Abstract
The efficacy of monoclonal antibodies (mAbs) used to treat solid tumors is limited by intercellular junctions which tightly link epithelial tumor cells to each another. In this study, we define a small, recombinant adenovirus serotype 3-derived protein, termed junction opener 1 (JO-1), which binds to the epithelial junction protein desmoglein 2 (DSG2). In mouse xenograft models employing Her2/neu- and EGFR-positive human cancer cell lines, JO-1 mediated cleavage of DSG2 dimers and activated intracellular signaling pathways which reduced E-cadherin expression in tight junctions. Notably, JO-1-triggered changes allowed for increased intratumoral penetration of the anti-Her2/neu mAb trastuzumab (Herceptin) as well as improved access to its target receptor, Her2/neu, which is partly trapped in tight junctions. This effect translated directly into increased therapeutic efficacy of trastuzumab in mouse xenograft models using breast, gastric, and ovarian cancer cells that were Her2/neu-positive. Furthermore, combining JO-1 with the EGFR-targeting mAb cetuximab (Erbitux) greatly improved therapeutic outcomes in a metastatic model of EGFR-positive lung cancer. Taken together, our findings offer preclinical proof of concept to employ JO-1 in combination treatments which enhance the efficacy of trastuzumab treatment, by generating a transient degradation of tumor stroma proteins that can elicit eradication of tumors.
Introduction
Trastuzumab (Herceptin) and cetuximab (Erbitux) are humanized monoclonal antibodies (mAbs) used for the therapy of Her2/neu- and EGFR-positive cancers, respectively. The mechanisms of trastuzumab and cetuximab action include the activation of antibody-dependent or complement-dependent cytotoxicity, and interference with tyrosine kinase receptor signaling (1). A unifying aspect among these mechanisms is that tumor cell growth inhibition is dependent on the binding of these mAbs to their corresponding receptors, i.e Her2/neu and EGFR. Therefore, molecules that prevent access and binding to the receptor, either by physically inhibiting intratumoral transport from blood vessels to malignant cells or masking of receptors, are predicted to block trastuzumab and cetuximab activity (2). Several studies demonstrated that the expression or upregulation of epithelial proteins correlated with increased resistance to trastuzumab (3) and cetuximab (4) therapy. Epithelial cells maintain several intercellular junctions (tight junctions, adherens junctions, gap junctions, and desmosomes), a feature which is often conserved in epithelial cancers in situ and in cancer cell lines (5). Epithelial junctions are composed of adhesive dimers consisting of cadherin molecules derived from two neighboring cells (6). Desmoglein 2 (DSG2), an epithelial catherin, is overexpressed in a series of epithelial malignancies, including breast cancer (7) (Suppl.Fig.1), ovarian cancer (7) (Suppl.Fig.1), lung cancer (7), gastric cancer (8), squamous cell carcinomas (9), melanoma (10), metastatic prostate cancer (11), and bladder cancer (12).
Recently, we demonstrated that a group of human adenoviruses (Ads) (Ad serotype 3, 7, 11, and 14) use DSG2 as a primary attachment receptor for the infection of cells (7). Importantly, in epithelial cells, Ad3 binding to DSG2 triggered activation of signaling pathways resulting in the transient opening of epithelial junctions (7). The opening of the epithelial junctions was also achieved with recombinant subviral particles, such as Ad3 penton-dodecahedra (PtDd) (Fig.1A). We subsequently generated a minimal Ad3-derived DSG2 ligand formed by two fiber knob domains (13). This protein, with a molecular weight of approximately 50 kDa, is produced in E. coli and can be easily purified. In a series of functional studies we demonstrated that this protein efficiently triggers the opening of junction. In the following study, we therefore refer to this protein as junction opener-1 (JO-1).
Figure 1. Transient opening of epithelial junctions by JO-1.
A) Structure of Ad3 viral particles. Left panel: complete, infectious Ad3 particle. The capsid proteins fiber and penton base are shown in green and blue, respectively. The trimeric fiber knob is shown in red. Middle panel: Ad3 penton-dodecahedra (PtDd) formed by spontaneous assembly of 12 recombinant pentons (fiber + penton base). Right panel: dimeric Ad3 fiber (JO-1). B) Schematic structure of JO-1 containing an N-terminal His-tag, a dimerization domain [K-coil (32)], a flexible linker, one fiber shaft motif, and the homotrimeric Ad3 fiber knob domain. C) Left panel: simplified structure of epithelial junctions with tight junctions, desmosomes, and adherens junctions. Confocal immunofluorescence microscopy of T84 cells. Shown are stacked XZ images. Cells were treated with JO-1 (5 μg/ml) for 1 h on ice. After removal of JO-1, cells were incubated at 37°C and analyzed 0, 30, and 60 min later. Upper panel: DSG2 (green) appears at the apical site of baso-lateral junctions marked by claudin 7 (red). Middle panel: within 30 min after adding JO-1, claudin 7 staining increases and DSG2 staining becomes visible along the upper part of the lateral membrane (yellow signals). Lower panel: By 60 minutes, lateral junctions resemble those of time point “0 min”. The scale bar is 40 μm. D) Transmission electron microscopy of junctional areas of polarized colon cancer T84 cells. Cells were either treated with PBS (left panel) or JO-1 (right panel) for 1 h on ice, washed, and then incubated for 1 h at 37 °C. At this time, the electron-dense dye ruthenium red (33) was added together with the fixative. The scale bar is 1μm. E) 14C-PEG-4,000 diffusion through monolayers of T84 cells at different time points after adding JO-1 or anti-DSG2 antibody 6D8 (directed against ECD3/4). F) Effect of various DSG2 ligands on the transepithelial electrical resistance (TEER) of polarized T84 epithelial cells. Cells were either treated as described in E).
In this study, we have partially delineated the in vivo mechanism of JO-1-mediated junction opening. Furthermore, we show that JO-1 treatment greatly increases the permeation of mAbs in tumors and significantly enhances the efficacy of trastuzumab and cetuximab therapy in a series of xenograft tumor models.
Material and Methods
Proteins
JO-1 (also known as Ad3-K/S/Kn) is produced in E-coli as described previously (13). Recombinant Ad3 penton-dodecahedral (PtDd) protein complexes were produced in insect cells and purified as described elsewhere (14).
Cell lines
BT474-M1 is a tumorigenic subclone of BT474 (ATTC, HTB-20) that was generously provided by Mien-Chie Hung (Department of Molecular and Cellular Oncology, University of Texas MD Anderson Cancer Center, Houston) in 2009 (15). BT474-M1 and HCC1954 cells (ATTC, CRL-2338) were cultured in RPMI-1640 with 10% FBS, 1% Pen/Strep and L-Glutamine. A549 (ATCC, CCL-185) and T84 (ATCC, CCL-248) were cultured in DMEM/F:12 with 10% FBS, 1% Pen/Strep and L-Glutamine. To achieve cell polarization, 1.4×105 T84 cells were cultured in collagen-coated 6.5 mm Transwell inserts (0.4 μm pore size) (Costar Transwell Clears) for a period of 14 to 20 days until transepithelial resistance was stable (7). Cell lines from the ATTC were obtained in December 2010. All cell lines have been passaged for fewer than 6 months. Cell surface expression of Her2/neu (BT474-M1, HCC1954) and/or EGFR1 (A549, T84) was confirmed by immunofluorescence analysis in January 2011.
Immunofluorescence analyses were performed as described recently (7).
Western Blots were performed as described recently (7).
Transepithelial electrical resistance (TEER) and PEG permeability assays
A total of 5×105 T84 cells were seeded on 12 mm transwell inserts [PET membrane, with 0.4 μm pore size (Corning, NY)] and cultured for 20 days. Culture medium was changed every 2-3 days. The cells were exposed to DSG2 ligands (20 μg/ml) in adhesion medium (DMEM, 1% FBS, 2 mM MgCl2, 20 mM HEPES) for 15 min at room temperature and TEER was measured and calculated as described elsewhere (16). For permeability assays 15 min after adding the DSG2 ligands, 1 mCi of [14C] polyethylene glycol-4000 (PEG-4000) (Perkin Elmer, Covina CA) was added to the inner chamber. Medium aliquots were harvested from the inner and outer chambers and measured by a scintillation counter. Permeability was calculated as described elsewhere (17).
Transmission Electron microscopy (TEM)
TEM was performed as described previously (13).
HSC-based relaxin expression
The protocol has been described elsewhere (18). Briefly, transplant recipients were 6- to 10-week old, female CB17 SCID-beige mice, sublethally irradiated with 350 cGy immediately before tail vein injection with 6×105 lentivirus vector– transduced bone marrow cells from 5-FU–treated mice. After engraftment of cells in the recipients’ bone marrow was confirmed, a total of 4×106 HCC1954 were injected into the mammary fat pad. The lentivirus vector expressing relaxin under the control of doxycycline (Dox) has been described previously (18).
Human IgG (Herceptin) ELISA
A polyclonal goat anti-human IgG antibody (G-101-C-ABS, R&D Systems, Minneapolis, MN) was used as a capture antibody. Tissues were lysed as for Western blots. Purified human IgG served as a standard. Binding was detected with a mouse monoclonal anti human IgG1 Fc antibody (MAB 110, R&D Systems), followed by an anti-mouse IgG-HRP conjugate.
Animal studies
Breast cancer xenografts were established by injecting 4×106 cancer cells into the mammary fat pad of CB17 SCID-beige mice. Trastuzumab was injected intraperitoneally (i.p.) at a dose of 10 mg/kg. PtDd or JO-1 was given intravenously (i.v.) at a dose of 2 mg/kg. Tumor volumes were measured as described previously (19). Mice were sacrificed when the tumor volume reached 1,000 mm3 or ulcerated. Lung cancer xenografts were established by injecting 4×106 A549 subcutaneously (s.c.) into the right flank of CB17 SCID beige mice. Cetuximab was injected at 10 mg/kg i.p. For the disseminated lung tumor model, mice were intravenously injected with 2×106 A549 cells. Animals were sacrificed when the first mouse of the control group was moribund. India ink (15% in PBS) was injected intratracheally prior to the removal of the lungs.
Statistical analysis
All results are expressed as mean +/- SD. Student`s t-test or 2-Way ANOVA for multiple testing, were applied when applicable. A p-value < 0.05 was considered significant.
Results
JO-1 triggers opening of epithelial junctions
As the large size of Ad3 or PtDd particles can affect their egress from blood vessels and tissue penetration, we attempted to generate smaller Ad3-derived DSG2 ligands that are functionally active as epithelial junction openers. We therefore designed JO-1 (aka Ad3-K/S/Kn) (13), a small, self-dimerizing Ad3 fiber derivative (Figs.1A, B) (13). JO-1 has a molecular weight of ~50 kDa and is produced in E. coli prior to purification by affinity chromatography. In contrast, PtDd have to be produced in insect cells and have a molecular weight of 4,860 kDa and a diameter of ~50nm. The functional activity of JO-1 was tested on polarized colon cancer T84 cells. Incubation of T84 cells with JO-1 triggered remodeling of epithelial junctions, as shown by confocal microscopy for claudin 7 and DSG2 (Fig.1C). Opening of the tight junctions, which are localized apical to the desmosomal and adherence junctions, is illustrated by electron microscopy (Fig.1D). Microphotographs of untreated epithelial cells show intact tight junctions as judged by the exclusion of the apically applied electrone-dense dye ruthenium red from basolateral space. Incubation of epithelial cells with JO-1 for 1 hour resulted in the disassembly of tight junctions and leakage of ruthenium red into the basolateral space (Fig.1D, right panel). Exposure of polarized epithelial cells to JO-1 also increased the transepithelial permeability, as shown by transflux of 14C-PEG-4000 with a molecular weight of 4000 Da (Fig.1E). Importantly, monoclonal antibodies against different regions of the extracellular domain of DSG2 did not significantly increase transepithelial permeability. We speculate that the ligation of several DSG2 molecules is required to trigger the opening of the junctions. Finally, transient opening of junction was confirmed by measuring the transepithelial electrical resistance (TEER) in polarized epithelial cells (Fig.1F). Notably, JO-1 had no significant effect on the TEER when studies were done in subconfluent cell cultures where mature junction had not yet formed (i.e. when TEER was not constant).
JO-1 triggers intracellular signaling and increases penetration of mAb in epithelial tumors in vivo
An orthotopic breast cancer xenograft model (HCC1954) was used to study the effect of JO-1 on epithelial junctions in vivo. HCC1954 xenograft tumors resembled the histology of breast cancer in humans (20), i.e. tumors were vascularized and contained nests of epithelial cells glued together by epithelial junctions and surrounded by extracellular matrixes (Suppl. Fig.2). JO-1 was injected intravenously into mice with pre-established tumors. JO-1 could be detected in the tumors by immunofluorescence microscopy as early as 1 hour post-injection. JO-1 accumulated in the tumors as is indicated by the increased immunofluorescence at 12 hours post injection (Fig.2A, left three panels). This is also confirmed by Western blot analysis of tumor lysates (Fig.2A, right panel). Analysis of DSG2 on tumor sections by immunofluorescence microscopy in PBS treated animals showed membrane localized signals (Fig.2B, left panel). One hour subsequent to JO-1 injection, DSG2 molecules were mostly found in the cytoplasm of the tumor cells (second panel). By 12 hours membrane localization of DSG2 appeared to be partly restored (third panel). Western blot analysis using anti-DSG2 antibodies against the extracellular domain of DSG2 revealed smaller fragments of the DSG2 (80 and 45kDa) at the 1 hour time point (Fig.2B, right panel). These fragments represent the extracellular domains (ECD) and proteolytic cleavage products of the ECD. Proteolytic cleavage of DSG2 to stable fragments in normal epithelial tissue and cancer has been reported before (21-23).
Figure 2. Analysis of mechanism of JO-1 action in tumors in an orthotopic (HCC1954) breast cancer model.
When tumors reached a volume of ~200 mm3, JO-1 (2 mg/kg in 200 μl PBS) was injected intravenously. Tumors were harvested either 1 or 12 h after JO-1 injection. Control mice received 200 μl PBS and tumors were collected 1 h later. A) Kinetics of JO-1 accumulation in tumors. Left panels: immunofluorescence analysis of tumor sections using anti-His tag antibodies (for visualization of JO-1). The scale bar is 20 μm. Right panel: Western blot analysis of tumor tissue using Ad3-fiber knob specific antibodies (7). B) Analysis of DSG2 in tumors. Left panel: immunofluorescence analysis of tumor sections using DSG2 antibodies (mAb 6D8 against extracellular domain 3/4 of DSG2). The inserts show a higher magnification. Right panel: The same anti-DSG2 antibody was used for Western blot analysis of tumor tissue. C) Intracelluar signaling in vivo. Left panel: Western blot analysis of tumor tissue for E-cadherin and phosphorylated E-cadherin, Erk 1/2, phosphorylated Erk1/2, claudin 7, and vimentin. Antibodies against γ-tubulin were used to assess sample loading (“loading control”). Right panels: immunofluorescence analysis using antibodies against E-cadherin and phosphorylated Erk1/2.
Recently, it was found in in vitro studies that Ad3 binding to DSG2 of epithelial cells triggered intracellular signaling including pathways that are involved in epithelial-to-mesenchymal transition (EMT) (7). Among the feature that characterize EMT are decreased expression of epithelial markers and activation of Erk1/2 (MAPK) (5). In our studies with xenograft tumors, we found less non-phosphorylated and phosphorylated forms of E-cadherin in tumors 12 hours after intravenous injection of JO-1 (Fig.2C, left panel). Preceding the changes in E-cadherin, was a transient increase in phosphorylated Erk1/2 (Fig.2C, compare pErk1/2 PBS vs. JO-1 (1h)). The decrease in E-cadherin and an increase in signals for phosphorylated Erk1/2 upon JO-1 injection were also observed by immunofluorescence microscopy (Fig.2C, right panels). These studies indicate that JO-1 triggers transient activation of Erk1/2 pathways in vivo.
Next, we tested whether JO-1-triggered opening of epithelial junctions in tumors would increase the penetration of mAbs in xenograft tumors. Trastuzumab, a humanized IgG1 mAb, was injected intraperitoneally at a dose of 10 mg/kg into HCC1954 tumor-bearing mice (24). In tumor sections and Western blot analyses, trastuzumab was detectable 1 hour post-injection and at higher levels 12 hours after injection (Figs.3A and B). Quantitative analysis of human IgG1 in tumor lysates by ELISA showed ~6 fold higher levels in mice that received JO-1 injection + trastuzumab (12 h time point) compared to mice that received trastuzumab alone (Fig. 3C).
Figure 3. JO-1 improves penetration of trastuzumab in HCC1954 breast cancer tumors in situ.
Tumor bearing mice were intravenously injected with PBS or JO-1 (2 mg/kg) followed by trastuzumab 1 h later. Tumors were harvested 1 h or 12 h after trastuzumab injection. A) Sections were stained for human IgG (i.e. trastuzumab). Positive staining appears green. The scale bar is 20 μm. B) Western blot analysis for human IgG (trastuzumab) in tumors. Heavy (HC) and light (LC) Ig chains are indicated by arrows. C) ELISA for human IgG1 in tumor lysates. Total protein concentration in all lysates was adjusted to 5 mg/ml. Shown is the ratio of human IgG1 concentrations in tumors of PBS treated mice vs mice that received JO-1 and/or trastuzumab treatment. n=3, * p<0.05
In conclusion, intravenous injection of JO-1 one hour prior to the administration of trastuzumab, significantly increased the amount of trastuzumab in the tumors, indicating either better egress from blood vessels, better intratumoral penetration, and/or longer intratumoral half-life.
mAb targets are trapped in epithelial junctions
In breast cancer xenograft sections and in cultured breast cancer cells we found co-staining of Her2/neu and the adherens junction protein claudin 7 (Fig.4A, Suppl.Fig.3). Confocal microscopy of breast cancer BT474 cells confirmed the trapping of Her2/neu in lateral junctions. Incubation of the Her2/neu positive breast cancer cell lines BT474 (Fig.4) or HCC1954s (Suppl.Fig.3) with JO-1 changed the composition of the lateral epithelial junctions within 1 hour. As a result of this, Her2/neu staining at the cells surface became more intense, while it faded in areas distal of the cell surface. This suggests that JO-1 mediated junction opening triggered a translocation of Her2/neu from lateral membranes to the cell surface. Being trapped in epithelial junctions also appears to be a problem for EGFR as co-staining for EGFR and the tight junction protein E-cadherin suggests (Fig.4B, Suppl.Fig.3B). In our studies with cetuximab we focused on a lung cancer model (A549 cells), as most colon cancer cell lines have mutations in K-ras, which confers resistance to cetuximab (25). Similar to what we observed for Her2/neu, incubation of A549 cells with JO-1 resulted in a translocation of EGFR to the cell surface.
Figure 4. JO-1 increases mAb killing of cells in which the target receptors are trapped in epithelial junctions.
A) Confocal microscopy of Her2/neu (green) and DSG2 (red) staining on polarized BT474 cell cultures (XY and XZ images). Cells treated with PBS are shown in the left panel. Middle and right panels: Cells were treated with JO-1 (20 μg/ml) for 1 h on ice. After removal of JO-1, cells were incubated at 37 °C and analyzed 1 h and 16 h later. XY images show the cell surface (left) and a section 2 μm below the cell surface. The scale bar is 40 μm. B) Confocal microscopy of EGFR (red) and the tight junction protein E-cadherin (green) on polarized A549 lung cancer cells. C) JO-1 enhances killing of Her2/neu-positive breast cancer cells by trastuzumab. BT474 cells were incubated with JO-1 (5 μg/ml) or PBS. Trastuzumab (15 μg/ml) was added 1 h later. Cell viability was measured after 3 h by WST-1 assays as described earlier (34). Viability of PBS-treated cells was taken as 100%. n = 5, D) JO-1 enhances cetuximab killing of EGFR-positive A549 cells. n = 5, * p<0.05
Release of mAb receptors from trapping is supported by the enhanced killing of cancer cells by trastuzumab and cetuximab. In vitro killing of BT474 breast cancer and A549 lung cancer cells by trastuzumab and cetuximab, respectively, was inefficient (Figs. 4C and D). Pretreatment of these cells with JO-1 significantly increased in vitro cytotoxicity of both antibodies in the corresponding cell lines, although the effect of JO-1 was relatively modest.
JO-1 improves trastuzumab therapy in vivo
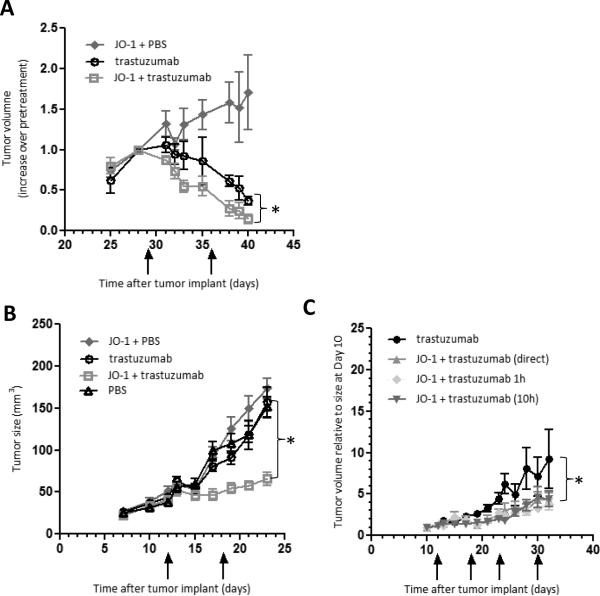
JO-1's potential enhancement of trastuzumab therapy was first tested in an orthotopic breast cancer model based on Her2/neu positive BT474-M1 cells. JO-1 injection alone had no significant effect on tumor growth (Fig.5A). BT474-M1 tumors initially responded well to trastuzumab, however pre-injection of JO-1 significantly enhanced the therapeutic efficacy of trastuzumab (Fig.5A). The enhancing effect of JO-1 pretreatment becomes more apparent when treated mice were followed long-term, i.e. for 136 days. While 60% of the animals that received trastuzumab monotherapy relapsed around day 100, none of the animals treated with JO-1+trastuzumab showed tumor re-growth (data not shown).
Figure 5. JO-1 improves trastuzumab therapy in Her2/neu positive breast cancer models.
A) BT474-M1 breast cancer model: When tumors reached a volume of ~100 mm3, mice received an intravenous injection of JO-1 or PBS, followed by an intraperitoneal injection of trastuzumab or PBS 10 h later. A second treatment cycle was started at day 36 (marked by arrows). Shown is the increase in tumor volume (compared to pretreatment levels at day 29). n=5. * p<0.05. B) HCC1954 breast cancer model: Mice were treated as in A). Mice received the first treatment at day 12. Treatment was repeated at day 18. n = 5. * p<0.05. C) Mice bearing HCC1954 breast cancer tumors were injected with a mixture of JO-1 and trastuzumab, JO-1 followed by trastuzumab 1 h later and, JO-1 followed by trastuzumab 10 h later. Injections were repeated weekly. n=5. All JO-1 cotherapies are significantly more effective than trastuzumab alone. There is not significant difference between the different cotherapy regimens.
A second breast cancer model involved HCC1954 cells. Tumors derived from these cells are more resistant to trastuzmab (Fig.5B). As seen in the BT474-M1 model, JO-1 pretreatment significantly improved trastuzumab therapy and stalled tumor growth. Based on our previous study with PtDd (7), we chose a time interval of 10 hours between JO-1 and trastuzumab injections. This regimen is supported by the kinetics of JO-1 accumulation in tumors and the kinetics of E-cadherin decrease (see Figs.2A and C). On the other hand, events that appear to be linked to junction opening, i.e. DSG2 cleavage or Erk1/2 activation, occur already within 1 hour after JO-1 injections. We therefore investigated how simultaneous JO-1/trastuzumab injection and injection of trastuzumab 1 hour after JO-1 application influenced the therapeutic outcome (Fig. 5C). In this study no significant difference was found when compared to the treatment approach used initially (trastuzumab 10 hours after JO-1). We speculate that this is due to the relative slow accumulation of the protein in the tumors. To further consolidate the clinical relevance of JO-1 as a co-therapeutic for trastuzumab, we performed efficacy studies in Her2/neu-positive gastric cancer (NCI-N87) and ovarian cancer (SKOV3-1ip) models (Suppl. Fig.4). Similar to the breast cancer model, we found co-staining of Her2/neu and claudin 7 in NCI-N87 cultures and xenografted tumors, suggesting trapping of Her2/neu in epithelial junctions. Pretreatment of NCI-N87 tumor-bearing mice with JO-1 significantly improved trastuzumab therapy as reflected by delayed tumor growth (Suppl.Fig.4A). To establish the ovarian cancer model, SKOV3-1ip, cells were injected intraperitoneally and survival was monitored after treatment (Suppl.Fig.4B). While all mice treated with trastuzumab alone had reached the endpoint by day 80, 80% of the animals that received the combination treatment JO-1 plus trastuzumab were still alive at this time.
JO-1 improves cetuximab therapy in vivo
Cetuximab treatment of mice with pre-established subcutaneous A549 tumors did not result in a significant delay of tumor growth when compared to treatment with PBS (Fig.6A). JO-1 was injected intravenously or intraperitoneally followed by cetuximab 12 hours later. Both treatment approaches had a significant therapeutic effect and resulted in a decrease of tumor volumes. An additional combination of intravenously injected JO-1 with an intratumoral application of the junction opener did not further increase the therapeutic efficacy. As seen in the breast cancer model, JO-1 treatment alone did not exert a significant anti-tumor effect. JO-1 pretreatment enhanced cetuximab therapy to a similar degree as seen with PtDd (Fig.6B).
Figure 6. JO-1 improves cetuximab therapy in lung cancer xenograft models.
A) Subcutaneous A549 tumor model: JO-1 was injected at day 12 and 15 intravenously (2 mg/kg) or intraperitoneally (4 mg/kg) followed by an intraperitoneal injection of cetuximab or PBS 10 h later. One group received 1 mg/kg of JO-1 intravenously and 1 mg/kg intratumorally. n = 5. All JO-1 cotherapies are significantly more effective than trastuzumab alone. The difference between JO-1 injection routes was not significant. B) Mice received an intravenous injection of 2 mg/kg PtDd followed by an intraperitoneal injection of cetuximab (10 mg/kg) or PBS 10 h. A second treatment cycle was started at day 14 (marked by arrows). n = 5. Cetuximab vs. PtDd plus cetuximab P < 0.001. C) Metastatic A549 lung cancer model: 10 days after intravenous injection of A549 cells, mice received an intravenous injection of 2 mg/kg JO-1 or PBS, followed by an intraperitoneal injection of cetuximab (10 mg/kg) or PBS 10 h later. The treatment was repeated every 3 days until day 38. n=10. Left panels: Lungs from individual mice stained with India ink. Healthy tissue appears black. Tumor tissue stains white. Right panel: representative sections of lungs stained with H&E.
Next, the co-therapy approach was tested in a metastatic lung cancer model. In this model, mice became morbid within 37 days of tumor cell transplantation with predominant tumor localization to the lung (Fig.6C, “PBS” group). Treatment of mice was started at day 10. All animals were sacrificed at day 40. While lung metastases were clearly visible in the control group, JO-1 group, as well as the cetuximab treated animals, 80% of the lungs in the JO-1+cetuximab-treated animals were free of tumor when inspected macroscopically. Microscopy of lung sections showed that in PBS treated-animals, tumor cells almost completely replaced normal lung tissue and also filled the broncioli. (Fig.6C, right panels). While cetuximab treated animals had considerable, infiltrating tumor growth, the majority of JO-1+ cetuximab injected animals showed only micrometastases.
Combined tumor stroma protein degradation and junction opening
Extracellular matrix proteins forming the tumor stroma tightly surround nests of malignant breast and colon cancer cells (26). We have recently shown that transient degradation of tumor stroma proteins by intratumoral expression of the peptide hormone relaxin significantly enhanced trastuzumab therapy (24). Here, we utilized the HCC1954 model to test whether additional transient tumor stroma protein degradation, would further increase the effect of JO-1 on trastuzumab therapy (Fig.7A). To deliver the relaxin gene to the tumor we employed an approach based on hematopoietic stem cells (HSCs) (26). The approach involved the ex vivo transduction of bone marrow derived HSCs with lentivirus vectors expressing relaxin under the control of a Doxycycline (Dox)-inducible transcription cassette, and the transplantation of these cells into myelo-conditioned recipients, where they engraft in the bone marrow and provide a long-term source of genetically modified cells that will home into tumors. This study showed that relaxin expression alone significantly delayed tumor growth and increased trastuzumab therapy (Fig.7B). The combination of relaxin expression and JO-1 treatment stopped tumor growth. Tumors did not re-grow when treatment was terminated, in contrast to groups that received either relaxin+trastuzmab or JO-1+trastuzumab therapy. Histological analyses of residual masses in the JO-1/relaxin/trastuzumab group at the end of the observation period, showed only connective tissue. In contrast, explanted tumors from the other groups contained tumor cells, which could be cultured in vitro upon protease digestion of tumors. Notably, no adverse side effects were observed in mice that received the triple combination (JO-1/relaxin/trastuzumab) treatment.
Figure 7. Combination therapy of JO-1 and relaxin in the HCC1854 breast cancer model.
A) Schematic illustration of the experiment. Lethally irradiated mice received either mock transduced or LV-EF1a/Rlx transduced Lin- bone marrow cells. Six weeks later, after engraftment of HSCs, mice were injected into the mammary fat pad with 4×106 HCC1954 cells. Relaxin expression was activated by Doxycyclin 7 days later. Mice were then given weekly treatment of PBS, PBS/trastuzumab or JO-1/trastuzumab and tumor volumes were measured. B) Tumor volumes of individual mice. n=5. trastuzumab vs. JO-1 + trastuzumab P < 0.001; Rlx + JO-1 + trastuzumab vs. Rlx + trastuzumab P < 0.001; Rlx + PBS vs. PBS P < 0.001; Rlx + PBS vs Rlx +JO-1 + PBS P<0.001; Rlx +PBS vs Rlx + JO-1 + trastuzumab P<0.001
Our data underscore that physical obstacles in tumors are involved in mediating resistance to trastuzumab therapy.
Discussion
JO-1 as new co-therapeutic
The epithelial phenotype of cancer, i.e. intercellular junctions, creates obstacles to mAb therapy. The small recombinant protein JO-1 increased the penetration of trastuzumab in the tumor and allowed for better access to mAb target receptors, which, in turn, facilitated mAb therapy in a series of xenograft models involving human epithelial tumor cells. Potentially, the combination of JO-1 with trastuzumab and cetuximab might allow for the reduction of the effective dose of these mAbs, thereby reducing critical side effects, i.e trastuzumab-associated cardiotoxity and acne-like rashes that often occur during cetuximab therapy.
Mechanisms of action
Our data suggest that JO-1 triggers junction opening in epithelial tumors through several, potentially connected, mechanisms: i) cleavage of the DSG2 ECD, and disruption of DSG2 dimers between neighboring cells; ii) intracellular signaling that leads to a transient decrease of E-cadherin and potentially other junction proteins; and iii) changes in the membrane distribution of Her2/neu. JO-1 treatment resulted in transient phosphorylation of Her2/neu in tumors (Suppl.Fig.5). However, trastuzumab treatment alone also triggered Her2/neu phosphorylation, a phenomenon that has been observed before (27), and JO-1 plus trastuzumab co-therapy did not further increase the levels of phosphorylated Her2/neu. This makes it unlikely that JO-1 enhances trastuzumab therapy through its effect on the biology of Her2/neu.
Side effects on normal epithelial tissues
Because the mouse orthologue of DSG2 is not recognized by Ad3 or JO-1 (7), we generated transgenic mice containing the human DSG2 locus. The expression pattern and level of human DSG2 in these animals were similar to those found in humans. Furthermore, we showed that JO-1 binding to human DSG2 in transgenic mouse epithelial cells triggered junction opening to a degree similar to data observed in human cells. In preliminary studies with DSG2-transgenic mice we did not find critical side effects of intravenous JO-1 injection (2 mg/kg) (28). We speculate that DSG2 in normal epithelial cells is not readily accessible to intravenously applied JO-1. On the other hand, greater leakage of tumor-associated blood vessels and the lack of strict cell polarization might make epithelial tumors more responsive to JO-1. Lack of toxicity after intravenous injection of JO-1 ligands is also underscored by studies with adenoviruses containing Ad3 fibers (29).
JO-1 immunogenicity
As JO-1 is a viral protein, adaptive immune responses might develop in humans, particularly after repeated injection. This might, however, not be a problem clinically because both trastuzumab and cetuximab are used in combination with immunosuppressive chemotherapeutic drugs.
Potential risk to enhance tumor invasion and metastasis
In agreement with other studies (8, 9), we found a higher DSG2 expression in malignant tissues than in the surrounding normal epithelial tissue. There are, however, also studies reporting a reduction in the amounts of DSG2 in invasive pancreatic or gastric cancer (23, 30). The latter, as well as the finding that JO-1 triggers EMT-like signaling, raises the question of whether JO-1 would facilitate metastasis. Notably, in all models used in this study, we did not see stimulation of tumor growth or macroscopic/microscopic signs of metastasis in animals treated with JO-1 alone. Tumor invasion and metastasis requires more than transient activation of EMT pathways. Detachment from epithelial cancers and migration of tumor cells is only possible after long-term crosstalk between malignant cells and the tumor microenviroment, resulting in changes in the tumor stroma and phenotypic reprogramming of epithelial cells into mesenchymal cells (31).
In summary, the epithelial junction opener JO-1 has the potential to improve mAb therapies of cancer both in term of efficacy and safety, i.e. by allowing lower therapeutic mAbs doses. This study also sheds light on the mechanisms of Ad3 infection of epithelial cells.
Supplementary Material
Acknowledgments
The work was supported by NIH grants R01 CA080192, R01 HLA078836 (AL), the Pacific Ovarian Cancer Research Consortium/Specialized Program of Research Excellence in Ovarian Cancer Grant P50 CA83636, FHCRC Breast Cancer Research Program Pilot Project Found, the Danish Cancer Society, the Danish National Research Foundation (RS and JB), and the European Commission (grant CZ.1.05/2.1.00/01.0030) (JB). I.B. is a recipient of a postdoctoral fellowship award from ”Deutsche Krebshilfe” (108988).
References
- 1.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesniak D, Xu Y, Deschenes J, Lai R, Thoms J, Murray D, et al. Beta1-integrin circumvents the antiproliferative effects of trastuzumab in human epidermal growth factor receptor-2-positive breast cancer. Cancer Res. 2009;69:8620–8. doi: 10.1158/0008-5472.CAN-09-1591. [DOI] [PubMed] [Google Scholar]
- 3.Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113–24. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 4.Oliveras-Ferraros C, Vazquez-Martin A, Cufi S, Queralt B, Baez L, Guardeno R, et al. Stem cell property epithelial-to-mesenchymal transition is a core transcriptional network for predicting cetuximab (Erbitux) efficacy in KRAS wild-type tumor cells. J Cell Biochem. 2011;112:10–29. doi: 10.1002/jcb.22952. [DOI] [PubMed] [Google Scholar]
- 5.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of Disease: epithelial-mesenchymal transition-does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008 doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeser J, Troyanovsky SM, Grund C, Franke WW. De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res. 2003;285:114–30. doi: 10.1016/s0014-4827(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biedermann K, Vogelsang H, Becker I, Plaschke S, Siewert JR, Hofler H, et al. Desmoglein 2 is expressed abnormally rather than mutated in familial and sporadic gastric cancer. J Pathol. 2005;207:199–206. doi: 10.1002/path.1821. [DOI] [PubMed] [Google Scholar]
- 9.Harada H, Iwatsuki K, Ohtsuka M, Han GW, Kaneko F. Abnormal desmoglein expression by squamous cell carcinoma cells. Acta Derm Venereol. 1996;76:417–20. doi: 10.2340/0001555576417420. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt CJ, Franke WW, Goerdt S, Falkowska-Hansen B, Rickelt S, Peitsch WK. Homo- and heterotypic cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glycoprotein. J Invest Dermatol. 2007;127:2191–206. doi: 10.1038/sj.jid.5700849. [DOI] [PubMed] [Google Scholar]
- 11.Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25:183–91. [PubMed] [Google Scholar]
- 12.Abbod MF, Hamdy FC, Linkens DA, Catto JW. Predictive modeling in cancer: where systems biology meets the stock market. Expert Rev Anticancer Ther. 2009;9:867–70. doi: 10.1586/era.09.47. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Li Z, Yumul R, Lara S, Hemminki A, Fender P, et al. Multimerization of adenovirus serotype 3 fiber knob domains is required for efficient binding of virus to desmoglein 2 and subsequent opening of epithelial junctions. J Virol. 2011;85:6390–402. doi: 10.1128/JVI.00514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fender P, Ruigrok RW, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat Biotechnol. 1997;15:52–6. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- 15.Lee C, Dhillon J, Wang MY, Gao Y, Hu K, Park E, et al. Targeting YB-1 in HER-2 overexpressing breast cancer cells induces apoptosis via the mTOR/STAT3 pathway and suppresses tumor growth in mice. Cancer Res. 2008;68:8661–6. doi: 10.1158/0008-5472.CAN-08-1082. [DOI] [PubMed] [Google Scholar]
- 16.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–99. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Horn M, Wang J, Shen DD, Ho RJ. Development and characterization of a recombinant madin-darby canine kidney cell line that expresses rat multidrug resistance-associated protein 1 (rMRP1). AAPS J. 2004;6:77–85. doi: 10.1208/ps060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer I, Li Z, Persson J, Liu Y, van Rensburg R, Yumul R, et al. Controlled extracellular matrix degradation in breast cancer tumors improves therapy by trastuzumab. Mol Ther. 2011;19:479–89. doi: 10.1038/mt.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuve S, Chen BM, Liu Y, Cheng TL, Toure P, Sow PS, et al. Combination of tumor site-located CTL-associated antigen-4 blockade and systemic regulatory T-cell depletion induces tumor-destructive immune responses. Cancer Res. 2007;67:5929–39. doi: 10.1158/0008-5472.CAN-06-4296. [DOI] [PubMed] [Google Scholar]
- 20.Li ZY, Ni S, Yang X, Kiviat N, Lieber A. Xenograft models for liver metastasis: Relationship between tumor morphology and adenovirus vector transduction. Mol Ther. 2004;9:650–7. doi: 10.1016/j.ymthe.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Kolegraff K, Nava P, Laur O, Parkos CA, Nusrat A. Characterization of full-length and proteolytic cleavage fragments of desmoglein-2 in native human colon and colonic epithelial cell lines. Cell Adh Migr. 2011;5 doi: 10.4161/cam.5.4.16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King IA, Wood MJ, Fryer PR. Desmoglein II-derived glycopeptides in human epidermis. J Invest Dermatol. 1989;92:22–6. doi: 10.1111/1523-1747.ep13070408. [DOI] [PubMed] [Google Scholar]
- 23.Ramani VC, Hennings L, Haun RS. Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer. BMC Cancer. 2008;8:373. doi: 10.1186/1471-2407-8-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyer I, Li Z, Persson J, Liu Y, van Rensburg R, Yumul R, et al. Controlled Extracellular Matrix Degradation in Breast Cancer Tumors Improves Therapy by Trastuzumab. Mol Ther. 2010 doi: 10.1038/mt.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Liu Y, Tuve S, Xun Y, Fan X, Min L, et al. Toward a stem cell gene therapy for breast cancer. Blood. 2009;113:5423–33. doi: 10.1182/blood-2008-10-187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Persson J, Wang H, Song H, Beyer I, Yumul R, et al. Biodistribution of DSG2 in humans, macaques, and DSG2 transgenic mice. 2011. in preparation.
- 29.Hemminki O, Bauerschmitz G, Hemmi S, Lavilla-Alonso S, Diaconu I, Guse K, et al. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 2011;18:288–96. doi: 10.1038/cgt.2010.79. [DOI] [PubMed] [Google Scholar]
- 30.Yashiro M, Nishioka N, Hirakawa K. Decreased expression of the adhesion molecule desmoglein-2 is associated with diffuse-type gastric carcinoma. Eur J Cancer. 2006;42:2397–403. doi: 10.1016/j.ejca.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–60. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Zeng Y, Pinard M, Jaime J, Bourget L, Uyen Le P, O'Connor-McCourt MD, et al. A ligand-pseudoreceptor system based on de novo designed peptides for the generation of adenoviral vectors with altered tropism. J Gene Med. 2008;10:355–67. doi: 10.1002/jgm.1155. [DOI] [PubMed] [Google Scholar]
- 33.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–4. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.