) The change in OCR of hepatocytes isolated from EtOH-fed rats pretreated with 0–1000 µM DetaNO is plotted as a function of the O2 tension of the media as it becomes hypoxic, as seen in Figure 4C. (C) The EC50 of the curves from panels A and B were calculated by fitting the data to a sigmoidal curve. Results are mean ± SEM. n=5 per group. *p<0.05 compared to respective vehicle-treated group.
3.5. EtOH-induced hypoxia in the liver is iNOS-dependent
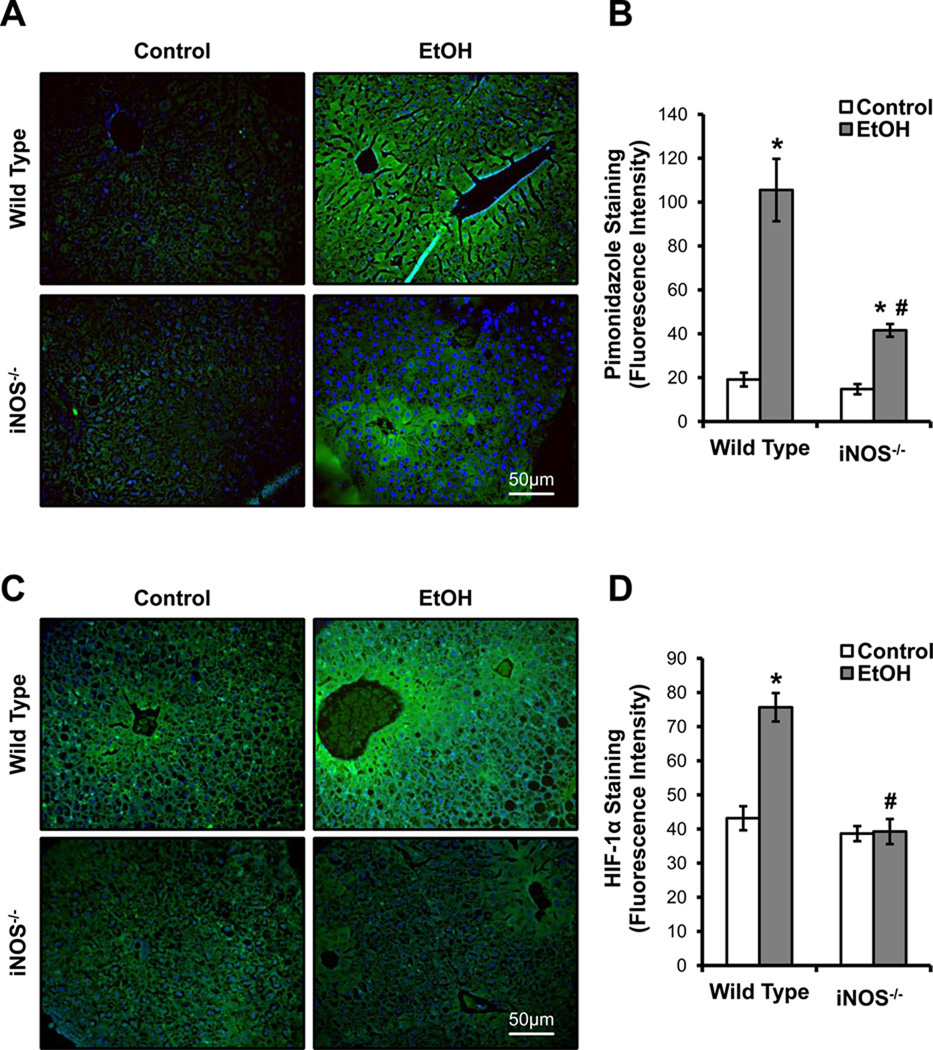
Because ˙NO inhibits O2 consumption in primary hepatocytes isolated from EtOH-fed rats, we determined the effect of endogenous ˙NO production on chronic EtOH-mediated hypoxia. In order to manipulate ˙NO levels in vivo, we fed wild-type C57BL/6 and iNOS−/− mice on a C57BL/6 background control and EtOH-containing diets[12]. Using the hypoxia marker pimonidazole, we used immunohistochemistry to visualize the in vivo O2 gradients in liver sections isolated from control and EtOH-fed wild type and iNOS−/− mice.
In normal, healthy liver, the most hypoxic zone is zone 3, the peri-central zone. A significant increase in pimonidazole binding was seen in the zone 3 region in liver of EtOH-fed wild-type mice, which extended into the mid-zonal and peri-portal regions compared to controls (Figure 6A). These data are indicative of the development of peri-portal and peri-central hypoxia due to chronic EtOH consumption. In contrast, livers from EtOH-fed iNOS−/− mice exhibited a significant decrease in pimonidazole binding (i.e., less zonal hypoxia) in the peri-portal regions, and when staining was present it was localized in the peri-central region of the liver lobule (Figure 6A). Livers from control-fed wild type and iNOS−/− mice showed minimal pimonidazole staining, indicating that there is a lower level of hypoxia in the absence of chronic EtOH exposure. Sections incubated with pre-immune sera or without the primary antibody for pimonidazole adducts showed less background staining as seen in control livers (data not shown). Quantitative analysis of images showed a 5.5 fold increase in pimonidazole staining in wild-type mice on the EtOH diet compared to their pair-fed controls, whereas the lack of iNOS expression attenuated the EtOH-dependent pimonidazole staining by 60% as compared to wild-type animal consuming EtOH (Figure 6B).
Figure 6. Inducible nitric oxide synthase (iNOS)-derived nitric oxide (˙NO) is required for chronic alcohol-induced liver hypoxia.
(A) Pimonidazole staining of formalin fixed liver sections from wild type and iNOS−/− mice with and without alcohol (EtOH) feeding was performed to assess liver hypoxia. (B) Quantification of the pimonidazole staining intensity from (A). Fluorescence microscopy was used to detect HIF-1α stabilization in liver sections from control and alcohol (EtOH)-fed wild type and iNOS−/− mice (C). The quantification of the immunofluorescence is shown in (D). Images are representative from each group and quantification results are mean ± SEM. n=6 per group. *p<0.05 compared to respective controls. #p<0.05 compared to EtOH-fed wild type mice.
Given that HIF-1α stabilization has also been shown to be modulated by ˙NO [42], we used immunohistochemistry to examine HIF-1α in liver from iNOS−/− and wild-type mice fed the control and EtOH diets. Consistent with pimonidazole staining, there was increased HIF-1α staining in the liver peri-central region from EtOH-fed wild-type mice as compared to pair-fed controls (Figure 6C). Interestingly, there was a significant decrease in HIF-1α staining in the liver peri-central region from EtOH-fed iNOS−/− mice as compared to EtOH-fed wild-type mice (Figure 6C). These results implicate a role of iNOS in the response to chronic EtOH consumption and the development of tissue hypoxia in vivo. Both controls had negligible background staining. Figure 6D depicts the quantitative analysis of HIF-1α levels in liver of all treatment groups.
EtOH is metabolized predominantly by the enzymes CYP2E1 and alcohol dehydrogenase; acetaldehyde, the main product of EtOH metabolism, is then metabolized by aldehyde dehydrogenase 2 (ALDH2) [43, 44]. We therefore examined the effect of chronic EtOH consumption on CYP2E1 and ALDH2 expression in iNOS−/−and wild-type mice to rule out any potential alterations in the expression of these enzymes. CYP2E1 expression was significantly increased after EtOH feeding in wild-type and iNOS−/− mice (Supplemental Figure 2A). ALDH2 protein was equal between control and EtOH groups and genotypes (Supplemental Figure 2B). As expected the expression of these proteins did not change in the iNOS−/− animals consuming ethanol, confirming that nitric oxide does not play a role in the metabolism of ethanol.
4. Discussion
Mitochondrial dysfunction is an important feature of chronic EtOH-induced hepatotoxicity and is associated with changes in the mitochondrial proteome and damage to mitochondrial DNA [22, 45]. These changes result in decreased mitochondrial ATP production which, when combined with an EtOH-dependent decrease in glycogen levels and utilization, lead to compromised liver bioenergetics [23–25]. While chronic EtOH consumption causes mitochondrial dysfunction[46, 47], the effects of EtOH toxicity on different parameters of mitochondrial function and the impact of ˙NO on mitochondrial function within intact hepatocytes remain unclear. Previously, we have shown that after chronic ethanol consumption respiration in isolated mitochondria is more sensitive to ˙NO-dependent inhibition [15]. Interestingly, this effect is likely a consequence of the long term exposure of mitochondria in vivo to ROS/RNS, particularly peroxynitrite, since increased mitochondrial sensitivity to ˙NO is absent in iNOS −/− mice after chronic alcohol consumption (23).
In the first series of experiments we demonstrated that the hepatocyte isolation procedure used did not preferentially select for a healthy population of cells because hepatocytes from ethanol-fed rats exhibited decreased mitochondrial function and induction of CYP2E1. In both cases, the decrease in CcOX and increased levels of CYP2E1 are essentially identical to those found in liver homogenates from ethanol-fed rats. In the current study, we found basal mitochondria respiration was unaffected by chronic EtOH consumption; however, hepatocytes from EtOH-fed rats exhibited a significant decrease in maximal OCR and reserve capacity in air-equilibrated buffer (Figure 2E,F). The fact that the basal OCR is not affected by EtOH consumption suggests that in the absence of an additional stressor such as ˙NO, the bioenergetic needs of the cell can be met, even though the mitochondria are in a more activated respiratory state with a state apparent much closer to State 3. It is now clear that in a cellular context, mitochondria function at an intermediate respiratory state which is below the maximal capacity of respiratory chain and in control hepatocytes this is close to State 4 [28, 29]. This bioenergetic reserve capacity can be used for any energetic process that makes demands upon the cell including protection against oxidative stress. We have shown that if reserve capacity is diminished then cells become more susceptible to toxicity mediated by RNS or toxic lipids such as 4-hydroxynonenal [28, 29]. Importantly, we and others have shown that mitochondria in the State 3 respiratory condition are more sensitive to ˙NO-dependent inhibition of respiration[16, 36, 37, 48]. Because chronic EtOH consumption is associated with increased ROS/RNS from different sources within the cell, this finding leads to the hypothesis that hepatocytes from EtOH-fed animals are less tolerant to the secondary stress of increased ˙NO generated by the activated Kupfer cells in the liver.
Importantly, increased iNOS leads to the increased production of ˙NO, which can inhibit mitochondrial respiration reversibly at CcOX and irreversibly once combined with superoxide to form peroxynitrite. This generation of peroxynitrite leads to post-translational modifications of other respiratory complex proteins, which can inhibit function and/or respiration [32, 49, 50]. We found that the inhibition of mitochondrial function by chronic EtOH consumption was further exacerbated by exposure to ˙NO (Figure 3). The combination of chronic EtOH and ˙NO decreased the total available mitochondrial function to the point that there was only negligible reserve capacity remaining (Figure 3E,F). Moreover, the basal OCR was also decreased significantly below the unstressed hepatocytes or those which were stressed with either ˙NO or chronic EtOH exposure alone (Figure 3B). We have previously reported the loss of reserve capacity after acute exposure of cells to ˙NO in endothelial cells and demonstrated that this is reversible and due to the inhibition at CcOX [29]. In the present study we did not test the reversibility of the loss of reserve capacity and cannot rule out an affect of peroxynitrite in irreversibly modifying the respiratory chain. With this said, these data supports the concept that the reserve capacity may indeed serve as a protective buffer of available mitochondrial function, enabling the cells to maintain the bioenergetic function necessary to maintain overall cellular function even after being exposed to acute stressors.
This loss of reserve capacity in EtOH-exposed hepatocytes upon treatment with ˙NO becomes even more important when hepatocytes are subjected to low O2 tensions, (Figure 4). Chronic EtOH consumption is known to cause liver hypoxia, particularly in zone 3 of the liver [51]. Importantly, the rate of oxygen consumption was sustained at near maximal basal rates in the hepatocytes from the EtOH-treated animals compared to control (Figure 4). This result would be consistent with increased hypoxia in response to EtOH consumption due to increased mitochondrial respiration. The mechanisms which contribute to this differential regulation of respiration in the EtOH treated animals are consistent with a decreased level of CcOX and the increased reductive stress which occurs as a consequence of alcohol consumption.
We hypothesized that since isolated mitochondria from chronic ethanol-fed rats are more sensitive to inhibition by ˙NO and that in the hepatocytes the mitochondria are closer to a State 3 condition, basal respiration will be more sensitive to ˙NO. While control hepatocytes were essentially resistant to the effects of ˙NO under decreasing O2 tensions (Figure 5A), the EtOH-exposed hepatocytes exhibited a significant inhibition of mitochondrial respiration by ˙NO in hypoxia (Figure 5B). This is consistent with the finding that hepatocytes isolated from EtOH-fed animals have less reserve capacity and respire closer to State 3 than those from control animals (Figure 2), which is then further diminished by exposure to ˙NO and enhanced at lower O2 tensions (Figure 3).
Cells respond to low O2 availability by initiating a series of adaptive responses through transcriptional activation and stabilization of HIF-1α [52]. Accumulation of HIF-1α is an important step in the activation of HIF-1 during hypoxia. Regulation of HIF-1α by ˙NO is an additional mechanism by which ˙NO might modulate cellular responses to hypoxia[3]. The modulation of the hypoxic response by ˙NO is believed to have wide pathophysiological significance [53]. The susceptibility of the peri-central region of the liver to low O2 tensions is predominantly due to an O2 gradient between the peri-portal and peri-central regions in vivo [54]. It is well established that EtOH consumption causes hypoxia and increased expression of iNOS in zone 3 of the liver lobule in vivo [12, 34, 55], and here we have shown that ˙NO inhibits mitochondrial function in EtOH-exposed hepatocytes. We therefore used iNOS−/− mice to test the role of ˙NO in the formation of hypoxia in the liver following chronic EtOH consumption. In agreement with earlier studies, chronic EtOH consumption significantly increased the binding of the hypoxia marker pimonidazole in liver tissue, predominantly in the O2-poor (zone 3) region of the liver lobule (Figure 6A,B) [9, 34, 55, 56]. In addition, we demonstrate that chronic EtOH consumption can lead to increased HIF-1α levels in the O2 deprived region of the liver lobule (Figure 6C,D). Interestingly, absence of iNOS prevented the accumulation of HIF-1α and pimonidazole staining in mice on the EtOH diet (Figure 6). In addition, we and others have also demonstrated that genetic ablation of iNOS attenuates EtOH-dependent hepatotoxicity [10, 12].
5. Conclusions
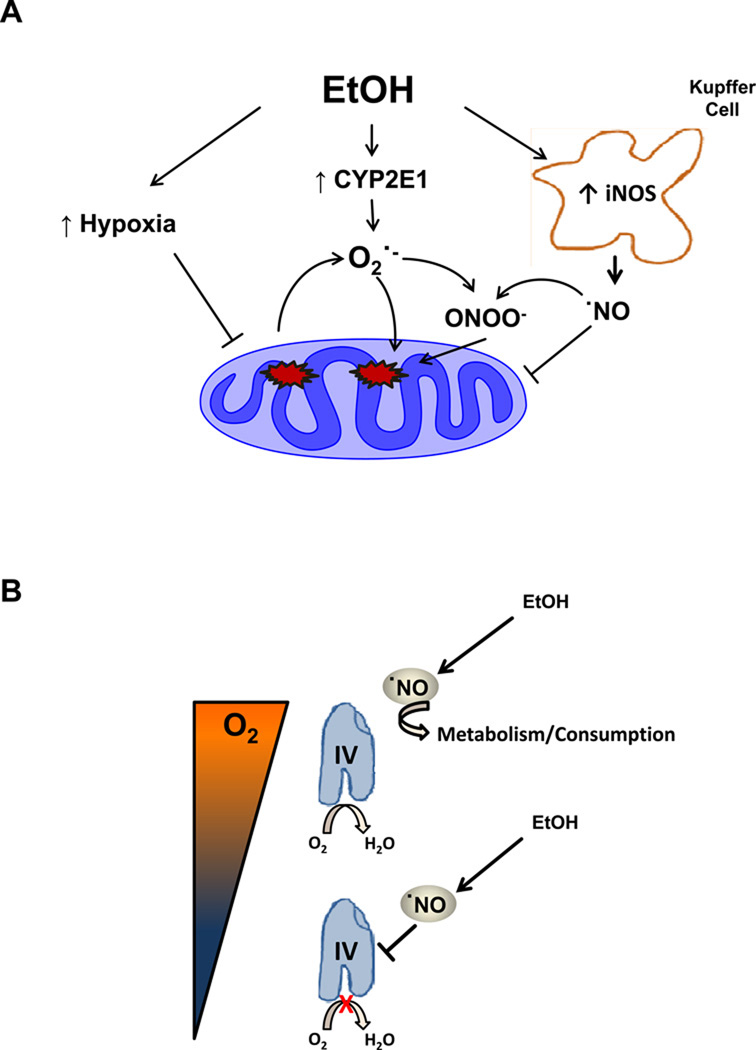
Taken together, these data provide evidence for the role of ˙NO as an important regulator of mitochondrial respiration at CcOX in EtOH-induced hepatotoxicity which is shown in the context of current concepts of alcohol-dependent hepatotoxicity in Figure 7. The microsomal ethanol oxidizing system, specifically, CYP2E1 is responsible, in part, for the oxidation of ethanol. Alcohol-dependent increases in CYP2E1 or NADPH oxidase have been shown to lead to oxidative stress from the production of ethanol derived radicals, O2·− and H2O2 [57–59]. The activation of resident macrophages or Kupffer cells are a result of gut derived endotoxin release from ethanol exposure causing an increase in iNOS expression and activity[10, 58]. The introduction of both ˙NO from iNOS and superoxide from several sources in this environment damages the mitochondrial respiratory chain and so contributes to hypoxia. In the present study we have shown that the addition of ˙NO to hepatocytes isolated from EtOH-fed rats exhibit diminished basal and ATP-linked OCR, increased proton leak, and decreased reserve capacity. We have also shown that under conditions of hypoxic stress, the addition of ˙NO to EtOH hepatocytes resulted in increased inhibition of respiration. Taken together these data integrate changes in mitochondrial bioenergetics that previously were only observed in isolated mitochondria with the pathological changes that occur in ethanol-dependent hepatotoxicity (Figure 7).
Figure 7. Chronic alcohol consumption induces mitochondrial dysfunction mediated by nitric oxide and hypoxia.
(A) Ethanol consumption causes increased cytochrome P450 2E1 (CYP2E1), NADPH oxidase (NOX) and inducible nitric oxide synthase (iNOS) via kupffer cell activation as well increased oxygen (O2) consumption thereby decreasing the O2 gradient within the liver leading to hypoxic regions, specifically the central lobular region. CYP2E1 and hypoxia causes increases in ROS production such as superoxide (O2·−) which inhibits mitochondrial respiration directly and through the production of peroxynitrite (ONOO−). Hypoxic signaling has been reported to be increased by ONOO- through mechanisms which are still unclear (B) At higher O2 tensions, in normal liver, the lower basal activity of CcOX results in less inhibition and ˙NO is metabolized. In response to ethanol the levels of ˙NO increase and the higher metabolic turnover of the enzyme increases the susceptibility of cytochrome c oxidase (CcOX) to inhibition by ˙NO. In addition at the lower O2 tensions caused by alcohol exposure to the hepatocyte, more ˙NO is available to able to bind to CcOX. The inhibition of electron flow results in increased production of superoxide at other sites in the respiratory chain and through damage mediated by RNS results in amplification of the bioenergetic deficit.
Highlights.
Mitochondrial damage and hypoxia are important features of alcohol-dependent hepatotoxicity.
We show hepatocytes isolated from chronically ethanol fed animals have decreased cellular bioenergetic reserve capacity.
This results in increased sensitivity to inhibition by nitric oxide.
Nitric oxide is a major contributor to hypoxic stress in vivo in response to chronic ethanol consumption.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Health grants HL007918 (to BR Zelickson), AA18841 and HL92857 (to SM Bailey), HL096638 (to A Landar) and AA013395 (to VM Darley-Usmar). We thank Mi Jung Chang, Elena Ulasova, and Amanda Wigley for their technical contribution to the early stages of this project.
List of Abbreviations
- iNOS
inducible nitric oxide
- ˙NO
nitric oxide
- HIF-1α
hypoxia-inducible factor-1α
- O2
oxygen
- ROS
reactive oxygen species
- EtOH
alcohol/ethanol
- OCR
oxygen consumption rate
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- CcOX
cytochrome c oxidase
- CYP2E1
cytochrome P450 2E1
- DetaNO
DetaNONOate
- ALDH2
aldehyde dehydrogenase 2
- RNS
reactive nitrogen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill BG, Dranka BP, Bailey S, Lancaster J, Darley-Usmar V. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 3.Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(1):S193–201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Charles IG, Moncada S. Nitric oxide: orchestrating hypoxia regulation through mitochondrial respiration and the endoplasmic reticulum stress response. Cell Res. 2005;15:63–65. doi: 10.1038/sj.cr.7290267. [DOI] [PubMed] [Google Scholar]
- 6.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 9.Arteel GE, Kadiiska MB, Rusyn I, Bradford BU, Mason RP, Raleigh JA, Thurman RG. Oxidative stress occurs in perfused rat liver at low oxygen tension by mechanisms involving peroxynitrite. Mol Pharmacol. 1999;55:708–715. [PubMed] [Google Scholar]
- 10.McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS. Ethanol consumption increases nitric oxide production in rats,and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin Exp Res. 2002;26:883–889. [PubMed] [Google Scholar]
- 12.Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, Bailey SM, Darley-Usmar VM. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 13.Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley-Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol. 2004;286:G521–527. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Venkatraman A, Shiva S, Davis AJ, Bailey SM, Brookes PS, Darley-Usmar VM. Chronic alcohol consumption increases the sensitivity of rat liver mitochondrial respiration to inhibition by nitric oxide. Hepatology. 2003;38:141–147. doi: 10.1053/jhep.2003.50293. [DOI] [PubMed] [Google Scholar]
- 16.Brookes PS, Kraus DW, Shiva S, Doeller JE, Barone MC, Patel RP, Lancaster JR, Jr, Darley-Usmar V. Control of mitochondrial respiration by NO*effects of low oxygen and respiratory state. J Biol Chem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 19.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Chandel NS. Mitochondrial regulation of oxygen sensing. Advances in experimental medicine and biology. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- 22.Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. The Journal of biological chemistry. 2004;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- 23.Ivester P, Lide MJ, Cunningham CC. Effect of chronic ethanol consumption on the energy state and structural stability of periportal and perivenous hepatocytes. Arch Biochem Biophys. 1995;322:14–21. doi: 10.1006/abbi.1995.1430. [DOI] [PubMed] [Google Scholar]
- 24.Baio DL, Czyz CN, Van Horn CG, Ivester P, Cunningham CC. Effect of chronic ethanol consumption on respiratory and glycolytic activities of rat periportal and perivenous hepatocytes. Arch Biochem Biophys. 1998;350:193–200. doi: 10.1006/abbi.1997.0514. [DOI] [PubMed] [Google Scholar]
- 25.Young TA, Bailey SM, Van Horn CG, Cunningham CC. Chronic ethanol consumption decreases mitochondrial and glycolytic production of ATP in liver. Alcohol Alcohol. 2006;41:254–260. doi: 10.1093/alcalc/agl017. [DOI] [PubMed] [Google Scholar]
- 26.Bailey SM, Cunningham CC. Effect of dietary fat on chronic ethanol-induced oxidative stress in hepatocytes. Alcohol Clin Exp Res. 1999;23:1210–1218. [PubMed] [Google Scholar]
- 27.Hill BG, Higdon AN, Dranka BP, Darley-Usmar VM. Regulation of vascular smooth muscle cell bioenergetic function by protein glutathiolation. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free radical biology & medicine. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls DG. Spare respiratory capacity oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- 31.Bailey SM. A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic Res. 2003;37:585–596. doi: 10.1080/1071576031000091711. [DOI] [PubMed] [Google Scholar]
- 32.Shiva S, Moellering D, Ramachandran A, Levonen AL, Landar A, Venkatraman A, Ceaser E, Ulasova E, Crawford JH, Brookes PS, Patel RP, Darley-Usmar VM. Redox signalling: from nitric oxide to oxidized lipids. Biochem Soc Symp. 2004:107–120. doi: 10.1042/bss0710107. [DOI] [PubMed] [Google Scholar]
- 33.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem. 1998;253:743–750. doi: 10.1046/j.1432-1327.1998.2530743.x. [DOI] [PubMed] [Google Scholar]
- 35.Shiva S, Brookes PS, Darley-Usmar VM. Methods for measuring the regulation of respiration by nitric oxide. Methods in cell biology. 2007;80:395–416. doi: 10.1016/S0091-679X(06)80020-8. [DOI] [PubMed] [Google Scholar]
- 36.Hollis VS, Palacios-Callender M, Springett RJ, Delpy DT, Moncada S. Monitoring cytochrome redox changes in the mitochondria of intact cells using multi-wavelength visible light spectroscopy. Biochim Biophys Acta. 2003;1607:191–202. doi: 10.1016/j.bbabio.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 38.Keefer LK, Nims RW, Davies KM, Wink DA. "NONOates" (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 39.Curran RD, Billiar TR, Stuehr DJ, Hofmann K, Simmons RL. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. The Journal of experimental medicine. 1989;170:1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billiar TR, Curran RD, West MA, Hofmann K, Simmons RL. Kupffer cell cytotoxicity to hepatocytes in coculture requires L-arginine. Archives of surgery. 1989;124:1416–1420. doi: 10.1001/archsurg.1989.01410120062013. discussion 1420–1411. [DOI] [PubMed] [Google Scholar]
- 41.Song BJ, Moon KH, Olsson NU, Salem N., Jr Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. Journal of hepatology. 2008;49:262–273. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi M, Lasker JM, Shimizu M, Rosman AS, Lieber CS. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10:437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki H, Nishiguchi K, Inoue K, Yasuyama T, Nakanishi S. Intralobular distribution of rat liver aldehyde dehydrogenase and alcohol dehydrogenase. Int J Biochem. 1988;20:435–437. doi: 10.1016/0020-711x(88)90212-1. [DOI] [PubMed] [Google Scholar]
- 45.Cahill A, Cunningham CC, Adachi M, Ishii H, Bailey SM, Fromenty B, Davies A. Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrion. Alcohol Clin Exp Res. 2002;26:907–915. [PMC free article] [PubMed] [Google Scholar]
- 46.Cahill A, Wang X, Hoek JB. Increased oxidative damage to mitochondrial DNA following chronic ethanol consumption. Biochem Biophys Res Commun. 1997;235:286–290. doi: 10.1006/bbrc.1997.6774. [DOI] [PubMed] [Google Scholar]
- 47.Bailey SM, Patel VB, Young TA, Asayama K, Cunningham CC. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res. 2001;25:726–733. [PubMed] [Google Scholar]
- 48.Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A. 2004;101:7630–7635. doi: 10.1073/pnas.0401723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 50.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey SM, Mantena SK, Millender-Swain T, Cakir Y, Jhala NC, Chhieng D, Pinkerton KE, Ballinger SW. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE(−/−) mouse: implications for a "multihit" hypothesis of fatty liver disease. Free Radic Biol Med. 2009;46:928–938. doi: 10.1016/j.freeradbiomed.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 53.Martinez RR, Setty S, Zong P, Tune JD, Downey HF. Nitric oxide contributes to right coronary vasodilation during systemic hypoxia. Am J Physiol Heart Circ Physiol. 2005;288:H1139–1146. doi: 10.1152/ajpheart.01139.2003. [DOI] [PubMed] [Google Scholar]
- 54.Sies H. Oxygen gradients during hypoxic steady states in liver. Urate oxidase and cytochrome oxidase as intracellular O2 indicators. Hoppe Seylers Z Physiol Chem. 1977;358:1021–1032. doi: 10.1515/bchm2.1977.358.2.1021. [DOI] [PubMed] [Google Scholar]
- 55.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–926. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 56.Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am J Physiol. 1996;271:G494–500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- 57.Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, Connor HD, Adachi Y, Wall C, Arteel GE, Raleigh JA, Forman DT, Mason RP. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42–46. doi: 10.2741/A478. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 59.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.