Abstract
Phthalates are ubiquitous compounds used in the manufacturing industry. Some are known endocrine disruptors, acting as xenoestrogens, others induce reproductive toxicity and damage to DNA among other effects. Studies on apoptosis induction and mitochondrial damage capacity of phthalates on the immune system are limited. This study aims to determine cell viability inhibition and apoptosis induction of diethylhexyl phthalate (DEHP) and monoethylhexyl phthalate (MEHP) on the human TK6 lymphoblast cell line at concentrations found in the environment. Key hallmark events, such as mitochondrial membrane permeability, generation of reactive oxygen species (ROS) and activation of caspase 3 and 7 were measured. Concentrations that inhibit viability of 50% (IC50) of the cells were determined at 24, 48 and 72 hours with doses ranging from 10μM to 500μM. Changes in mitochondrial membrane permeability, ROS generation and activation of caspases 3 and 7, were measured as part of the cell death mechanism. The IC50 at 24 hours was approximately 250 μM for both phthalates; at 48 hours were 234μM and 196μM for DEHP and MEHP, respectively and at 72 hours IC50s were 100 μM and 80 μM for DEHP and MEHP respectively. Overall the longer the time of exposure the lower the IC50's for both compounds. Both compounds affected mitochondrial membrane potential, promoted ROS generation and activated caspases 3 and 7. MEHP is more toxic, promotes higher level of ROS production and caspases activation. Our findings suggest that DEHP and MEHP have the capacity to induce apoptosis in cells of the immune system at concentrations found in the environment.
Keywords: phthalates, apoptosis, mitochondrial membrane, Tk6 lymphoblast, immune system, ROS, caspases
Introduction
Phthalates are a family of compounds widely used in the manufacturing industry and the production of plastics. Exposure to these substances can occur through consumer items, medical devices, and beauty products (Wahl et al. 2004; Koo et al. 2004). There are a high number of products containing phthalates that are easily released into the environment. Some studies at industrialized countries have pointed out that a large extent of the population are exposed to various phthalates including DEHP and MEHP (Fromme, H., et al. 2007; Swan, S.H., 2008; Suzuki et al. 2009; Wormuth et al. 2006). Phthalate residues have been detected in households, food, plastic containers, Superfund sites, in adult and children urine samples, in blood samples of women with endometriosis, and in placental samples (Nakamiya et al. 2005; U.S. Environmental Protection Agency 2002; Silva et al. 2007; Cobellis et al. 2003; Jen and Liu, 2006; Sax, 2010; Latini et al. 2003). Infant exposure has also been demonstrated through the detection of phthalates in urine, potentially from the use of personal care products such as lotions, shampoo and powders (Sathyanarayana et al. 2008).
Phthalate exposure and some of its effects have been determined partially through research with animal models including rats, mice, hamsters, and tissue culture. Some of the observed effects are hepatocarcinogenesis, tumors, DNA mutations, alterations in embryonic development, reproductive toxicity, and infertility in animal models as well (Cobellis et al. 2003). Other studies have indicated relationships between exposure to phthalates and adverse health effects in humans such as the report of exposure to MEHP causing alteration of thyroid hormone level in men where an inverse relation was found between the MEHP concentrations in urine samples and the serum levels of T3 and T4 (triiodothyronine). MEHP exposure has been linked to up and down regulation of genes such as PAFAH1B1 related with the cortical development as well as androgen antagonist activity has been reported (Meeker et al. 2007; Hokanson et al. 2006), additionally, effects on the cardiovascular, hepatic, urologic and genital systems have been reported. A dose-response relationship was observed between phthalate concentrations, asthma and allergic symptoms in children (Singh and Li, 2010; Bornehag et al. 2004). An inverse significant relation was found between sperm concentration, percentage of abnormal sperm and DEHP concentrations (Pant et al. 2008).
Limited information has been reported on the effect of phthalates on the immune system, where phthalate ester metabolites have been reported to be peroxisome proliferator-activated receptor γ (PPARγ) agonists capable of inducing apoptosis on primary bone marrow B cells (Schlezinger et al. 2004). Cells from the immune system such as lymphoblasts are crucial to the adaptive cellular immune response based in a dynamic antigen pathogen recognition system. The ubiquitous presence of phthalates in human daily activities implies a constant exposure to the immune system including changes in the viability and function of lymphoblast cells suggesting that phthalates may have adverse effects on the immune system (Jaakkola and Knight, 2008). Studying the response of human lymphoblast cells to DEHP and MEHP can bring a new perspective on the toxicity and cellular effects of these compounds on the immune system.
It is important to emphasize that the experimental doses used in this study are consistent with levels encountered in the environment and that the selected time of exposures, although longer than in previous reported studies, respond to the need to better understand the effects of a chronic, rather than an acute exposure. The dose responses observed in this study provides valuable information specifically on the capacity of phthalates, to induce change on the mitochondrial membrane potential, generation of ROS and activation of caspases, which are hallmark events in cellular apoptosis.
Materials and Methods
Stock solutions and Reagents
DEHP and reduced L-glutathione where obtained from Sigma Aldrich (St Louis, MO). MEHP was obtained from Accu Standard (New Haven, CT). Stock solutions of both DEHP and MEHP were prepared at concentrations of 2mM in dimethyl sulfoxide (DMSO) biotechnology grade, obtained from Sigma Aldrich, St. Louis, MO. Stock solutions were kept in sterile glass vials and stored at 4°C. The following positive controls where obtained from Sigma Aldrich, St. Louis, MO: cis-diammineplatinum (II) dichloride (cisplatin) for ROS determination; Valinomycin for the mitochondrial membrane permeability and Staurosporine for caspases 3 and 7 activation.
Cell Culture
The Human TK-6 lymphoblasts cells from American Type Culture Collection (ATCC), Manassas, VA (ATCC CRL-8015) were cultured to confluence on RPMI 1640 culture media (ATCC, Manassas, Virginia) with 10% fetal bovine serum (ATCC, Manassas, Virginia). Cell cultures were maintained at 37°C and 5% CO2.
Determination of IC50
Prior to treatment, TK6 cells were subcultured and kept at a density of 1 × 106 cells per 3.5mL of culture media plus additives in 25cm2 flasks to assure stable metabolic state and exponential growth. TK6 cells were exposed to DEHP and MEHP for 24, 48 and 72 hours to experimental doses ranging from 10μM to 500μM. Cell viability and the IC50 of each phthalate were determined by trypan blue exclusion with the Countess™ automated cell counter (Invitrogen Corp. Carlsbad, California).
Mitochondrial Membrane Permeability
TK6 cells in culture at a density of 1×106 cells per 3.5mL of media were exposed to the experimentally determined IC50 of DEHP and MEHP at 24 and 48 hours. DMSO (vehicle) and valinomycin (11 uM) were used as the negative control and positive controls respectively. To evaluate the effect of antioxidants on mitochondrial membrane permeability Tk6 cells were also exposed for 48 hours to DEHP and MEHP in the presence of L-glutathione reduced at a concentration of 500μM.
Mitochondrial membrane permeability was indirectly assessed using Mito PT™ (Immunochemistry Technologies LLC, Bloomington, MN) following the manufacturer specifications. This assay permits detection of changes in mitochondrial membrane potential by means of fluorescence produced by the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1) stain. In healthy mitochondria, JC-1 is up taken forming a series of complexes known as J aggregates. Upon alteration of the membrane potential the dye will be dispersed in the cytosol in its monomeric form causing the cells to fluoresce green, measured in fluorescence standard units (FSU) with the Modulus fluorometer (Promega, Sunnyvale, CA).
Generation of reactive oxygen species
The generation of reactive oxygen species (ROS) from mitochondria can occur before or after mitochondrial membrane depolarization. For the determination of an increase in the generation of ROS due to the exposure to DEHP and MEHP, TK6 cells in cultures were exposed for 24 and 48 hours to the IC50 doses at the conditions previously described. Determination of ROS generation was performed through the application of the reagent 2,7-dichlorofluorescein diacetate (DCFH-DA) as described by Park (2007). The reagent DCFH-DA indicates the generation of reactive oxygen species by producing green fluorescence proportional to the ROS generation level.
Cultures with reduced L-glutathione as an antioxidant, at a concentration of 500 μM was also included to evaluate the effect on the levels of ROS generated. Cultures treated with DMSO were included as negative controls and cis-diammineplatinum (II) dichloride (cisplatin) at a concentration of 13uM as the positive control. ROS levels were measured in fluorescence standard units (FSU) with the Modulus fluorometer (Promega, Sunnyvale, CA).
Activation of caspases 3 and 7
TK6 cultures were exposed to DEHP and MEHP at their respective IC50 at conditions previously described. For determination of caspases 3 and 7 activation, the Magic Red™ assay from Immunochemistry Technologies LLC, Bloomington, MN, was implemented following the manufacturer recommended protocol. The assay detects activation of the effector caspases 3 and 7 by the production of red fluorescence. Included controls were DMSO and reduced L- glutathione at a concentration of 500μM as negative controls and staurosporine (1μM) as positive control. Quantitative measures of caspase activation were obtained in fluorescence standard units (FSU) with the Modulus fluorometer (Promega, Sunnyvale, CA).
Statistical Analysis
To assess significance in the average changes of TK6 human lymphoblasts cells according to the concentration of DEHP and MEHP, one way ANOVA with fixed effect was performed. In case significant results were found in the one way ANOVA, a Post Hoc Test Tukey honestly significant difference (HSD) was also performed.
Results
Determination of IC50
The IC50 of DEHP and MEHP on TK6 cells were determined at 24, 48 and 72 hours. Treatments with DEHP indicated an IC50 of approximately 250μM after 24 hours of exposure, 234μM (91.4mg/L) after a 48 hour exposure and 100 μM (39.06mg/L) after 72 hours (Figure 1A). The determined IC50 dose for MEHP was 250μM for the exposure period of 24 hours, 196μM (54.55mg/L) after a 48 hours and 80μM (22.27mg/L) at 72 hours (Figure 1B). A stimulus in cell growth was observed at low doses (20μM) but a clear dose response was observed at higher concentration.
Figure 1.
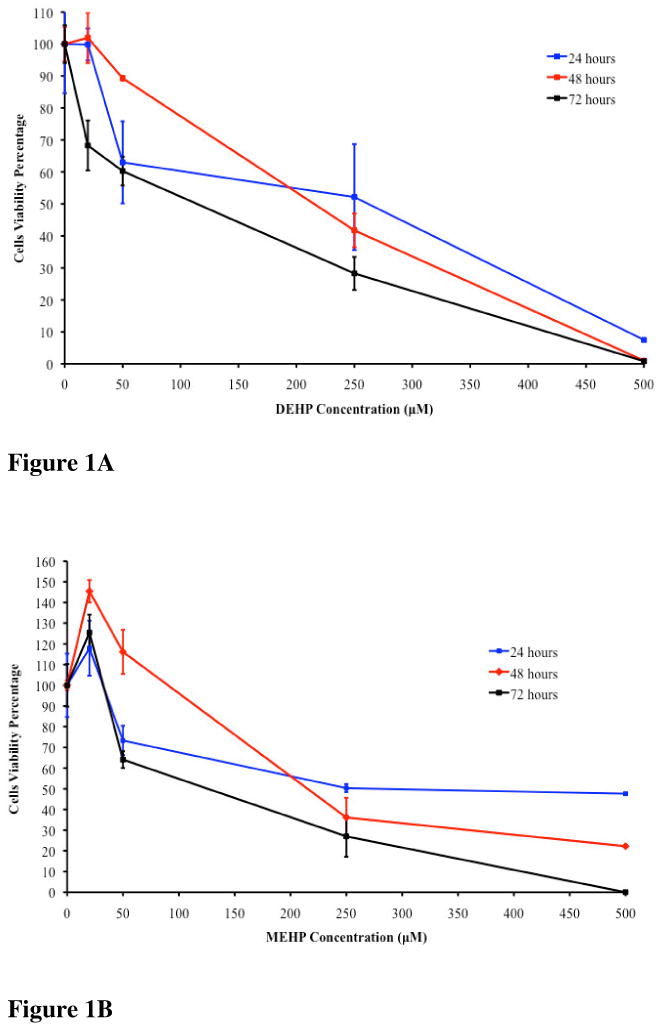
Figure 1A. TK6 cells viability after 24, 48 and 72 hours of exposure to DEHP. The 24 hours IC50 was 250μM, the 48 hours IC50 was 234μM and the 72 hours IC50 was 100μM.
Figure 1B. TK6 cells viability after 24, 48 and 72 hours exposure to MEHP. The 24 hours IC50 was 250μM, the 48 hours IC50 was 196μM and the 72 hours IC50 was 80μM.
Mitochondrial Membrane Permeability
Figure 2 presents the comparison of mitochondrial membrane permeability (measured in FSU) among the tested compounds including the positive and negative controls after 24 and 48 hours of exposure. Comparison of the mitochondrial membrane permeability after 24 hours exposure (measured in FSU) demonstrated the following levels of membrane permeability: DMSO negative control (431.51FSU) < valinomycin positive control (1079.18FSU ± 1.39) < DEHP (1100.85FSU ± 40) < MEHP (1630.94FSU ± 1.39). At the 48 hours exposure period however, similarities where observed among DEHP (2505.58FSU ± 84.59), MEHP (2210.09FSU ± 58.07) and valinomicyn (2327.65FSU ± 131.7) in their capacity to induce mitochondrial damage; in contrast to the negative control DMSO (1146.44FSU ± 179.96). The Post Hoc Test Tukey (HSD) indicated significant difference on the average level of mitochondrial membrane permeability caused by DEHP and MEHP (P-values of 0.002 and 0.024, respectively) in comparison with the negative control. No significant difference however, was observed among the positive control, MEHP and DEHP (P-value >0.10).
Figure 2.
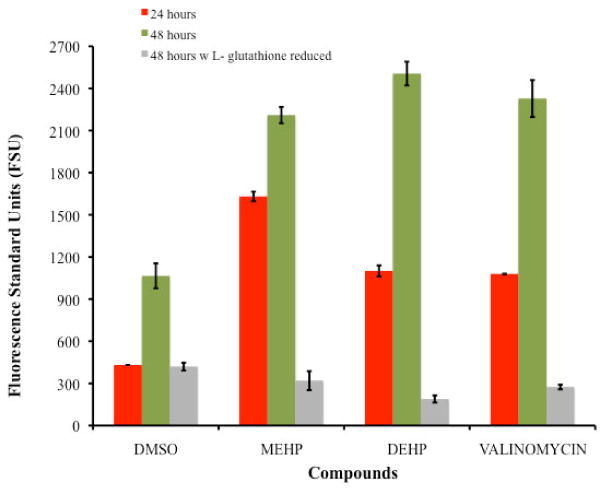
Comparison of changes in the mitochondrial membrane potential among TK6 cells exposed for 24 and 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM). For the 48 hours exposure assay the average level of mitochondrial membrane permeability caused by DEHP and MEHP presented P-values of 0.002 and 0.024, respectively when compared with the negative control. Also no significant difference was observed among the positive control (valinomycin) with MEHP and DEHP (P-value >0.10). Comparison of the changes in the mitochondrial membrane potential generated on TK6 cells exposed for 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM) with 500μM of L- glutathione is also presented an illustrates the reduction effect.
These phthalate compounds demonstrated capacity to induce alterations to the mitochondrial membrane potential. Confirmatory evidence was obtained when cultures where exposed to DEHP and MEHP in the presence of the antioxidant reduced L-glutathione for 48 hours (Figure 2) where the effect in the mitochondrial membrane permeability was clearly diminished. In DMSO cultures in the presence of L-glutathione the reduction resulted in ROS levels after 48 hours of treatment similar to the 24 hours treatment proving to be a protecting factor.
Generation of reactive oxygen species
Figure 3 shows the buildup of ROS in the TK6 after 24 and 48 hours exposed to DEHP or MEHP and subsequent staining with the ROS reactive agent DCFH-DA. After 24 hours of exposure, the observed levels of ROS generated was as follow: Cisplatin as positive control (1348.85FSU ± 20.05) > MEHP (816.53FSU ± 14.55) > DEHP (458.54FSU ± 5.56) > DMSO (399.08FSU ± 10.55). At the 48 hours exposure both compounds reported an increase in the ROS generation in comparison with the 24 hours treatment but the order of highest to lowest ROS inductors remained. The 48 hours ROS generated levels was as follow: Cisplatin (1575.35FSU ± 108.4) > MEHP (1518.75FSU ± 3.66) > DEHP (793.27FSU ± 108.74) > DMSO (39.62FSU ± 8.14). Comparison among phthalates revealed that the metabolite MEHP induced cells to produce twice as much ROS than the parent DEHP. The Post Hoc Test Tukey (HSD) showed significant difference (p<0.05) in the level of ROS species generated by the cells exposed to DEHP compared with the positive control and MEHP. Comparison of the level of ROS generated by the cells exposed to DEHP and MEHP showed significant differences (P-value = 0.009). No significant difference was observed among the positive control (cisplatin) and MEHP (P-value >0.1), suggesting similarities in their capacity to induce the generation of ROS.
Figure 3.
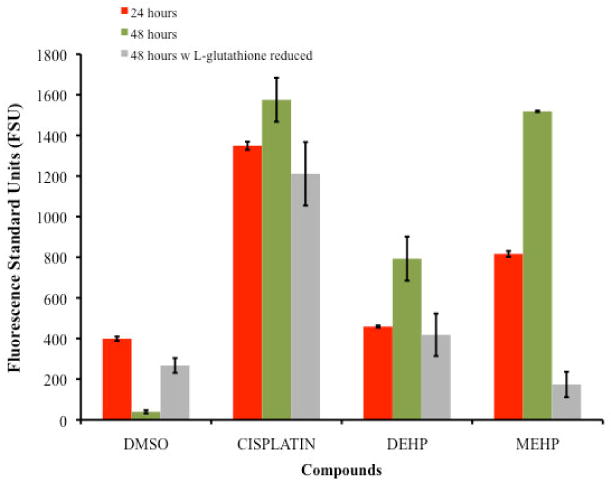
Comparison of the Generation of Reactive Oxygen Species as detected by DCFH-DA of TK6 cells exposed for 24 and 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM). For the 48 hours exposure assay there is significant difference (p<0.05) in the level of ROS generated by the cells exposed to DEHP compared with the positive control and MEHP. The cells exposed to DEHP and MEHP also showed significant differences (P-value = 0.009). But no significant difference was observed among the positive control (cisplatin) and MEHP (P-value >0.1). Comparison of the ROS generated of TK6 cells exposed for 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM) with 500μM of L- glutathione reduced are also presented also presented an illustrates the reduction in ROS generation.
Cultures treated in the presence of the antioxidant L-glutathione for 48 hours resulted in a reduced level of ROS (Figure 3). The highest level of ROS was observed on cells exposed to the positive control, Cisplatin (1210. 85FSU ± 156.37), followed by DEHP (418.42FSU ± 104.57), DMSO (267.27FSU ± 36.44) and MEHP (173.55FSU ± 62.47). Although ROS levels in the negative control resulted higher than expected, the decrease in the level of ROS in the presence of L-glutathione was clearly observed for the DEHP and MEHP treated cultures.
Activation of caspases 3 and 7
Figure 4 presents activation of caspases 3 and 7 after 24 and 48 hours of treatment at the IC50's of DEHP and MEHP followed by the Magic Red™ protocol. Staurosporine (1 uM), a known caspase activator was used as the positive control and DMSO as negative control. For the 24 hours exposure period Staurosporine was the highest caspases activator (1390.92FSU ± 151.25), followed by MEHP (292.80FSU ± 19.71), and DEHP (122.13FSU ± 4.68).
Figure 4.
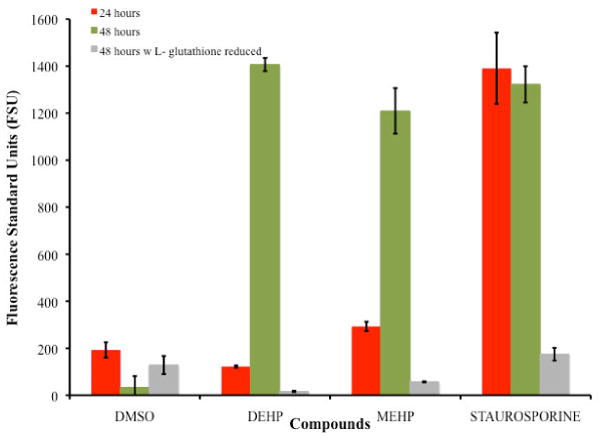
Comparison of Caspases 3 and 7 Activation of TK6 cells exposed for 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM). For the 48 hours exposure period the comparison of the positive control staurosporine with DEHP produced a significance value of 1.0, and with MEHP a value of 0.999 indicating no significant difference in the level of caspases 3 and 7 activation between DEHP, MEHP and the positive control. Comparison of Caspases 3 and 7 Activation on TK6 cells exposed for 48 hours to the IC50's of DEHP (234μM) and MEHP (196μM) with 500μM of L- glutathione reduced are also presented an illustrates the reduction effect.
At 48 hours DEHP (1406.94FSU ± 28.30) and MEHP (1209.72FSU ± 97.15) presented a strong activation of caspases 3 and 7 in contrast to the negative control DMSO (33.21 ± 48.04). The Post Hoc Test Tukey (HSD) showed the comparison with the positive control staurosporine (1322.55FSU ± 77.14) with DEHP to produced a significance value of 1.0, and with MEHP a value of 0.999 indicating lack of significant difference in the level of caspases 3 and 7 activation between the phthalates and the positive control. The effect on the activation of caspases 3 and 7 was tested in the presence of the antioxidant reduced L-glutathione. The decrease in activation in the presence of the antioxidant demonstrated the capacity of DEHP and MEHP to activate caspases 3 & 7. The order in activation was as follows: staurosporine positive control (174.48FSU ± 27.11) > DMSO (128.55FSU ± 38.54) > MEHP (56.99FSU ± 2.53) > DEHP (16.33FSU ± 3.25).
Discussion
Phthalates and their derivatives are a common group of widely used chemicals in manufacture; however their effects upon human health continue to present controversy because relatively little is known about their effects on the immune system at a cellular level. DEHP and MEHP are known to affect some immune cells and interfere with immune functions but the mechanism remains elusive (Palleschi et al. 2009; Koike et al. 2009; Kleinsasser et al. 2004). Our data shows the effects of these compounds upon a normal lymphoblast cells line in which several key toxicity checkpoints were tested.
A DEHP median (maximal) human intake concentration of 2.7(42.2) μg/kg has been reported (Wittassek and Angerer, 2008). This environmental level generates strong concern since DEHP is one of the most widely used phthalates and one can argue that exposure to DEHP leads to formation of more toxic metabolites such as MEHP and 2-ethylhexanol. In this study the observed concentrations at which DEHP inhibited the 50% of the cell replication are comparable with the concentrations found in sludge from several sewage treatment plants (STPs) that range from 15 to 346mg/Kg in Quebec, Canada and in blood patient samples with a range between 50 to 350μM (Beauchesne et al. 2008; Plonait, et al. 1993; Tickner et al. 2001).
In urban areas of Montreal, Canada, DEHP was detected at concentrations around 180μg/L in river water and 4.6μg/L in samples of tap water (Horn et al. 2004). It has also been found in plasma samples of pubertal gynecomastia at an average DEHP concentration of 4.66 ± 1.58μg/mL and MEHP at a concentration of 3.19 ± 1.41 μg/mL. In human breast milk, DEHP concentration has been found with a mean of 17± 47ng/mL; and in human blood and serum at a mean level of 5.9 ± 21ng/ (Durmaz et al. 2010; Högberg et al. 2008) as well as in semen at concentration levels of 0.77± 1.20μg/mL (Pant et al. 2008). The wide range of levels of phthalates found in different scenarios demonstrates the need to better understand their toxicity at the molecular levels.
In this study the metabolite MEHP was found to be more toxic to TK6 lymphoblast cells than the parent compound DEHP, consistent with studies with other cell types (Schlezinger et al. 2004). A clear dose response was observed for both compounds as a function of exposure time. MEHP induced stronger survival reduction at 48 and 72 hours in contrast to the parent DEHP. That is, at 72 hours the IC50 was higher than at 48 and 24 hours of treatment revealing the incapacity of the lymphoblast to overcome the damage and therefore affecting cell replication and viability.
The difference in cell viability effect between both compounds at 48 hours can be a combination of the greater toxicity of MEHP and the time it takes to initiate the biotransformation of DEHP into MEHP, which appears to be a more toxic species. Lymphocytes are most of the time in quiescent state and just shift to a highly active state within hours after being stimulated (Frauwirth and Thompson, 2004). This may suggest a slower biotransformation of DEHP and MEHP by TK6 at the beginning of the exposure period, and which may include the formation of the metabolite 2-ethylhexanol for both compounds (Carter, 1974; Wahl, 2004).
Our study also reports that both DEHP and MEHP cause alterations in the mitochondrial membrane potential, which is a hallmark of apoptosis (Brunelle et al. 2009; Elmore, 2007; Fink and Cookson, 2005). This result is consistent with previous reports, since mitochondria are the sites where these compounds are hydrolyzed and modified (Carter, 1974; Melnick and Schiller, 1982). These alterations in the membrane potential may promote the formation of ROS, which was particularly high in cultured cells exposed to MEHP.
The ROS generated may contribute as positive feedback to induce alterations in the mitochondrial membrane potential as suggested by the results of the mitochondrial membrane permeability assay in the presence of the antioxidant L-glutathione reduced in which the permeability is drastically reduced as well as the activation of caspase 3 and 7. Although not tested in this study phthalates have shown to stimulate Ca2+ entry in the cells and mitochondria which increases ROS production as well as significant crossover between the two principal apoptosis pathways suggesting the existence of positive feedback loops (Palleschi et al. 2009; Bissonnette et al. 2008). The difference between the ROS generated by MEHP and DEHP may be caused also by the release and/or activation of specific mitochondrial proteins such as Prx3, COX-2 and cytochrome c oxidase. Figure 5 proposes the cell death mechanism induced on TK6 cells after treatment with DEHP and MEHP.
Figure 5.
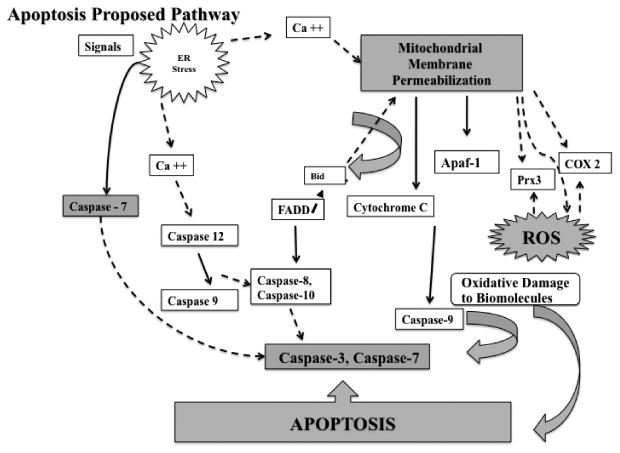
Proposed cell death mechanism induced on TK6 cells after treatment with DEHP and MEHP. Events presented in this pathway are a summary of general events involved in apoptosis. As demonstrated in this study the cell death mechanism induced by DEHP and MEHP includes mitochondrial membrane permeability, generation of ROS and activation of caspases 3 and 7 (shaded shapes). Segmented lines indicate apoptotic contributing events that were not measured in this study but are proposed for DEHP and MEHP based on our findings. The differences observed in mitochondrial permeability, ROS and caspase activation levels generated by MEHP and DEHP imply that different factors are influencing their cell death mechanism. Although not tested in this study phthalates have shown to stimulate Ca2+ entry in the cells and mitochondria which increases ROS production as well as significant crossover between the two principal apoptosis pathways suggesting the existence of positive feedback loops (Palleschi et al. 2009; Bissonnette et al. 2008). The difference between the ROS generated by MEHP and DEHP may be caused also by the release and/or activation of specific mitochondrial proteins such as Prx3, COX-2 and cytochrome c oxidase.
Relevant to our study is the effect that MEHP inside the mitochondria could exert over antiapoptotic proteins such as peroxiredoxin 3 (Prx3) which under short term exposure to MEHP could exert a cytoprotective role (Onorato et al. 2008). Acute and chronic oxidative injuries can lead to reduced Prx3 levels resulting in diminished protective responsiveness and increased susceptibility to oxidative stressors (Wood-Allum et al. 2006; Onorato et al. 2008). It has also been reported that cytochrome c oxidase can be stimulated by MEHP and ethyl hexanol (EH) but not by DEHP in hepatocytes (Ganning et al. 1982; Ganning et al. 1983; Ganning and Dallner, 1981).
It is the cascade of events and degeneration of the mitochondrial machinery that may trigger the activation of effector caspases such as caspases 3 and 7. Although apoptosis involves the activation and participation of multiple proteins the activation of caspases 3 and 7 are considered early stages changes of apoptosis. Our data shows the activation of these enzymes after exposure to DEHP and MEHP providing additional information indicating the capacity of these compounds to trigger cellular activities and to generate cellular damage. The role of mitochondria and the release of ROS are also key indicators of a program cell death.
Conclusions
This research provides novel information regarding the cellular effects of DEHP and MEHP in lymphoblast cells. Our data demonstrate that these compounds affect the viability of the lymphoblast cells including increased mitochondrial membrane permeability, generation of ROS and caspase 3 and 7 activation, hallmark events in apoptosis. The toxicity of these phthalates in lymphoblast cells is clear and the constant human exposure and absorption is a factor that could compromise the human immune system in a way similar to what was observed in this study where the viability of the cells was clearly reduced with constant presence and a long exposure period. It is important to emphasize that the experimental doses used in this study are consistent with levels encountered in the environment and that the selected time of exposures, although longer than in previous reported studies, respond to the interest of modeling a chronic, rather than an acute exposure. The abundance of these compounds as part of human daily activities implies that more research is needed and that it is important to further and clearly understand their effects at the organ system level specially for immune-compromised subjects, children, infants, and advance aged persons in order to prevent the health hazards posed by these substances at levels encountered in the environment.
Highlights.
Study provides evidence on the capacity of phthalates to induce hallmark events in apoptosis
Experimental doses consistent with levels of DEHP and MEHP encountered in the environment
Longer experimental time of exposures resembling continues environmental exposure
Acknowledgments
The authors want to thanks Dr. Erick Suarez at University of Puerto Rico Medical Sciences Campus, for his review on the statistical analysis and Dr. Osvaldo Cox at Universidad Metropolitana School of Environmental Affairs for his insightful review and suggestions.
Financial Support: Provided by the NIH/NCRR INBRE program grant: 2P20RR-0016470-10
Abbreviations
- DEHP
diethylhexyl phthalate
- MEHP
monoethylhexyl phthalate
- DMSO
dimethyl sulfoxide
- JC -1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide
- ROS
Reactive Oxygen Species
- FSU
Fluorescence standard units
- IC50
Inhibition concentration of 50 % of cell population
- DCFH-DA
2,7 – dichlorodihydrofluorescein-diacetate
Footnotes
Conflict of Interests: The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beauchesne I, Barnabe S, Cooper DG, Nicell JA. Plasticizers and related toxic degradation products in wastewater sludges. Water Science & Technology. 2008;57(3):367–374. doi: 10.2166/wst.2008.001. [DOI] [PubMed] [Google Scholar]
- Bissonnette SL, Teague JE, Sherr DH, Schlezinger JJ. An endogenous prostaglandin enhances environmental phthalate-induced apoptosis in bone marrow B cells: Activation of distinct but overlapping pathways. Journal of Immunology. 2008;181:1728–1736. doi: 10.4049/jimmunol.181.3.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, Hägerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environmental Health Perspective. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. Journal of Cell Science. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JE, Roll DB, Petersen RV. The in Vitro hydrolysis of di-(2- ethylhexyl) phthalate by rat tissues. Drug Metabolism and Disposition. 1974;2(4):341–344. [PubMed] [Google Scholar]
- Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, et al. High plasma concentration of di-(2-ethylhexyl) phthalate in women with endometriosis. Human Reproduction. 2003;18:1512–1515. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- Durmaz E, Özmert EN, Erkekoğlu P, Giray B, Derman O, Hincal F, Yurdakök K. Plasma phthalate levels in pubertal gynecomastia. Pediatrics. 2010;125:e122–e129. doi: 10.1542/peds.2009-0724. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: A review of programmed cell death. Toxicological Pathology. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency. Documentation of Environmental Indicator Determination, RCRA Corrective Action Environmental Indicator (EI) RCRA Info code (CA725) Current Human Exposure Under Control. Chevron Phillips Chemical Puerto Rico Core, Inc. U.S. Environmental Protection Agency; 2002. [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infection and Immunity. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Thompson CB. Regulation of T lymphocytes metabolism. The Journal of Immunology. 2004;172:4661–4666. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, Mayer R, Liebl B. Ocurrence and daily variation of phthalates metabolites in the urine of an adult population. Int J Hyg Environ-Health. 2007;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Ganning AE, Dallner G. Induction of peroxisomes and mitochondria by di-(2-ethylhexyl) phthalate. FEBS Letters. 1981;130(1):77–79. doi: 10.1016/0014-5793(81)80669-2. [DOI] [PubMed] [Google Scholar]
- Ganning AE, Klasson E, Bergman A, Brunk U, Dallner G. Effect of phthalate ester metabolites on rat liver. Acta Chem Scand B. 1982;36:563–565. doi: 10.3891/acta.chem.scand.36b-0563. [DOI] [PubMed] [Google Scholar]
- Ganning AE, Brunk U, Dallner G. Effects of dietary di-(2-ethylhexyl) phthalate on the structure and function of rat hepatocytes. Biochim Biophys Acta. 1983;763(1):72–82. doi: 10.1016/0167-4889(83)90027-7. [DOI] [PubMed] [Google Scholar]
- Hokanson R, Hanneman W, Hennessey M, Donnelly KC, McDonald T, Chowdhary R, et al. DEHP, bis-(2)-ethylhexyl phthalate, alters gene expression in human cells: possible correlation with initiation of fetal developmental abnormalities. Human & Experimental Toxicology. 2006;25:687–695. doi: 10.1177/0960327106071977. [DOI] [PubMed] [Google Scholar]
- Högberg J, Hanberg A, Berglund M, Skerfvin S, Remberger M, Calafat AM, Falk Filipsson A, Jansson B, Johansson N, Appelgren M, Hăkansson H. Phthalates diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers exposure in vulnerable populations. Environmental Health Perspective. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn O, Nalli S, Cooper D, Nicell J. Plasticizers metabolites in the environment. Water Research. 2004;38:3693–3698. doi: 10.1016/j.watres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJK, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ Health Perspect. 2008;116(7):845–853. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JF, Liu TC. Determination of phthalate esters from food-contacted materials by on-line microdialysis and liquid chromatography. J Chromatogr A. 2006;1130:28–33. doi: 10.1016/j.chroma.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, et al. Role of oxidative stress in germ cell apoptosis induced by Di(2-ethylhexyl) phthalate. Biochemical Journal. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsasser NH, Harreus UA, Kasternbauer ER, Wallner BC, Sassen AW, Staudenmaier R, et al. Mono (2-ethylhexyl) phthalate exhibit genotoxic effects in human lymphocytes and mucosal cells of the upper aerodigestive tract in the comet assay. Toxicol Lett. 2004;148:83–90. doi: 10.1016/j.toxlet.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Koike E, Inoue Ken-ichiro, Yanagisawa R, Takano H. Di-(2-ethylhexyl) phthalate affects immune cells from atopic prone mice in vitro. Toxicology. 2009;259:54–60. doi: 10.1016/j.tox.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. Journal of Toxicology and Environmental Health, part A. 2004;67:1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. In utero exposure to Di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di (2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick R, Schiller C. Mitochondrial toxicity of phthalate esters. Environ Health Perspect. 1982;45:51–56. doi: 10.1289/ehp.824551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamiya K, Takagi H, Nakayama T, Ito H, Tsuruga H, Edmonds JS, et al. Microbial production and vaporization of mono-(2)-ethylhexyl phthalate from di-(2)-ethylhexyl phthalate by microorganisms inside houses. Arch Environ Occup Health. 2005;60:321–325. doi: 10.3200/AEOH.60.6.321. [DOI] [PubMed] [Google Scholar]
- Onorato TM, Brown PW, Morris PL. Mono (2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. Journal of Andrology. 2008;29(3):293–303. doi: 10.2164/jandrol.107.003335. [DOI] [PubMed] [Google Scholar]
- Palleschi S, Rossi B, Diana L, Silvestroni Di(2-ethylhexyl) phthalate stimulates Ca2+ entry, chemotaxis and ROS production in human granulocytes. Toxicol Lett. 2009;187:52–57. doi: 10.1016/j.toxlet.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, Krishna Saxena D. Correlation of phthalates exposure with semen quality. Toxicology and Applied Pharmacology. 2008;231:112–116. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Park EJ, Park K. Induction of reactive oxygen species and apoptosis in BEAS-2B cells by mercuric chloride. Toxicol In Vitro. 2007;21(5):789–94. doi: 10.1016/j.tiv.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Plonait SL, Nau H, Maier HF, Wittfoht W, Obladen M. Exposure of newborn infants to di-(2-ethylhexyl)-phthalate and 2-ethylhexanoic acid following exchange transfusion with polyvinylchloride catheters. Transfusion. 1993;33:598–605. doi: 10.1046/j.1537-2995.1993.33793325058.x. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–e268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Sax L. Polyethylene terephthalate may yield endocrine disruptors. Environ Health Perspect. 2010;118:445–448. doi: 10.1289/ehp.0901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger JJ, Howard GJ, Hurst CH, Emberley JK, Waxman DJ, Webster T, et al. Environmental and endogenous peroxisome proliferator-activated receptor γ agonist induce bone marrow B cell growth arrest and apoptosis: interactions between Mono (2-ehtylhexyl)phthalate, 9-cis-Retinoic Acid, and 15-Deoxy-Δ12,14-prostaglandin J2. The Journal of Immunology. 2004;173:3165–3177. doi: 10.4049/jimmunol.173.5.3165. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Singh S, Li SS. Phthalates: toxicogenomics and inferred human diseases. Genomics. 2010 doi: 10.1016/j.ygeno.2010.11.008. In Press, Accepted Manuscript: http://www.sciencedirect.com/science/article/B6WG1-51P9WY02/2/3113e70ff5c3770c02075516d8263a69. [DOI] [PubMed]
- Suzuki Y, Niwa M, Yoshinaga J, Watanabe C, Mizumoto Y, Serizawa S, Shiraishi H. Exposure assessment of phthalate esters in Japanese pregnant women by using urinary metabolite analysis. Environ Health Prev Med. 2009;14:180–187. doi: 10.1007/s12199-009-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental Phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental Research. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wahl HG, Hong Q, Hildenbrand S, Risler T, Luft D, Liebich H. 4-Heptanone is a metabolite of the plasticizer di-(2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrol Dial Transplant. 2004;19:2576–2583. doi: 10.1093/ndt/gfh425. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–138. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Analysis. 2006;26(3):803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Wood-Allum CA, Barber SC, Kirby J, Heath P, Holden H, Mead R, Higginbottom A, Allen S, Beaujeux T, Alexson SE, Ince PG, Shaw PJ. Impairment of mitochondrial anti-oxidant defense in SODI- related motor neuron injury and amelioration by ebselen. Brain. 2006;129:1693–1709. doi: 10.1093/brain/awl118. [DOI] [PubMed] [Google Scholar]


