Abstract
During early embryogenesis, the formation of the cardiac atrioventricular canal (AVC) facilitates the transition of the heart from a linear tube into a chambered organ. However, the genetic pathways underlying this developmental process are poorly understood. The T-box transcription factor Tbx20 is expressed predominantly in the AVC of early heart tube. It was shown that Tbx20 activates Nmyc1 and suppresses Tbx2 expression to promote proliferation and specification of the atrial and ventricular chambers, yet it is not known if Tbx20 is involved in early AVC development. Here, we report that mice lacking Tbx20 in the AVC myocardium fail to form the AVC constriction, and the endocardial epithelial-mesenchymal transition (EMT) is severely perturbed. Tbx20 maintains expression of a variety of genes, including Bmp2, Tbx3 and Hand1 in the AVC myocardium. Intriguingly, we found Bmp2 downstream genes involved in the EMT initiation are also downregulated. In addition, re-expression of Bmp2 in the AVC myocardium substantially rescues the EMT defects resulting from the lack of Tbx20, suggesting Bmp2 is one of the key downstream targets of Tbx20 in AVC development. Our data support a complex signaling network with Tbx20 suppressing Tbx2 in the AVC myocardium but also indirectly promoting Tbx2 expression through Bmp2. The spatiotemporal expression of Tbx2 in the AVC appears to be balanced between these two opposing signals. Overall, our study provides genetic evidence that Tbx20 has essential roles in regulating AVC development that coordinate early cardiac chamber formation.
Keywords: Heart development, atrioventricular canal, epithelial-mesenchymal transition, mouse, Tbx20
Introduction
Cardiac chamber formation represents an essential milestone in evolution and is profoundly important for proper cardiac function (Chi et al., 2008). In vertebrates, the emergence and subsequent development of the atrioventricular canal (AVC), a cardiac component that joins the primitive atria and ventricles, facilitates the transition of the heart from a linear tube into a chambered organ. Along with evolution, the high-volume low-pressure cardiovascular system of the tunicates developed into a low-volume high-pressure system in vertebrates (Moorman and Christoffels, 2003). Therefore, a proper development of the AVC is crucial for cardiac chamber formation.
T-box genes encode transcription factors that have vital roles in organogenesis (Naiche et al., 2005). Tbx20 is an ancient T-box family member with orthologues conserved from Drosophila to human (Plageman and Yutzey, 2005), and is essential for heart development in different species (Brown et al., 2005; Qian et al., 2008; Szeto et al., 2002). In mammals, Tbx20 is expressed in the heart throughout gestation (Carson et al., 2000; Kraus et al., 2001). Mice lacking Tbx20 (Tbx20−/−) die at E9.5–E10.5 with severely hypoplastic heart tubes. Tbx20 promotes Nmyc1 and suppresses Tbx2 in the chamber myocardium, which is crucial for the chamber myocardial proliferation and specification (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005). Recent studies also revealed that TBX20 mutations are associated with human congenital heart disease (CHD) (Kirk et al., 2007; Liu et al., 2008; Qian et al., 2008).
Previous studies indicated that vertebrate AVC development is regulated by bone morphogenetic proteins (Bmps). In mouse, Bmp2 is the earliest known marker expressed in the precursors of AVC myocardium. Bmp2 regulates early AVC development by activating Tbx2, Msx2 and Has2 in the AVC myocardium (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). In addition, Bmp2 signals mediate Twist1, Msx1 and Smad6 expression in the AVC endocardium to modulate EMT and AVC cushion formation. Another Bmp gene, Bmp4, is expressed in the outflow tract (OFT) and atrioventricular valves. Disruption of Bmp4 in mouse heart leads to OFT and AVC septation defects at mid-late gestation (Jiao et al., 2003; Liu et al., 2004; McCulley et al., 2008).
Transcriptional signaling also plays fundamental roles in the AVC development. Murine Tbx2 and Tbx3 are specifically expressed in the AVC of the early heart. Tbx2 expression is activated by Bmp2 whereas the signals activating Tbx3 are unknown. Tbx2 and Tbx3 suppress the chamber specification program in AVC and OFT cells (Aanhaanen et al., 2009; Christoffels et al., 2004; Habets et al., 2002; Harrelson et al., 2004; Hoogaars et al., 2007; Ribeiro et al., 2007). Disruption of Tbx2 or Tbx3 in mouse embryos cause variable defects, including affects in AVC and OFT development (Aanhaanen et al., 2009; Christoffels et al., 2004; Habets et al., 2002; Harrelson et al., 2004; Hoogaars et al., 2007; Ribeiro et al., 2007). The basic helix-loop-helix transcription factors Hesr1 (Hey1) and Hesr2 (Hey2) are expressed in the atrium and ventricle, respectively. They regulate AV boundary formation by suppressing Bmp2 and Tbx2 expression in the cardiac chambers (Kokubo et al., 2007; Rutenberg et al., 2006). As in mouse, zebrafish Tbx2a (orthologue of murine Tbx2) and Tbx3b (orthologue of murine Tbx3) are expressed in the AVC and OFT. They inhibit cell proliferation and repress the chamber genetic program during heart remodeling (Ribeiro et al., 2007). In addition, zebrafish Forkhead transcription factor Foxn4 activates Tbx2b in the AVC to contribute atrial and ventricular septation (Chi et al., 2008).
During early embryogenesis in mouse, Tbx20 suppresses Bmp mediated Tbx2 activity in the cardiac chambers by interacting with Smad1/5 (Singh et al., 2009). Although this mechanism may explain why Tbx2 expression is confined to the AVC, it does not address the role of Tbx20 itself in AVC development. This is even more pertinent as in mouse and chicken, Tbx20 is expressed at strikingly higher level in the AVC and OFT than in the chamber cardiac cells (Dupays et al., 2009; Shelton and Yutzey, 2007; Stennard et al., 2003; Takeuchi et al., 2005). In this study, we specifically deleted Tbx20 in the AVC myocardium by crossing Tbx20-floxed mice (Tbx20flox/flox) to Tbx2-Cre mice (Tbx2Cre/+). We found that mutant embryos display severe AVC constriction defects with a lack of cushion mesenchymal cells. Tbx20 regulates EMT through Bmp2 expression, whereas a role of endocardial Tbx20 is excluded. Our data show that, in addition to promoting chamber myocardial proliferation and specification (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005), Tbx20 is also essential for mediating AVC patterning. Therefore, Tbx20 plays crucial roles in these two important aspects of early heart development.
Material and methods
Animals
Tbx20-floxed mice (Tbx20flox/flox or Tbx20f/f) were generated by flanking exon 2 with two LoxP sites. Cre-mediated excision of exon 2 shifts Tbx20 reading frame and obliterates the T-box DNA binding domain (Cai et al., 2005). Tbx2-Cre (Tbx2Cre/+) knock-in mice and Tie2-Cre (Tie2Cre) transgenic mice were as described (Aanhaanen et al., 2009; Kisanuki et al., 2001). Bmp2 transgenic mice were generated with a vector containing a ubiquitously expressing CAG promoter (CMV-βactin-intron), followed by a LoxP-eGFP-STOP-LoxP cassette (Collombat et al., 2009). The full-length Bmp2 cDNA was inserted with an IRES-LacZ cassette. The purified DNA fragments were microinjected into B6C3 mouse zygotes to generate transgenic animals. All mice were bred in a mixed genetic background (Black Swiss) for analysis.
Whole-mount RNA in situ hybridization and histology
Whole-mount RNA in situ hybridization of mouse embryos was carried out according to Wilkinson’s protocol (Wilkinson, 1992). For histology, mouse embryos were fixed in 4% paraformaldehyde, dehydrated through graded ethanol and embedded in paraffin. Paraffin sections were cut at 8 µm thickness and stained with hematoxylin-eosin as needed.
X-gal and Alcian blue staining
Mouse embryos were fixed in 4% paraformaldehyde for 30 min. After permeabilization (10% Na deoxycholate, 10% NP40 in PBS), embryos were stained in X-gal solution (50 mM K-ferricyanide, 50 mM K-ferrocyanide, 200 mM MgCl2, 100 mg/ml X-gal in PBS) for 12 h. For Alcian blue staining, tissues from paraffin sections were rehydrated and stained in 3% Alcian blue solution (pH 2.5) for 30 min, washed in tap water and counterstained with nuclear fast red by a standard procedure (Bancroft and Gamble, 2008).
Proliferation and apoptosis analysis
Mitotic and apoptotic cells were examined with anti-phosphohistone H3 (pH3) antibody (Millipore, 1:100 dilution) and an in situ cell death detection kit (Roche). Alexa 488 conjugated anti-rabbit antibody (Invitrogen, 1:500 dilution) and DAPI were used to detect pH3 and nuclei, respectively. Embryos were embedded in paraffin, sectioned, and immunostained.
Statistic analysis of AVC mesenchymal cells
At least three embryos of each genotype were embedded in paraffin. Sagittal sections were performed to examine AVC mesenchymal cells. Five sections of each embryo were counted. Independent counting and statistic assays were carried out by two different individuals. The results are shown as mean±S.D. values. A Student’s t-test was used to determine differences between every two groups.
Quantitative Real-Time PCR analysis
Heart tissues from E9.5 embryos were collected and total RNA was isolated with Trizol (Invitrogen). First-strand cDNA was synthesized by QuantiTect Reverse Transcription Kit (Qiagen). Real-time PCR was performed using QuantiTect SYBR Green PCR Kit (Qiagen), and was normalized to β-actin with the following primers: β-actin 5’-TGAACCCTAAGGCCAACCGTGAAA-3’ and 5’-CAGGATGGCGTGAGGGAGAGCATAG-3’, αMhc 5’-GTATGTTAAGGCCAAGGTCGTG-3’ and 5’-GTGCAGGAAGGTCAGCATG-3’, βMhc 5’-ACTGCTGAGACGGAGAATGG-3’ and 5’-CAGAAGAGGCCCGAGTAGG-3’, Mlc2a 5’-GGCACAACGTGGCTCTTCTAA-3’ and 5’-GATTTGCAGATGATCCCATCCC-3’, Tbx2 5’-TTCCACAAACTGAAGCTGAC-3’ and 5’-GCTGTGTAATCTTGTCATTCTG-3’. Real-Time PCR results represent three independent experiments with reactions performed in triplicate. A Student’s t-test was applied for statistical analysis.
Results
Myocardial Tbx20 is required for early AVC development
Tbx20 is differentially expressed in the early heart tube of mouse embryos, exhibiting much higher levels of expression in the AVC and OFT (including myocardial, endocardial and cushion mesenchymal cells) than in chamber cardiac cells (Dupays et al., 2009; Stennard et al., 2003; Takeuchi et al., 2005). We confirmed this observation by whole-mount RNA in situ hybridization of mouse embryos (E9.0–9.5 with short time staining) (Figure 1 A–D).
Fig. 1.
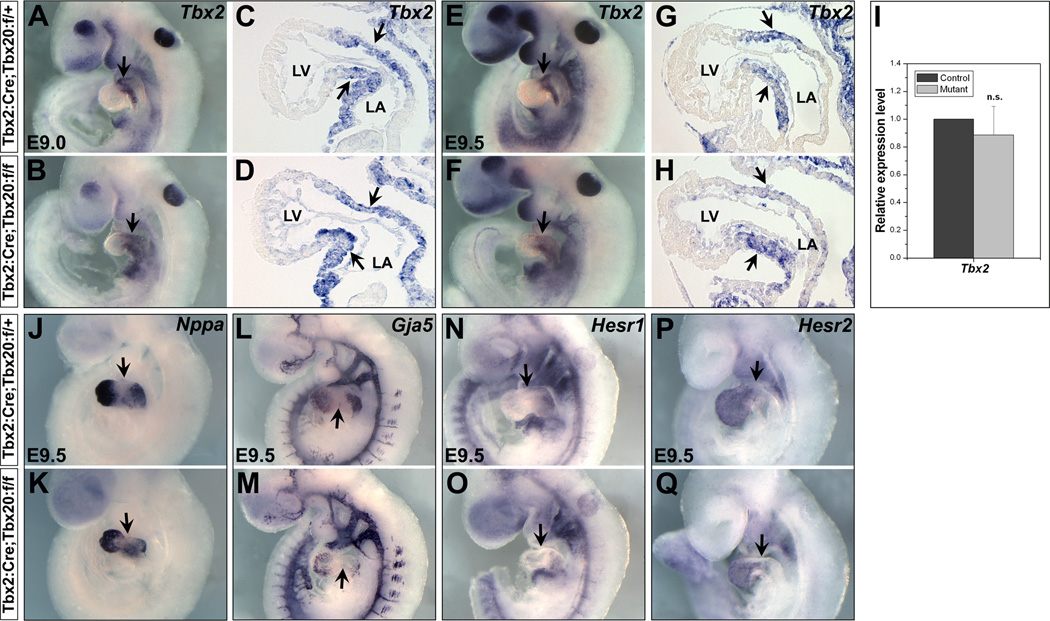
Myocardial Tbx20 is required for early AVC development. (A–D) Whole-mount RNA in situ hybridization with a brief staining time shows Tbx20 is unevenly expressed in the heart at E9.0–9.5, exhibiting much higher levels of expression in the AVC and OFT (arrows in A, C) than in the chamber cardiac cells (atria and ventricles). Sagittal sections confirm the stronger expression of Tbx20 in the AVC and OFT in both myocardium (arrows in B, D) and endocardium, as well as cushion mesenchymal cells (asterisks in B, D). (E,F) Left lateral views of Tbx20 CKO mutants (F, Tbx2:Cre;Tbx20:f/f) and control littermates (E, Tbx2:Cre;Tbx20:f/+) at E9.5. Less AVC constriction is observed in the mutants (arrows in E–H). G/H are sagittal sections for E/F, respectively. While AVC cushion mesenchymal cells are apparent at E9.5 (asterisk in G and arrows in I), very few mesenchymal cells are detected in the mutants (asterisk in H and arrows in J). I/J are high magnification images for G/H in the AVC (squares), respectively. LV, left ventricle; LA, left atrium.
To investigate a role for Tbx20 in the AVC formation, we crossed Tbx20f/f mice to Tbx2Cre/+ mice. Tbx2Cre/+ is a knock-in mouse model that specifically drives Cre expression in the AVC and OFT myocardium as early as E8.5 (Aanhaanen et al., 2009 and Figure S1). Mice with heterozygous deletion of Tbx20 (Tbx2Cre/+;Tbx20f/+) were viable and normal, while Tbx2Cre/+;Tbx20f/f mice (Tbx20 conditional knockout or Tbx20 CKO) did not survive to E10.5. Mutant hearts displayed a reduced AVC constriction when compared to their heterozygous littermates at E9.5 (Figure 1 E,F). The defects could be detected as early as E9.0 (data not shown). In addition, while AVC cushion mesenchymal cells were present in the control hearts from E9.5, very few mesenchymal cells were observed in the mutants (Figure 1 G–J, and data not shown for later stages).
Myocardial differentiation and chamber specification occurred in Tbx20 CKO mutants
To determine if the AVC defects in Tbx20 CKO embryos reflected abnormal myocardial differentiation, we assayed expression of markers for differentiated myocardium, including αMhc (Myh6), βMhc (Myh7) and Mlc2a (Myl7). αMhc was restricted to the AVC and forming atria at early stages, and βMhc and Mlc2a were expressed throughout the myocardium. We examined expression of these genes with RNA in situ hybridization and quantitative RT-PCR. No significant difference in expression localization was observed from the RNA in situ hybridization assays (Figure S2 A–F). Quantitative RT-PCR detected that αMhc was downregulated (13.3%) and βMhc was upregulated (19.9%), both significant but very modest differences (Figure S2 G); Mlc2a expression level was unchanged. These observations demonstrate that the myocardial differentiation program within the AVC area had occurred in Tbx20 CKO mutants but was slightly perturbed.
We further examined the expression of key cardiac transcription factors, including Nkx2.5, Gata4, Tbx5, Mef2c and Hand1, to determine if Tbx20 regulates AVC myocardial formation by acting upstream of them. Expression of most of these genes was unaffected in the Tbx20 CKO mutants (Figure S3 A–H). In contrast, Hand1, the basic helix-loop-helix (bHLH) transcription factor that is normally expressed in the AVC and left ventricular myocardium at E9.0, was downregulated in the AVC region of mutant embryos (Figure S3, I,J). We speculate, however, that reduced Hand1 expression is not the main cause for the AVC defects observed in the mutants, given that cardiac deletion of Hand1 results in much less perturbation of early heart development (McFadden et al., 2005). We also examined the proliferating and apoptotic cells in the AVC myocardium and did not detect significant differences between the mutant and control embryos (Figure S4).
During early cardiogenesis, Tbx2 is specifically expressed in the myocardial layer of the AVC and OFT (Harrelson et al., 2004 and Figure S1). In chamber myocardium, Tbx20 represses Tbx2 expression (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005). Because Tbx20 and Tbx2 are co-expressed in the AVC region, we wanted to determine if Tbx20 alters (activates) Tbx2 expression in the AVC myocardium. As shown in Figure 2 A–I, Tbx2 expression was not significantly changed in the AVC region of the mutant embryos. These data indicate that Tbx20 does not activate Tbx2 in the AVC myocardium.
Fig. 2.
Cardiac chamber specification is not perturbed in Tbx20 CKO embryos. Saggital sections of embryos showing expression of genes. During early cardiogenesis, Tbx2 is expressed in the AVC myocardium (arrows in A,C,E,G). Expression of Tbx2 is maintained in the AVC of Tbx20 CKO embryos (arrows in B,D,F,H). C/D and G/H are sagittal sections of A/B and E/F, respectively. (I) Quantitative RT-PCR revealed Tbx2 expression level is not significantly changed (n.s., not significant). Expression of chamber specification genes, Nppa (J/K, atrium and ventricle specific), Gja5 (L/M, atrium and ventricle specific), Hesr1 (N/O, atrium specific) and Hesr2 (P/Q, ventricle specific), is unchanged in the chamber myocardium of Tbx20 CKO mutants. Arrows indicate the AVC region. LV, left ventricle; LA, left atrium.
To further investigate the chamber specification of Tbx20 CKO embryos, particularly whether the mutant hearts retained proper chamber and AVC identities, we examined the expression of the chamber-specific genes, Nppa (Anf) and Gja5 (Cx40). Nppa and Gja5 maintained their “localized” expression in the chamber myocardium (atria and ventricles), and neither was ectopically expressed in the AVC (Figure 2 J–M), indicating that the mutant embryos still possessed proper chamber and AVC identities. The basic helix-loop-helix transcription factors Hesr1 and Hesr2 were specifically expressed in the atrium and ventricle, respectively, and they play crucial roles in the AV boundary formation by suppressing Bmp2 and Tbx2 in the chamber myocardium (Kokubo et al., 2007; Rutenberg et al., 2006). We found Hesr1 and Hesr2 expression was unaffected in the AVC and chamber regions (Figure 2 N–Q). The relatively normal expression of the chamber-specific genes suggests that the chamber specification still occurs in the Tbx20 CKO embryos, and unlike Tbx2, Tbx20 does not repress chamber-specific gene in the AVC.
Bmp2 expression is downregulated in the AVC myocardium of Tbx20 CKO embryos
Bmp2 is the earliest known marker expressed in the AVC myocardium and is critical for early AVC development (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000). In chick and mouse, Bmp2 induces Tbx2 in the AVC myocardium (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000). Mice with cardiac deletion of Bmp2 exhibit severe AVC constriction defects, lack of endocardial cushion EMT, and loss of Tbx2 expression (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000). Although Bmp2 acts as an upstream activator for Tbx2 expression, the signals inducing Bmp2 expression in the AVC myocardium are unknown.
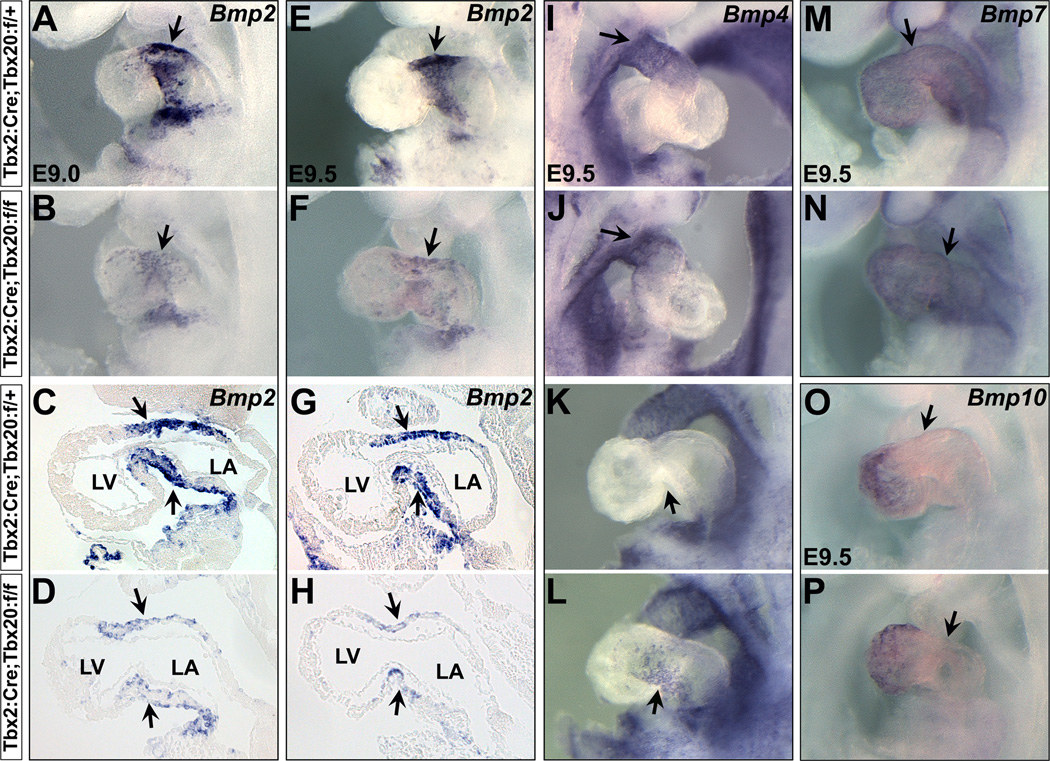
We determined if Tbx20 controls Bmp2 expression in the AVC myocardium. Interestingly, Bmp2 expression was dramatically downregulated in the Tbx20 CKO hearts (Figure 3 A–H). We further checked the expression of additional Bmp genes as they share important roles in heart development. Bmp4 expression was unchanged in the OFT (Figure 3 I,J), but was ectopically expressed at very low level in the AVC (Figure 3 K,L). Expression of Bmp7 and Bmp10 in the mutant hearts was unchanged (Figure 3 M–P).
Fig. 3.
Bmp signaling in Tbx20 CKO embryos. (A–H) Bmp2 expression is significantly downregulated in the AVC myocardium of Tbx20 CKO mutants at E9.0–9.5 (arrows). C/D and G/H are sagittal sections for A/B and E/F in the heart, respectively. (I–L) Bmp4 is specifically expressed in the OFT in the early heart tube (arrow in I). While Bmp4 expression is unchanged in the OFT (arrow in J), it is ectopically expressed at very low level in the AVC of mutant hearts (arrows in K,L). Bmp7 (M,N) and Bmp10 (O,P) expression are unaffected in the mutants. Arrows indicate the AVC region. LV, left ventricle; LA, left atrium.
Tbx20 is a key upstream regulator for AVC development
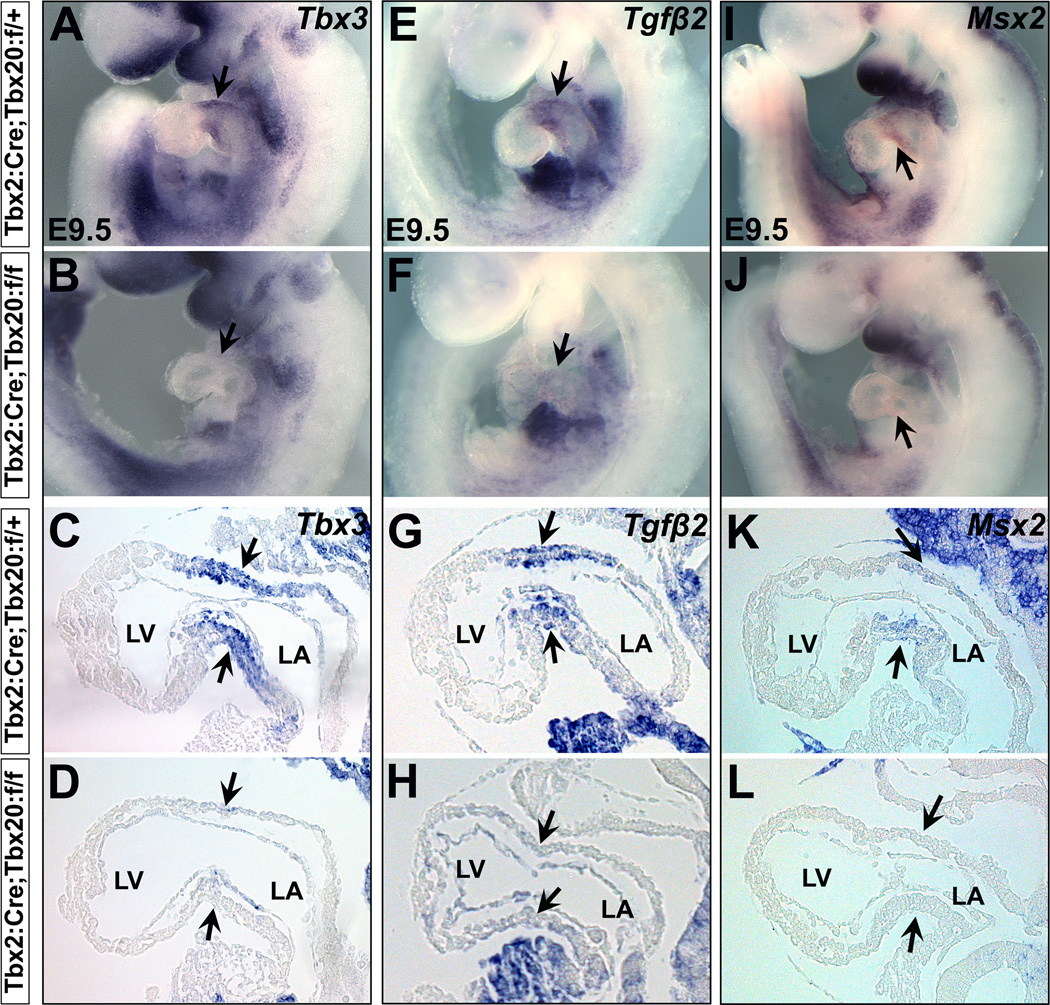
During early heart development, Tbx3 is co-expressed with Tbx2 in the AVC myocardium (Hoogaars et al., 2007; Mesbah et al., 2008). Tbx3 and Tbx2 suppress AVC myocardial proliferation to promote cardiac chamber formation (Aanhaanen et al., 2009; Christoffels et al., 2004; Habets et al., 2002; Harrelson et al., 2004; Hoogaars et al., 2007; Ribeiro et al., 2007). We found Tbx3 expression was remarkably downregulated in Tbx20 CKO embryos (Figure 4 A–D). Transforming growth factor β2 (Tgfβ2) and the msh homeobox transcription factor Msx2 are specifically expressed in the AVC myocardium. They act as downstream of Bmp2 and are activated by Bmp2 signals (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). Tgfβ2 induces EMT initiation (Person et al., 2005), and Msx2 is essential for AVC endocardial EMT and myocardial patterning (Chen et al., 2008). Expression of Tgfβ2 and Msx2 was completely absent in the AVC myocardial cells of Tbx20 CKO embryos (Figure 4 E–L).
Fig. 4.
Aberrant AVC myocardium development programs in Tbx20 CKO embryos. At E9.5, Tbx3, Tgfβ2 and Msx2 are specifically expressed in the AVC myocardium (arrows in A/C, E/G and I/K). Expression of these genes is severely downregulated in Tbx20 CKO mutants (arrows in B/D, F/H and J/L). C/D, G/H and K/L are sagittal sections for A/B, E/F and I/J in the hearts, respectively. LV, left ventricle; LA, left atrium.
Extracellular matrix (ECM) formation is crucial for EMT (Camenisch et al., 2000). To determine whether the AVC cushion development of the mutant embryos results from defective ECM, we performed Alcian blue staining to examine ECM integrity. As shown in Figure 5, ECM in the cardiac jelly was intact in the mutants (Figure 5, A,B), suggesting the EMT defects of Tbx20 CKO embryos were not due to a defective ECM formation. Hyaluronic acid (HA) synthase 2 (Has2), a major enzyme for HA synthesis, is expressed in both AVC myocardium and endocardium (Figure 5, C,E) and is essential for ECM formation. In agreement with the Alcian blue staining, Has2 expression was unaltered in the endocardial cells of the mutant embryos (Figure 5 C–F), yet the myocardial expression was missing, suggesting that Has2 expression in the AVC myocardium is also maintained by Tbx20.
Fig. 5.
ECM integrity of AVC endocardial cushion is unaffected in Tbx20 CKO hearts. Alcian blue staining reveals ECM integrity of the AVC cardiac jelly is unaffected in the mutant hearts (arrows in A,B). Has2, a synthetic enzyme for hyaluronic acid (HA), is specifically expressed in the AVC myocardium (arrows in C,E) and endocardium (asterisks in E). Although Has2 expression is absent in the AVC myocardium of Tbx20 CKO mutant (arrows in D,F), the endocardial expression is maintained (asterisks in F), suggesting HA synthesis still occurs in the ECM of mutant embryos. LV, left ventricle; LA, left atrium.
Tbx20 regulates EMT in a non-cell-autonomous manner
The transcription factors Sox9, Twist1 and Msx1 are specifically expressed in the AVC cushion mesenchyme and crucial for the proliferation and migration of these cells (Akiyama et al., 2004; Chen et al., 2008; Shelton and Yutzey, 2008). Expression of Sox9, Twist1 and Msx1 was absent in the Tbx20 CKO mutants (Figure 6 A–L), consistent with the prior observation that mutant embryos lack AVC cushion mesenchymal cells (Figure 1 G–J). The early endocardial markers Notch1 and VE-Cadherin were still expressed in Tbx20 CKO hearts, suggesting that early AVC endocardium development was unaffected in the mutant embryos (Figure 6 M–T).
Fig. 6.
Disrupted AVC cushion mesenchyme formation in Tbx20 CKO embryos. Sox9, Twist1 and Msx1 are specifically expressed in the AVC cushion mesenchymal cells at E9.5 (A/C,E/G,I/K). Expression of these genes is nearly absent in Tbx20 CKO hearts (B/D,F/H,J/L). Notch1 (M–P) and VE-Cadherin (Q–T) expression is maintained in the AVC endocardium of Tbx20 CKO embryos. Arrows indicate the AVC region. LV, left ventricle; LA, left atrium.
As Tbx20 has been implicated for promoting endocardial cushion cell proliferation during heart development (Shelton and Yutzey, 2007), and Tbx20 is strongly expressed in the AVC endocardium and cushion mesenchyme (Figure 1 A–D), we asked whether loss of Tbx20 in these cells could also affect early EMT and AVC formation. By crossing Tbx20f/f mice to Tie2Cre mice, specifically deleting Tbx20 in the endocardium and cushion mesenchyme (Figure 7 G,I) (Kisanuki et al., 2001), we found mutant embryos (Tie2Cre;Tbx20f/f) had grossly normal cardiac shape at E9.5–10.0 (Figure 7 A–F), and the number of AVC cushion mesenchymal cells was similar to their control littermates at E10.0 (Figure 7 H,J), indicating that loss of Tbx20 in the endocardium and cushion mesenchyme did not inhibit EMT. These observations further suggest that the myocardial Tbx20 expression modulates EMT during early AVC development.
Fig. 7.
EMT initiation in AVC is independent of endocardial Tbx20. (A–F) Whole-mount views of mouse embryos with Tbx20 deletion in the endocardium and cushion mesenchyme by Tie2Cre. Unlike Tbx20 CKO embryos, Tie2Cre;Tbx20f/f hearts do not display morphogenetic defects at E9.5–E10.0. Arrows in A–F indicate OFT or AVC. (G–J) Sagittal sections of mouse hearts at E10.0. Embryos contain one copy of R26RlacZ to determine Tie2Cre lineage cells with disrupted Tbx20 allele. X-gal staining shows Tie2Cre lineage cells including endocardium and cushion mesenchyme (G,I). As in the controls (arrows in I), AVC endocardium and cushion mesenchymal cells are still well formed in the mutants at E10.0 (arrows in J). I/J are high magnification images for G/H in the AVC (square area), respectively. LV, left ventricle; LA, left atrium.
Re-expression of Bmp2 substantially rescues EMT defects in Tbx20 CKO hearts
Our results revealed that two categories of genes were altered in Tbx20 CKO hearts: 1) Tgfβ2, Msx2 and Has2 expression was missing in the AVC myocardium; 2) Sox9, Twist1 and Msx1 expression was missing in the AVC cushion mesenchyme (or absence of AVC cushion mesenchymal cells). Intriguingly, these altered gene expressions are completely reminiscent of that observed in Bmp2 cardiac deletion mouse embryos (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). In addition, Tbx20 CKO and Bmp2 cardiac deletion embryos displayed similar AVC constriction and cushion development defects (Figure 1 E–J and Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). These observations led us to hypothesize that Tbx20 regulates early AVC and EMT development through Bmp2.
To further characterize the regulatory interaction between Tbx20 and Bmp2, particularly whether Bmp2 is a key downstream factor of Tbx20 during the AVC formation and EMT initiation, we generated a transgenic mouse model with a conditional Bmp2 expression vector (CAG-LoxP-eGFP-STOP-LoxP-Bmp2-IRES-LacZ), to force re-expression of Bmp2 in the AVC myocardium of Tbx20 CKO embryos (Figure 8 A). In this model, eGFP expression is under the control of a ubiquitously expressed CAG promoter. Bmp2 expression is initiated when Cre excises the eGFP-STOP cassette. We applied Tbx2Cre to efficiently re-express Bmp2 in the AVC myocardium (Figure 8 A3/4). Compared with the Tbx20 CKO mutants, we found a significantly increased number of AVC cushion mesenchymal cells in the mutant embryos expressing Bmp2 in the Tbx2 domain (17.8±3.9 cells, n=5, Figure 8 J,K), with numbers approaching those seen in the control littermates (22.3±0.9 cells, n=5, Figure 8 H,K), while very few mesenchymal cells were observed in the mutant embryos (2.8±1.4 cells, n=3, Figure 8 I,K). We further performed RNA in situ hybridization with Sox9, a specific marker for AVC mesenchymal cells. Sox9 was re-expressed in the AVC region of the “rescued” embryos (Figure 8 L–N), indicating a recovery formation of AVC mesenchymal cells. We also checked Tbx2 expression in the “rescued” embryos, and found it was still expressed in the AVC region (Figure S5). These results show that re-expression of Bmp2 rescues EMT defects of Tbx20 CKO embryos, supporting the idea that downregulation of Bmp2 is the main cause of the EMT defects in the mutant embryos. In addition, the “rescued” embryos displayed mitigated AVC constriction (Figure 8 B–D). Notably, re-expression of Bmp2 did not fully rescue the cardiac development, and the resulting embryos could not survive beyond E10.5. These findings indicate that the regulation of Bmp2 by Tbx20 is a critical, but not the exclusive, factor in AVC development.
Fig. 8.
Genetic rescue of EMT defects by re-expression of Bmp2 in Tbx20 CKO embryos. (A) Diagram for Bmp2 conditional expression transgenic construct. The floxed eGFP (LoxP-eGFP-STOP-LoxP) followed by a Bmp2-IRES-lacZ cassette is driven by a ubiquitously expressing CAG promoter (A1). Visualization of GFP expression in heart on an E9.5 transgenic embryo (A3). Bmp2 expression is activated when Cre excises the eGFP-STOP cassette. The transgenic mouse line is crossed to Tbx2:Cre mice for re-expression of Bmp2 in Tbx2+ cells (A2). X-gal staining is performed to evaluate Bmp2 expression in Tbx2Cre;Bmp2-Tg+ embryos (A4). (B–D) Left lateral views of the control (B, Tbx2Cre;Tbx20f/+;Bmp2-Tg−), mutant (C, Tbx2Cre;Tbx20f/f;Bmp2-Tg−) and “rescued” (D, Tbx2Cre;Tbx20f/f;Bmp2-Tg+) embryos at E9.5. The defects of AVC constriction are slightly attenuated in the “rescued” embryos (comparisons for B/C/D, arrows). (E–J) Sagittal sections reveal that the migrating cushion mesenchymal cells are obviously formed in the “rescued” embryos (G, arrows in J), resembling the control littermates (E, arrows in H). Very few mesenchymal cells are observed in the mutant littermates (F, arrows in I). H/I/J are high magnification images for E/F/G in the AVC region (squares), respectively. (K) Statistic analysis indicates a significantly increased number of mesenchymal cells in the AVC region of “rescued” embryos. Separate counting and statistic assays are confirmed by two analysts. Results are obtained from five control, three mutant and five “rescued” littermate embryos. *p<0.01 vs. mutant. (L–N) Sox9, a marker of mesenchymal cells, is re-expressed in the AVC region of “rescued” embryos. Arrows indicate the AVC region. LV, left ventricle; LA, left atrium.
Discussion
In this study, we deleted Tbx20 in the AVC myocardium by crossing Tbx20-floxed mice to Tbx2-Cre mice. The mutant embryos displayed severely underdeveloped AVC constriction with loss of cushion mesenchymal cells (Figure 1 E–J), demonstrating that Tbx20 is essential for early AVC formation. Given that Tbx20 is highly expressed in the AVC endocardium and cushion mesenchymal cells (Figure 1 A–D), we further eliminated Tbx20 in these cells by crossing Tbx20-floxed mice to Tie2Cre mice. The grossly normal cardiac morphology of Tie2Cre;Tbx20f/f embryos (E9.5–10.0) indicates that the expression of Tbx20 in the AVC myocardium, not in the endocardium or cushion mesenchyme, controls EMT initiation (Figure 7). Re-expression of Bmp2 in the AVC myocardium rescued the EMT defects of Tbx20 CKO hearts, suggesting that Tbx20 regulates EMT through Bmp2 (Figure 8). It is also important to note that, although EMT defects were not observed in Tie2Cre;Tbx20f/f hearts by E10.0 (Figure 7), Tbx20 may still be important for AVC cushion and valve formation at later gestational stages in mice, as suggested in studies of chick embryos (Shelton and Yutzey, 2007).
Several studies have indicated that Bmp2 is the earliest gene expressed in the AVC myocardium, and Bmp2 induces Tbx2 expression during AVC formation (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000) (Bmp2→Tbx2, Figure 9). Bmp2 also activates Tgfβ2, Msx2 and Has2 in the AVC myocardium, and initiates EMT by regulating Sox9, Twist1 and Msx1 expression in the AVC cushion mesenchyme (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006) (Figure 9). A recent study also revealed that Bmp2 integrates with endocardial Notch signals to promote EMT (Luna-Zurita et al., 2010). Although the genes downstream of Bmp2 are well characterized, the upstream signals for induction of Bmp2 expression are largely unknown. We found that Tbx20 is important to maintain Bmp2 expression in the AVC myocardium, identifying Tbx20 as the earlier regulator for this key aspect of heart development (Tbx20→Bmp2, Figure 9). Additional as-yet-unidentified factors may also contribute to Bmp2 activation, because Bmp2 transcripts were not completely eliminated (although mostly) in Tbx20 CKO hearts (Figure 3 A–H, Figure 9). In addition, we found that Tbx20 promotes other key transcription factors, including Tbx3 and Hand1 expression in the AVC myocardium (Figure 3 A–D, Figure S3 I,J, and Figure 9). Although individual disruption of these genes does not cause severe defects reminiscent of Tbx20 CKO embryos (McFadden et al., 2005; Mesbah et al., 2008), their dysregulation may still contribute to certain aspects of cardiac malformations in the mutant embryos. This may also explain why re-expression of Bmp2 cannot fully rescue AVC constriction defects of Tbx20 CKO embryos, although the EMT and cushion development is largely recovered.
Fig. 9.
Schematic illustration of Tbx20 regulatory cascades and genetic interactions of Tbx20, Bmp2 and Tbx2 during early AVC development. During early cardiogenesis, Tbx20 is predominantly expressed in the AVC myocardium. Upon regulation by Tbx20 as well as other factors, Bmp2 is expressed and it further activates Tgfβ2, Msx2 and Has2 in the myocardium (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). Bmp2 also regulates EMT in AVC through promoting Sox9, Twist1 and Msx1 expression in the cushion mesenchyme (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006). Tbx20 is required for EMT initiation through regulation of Bmp2 in the AVC myocardium. Tbx20 also maintains Tbx3 and Hand1 expression in the AVC myocardium. Tbx2 expression is induced by Bmp2 (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000), and is possibly still repressed by Tbx20. Tbx2 represses chamber-specific genes expression (e.g., Nppa, Gja5) in the AVC myocardium (Christoffels et al., 2004; Harrelson et al., 2004). LV, left ventricle; LA, left atrium.
During early cardiogenesis, Tbx2 is specifically expressed in the AVC to repress chamber-specific gene expression (Christoffels et al., 2004; Harrelson et al., 2004) (Tbx2–|Nppa/Gja5, Figure 9). In the chamber myocardium, Tbx20 acts as a transcriptional suppressor for Tbx2 such that its expression is not detectable (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005). Tbx20 may interfere with Bmp/Smad signaling to inhibit Tbx2 expression in the cardiac chambers to confine Tbx2 expression in the AVC (Singh et al., 2009). Although Tbx20 suppressing Tbx2 transcription in the chamber myocardium has been well recognized, it was not known whether and how Tbx20 regulates Tbx2 expression in the AVC. In Tbx20 CKO embryos, we detected a relatively normal Tbx2 expression and a dramatically downregulated Bmp2 expression in the AVC myocardium. As Bmp2 is essential for Tbx2 expression in the AVC (Bmp→Tbx2) (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Yamada et al., 2000), we anticipated a downregulated Tbx2 expression in the AVC of mutant hearts. In fact, Tbx2 expression was not significantly decreased in the AVC region (Figure 2 A–I). Based on these observations, we speculate that Tbx20 still acts as a suppressor for Tbx2 expression in the AVC myocardium (Figure 9). The reason that Tbx2 expression was not downregulated in the mutant embryos (which was expected due to loss of Bmp2 activation) was because it may be mitigated by loss of Tbx20’s direct repression. Therefore, the spatiotemporal expression of Tbx2 may be regulated by two antagonistic signals: Tbx20–|Tbx2 and Tbx20→Bmp2→Tbx2 (Figure 9), and the high-level expression of Tbx20 in the AVC myocardium may play an essential role for balancing of the antagonistic signals that further affect early heart development.
Interestingly, a recent study revealed that Bmp/Smad1 signals directly activate Tbx20 cardiac expression in Xenopus (Mandel et al., 2010). Mouse Bmp10 also induces Tbx20 expression in the ventricular wall (Wenjun Zhang, 2011). It is unclear, however, whether Bmps still act as upstream regulators for activating Tbx20 expression in the AVC region. In Bmp2 cardiac deletion mouse embryos, Tbx20 is still normally expressed (Ma et al., 2005), indicating that Bmp2 itself is not an upstream activator for Tbx20 expression in the AVC, and that regulation of Bmp2 by Tbx20 is unidirectional. Collectively, these observations suggest that Bmp signals may act as either upstream or downstream of Tbx20 during early heart formation.
It was shown that Tbx2 directly activates Has2 and Tgfβ2 activity to mediate AVC endocardial cushion formation (Shirai et al., 2009). In Tbx20 CKO embryos, Has2 and Tgfβ2 expression is absent whereas Tbx2 is still expressed in the AVC myocardium. Thus, we suspect that Has2 and Tgfβ2 may be regulated by both Tbx2 (Shirai et al., 2009) and Tbx20/Bmp2 (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006) signals, and that Tbx20→Bmp2 pathways seem more effectively to manipulate Has2 and Tgfβ2 activity during AVC formation.
Taken together, our studies provide genetic evidence that Tbx20 acts as a key upstream regulator for a variety of genes, especially Bmp2, in the regulation of early AVC development. Our data also suggest that, in addition to promoting chamber myocardial proliferation and specification (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005), Tbx20 is essential for AVC patterning and cushion formation, therefore playing crucial roles in these two important aspects to coordinate early heart development.
Highlights.
-
>
Myocardial Tbx20 is required for early AVC formation and endocardial EMT
-
>
Tbx20 maintains expression of Bmp2, Tbx3 and Hand1 in the AVC myocardium
-
>
Bmp2 downstream genes involved in the EMT initiation are downregulated in mutant embryos
-
>
EMT initiation in AVC is independent of endocardial Tbx20
-
>
Re-expression of Bmp2 in the AVC myocardium substantially rescues the EMT defects
Supplementary Material
Fig. S1. Tbx2 is specifically expressed in the AVC and OFT myocardium during early cardiogenesis in mouse. A Tbx2:nLacZ knock-in mouse model was generated to determine Tbx2 expression pattern. Details for generation of the mouse model will be described elsewhere. In summary, a nLacZ-H2B-GFP cassette (LoxP-nLacZ-polyA-LoxP-H2B-GFP-polyA-FRT-Neo-FRT) is inserted into Tbx2 exon1 (3 bp upstream of the endogenous ATG with disruption of start condon). Neo cassette is removed by crossing the knock-in mice to Flippase deleter mice. nLacZ is expressed under control of the endogenous Tbx2 promoter. X-gal staining of Tbx2:nLacZ embryos reveal it mirrors endogenous Tbx2 expression. Tbx2 is specifically expressed in the myocardium of AVC (notched arrows) and OFT (unnotched arrows) at E8.75 (A), E9.25 (B–D) and E10.25 (E–G). A,E,C, left lateral view; B, right lateral view; F,G, ventral and dorsal view of the heart. D and H, sagital section for C and E, respectively.
Fig. S2. AVC myocardial differentiation in Tbx20 CKO embryos. (A–F) Whole-mount RNA in situ hybridization with differentiated myocardial markers on Tbx20 CKO and littermate controls. αMhc (A,B), βMhc (C,D) and Mlc2a (E,F) are expressed in the mutant AVC myocardium. Arrows indicate the AVC region. (G) Quantitative RT-PCR revealed αMhc expression is significantly downregulated, while βMhc is upregulated. No significant change is found for Mlc2a expression. *p<0.05 vs. control. n.s., not significant.
Fig. S3. Expression of key cardiac transcription factors in the AVC of Tbx20 CKO embryos. Whole-mount RNA in situ hybridization reveal that the expression of key cardiac transcription factors, including Nkx2.5 (A,B), Gata4 (C,D), Tbx5 (E,F) and Mef2c (G,H) is unaffected in the AVC of Tbx20 CKO embryos. Hand1, however, is downregulated in the AVC of mutant embryos (I,J). Arrows indicate the AVC region.
Fig. S4. Apoptosis and proliferation assay of Tbx20 CKO embryos. TUNEL and anti-phosphohistone H3 (pH3) immunostaining are performed at E9.25. (A,B) No apoptotic cells are observed in the AVC of mutants or control littermates (arrows). Asterisks indicate apoptotic cells in the pharyngeal region (green fluorescent signals). (C,D) Proliferating cells are examined by pH3 in the AVC (green fluorescent signals, arrows). No significant differences are detected between the mutants and controls.
Fig. S5. Expression of Tbx2 in Tbx20 CKO embryos with re-expression of Bmp2. Whole-mount RNA in situ hybridization reveal that Tbx2 expression is unaffected in the AVC area of “rescued” embryos (C), similar to Tbx20 CKO (B) and heterozygous deletion embryos (A). Arrows indicate AVC region.
Acknowledgments
The authors thank Drs. Bruce Gelb and Marek Mlodzik for critical reading of the manuscript, and Dr. Kevin Kelley in the Mouse Genetics Resource Facility at Mount Sinai School of Medicine for production of Bmp2 transgenic mice. We also thank Dr. Ahmed Mansouri (Max-Planck) for providing the plasmid to generate the Bmp2 transgenic construct, and Drs. James Martin (Baylor College of Medicine) and Hiroki Kokubo (National Institute of Genetics, Japan) for providing RNA in situ probes. This work is supported by grants to C.L.C. from the NIH/NHLBI (1K02 HL094688 and 1R01HL095810), the American Heart Association (0855808D) and the March of Dimes Foundation (5-FY07-642).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- Aanhaanen WT, Brons JF, Dominguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, Moorman AF, Christoffels VM. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res. 2009;104:1267–1274. doi: 10.1161/CIRCRESAHA.108.192450. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. Philadelphia: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- Brown DD, Martz SN, Binder O, Goetz SC, Price BM, Smith JC, Conlon FL. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development. 2005;132:553–563. doi: 10.1242/dev.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Zhou W, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S. T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development. 2005;132:2475–2487. doi: 10.1242/dev.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson CT, Kinzler ER, Parr BA. Tbx12, a novel T-box gene, is expressed during early stages of heart and retinal development. Mech Dev. 2000;96:137–140. doi: 10.1016/s0925-4773(00)00376-2. [DOI] [PubMed] [Google Scholar]
- Chen YH, Ishii M, Sucov HM, Maxson RE., Jr. Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium. BMC Dev Biol. 2008;8:75. doi: 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn. 2004;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupays L, Kotecha S, Angst B, Mohun TJ. Tbx2 misexpression impairs deployment of second heart field derived progenitor cells to the arterial pole of the embryonic heart. Dev Biol. 2009;333:121–131. doi: 10.1016/j.ydbio.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EP, Sunde M, Costa MW, Rankin SA, Wolstein O, Castro ML, Butler TL, Hyun C, Guo G, Otway R, Mackay JP, Waddell LB, Cole AD, Hayward C, Keogh A, Macdonald P, Griffiths L, Fatkin D, Sholler GF, Zorn AM, Feneley MP, Winlaw DS, Harvey RP. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am J Hum Genet. 2007;81:280–291. doi: 10.1086/519530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Tomita-Miyagawa S, Hamada Y, Saga Y. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development. 2007;134:747–755. doi: 10.1242/dev.02777. [DOI] [PubMed] [Google Scholar]
- Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene Tbx18. Mech Dev. 2001;100:83–86. doi: 10.1016/s0925-4773(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Liu C, Shen A, Li X, Jiao W, Zhang X, Li Z. T-box transcription factor TBX20 mutations in Chinese patients with congenital heart disease. Eur J Med Genet. 2008 doi: 10.1016/j.ejmg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci U S A. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Zurita L, Prados B, Grego-Bessa J, Luxan G, del Monte G, Benguria A, Adams RH, Perez-Pomares JM, de la Pompa JL. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest. 2010;120:3493–3507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mandel EM, Kaltenbrun E, Callis TE, Zeng XX, Marques SR, Yelon D, Wang DZ, Conlon FL. The BMP pathway acts to directly regulate Tbx20 in the developing heart. Development. 2010;137:1919–1929. doi: 10.1242/dev.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Theveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr., Yutzey KE. T-box genes and heart development: putting the "T" in heart. Dev Dyn. 2005;232:11–20. doi: 10.1002/dvdy.20201. [DOI] [PubMed] [Google Scholar]
- Qian L, Mohapatra B, Akasaka T, Liu J, Ocorr K, Towbin JA, Bodmer R. Transcription factor neuromancer/TBX20 is required for cardiac function in Drosophila with implications for human heart disease. Proc Natl Acad Sci U S A. 2008;105:19833–19838. doi: 10.1073/pnas.0808705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro I, Kawakami Y, Buscher D, Raya A, Rodriguez-Leon J, Morita M, Rodriguez Esteban C, Izpisua Belmonte JC. Tbx2 and Tbx3 regulate the dynamics of cell proliferation during heart remodeling. PLoS ONE. 2007;2:e398. doi: 10.1371/journal.pone.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol. 2007;302:376–388. doi: 10.1016/j.ydbio.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol. 2008;317:282–295. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai M, Imanaka-Yoshida K, Schneider MD, Schwartz RJ, Morisaki T. T-box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgfbeta2 expression and endocardial cushion formation. Proc Natl Acad Sci U S A. 2009;106:18604–18609. doi: 10.1073/pnas.0900635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Christoffels VM, Dias JM, Trowe MO, Petry M, Schuster-Gossler K, Burger A, Ericson J, Kispert A. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development. 2005;132:2697–2707. doi: 10.1242/dev.01854. [DOI] [PubMed] [Google Scholar]
- Singh R, Horsthuis T, Farin HF, Grieskamp T, Norden J, Petry M, Wakker V, Moorman AF, Christoffels VM, Kispert A. Tbx20 interacts with smads to confine tbx2 expression to the atrioventricular canal. Circ Res. 2009;105:442–452. doi: 10.1161/CIRCRESAHA.109.196063. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Elliott DA, Rankin S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau BG, Zorn AM, Harvey RP. Cardiac T-box factor Tbx20 directly interacts with Nkx2–5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev Biol. 2003;262:206–224. doi: 10.1016/s0012-1606(03)00385-3. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OW, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Griffin KJ, Kimelman D. HrT is required for cardiovascular development in zebrafish. Development. 2002;129:5093–5101. doi: 10.1242/dev.129.21.5093. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Mileikovskaia M, Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M, Georges R, Davidson L, Mo R, Hui CC, Henkelman RM, Nemer M, Black BL, Nagy A, Bruneau BG. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development. 2005;132:2463–2474. doi: 10.1242/dev.01827. [DOI] [PubMed] [Google Scholar]
- Wenjun Zhang HC, Wang Yong, Yong Weidong, Zhu Wuqiang, Liu Yunlong, Randall Gregory, Wagner, Payne R Mark, Field Loren J, Xin Hongbo, Shou Weinian. Tbx20 a downstream mediator for Bmp10 signaling in regulating ventricular wall development and function. Weinstein Cardiovascular Development Conference. 2011 doi: 10.1074/jbc.M111.279679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. In Situ Hybridization: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- Yamada M, Revelli JP, Eichele G, Barron M, Schwartz RJ. Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev Biol. 2000;228:95–105. doi: 10.1006/dbio.2000.9927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Tbx2 is specifically expressed in the AVC and OFT myocardium during early cardiogenesis in mouse. A Tbx2:nLacZ knock-in mouse model was generated to determine Tbx2 expression pattern. Details for generation of the mouse model will be described elsewhere. In summary, a nLacZ-H2B-GFP cassette (LoxP-nLacZ-polyA-LoxP-H2B-GFP-polyA-FRT-Neo-FRT) is inserted into Tbx2 exon1 (3 bp upstream of the endogenous ATG with disruption of start condon). Neo cassette is removed by crossing the knock-in mice to Flippase deleter mice. nLacZ is expressed under control of the endogenous Tbx2 promoter. X-gal staining of Tbx2:nLacZ embryos reveal it mirrors endogenous Tbx2 expression. Tbx2 is specifically expressed in the myocardium of AVC (notched arrows) and OFT (unnotched arrows) at E8.75 (A), E9.25 (B–D) and E10.25 (E–G). A,E,C, left lateral view; B, right lateral view; F,G, ventral and dorsal view of the heart. D and H, sagital section for C and E, respectively.
Fig. S2. AVC myocardial differentiation in Tbx20 CKO embryos. (A–F) Whole-mount RNA in situ hybridization with differentiated myocardial markers on Tbx20 CKO and littermate controls. αMhc (A,B), βMhc (C,D) and Mlc2a (E,F) are expressed in the mutant AVC myocardium. Arrows indicate the AVC region. (G) Quantitative RT-PCR revealed αMhc expression is significantly downregulated, while βMhc is upregulated. No significant change is found for Mlc2a expression. *p<0.05 vs. control. n.s., not significant.
Fig. S3. Expression of key cardiac transcription factors in the AVC of Tbx20 CKO embryos. Whole-mount RNA in situ hybridization reveal that the expression of key cardiac transcription factors, including Nkx2.5 (A,B), Gata4 (C,D), Tbx5 (E,F) and Mef2c (G,H) is unaffected in the AVC of Tbx20 CKO embryos. Hand1, however, is downregulated in the AVC of mutant embryos (I,J). Arrows indicate the AVC region.
Fig. S4. Apoptosis and proliferation assay of Tbx20 CKO embryos. TUNEL and anti-phosphohistone H3 (pH3) immunostaining are performed at E9.25. (A,B) No apoptotic cells are observed in the AVC of mutants or control littermates (arrows). Asterisks indicate apoptotic cells in the pharyngeal region (green fluorescent signals). (C,D) Proliferating cells are examined by pH3 in the AVC (green fluorescent signals, arrows). No significant differences are detected between the mutants and controls.
Fig. S5. Expression of Tbx2 in Tbx20 CKO embryos with re-expression of Bmp2. Whole-mount RNA in situ hybridization reveal that Tbx2 expression is unaffected in the AVC area of “rescued” embryos (C), similar to Tbx20 CKO (B) and heterozygous deletion embryos (A). Arrows indicate AVC region.