Abstract
A single dose of florfenicol (Nuflor®) was administered to eight healthy adult alpacas, at 20mg/kg IM (intramuscular) and 40mg/kg SC (subcutaneous) using a randomized, cross-over design and 28-day washout period. Subsequently, 40mg/kg florfenicol was injected SC every other day for 10 doses to evaluate long-term effects. Maximum plasma florfenicol concentrations (Cmax, measured via high-performance-liquid-chromatography) were achieved rapidly, leading to a higher Cmax of 4.31+/−3.03 μg/ml following administration of 20mg/kg IM than 40mg/kg SC (Cmax: 1.95+/−0.94 μg/ml). Multiple SC dosing at 48hr intervals achieved a Cmax of 4.48+/−1.28 μg/ml at steady state. The area under the curve and terminal elimination half-lives were 51.83+/−11.72μg/ml.h and 17.59+/−11.69 hours after single 20mg/kg IM administration, as well as 99.78+/−23.58μg/ml.h and 99.67+/−59.89 hours following 40mg/kg injection of florfenicol SC, respectively. Florfenicol decreased the following hematological parameters after repeated administration between weeks 0 and 3: total protein (6.38 vs. 5.61 g/dL, P<0.0001), globulin (2.76 vs. 2.16 g/dL, P<0.0003), albumin (3.61 vs. 3.48 g/dL, P=0.0038), white blood cell count (11.89 vs. 9.66 ×10^3/μL, P<0.044), and hematocrit (27.25 vs. 24.88%, P<0.0349). Significant clinical illness was observed in one alpaca. The lowest effective dose of florfenicol should thus be used in alpacas and limited to treatment of highly susceptible pathogens.
Keywords: camelids, Nuflor, adverse effects
INTRODUCTION
Alpacas significantly contribute to the non-food producing livestock population in the United States [with 211,413 registered alpacas by 2011] (Alpaca-Registry, 2011) and constitute an important percentage of patients in large-animal veterinary practice. Bacterial infections requiring long-term antibiotic therapy [such as pneumonia, peritonitis, sepsis, uterine and dental infection] (Smith, 1989; Niehaus & Anderson, 2007) are a significant cause of morbidity and mortality in this species. However, until now only six antimicrobials have been evaluated and subsequently proven to have favorable pharmacokinetics for therapeutic use (Christensen, Smith et al., 1996; Lackey, Belknap et al., 1996; Junkins, Boothe et al., 2003; Drew, Johnson et al., 2004; Gandolf, Papich et al., 2005). This paucity of pharmacological data currently hampers effective patient care and routinely causes practitioners to empirically treat camelids on the basis of dosage extrapolation from other species.
Bacterial isolates obtained from both llamas and alpacas have demonstrated favorable sensitivity patterns to the antimicrobial florfenicol (Anderson, 2009). For example, isolates from tooth root abscesses commonly include Actinomyces spp and Actinobacillus spp (Niehaus & Anderson, 2007), which are considered susceptible to the antibiotic florfenicol, based on breakpoints used for cattle (Clinical Laboratory Standards Institute, 2008). Furthermore, the bacteriostatic activity of florfenicol may prevail at relatively low drug concentrations, even in the face of chloramphenicol resistance (Graham, Palmer et al., 1988). Florfenicol is a broad-spectrum antibiotic with penetration into most body tissues including internal organs (Adams, Varma et al., 1987), skeletal muscle, milk (Soback, Paape et al., 1995), synovial fluid (Gilliam, Streeter et al., 2008) and to a lesser extent aqueous humor (Adams, Varma et al., 1987) and spinal fluid (de Craene, Deprez et al., 1997). Florfenicol has produced few adverse effects in calves, which included transiently decreased feed and water consumption, depression and soft stool consistency at high drug levels (3–5 × recommended dosage) (NuFlor®, 2009). Although similar safety studies have not been conducted in alpacas, florfenicol is potentially an effective choice for clinical patients and could fill a current void in veterinary camelid care.
The clinical use of florfenicol is based on studies in cattle (Lobell, Varma et al., 1994), sheep (Ali, Al-Qarawi et al., 2003), goats (Ali, Al-Qarawi et al., 2003), camels (Ali, Al-Qarawi et al., 2003), and elk (Alcorn, Dowling et al., 2004) and remains empirical in camelids. Florfenicol was administered to 6 of 123 alpacas with dental disease (20 mg/kg SC every 48 hours for 1 week), evaluated by Niehaus et al in a retrospective study (Niehaus & Anderson, 2007). Additionally, florfenicol was administered at 20 mg/kg SC every other day for 20 days for the treatment of tooth root abscesses in alpacas. The latter author considered this protocol superior to a 30-day standard 33,000 units/kg treatment with procaine penicillin G (Niehaus, 2009). However, species comparisons have documented lower serum concentrations of florfenicol in camels (Cmax=0.84μg/mL), a closer relative to the alpaca, versus feeder calves (Cmax=3.1μg/mL) and goats (Cmax=1.04μg/mL) after a single IM injection of 20 mg/kg florfenicol (Ali, Al-Qarawi et al., 2003; NuFlor®, 2009). Pharmacokinetic data may also be affected by the larger extracellular fluid compartment of alpacas, which is estimated to be 37% greater than in llamas, an even closer phylogenetic relative to alpacas than the camel. Unless there are specific pharmacokinetic studies to determine the optimum therapeutic dose there is a risk of producing subtherapeutic concentrations of florfenicol in alpacas if dosages are simply extrapolated from other animals. Subinhibitory concentrations of florfenicol have been shown to cause changes in bacterial morphology, (Blickwede, Valentin-Weigand et al., 2004) potentially contributing to greater antibacterial resistance. A species specific pharmacokinetic-pharmacodynamic [PK-PD] analysis is therefore needed to determine the optimum florfenicol dose necessary for therapeutic success and to prevent emergence of resistant bacteria.
The activity of florfenicol is believed to be time-dependent. Therefore, a long terminal half-life is favorable for treatment. In cattle, the half-life is longer after SC injection (64–80 hrs (Alcorn, Dowling et al., 2004)) than after IM injection (12.5–18.3 hrs (Soback, Paape et al., 1995), although IM administration may produce more complete absorption than SC delivery. Because alpacas have limited muscle mass and a potentially fractious temperament, SC injection would be preferred in the field. However, it currently remains speculative whether intramuscular administration may be necessary to optimize bioavailability and reduce variability in drug absorption. The purpose of this study is, therefore, to evaluate the pharmacokinetics and hematological effects of florfenicol (NuFlor®; Schering-Plough) following subcutaneous and intramuscular administration in healthy adult alpacas, following IM (20mg/kg) and SC (40mg/kg) dosing.
MATERIALS AND METHODS
Animals
Eight clinically healthy, client-owned adult alpacas (six males and two females; mean age: 4.25 ± 2.9 years) weighing 55 to 98 kg (mean weight ± SD = 70 ± 13.7 kg) were enrolled in this study. All animals were considered healthy based on clinical history, physical examination and hematology (complete blood count and serum chemistry analysis).
The alpacas were housed indoors during sample collection, with pasture turnout throughout the washout periods. Timothy hay and water were available ad libitum. Clinical parameters (attitude, appetite, fecal output, vital signs) were recorded twice daily throughout the study period. None of the alpacas were treated with antibiotics for one month prior to study enrolment. All procedures were approved by the Animal Care and Use Committee of the Cummings School of Veterinary Medicine at Tufts University and written client consent was obtained for each animal.
Animal Procedures
The alpacas were acclimated to the hospital for 12–18 hours prior to first sample collection. During this time all animals were outfitted with an aseptically placed long term jugular catheter (Milacath® Extended Use, Mila International, Inc., Erlanger, KY, USA), following IV xylazine sedation (AnaSed® Injection, Lloyd Laboratories, Shenandoah, IA; 0.1 mg/kg). All catheters were flushed with heparinized 0.9% NaCl solution (0.9% Sodium Chloride Injection USP, Baxter Healthcare Corporation, Deerfield, IL; with Heparin Sodium Injection, USP, 1000 USP Units/mL, APP Pharmaceuticals LLC, Schaumburg, IL; 1cc Heparin per 500 mL solution) every 6 hours and remained in position until the final sampling of each project phase.
Drug administration
Florfenicol injectable solution (300 mg/mL; Schering-Plough Corp., Kenilworth, NJ, USA) was administered once to 8 adult alpacas at 20 mg/kg IM (intramuscular) and 40 mg/kg SC (subcutaneous) using a randomized, cross-over design with a 28 day washout period between treatments. Following the single-dose studies, florfenicol was administered at 40 mg/kg SC every 48 hours for ten doses, in order to evaluate the effect of long term drug administration on hematological and clinical variables.
All injections were performed using a 20 gauge, 1 inch needle inserted SC over the lateral thorax or IM into the semimembranosus or semitendinosus muscle. The dose did not to exceed 10 ml per injection site.
Sample collection
Blood samples (5 mL) were collected from the jugular catheter after 20 mL of heparinized blood were withdrawn prior to each sample collection and subsequently returned to the subjects to prevent dilution effects. All samples were immediately placed into heparinized tubes (6mLVacuette® NH Sodium Heparin, Greiner Bio-One North America, Inc., Monroe, NC) and processed within one hour. Blood samples were collected prior to, and at 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, 18.0, 24.0, 36.0, 48.0, 60.0, 72.0, 96.0, 120.0 hrs post-injection for single-dose studies. Following long term administration of florfenicol at 40 mg/kg SC every 48 hours for 10 doses, samples were collected prior to the first and after the last dose. Post treatment samples were obtained at times 0 (prior to the last injection), 2, 4, and 8 hours after the last injection. These samples were collected to identify the plasma concentrations at steady-state. Plasma was separated following centrifugation (3000 g for 10 min), placed in 2.0 mL screw-top Cryogenic vials (Cryogenic Vials 2.0 mL, Sterile, Fisherbrand, Pittsburgh, PA, USA) and frozen at −80 °C until high-pressure liquid chromatography (HPLC) analysis. Blood (5 mL) was also collected on days 0 (day of drug administration), 2 and 4 following single dose Florfenicol injections, for complete blood count and chemistry analysis (IDEXX laboratories; North Grafton, MA). Similarly, the latter samples were obtained on days 0, 8, 16 and 24 after the initiation of long term drug administration.
Sample analysis
Plasma samples were assayed for florfenicol via HPLC, using methods developed in one of the author's (MGP) laboratory and modified from a validated assay used in a previous study (Gilliam, Streeter et al., 2008). The HPLC system consisted of a quaternary pump and degasser (Agilent 1100 series Quarternary Pump, Agilent Technologies, Wilmington, DE), automated sampler (Agilent 1100 series Autosampler, Agilent Technologies, Wilmington, DE), and UV detector (Agilent 1100 series Variable Wavelength Dectector, Agilent Technologies, Wilmington, DE). Plasma extraction was accomplished with solid phase hydrophilic-lipophilic balanced (HLB) extraction cartridges (Oasis extraction cartridges, Waters Corporation, Milford, MA), conditioned with 1 mL methanol followed by 1 mL distilled water. After addition of 500 μL of plasma sample to the cartridge, it was washed with 1.0 mL distilled water:methanol (95:5). The eluent was discarded. The final elution was achieved with the addition of 1.0 mL methanol into a clean glass tube. The eluate was evaporated in a hot water bath (45°C) for 20–25 minutes and reconstituted with 200 μL of mobile phase.
A reverse phase stable bond 4.6 mm × 15 cm C-8 column (Zorbax Eclipse XDB-C8 4.6 mm × 15 cm column; Agilent Technologies Wilmington, DE) heated to 40°C, achieved separation. The mobile phase consisted of 65% distilled water, and 35% acetonitrile. The UV detector was set to a wavelength of 223 nm. The volume for each injection was 30 μL. Retention time for florfenicol was 3.7–3.8 minutes. Chromatograms were integrated with computer software (1100 Series Chemstation software, Agilent Technologies, Wilmington, DE).
A stock solution of florfenicol was prepared by dissolving a pure analytical reference standard of florfenicol (Florfenicol reference standard donated by Intervet-Schering Plough Corporation) in acetonitrile at a concentration of 1 mg/mL and stored in the refrigerator. The analytical reference standard solution was used to make calibration standards and to fortify quality control (QC) samples. The 1 mg/mL stock solution was further diluted serially with distilled water to prepare spiking solutions for the calibration curve samples. The calibration curves for plasma analysis were prepared by fortifying pooled alpaca plasma with 20 μL of the diluted stock solutions to make seven calibration standards (including zero) of florfenicol. Concentrations in the calibration curve covered a linear dynamic range of 10 μg/mL to 0.05 μg/mL. Unfortified alpaca plasma was used as a blank, to verify that the assay contained no interfering compounds and to determine the background noise for the assay. The fortified calibration samples were processed and prepared exactly as described for the incurred samples. For each day's run a fresh set of calibration and blank samples were prepared. Calibration curves of peak height versus concentration were calculated by use of linear-regression analysis. All calibration curves were linear with an R2 value of 0.99 or higher. Limit of quantification (LOQ) for florfenicol in alpaca plasma was 0.05 μg/mL, which was determined from the lowest point on a linear calibration curve that produced an acceptable signal-to-noise ratio. The laboratory used guidelines published by the United States Pharmacopeia (2010).
Pharmacokinetic and statistical analysis
The florfenicol plasma concentration vs. time plot of each animal was analyzed using a non-compartmental analysis and commercial software (Phoenix WinNonlin 6.0, Pharsight Inc. Mountain View, CA). The area under the plasma concentration - time curve (AUC) was determined using the trapezoidal method. Peak plasma concentrations of florfenicol (Cmax), and times to reach peak concentration (Tmax) were obtained from the individual plasma concentration - time curves. The terminal half-life was calculated from the slope of points after Cmax was attained. The systemic clearance was calculated from the AUC and the mean residence time (MRT) was obtained from the ratio of area under the first moment curve (AUMC) to AUC. The apparent steady-state volumes of distribution (VDSS) were calculated from the AUMC and AUC. The comparative bioavailability (F) of the SC dose was determined by the ratio of the AUCSC / AUCIM and normalized for dose. Furthermore, the multiple dosing half-life (also referred to as the effective half-life) was calculated from: T ½MD = ln(2) · MRT. The accumulation index (AI), which predicts the accumulation after multiple doses at stead-state was calculated as: AI = 1/(1-e−k τ), where e is the base of the natural logarithm and τ is the dosing interval (48 hours). The actual observed accumulation index was determined from the ratio of Cmax at steady state to the Cmax after the first dose.
All results are presented as mean ± SD. Complete blood count and chemistry variables were compared between time points using repeated measures ANOVA and paired samples T-test post hoc. All statistical analyses were performed using statistical software (SPSS version 12, SPSS Inc, Chicago, Ill).
RESULTS
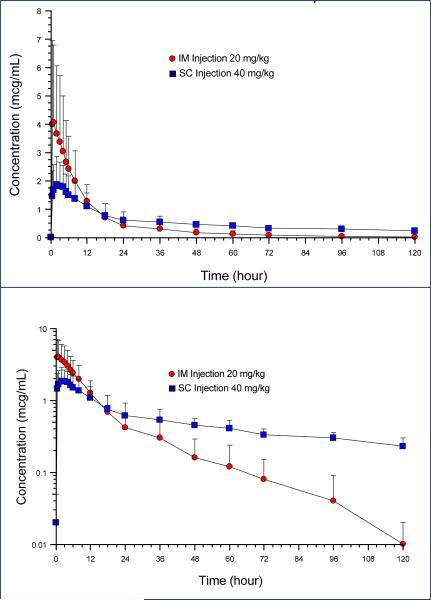
Maximum plasma florfenicol concentrations (Cmax) were reached rapidly, irrespective of route of administration, leading to a higher Cmax of 4.31+/−3.03 μg/ml following single 20mg/kg IM injection than 40mg/kg SC (Cmax: 1.95+/-0.94 μg/ml). Furthermore, a significantly shorter terminal half-life (T1/2) of florfenicol was observed subsequent to IM (17.59+/−11.69 hrs) versus SC administration (99.67+/−59.89 hrs). The plasma florfenicol levels consequently decreased below 1μg/ml within 14 hrs and 18 hrs after single injection of 20mg/kg IM and 40 mg/kg SC, respectively. All mean (+/− standard deviation) single-dose pharmacokinetic parameters are listed in Table 1 and displayed graphically in Figure 1. Based on the data collection interval, a higher percent of AUC was extrapolated after the SC dosing (33.8%) than after IM drug administration (3.5%). The comparative bioavailability (F) determined by the AUC ratios and normalized for the dose was 0.98 (±0.22).
Table 1.
Pharmacokinetic parameters of florfenicol administration in adult alpacas
| Florfenicol dose | 20 mg/kg IM | 40 mg/kg SQ | |
|---|---|---|---|
| Units | Mean (+/− SD) | Mean (+/− SD) | |
| Single dose parameters: | |||
| Maximum concentration (Cmax) | ug/mL | 4.31 (3.03) | 1.95 (0.94) |
| Time to Cmax (Tmax) | h | 1.00 (0.65) | 2.50 (1.07) |
| Area under the curve (AUC0-inf) | h*ug/mL | 51.83(11.72) | 99.78(23.58) |
| Volume of distribution (VD/F) | L/kg | 11.07 (8.14) | 55.74 (25.88) |
| Terminal Half-life (T1/2) | h | 17.59 (11.69) | 99.67 (59.89) |
| Mean residence time (MRT) | h | 21.01 (11.70) | 130.19 (80.00) |
| Clearance (CL/F) | mL/h/kg | 403.79 (92.23) | 422.33 (105.26) |
| Terminal rate constant (k) | 1/h | 0.10 (0.12) | 0.01 (0.01) |
| Multiple dose parameters: | |||
| Multiple dose half-life (T ½ MD) | h | 14.56 (8.11) | 90.24 (55.45) |
| Accumulation Index (AI) from 48 hr dosing interval | not done | 3.54 (1.78) |
Figure 1.
Pharmacokinetics of florfenicol in adult alpacas. Linear axis shown in the top panel and semi-logarithmic axis shown in the bottom panel.
Steady state pharmacokinetic parameters were obtained after repeated drug administration (40 mg/kg florfenicol, every 48 hrs SC for 10 doses), achieving a mean Cmax of 4.48 μg/mL (± 1.28) and Tmax of 2.5 hours (± 0.93). The calculated mean T ½MD was 90.24 (± 55.45) and 14.56 (± 8.11) after florfenicol administration of 40 mg/kg SC and 20 mg/kg IM, respectively. The AI predicted from the first dose of 40 mg/kg florfenicol SC was 3.54 (± 1.78); whereas the actual accumulation index calculated from Cmax ratios was 2.64 (± 1.11).
Transient, palpable swelling was noted following subcutaneous injection of florfenicol in all animals, with focal, dark discoloration of fleece at the site of needle placement in some light colored alpacas. Florfenicol was associated with significant changes in hematological parameters following single and repeated dosing (Table 2 and 3). Repeated drug administration (40 mg/kg florfenicol SC every 48 hrs for 10 doses) induced a significant reduction in the following hematological parameters between week 0 (baseline) and week 3 (time of last injection): total protein (6.38 vs. 5.61 g/dL, P<0.0001), globulin (2.76 vs. 2.16 g/dL, P<0.0003), albumin (3.61 vs. 3.48 g/dL, P=0.0038), white blood cell count (WBC: 11.89 vs. 9.66 ×10^3/μL, P<0.044), and hematocrit (Hct: 27.25 vs. 24.88%, P<0.0349).
Table 2.
Hematological parameters following single dose Nuflor injection
| [40mg/kg SC] Mean (+/− SD) | [20mg/kg IM] Mean (+/− SD) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 0 | Day 2 | Day 4 | |
| AST (U/L) | 168.1 (26.8) | 198.4 (60.3) | 308.5 (357.5) | 163.5 (35.8) | 212.3 (35.8) * | 411.4 (627.1) |
| Albumin (g/dL) | 3.81 (0.25) | 3.84 (0.27) | 3.84 (0.27) | 3.8 (0.47) | 3.65 (0.43) * | 3.7 (0.46) |
| Globulin (g/dL) | 2.59 (0.49) | 2.68 (0.54) | 2.79 (0.60) | 2.9 (0.67) | 2.86 (0.79) | 2.8 (0.68) |
| WBC (10^3/μL) | 14.81 (1.9) | 11.54 (2.7) * | 10.81 (1.9) * | 12.96 (2.74) | 11.98 (2.18) | 11.52 (1.80) |
| RBC (10^6/μL) | 10.91 (0.97) | 10.18 (0.95) | 11.38 (1.16) | 10.91 (0.74) | 10.23 (0.66) * | 10.1 (1.01) * |
| HCT (%) | 29.13 (2.59) | 26.88 (3.00) | 30.83 (3.60) | 28.25 (2.43) | 26.75 (2.38) | 29.52 (4.55) |
| Neutrophils | 9.05 (2.33) | 6.08 (2.8) * | 6.25 (1.39) * | 7.46 (2.66) | 6.37 (1.59) | 6.96 (1.34) |
| Lymphocytes | 3.97 (1.13) | 3.01 (0.97) * | 3.19 (1.61) | 3.53 (1.02) | 2.95 (0.56) | 2.89 (1.52) |
significant change from Day0 (P<0.05)
Table 3.
Hematological parameters following repeated 40 mg/kg SC Nuflor injection
| Week 0 | Week 1 | Week 2 | Week 3 (last injection) | Week 4 | |
|---|---|---|---|---|---|
| ALT (U/L) | 15.8 (7.5) | 18.8 (9.3) | 19.3 (9.1) | 19.3 (6.8) | 18.6 (6.1) |
| AST (U/L) | 183.5 (16.1) | 197.8 (44.8) | 217.6 (52.5) | 276 (170.5) | 288.3 (349.9) |
| Protein (g/dL) | 6.38 (0.58) | 5.40 (0.39) | 5.70 (0.34) | 5.61 (0.43) * | 5.06 (0.87) * |
| Albumin (g/dL) | 3.61 (0.41) | 3.51 (0.45) | 3.55 (0.31) | 3.48 (0.40) * | 3.19 (0.65) |
| Globulin (g/dL) | 2.76 (0.71) | 2.56 (0.64) | 2.40 (0.61) | 2.16 (0.48) * | 2.05 (0.59) * |
| Creatinine (mg/dL) | 1.39 (0.25) | 1.63 (0.38) | 1.56 (0.35) | 1.55 (0.33) | 1.64 (0.59) |
| HCT (%) | 27.25 (3.38) | 27.63 (2.13) | 27.00 (2.02) | 24.88 (2.23) * | 23.38 (3.07) * |
| WBC (10^3/μL) | 11.89 (1.95) | 10.83 (1.57) | 10.51 (1.93) | 9.66 (2.52) * | 11.39 (3.02) |
| Neutrophils (10^3/μL) | 6.32 (0.83) | 5.48 (0.86) | 6.05 (1.11) | 5.18 (1.73) | 6.0 (1.53) |
| Lymphocytes (10^3/μL) | 3.41 (1.8) | 3.21 (0.80) | 2.64 (1.49) | 2.6 (0.84) | 3.5 (1.16) |
AST: aspartate aminotransferase; ALT: Alanine transaminase
significant change between week 0 and week 3 [time of last injection], or week 0 and week 4 (P<0.05)
A mean weight loss of 1.12 kg (+/− 3.09) occurred between weeks 0 and 4 in alpacas following repeated SC florfenicol injections. Mucoid covered feces were noted on a single occasion in two animals during the last week of drug administration. Notable clinical illness was also observed in one alpaca during this time, including anorexia, volume depletion, lipemia and azotemia. The animal showed profound elevation in AST in the face of significant anemia and hypoproteinemia, but fully recovered following 2 weeks of supportive care. Proteinuria was not identified in any animals for which a free catch urine analysis was available during long term drug administration (6/8 alpacas; weeks 2–3).
DISCUSSION
The pharmacokinetic behavior of florfenicol differed significantly following injection of 20 mg/kg IM versus 40 mg/kg SC in adult alpacas. A higher mean plasma level and shorter terminal half life were observed following IM administration of the lower dose. These findings are similar to reported observations in cattle (Varma KJ, 1998; Schering-Plough-Animal-Health, 2008; NuFlor®, 2009) and are most likely related to slower drug absorption from the SC vs. IM space. In pharmacokinetic terms this is known as the “flip-flop” effect in which the terminal half-life is determined by the absorption from the injection site.
The observed maximum plasma concentration (mean Cmax) following SC drug administration was lower in alpacas than reported for other large animal species using comparable dosing regiments. The mean Cmax of previous pharmacokinetic analyses ranged from 5.36 μg/ml in beef calves (Varma KJ, 1998) to 4.69 μg/ml in feeder calves (Schering-Plough-Animal-Health, 2008), 3.7 μg/mL in elk (Alcorn, Dowling et al., 2004), and 2.6 μg/mL in sheep (Lane, Villarroel et al., 2004). Maximum plasma concentrations in alpacas following single-dose IM administration (Cmax: 4.31+/−3.03 μg/ml) compared more closely to observations in beef cattle (Cmax: 3.07 μg/ml [1.43–5.6]; (Lobell, Varma et al., 1994)). In comparison, off-label IM administration in other species has shown a lower mean Cmax in camels (0.84 μg/ml) (Ali, Al-Qarawi et al., 2003), goats (1.21 μg/ml) (Ali, Al-Qarawi et al., 2003) and sheep (1.04 μg/ml via microbiological assay) (Ali, Al-Qarawi et al., 2003). However, comparison of peak plasma drug concentrations among studies may not be reliable, because the determination of Cmax is dependent on the time of sampling after the dose is administered.
Elimination half lives of florfenicol after IM administration in alpacas (T½: 17.59+/−11.69 hrs) appeared similar to those reported in cattle (T½: 18.3 [8.3–44] hrs, (Lobell, Varma et al., 1994)). In contrast, florfenicol elimination following SC dosing (T½: 99.67+/−59.89 hrs) was prolonged in comparison to previous reports in sheep (T½: 34.7+/−9.6 hrs (Lane, Villarroel et al., 2004)) and elk (T½: 44+/−15 hrs (Alcorn, Dowling et al., 2004)) and demonstrated significant variability. Without further study, we cannot explain the long terminal half-life in this study following SC administration compared to the IM injection in alpacas. As this finding is most likely caused by the flip-flop phenomenon, we can only speculate that the composition of the SC space in alpacas may produce an extensive delay in absorption. It is unlikely that the extent of absorption was inhibited, as florfenicol bioavailability was comparable between SC and IM dosing, as determined by near unison of AUC ratios (F = 0.98 ±0.22). Instead, these data suggest that florfenicol is slowly absorbed from the SC space in alpacas, delaying and reducing peak plasma concentrations.
Florfenicol accumulated following repeated SC administration of 40 mg/kg every 48 hours for 10 doses. The accumulation index determined from the ratio of Cmax at steady-state to Cmax after the respective single dose was somewhat shorter than the calculated accumulation index (AI, 2.64 ±1.11 vs. 3.54 ±1.78). The reason for this discrepancy may be that the multiple dosing half-life (T ½ MD) was shorter than the single dose half-life (90.24 hours vs. 99.67 hours). Accumulation of florfenicol was expected at a dosing interval of 48 hours, as it was shorter than the drug's half-life. However, Cmax at steady state (4.48 ±1.28 μg/mL) following repeated 40 mg/kg SC administration in alpacas was comparable to maximum plasma concentrations observed following single 40 mg/kg SC dosing in beef calves (Cmax = 5.36 μg/mL; range, 2.7–18.7) and feeder calves (Cmax = 4.69 μg/mL, coefficient of variation 47.3%), which serve as a basis for the manufacturer's dosing recommendations in cattle (Varma KJ, 1998; Schering-Plough-Animal-Health, 2008).
Although cultures from camelids have been evaluated for sensitivity to florfenicol, (Anderson, 2009)(IDEXX laboratories, personal communication 2010) minimum inhibitatory concentrations (MICs) have not been published to date. However, several bacteria commonly cultured from cattle are also found in alpacas (Adams & Garry, 1992; Tibary, Fite et al., 2006; Dwan, Thompson et al., 2008), such as Staphylococcus aureus (MIC90: 6.25 μg/ml) (Yoshimura, Ishimaru et al., 2002), Mannheimia haemolytica (MIC90: 1–2 μg/ml) (Priebe & Schwarz, 2003), Salmonellae spp. (MIC: 4–8 μg/ml), Escherichia coli (MIC: 4–8 μg/ml), Streptococcus spp. (MIC: 1–4 μg/ml), Moraxella (MIC: 0.5–1 μg/ml) (Graham, Palmer et al., 1988) and Actinobacillus spp (MIC50: 0.39 μg/ml) (Yoshimura, Takagi et al., 2002), for which MICs have been established in ruminants. The CLSI breakpoint for bovine respiratory pathogens susceptible to florfenicol is ≤ 2 μg/mL (Clinical Laboratory Standards Institute, 2008). Therefore, isolates cultured with a MIC above 2 μg/mL would not be considered susceptible to florfenicol. Antimicrobial dosage regimen recommendations for bacteriostatic drugs are typically directed at maintaining plasma concentrations above MIC for bacterial pathogen throughout the dosing interval. The low mean Cmax of 1.95+/−0.9 μg/ml following single 40mg/kg SC florfenicol administration in alpacas indicates that effective concentrations may not be achieved from this dose and route for some bacteria. Repeated SC dosing at 48 hour intervals may be necessary to maintain the concentrations above the MIC for some bacteria. Intramuscular administration may be preferred to attain an initial high Cmax, as we demonstrated in this study. A dosing regimen that has not been explored, but deserves study, is a regimen of a single dose IM initially, followed by subsequent doses SC to maintain steady-state. Simply administering a higher dose is not recommended until it can be shown to be safe. As discussed below, high drug concentrations may lead to clinical side effects in some alpacas.
Repeated 40mg/kg SC florfenicol administration in alpacas was associated with a statistically significant reduction in total serum protein, albumin, globulin, white blood cell count (WBC) and hematocrit (Hct) over time. These findings may potentially indicate mild bone marrow suppression as reported for this drug's analogue, chloramphenicol (Yunis, 1973). Chloramphenicol is also known to inhibit protein synthesis in animals. We eliminated renal protein loss as a cause of the low protein because of the observed lack of proteinuria. The significant decrease in total protein and albumin may also indicate gastro-intestinal protein loss. These findings were supported by the development of mucoid covered feces in two alpacas and clinical illness (anorexia, lipemia, azotemia, depression, volume depletion) in one animal following multiple doses of florfenicol. The latter alpaca also achieved the highest Cmax of 6.8 μg/ml after 10 doses of 40 mg/kg florfenicol SC every 48 hours. These data suggest that florfenical may be unsafe for some alpacas at the tested dosing regimens of this study.
In conclusion, it is difficult to recommend an optimum dose and route for florfenicol based on the current investigation. Our data suggests that subcutaneous dosing of 40mg/kg florfenicol may be inadequate for some bacterial pathogens unless multiple doses are administered. On the other hand, there is evidence of adverse effects caused by florfenicol after repeated doses. Clearly, more information is needed on the susceptibility (MIC) of bacterial pathogens common to alpacas so that optimum doses based on PK-PD principles can be calculated from these data. Until such data is available, florfenicol therapy should be limited to highly susceptible pathogens in alpacas. Repeated SC administration of high drug doses is not recommended as significant adverse effects may be observed in some alpacas. Close hematological and clinical monitoring is advised in clinical patients.
ACKNOWLEDGMENTS
This project was funded by the Morris Animal Foundation, New England Alpaca Tours (private donors) and NIH summer student grant (T35DK07635). The authors would like to thank Dawn Meola, Brooke Bricen, Jen Quinones and Mike Anderson for providing technical assistance.
REFERENCES
- Adams PE, Varma KJ, Powers TE, Lamendola JF. Tissue concentrations and pharmacokinetics of florfenicol in male veal calves given repeated doses. Am J Vet Res. 1987;48(12):1725–1732. [PubMed] [Google Scholar]
- Adams R, Garry FB. Gram-negative bacterial infection in neonatal New World camelids: six cases (1985–1991) J Am Vet Med Assoc. 1992;201(9):1419–1424. [PubMed] [Google Scholar]
- Alcorn J, Dowling P, Woodbury M, Killeen R. Pharmacokinetics of florfenicol in North American elk (Cervus elaphus) J Vet Pharmacol Ther. 2004;27(5):289–292. doi: 10.1111/j.1365-2885.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- Ali BH, Al-Qarawi AA, Hashaad M. Comparative plasma pharmacokinetics and tolerance of florfenicol following intramuscular and intravenous administration to camels, sheep and goats. Vet Res Commun. 2003;27(6):475–483. doi: 10.1023/a:1025741724701. [DOI] [PubMed] [Google Scholar]
- Alpaca-Registry I. 2011 http://www.alpacaregistry.com/public/reports/alpacaus.
- Anderson D. Analysis of Antimicrobial Cultures from Llamas and Alpacas: A Review of 1821 Cultures (2001–2005). Proc Int. Camelid Health Care Conference; Corvallis, Oregon. 2009. [Google Scholar]
- Blickwede M, Valentin-Weigand P, Rohde M, Schwarz S. Effects of subinhibitory concentrations of florfenicol on morphology, growth, and viability of Staphylococcus aureus. J Vet Med B Infect Dis Vet Public Health. 2004;51(6):293–296. doi: 10.1111/j.1439-0450.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Christensen JM, Smith BB, Murdane SB, Hollingshead N. The disposition of five therapeutically important antimicrobial agents in llamas. J Vet Pharmacol Ther. 1996;19(6):431–438. doi: 10.1111/j.1365-2885.1996.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Clinical Laboratory Standards Institute, C. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals - Approved Standard. Wayne; PA: 2008. CLSI document M31-A3. [Google Scholar]
- De Craene BA, Deprez P, D'Haese E, Nelis HJ, Van den Bossche W, De Leenheer P. Pharmacokinetics of florfenicol in cerebrospinal fluid and plasma of calves. Antimicrob Agents Chemother. 1997;41(9):1991–1995. doi: 10.1128/aac.41.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew ML, Johnson L, Pugh D, Navarre CB, Taylor IT, Craigmill AL. Pharmacokinetics of ceftiofur in llamas and alpacas. J Vet Pharmacol Ther. 2004;27(1):13–20. doi: 10.1046/j.0140-7783.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- Dwan LW, Thompson H, Taylor DJ, Philbey AW. Laryngeal abscessation due to Mannheimia haemolytica in an alpaca (Vicugna pacos) cria. Vet Rec. 2008;163(4):124–125. doi: 10.1136/vr.163.4.124. [DOI] [PubMed] [Google Scholar]
- Gandolf AR, Papich MG, Bringardner AB, Atkinson MW. Pharmacokinetics after intravenous, subcutaneous, and oral administration of enrofloxacin to alpacas. Am J Vet Res. 2005;66(5):767–771. doi: 10.2460/ajvr.2005.66.767. [DOI] [PubMed] [Google Scholar]
- Gilliam JN, Streeter RN, Papich MG, Washburn KE, Payton ME. Pharmacokinetics of florfenicol in serum and synovial fluid after regional intravenous perfusion in the distal portion of the hind limb of adult cows. Am J Vet Res. 2008;69(8):997–1004. doi: 10.2460/ajvr.69.8.997. [DOI] [PubMed] [Google Scholar]
- Graham R, Palmer D, Pratt BC, Hart CA. In vitro activity of florphenicol. Eur J Clin Microbiol Infect Dis. 1988;7(5):691–694. doi: 10.1007/BF01964257. [DOI] [PubMed] [Google Scholar]
- Junkins K, Boothe DM, Jensen J, Herzog T, Chatfield J. Disposition of sulfadimethoxine in male llamas (Llama glama) after single intravenous and oral administrations. J Zoo Wildl Med. 2003;34(1):9–15. doi: 10.1638/1042-7260(2003)34[0009:DOSIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lackey MN, Belknap EB, Greco DS, Fettman MJ. Single intravenous and multiple dose pharmacokinetics of gentamicin in healthy llamas. Am J Vet Res. 1996;57(8):1193–1199. [PubMed] [Google Scholar]
- Lane VM, Villarroel A, Wetzlich SE, Clifford A, Taylor I, Craigmill AL. Intravenous and subcutaneous pharmacokinetics of florfenicol in sheep. J Vet Pharmacol Ther. 2004;27(4):191–196. doi: 10.1111/j.1365-2885.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- Lobell RD, Varma KJ, Johnson JC, Sams RA, Gerken DF, Ashcraft SM. Pharmacokinetics of florfenicol following intravenous and intramuscular doses to cattle. J Vet Pharmacol Ther. 1994;17(4):253–258. doi: 10.1111/j.1365-2885.1994.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Niehaus A. Dental disease in llamas and alpacas. Vet Clin North Am Food Anim Pract. 2009;25(2):281–293. doi: 10.1016/j.cvfa.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Niehaus AJ, Anderson DE. Tooth root abscesses in llamas and alpacas: 123 cases (1994–2005) J Am Vet Med Assoc. 2007;231(2):284–289. doi: 10.2460/javma.231.2.284. [DOI] [PubMed] [Google Scholar]
- NuFlor® 2009 http://intervetus.naccvp.com/?m=product_view&u=intervetus&p=intervetus&id=1047137.
- Priebe S, Schwarz S. In vitro activities of florfenicol against bovine and porcine respiratory tract pathogens. Antimicrob Agents Chemother. 2003;47(8):2703–2705. doi: 10.1128/AAC.47.8.2703-2705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schering-Plough-Animal-Health . Freedom of information summary. Original new animal drug application: Nuflor Gold injectable solution. Florfenicol (with 2-pyrrolidone and triacetin): Beef and Non-Lactating Dairy Cattle. Summit, NJ: 2008. Freedom of information summary. Original new animal drug application: Nuflor Gold injectable solution. Florfenicol (with 2-pyrrolidone and triacetin): Beef and Non-Lactating Dairy Cattle. [Google Scholar]
- Smith J. Noninfectious Diseases, Metabolic Diseases, Toxicities, and Neoplastic Diseases of South American Camelids. Vet Clin North Am: Food Animal Practice. 1989;5:101–143. doi: 10.1016/s0749-0720(15)31006-9. [DOI] [PubMed] [Google Scholar]
- Soback S, Paape MJ, Filep R, Varma KJ. Florfenicol pharmacokinetics in lactating cows after intravenous, intramuscular and intramammary administration. J Vet Pharmacol Ther. 1995;18(6):413–417. doi: 10.1111/j.1365-2885.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Tibary A, Fite C, Anouassi A, Sghiri A. Infectious causes of reproductive loss in camelids. Theriogenology. 2006;66(3):633–647. doi: 10.1016/j.theriogenology.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma KJ, L.P., Cosgrove SB, Rogers ER, Schering-Plough Animal Health Pharmacology Safety and Clinical Efficacy of Nuflor® (florfenicol) Following Subcutaneous Administration to Cattle. Cattle Practice. 1998;6(4):281–286. [Google Scholar]
- Yoshimura H, Ishimaru M, Kojima A. Minimum inhibitory concentrations of 20 antimicrobial agents against Staphylococcus aureus isolated from bovine intramammary infections in Japan. J Vet Med B Infect Dis Vet Public Health. 2002;49(9):457–460. doi: 10.1046/j.1439-0450.2002.t01-1-00593.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Takagi M, Ishimura M, Endoh YS. Comparative in vitro activity of antimicrobial agents against Actinobacillus pleuropneumoniae. Vet Res Commun. 2002;26(1)):11–19. doi: 10.1023/a:1013397419995. [DOI] [PubMed] [Google Scholar]
- Yunis AA. Chloramphenicol-induced bone marrow suppression. Semin Hematol. 1973;10(3):225–34. [PubMed] [Google Scholar]