Abstract
Background
Irreversible muscle changes following rotator cuff tears is a well-known negative prognostic factor after shoulder surgery. Currently, little is known about the pathomechanism of fatty degeneration of the rotator cuff muscles after chronic cuff tears.
Hypothesis/Purpose
The purposes of this study were: 1) to develop a rodent animal model of chronic rotator cuff tears that can reproduce fatty degeneration of the cuff muscles seen clinically, 2) to describe the effects of tear size and concomitant nerve injury on muscle degeneration, and 3) to evaluate the changes in gene expression of relevant myogenic and adipogenic factors following rotator cuff tears using the animal model.
Methods
Rotator cuff tears were created in rodents with and without transection of the suprascapular nerve. The supraspinatus and infraspinatus muscles were examined 2, 8, and 16 weeks after injury for histological evidence of fatty degeneration and expression of myogenic and adipogenic genes.
Results
Histological analysis revealed adipocytes, intramuscular fat globules, and intramyocellular fat droplets in the tenotomized and neurotomized supraspinatus and infraspinatus muscles. Changes increased with time and were most severe in the muscles with combined tenotomy and neurotomy. Adipogenic and myogenic transcription factors and markers were upregulated in muscles treated with tenotomy or tenotomy combined with neurotomy compared to normal muscles.
Conclusions
The present study describes a rodent animal model that produces fatty degeneration of the rotator cuff muscles similar to human muscles after chronic cuff tears. The severity of changes was associated with tear size and concomitant nerve injury.
Keywords: adipogenesis, myogenesis, tenotomy, neurotomy, tendon
INTRODUCTION
Rotator cuff disease is a degenerative condition that leads to significant shoulder pain, muscle atrophy, and tendon rupture, severely limiting upper extremity function. Rotator cuff repair to treat shoulder pain and restore function is one of the most common orthopedic surgical procedures, with over 75,000 repairs performed each year in the United States39. However, rotator cuff tendon healing is unpredictable, with short to mid-term failure rates ranging from 30–94%.10,14 The majority of rotator cuff disease is related to chronic degeneration of the cuff tissues.15,24 Degenerative changes can lead to massive, irreparable rotator cuff tears through attritional changes in the tendons and fatty degeneration of the rotator cuff muscles.10 To date, most rotator cuff experimental studies have used acute injury and repair animal models (i.e., a healthy muscle and tendon is injured and then immediately repaired).3,18,33,35,37,38 These studies, while valuable to answer certain questions, are only relevant to a small percentage of the rotator cuff tear cases seen clinically.
Clinical observations of chronic rotator cuff tears show significant changes in the muscle such as atrophy, fat accumulation, and fibrosis.9,13 Muscle degeneration has a highly negative influence on the outcome of a rotator cuff repair, and can often render a tear irreparable. In some clinical settings, early repair is recommended before irreversible muscular damage takes place.13 Most of the clinical literature refers to this muscle damage as “fatty infiltration” in the affected rotator cuff muscle(s). However, it is unclear if the presence of fat in these atrophied muscles is due to infiltration of adipocytes, differentiation of muscle satellite cells, or transdifferentiation of local cells. A number of animal models have been proposed for the study of chronic rotator cuff disease.2,5,11,12,29,30 A rodent model of chronic rotator cuff tears would provide investigators the opportunity to study the mechanism of irreversible muscle degeneration - potentially using genetically modified mice - in an inexpensive and highly efficient model system.
The purposes of this study were: 1) to develop a rodent animal model of chronic rotator cuff tears that can reproduce fatty degeneration of the cuff muscles seen clinically, 2) to describe the effects of tear size, tear duration, and concomitant nerve injury on muscle degeneration, and 3) to evaluate the changes in gene expression of relevant myogenic and adipogenic factors following rotator cuff tears using the animal model. We hypothesized that: 1) surgically created chronic rotator cuff tears in rodents would produce muscle fatty degeneration similar to the degeneration seen after chronic rotator cuff tears in humans, 2) the severity of muscle degeneration would be related to cuff tear size and concomitant muscle denervation, and 3) the expression of myogenic and adipogenic genes would be upregulated following surgically created rotator cuff tears.
MATERIALS AND METHODS
Animal Model – Rats
The institutional Animal Studies Committee approved this study. Forty-five Sprague-Dawley rats were assigned to three treatment groups and three time points (n=5 per group). The first group of rats underwent tenotomy of the supraspinatus tendon at the greater tuberosity of the humerus to simulate a small cuff tear (“SS” group). A second group of rats underwent tenotomy of the supraspinatus and infraspinatus tendons to simulate a large cuff tear (“SS/IS” group). A third group of rats underwent suprascapular nerve neurotomy in addition to tenotomy of the supraspinatus and infraspinatus tendons to simulate a large cuff tear complicated by suprascapular neuropathy (“SS/IS Neurotomy” group). A group of five normal rats were used as a non-surgical control group (“Normal” group). The rats were sacrificed 2, 8, and 16 weeks after the procedure, and the Normal group rats were sacrificed 10 weeks after their arrival at our institution. Gene expression analysis was performed at all three timepoints and histological analysis was performed only at 8 and 16 weeks.
Animal Model – Mice
In order to validate the experiments performed in rats and provide the opportunity for future studies using transgenic animals, a subset of groups and assays were examined in a small number of mice. Six adult CD1 mice were divided into two groups. CD1 mice were chosen to provide consistency with prior studies examining the effect of loading on the development of the shoulder.7,17,36 One group of mice (n=3) underwent unilateral surgical detachment of the supraspinatus and infraspinatus tendons at the greater tuberosity of the humerus to simulate a large rotator cuff tear (“SS/IS” group). The other group of mice (n=3) underwent unilateral tenotomy of the supraspinatus and infraspinatus and also underwent suprascapular nerve transection at a location just proximal to the suprascapular notch (“SS/IS Neurotomy” group). The mice were sacrificed 8 weeks after the procedures.
Surgical Procedure
For detailed surgical methods, see Supplemental Document. The procedure for surgical detachment of the supraspinatus and infraspinatus tendons was identical in mice and rats. The supraspinatus and infraspinatus tendons were sharply detached from the greater tuberosity using a surgical blade. For the SS group rats, only the supraspinatus tendon was detached. For the animals assigned to the Neurotomy group, the suprascapular nerve was transected using microsurgical scissors at a location just anterior to the suprascapular notch. The animals were allowed normal cage activity postoperatively. The infraspinatus muscles of the animals in the SS group were not harvested at the time of dissection as it was expected that the infraspinatus would show minimal changes following tenotomy of only the supraspinatus.
Histology
For detailed histology methods, see Supplemental Document. The rat muscle specimens were processed for frozen sections and stained with Hematoxylin and Eosin (H & E) and Oil Red O; whereas the mouse specimens were processed with paraffin embedding and stained only with H & E. Specimens were sectioned in the longitudinal (coronal) plane at 10μm thickness. Intramyocellular fat “droplets” and intramuscular fat (i.e., fat globules between muscle fibers) were visualized using Oil red O stain, in which fat (triglyceride) was stained in distinct red color. Counter-staining with Hematoxylin was performed to visualize nuclei. The amount of intramuscular fat was graded semi-quantitatively with a 5-scale system (0, 1, 2, 3, or 4), where a grade 0 = no fat deposits and 4 = numerous fat deposits. The amount of intramyocellular fat droplets was graded similarly with a 5-scale system (0, 1, 2, 3, or 4), where a grade 0 = no fat droplets in muscle fibers and 4 = fat droplets found in most fibers. H & E stained histology was graded semi-quantitatively for the amount of intramuscular adipocytes, inflammatory cells in the endomysium, and atrophy of muscle fibers. Muscle atrophy was identified based on a few suggestive findings, such as the decreased muscle fiber size, an angular shape of muscle fibers as opposed to a round shape, decreased distance between myonuclei, centralization of myonuclei, etc. The muscle fiber size was not measure. Sections were graded by two independent investigators (an orthopaedic surgery fellow and a pathologist). The H & E histology grading scales are shown in Table I with the histology results. Mouse muscle sections were examined for intramuscular adipocytes, inflammatory cells in the endomysium, and muscle fiber atrophy, but no formal histology grading was performed on the mouse specimens.
Table 1.
Grading of H & E stained histology for rat rotator cuff muscles
| 8 weeks | ||||
|---|---|---|---|---|
| Groups | Muscle | Adipocytes (grade 0–3)* | Atrophy (grade 0–4)† | Endomysial inflammatory cells (grade 0–3)§ |
| Normal | Supraspinatus | 0 | 0 | 0 |
| Infraspinatus | 0 | 0 | 0 | |
| SS Tenotomy | Supraspinatus | 0 | 0 | 0 |
| Infraspinatus | 0 | 0 | 0 | |
| SS/IS Tenotomy | Supraspinatus | 0 | 0 | 0 |
| Infraspinatus | 0 | 0.8 | 0 | |
| SS/IS Neurotomy | Supraspinatus | 0 | 1.6 | 0.8 |
| Infraspinatus | 0.6 | 2 | 0.8 | |
| 16 weeks | ||||
| Groups | Muscle | Adipocytes (grade 0–3)* | Atrophy (grade 0–4)† | Endomysial inflammatory cells (grade 0–3)§ |
| Normal | Supraspinatus | 0 | 0 | 0 |
| Infraspinatus | 0 | 0 | 0 | |
| SS Tenotomy | Supraspinatus | 0 | 1.2 | 0.4 |
| Infraspinatus | 0 | 0 | 0 | |
| SS/IS Tenotomy | Supraspinatus | 0 | 1 | 0.6 |
| Infraspinatus | 0.4 | 1.7 | 0.5 | |
| SS/IS Neurotomy | Supraspinatus | 0 | 1.4 | 0.5 |
| Infraspinatus | 1.3 | 2.6 | 1.6 | |
Adipocytes grading: Grade 0 = no adipocyte, 1 = a few (< 5 in the entire field), 2 = some (5 to 20), 3 = many (> 20)
Atrophy grading: Grade 0 = no atrophy, 1 = minimal atrophy, 2 = mild atrophy, 3 = moderate atrophy, 4 = severe atrophy
Endomysial inflammatory cell grading: Grade 0 = no inflammatory cell, 1 = some, 2 = many, 3 = numerous
Data are shown as the means of the two investigators’ grading scores.
Gene Expression
For detailed gene expression methods, see Supplemental Document. Only rat specimens were used for gene expression analysis. The supraspinatus and infraspinatus muscles were harvested immediately post-mortem and total RNA was isolated using the TRIspin method.26 RNA extraction and DNase treatment was performed using the RNeasy mini-kit and DNase I (Qiagen, CA, USA). RNA yield was quantified using a NanoDrop spectrophotometer (Thermo Scientific, DE, USA). Five hundred nanograms of RNA were reverse-transcribed to cDNA using Superscript III RT kit (Invitrogen, CA, USA). Real-time PCR reactions were performed using Sybr Green chemistry on a 7300 sequence detection system (Applied Biosystems, CA, USA). A number of quality control steps were taken to ensure that results were accurate (see Supplemental Document. The target genes examined were: myogenic transcription factors (Myogenin, MyoD1, and Myf5), muscle fiber type markers (Myh4, fast myosin heavy chain type IIb isoform, and Myh7, slow myosin heavy chain type I isoform), adipogenic transcription factors (C/EBPα, CCAAT/enhancer binding protein alpha, and PPARγ2, Peroxisome Proliferator-Activated Receptor gamma2), adipogenic marker (Leptin), and myostatin. GAPDH was used as a housekeeping gene. Primers were purchased from Qiagen (Valencia, CA, USA). Results were expressed as fold differences relative to GAPDH and were calculated using the Delta Ct method.31
Statistical Methods
Muscle weight was compared between groups using an unpaired t-test. For gene expression data, groups were compared using a two-factor ANOVA (factors: time, injury group) followed by a least-square differences post-hoc test when the ANOVA demonstrated significance. Statistical significance was set at p < 0.05. Statistical analysis was not performed for the histology results, as they were semi-quantitative in nature.
RESULTS
Gross observation at dissection
All detached tendon stumps were found retracted medially at the time of dissection. In most animals from the SS/IS group, the tendon stumps were found adhered to the perimeter of the glenoid. In the animals from the SS group, the supraspinatus tendon stumps were found adhered to the glenoid with its end located slightly lateral to the glenoid. There was fibrous tissue filling the gap between the great tuberosity and the retracted tendon stump in all animals. Both the supraspinatus and infraspinatus muscles showed severe atrophy when there had been both tenotomy and neurotomy in that shoulder. The atrophy was less severe when there had been only tenotomy. None of the normal group animals showed muscle atrophy. Changes were similar when comparing mouse to rat. The mean supraspinatus muscle weight at 8 weeks was 0.64 ± 0.13g for the normal group, 0.58 ±0.08g for the SS group, 0.58 ± 0.05g for the SS/IS group, and 0.38 ± 0.09g for SS/IS Neurotomy group. The mean infraspinatus muscle weight was 0.64 ± 0.08g for the normal group, 0.55 ± 0.05g for the SS/IS group, and 0.33 ± 0.05g for the SS/IS Neurotomy group. Both muscles showed a significant weight decrease in the SS/IS Neurotomy group compared to the other groups (p < 0.01).
Histology
Mouse muscle histology
Frank adipocytes with the characteristic signet-ring shape were observed in both the supraspinatus and infraspinatus muscles of all mice that had received tenotomy of the supraspinatus and infraspinatus with/without suprascapular neurotomy (Figures 1 and 2). The adipocytes were found as clusters between muscle fibers. The amount of adipocytes was greater in the infraspinatus than in the supraspinatus and greater in the SS/IS Neurotomy group than in the SS/IS group. Atrophy and degenerative changes (e.g., inflammatory cells in the endomysium, centralization of myonuclei, etc) were also observed in these muscles, which were found to be more severe in the infraspinatus than in the supraspinatus and more severe in the SS/IS Neurotomy group than in the SS/IS group. There was no apparent trend for the distribution of adipocytes when comparing the lateral to the medial portion.
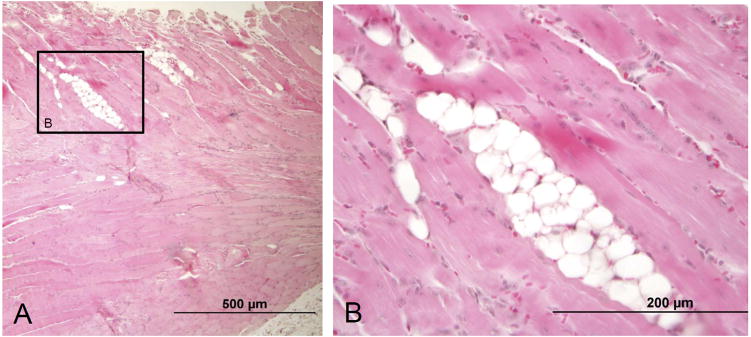
Figure 1.
(A) A mouse supraspinatus muscle at 8 weeks following tenotomy of both the supraspinatus and infraspinatus tendons shows several clusters of adipocytes within the muscle. (B) Adipocytes with the characteristic signet ring shape are clearly seen in a magnified view of the outlined area in (A). The muscle fibers show atrophy and degeneration, as evidenced by the small fiber size, centralized myonuclei, and rouleaux formation of myonuclei. [10X objective used in (A) and 40X objective used in (B); H & E stain.]
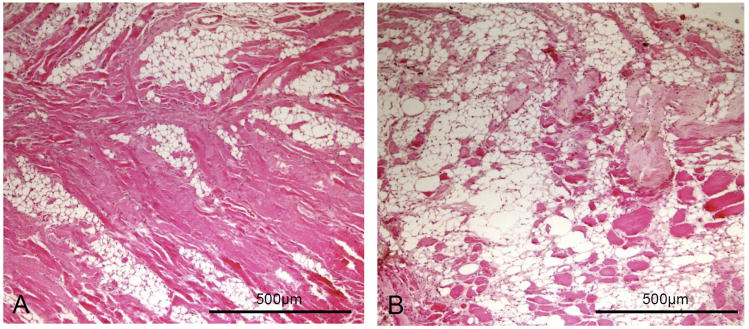
Figure 2.
(A) A mouse supraspinatus muscle at 8 weeks following tenotomy of the two tendons and neurotomy of the suprascapular nerve shows a substantial amount of adipocytes formed within the muscle with a substantial decrease of muscle fiber size. (B) A mouse infraspinatus muscle following tenotomy and neurotomy shows severe fatty degeneration with adipocytes occupying most of the space within the muscle. There are only a small number of degenerative muscle fibers left. [10X objective; H & E stain.]
Rat muscle histology
In H & E stained histology, adipocytes were observed in the infraspinatus of the SS/IS group and in the infraspinatus of the SS/IS Neurotomy group (Table 1). The 16-week specimens appeared to have more adipocytes than the 8-week specimens. Atrophic muscle fibers and increased endomysial inflammatory cells were also observed in the tenotomized and neurotomized muscles. Based on semi-quantitative grading, the SS/IS Neurotomy group had more severe changes than the SS and SS/IS groups, the infraspinatus had more severe changes than the supraspinatus, and the 16-week specimens had more severe changes than the 8-week specimens.
In Oil red O stain histology, positive staining for fat was observed in all of the muscles that received tenotomy only or tenotomy plus neurotomy (Figure 3). The quantity of fat was greater in the 16-week specimens compared to the 8-week specimens. The normal group showed no staining for fat. Therefore, there was an absolute increase in fat rather than a relative increase in fat. The positive staining occurred in two distinct distributions - intramuscular fat (i.e., fat located between muscle fibers) and intramyocellular fat droplets (i.e., fat located inside muscle fibers). There were more intramuscular fat globules in the infraspinatus than in the supraspinatus in the SS/IS group. This phenomenon was also observed in the SS/IS Neurotomy group. Intramyocellular fat droplets within muscle fibers were consistently found in the muscles that had received tenotomy only or tenotomy plus neurotomy (Figure 4). There were more intramyocellular fat droplets in the supraspinatus than in the infraspinatus within each animal. There were more intramyocellular fat droplets in the 16-week specimens than in the 8-week specimens. There was no apparent trend for the distribution of adipocytes or fat when comparing the lateral to the medial portion.
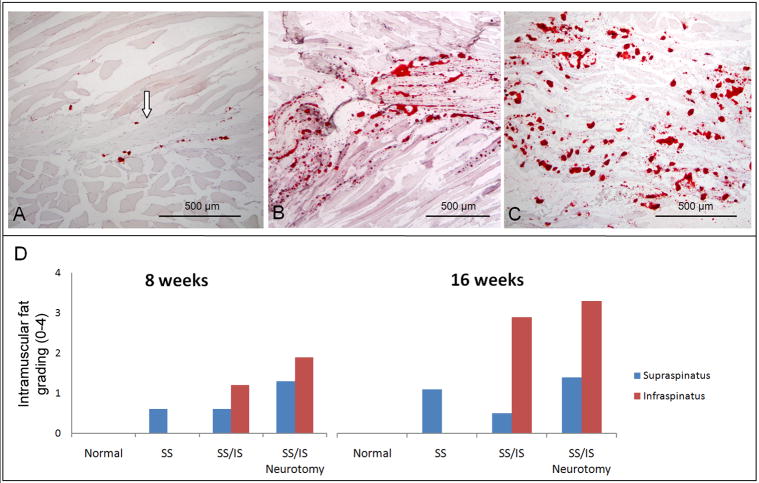
Figure 3.
(A) A normal rat supraspinatus muscle stained with Oil red O showing very few intramuscular fat deposits and intramyocellular fat droplets. The supraspinatus tendon can be seen at the center of the muscle (arrow) and the muscle fibers can be seen above and below the tendon. (B) The infraspinatus muscle of a rat 16 weeks following tenotomy of the supraspinatus and infraspinatus tendons. There are high numbers of fat deposits (seen as red dots). (C) The infraspinatus muscle of a rat 16 weeks following tenotomy plus neurotomy showing high levels of intramuscular fat. [10X objective; Oil red O stain.] (D) Histology grading results are shown for intramuscular fat on Oil red O stained histology sections. Normal muscles showed no fat. After tenotomy of the supraspinatus and infraspinatus tendons, the infraspinatus muscle had more intramuscular fat than the supraspinatus muscle. The 16-week specimens had more intramuscular fat than the 8-week specimens within each group.
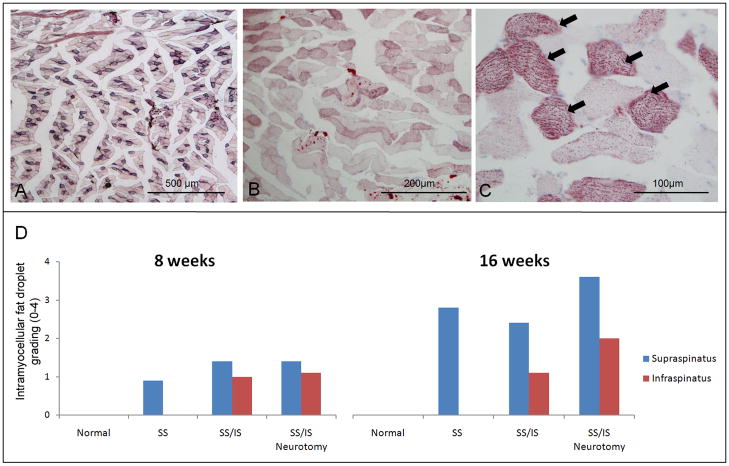
Figure 4.
(A) The supraspinatus muscle of a rat at 16 weeks following tenotomy of the supraspinatus tendon showed a number of muscle fibers stained with Oil red O. However, there was almost no intramuscular fat observed. (B) A micrograph of the supraspinatus muscle of a rat at 16 weeks following tenotomy of the supraspinatus and infraspinatus shows higher levels of intramyocellular fat droplets in the muscle fibers. (C) A higher magnification of (B) shows fine, granule-shaped fat droplets within muscle fibers. A subset of muscle fibers showed high levels of fat droplets (arrows), making them appear as darkly stained fibers at low magnification. [10X objective used in (A), 20X objective used in (B), and 40X objective used in (C); Oil red O stain.] (D) Histology grading for intramyocellular fat droplets on Oil red O stained sections is shown. The normal muscles showed no fat droplets while the muscle that had received tenotomy only or tenotomy plus neurotomy showed intramyocellular fat droplets. The supraspinatus muscle had more fat droplets than the infraspinatus muscle when there had been tenotomy of both tendons. Overall, the 16-week specimens showed more fat droplets than the 8-week specimens.
Gene expression
Myogenic markers
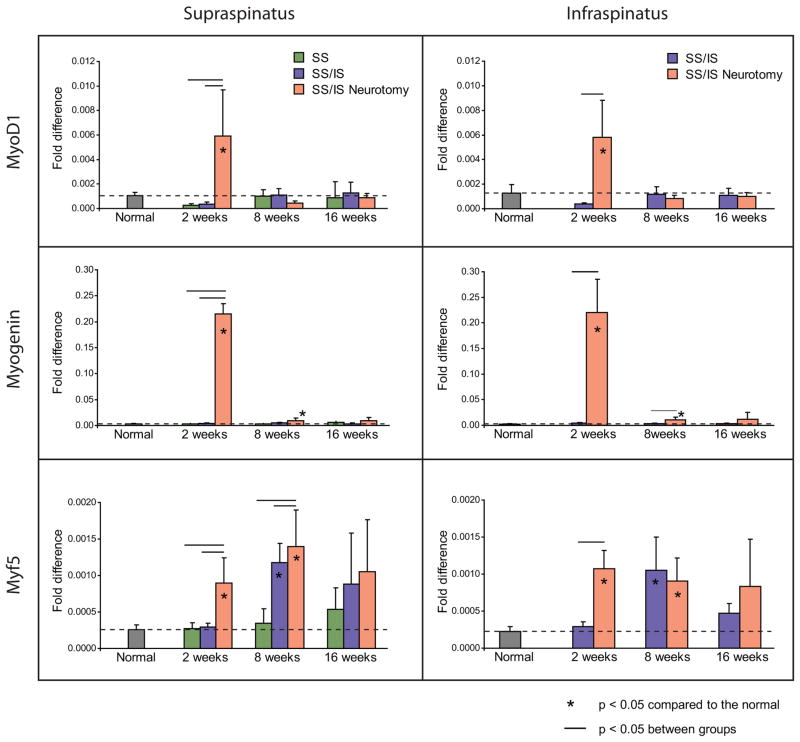
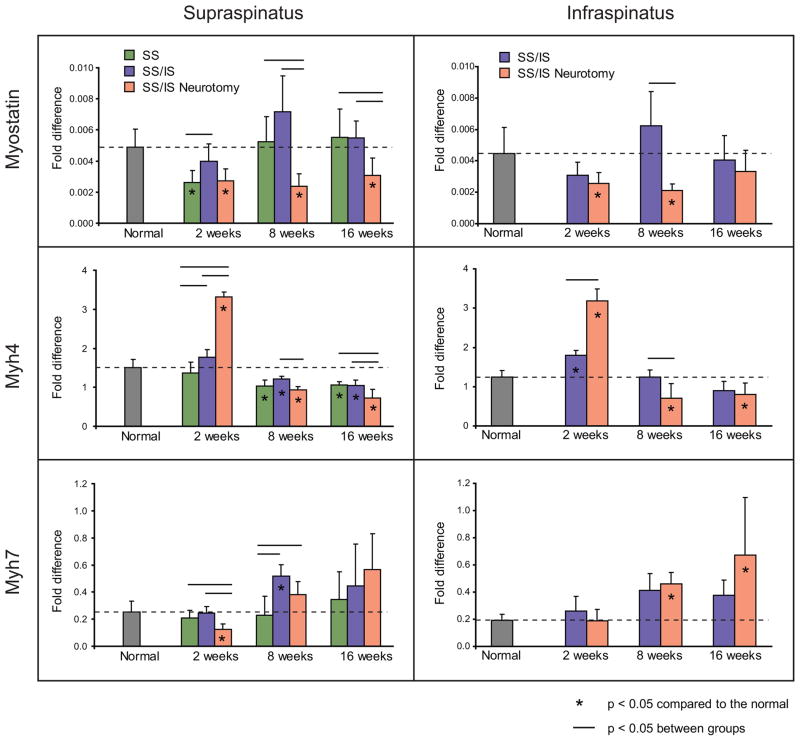
The myogenic transcription factors MyoD1, myogenin, and Myf5 were significantly upregulated at 2 weeks in the supraspinatus and infraspinatus muscles of the SS/IS Neurotomy group compared to the other groups (p < 0.05) (Figure 5). Myf5 was also significantly upregulated in both muscles at 8 weeks in the SS/IS and SS/IS Neurotomy groups compared to the normal and SS groups (p < 0.05) (Figure 5). Myostatin, a negative regulator of muscle development, was significantly down-regulated in both muscles at most time points in the SS/IS Neurotomy group (p < 0.05) (Figure 6). Initially, Myh4 (fast twitch muscle fiber isoform) was found significantly upregulated in the SS/IS Neurotomy group compared to the other groups at 2 weeks (p < 0.05), but this trend was reversed at the later time points showing significant downregulation at 8 and 16 weeks (p < 0.05). On the other hand, Myh7 (slow twitch muscle fiber isoform) was found initially significantly down-regulated in the supraspinatus of the SS/IS Neurotomy group compared to the other groups at 2 weeks (p < 0.05). This trend was reversed at the later time points showing slight upregulation in the supraspinatus (p > 0.05) and significant upregulation in the infraspinatus (0 < 0.05) at 8 and 16 weeks (Figure 6).
Figure 5.
The myogenic transcription factors MyoD1, myogenin, and Myf5 were upregulated at 2 weeks in supraspinatus and infraspinatus muscles of the SS/IS Neurotomy group compared to normal muscles and the muscles in the SS and SS/IS groups. Myf5 was also upregulated in both muscles at 8 weeks in the SS/IS and SS/IS Neurotomy groups. Means and standard deviation error bars are shown. Results are reported as fold-changes relative to the expression of the housekeeping gene GAPDH. [*p < 0.05 compared to normal; horizontal lines above bars indicate p < 0.05 between groups.]
Figure 6.
Myostatin was down-regulated in both muscles at most timepoints in the SS/IS Neurotomy group. At the earliest timepoint, Myh4 (fast twitch muscle isoform) was upregulated and Myh7 (slow twitch isoform) was down-regulated in the SS/IS Neurotomy group. This trend was reversed at the later timepoints. Means and standard deviation error bars are shown. Results are reported as fold-changes relative to the expression of the housekeeping gene GAPDH. [*p < 0.05 compared to normal; horizontal lines above bars indicate p < 0.05 between groups.]
Adipogenic markers
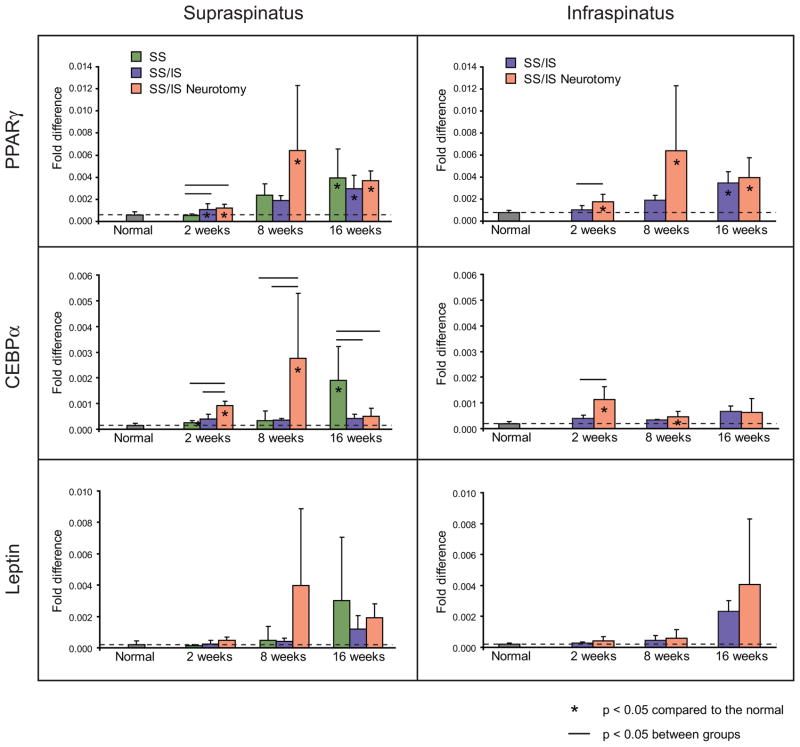
Adipogenic transcription factors were upregulated in the muscles of the SS/IS and SS/IS Neurotomy groups (Figure 7). PPARγ2 was significantly upregulated in both muscles at all time points in the SS/IS Neurotomy group (p < 0.05). C/EBPα was significantly upregulated in both muscles at 2 and 8 weeks in the SS/IS Neurotomy group (p < 0.05). Expression of leptin was increased at the later time points in most groups, but these increases were not statistically significant compared to the normal group (p > 0.05).
Figure 7.
Adipogenic transcription factors were upregulated in the muscles of the SS/IS and SS/IS Neurotomy groups. PPARγ2 was upregulated in both muscles at all timepoints in the SS/IS Neurotomy group. C/EBPα was significantly upregulated in both muscles at 8 and 16 weeks in the SS/IS Neurotomy group. Expression of leptin was increased at the later timepoints in most groups, but these increases were not statistically significant. Means and standard deviation error bars are shown. Results are reported as fold-changes relative to the expression of the housekeeping gene GAPDH. [*p < 0.05 compared to normal; horizontal lines above bars indicate p < 0.05 between groups.]
DISCUSSION
The present study investigated the feasibility of a rodent animal model for fatty degeneration of the rotator cuff muscles following chronic rotator cuff tears. We hypothesized that rotator cuff tears in rodents would result in muscle fatty degeneration and that the severity of muscle degeneration would be related to tear size and concomitant muscle denervation. The results of the present study supported both hypotheses, demonstrating that unloading a healthy adult rotator cuff through tenotomy and denervation leads to muscle degeneration that is similar to the atrophy and fatty degeneration seen clinically and that these changes are more severe in larger tears and tears complicated with a nerve injury.
Given the negative impact of fatty degeneration on the functional and anatomical outcomes following shoulder surgery13, understanding the mechanisms leading to degeneration is critical in order to develop effective prevention and treatment interventions. In this regard, animal models have been developed to reproduce rotator cuff muscle degeneration. These studies were performed on sheep, dogs, and rabbits, and various surgical methods were used including barriers wrapping the detached tendon stumps to prevent spontaneous healing and detachment of the subscapularis rather than the supraspinatus or infraspinatus.5, 28, 29, 30 In contrast to other animals, rodent have important advantages for studying rotator cuff disease because they have a similar anatomy to humans32, are easy to handle, are inexpensive, and most importantly, have the potential for targeted genetic manipulation. Attempts have been made to extend the rat rotator cuff model to include chronic degeneration.2,11,12 However, fatty degeneration, the hallmark of chronic degeneration seen clinically, has not previously been demonstrated.
In our study, adipocytes with the characteristic signet-ring shape were observed in the mouse supraspinatus and infraspinatus muscles following detachment of the tendons. These adiopocytes were found as clusters between muscle fibers and did not appear to have a specific distribution pattern within a muscle. Muscle fiber atrophy and inflammatory changes in the endomysium were also observed. All these changes were more severe when there was concomitant denervation of the muscle. The rat supraspinatus and infraspinatus muscles also showed adipocytes, but fewer than the mouse muscles. Although no quantitative comparison was made between the mouse and rat specimens, it appeared that the mouse muscles developed more severe changes than the rat muscles at the 8-week time point.
Fatty degeneration in the human rotator cuff is most commonly observed in large tears and is seldom observed in small tears with minimal retraction. In the present study, the small tears involving only the supraspinatus showed no adipocytes, suggesting that tear size and subsequent muscle unloading is associated with the development of fatty degeneration. Additionally, there is a growing amount of clinical evidence that suprascapular neuropathy may be associated with large rotator cuff tears.6,21 Based on this, the presence of a nerve injury in the setting of a massive tear might play important roles in the development of intramuscular adipocytes.
The infraspinatus had more severe fatty degeneration and more intramuscular fat globules than the supraspinatus following tendon detachment and denervation. We do not have clear explanation for this, but it may be that each muscle has a different reaction pattern to tendon and nerve injuries. For instance, the amount of mesenchymal stem cells capable of adipogenesis and myogenesis may be different between muscles. It was also noted that the supraspinatus had more intramycellular fat droplets than the infraspinatus following tendon detachment and denervation. The mechanism for this phenomenon is unclear. According to the study of Barton et al2, the rat infraspinatus is almost exclusively composed of fast twitch fibers whereas the supraspinatus has a mixture of fast and slow fibers. Slow fibers are known to primarily use an oxidative pathway for their energy metabolism and have more intracellular fat than fast fibers, which use a glycolytic pathway.19,20,34 Thus, the rat supraspinatus may have a higher propensity for fat droplet accumulation than the infraspinatus following tendon and nerve injuries.
Myogenic transcription factors are upregulated and the negative regulator, myostatin, is down-regulated after tendon detachment, especially when combined with denervation. MyoD1 and Myogenin showed a dramatic increase at 2 weeks and returned close to normal levels at the later time points. Myf5 showed a relatively consistent increase throughout the time points. The downregulation of myostatin may be related to the upregulation of the myogenic factors as myostatin is known to downregulate myogenic factors. The increase of the myogenic factors Myf5 has been reported after tenotomy in a sheep animal model study.8 The upregulation of the myogenic transcription factors associated with tenotomy may indicate a failed attempt at muscle repair in the injured tissue (i.e., although myogenic factors are increased at the mRNA level, the muscle tissue atrophies overall). The adipogenic transcription factors (PPARγ2 and C/EBPα) were also found to be upregulated, especially in the muscles with both tendon detachment and denervation. Our findings are consistent with those of Frey et al who reported the upregulation of PPARγ following detachment of the infraspinatus tendon in sheep.8
The present study showed that the gene expression of muscle isoforms changed in a certain pattern following tendon detachment and denervation. At the early time points, the gene for the fast twitch fiber (Myh4) was upregulated while the gene for the slow twitch fiber (Myh7) was down-regulated. This trend was reversed at later time points showing downregulation of the fast fiber gene and upregulation of the slow fiber gene. This finding is consistent with our observation that the content of intramycellular fat droplets increased substantially at the later time points because slow fibers have more intracellular fat than fast fibers.19,20,34 It has been reported in the literature that skeletal muscles undergo a fiber type shift from slow to fast type following muscle unloading such as disuse, weightlessness, tenotomy, or denervation.16,23,25 Our finding is slightly different in that the initial slow-to-fast shift was reversed to the fast-to-slow shift at the later time points. Further investigation is necessary to determine whether the changes in gene expression reflect true fiber type changes or simply the changes of gene expression levels without actual fiber type changes.
Fat accumulated within the injured rotator cuff muscles via two distinct mechanisms. Intramyocellular fat droplets increased within the sarcoplasma, and fat globules appeared outside the muscle cells. These processes, while occurring simultaneously, are likely due to different mechanisms. Intramyocellular fat is known to be a source of energy for muscle cells via the oxidative pathway, which is the primary method of energy production in slow twitch fibers. This fat also accumulates pathologically in the setting of a high fat diet, contributes to insulin insensitivity, and is seen in lipid storage diseases. Therefore, the accumulation could be due to an alteration in metabolism or a shift in muscle isoform in response to unloading. Adipocytes outside the muscle cells may be present secondary to differentiation of cells.
There are several important limitations to our study. First, the histological analysis was semi-quantitative. Because of the qualitative and descriptive nature of histological outcome measures, it was not appropriate to treat our grading scales as quantitative for statistical analysis. To minimize the potential for incorrect conclusions, two investigators with extensive experience in muscle histopathology independently reviewed the histology specimens, blinded to groups and time points. Second, because the equivocal time points between rodents and humans are uncertain, it is not known how the time points that we examined relate to temporal changes seen in humans. Clinically, the definition of an acute vs. chronic rotator cuff tear is debatable and unclear. Regardless, knowledge of the degeneration process and associated pathology will contribute to the information base relevant to the treatment of muscle disorders. Third, although little is known clinically about the contribution of suprascapular neuropathy to rotator cuff disease, there has been a surge of interest and evidence to support a role. The nerve injuries we created in the present animal study were acute transections of the nerve immediately following tendon detachment. Thus, the neurotomized muscles in our study may not have undergone the same degenerative process as human rotator cuff tears with concomitant nerve injuries. Fourth, the effect of neurotomy alone was not investigated in this study, and this may make it difficult to interpret the more severe degenerative changes seen in the muscles with both neurotomy and tenotomy. However, there have been a number of studies that reported the radiographic absence of fatty degeneration in patients with isolated suprascapular neuropathy.1,4,22,27,40 Typically, patients with suprascapular neuropathy show only atrophy of the cuff muscles without fatty degeneration unless there is a concomitant large rotator cuff tear. Fifth, functional assessment of the muscle was not performed. Although muscle atrophy and fatty degeneration relate directly to muscle function7, further study is necessary to determine how rotator cuff degeneration affects muscle contractile ability. Future studies will examine the passive mechanics and the electromyographic activity of the muscles. Sixth, we did not include age-matched controls for the three different injury time points. Based on ethical considerations and available resources, a single adult time-point was used for the normal controls. However, we expect that muscle histology and muscle gene expression remains relatively unchanged during this time period, as the rats were neither adolescent nor aged. Seventh, while most degenerative rotator cuff tears in humans are chronic and gradual in nature, tenotomy and neurotomy of the present study represent an acute and complete event. Thus, there may be some differences between the fatty degeneration observed in the present study and the fatty degeneration seen in human rotator cuff tears. Lastly, the results of this study included a limited sample size and focused on a predetermined set of genes. This necessitates future studies with a larger sample size and expansive gene expression analysis
CONCLUSION
In summary, the present study describes a rodent animal model that produced fatty degeneration of the rotator cuff muscles similar to the fatty degeneration seen in torn human rotator cuffs. Fatty degeneration was evidenced by the histological observation of apparent adipocytes, intracellular and extracellular fat accumulation, atrophy, and inflammatory cells in the endomysium. Mice developed more severe fatty degeneration, in a shorter time, than rats. Adiopocytes were observed only in large tears involving both the supraspinatus and infraspinatus while no adipocytes were observed in small tears involving only the supraspinatus, suggesting the association of tear size with fatty degeneration. Fatty degeneration appeared more severe in tears with both tendon detachment and denervation than those with only tendon detachment, suggesting a possible role of nerve injury in fatty degeneration. This new animal model provides the opportunity for studying the pathomechanisms of rotator cuff disease in a highly efficient system. In particular, the use of transgenic mice in future studies provides the opportunity for mechanistic studies of the degenerative process.
Supplementary Material
Acknowledgments
Funding sources: This study was funded by a National Institutes of Health grant (R01 AR055580) and an Orthopaedic Research and Education Foundation Career Development award.
Footnotes
Animal studies committee approval: The Animal Studies Committee of Washington University approved the protocol for the use of animals in conjunction with the research project related to this paper. The protocol was approved on 1/25/2010 (approval #20090368) and expired on 1/25/2013.
Level of evidence: Basic Science Study, Laboratory Study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antoniou J, Tae SK, Williams GR, Bird S, Ramsey ML, Iannotti JP. Suprascapular neuropathy. Variability in the diagnosis, treatment, and outcome. Clin Orthop Relat Res. 2001:131–8. [PubMed] [Google Scholar]
- 2.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259–65. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen AL, Ong BC, Rose DJ. Arthroscopic management of spinoglenoid cysts associated with SLAP lesions and suprascapular neuropathy. Arthroscopy. 2003;19:E15–21. doi: 10.1016/s0749-8063(03)00381-5. S0749806303003815 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Coleman SH, Fealy S, Ehteshami JR, MacGillivray JD, Altchek DW, Warren RF, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85-A:2391–402. doi: 10.2106/00004623-200312000-00018. doi: not available. [DOI] [PubMed] [Google Scholar]
- 6.Costouros JG, Porramatikul M, Lie DT, Warner JJ. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007;23:1152–61. doi: 10.1016/j.arthro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Das R, Rich J, Kim HM, McAlinden A, Thomopoulos S. Effects of botulinum toxin-induced paralysis on postnatal development of the supraspinatus muscle. J Orthop Res. 2011;29:281–8. doi: 10.1002/jor.21234. BLINDED FOR REVIEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, et al. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res. 2009;27:504–9. doi: 10.1002/jor.20695. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 10.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–24. doi: 10.2106/00004623-200402000-00002. doi: not available. [DOI] [PubMed] [Google Scholar]
- 11.Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004:258–65. doi: 10.1097/01.blo.0000136831.17696.80. [DOI] [PubMed] [Google Scholar]
- 12.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–49. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 14.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–9. [PubMed] [Google Scholar]
- 15.Iannotti JP, Naranja RJ, Gartsman GM. Surgical treatment of the ifact cuff and reparable cuff defect: Arthroscopic and open techniques. In: Norris TR, editor. Orthopaedic Knowledge Update: Shoulder and Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1994. pp. 151–55. [Google Scholar]
- 16.Jiang B, Roy RR, Edgerton VR. Expression of a fast fiber enzyme profile in the cat soleus after spinalization. Muscle Nerve. 1990;13:1037–49. doi: 10.1002/mus.880131107. [DOI] [PubMed] [Google Scholar]
- 17.Kim HM, Galatz LM, Das R, Patel N, Thomopoulos S. Musculoskeletal deformities secondary to neurotomy of the superior trunk of the brachial plexus in neonatal mice. J Orthop Res. 2010 doi: 10.1002/jor.21128. BLINDED FOR REVIEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike Y, Trudel G, Uhthoff HK. Formation of a new enthesis after attachment of the supraspinatus tendon: A quantitative histologic study in rabbits. J Orthop Res. 2005;23:1433–40. doi: 10.1016/j.orthres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochemistry and cell biology. 2001;116:63–8. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 20.Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1316–21. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- 21.Mallon WJ, Wilson RJ, Basamania CJ. The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg. 2006;15:395–8. doi: 10.1016/j.jse.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 22.McCluskey L, Feinberg D, Dolinskas C. Suprascapular neuropathy related to a glenohumeral joint cyst. Muscle Nerve. 1999;22:772–7. doi: 10.1002/(sici)1097-4598(199906)22:6<772::aid-mus16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Musacchia XJ, Steffen JM, Fell RD. Disuse atrophy of skeletal muscle: animal models. Exerc Sport Sci Rev. 1988;16:61–87. [PubMed] [Google Scholar]
- 24.Naranja RJ, Iannotti JP, Gartsman GM. Complications in rotator cuff surgery. In: Norris TR, editor. Orthopaedic Knowledge Update: Shoulder and Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1994. pp. 157–66. [Google Scholar]
- 25.Ohira Y, Yoshinaga T, Nomura T, Kawano F, Ishihara A, Nonaka I, et al. Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv Space Res. 2002;30:777–81. doi: 10.1016/s0273-1177(02)00395-2. Pii S0273-1177(02)00395-2. [DOI] [PubMed] [Google Scholar]
- 26.Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–6. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 27.Romeo AA, Rotenberg DD, Bach BR., Jr Suprascapular neuropathy. The Journal of the American Academy of Orthopaedic Surgeons. 1999;7:358–67. doi: 10.5435/00124635-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R. Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg Am. 2010;92:2270–8. doi: 10.2106/JBJS.I.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubino LJ, Stills HF, Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23:717–22. doi: 10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87:2662–70. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. Journal of Shoulder & Elbow Surgery. 1996;5:383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 33.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Annals of Biomedical Engineering. 2002;30:1057–63. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 34.Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–28S. doi: 10.1093/jn/135.7.1824S. 135/7/1824S [pii] [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–63. doi: 10.1016/S0736-0266(01)00144-9. BLINDED FOR REVIEW. [DOI] [PubMed] [Google Scholar]
- 36.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–63. doi: 10.1002/jor.20418. BLINDED FOR REVIEW. [DOI] [PubMed] [Google Scholar]
- 37.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125:106–13. doi: 10.1115/1.1536660. BLINDED FOR REVIEW. [DOI] [PubMed] [Google Scholar]
- 38.Uhthoff HK, Sano H, Trudel G, Ishii H. Early reactions after reimplantation of the tendon of supraspinatus into bone. A study in rabbits. J Bone Joint Surg Br. 2000;82:1072–6. doi: 10.1302/0301-620x.82b7.9986. [DOI] [PubMed] [Google Scholar]
- 39.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181–7. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Westerheide KJ, Dopirak RM, Karzel RP, Snyder SJ. Suprascapular nerve palsy secondary to spinoglenoid cysts: results of arthroscopic treatment. Arthroscopy. 2006;22:721–7. doi: 10.1016/j.arthro.2006.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.