Abstract
Membrane fusion between the lamellar bodies and plasma membrane is an obligatory event in the secretion of lung surfactant. Previous studies have postulated a role for annexin A7 (A7) in membrane fusion during exocytosis in some cells including alveolar type II cells. However, the intracellular trafficking of A7 during such fusion is not described. In this study, we investigated association of endogenous A7 with lamellar bodies in alveolar type II cells following treatment with several secretagogues of lung surfactant. Biochemical studies with specific antibodies showed increased membrane-association of cell A7 in type II cells stimulated with agents that increase secretion through different signaling mechanisms. Immuno-fluorescence studies showed increased co-localization of A7 with ABCA3, the lamellar body marker protein. Because these agents increase surfactant secretion through activation of PKC and PKA, we also investigated the effects of PKC and PKA inhibitors, bisindolylmaleimideI (BisI) and H89, respectively, on A7 partitioning. Western blot analysis showed that these inhibitors prevented secretagogue-mediated A7 increase in the membrane fractions. These inhibitors also blocked increased co-localization of A7 with ABCA3 in secretagogue-treated cells, as revealed by immuno-fluorescence studies. In vitro studies with recombinant A7 showed phosphorylation with PKC and PKA. The cell A7 was also phosphorylated in cells treated with surfactant secretagogues. Thus, our studies demonstrate that annexin A7 relocates to lamellar bodies in a phosphorylation-dependent manner. We suggest that activation of protein kinase promotes phosphorylation and membrane-association of A7 presumably to facilitate membrane fusion during lung surfactant secretion.
Keywords: Lung surfactant secretion, exocytosis, membrane fusion, lamellar bodies, protein kinase inhibitors, protein kinase C, cAMP-dependent protein kinase, A7 phosphorylation
Lung surfactant is essential for normal gas-exchange as it prevents lung collapse at low volumes by lowering surface tension at air-liquid interface during end-expiration. The principal surface-active component, phosphatidylcholine, and other components of lung surfactant are synthesized and secreted by lung epithelial type II cells by exocytosis of stored surfactant in lamellar bodies. One of the obligatory events during secretion is the membrane fusion between lamellar bodies and plasma membrane in type II cells. Although several agents promote lung surfactant secretion, and intracellular signaling mediating secretion has been extensively investigated [1–3], the mechanisms that regulate such membrane fusion have been relatively poorly investigated. We have previously shown that one of the annexin proteins, annexin A7 (A7), can facilitate membrane fusion between lamellar bodies and plasma membrane in vitro[4] and that it can promote surfactant secretion in semi-permeable alveolar type II cells [5]. More recently, our in vitro studies suggested that diacylglycerol could regulate A7 function during membrane fusion, since lamellar body enrichment with diacylglycerol increased the A7-mediated membrane fusion activity [6].
In the scheme of A7-mediated membrane fusion during surfactant secretion, we have postulated that binding of A7 to lamellar bodies or plasma membrane would facilitate the membrane fusion [7]. Several studies have suggested involvement of soluble N-ethylmaleimide-sensitive fusion protein attachment receptors (SNARE) proteins in membrane fusion during exocytosis (reviewed in [8–11]). The assembly of SNARE protein complex is best characterized in exocytosis at the synapse or intracellular cargo delivery in the Golgi. The fusion complex formation involves members of the vesicle (v)-SNARE (synaptic vesicle-associated membrane protein, VAMP) and target (t)-SNARE (members of syntaxin and snap families) proteins. The pairing of cognate SNARE members allows ‘zippering’ to affect close apposition of the two fusing membranes [12]. Although it is unclear if tight apposition between fusing membranes is sufficient to allow fusion, the presence of SNAREs in liposome preparation is shown to achieve membrane fusion [13]. Nevertheless, a role for SNARE proteins in, at least, docking of secretory vesicles on target membrane has been proposed in several studies. In addition, other proteins have been invoked to facilitate membrane fusion in type II cells. Lipid vesicle fusion is facilitated by at least two of the annexin proteins, annexin A2 [14, 15] and A7 [7, 16, 17]. Some members of the SNARE family are present in type II cells and have been suggested to play a role in lung surfactant secretion [18]. Although it is proposed that annexin A2 and some of the SNARE proteins can influence surfactant secretion, the regulation of interactions between A2 and SNARE proteins has not been investigated.
We and others have suggested that protein phosphorylation may be one mechanism for membrane-association of annexin A7 [17, 19]. In our previous studies, we have demonstrated preferential binding of purified bovine A7 to isolated lamellar bodies and plasma membrane fractions [19]. The in vitro binding to plasma membrane and lamellar body fractions from secretagogue-stimulated cells was higher [19]. These studies also demonstrated that stimulation of cells in presence of PKC inhibitor blocked the increased in vitro binding. In chromaffin cells, phosphorylation of A7 with carbachol was increased in the absence but not in the presence of PKC inhibitors [17]. However, intracellular trafficking of endogenous A7 and targets of A7 relocation has not been identified. In the current study, we determined the ‘in vivo’ partitioning of cell A7 between the membrane and cytosol fractions. Immuno-staining with specific antibodies was employed to determine A7 trafficking to lamellar bodies, since these are involved in surfactant secretion. Our studies demonstrate that all of the established secretagouges for lung surfactant increase membrane partitioning of cell A7 in type II cells. Some of this increase appears to be due to partitioning into lamellar body membranes as determined from co-localization of A7 with ABCA3 also known as ABCA3, a lamellar body specific protein [20, 21]. Since protein kinase activation with various secretagogues is important for stimulated secretion in type II cells, we also investigated membrane-association of endogenous A7 in presence of PKC and PKA inhibitors. Our studies support that increased membrane-association of A7 in domains containing ABCA3, i.e., lamellar bodies, is facilitated by protein kinase activation, which could be permissive for membrane fusion during secretion.
METHODS
Preparation of Annexin A7 antibodies
Bacterially expressed purified A7 [22] was used to raise antibodies in rabbits through a commercial source. The pooled antiserum from two rabbits was cleaned by passing through rat serum proteins coupled to CNBR-activated Sepharose 4B, before use for studies reported here.
Isolation of alveolar type II cells
Lungs of male Sprague-Dawley rats (200 – 300 g, body weight) were used for isolation of alveolar type II cells following protease digestion as described previously [23]. The institutional animal care and use committee had approved the handling and use of rats for studies reported here. Briefly, rats were anesthetized with Nembutal (50 mg/kg, ip) and sacrificed by exsanguinations after severing major abdominal blood vessels. The lungs were ventilated with a rodent respirator and visibly cleared by perfusion through pulmonary artery. Lungs were excised following intra-tracheal instillation of porcine elastase (3 units/ml) and incubated to partially digest the lung tissue. The lung lobes were minced on a tissue-chopper, and the lung mince was mixed vigorously to release free cells that show enrichment with alveolar type II cells. The major contaminating cells, the macrophages, were removed by adherence to bacteriological plates coated with rat serum IgG, which facilitates adherence of macrophages through the Fc receptors. The free cells showing ~90% purity of type II cells (by acridine orange staining [24]) were suspended in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) and cultured for 20–22 h in tissue culture plastic dishes. The adherent cells at the end of this culture period showed >95% purity of type II cells and >97% routinely exclude vital dye Trypan Blue. The adherent cells were used for all studies reported here.
Cell treatment with agents
Following culture of type II cells for indicated periods, the cells were washed repeatedly and equilibrated in fresh MEM for 30 min before addition of secretagogues and incubated for specified periods. Various agents were added from (100x) stock solutions. A stock solution of ATP (100mM) was prepared in ice-cold water. In case of phorbol myristate acetate or A23187, the stock solutions were prepared in ethanol and diluted 10-fold in MEM before addition so that the medium contained 0.1% ethanol. Following incubations, the medium was removed and the cells harvested by scarping in homogenization buffer (50mM Tris-100mM NaCl-0.5mM EDTA-0.5mM EGTA, pH 7.4) containing protease and phosphatase inhibitors (1mM PMSF and a mixture of leupeptin, pepstatin and aprotinin, each 5μg/ml, 1mm Na orthovanadate). The cells were disrupted by sonication on ice (3 × 10sec) followed by passage through 27 1/2G needle (×5) and the membrane and the cytosol fractions were isolated by differential centrifugation (100,000g for 60min) where applicable.
Isolation of lung lamellar bodies
Rat lungs were cleared of residual blood by perfusion as described above for isolation of alveolar type II cells. The visibly cleared lungs were then given a bolus of 5ml 1M sucrose and the lung lobes harvested, dissected free of airways and blotted on gauze pads. For isolation of lamellar bodies by previously described methods [25], lung lobes were disrupted with a Polytron® type tissue disruptor and homogenized in 1M sucrose in Potter-Elvehjam type glass homogenizer. The lamellar body enriched fraction from lung homogenate was obtained by upwards flotation following centrifugation on a discontinuous gradient of 0.8 to 0.2M sucrose. The lamellar body enriched fraction at the 0.5 and 0.4M sucrose interface was diluted to 0.2M sucrose and the purified lamellar bodies were isolated by differential centrifugation. We have previously carried out extensive characterization of thus isolated lamellar bodies in terms of phospholipids composition, ultra-structure, and physiological properties of maintenance of acidic pH and transport of other ions [24–26].
Immunoprecipitation of annexin A7
In some experiments, the adherent cells were harvested by scraping in ice-cold non-denaturing lysis buffer (50mM Tris-HCl, pH 7.4, 0.3M NaCl, 5mM EDTA, 0.02% Na azide and 1% octyl β-D-glucoside) to which 10mM iodoacetamide, 1mM PMSF, 1μg/ml aprotinin and 2μg/ml leupeptin were added before use. All processing was done at or below 4° C unless indicated. The suspension was transferred to pre-cooled centrifuged tubes, vortexed gently and lysed on ice for at least 30 min. The suspension was cleared by centrifugation (16000g × 15min) and used for immuno-precipitation with purified antibodies to A7 that were bound to protein A-Sepharose beads. The beads were pre-treated with preimmune serum before binding of antibodies. Pilot studies were carried out to verify that preimmune serum did not precipitate A7 from cell lysate. The antibody-bound beads (10μl) were incubated with bovine serum albumin (10μl of 10%) to block non-specific binding sites. The clarified supernatant was added and the beads incubated overnight with end-over-end mixing. The beads were centrifuged (100g for 150sec), washed (x4) with lysis buffer and once with ice-cold phosphate-buffered saline. The proteins bound to the beads were eluted and analyzed by immuno-blotting using appropriate primary and HRP-conjugated secondary antibodies.
Western blot analysis
Equal proportions of isolated fractions from cells or immuno-precipitated proteins were resolved on SDS-PAGE (4–20%), transferred to PVDF membranes, blocked with blocking solution containing 5% bovine serum albumin or fat-free dry milk, and reacted with primary antibodies (1:1000 dilution unless indicated). The membranes were then reacted with species-specific secondary antibodies (1:10,000 dilution) and the antigen(s) detected by chemiluminiscence using Super-Signal® reagent (Pierce). In experiments determining A7 distribution into the membrane and cytosol fractions, equal proportions of both fractions were analyzed for the presence of A7 by Western blot analysis. Blots were imaged by exposing to X-ray films or with a photo-imager (SynGene). Relative proportions of protein in each fraction were determined by densitometry and expressed as percent distribution.
Immuno-staining of cells
In studies designed to investigate A7 distribution by fluorescence imaging, type II cells were cultured for 20–22h on glass cover slips. The cover slips with adherent cells were washed and incubated with agents as indicated, washed and fixed in ice-cold methanol for 15min at −20° C. In some experiments, the cells were fixed in 2% paraformaldehyde and then permeabilized by a 10min-treatment with 0.1% Triton X-100. Both techniques yielded comparable results. The cells were washed with PBS and stored at 4° C in PBS until processing. For immuno-staining, the fixed cells were blocked with donkey serum and treated for two hours with the primary antibodies (rabbit anti-annexin A7 antibodies and monoclonal anti-ABCA3 from Covance, 1:500, each). The cells were washed and treated with FITC- or Alexa568-labeled species-specific secondary antibodies (1:500, each). The immuno-stained cells were viewed and imaged using a laser confocal microscope.
Phosphorylation of recombinant annexin A7
Commercially available PKC and catalytic subunit of cAMP-dependent protein kinase were employed to verify phosphorylation of rA7 according to previous published reports [17]. Briefly, purified A7 (1μg) was incubated in a total volume of 20μl containing 20μg PS and 120ng PMA suspension in 0.1% Triton X-100, 1mM Ca2+, 10ng rat brain PKC, specific activity 1.3units/μg protein (Calbiochem) in 25mM Tris buffer (pH 6.8). For phosphorylation with PKA, the reaction was conducted in 20μl total volume containing 1μg annexin A7 and 10units of catalytic subunit of PKA (Promega) in 25mM Tris buffer (pH 6.8). The phosphorylation reaction was started with the addition of 100μM γ-ATP32 (sp activity, 2–5mCi/mmole) plus 10mM MgCl2. The reaction at 30°C was terminated at indicated period by adding the loading buffer for SDS-PAGE. Proteins were separated by SDS-PAGE and stained with Coomassie Blue. The gels were air-dried and radioactivity detected with a phosphorimager.
Other analyses
Proteins were quantified according to Bradford [27] using protein-binding dye reagent (Bio-Rad) and bovine-γ-globulin as standard. Results of western blot analysis were analyzed for statistical significance (P < 0.05) by ANOVA followed by Tukey’s post-hoc test according to the GraphPad software. Results of immuno-fluorescence were analyzed for co-localization using the Zeiss LSM 510, version2, software with co-localization function (Zeiss Corporation). The fluorescence intensities for different conditions were normalized to the same pixel range for individual fluorophore. Scatter plots were obtained for the intensities of two probes and their co-localization of the two probes was derived according to this program.
RESULTS
A7 antibody
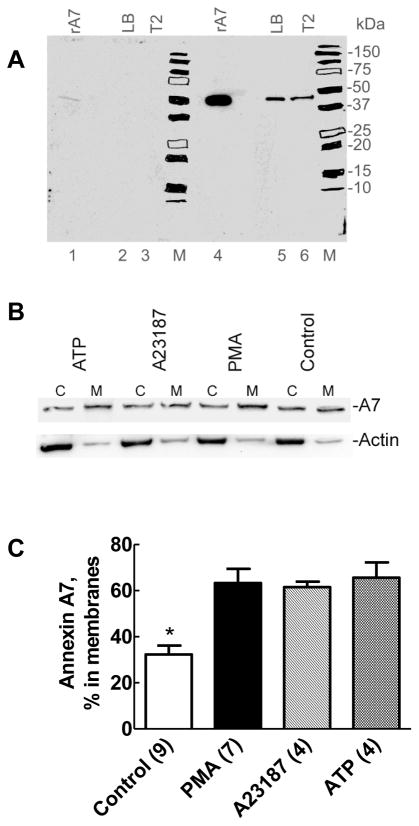
Western blot analysis for A7 showed a single band at ~47kDa in 24h-cultured type II cells and in lamellar bodies isolated from rat lung homogenate (Figure 1A). The recombinant protein also migrated with a similar molecular weight. The blot shows reactivity with 1μg of recombinant A7, but as low as 10ng of A7 could be detected with this antibody (at 1:3000, not shown). Antigen inhibition of antibody reaction (lanes 1 – 3) was used to verify the specificity of antibody. Overnight incubation of 10μl of antibody with 10μg of purified recombinant A7 strongly diminished reactivity with the recombinant protein and with the A7 in type II cells or in lung lamellar bodies demonstrating the specificity of antibody reaction.
Figure 1. Surfactant secretagogues cause increased membrane-association of endogenous annexin A7.
A. Western blot demonstrates the specificity of antibodies to recombinant rat annexin A7. Proteins (10μg) in isolated lung lamellar bodies, lysates of isolated type II cells cultured for 20–22h, and 1μg of recombinant rat annexin A7 (rA7) were probed for annexin A7 using antibodies preincubated for overnight without (panel containing lanes 4–6 and M) or with (panel with lanes 1–3 and M) 10μg of recombinant rat annexin A7 (rA7). Chemiluminiscence was recorded by autoradiography on an X-ray film with both panels exposed simultaneously. The blot demonstrates the presence of annexin A7 in lamellar bodies and isolated type II cells. Preincubation of antibodies with excess antigen decreased the signal intensity in each lane. M – Molecular weight markers with each position highlighted with a marker pen. B. Isolated rat alveolar type II cells were incubated for 30min without or with ATP (1mM), A23187 (250nM) or PMA (80nM). All of the recovered cytosol (c) and the membrane (M) fractions were probed for annexin A7. The membranes were stripped and probed for actin. C. Densitometry results of western blots for the membranes and cytosol fractions from several experiments showing increased membrane-association of annexin A7 in cells treated for 30min with all three agents used, in comparison to the controls. For these experiments, equal proportions of the cytosol and membrane fractions were probed for annexin A7 levels by western blot analysis followed by densitometry analysis to determine relative distribution of annexin A7. Only membrane-associated A7 levels are shown in each case for better clarity. Each secretagogue increased membrane-association of annexin A7. Results are expressed as mean ± SE of number of cell preparations shown in parentheses. * P < 0.05 – % distribution in control membranes was different in comparison to all others.
Membrane-association of A7
The next series of studies were aimed to determine if stimulation of type II cells with surfactant secretagogues increased membrane-association of A7. In these studies, isolated type II cells were incubated for 30min without or with 80nM PMA, 1mM ATP or 250nM A23187, all established secretagogues for lung surfactant [2, 3]. Western blots of the cytosol and membrane fractions for the control and treated cells showed that A7 was present in both fractions (Figure 1B). Each agent caused higher levels of A7 in the membrane fraction relative to the cytosol fraction in comparison to the control, which showed that the membrane-associated A7 levels were lower than in the cytosol. Since all of the recovered cytosol and membrane fractions from each cell sample were probed for A7, the requirement of conducting additional assay for total protein in each fraction was not necessary for calculations of relative distribution of A7 in the two fractions of each sample (Figure 1B). Nevertheless, we confirmed that the distribution of actin was unchanged by reprobing the membrane with actin antibodies. The A7 distribution between the two fractions showed some variation between different cell preparations. However, the percent distribution in the membrane fraction was always higher in stimulated cells when compared with the control cells (also see Figure 3). The results of densitometric analyses from several such experiments with the indicated secretagogues are shown in figure 1C. The total A7 levels in the membrane and the cytosol fractions are 100% and the distribution in the membrane fraction is expressed relative to the total. Even with the above-described variability in A7 distribution between the two fractions, our analysis showed that all secretagogues increased the relative content of A7 in the membrane fraction, in comparison to the controls. Similar results were observed with the β-adrenegic agonist, isoproterenol (see below). Thus, increased A7 partitioning into membranes in stimulated type II cells appears to be a common response to cell treatment with agents that increase surfactant secretion through the protein kinase C pathway (PMA and ATP), increasing cell calcium (A23187) and through cAMP-dependent pathway (isoproterenol or terbutaline).
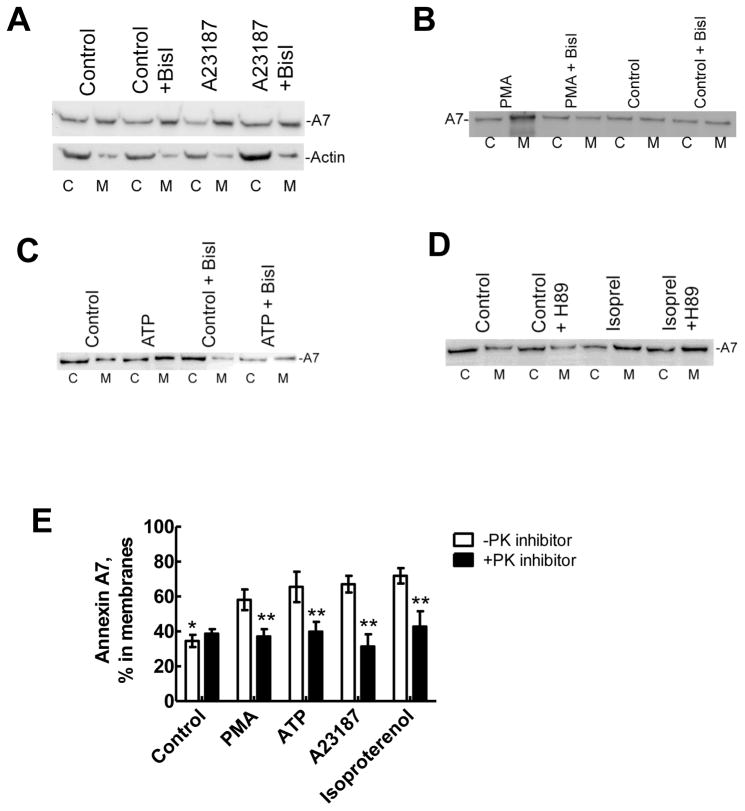
Figure 3. Membrane-association of annexin A7 is inhibited by protein kinase inhibitors.
Alveolar type II cells were incubated for 30min without or with protein kinase inhibitors, bisindolylmaleimideI (BisI) or H-89 before treatment for 30min without or with indicated secretagogues. Equal proportions of the membrane (M) and cytosol (c) fractions were probed for the annexin A7 levels by western blot analysis. Western blot for fractions from cells treated with A23187 (A) shows increased membrane-association of A7, which was inhibited in the presence of BisI. The distribution of actin was not affected by either A23187 or BisI. Similarly, PMA (B), ATP (C) and isoproterenol (D) increased membrane-association of A7, which was inhibited by respective protein kinase inhibitor. E. Results of densitometry analysis of western blot images from all experiments. Results are relative distributions in the membrane fraction as a percent of the total and are mean ± SE of 3 –12 cell preparations. * P < 0.05 in comparison to all other conditions in the absence of protein kinase (PK) inhibitor, ** P < 0.05 in comparison to respective condition in the absence of PK inhibitor (BisI or H-89, as appropriate).
Membrane-partitioning of A7 by immuno-staining
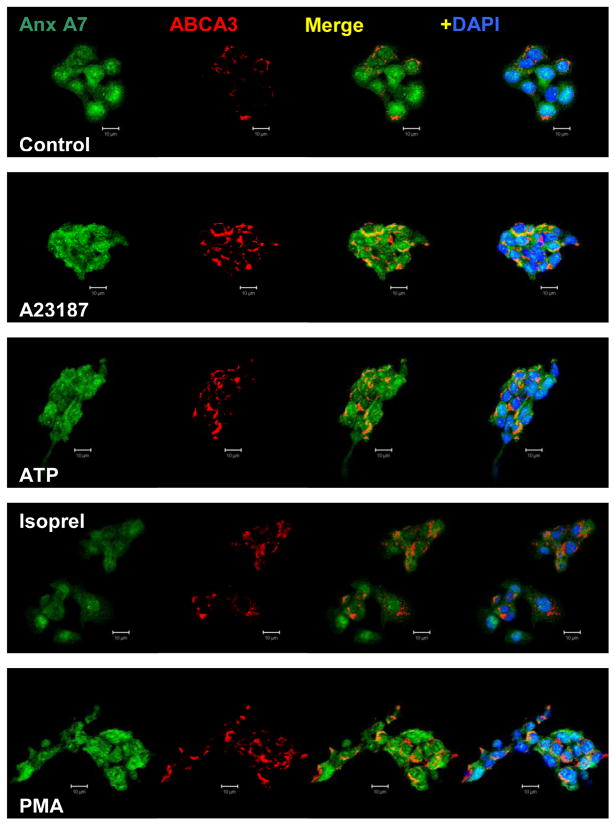
The membrane-association of A7 in secretagogue-treated type II cells was also investigated by immuno-staining. For these studies, type II cells were allowed to adhere to glass cover slips during the 20–22h cell culture. The adherent cells were washed and treated for indicated periods with each secretagouge, fixed in ice-cold methanol and stained for the described proteins using appropriate antibodies. Immuno-staining for A7 revealed its presence in the cytoplasmic, nuclear and membrane compartments. Nuclear localization of A7 has been previously reported in astroglial cells by immunostaining and in astrocyte derived C6 rat glioblastoma cells by localization of green fluorescence protein (GFP)-A7 [28]. As observed in our study, this report also demonstrated that calcium ionophore A23187 increased membrane association of GFP-A7. Because immuno-staining for A7 alone did not reveal continuous portioning along the cell periphery in treated cells, we employed the co-localization strategy to determine if A7 is localized to lamellar bodies, which can be identified by the marker protein ABCA3. In control cells, annexin A7 staining was weak possibly because of its diffused distribution throughout the cells (Figure 2). Most cells expressed both A7 and ABCA3, as determined from lower magnification images (not shown). In initial studies, cell-stimulation with A23187 (250nM) and PMA (80nM) showed a time-dependent increase in annexin A7 association with the lamellar bodies, as indicated by its co-localization with ABCA3 (not shown). In control cells, the co-localization coefficients for A7 and ABCA3 were 0.03 and 0.02, respectively. The coefficients increased as a function of time and were 0.25 and 0.54 with A23187 and 0.29 and 0.42 with PMA after 30min stimulation. In subsequent experiments, cells were stimulated for 30min with 80nM PMA, 250nM A23187, 1mM ATP or 10μM isoproterenol. In each case, annexin A7 co-localization with ABCA3 was greater (Figure 2). Thus, our studies show that lamellar bodies were one of the target membranes for annexin A7 in stimulated cells and that the increased membrane-association of annexin A7 (Figure 1B and C) was, in part, due to association with the lamellar bodies.
Figure 2. Immuno-staining for annexin A7 and ABCA3 shows co-localization with surfactant secretagogues.
Adherent type II cells were incubated for 30min in the absence (Control) or presence of 250nM A23187, 1mM ATP, 10μM Isoproterenol or 80nM PMA. Cells were then fixed and stained for annexin A7 and ABCA3. Confocal fluorescence microscopic images are shown after adjusting for fluorescence intensities to the same range. The DAPI-stained nuclei are shown only in the last panels to clearly show the fluorescence due to annexin A7 and ABCA3 reactivity. All agents increased co-localization of annexin A7 with ABCA3. Representative images are shown from experiments that were repeated at least two times.
Annexin A7 relocation is dependent upon protein kinase activation
Stimulus-dependent activation of PKC or PKA regulates lung surfactant secretion. In vitro studies showed that augmented binding of purified bovine annexin A7 to the plasma membrane or lamellar body fractions from type II cells stimulated with PMA or A23187 could be prevented by pretreatment of cells with PKC inhibitor calphostin C [19]. In the current study, type II cells were pre-treated for 30 min with bisindolylmaleimideI (BisI) and H-89, specific inhibitors of PKC and PKA, respectively, before treatment with indicated secretagogues for lung surfactant. Western blot analysis of the cytosol and membrane fractions from control and stimulated cells demonstrated that A23187 increased membrane-association of cell A7 (Figure 3A), which was blocked in cells pre-treated with BisI. As shown in Figure 1B, the distribution of actin was unchanged was unchanged with A23187 treatment with or without the presence of BisI. Similarly, PMA (Figure 3B), or ATP (Figure 3C) stimulation caused relative increase of endogenous annexin A7 in the membrane fraction, which was also diminished by BisI pretreatment. Cell-stimulation with isoproterenol also increased membrane-association of A7, which was inhibited in cells pre-treated with PKA inhibitor, H89 (Figure 3D), but not with BisI (not shown). Thus, phosphorylation through PKC or PKA activation caused A7 association with the membranes. Some variability in percent distribution of A7 in the cytosol and membrane fractions, as discussed under Figure 1, can also be seen here. Nevertheless, increased distribution in membrane fraction and the inhibition of such increase in presence of protein kinase inhibitor was seen in every cell preparation. Densitometry analyses of western blots from several experiments with all four secreatagogues are summarized in Figure 3E. Only the percent of total cellular A7 (cytosol plus membrane fractions) in the membrane fraction without or with the protein kinase inhibitor (BisI or H-89) is shown in each case. These results indicate that all of the secretagogues caused a common response of increased A7 association with the membranes in a protein kinase-dependent manner.
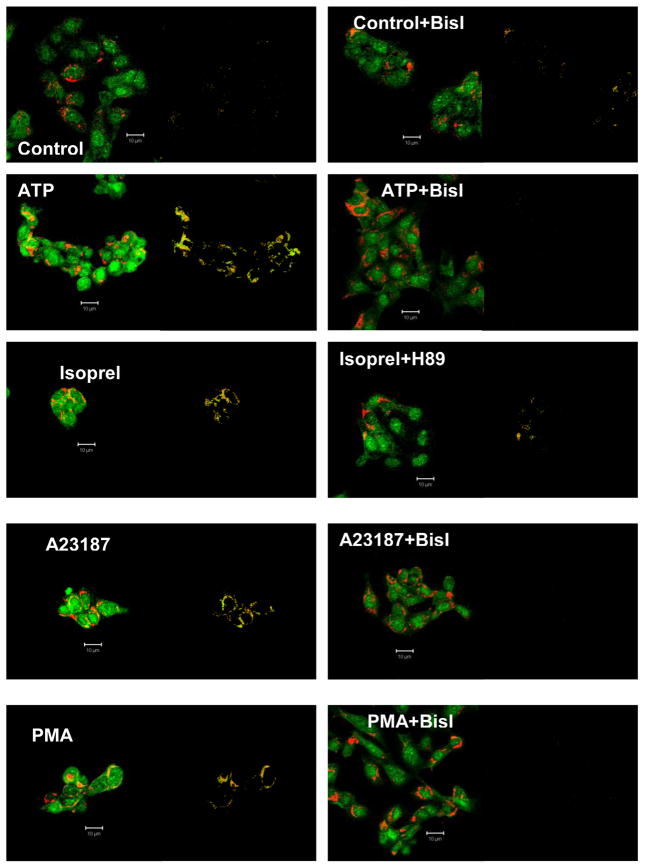
That protein kinase activation is required for A7 relocation to the lamellar bodies was also demonstrated by immuno-staining studies. As shown above, all of the indicated secretagogues increased the co-localization of ABCA3 and annexin A7 (Figure 4, left panels). The pattern of co-localization for each condition is shown in the adjacent panel, as derived by the program. The co-localization was, however, diminished if the cells were pre-treated with BisI (in case of ATP, A23187 or PMA) or with H89 (in case of isoproterenol) prior to the 30min treatment with indicated secretagogue (Figure 4, right panels). Analysis of confocal images shows that the co-localization coefficients for both proteins were higher in the absence than in the presence of protein kinase inhibitor (Table 1). The coefficients were higher for ABCA3 in comparison to those for A7 suggesting that not all of the A7 was directed to the lamellar bodies in stimulated cells. Thus, the immuno-staining studies support the results of cell fractionation studies in terms of protein kinase-dependent relocation of A7 to the membranes.
Figure 4. Protein kinase inhibitors diminish co-localization of annexin A7 and ABCA3 in secretagogue-stimulated cells.
Isolated rat type II cells were pre-incubated for 30min without or with PKC inihibitor, BisI (for ATP, A23187 or PMA), or PKA inhibitor, H89 (for isoproterenol), before incubation for 30min without or with indicated secretagogue. Confocal microscope fluorescence images are shown for each agent. The adjacent image for each condition shows co-localization pattern as determined using the Zeiss LSM co-localization software. The analysis showed that prior treatment with protein kinase inhibitor prevented the increase in co-localization of annexin A7 and ABCA3. Representative images from several experiments are shown. Isoprel – isoproterenol.
Table 1.
Co-localization coefficients of annexin A7 and ABCA3 in alveolar type II cells in the absence or presence of protein kinase inhibitors
| Co-localization Coefficient | ||||
|---|---|---|---|---|
| Without PK Inhibitor | With PK Inhibitor* | |||
| A7 with ABCA3 | ABCA3 with A7 | A7 with ABCA3 | ABCA3 with A7 | |
| Control | 0.05 | 0.06 | 0.12 | 0.14 |
| ATP | 0.17 | 0.32 | 0.01 | 0.00 |
| PMA | 0.33 | 0.63 | 0.01 | 0.00 |
| A23187 | 0.21 | 0.54 | 0.03 | 0.00 |
| Isoproterenol | 0.25 | 0.67 | 0.09 | 0.15 |
Co-localization coefficient (ratio of co-localizing pixels to the total number of pixels in each channel) was determined from confocal image analysis using the Zeiss LSM 510 Software Co-localization (Function) program.
0: denotes the absence of co-localization.
BisI or H89.
Secretagogues cause annexin A7 phosphorylation
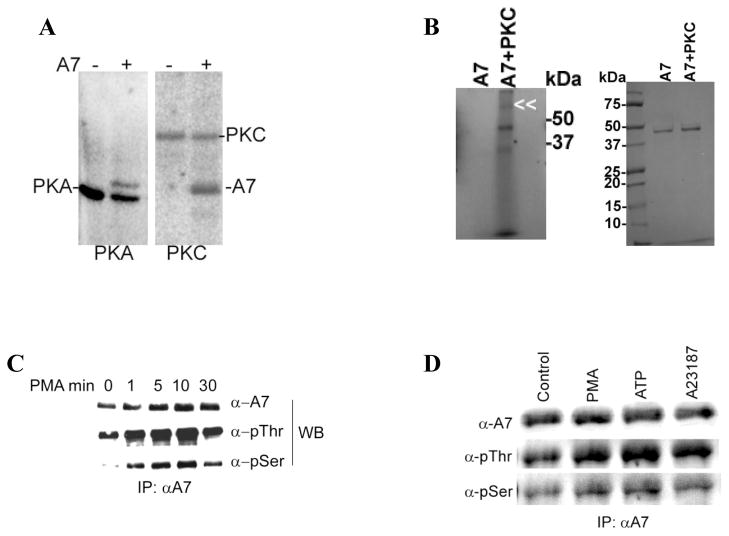
In vitro studies with recombinant A7 were conducted to determine phosphorylation with purified protein kinase enzymes, PKC and cAMP-dependent protein kinase. The in vitro phosphorylation for 60min clearly showed that in addition to autophosphorylation of protein kinase enzyme, annexin A7 was also phosphorylated (Figure 5A). As against autophosphorylation of PKC, A7 did not show autophosporylation when incubated in presence of 32P-ATP in the absence of PKC (Figure 5B). The phosphorylation was time-dependent (not shown). Next, we evaluated in vivo phosphorylation of annexin A7 without or with stimulation of type II cells with various secretagogues. Initial studies evaluated the cell lysate by western blot analysis using antibodies for phosphoamino acids from cells treated without or with several secretagogues. All agents (A23187, PMA, ATP and terbutaline) caused increased phosphorylation of several proteins, as determined by reaction with these antibodies, including those that migrated to the same position as annexin A7, as verified by reprobing of blots with annexin A7 antibodies (not shown). Therefore, next we determined the phosphorylation of A7 by immuno-precipitation (Figure 5C and 5D) with A7 antibodies. The A7 antibodies specifically precipitated A7, since the non-immune serum did not show immuno-precipitation of A7 (not shown). Subsequent western blots analysis of immunoprecipitates from lysates of PMA-treated cells showed increased phosphorylation of A7 at the serine and threonine residues as analyzed with antibodies for phosphoserine (Santa Cruz) and phosphothreonine (Sigma). The phosphorylation was increased at 1min and reached a maximum between 5–10min after cell stimulation (Figure 5C). Increased phosphorylation of A7 was also observed in cell treated for 10 min with ATP or A23187 (Figure 5D). Thus, our studies indicate increased phosphorylation of A7 in secretagogue-stimulated type II cells.
Figure 5. Annexin A7 phosphorylation in vitro and in vivo in alveolar type II cells.
A. Phosphorylation of purified recombinant rat annexin A7 (rA7) by commercially available catalytic subunit of PKA and by purified rat brain PKC. Purified A7 (1μg) was incubated with ATP-γ-32P in presence of required co-factors for 1h. The proteins were separated by SDS-PAGE, stained to localize A7, and imaged with a phosphor-imager to determine phosphorylation. Both the kinase enzyme and rA7 were phosphorylated. B. Phosphorylation of purified A7 in the absence or presence of PKC. Purified A7 was incubated in the described assay mixture in the absence or presence of PKC and in the presence of ATP-γ-32P. Left panel shows phosphorylation of A7 as determined by imaging of radioactivity using a phosphorimager. The phosphorylation of PKC is indicated by double arrowhead. A7 position is just below the 50kDa maker. Right panel shows Coomassie Blue-stained gels showing comparable amounts of A7 in two lanes. Results show no authophosphorylation of A7 under these conditions. C. Type II cells were incubated for indicated periods without or with 100nM PMA and the immuno-precipitates (IPs) were obtained with A7 antibodies. Western blots of IPs with indicated antibodies showed a time-dependent increase in A7 phosphorylation at the serine and threonine residues. D. Type II cells were incubated for 30min without or with indicated agents and harvested in lysis buffer. Equal amounts of cell lysate proteins were immunoprecipitated using A7 antibodies. Western blots of IPs with A7, p-thr and p-ser antibodies showed equal levels of A7 but increased reactivity with p-ser and p-thr antibodies in stimulated cells in comparison to the controls.
DISCUSSION
In this study, we provide new evidence suggesting a role for A7 in the regulation of lung surfactant secretion. Our studies using cell fractionation and immuno-colocalization with compartment-specific markers demonstrate that established secretagogues of lung surfactant increase association of endogenous A7 with the lamellar bodies, a surfactant storage compartment which must fuse with plasma membrane for the release of lung surfactant. Several studies have previously demonstrated the importance of protein kinase in the regulation of lung surfactant secretion (reviewed in [1–3]. Along similar lines, our studies show that protein kinase activation is important for A7 relocation to specific membranes in stimulated cells, where it possibly interacts with the lamellar body marker protein ABCA3. We have previously suggested a role for annexin A7 in membrane fusion during surfactant secretion since this protein increased surfactant secretion in permeabilized type II cells [5]. Our present studies demonstrating A7 co-localization with lamellar bodies further support a role for A7 in membrane fusion during surfactant secretion.
Although all annexin proteins share the common property of calcium-dependent binding to phospholipid membranes [29, 30], only annexin A2 and A7 have been demonstrated to facilitate membrane fusion and have been postulated to play a role in membrane fusion during exocytosis of lung surfactant. The findings that both proteins relocate to membranes upon cell-stimulation with secretagogues support their postulated role. However, it is not clear if protein kinase-dependent phosphorylation is important for relocation of annexin A2 to the membranes, although annexin A2 knock-down with siRNA was shown to lower stimulated secretion of lung surfactant in type II cells. Our study shows that protein kinase activation is important for relocation of A7 to the membranes including the lamellar bodies. Whereas our previous studies on in vitro membrane binding of bovine A7 showed that PKC activation was required for increased binding [19], our current study shows that ‘in vivo’ binding of cell A7 required PKC or PKA activation in a stimulus-dependent manner. Clearly, there is an overall similarity in membrane binding properties (protein kinase dependence) of purified and endogenous A7.
Previous studies have demonstrated in vitro phosphorylation of recombinant annexin A7 and ‘in vivo’ phosphorylation of endogenous annexin A7 in isolated chromaffin cells [17]. The phosphorylation of recombinant A7 was observed with several classes of commercially available protein kinase enzymes, but only phosphorylation with PKC increased its membrane aggregation property[17]. In comparison, phosphorylation of annexin A2 diminishes its membrane aggregation properties [31]. Nevertheless, the endogenous proteins (A7 and A2) could behave differently from recombinant or purified proteins because of cellular environment. Our studies, however, cannot determine if the increase in membrane-association of annexin A7 in stimulated cells occurs only due to phosphorylation of membrane proteins, A7 or both. Future studies with phosphorylation defective mutants could provide such answer.
Increased membrane-association of A7 in stimulated cells implies the presence of specific docking domains in the target membranes where specific proteins may interact with the phosphorylated A7. These docking domains are present in the lamellar bodies as demonstrated by protein kinase-dependent co-localization of A7 with ABCA3. Although not investigated here, the plasma membrane is reported to contain the t-SNARE proteins that may facilitate docking of secretion vesicles prior to membrane fusion. Our in vitro studies showed that surfactant secretagogues increase binding of purified bovine A7 (non-phosphorylated) to type II cell plasma membranes, suggesting that phosphorylation of membrane proteins facilitated interaction with A7 [19]. Although PKC-dependent phosphorylation of ABCA3 has not been reported, the ABC transporters contain a cytoplasmic nucleotide-binding domain and the regulatory domain of ABCA1 has several putative phosphorylation sites [32, 33]. Increased phosphorylation of SNARE proteins, syntaxin [34] and snap [35] by PKC has been associated with increased exocytosis and secretion in other systems. In type II cells, the importance of syntaxin2 and snap23 in surfactant secretion has been implicated [18], but their phosphorylation with PKC or PKA activation remains to be determined. It is likely that phosphorylation of syntaxin and snap homologues facilitates interaction with A7 and promotes membrane fusion during surfactant secretion.
The lipid composition of membranes can regulate their interaction with proteins. Our previous studies have demonstrated that molecular organization of A7 can be modified by membrane lipid composition and that PIP2, which is mostly present in the plasma membrane, has a dramatic influence on annexin A7 properties [6]. Similarly, membrane insertion of synaptotagmin, which acts as a calcium sensor in SNARE-mediated release of synaptic vesicles, is facilitated by PIP2 in the membranes [36]. Although this study did not investigate A7 co-localization with PIP2, but A7 interaction with PIP2 and consequent changes in protein conformation [6] would suggest that A7 possibly binds to PIP2-rich domains in the plasma membrane. Previous studies have demonstrated activation of exocytic sites by microdomains of PIP2 and syntaxin [37]. In case of A7 also, phospholipase C-mediated hydrolysis of PIP2 could increase diacylglycerol content in the membranes that may facilitate the membrane-fusion function of A7 [6].
Others have postulated that A7 can function as a Ca2+ channel [38] and act as Ca2+- dependent GTPase [39, 40]. A7 phosphorylation with PKC can be increased in presence of GTP leading to increased membrane aggregation activity of A7 [39]. Thus, A7 can function as a Ca2+ and GTP sensor. Annexin A7 together with PKC and GTP could function like a G-protein during exocytosis [39]. Our preliminary studies show that A7 also relocates to t-SNARE containing compartments in stimulated type II cells. In this respect, A7 appears to function like synaptotagmin I during synaptic transmission where it may act as a Ca2+ sensor [41]. Our studies support A7 localization in domains enriched with t-SNARE proteins in stimulated type II cells. Similar to A7, several SNARE-interacting proteins including calmodulin [42], CaMKII [43], ENaC [44], synaptotagmins [45] and synaptophysin [46] have been described (reviewed in [47]). While most of these interact with either t- or v-SNARE proteins [45, 48], synaptotagmin I has been reported to interact with both the t- and v-SNARE proteins. Similarly, A7 interaction with t-SNARE proteins and ABCA3, the secretory vesicle protein in type II cells, may facilitate close juxtaposition of lamellar bodies and the exocytic sites to promote membrane fusion, in co-operation with SNARE proteins, during surfactant secretion. This speculation is also supported by the presence of Ca2+ and phospholipids binding domains in A7 and in synaptotagmins [41, 49].
Research Highlights.
Specific antibody is used for annexin A7 trafficking in alveolar type II cells.
Cell-stimulation increased annexin A7 in cell membranes and lamellar bodies.
Membrane-association or co-localization required protein kinase activation.
Stimulation increases annexin A7 phosphorylation at serine and threonine residues.
Phosphorylation of annexin A7 may regulate its function during membrane fusion.
Acknowledgments
The authors are thankful for excellent technical assistance of Delon Callender, Pavan Kumar Vasa and Kelly Holbrook. Parts of these studies have been presented at the annual meeting of Experimental Biology 2010. These studies were supported by a grant (HL49959 to AC) from NHLBI, NIH, Bethesda, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259–271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 3.Chander A, Fisher AB. Regulation of lung surfactant secretion. The American journal of physiology. 1990;258:L241–253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 4.Chander A, Wu RD. In vitro fusion of lung lamellar bodies and plasma membrane is augmented by lung synexin. Biochimica et biophysica acta. 1991;1086:157–166. doi: 10.1016/0005-2760(91)90003-z. [DOI] [PubMed] [Google Scholar]
- 5.Chander A, Sen N, Spitzer AR. Synexin and GTP increase surfactant secretion in permeabilized alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L991–998. doi: 10.1152/ajplung.2001.280.5.L991. [DOI] [PubMed] [Google Scholar]
- 6.Chander A, Chen XL, Naidu DG. A role for diacylglycerol in annexin A7-mediated fusion of lung lamellar bodies. Biochimica et biophysica acta. 2007;1771:1308–1318. doi: 10.1016/j.bbalip.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen N, Spitzer AR, Chander A. Calcium-dependence of synexin binding may determine aggregation and fusion of lamellar bodies. The Biochemical journal. 1997;322(Pt 1):103–109. doi: 10.1042/bj3220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich LE, Boeddinghaus C, LaGrassa TJ, Ungermann C. Control of eukaryotic membrane fusion by N-terminal domains of SNARE proteins. Biochimica et biophysica acta. 2003;1641:111–119. doi: 10.1016/s0167-4889(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 9.Fasshauer D. Structural insights into the SNARE mechanism. Biochimica et biophysica acta. 2003;1641:87–97. doi: 10.1016/s0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 10.Gerst JE. SNARE regulators: matchmakers and matchbreakers. Biochimica et biophysica acta. 2003;1641:99–110. doi: 10.1016/s0167-4889(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 11.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 12.Mayer A. Membrane fusion in eukaryotic cells. Annual review of cell and developmental biology. 2002;18:289–314. doi: 10.1146/annurev.cellbio.18.032202.114809. [DOI] [PubMed] [Google Scholar]
- 13.Paumet F, Rahimian V, Rothman JE. The specificity of SNARE-dependent fusion is encoded in the SNARE motif. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3376–3380. doi: 10.1073/pnas.0400271101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Wang M, Fisher AB, Zimmerman UJ. Involvement of annexin II in exocytosis of lamellar bodies from alveolar epithelial type II cells. The American journal of physiology. 1996;270:L668–676. doi: 10.1152/ajplung.1996.270.4.L668. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay S, Sun P, Wang P, Abonyo B, Cross NL, Liu L. Fusion of lamellar body with plasma membrane is driven by the dual action of annexin II tetramer and arachidonic acid. J Biol Chem. 2003;278:39675–39683. doi: 10.1074/jbc.M212594200. [DOI] [PubMed] [Google Scholar]
- 16.Hong K, Duzgunes N, Ekerdt R, Papahadjopoulos D. Synexin facilitates fusion of specific phospholipid membranes at divalent cation concentrations found intracellularly. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:4642–4644. doi: 10.1073/pnas.79.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caohuy H, Pollard HB. Activation of annexin 7 by protein kinase C in vitro and in vivo. J Biol Chem. 2001;276:12813–12821. doi: 10.1074/jbc.M008482200. [DOI] [PubMed] [Google Scholar]
- 18.Abonyo BO, Gou D, Wang P, Narasaraju T, Wang Z, Liu L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry. 2004;43:3499–3506. doi: 10.1021/bi036338y. [DOI] [PubMed] [Google Scholar]
- 19.Chander A, Sen N, Naidu DG, Spitzer AR. Calcium ionophore and phorbol ester increase membrane binding of annexin a7 in alveolar type II cells. Cell calcium. 2003;33:11–17. doi: 10.1016/s0143-4160(02)00177-x. [DOI] [PubMed] [Google Scholar]
- 20.Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS letters. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 21.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 22.Naidu DG, Raha A, Chen XL, Spitzer AR, Chander A. Partial truncation of the NH2-terminus affects physical characteristics and membrane binding, aggregation, and fusion properties of annexin A7. Biochimica et biophysica acta. 2005;1734:152–168. doi: 10.1016/j.bbalip.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Chander A, Sen N. Inhibition of phosphatidylcholine secretion by stilbene disulfonates in alveolar type II cells. Biochem Pharmacol. 1993;45:1905–1912. doi: 10.1016/0006-2952(93)90450-b. [DOI] [PubMed] [Google Scholar]
- 24.Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem. 1986;261:6126–6131. [PubMed] [Google Scholar]
- 25.Chander A, Dodia CR, Gil J, Fisher AB. Isolation of lamellar bodies from rat granular pneumocytes in primary culture. Biochimica et biophysica acta. 1983;753:119–129. doi: 10.1016/0005-2760(83)90105-4. [DOI] [PubMed] [Google Scholar]
- 26.Wadsworth SJ, Spitzer AR, Chander A. Ionic regulation of proton chemical (pH) and electrical gradients in lung lamellar bodies. The American journal of physiology. 1997;273:L427–436. doi: 10.1152/ajplung.1997.273.2.L427. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Clemen CS, Herr C, Lie AA, Noegel AA, Schroder R. Annexin VII: an astroglial protein exhibiting a Ca2+-dependent subcellular distribution. Neuroreport. 2001;12:1139–1144. doi: 10.1097/00001756-200105080-00018. [DOI] [PubMed] [Google Scholar]
- 29.Gerke V, Moss SE. Annexins and membrane dynamics. Biochimica et biophysica acta. 1997;1357:129–154. doi: 10.1016/s0167-4889(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 30.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochimica et biophysica acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone SA, Hubaishy I, Waisman DM. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J Biol Chem. 1992;267:25976–25981. [PubMed] [Google Scholar]
- 32.See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–47897. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 34.Nagy B, Jr, Bhavaraju K, Getz T, Bynagari YS, Kim S, Kunapuli SP. Impaired activation of platelets lacking protein kinase C-theta isoform. Blood. 2009;113:2557–2567. doi: 10.1182/blood-2008-07-169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature structural & molecular biology. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 37.Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 38.Burns AL, Magendzo K, Shirvan A, Srivastava M, Rojas E, Alijani MR, Pollard HB. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3798–3802. doi: 10.1073/pnas.86.10.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caohuy H, Pollard HB. Protein kinase C and guanosine triphosphate combine to potentiate calcium-dependent membrane fusion driven by annexin 7. J Biol Chem. 2002;277:25217–25225. doi: 10.1074/jbc.M202452200. [DOI] [PubMed] [Google Scholar]
- 40.Caohuy H, Srivastava M, Pollard HB. Membrane fusion protein synexin (annexin VII) as a Ca2+/GTP sensor in exocytotic secretion. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10797–10802. doi: 10.1073/pnas.93.20.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 42.Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. The EMBO journal. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohyama A, Hosaka K, Komiya Y, Akagawa K, Yamauchi E, Taniguchi H, Sasagawa N, Kumakura K, Mochida S, Yamauchi T, Igarashi M. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J Neurosci. 2002;22:3342–3351. doi: 10.1523/JNEUROSCI.22-09-03342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Condliffe SB, Zhang H, Frizzell RA. Syntaxin 1A regulates ENaC channel activity. J Biol Chem. 2004;279:10085–10092. doi: 10.1074/jbc.M313592200. [DOI] [PubMed] [Google Scholar]
- 45.Rickman C, Davletov B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J Biol Chem. 2003;278:5501–5504. doi: 10.1074/jbc.C200692200. [DOI] [PubMed] [Google Scholar]
- 46.Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. The EMBO journal. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion? Am J Physiol Cell Physiol. 2003;285:C237–249. doi: 10.1152/ajpcell.00091.2003. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda M. Vesicle-associated membrane protein-2/synaptobrevin binding to synaptotagmin I promotes O-glycosylation of synaptotagmin I. J Biol Chem. 2002;277:30351–30358. doi: 10.1074/jbc.M204056200. [DOI] [PubMed] [Google Scholar]
- 49.Rhee JS, Li LY, Shin OH, Rah JC, Rizo J, Sudhof TC, Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]