Abstract
An increasing body of evidence suggests that Endothelin-converting enzyme-2 (ECE-2) is a non-classical neuropeptide processing enzyme. Similar to other neuropeptide processing enzymes, ECE-2 exhibits restricted neuroendocrine distribution, intracellular localization, and an acidic pH optimum. However, unlike classical neuropeptide processing enzymes, ECE-2 exhibits a non-classical cleavage site preference for aliphatic and aromatic residues. We previously reported that ECE-2 cleaves a number of neuropeptides at non-classical sites in vitro; however its role in peptide processing in vivo is poorly understood. Given the recognized roles of neuropeptides in pain and opiate responses, we hypothesized that ECE-2 knockout (KO) mice might show altered pain and morphine responses compared to wild-type (WT) mice. We find that ECE-2 KO mice show decreased response to a single injection of morphine in hot-plate and tail-flick tests. ECE-2 KO mice also show more rapid development of tolerance with prolonged morphine treatment and fewer signs of naloxone-precipitated withdrawal. Peptidomic analyses revealed changes in the levels of a number of spinal cord peptides in ECE-2 KO as compared to WT mice. Taken together, our findings suggest a role for ECE-2 in the non-classical processing of spinal cord peptides and morphine responses; however, the precise mechanisms through which ECE-2 influences morphine tolerance and withdrawal remain unclear.
Keywords: opioid, morphine tolerance, morphine withdrawal, neuropeptide biosynthesis, peptidomics, differential isotopic labeling
INTRODUCTION
The majority of neuropeptides are synthesized as prepropeptide precursors that undergo cleavage to bioactive peptides while transiting from the Golgi apparatus to the cell membrane in secretory vesicles. This post-translational processing serves as an important regulatory step in peptide synthesis, as the precursor propeptide may be processed to different peptides depending upon the peptidases expressed in a particular tissue (Rouille et al. 1997; Rouille et al. 1997). For example, proenkephalin can be “classically” processed at dibasic residues by prohormone convertases and carboxypeptidases to yield Met- and Leu-enkephalin. Alternatively, this precursor can be “non-classically” processed at non-basic residues to yield longer bovine adrenal medulla (BAM) peptides (Mizuno et al. 1980; Mizuno et al. 1980; Mzhavia et al. 2003), which exhibit higher analgesic potency relative to enkephalins and can decrease morphine tolerance (Hollt et al. 1982; Jiang et al. 2006). Alternative processing of peptide precursors could thus have important physiological implications.
While the endopeptidases (prohormone convertase 1 and 2) and carboxypeptideases responsible for classical neuropeptide processing have been well-characterized, less is known about the peptidases that cleave at non-classical sites (Fricker 2005). To date, endothelin-converting enzyme-2 (ECE-2) has been suggested as a non-classical neuropeptide processing enzyme based on its biochemical properties, tissue distribution, and cleavage-site selectivity (Fricker 2005). ECE-2 belongs to the Neprilysin (NEP) family (M13) of zinc metalloproteases, sharing 59% sequence homology with ECE-1 and 37% with NEP. Homology modeling has revealed that the substrate binding pocket is well conserved between these enzymes (Gagnidze et al. 2008). Despite this fact, ECE-2’s distribution and biochemical properties suggests that it plays a unique role in neuropeptide processing within the secretory pathway. While ECE-1 and NEP show predominant cell-surface expression and are optimally active at neural pH (Xu et al. 1994; Turner and Murphy 1996; Turner et al. 2001), ECE-2 is present intracellularly and it exhibits an acidic pH optimum, consistent with processing that takes place within the acidic secretory pathway (Emoto and Yanagisawa 1995; Russell and Davenport 1999; Mzhavia et al. 2003). ECE-2 also has a restricted neuroendocrine distribution, with relatively high levels in regions rich in neuropeptides, such as the hypothalamus, spinal cord, and midbrain (Emoto and Yanagisawa 1995; Rodriguiz et al. 2007). In contrast, NEP and ECE-1 show a broad tissue distribution, with highest expression in the kidney and liver, respectively (Xu et al. 1994; Rodriguiz et al. 2007). Hence, the distribution and biochemical properties of ECE-2 support a unique role in neuropeptide processing.
ECE-2 was named for its role in generating the potent vasoconstrictor endothelin-1 (ET-1) from its precursor big-ET-1 (Emoto and Yanagisawa 1995). We have shown that ECE-2 processes a number of neuroendocrine peptides at non-classical sites at acidic pH in vitro (Mzhavia et al. 2003). Of the 42 peptides screened using mass spectrometry (MS), 10 were found to be substrates for ECE-2, including three proenkephalin-derived peptides (i.e., Peptide E, BAM22 and BAM18), dynorphin B, and substance P. Comparisons of these substrates revealed that ECE-2 prefers aromatic or aliphatic residues in the P1’ position (C-terminal aspect of the peptide bond), like ECE-1 and NEP. However, ECE-2 can cleave some, but not all substrates of ECE-1 and NEP, indicating there are cleavage-site preferences beyond these residues. This ability to cleave selectively at non-basic residues further supports the contention that ECE-2 functions as a non-classical neuropeptide processing enzyme.
Recently, a viable and fertile knockout (KO) mouse model has aided investigation of ECE-2’s role in vivo. These mice show no gross developmental defects, and behavioral analysis has revealed no impairments in sensory or motor function or signs of depressive- or anxiety-like responses (Yanagisawa et al. 2000; Rodriguiz et al. 2007). However, we did observe impairments in learning and memory, which, together with its relatively high expression in the hippocampal dentate gyrus, suggests that ECE-2 may processes as-yet-unidentified peptides important for mediating these behaviors (Rodriguiz et al. 2007). Alternatively, these deficiencies in learning and memory processes could reflect an Alzheimer’s disease-like phenotype, as ECE-2 KO mice show increased amyloid β-protein accumulation in the brain (Eckman et al. 2003).
Given ECE-2’s relatively high expression in the spinal cord (Rodriguiz et al. 2007) and the roles of spinal cord peptides in pain processing and responses to opiates (Wiesenfeld-Hallin and Xu 1996; Gaveriaux-Ruff and Kieffer 2002; Nitsche et al. 2002; Komatsu et al. 2009), we hypothesized that ECE-2 KO mice would show altered pain thresholds and opiate responses. We have found that ECE-2 KO mice display altered opiate responses, namely faster development of tolerance to the analgesic effects of morphine and decreased signs of naloxone-precipitated withdrawal. We also investigated the role of ECE-2 in processing spinal cord peptides using MS-based peptidomics and found that a number of peptides are altered in ECE-2 KO mice. Taken together, our findings support a role for ECE-2 in non-classical peptide processing and morphine responses.
MATERIALS AND METHODS
Animals
Adult age-matched and sex-matched ECE-2 KO mice and wild-type (WT) littermate controls were used for these experiments. Animals were generated and propagated by heterozygous C57BL6/J-129/SvJ matings at Mount Sinai School of Medicine and at Duke University as described (Rodriguiz et al. 2007). Efforts were made to minimize animal suffering, and all experimental procedures were approved by the Institutional Animal Care and Use Committees at Mount Sinai School of Medicine and Duke University. ARRIVE guidelines were followed. Mice were anesthetized and euthanized by CO2 for biochemical experiments. The spinal cords were removed, immediately frozen on dry ice, and stored at −80°C.
Behavioral Studies
1. Baseline thermal thresholds
To examine sensitivity to thermal stimulation, 10–12 naïve ECE-2 KO and WT animals were injected with vehicle (4 ml/kg saline; s.c.) and individually tested 30 min later for their responses on the hot-plate (Columbus Instruments, Columbus, OH) and ~10 sec later for tail-flick responses (Columbus Instruments) as described (Bohn et al. 2002). For the hot-plate, the metal plate was enclosed by 20 cm Plexiglas walls and maintained at 52.0 ±0.2°C. Mice remained on the hot-plate until they engaged in fore- or hind-paw licking, paw flicking, or jumping. Animals were immediately removed from the hot-plate after the response and the latency was scored. If they did not respond, they were removed after 20 sec and assigned that time. For tail-flick, the heat source was set to 13 (range 1–25) which produced a 56.0 ±0.1°C beam on a digital thermometer. Mice were gently wrapped in a clean terry-cloth towel and the tail was illuminated by the light-beam 4–5 cm from the rump of the animal. The latency to flick the tail was recorded. In the absence of response, the tail was removed from the beam after 20 sec and assigned that time.
2. Responses to Morphine
Responses to morphine were examined as previously described (Bohn et al. 1999; Bohn et al. 2000; Bohn et al. 2000; Bohn et al. 2002). For all response latencies, the data were converted to a ratio reflecting the maximum possible effect (MPE) where MPE = [(drug response-time – basal response-time) / (20 sec – basal response-time)]* 100%.
a. Acute tolerance
Naïve ECE-2 KO and WT mice were tested for hot-plate and tail-flick responses at baseline (time 0) and then given 10 mg/kg morphine (s.c.; Sigma-Aldrich, St. Louis, MO) and responses were evaluated at 15, 30, 60, 90, and 120 min later.
b. Chronic tolerance
Naïve ECE-2 KO and WT mice were examined at baseline (no morphine). Subsequently, animals received 10 mg/kg morphine (s.c.) daily for 6 consecutive days; hot-plate and tail-flick responses were analyzed 30 min after drug administration.
c. Morphine withdrawal
The same mice that were used for the chronic tolerance study were used for the withdrawal experiment. Animals received 10 mg/kg morphine (s.c.) on day 7, and on day 8 they were given two injections of 10 mg/kg morphine spaced 6 hrs apart. On day 8, 30 min after the last morphine injection, mice were given 1 mg/kg naloxone (s.c.; Sigma-Aldrich) and responses were followed for 40 min. During this time, all behaviors were scored continuously by trained observers using the Noldus Observer XT (Noldus Information Technologies, Leesburg, VA). The behaviors included oral stereotypes, tremor, paw-wringing or rubbing, and wet-dog shakes, and were reported as the percent time engaged in each specific behavior.
3. Statistical analysis
All data are presented as mean ± SEM. The results were analyzed with independent measures t-tests (baseline nociceptive responses and withdrawal behaviors), or ANOVA or repeated measures ANOVA (RMANOVA) for time-dependent nociceptive responses to morphine or vehicle. For RMANOVA, time (days or minutes) was the within-subjects effect, and genotype and drug treatment were the between-subject effects. Decomposition of the significant interaction was conducted with Bonferroni corrected pair-wise comparisons. In all cases, p<0.05 was considered significant. .
Spinal cord peptide extraction and radioimmunoassay (RIA) for Leu-enkephalin
ECE-2 KO and WT spinal cords samples (three each) were individually sonicated in 0.1N glacial acetic acid preheated to 100°C. Samples were incubated for 7 min at 100°C, sonicated again, and centrifuged at 14,000 rpm for 30 min at 4°C. The supernatants were collected and dried overnight using vacuum centrifugation. Pellets were then washed with water and dried again by vacuum centrifugation.
Samples were resuspended in 300 uL radioimmunoassay (RIA) buffer (50mM Tris-HCl pH 7.5, 0.1% bovine serum albumin (BSA), 0.1% Triton X-100, 0.1% gelatin, 0.02% sodium azide). Half of the samples were then treated with TPCK-trypsin and carboxypeptidase B (CPB). Briefly, 60 µL of each sample was incubated at 37°C with 10 µL of 0.1 µg/µL TPCK-trypsin for 3 hrs in 20 µL buffer (0.1M Tris-HCl, pH 8.0, containing 100 µg/µL protease-free BSA) in a total volume of 90 µL. Next, 10 µL of 10 ng/µl CPB was added (total volume of 100 µL) and incubated at 37°C for 40 min. Reactions were terminated by incubating tubes at 100°C for 2 min, samples were aliquoted in duplicate and RIA buffer added for a final volume of 100 µL. Sixty µL of non-digested sample was similarly aliquoted to duplicate tubes and RIA buffer added for a final volume of 100 µL. Concentrations (10−6 M to 10−10 M) of Leu-enkephalin peptide (100 µL), were used as standards. Samples were incubated overnight at 4°C with 100 µL of 1:100 rabbit anti-Leu-enkephalin IgG antibody (Phoenix Pharmaceuticals, Inc., Burlingame, CA). The next day 100 µL of [125I]-Leu-enkephalin (Phoenix Pharmaceuticals) was added (for a final concentration of 10,000 counts/100 µL), and samples were incubated at 4°C overnight. Nonspecific binding was assessed by adding [125I]-Leu-enkephalin in the absence of sample, standard, or Leu-enkephalin antibody. The following day 100 µL of goat anti-rabbit IgG and 100 µL of normal rabbit serum (Phoenix Pharmaceuticals) were added, followed by a 90 min incubation at room temperature (27 °C). Next, 250 µL termination buffer was added (50 mM Trizma base, 0.1% Triton X-1000, 0.02%, sodium azide, pH 7.5), samples were centrifuged at 3700 rpm for 20 min, and the supernatant was aspirated. Radioactivity was measured by gamma counter. Levels of immunoreactive (ir) Leu-enkephalin were calculated by subtracting the non-specific binding and extrapolating from the standard curve values using Prism software (GraphPad, San Diego, CA).
GTPγS Binding Assays
ECE-2 KO and WT spinal cord membranes were prepared and analyzed using GTPγS binding assays as previously described (Gomes et al. 2003). Maximal agonist stimulation (using 10 µM of DOR agonist DPDPE) of [35S]GTPγS binding was expressed as a percentage of basal values.
Peptidomics
Peptide extraction and isotopic labeling
A multistage extraction protocol that allows for efficient extraction of peptides was used (Bora et al. 2008). Briefly, spinal cord tissues from three WT or ECE-2 KO mice were preheated at 90°C for 1 min to inactivate endogenous enzymes. Samples were then partially homogenized in ~5 volumes of deionized water, sonicated for 30 sec, and heated to 90°C for 20 min. After centrifugation and supernatant collection, the remaining pellets were cooled on ice for 20 min and then fully homogenized two times in ~10 volumes of acidified acetone (40:6:1 acetone/water/HCl) twice. Following sonication, vortexing, and centrifugation, the supernatants were collected. The combined two-stage extracts were dried using a SpeedVac concentrator (Thermo Scientific, San Jose, CA), and reconstituted in solvent A [95% water and 5% acetonitrile (ACN) with 0.1% formic acid (FA) and 0.01% trifluoroacetic acid (TFA)]. Prior to tandem MS/MS studies, the samples were filtered through a Microcon centrifugal filter with a 10 kDa cutoff (Millipore, Billerica, MA). After pH adjustment to ~9 using 1M phosphate buffer or 1M NaOH if needed, each spinal cord was divided into half and labeled with 2 µL of 4 M [H4] or [D4] succinic anhydride (SA) in dimethylsulfoxide. After vortexing, centrifugation, and incubation at room temperature for 15 min, the sample pH was readjusted to ~9 with 1 M NaOH. The labeling procedure was repeated 4 times with subsequent pH adjustment. Any excess free labeling was quenched by 15 µl of 2.5 M glycine for at least 1 hr. The combined isotope-labeled samples were then filtered through a 10 kDa Microcon centrifugal filter (Millipore), followed by the addition of 15 µL of 2.5 M hydroxylamine solution, as described (Che et al. 2006; Brockmann et al. 2009). For each replicate, there were two technical replicates: the combination of [H4]-SA labeled WT and [D4]-SA labeled ECE-2 KO samples as forward replicates and the combination of [D4]-SA labeled WT and [H4]-SA labeled ECE-2 KO samples as reverse replicates. The samples were desalted by PepClean™ C18 spin columns (Pierce, Rockford, IL), and eluted with 70% aqueous ACN solution.
Peptide measurement and identification by tandem MS
Extracts were passed through the first stage of fractionation using a microbore HPLC system Magic 2000 (Michrom BioResources, Inc., Aubum, CA) equipped with a C18 reverse phase column. A 70 min run was conducted at a 20 µL/min flow rate, with a five-step linear gradient generated by mixing mobile phases A (95% water and 5% ACN, 0.1% FA and 0.01% TFA) and B (95% ACN, 5% water, 0.1% FA and 0.01% TFA) as previously described (Collins et al. 2010). Fractions were manually collected, and subjected to second stage liquid chromatography (LC) separation coupled to MS.
Two LC-MS/MS systems were employed for more comprehensive peptide identification. The first was a NanoAcquity ultra performance LC (UPLC) system (Waters, Milford, MA) equipped with a C18 reverse phase column (75 µm i.d., particle size 3 µm, and pore size 100 Å), connected to an electrospray ionization (ESI) source and quadrupole time-of-flight (Q-TOF) mass spectrometer (Waters). The solvent gradient over a 90 min run was generated by water/ACN with 0.1% FA at a flow rate of 400 nl/min. MS detection of peptides was controlled by MassLynx 4.1 software (Waters) in a data-dependent manner. The MS/MS precursor ion selection was set at four ions of the highest intensity per MS scan, and dynamic exclusion of previously fragmented precursor ions was enabled. The mass-to-charge (m/z) ranges for the MS and MS/MS scans were 200–2000 and 50–2000 Da, respectively. The second LC-MS/MS system was a Micromass capillary HPLC system (Manchester, U.K.) with a C18 reversed phase column (300 µm i.d., particle size 3 µm, and pore size 100 Å; Dionex, Sunnyvale, CA), coupled to an ESI source and ion-trap mass spectrometer (HCTUltra-PTM Discovery system, Bruker Daltonics Inc, Billerica, MA ). The solvent gradient over a 60 min run was generated by water/methanol with 0.1% FA at a flow rate of 2.5 µl/min; the Esquire software (Bruker Daltonics Inc) controlled the MS detection of peptides in a data-dependent manner as described (Collins et al. 2010). The m/z ranges for MS and MS/MS scans were 300–1800 and 50–3000 Da, respectively.
After data collection, the MS/MS spectra were converted to either .pkl or .mgf file formats and sequenced automatically against an in-house mouse precursor database using the PEAKS Studio 4.5 software (Bioinformatics Solutions, Inc., Waterloo, CA). The search parameters for PEAKS were: cleavage sites, variable post-translational modifications (including N-terminal pyro-Glu and pyro-Gln, acetylation, methylation, phosporylation, C-terminal amidation, and disulfide bond), mass tolerance 0.5 Da for the precursor ion, and 0.5 Da for fragments. The criteria for peptide assignments include the PEAKS score, mass error, and manual verification as described in previous studies (Collins et al. 2010).
Peptide measurement and quantitation by MS
Two mass spectrometric platforms were implemented for peptide quantitation. The first was the Waters NanoAcquity UPLC-ESI Q-TOF instrument described above. The solvents and instrument settings/parameters were the same as those for the peptide identification studies, except that the MS spectra were collected from a scan range of m/z 200–2000 Da. The second instrument used was a matrix-assisted laser desorption/ionization (MALDI) coupled to TOF mass spectrometer (Ultraflex MALDI TOF/TOF, Bruker Daltonics). For these studies, 0.5 µL of sample was spotted onto a Bruker plate with 0.5 µl of saturated 2,5-dihydroxybenzoic acid as the matrix. The spectra were acquired across the spot, and comprised of 10 acquisitions with 500 laser shots for each.
After identifying the labeled peptide pairs, an extracted ion chromatography from the UPLC-ESI-Q-TOF MS scans was created for both light and heavy labeled peptides, and the mass spectra across the entire elution period for both peaks were summed. For both platforms, the relative abundance of peptides in WT versus ECE-2 KO mice was determined by the ratio of peak intensities between light and heavy labeled peptides. The data were then analyzed by student’s t-test to identify peptides showing statistically significant differences between genotypes.
RESULTS
1. ECE-2 KO mice show altered responses to morphine
Our previous studies revealed that ECE-2 can cleave a number of neuropeptides implicated in pain and opiate responses in vitro, including proenkephalin- and prodynorphin-derived peptides and substance P (Mzhavia et al. 2003). To investigate the role of ECE-2 in the processing of these and other neuropeptides in vivo, we examined pain and opiate responses in WT and ECE-2 KO mice.
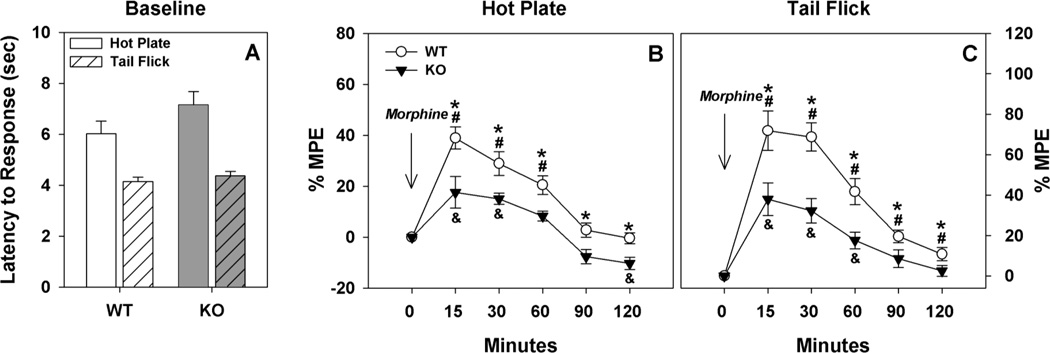
We first examined sensitivity to thermal stimulation using the hot-plate and tail-flick assays. No significant genotype differences were observed in either assay (Fig. 1A). Acute tolerance to morphine was next examined by measuring hot-plate and tail-flick responses up to two hours after a single injection of morphine (Fig. 1B and C). Percent maximum possible effect (MPE) was calculated for each time-point based on the latency to respond to morphine treatment compared to the latency to respond at baseline; the greater the %MPE, the more prolonged the response latency to morphine compared to baseline. The RMANOVA of hot-plate responses to morphine revealed significant main effects of time [F(5,90) = 39.292, p<0.001] and a significant genotype by time interaction [F(5,90) = 2.721, p<0.025]. Bonferroni pair-wise comparisons revealed that ECE-2 KO mice had reduced %MPE at all time-points assessed compared to WT controls (ps<0.017). As expected, WT mice had higher %MPE at 15, 30 and 60 min after morphine treatment than that at baseline (ps<0.001); their responses returned to baseline after this time (Fig. 1B). By contrast, ECE-2 KO mice showed a significant increase in %MPE only at 15 and 30 min (ps<0.015); however, their %MPE was reduced to below baseline at 120 min (p<0.004). Similarly RMANOVA of tail-flick responses to morphine demonstrated significant main effects of time [F(5,90) = 44.390, p<0.001] and a significant genotype by time interaction [F(5,90) = 3.982, p<0.003]. Bonferroni pair-wise comparisons revealed that WT mice had higher %MPE at all time-points relative to ECE-2 KO animals (ps<0.053). In the tail-flick test, WT animals also showed increased %MPE relative to their baseline at all time-points (ps<0.031), whereas ECE-2 KO mice had significant increases above their baseline only at 15, 30 and 60 min post-injection (ps<0.054). Taken together, both WT and KO mice show acute tolerance to morphine ton he hot-plate and tail-flick tests. However, acute tolerance is lower in KO mice for up to two hours after drug administration.
Figure 1. Baseline pain thresholds and acute tolerance to morphine in ECE-2 WT and KO mice.
A. No significant genotype differences were observed for baseline tail-flick or hot-plate responses. N=10 mice/genotype. B. ECE-2 KO mice showed a lower analgesic response to a single injection of morphine in the hot-plate test compared to WT controls; this was observed up to 2 hr after drug administration. C. ECE-2 KO mice similarly demonstrated decreased responsiveness to a single injection of morphine in the tail-flick test. N=10 mice/genotype. MPE, maximum possible effect. *p<0.05, WT versus KO mice; #p<0.05, WT response to morphine compared to WT baseline; &p<0.05, KO response to morphine compared to KO baseline.
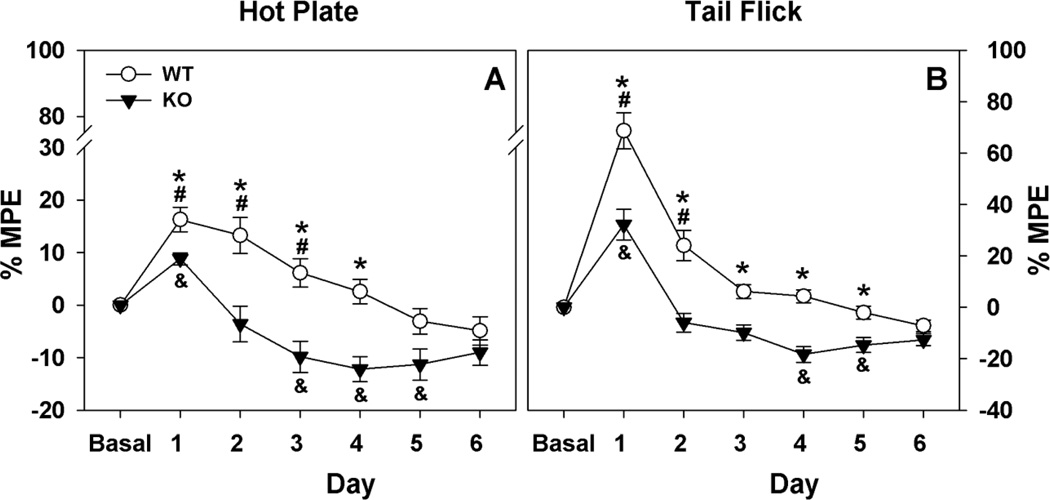
Next, we examined hot-plate and tail-flick responses to morphine (10 mg/kg, s.c.) when it was administered daily over six consecutive days (Fig. 2A & 2B). While sensitivity to morphine injections declined across days for both WT and ECE-2 KO animals, tolerance appeared more quickly in ECE-2 KO mice on both tests. The RMANOVA of the hot-plate assay showed significant main effects of test day [F(6,108) = 39.883, p<0.001] and a significant test day by genotype interaction [F(6,108) = 5.292, p<0.001]. Bonferroni pair-wise comparisons showed significant genotype differences for %MPE on days 1 to 4 (ps<0.013). The within genotype analyses revealed that WT animals had higher %MPE relative to their baseline on days 1 to 3 of testing (ps<0.022), whereas ECE-2 KO mice had increased %MPE relative to baseline response only on day 1 (p<0.020); reductions in %MPE fell significantly below baseline on days 3 to 5 (ps<0.028). The RMANOVA of the tail-flick responses demonstrated significant main effects of test day [F(6,108) = 73.397, p<0.001] and the test day by genotype interaction was significant [F(6,108) = 6.123, p<0.001]. Bonferroni pair-wise comparisons found that %MPE for WT mice was significantly higher than that for ECE-2 KO animals on days 1 to 5 (ps<0.002). WT mice had marked increases in %MPE compared to baseline values on days 1 and 2 (ps<0.003). By comparison, ECE-2 KO mice showed increases in %MPE relative to baseline only on day 1 (p<0.002); on days 4 and 5 %MPE was reduced below baseline (ps<0.013). By day 6, no genotype differences were observed. Taken together, both the hot-plate and tail-flick results reveal that ECE-2 KO mice develop tolerance to morphine-induced analgesia more rapidly across days than the WT controls.
Figure 2. ECE-2 WT and KO responses to repeated daily morphine injections.
A. Both WT and ECE-2 KO animals developed tolerance to daily morphine administration on the hot-plate; however, tolerance appeared more rapidly in KO mice. By day 6, no genotype differences were evident. B. Development of tolerance to daily injections of morphine on tail-flick was again significantly slower in WT than KO animals. By day 6, no genotype differences were apparent. MPE, maximum possible effect. N=9 mice/genotype. *p<0.05, WT versus KO mice; #p<0.05, WT response to morphine compared to WT baseline; &p<0.05, KO response to morphine compared to KO baseline.
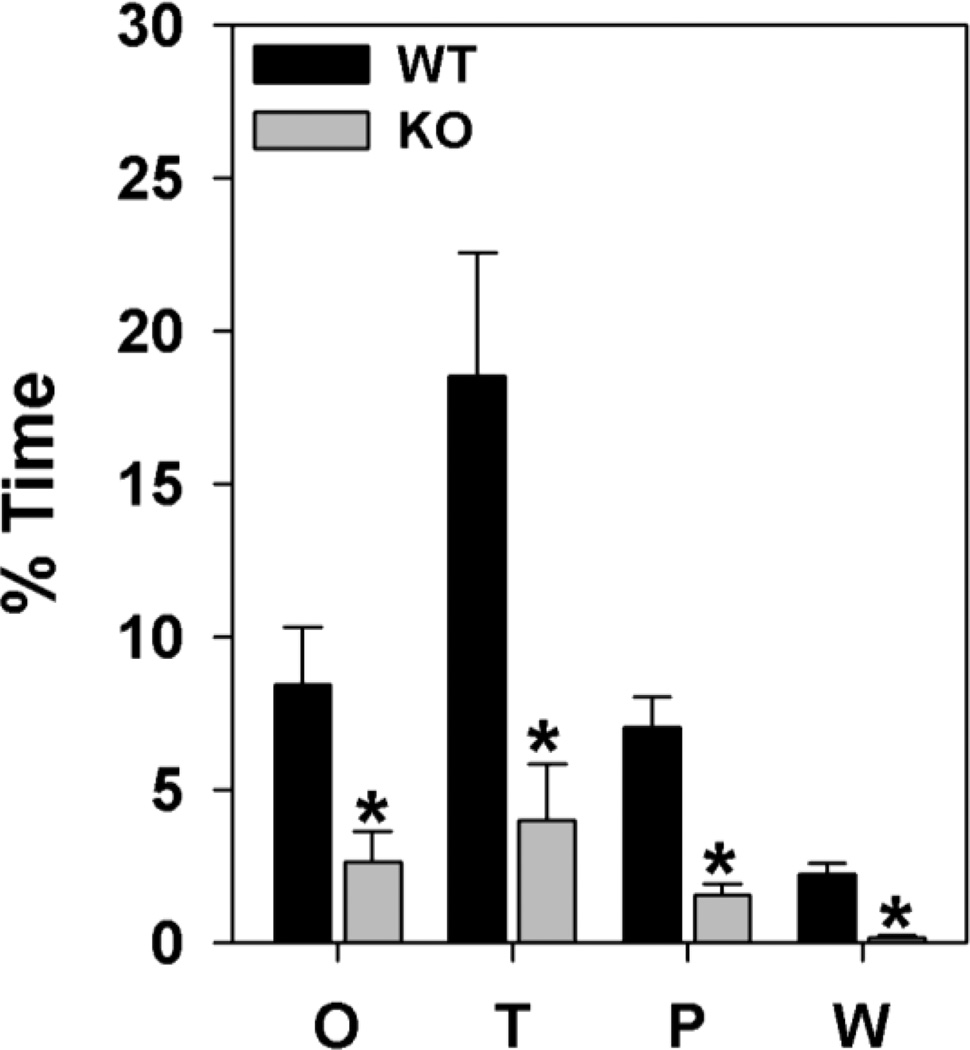
To examine morphine dependence, the same mice that were used in the chronic tolerance study were given two daily injections of 10 mg/kg morphine (s.c.) for two additional days (Fig. 3) followed by 1 mg/kg naloxone (s.c.) to precipitate withdrawal. WT mice showed jumping, loss of balance, ptosis, flat posture, and swallowing, whereas ECE-2 KO animals rarely displayed these responses (data not shown). Both genotypes displayed various oral behaviors (mastication, teeth-chattering, etc.), paw-tremor and paw-wringing, body tremor, and wet-dog shakes; however, the expression of the following naloxone-precipitated withdrawal responses were reduced in ECE-2 KO mice: oral behaviors [t(1,18) = 2.705, p<0.016], tremor [t(1,18) = 3.273, p<0.005], paw-tremor or paw-wringing [t(1,18) = 2.583, p<0.020], and wet-dog shakes [t(1,18) = 2.852, p<0.012]. Taken together, these results reveal that the morphine withdrawal responses of ECE-2 KO mice are less severe than those of WT controls.
Figure 3. Morphine withdrawal in ECE-2 WT and KO mice.
The expression of naloxone-precipitated withdrawal responses were significantly lower in ECE-2 KO than in WT mice: O=oral behaviors, T=tremor, P=paw-tremor or paw-wringing W=wet-dog shakes. N=9 mice/genotype. *p<0.05, WT versus KO mice.
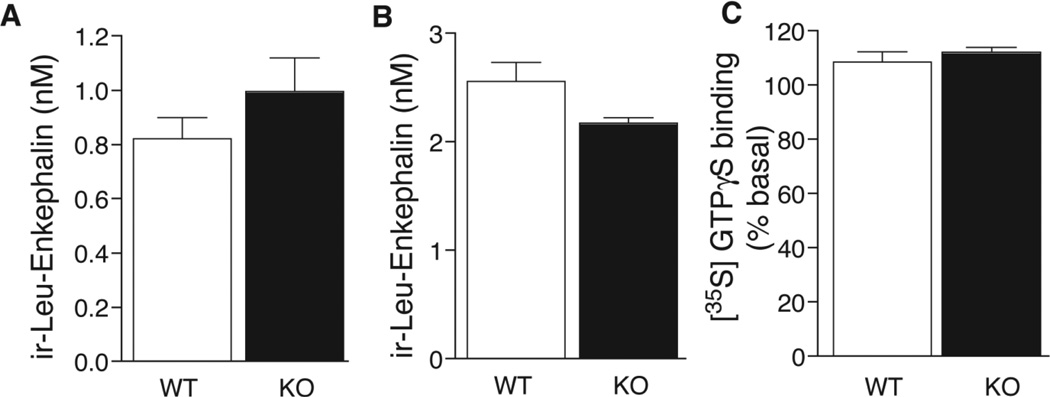
2. Effects of ECE-2 deletion on the Leu-enkephalin system in the spinal cord
Given that enkephalins have been implicated in morphine responses (Lee et al. 1980; Rady et al. 2001; Nitsche et al. 2002), and that ECE-2 was previously found to process proenkephalin-derived peptides in vitro (Mzhavia et al. 2003), we investigated the enkephalin system in the spinal cord of ECE-2 KO mice. First we measured the levels of free Leu-enkephalin by RIA. Initial RIA analysis revealed similar levels of free ir-Leu-enkephalin in the spinal cords of ECE-2 KO and WT mice (Fig. 4A). To further investigate whether the processing of Leu-enkephalin-containing intermediates was altered, spinal cord extracts were treated with trypsin and CPB, which cleave at dibasic sites and therefore release Leu-enkephalin from larger peptides. These treatments resulted in an increase in free ir-Leu-enkephalin by about two-fold in both ECE-2 KO and WT tissues (Fig 4B). These data suggest that the lack of ECE-2 does not affect the processing of Leu-enkephalin precursors in the spinal cord. Next, we compared opioid receptor activity in ECE-2 KO and WT spinal cord using the delta opioid receptor (DOR) agonist DPDPE (D-Penicillamine(2,5)-enkephalin), because enkephalins have the greatest affinity for DOR among the opioid receptors (Oswald and Wand 2004). No significant differences in DPDPE-induced GTPγS binding in spinal cord membranes were found, indicating no differences in DOR activity between ECE-2 KO and WT mice (Fig. 4C). This finding is consistent with the above observation that Leu-enkephalin levels are not different between ECE-2 KO and WT spinal cords (Clarke et al. 2003), and further suggests that the lack of ECE-2 does not significantly alter the enkephalin system in this region of the CNS. However, additional studies of Met-enkephalin levels and activity of other targets of the enkephalins (namely mu opioid receptors) are needed to further elucidate the role of ECE-2 in the enkephalin system.
Figure 4. Leu-enkephalin levels and delta opioid receptor activity in ECE-2 WT and KO spinal cord.
Levels of ir-Leu-enkephalin were examined by radioimmunoassay of spinal cord extracts from WT and KO mice without (A) and with (B) treatment with trypsin/carboxypeptidase B; no significant differences were observed. The levels of DPDPE-induced GTPγS binding in membranes from spinal cord extracts (C) were similar between genotypes. Data are represented as mean ±SEM from quadruplicate determinations from three individual animals (N=3 mice/genotype). DPDPE (D-Penicillamine(2,5)-enkephalin) is a delta opioid receptor agonist.
3. Peptidomic analysis of spinal cord extracts
Intrigued by these observations, we initiated studies to examine the role of ECE-2 in processing the broader repertoire of spinal cord peptides. First, we performed MS/MS analysis using WT mouse spinal cord following an extraction protocol optimized for peptide recovery from the CNS (Bora et al. 2008). We identified a number of peptides known or presumed to be present in WT spinal cord (Monroe et al. 2008) and implicated in pain processing and/or opiate responses. These peptides include: Met-enkephalin, neurokinins A and B, and cholecystokinin (CCK; Table 1). Parenthetically, the inability to identify Leu-enkephalin may have been due to its relatively low abundance in the complex mixture of spinal cord proteins; each proenkephalin is processed to four Met-enkephalins and only one Leu-enkephalin peptide. Nevertheless, we were able to detect other known neuropeptides in the spinal cord, including little SAAS, PEN, somatostatin, secretogranin I, and secretogranin II (Table 1), with peptides from nonprohormone-related proteins listed in the Supplemental Table S1.
Table 1.
Neuropeptides identified from wild type mice spinal cord by MS/MS.
| Precursor | Peptide name |
Peptide sequence | Obs. Mass |
Theor. Mass |
Error (ppm) |
PEAKS Score |
LC-ESI-IT | LC-ESI-Q- TOF |
|---|---|---|---|---|---|---|---|---|
| Procholecystokinin | 23–32 | *QP.WPAEATDPV.E | 996.82 | 996.51 | 311 | 78 | X | |
| Proenkephalin | Met-enkephalin | KK.YGGFM.KR | 573.4 | 573.23 | 296 | 54 | X | |
| Proenkephalin | 197–208 | KR.SPQLEDEAKELQ.KR | 1385.99 | 1385.67 | 231 | 99 | X | |
| ProSAAS | little SAAS | R.SLSAASAPLVETSTPLRL.RR | 1812.66 | 1812.0 | 364 | 99 | X | |
| ProSAAS | PEN | RR.SVDQDLGPEVPPENVLGALLRV.KR | 2316.84 | 2316.23 | 263 | 99 | X | |
| Prosomatostatin | Somatostatin 28–14 | R.SANSNPAMAPRE.RK | 1243.79 | 1243.56 | 185 | 98 | X | |
| Protachykinin A | Neurokinin A | KR.HKTDSFVGL(AmideM).GKR | 1132.54 | 1132.57 | 26 | 45 | X | |
| Protachykinin B | Neurokinin B | KR.DMHDFFVGLMa.GKR | 1209.61 | 1209.52 | 74 | 97 | X | |
| Secretogranin 1 | 588–597 | KR.SFARAPQLDL.KR | 1117.01 | 1116.59 | 376 | 86 | X | |
| Secretogranin II | 569–581 | KR.IPVGSLKNEDTPN.R | 1382.59 | 1382.7 | 80 | 80 | X | X |
(.) indicates a cleavage site;
indicates the presence of signal peptide before the sequence;
“X” indicates the MS/MS platforms used to identify the peptide; Obs. mass, observed monoisotopic mass; Theor. mass, theoretical monoisotopic mass; putative PTM includes C-terminal amidation (“Amide”).
Changes in ECE-2 KO versus WT spinal cord peptides were quantified by differential isotopic labeling followed by analyses on two different MS platforms: LC-ESI-Q-TOF MS and MALDI-TOF MS (Figure 5). Fourteen masses were found to be significantly different between ECE-2 KO and WT mice. Five showed a significant increase, while nine showed a significant decrease in ECE-2 KO spinal cords compared to WT (Table 2). Two peptides that showed an increase in ECE-2 KO mice were derived from myelin basic protein (MBP). Notably, we did not detect significant changes in any of the classically-processed peptides that were identified in WT spinal cord (see Table 1). Collectively, these peptidomics results support a role for ECE-2 in the non-classical processing of a range of spinal cord peptides and/or precursors in vivo.
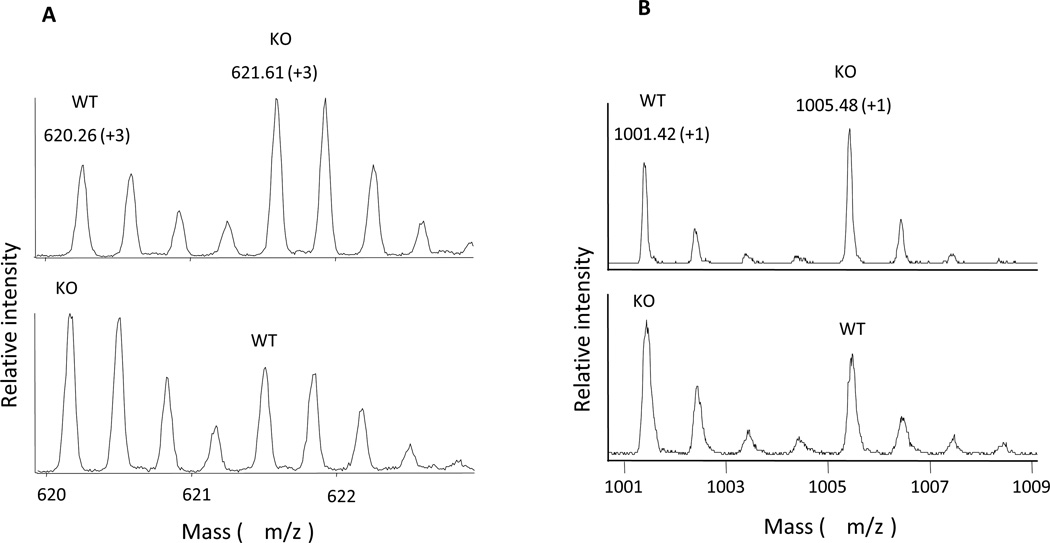
Figure 5. Representative mass spectra of succinic anhydride (SA)-labeled peptides from mice spinal cord obtained by MS analysis.
A. LC-ESI-Q-TOF mass spectra of a labeled peptide pair (MW 1757.78, charge state +3) after the combination of [H4]-SA labeled WT and [D4]-SA labeled KO samples (upper panel), or [D4]-SA labeled WT and [H4]-SA labeled KO samples (lower panel). B. MALDI-TOF mass spectra of a labeled peptide pair (MW 900.42, charge state +1) after the combination of [H4]-SA labeled WT and [D4]-SA labeled KO samples (upper panel), or [D4]-SA labeled WT and [H4]-SA labeled KO samples (lower panel).
Table 2.
Quantitative peptidomics of mouse spinal cord: comparison of ECE-2 WT and KO mice
| Precursor | Peptide sequence | Obs. mass |
Theor. mass |
Z | #T | KO : WT Ratio±SD |
MS platform |
|---|---|---|---|---|---|---|---|
| Unknown | 658.18 | 1 | 1 | 0.81±0.09* | LC-ESI-Q-TOF | ||
| Unknown | 768.38 | 2 | 1 | 1.25±0.11* | LC-ESI-Q-TOF | ||
| Unknown | 900.42 | 1 | 1 | 1.74±0.38* | MALDI-TOF | ||
| Unknown | 1174.52 | 3 | 1 | 0.65±0.10** | LC-ESI-Q-TOF | ||
| Unknown | 1174.52 | 4 | 1 | 0.62±0.15* | LC-ESI-Q-TOF | ||
| Unknown | 1174.57 | 1 | 1 | 0.59±0.17* | MALDI-TOF | ||
| Unknown | 1423.34 | 2 | 2 | 0.86±0.02* | LC-ESI-Q-TOF | ||
| Myelin basic protein | N.PVVHFFKNIVTPR.T | 1552.36 | 1552.88 | 2 | 2 | 1.15±0.02** | LC-ESI-Q-TOF |
| Unknown | 1713.48 | 4 | 1 | 0.78±0.04** | LC-ESI-Q-TOF | ||
| Unknown | 1757.78 | 3 | 1 | 0.59±0.17* | LC-ESI-Q-TOF | ||
| Myelin basic protein | F.SWGGRDSRSGSPMARR.# | 1761.47 | 1761.83 | 3 | 1 | 1.24±0.11* | LC-ESI-Q-TOF |
| Unknown | 1777.52 | 4 | 1 | 0.84±0.07* | LC-ESI-Q-TOF | ||
| Unknown | 1777.4 | 3 | 1 | 0.84±0.08* | LC-ESI-Q-TOF | ||
| Unknown | 1990.51 | 3 | 2 | 0.86±0.03** | LC-ESI-Q-TOF | ||
| Unknown | 2104.51 | 3 | 2 | 0.86±0.05* | LC-ESI-Q-TOF | ||
| Unknown | 2298.15 | 1 | 2 | 1.29±0.01** | MALDI-TOF | ||
| Unknown | 2367.60 | 4 | 1 | 0.77±0.11* | LC-ESI-Q-TOF |
Two peptides (MW 1174.52 or 1777.52/1777.4 in bold) were quantified at different charge states (+3 or +4) by LC-ESI-Q-TOF MS, and the results from two charge states are consistent. Peptides were quantified by either LC-ESI-Q-TOF MS or MALDI-TOF MS, with one peptide (MW 1174.52/1174.57 in italic) showing consistent quantitation results between two instruments. Two peptides from myelin basic protein were identified by tandem MS.
(.) indicates a cleavage site;
(#) indicates the end of a precursor sequence;
(_) highlights amino acids W, I and V; Obs. mass, observed monoisotopic mass (after subtraction of the labeling tags); Theor. mass, theoretical monoisotopic mass; Z, charge state; #T, number of tags labeling the peptide; WT, wild type; KO, knockout; SD, standard deviation; n, number of biological replicates observed for a peptide. Student’s t-test was carried out to determine the statistically significant difference between the peptide level of WT and KO mice.
p<0.01,
p<0.05.
DISCUSSION
ECE-2 has recently been proposed as a novel non-classical neuropeptide processing enzyme based on its tissue distribution, biochemical properties, and ability to cleave a number of neuropeptides at non-basic sites in vitro. Its role in vivo is beginning to be revealed with the use of the viable and fertile ECE-2 KO mouse. We have previously found that these mice exhibit deficits in learning and memory processes, which given ECE-2’s relatively high expression in the hippocampal dentate gyrus, suggests a role for this enzyme in processing peptides important in learning and memory (Rodriguiz et al. 2007). Here, we find that ECE-2 KO mice exhibit aberrant responses to morphine. Together with the relatively high expression of ECE-2 in the spinal cord (Rodriguiz et al. 2007) and altered levels of spinal cord peptides in ECE-2 KO mice, these findings imply a role for ECE-2 in the processing of neuropeptides that mediate morphine responses.
We have previously shown that ECE-2 can cleave a number of neuropeptides at non-classical sites in vitro, including opioid peptides and substance P (Mzhavia et al. 2003). Given that these and other neuropeptides play critical roles in pain and opiate responses (Wiesenfeld-Hallin and Xu 1996; Foran et al. 2000; Gaveriaux-Ruff and Kieffer 2002; Nitsche et al. 2002; Komatsu et al. 2009), we compared thermal thresholds and morphine responses in ECE-2 KO and WT mice. Although baseline pain responses in the hot-plate and tail-flick tests did not differ between genotypes, we found that deletion of ECE-2 led to altered morphine-induced analgesia. Specifically, the naive ECE-2 KO animals showed acute tolerance to a single injection of morphine compared to their WT controls. The mutants also demonstrated more rapid development of tolerance to repeated morphine injections. Thus, ECE-2 KO mice showed enhanced tolerance to acute and repeated morphine injections compared to WT controls, suggesting that physical dependence developed more early and rapidly in these mutants. However, after 6 consecutive days of repeated morphine injections the %MPE was virtually identical between the two genotypes, indicating that ECE-2 KO and WT mice had developed similar levels of tolerance to morphine by this time. Interestingly, withdrawal from morphine was much less pronounced in ECE-2 KO mice than WT. These findings suggest that physical dependence to morphine may be not be long-lasting in the ECE-2 KO mice, perhaps due preexisting alterations in the neuropeptide systems that mediate morphine tolerance and withdrawal responses. Alternatively, tolerance and withdrawal could occur as independent processes in ECE-2 KO mice. Future experiments will address these possibilities.
Multiple opioid-modulatory neuropeptides have been identified, and these include CCK (Wiesenfeld-Hallin and Xu 1996; Wiesenfeld-Hallin et al. 2002), neuropeptide FF (NPFF) (Yang et al. 2008; Mouledous et al. 2010), substance P (Foran et al. 2000; Komatsu et al. 2009), proenkephalin-derived peptides (Rady et al. 2001; Nitsche et al. 2002), nociceptin and dynorphin (Mika et al. 2011). Of these, ECE-2 is capable of cleaving substance P, proenkephalin- and prodynorphin-derived peptides in vitro (Mzhavia et al. 2003); cleavage of the other peptides has not yet been examined. Opioid-modulatory peptides have been found to regulate sensitivity and/or tolerance to opiates at spinal (based on intrathecal administration of peptides or analogs) and/or at supraspinal sites [intracerebroventricular or region-specific administration in the periaqueductal grey (PAG) or rostroventral medulla (RVM)]. Interestingly, the nature of the opioid-modulatory effect can vary with CNS region. For instance, NPFF and nociceptin potentiate morphine-induced analgesia in the spinal cord and attenuate the analgesia in the brain (Yang et al. 2008; Mouledous et al. 2010; Mika et al. 2011). By comparison Leu-enkephalin potentiates morphine-induced analgesia in the brain and attenuates it in the spinal cord (Lee et al. 1980; Rady et al. 2001). In contrast, CCK exters anti-opioid effects at both the spinal and supraspinal levels (Wiesenfeld-Hallin and Xu 1996; Wiesenfeld-Hallin et al. 2002). Although the pro-opioid effects of substance P are well known at the spinal cord (Foran et al. 2000; Komatsu et al. 2009), such effects have not yet been examined at supraspinal sites.
Intrigued by the alterations in morphine-induced analgesia in ECE-2 KO mice, we examined the levels of spinal cord peptides in ECE-2 KO mice using MS-based peptidomics. We identified fourteen peptides that were altered in KO spinal cords compared to WT, spinal cords suggesting that ECE-2 may play a role in the processing of these peptides. Two of these peptides were derived from myelin basic protein (MBP), which has previously been found to be processed at non-classical sties in vitro and in vivo by other enzymes, including calpain (Schaecher et al. 2001; Ottens et al. 2008) and cathepsin (Beck et al. 2001; Burster et al. 2004; Burster et al. 2007). Our results suggest that ECE-2 may have a role in processing MBP in the spinal cord. Since the two MBP-derived peptides showed increases in ECE-2 KO mice, implying reduced cleavage within the sequences; the cleavage site preferences of ECE-2 could not be inferred. However, both peptides contain aliphatic and/or aromatic residues previously found at P1’ sites of ECE-2 cleavage in vitro (namely Ile, Val, Trp) (Mzhavia et al. 2003). Notably, we did not detect any significant changes in classically-processed peptides in the spinal cord, supporting a role for ECE-2 in non-classical peptide processing. Work is currently underway to identify the unidentified peaks that showed significant differences between ECE-2 KO and WT animals; these results should lead to a better understanding of ECE-2 cleavage site preferences. The difficulty in identifying the majority of the peptides showing altered levels may be due to their low abundance, interference by other high-abundance proteins in the spinal cord, and/or the difficulty in fragmenting some peptides and generating high quality spectra by MS.
Myelin basic protein is known to be a cytosolic/nuclear protein, which raises the intriguing question as to the compartment in which ECE-2 might process MBPs. MBP has recently been shown to exhibit additional subcellular localization. Exosomes are small vesicles derived from the endocytic pathway that lead of secretion of proteins; release is regulated by potassium or calcium levels (Faure et al. 2006). Besides playing a role in discarding proteins, exosomes may also allow intercellular transport of proteins (Record et al. 2011). Oligodendrocytes have been shown to secrete major myelin proteins (such as MBP) through exosomes (Kramer-Albers et al. 2007). Exosomes could therefore be a possible site of MBP processing by ECE-2. While ECE-2 has been localized to endocytic compartments (Miller and Devi, unpublished), its localization in exosomes has yet to be investigated. ECE-2’s localization in endosomes also raises the possibility that it processes larger proteins (in addition to neuropeptides) in vesicles. Altered processing of larger proteins in the analgesia pathways (e.g. ion channels, receptors) could contribute to the altered morphine responses seen in ECE-2 KO mice.
Since Leu-enkephalin is known to affect morphine-induced analgesia (Lee et al. 1980; Rady et al. 2001), we specifically investigated levels in ECE-2 KO mice using RIA. No differences in the levels of Leu-enkephalin or its precursors were found in the spinal cords from ECE-2 KO and WT mice. Therefore, it appears that ECE-2 does not have a significant role in processing Leu-enkephalin precursors, at least under normal conditions. In addition, agonist-induced GTPγS binding studies failed to reveal any alterations in DOR activity in ECE-2 KO and WT spinal cords, in support of unchanged Leu-enkephalin levels.
It should be emphasized that our biochemical and proteomic studies were limited to the spinal cord; ECE-2 could also regulate morphine responses through its effects on neuropeptide levels in supraspinal regions. As stated previously, multiple opioid-modulatory peptides exist in supraspinal sites, including the PAG and RVM. Given that ECE-2 is widely expressed in the brain (Rodriguiz et al. 2007), it would be interesting in the future to investigate changes in the levels of opioid-modulatory peptides in additional brain regions of ECE-2 KO mice, particularly in the PAG and RVM.
Another point to be emphasized is that in these studies were conducted using “healthy” mice; ECE-2 could take on a greater role in pathological states where release of its peptide substrates might be increased or its expression or activity altered. Basal release of opioid-modulating neuropeptides is likely to be low in normal animals. For example, extracelluar levels of CCK are nearly undetectable in normal animals, but are increased up to three-fold in animals with chronic pain (Wiesenfeld-Hallin et al. 2002). ECE-2 expression or activity could also be altered in pain states. ECE-2 expression was found to be downregulated in late-onset Alzheimer’s patients (Weeraratna et al. 2007); however its levels and activity in other CNS pathologies have yet to be investigated. A unique role for ECE-2 in pathological states is also suggested by its acidic pH optimum, which would allow it to cleave peptides after secretion in acidified extracellular spaces (Emoto and Yanagisawa 1995). It would be interesting to repeat our biochemical and peptidomics studies in ECE-2 KO mice in an acute or chronic pain model, while simultaneously examining peptide levels at spinal and supraspinal sites (e.g. PAG and RVM).
In the present study we have continued to investigate of the role of the putative non-classical processing enzyme ECE-2 in vivo. We find striking differences in morphine-induced analgesia in ECE-2 KO mice, including more rapid development of tolerance and decreased withdrawal responses. The results of our peptidomics analyses revealed altered levels of a number of spinal cord peptides in ECE-2 KO mice, the first such evidence of ECE-2 peptide processing in vivo. Taken together with our previous findings of the learning and memory deficits in ECE-2 KO mice, the present results further support an emerging and important role for ECE-2 in non-classical peptide processing and behavior.
Supplementary Material
Acknowledgements
We wish to thank Ms. Jiechun Zhou and Liping Du for helping to maintain the mice at Duke University. This work was supported in part by grants from NIH NS026880 and DA0019521 (to LAD), P30 DA018310 to (XH and JSW). LKM is a trainee in the Integrated Pharmacological Sciences Training Program supported by grant T32GM062754 from the National Institute of General Medical Sciences. The authors have no actual or potential conflict of interest to disclose; this includes any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Abbreviations
- ECE-2
endothelin converting enzyme-2
- BAM
bovine adrenal medulla
- NEP
neprilysin
- ET-1
endothelin-1
- MS
mass spectrometry
- KO
knockout
- WT
wild-type
- MPE
maximal possible effect
- RIA
radioimmunoassay
- CPB
carboxypeptidase B
- ir
immunoreactive
- DOR
δ-opioid receptor
- ACN
acetonitrile
- FA
formic acid
- TFA
trifluoroacetic acid
- SA
succinic anhydride
- ESI
electrospray ionization source
- Q-TOF MS
quadrupole time-of-flight MS
- MALDI-TOF MS
matrix-assisted laser desorption/ionization coupled to time-of-flight MS
- DHB
2,5-dihydroxybenzoic acid
- CCK
cholecystokinin
- NPFF
neuropeptide FF
REFERENCES
- Beck H, Schwarz G, Schroter CJ, Deeg M, Baier D, Stevanovic S, Weber E, Driessen C, Kalbacher H. Cathepsin S and an asparagine-specific endoprotease dominate the proteolytic processing of human myelin basic protein in vitro. Eur J Immunol. 2001;31(12):3726–3736. doi: 10.1002/1521-4141(200112)31:12<3726::aid-immu3726>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22(23):10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20(24):9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora A, Annangudi SP, Millet LJ, Rubakhin SS, Forbes AJ, Kelleher NL, Gillette MU, Sweedler JV. Neuropeptidomics of the supraoptic rat nucleus. J Proteome Res. 2008;7(11):4992–5003. doi: 10.1021/pr800394e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann A, Annangudi SP, Richmond TA, Ament SA, Xie F, Southey BR, Rodriguez-Zas SR, Robinson GE, Sweedler JV. Quantitative peptidomics reveal brain peptide signatures of behavior. Proc Natl Acad Sci U S A. 2009;106(7):2383–2388. doi: 10.1073/pnas.0813021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burster T, Beck A, Poeschel S, Oren A, Baechle D, Reich M, Roetzschke O, Falk K, Boehm BO, Youssef S, Kalbacher H, Overkleeft H, Tolosa E, Driessen C. Interferon-gamma regulates cathepsin G activity in microglia-derived lysosomes and controls the proteolytic processing of myelin basic protein in vitro. Immunology. 2007;121(1):82–93. doi: 10.1111/j.1365-2567.2007.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burster T, Beck A, Tolosa E, Marin-Esteban V, Rotzschke O, Falk K, Lautwein A, Reich M, Brandenburg J, Schwarz G, Wiendl H, Melms A, Lehmann R, Stevanovic S, Kalbacher H, Driessen C. Cathepsin G, and not the asparagine-specific endoprotease, controls the processing of myelin basic protein in lysosomes from human B lymphocytes. J Immunol. 2004;172(9):5495–5503. doi: 10.4049/jimmunol.172.9.5495. [DOI] [PubMed] [Google Scholar]
- Che FY, Vathy I, Fricker LD. Quantitative peptidomics in mice: effect of cocaine treatment. J Mol Neurosci. 2006;28(3):265–275. doi: 10.1385/JMN:28:3:265. [DOI] [PubMed] [Google Scholar]
- Clarke S, Zimmer A, Zimmer AM, Hill RG, Kitchen I. Region selective up-regulation of micro-, delta- and kappa-opioid receptors but not opioid receptor-like 1 receptors in the brains of enkephalin and dynorphin knockout mice. Neuroscience. 2003;122(2):479–489. doi: 10.1016/j.neuroscience.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Collins JJ, 3rd, Hou X, Romanova EV, Lambrus BG, Miller CM, Saberi A, Sweedler JV, Newmark PA. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 2010;8(10):e1000509. doi: 10.1371/journal.pbio.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer's disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278(4):2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270(25):15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Foran SE, Carr DB, Lipkowski AW, Maszczynska I, Marchand JE, Misicka A, Beinborn M, Kopin AS, Kream RM. Inhibition of morphine tolerance development by a substance P-opioid peptide chimera. J Pharmacol Exp Ther. 2000;295(3):1142–1148. [PubMed] [Google Scholar]
- Fricker LD. Neuropeptide-processing enzymes: applications for drug discovery. AAPS J. 2005;7(2):E449–E455. doi: 10.1208/aapsj070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnidze K, Sachchidanand R, Rozenfeld M, Mezei M, Zhou M, Devi LA. Homology modeling and site-directed mutagenesis to identify selective inhibitors of endothelin-converting enzyme-2. J Med Chem. 2008;51(12):3378–3387. doi: 10.1021/jm7015478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Opioid receptor genes inactivated in mice: the highlights. Neuropeptides. 2002;36(2–3):62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- Gomes I, Filipovska J, Devi LA. Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods Mol Med. 2003;84:157–183. doi: 10.1385/1-59259-379-8:157. [DOI] [PubMed] [Google Scholar]
- Hollt V, Tulunay FC, Woo SK, Loh HH, Herz A. Opioid peptides derived from pro-enkephalin A but not that from pro-enkephalin B are substantial analgesics after administration into brain of mice. Eur J Pharmacol. 1982;85(3–4):355–356. doi: 10.1016/0014-2999(82)90226-6. [DOI] [PubMed] [Google Scholar]
- Jiang J, Huang J, Hong Y. Bovine adrenal medulla 22 reverses antinociceptive morphine tolerance in the rat. Behav Brain Res. 2006;168(1):167–171. doi: 10.1016/j.bbr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Sasaki M, Sanai K, Kuwahata H, Sakurada C, Tsuzuki M, Iwata Y, Sakurada S, Sakurada T. Intrathecal substance P augments morphine-induced antinociception: possible relevance in the production of substance P N-terminal fragments. Peptides. 2009;30(9):1689–1696. doi: 10.1016/j.peptides.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1(11):1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- Lee NM, Leybin L, Chang JK, Loh HH. Opiate and peptide interaction: effect of enkephalins on morphine analgesia. Eur J Pharmacol. 1980;68(2):181–185. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- Mika J, Obara I, Przewlocka B. The role of nociceptin and dynorphin in chronic pain: Implications of neuro-glial interaction. Neuropeptides. 2011 doi: 10.1016/j.npep.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Minamino N, Kangawa K, Matsuo H. A new endogenous opioid peptide from bovine adrenal medulla: isolation and amino acid sequence of a dodecapeptide (BAM-12P) Biochem Biophys Res Commun. 1980;95(4):1482–1488. doi: 10.1016/s0006-291x(80)80064-7. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Minamino N, Kangawa K, Matsuo H. A new family of endogenous big Met-enkephalins from bovine adrenal medulla: purification and structure of docosa- (BAM-22P) and eicosapeptide (BAM-20P) with very potent opiate activity. Biochem Biophys Res Commun. 1980;97(4):1283–1290. doi: 10.1016/s0006-291x(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Monroe EB, Annangudi SP, Hatcher NG, Gutstein HB, Rubakhin SS, Sweedler JV. SIMS and MALDI MS imaging of the spinal cord. Proteomics. 2008;8(18):3746–3754. doi: 10.1002/pmic.200800127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Mollereau C, Zajac JM. Opioid-modulating properties of the neuropeptide FF system. Biofactors. 2010;36(6):423–429. doi: 10.1002/biof.116. [DOI] [PubMed] [Google Scholar]
- Mzhavia N, Pan H, Che FY, Fricker LD, Devi LA. Characterization of endothelin-converting enzyme-2. Implication for a role in the nonclassical processing of regulatory peptides. J Biol Chem. 2003;278(17):14704–14711. doi: 10.1074/jbc.M211242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22(24):10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81(2):339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ottens AK, Golden EC, Bustamante L, Hayes RL, Denslow ND, Wang KK. Proteolysis of multiple myelin basic protein isoforms after neurotrauma: characterization by mass spectrometry. J Neurochem. 2008;104(5):1404–1414. doi: 10.1111/j.1471-4159.2007.05086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady JJ, Holmes BB, Tseng LF, Fujimoto JM. Inverse agonist action of Leu-enkephalin at delta (2)-opioid receptors mediates spinal antianalgesia. J Pharmacol Exp Ther. 2001;297(2):582–589. [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Gadnidze K, Ragnauth A, Dorr N, Yanagisawa M, Wetsel WC, Devi LA. Animals lacking endothelin-converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2007 doi: 10.1111/j.1601-183X.2007.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouille Y, Bianchi M, Irminger JC, Halban PA. Role of the prohormone convertase PC2 in the processing of proglucagon to glucagon. FEBS Lett. 1997;413(1):119–123. doi: 10.1016/s0014-5793(97)00892-2. [DOI] [PubMed] [Google Scholar]
- Rouille Y, Kantengwa S, Irminger JC, Halban PA. Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1. J Biol Chem. 1997;272(52):32810–32816. doi: 10.1074/jbc.272.52.32810. [DOI] [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Evidence for intracellular endothelin-converting enzyme-2 expression in cultured human vascular endothelial cells. Circ Res. 1999;84(8):891–896. doi: 10.1161/01.res.84.8.891. [DOI] [PubMed] [Google Scholar]
- Schaecher KE, Shields DC, Banik NL. Mechanism of myelin breakdown in experimental demyelination: a putative role for calpain. Neurochem Res. 2001;26(6):731–737. doi: 10.1023/a:1010903823668. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23(3):261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51(2):91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Kalehua A, Deleon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, 3rd, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer's disease tissues. Exp Cell Res. 2007;313(3):450–461. doi: 10.1016/j.yexcr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regul Pept. 1996;65(1):23–28. doi: 10.1016/0167-0115(96)00068-7. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Hokfelt T. The role of spinal cholecystokinin in chronic pain states. Pharmacol Toxicol. 2002;91(6):398–403. doi: 10.1034/j.1600-0773.2002.910619.x. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105(10):1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides. 2008;42(1):1–18. doi: 10.1016/j.npep.2007.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.