Abstract
Objective
To determine the frequency and risk factors of post-dural puncture headache (PDPH) in research volunteers.
Background
Despite increasing interest in measuring cerebrospinal fluid biomarkers to investigate disease pathogenesis and diagnosis, previous case series have evaluated lumbar puncture (LP) safety only in clinical care. PDPH is a common complication after LP.
Methods
We determined the frequency of post-dural puncture headache (PDPH) in neurologically unselected HIV seropositive and seronegative adults volunteering for research, as well as the variables associated with the development of (PDPH). Variables studied were BMI, HIV serostatus, volume of CSF removed, number of previous LPs, use of pre-medication, LP position, lumbar space, number of needle passes, whether or not aspiration was used, CSF WBC, CSF RBC, CD4 count, CD4 nadir, CSF HIV viral load, plasma HIV viral load, and race.
Results
Of 675 LPs performed over one year, headache developed in 38 (5.6%; 95% CI 4.2, 7.1)). Most PDPH (92%) resolved spontaneously or with conservative medical management; 3 required epidural blood patch. Greater headache risk was associated with lower body mass index (BMI ≤ 25 versus > 25) (OR 3.3; CI 95% 1.5, 7.0; p=0.001) and less prior LP experience (previous LPs ≤ 2 vs > 2) (OR 2.1; CI 95% 1.1, 4.1; p=0.03). PDPH was not significantly (p > .05) related to HIV serostatus, CSF volume, or gender.
Conclusion
In this study, where tolerance to risk was low because LPs were done for research rather than clinical purposes and healthy controls were included, adverse effects were mild and self-limited.
Keywords: dural puncture headache (PDPH), Post-lumbar puncture headache, CSF, HIV
INTRODUCTION
Analysis of biomarkers in cerebrospinal fluid (CSF) from neurologically affected research volunteers and from healthy controls has provided substantial diagnostic and pathogenic insights in a number of neurological diseases with degenerative, neoplastic or infectious etiologies such as Alzheimer’s disease, tauopathies, meningitis and HIV CNS complications (1–4). Post-dural puncture headache (PDPH) is a frequent complication of lumbar puncture, performed for diagnostic or therapeutic purposes or accidentally, as a complication of epidural anesthesia. The frequency of PDPH following LP varies with characteristics of individual patients (nonmodifiable risk factors), the type of needle and technique used (modifiable risk factors), as well as the diagnostic definition of PDPH and method of follow-up (5, 6).
The present study was performed to evaluate headaches following lumbar puncture (LP) performed solely for research purposes on HIV positive and negative research volunteers. We assessed a number of headache risk factors demonstrated in previous studies such as, BMI, age, gender, volume of CSF removed and number of prior LPs.
METHODS
Study Design
This was a cross-sectional analysis of data collected on consecutive LPs performed for multiple prospective research cohort studies over one year (from 2003 to 2004) at a single research center.
Subjects
Research volunteers were enrolled in prospective, longitudinal, observational cohort studies at the HIV Neurobehavioral Research Center (HNRC) at the University of California, San Diego. The protocols were approved by the local Institutional Review Board and informed consent was obtained from each research volunteer. All research volunteers were selected for their willingness to participate in observational clinical research studies at the HNRC and for their willingness to undergo LP for research purposes. LPs were deferred when factors associated with increased risk of bleeding were present, such as clinical history of bleeding diathesis, impaired hemostasis, ongoing treatment with anticoagulants, platelet counts <50,000/mm3, INR >1.4, and clinically evident abnormal bleeding. LPs were also deferred when signs of untreated systemic or local infection were present. If the research participant reported procedure-related anxiety prior to the LP, lorazepam 0.25 - 0.5 mg was given. Individuals with suspected central nervous system opportunistic infection were not specifically excluded. However, since all were ambulatory research volunteers, active CNS opportunistic disease was unlikely.
Procedures
LPs were performed in the outpatient setting by trained physicians (MD neurologists and infectious disease specialists), RNs and NPs. All RNs and NPs were trained and credentialed to perform LPs in the research setting according to scope of practice as outlined in the California Nursing Practice Act. The certification process met the Standardized Procedure Guidelines of The Board of Registered Nursing (Title 16, Article 7, 1474)(7) and included viewing an educational video and 5 procedures performed by credentialed clinicians and physicians, followed by performing 10 LPs on research volunteers under the direct supervision of a credentialed physician, after obtaining a thorough pre-procedure history and neurological exam to rule out contraindications.
LPs were performed under aseptic conditions using a 22-gauge atraumatic spinal needle. Local back pain was minimized by injection of an anesthetic, 1% lidocaine, into the subcutaneous tissue surrounding the needle puncture site. If a subject became uncomfortable during the procedure, it was discontinued. Following LP, research volunteers were observed for 10–30 minutes before being permitted to ambulate.
Definition and Management of PDPH
For the purposes of this study, a conservative definition of PDPH comprised any research volunteer reporting any headache following the LP. Secondary analyses separately evaluated headaches that fulfill the criteria for PDPH of the International Headache Society Classification (ICHD-II) (8). A nurse or physician was available by pager 24 hours a day to respond to any post-LP complications, including headache and back pain. The diagnosis of PDPH was done by trained professionals (RN, NP) supervised by a neurologist.
The participants were contacted, usually with in 48 hours of their LP, and were asked if they were experiencing any headache. Those experiencing headache were managed according to a structured algorithm with initial recommendations for bed rest enhanced fluid intake including caffeinated beverages, and over-the-counter and then prescription analgesics, as needed. Subjects who experienced a headache that did not respond to conservative management underwent a blood patch performed by an anesthesiologist at the University Hospital.
PDPH Risk Factors
For each LP, the following data were collected: operator (RN, NP or MD), body mass index, HIV serostatus, age, gender, volume of CSF removed, whether the resulting headache was positional, whether a blood patch was needed and number of reported prior LPs. Additional secondary factors assessed were CSF white and red blood cell counts (WBC, RBC), current CD4, CD4 nadir (lowest CD4 count in the clinical history), CSF HIV viral load, race, education, whether premedication was used, position (seated versus lateral decubitus), lumbar space, and number of needle passes required to obtain CSF.
Statistical Analysis
Results are shown as mean plus standard deviation, median and IQR or frequencies and percentage, as appropriate. PDPH risk factors were evaluated by calculating odds ratios that compared headache frequency according to the presence or absence of the risk factor. Because some participants contributed more than one datapoint to the analysis, odds ratios for patient-specific risk factors such as age, gender and BMI were calculated using a single datapoint per subject (the first LP), whereas for procedure-specific factors, such as operator and position, individual subjects could contribute more than one datapoint. For ease of interpretation and to allow comparison between risk factors by continuous variables we calculated odds ratios based on empirical or clinically validated cutoffs (e.g., BMI ≤ 25). Statistical analyses were performed using JMP 8.0 (Copyright © 2009 SAS Institute Inc.). A p value ≤ 0.05 was considered significant.
RESULTS
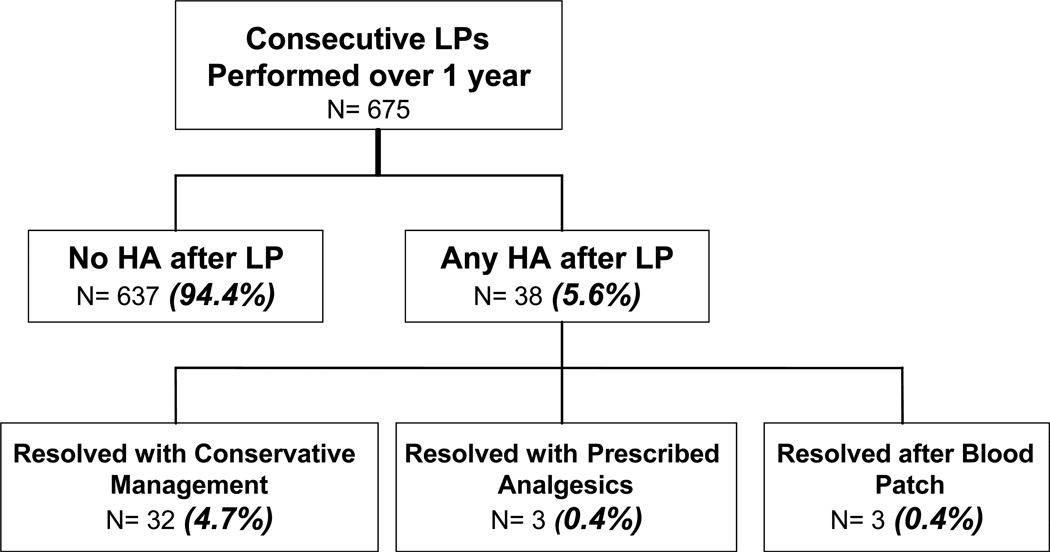
Of 675 LPs performed on 477 research volunteers, 575 were done on HIV+ subjects and 100 (14.8%) on healthy, HIV negative controls. Subjects were typically white (53%) men (80%) with a mean (+/−SD) age and education of 42 (±9) and 13 (±3) years, respectively. Of the 477 subjects, 144 (30%) reported 0 prior LPs, and 73 (15%) reported 1, 54 (11%) 2, and 206 (43%) >2 prior LPs. Of the 675 LPs, 38 (5.6%; 95% CI 4.2, 7.1) resulted in a post-lumbar headache (Figure 1). Of these 38, 32 headaches (4.7%) resolved after rest, hydration and over-the-counter analgesics. Three (0.4%) required prescribed analgesics following the procedure and another 3 (0.4%) resolved only after blood patch. There were no instances in which a repeat blood patch was required. With the exception of transient back pain and bleeding, no other adverse effects of LP were seen. Figure 1 illustrates the breakdown of consecutive LP procedures.
Figure 1.
Breakdown of consecutive lumbar puncture procedures.
PDPH Risk Factors
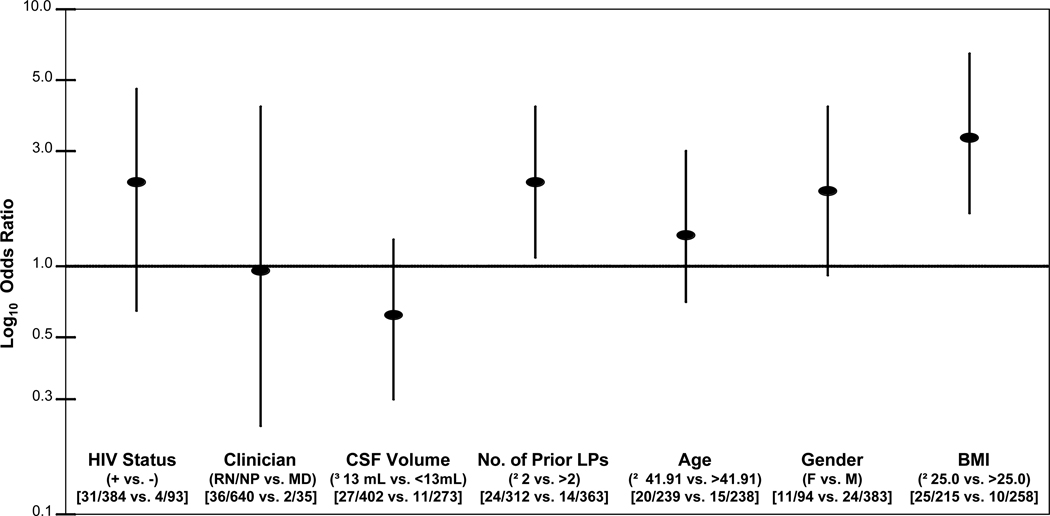
As shown in Figure 2, HIV infected individuals were no more likely to develop headache after LP than HIV negative individuals (OR: 1.95; CI 95% 0.67, 5.68; p=0.18). Of the 675 total LPs performed, 502 (74.4%) were done by nurses, 138 (20.4%) were done by nurse practitioners, and 35 (5.2%) were done by physicians. LP procedures performed by an RN or an NP were no more likely to result in headache than procedures performed by an MD (OR: 0.98; CI 95% 0.23, 4.26; p=0.98). The median amount of CSF removed was 13 mL (IQR 12–14). Research volunteers who had greater than or equal to the median volume withdrawn (13ml) were not significantly more likely to develop headache than research volunteers who had less than the median volume withdrawn (OR: 0.58; CI 95% 0.28, 1.20; p=0.13). The median number of reported prior LPs was 2 (IQR, 1–6). Research volunteers reporting 0, 1, or 2 prior LPs developed headache significantly more frequently than research volunteers reporting greater than two LPs (OR: 2.08; CI 95% 1.06, 4.09; p=0.03).
Figure 2.
Odds ratios (points) and 95% confidence intervals (bars) for post-dural puncture headache (PDPH) risk factors. For subject-related risk factors (i.e., HIV status, age, gender, BMI), the proportions developing headache are compared for number of individuals (477) with or without the risk factor. For procedure-related risk factors (i.e., clinician, CSF volume, number of prior LPs), the proportions developing headache are compared for number of total procedures (675) with or without the risk factor. R.N., registered nurse. N.P., nurse practitioner. M.D., physician. BMI, body mass index.
Subjects less than or equal to the median age of 41.9 years were no more likely to develop headache than individuals older than the median (OR: 1.36; CI 95% 0.68, 2.72; p=0.39). Women were not more likely to develop headache than men (OR: 1.98; CI 95% 0.93, 4.21; p=0.09). Subjects with a BMI less than or equal to the clinical validated cutoff of 25.0 were 3.26 times more likely to experience PDPH than those greater than 25.0 (OR: 3.26; CI 95% 1.53, 6.96; p=0.001).
The use of pre-medication (lorazepam 0.25 - 0.5 mg) for anxiety prior to the LP was not associated with headache incidence. Other secondary risk factors that did not reach statistical significance were, LP position, lumbar space, number of passes, whether or not aspiration was used, CSF WBC, CSF RBC, CD4 count, CD4 nadir, CSF viral load, plasma viral load, and race.
DISCUSSION
In this large series of LPs performed in research volunteers, no instances of epidural hematoma, infection or radiculopathy were identified, and headaches were infrequent (5.6%), generally mild and self-limited, with only 3 (0.4%) requiring a blood patch. Both of these values compare favorably to published studies of PDPH in clinical series, where PDPH rates range between 2.6 (9) and 30% (5, 10) According to the ICHD-II criteria PDPH occurred in this study in only 0.9%, which is a figure well below those previously reported. This low incidence of PDPH might be attributable in part to the use of a 22G atraumatic needle. Other papers reported an incidence of 3% for PDPH in LPs done using 22G Whitacre needles (11). Additionally, the high level of experience of operators in this study might explain the low incidence of PDPH. In a previous study the incidence of PDPH among anesthesiologists ranged from 2.5% in less experienced operators to 1.2% in more experienced operators (12).
Risk factors for PDPH in this research cohort were similar to those seen in previous clinical series. Thus, we found that lower body mass index was associated with increased risk, similar to prior reports (13). Women were at greater risk as in previous studies, although this did not reach statistical significance in our study, possibly because only 20% of our research volunteers were women. Similarly, age was not a significant risk factor, possibly because few of our subjects were over 50 years old, an age at which PDPH headache frequency diminishes in some studies (13, 14).
Post-dural puncture headache is frequently diagnosed on the basis of clinical features. The cardinal feature of PDPH is orthostatic or postural headache (16). Headache typically appears or worsens when the patient moves from a supine to an upright position and is typically relieved when the patient lies down. Headache generally appears or worsens within 20 seconds of a postural change and reaches its maximum within a minute. Headache characteristically subsides within 20 seconds of recumbency (17). Headache features are variable. The pain is usually severe, but may be mild or moderate (18). The quality may be burning, dull and/or throbbing (18, 19). PDPH may have associated features including low back pain, vertigo, tinnitus, hearing changes, cranial nerve palsies, diplopia, and even cortical blindness. In addition, the associated features of migraine such as nausea, photophobia, and phonophobia may occur (15, 19, 20).
Our study design permitted the evaluation of some unique potential risk factors not previously assessed in research. These included employing LP operators of different training backgrounds (RNs, NPs, MDs) and performing serial LPs. Although LP is a procedural skill typically practiced by physicians, we found that registered nurses and nurse practitioners performed LPs with no greater adverse event rate than physicians. This observation is particularly important for research in resource-limited settings, where the availability of qualified clinicians, especially physicians, is often very limited. Thus, research may be facilitated by having a variety of trained personnel available to perform the procedure. Additionally, we performed multiple LPs in some individuals; these included some volunteers who reported having had LPs prior to the year in which this analysis was performed, as well as some who volunteered two or more times during the year of the analysis. Serial LPs conferred no greater risk of adverse events. In fact, individuals undergoing greater numbers of LPs were significantly less likely to experience PDPH. We interpret this as a type of self-selection bias; a research volunteer who has previously experienced a PDPH may be less likely to submit to additional voluntary LPs.
LP is undertaken frequently in HIV positive patients for clinical reasons. HIV positive participants are not more susceptible to develop PDPH than HIV negative participants. This is the first study evaluating the association between PDPH and specific HIV parameters as CD4 count, CD4 nadir, CSF HIV viral load and plasma HIV viral load. We found no significant association between PDPH and these variables. Different from previous studies that reported lower frequency of PDPH in the presence of an inflammatory CSF, in this study there was no statistical significance with CSF WBC.
Emotional status is a controversial issue in PDPH. In this study, the use of anxiolytic medication was not related to the risk of post-LP headache.
A variety of other factors were assessed to ascertain whether they influenced risk for PDPH. We found no correlation of PDPH with CSF white or red blood cell counts. There was no difference in headache risk for procedures performed in the seated versus the lateral decubitus position. PDPH did not correlate with the number of needle passes required to obtain CSF return. We found no significant association between larger CSF volumes and increased PDPH risk. This is particularly important for research studies, since they may specify reduced volumes in attempt to limit headache risk. Our finding contrasts with previous studies suggesting that larger CSF volumes increase PDPH risk (5, 6, 10).
This study's findings are limited in several ways. The statistical significance and confidence intervals for odds ratios depend not only on the total number of subjects, but also on the number and proportion of those who suffer an event (post-LP headache), which in this case is small (38/675). With few events, apparently large odds ratios (e.g., 1.95 for HIV status) may be nonsignificant due to the uncertainty of the estimate.
As these were ambulatory outpatients, and PDPH can be delayed for up to up to 12 days, some PDPH may have gone unreported. This is unlikely, since participants were proactively contacted by phone 1–2 days after the LP, and a 24-hour pager was available at all times. About 90% of the PDPH start within the first 72 hours and 66% within the first 48 hours of LP (22, 23).
Since we did not collect information about headache history prior to the LP, we could not evaluate the influence of this factor on PDPH recurrence. Similarly, because we did not require rest in the supine position after LPs, we could not evaluate the potential influence of this intervention. Other studies have found that rest after LP does not prevent PDPH.(24–27) All LPs in our study were performed with 22-gauge, atraumatic needles, as opposed to the traditional, cutting-edge (Quinke) needle. Atraumatic spinal needle was already shown in previous studies to reduce PDPHs (10, 28). Needle size may be the most important factor in determining the risk of PDPH (6, 18, 27, 29). All the LPs in this study were performed using 22 gauge needles, so we could not evaluate the impact of needle size on adverse event rates. Because the range of CSF volumes collected in this study was restricted, our findings cannot be generalized to volumes over 15mL.
ACKNOWLEDGEMENTS
We thank Elcio Piovesan, M.D., PhD for the careful review of this paper and the valuable suggestions.
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from NIMH.
Footnotes
Conflict of interest: No conflict
* The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Shannon LeBlanc; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S., Tanya Wolfson, M.A.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
REFERENCES
- 1.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 2.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 3.Letendre SL, van den Brande G, Hermes A, Woods SP, Durelle J, Beck JM, et al. Lopinavir with Ritonavir Reduces the HIV RNA Level in Cerebrospinal Fluid. Clin Infect Dis. 2007;45(11) [Google Scholar]
- 4.de Almeida SM, Faria FL, de Goes Fontes K, Buczenko GM, Berto DB, Raboni SM, Vidal LR, Nogueira MB. Quantitation of cerebrospinal fluid lactic acid in infectious and non-infectious neurological diseases. Clin Chem Lab Med. 2009;47(6):755–761. doi: 10.1515/CCLM.2009.160. [DOI] [PubMed] [Google Scholar]
- 5.Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology and pathology. Headache. 2010;50:1144–1152. doi: 10.1111/j.1526-4610.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 6.Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part II prevention, management and prognosis. Headache. 2010;50:1144–1152. doi: 10.1111/j.1526-4610.2010.01758.x. [DOI] [PubMed] [Google Scholar]
- 7.Regulations. The Board of Registered Nursing California Code of Regulations Standardized Procedure Guidelines. Division 14, Title 16, Article 7. 2007 [Google Scholar]
- 8.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Zetterberg H, Tullhög K, Hansson O, Minthon L, Londos E, Blennow K. Low incidence of post-lumbar puncture headache in 1,089 consecutive memory clinic patients. Eur Neurol. 2010;63(6):326–330. doi: 10.1159/000311703. [DOI] [PubMed] [Google Scholar]
- 10.Muller B, Adelt K, Reichmann H, Toyka K. Atraumatic needle reduces the incidence of post-lumbar puncture syndrome. J Neurol. 1994;241:376–380. doi: 10.1007/BF02033354. [DOI] [PubMed] [Google Scholar]
- 11.Lavi R, Yarnitsky D, Rowe JM, Weissman A, Segal D, Avivi I. Standard vs atraumaticWhitacre needle for diagnostic lumbar puncture: A randomized trial. Neurology. 2006;67:1492–1494. doi: 10.1212/01.wnl.0000240054.40274.8a. [DOI] [PubMed] [Google Scholar]
- 12.MacArthur C, Lewis M, Knox EG. Accidental dural puncture in obstetric patients and long term symptoms. BMJ. 1993;306:883–885. doi: 10.1136/bmj.306.6882.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuntz KM, Kokmen E, Stevens JC, Miller P, Offord KP, Ho MM. Post-lumbar puncture headaches: experience in 501 consecutive procedures. Neurology. 1992;42:1884–1887. doi: 10.1212/wnl.42.10.1884. [DOI] [PubMed] [Google Scholar]
- 14.Evans RW, Armon C, Frohman EM, Goodin DS. Assessment: prevention of post-lumbar puncture headaches: report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2000;55:909–914. doi: 10.1212/wnl.55.7.909. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: Diagnosis and management. Postgrad Med J. 2006;82:713–716. doi: 10.1136/pgmj.2006.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Kaye A, Comarda N, Li M. Paradoxical postural cerebrospinal fluid leak-induced headache: Report of two cases. J Clin Anesth. 2008;20:383–385. doi: 10.1016/j.jclinane.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Vilming ST, Kloster R. Post-lumbar puncture headache: Clinical features and suggestions for diagnostic criteria. Cephalalgia. 1997;17:778–784. doi: 10.1046/j.1468-2982.1997.1707778.x. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull DK, Shepherd DB. Post-dural puncture headache: Pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–721. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 19.Kuczkowski KM. Postdural puncture headache after lumbar puncture: Do the gauge and the design of a spinal needle matter? Am J Emerg Med. 2006;24:757. doi: 10.1016/j.ajem.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Clark JW. Substance P concentration and history of headache in relation to postlumbar puncture headache: Towards prevention. J Neurol Neurosurg Psychiatry. 1996;60:681–683. doi: 10.1136/jnnp.60.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raskin NH. Lumbar puncture headache: a review. Headache. 1990;30:197–200. doi: 10.1111/j.1526-4610.1990.hed3004197.x. [DOI] [PubMed] [Google Scholar]
- 22.Weir EC. The sharp end of the dural puncture. Br Med J. 2000;320:127–128. [PMC free article] [PubMed] [Google Scholar]
- 23.Leibold RA, Yealy DM, Coppola M, Cantees KK. Post-dural puncture headache: Characteristics, management and prevention. Ann Emerg Med. 1993;22:1863–1870. doi: 10.1016/s0196-0644(05)80416-0. [DOI] [PubMed] [Google Scholar]
- 24.Carbaat PA, van Crevel H. Lumbar puncture headache: controlled study on the preventive effect of 24 hours' bed rest. Lancet. 1981;2:1133–1135. doi: 10.1016/s0140-6736(81)90586-9. [DOI] [PubMed] [Google Scholar]
- 25.Cook PT, Davies MJ, Beavis RE. Bed rest and postlumbar puncture headache. The effectiveness of 24 hours' recumbency in reducing the incidence of postlumbar puncture headache. Anaesthesia. 1989;44:389–391. doi: 10.1111/j.1365-2044.1989.tb11334.x. [DOI] [PubMed] [Google Scholar]
- 26.Hilton-Jones D, Harrad RA, Gill MW, Warlow CP. Failure of postural manoeuvres to prevent lumbar puncture headache. J Neurol Neurosurg Psychiatry. 1982;45:743–746. doi: 10.1136/jnnp.45.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tourtellotte WW, Somers JF, Parker JA, Itabashi HH, Dejong RN. A study on traumatic lumbar punctures. Neurology. 1958;8:129–134. doi: 10.1212/wnl.8.2.129. [DOI] [PubMed] [Google Scholar]
- 28.Strupp M, Schueler O, Straube A, Von Stuckrad- Barre S, Brandt T. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2001;57:2310–2312. doi: 10.1212/wnl.57.12.2310. [DOI] [PubMed] [Google Scholar]
- 29.Carson D, Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47:33–37. doi: 10.1212/wnl.47.1.33. [DOI] [PubMed] [Google Scholar]