Abstract
Mantle cell lymphoma (MCL) is a malignancy of mature B cells characterized by aberrant expression of cyclin D1 due to the translocation t(11;14). Epigenomic and genomic lesions in pathways regulating B-cell activation, cell cycle progression, protein homeostasis, DNA damage response, cell proliferation and apoptosis contribute to its pathogenesis. While patients typically respond to first-line chemotherapy, relapse is the rule resulting in a median survival of 5–7 years. The PI3K/AKT/mTOR appears as a key pathway in the pathogenesis and can be targeted with small molecules. Most experience is with mTOR inhibitors of the rapamycin class. Second-generation mTOR inhibitors and the PI3K inhibitor CAL-101 are novel options to more effectively target this pathway. Bruton’s tyrosine kinase inhibition by PCI-32765 has promising activity and indicates immunoreceptor signaling as a novel therapeutic target. Up to 50% of relapsed patients respond to the proteasome inhibitor bortezomib suggesting that MCL may be particularly sensitive to disruption of protein homeostasis and/or induction of oxidative stress. Recent work has focused on elucidating the mechanism of bortezomib-induced cytotoxicity and the development of second-generation proteasome inhibitors. DNA hypomethylating agents and histone deacetylase inhibitors effect epigenetic de-repression of aberrantly silenced genes. These epigenetic pharmaceuticals and HSP90 inhibitors can synergize with proteasome inhibitors. Finally, BH3 mimetics are emerging as tools to sensitize tumor cells to chemotherapy. Participation in clinical trials offers patients a chance to benefit from these advances and is essential to maintain the momentum of progress. Innovative trial designs may be needed to expedite the clinical development of these targeted agents.
Introduction
Mantle cell lymphoma represents a challenge for designing therapeutics targeting the causative lesions associated with its pathogenesis. First, there is considerable disease heterogeneity in both tumor biology and clinical outcome. This is further compounded by the variety of first-line treatments used in the absence of a commonly agreed upon standard of care. Newly diagnosed patients respond well to first-line therapy, but relapse is virtually certain, resulting in a median survival for most patients of 5–7 years1. Secondly, for many years the focus of investigation in MCL has been on cyclin D1-driven cell cycle dysregulation and aberrations in DNA damage pathways. However, recently multiple novel aberrant cellular and extracellular pathways have been identified at both genomic and epigenomic levels. There exists a pressing need for specific and well-tolerated agents to improve the depth of remission that could eventually lead to cure. Equally important is the development of agents that are effective in relapsed/refractory patients. Current preclinical and clinical trials are exploring an impressive breadth of agents targeting pathogenic pathways in the tumor as well as its micro-environment. Although the majority of these agents are designed to target a specific molecular lesion, off-target effects and cross-talk between molecular pathways are often unavoidable. Improvements in our understanding of the molecular biology of MCL will help in the precise application of these non-traditional agents and in the development of rational combination therapies. This review discusses many of the novel agents that target aberrant intracellular pathways while agents targeting the tumor micro-environment are covered elsewhere in this series.
Pathogenic lesions in MCL
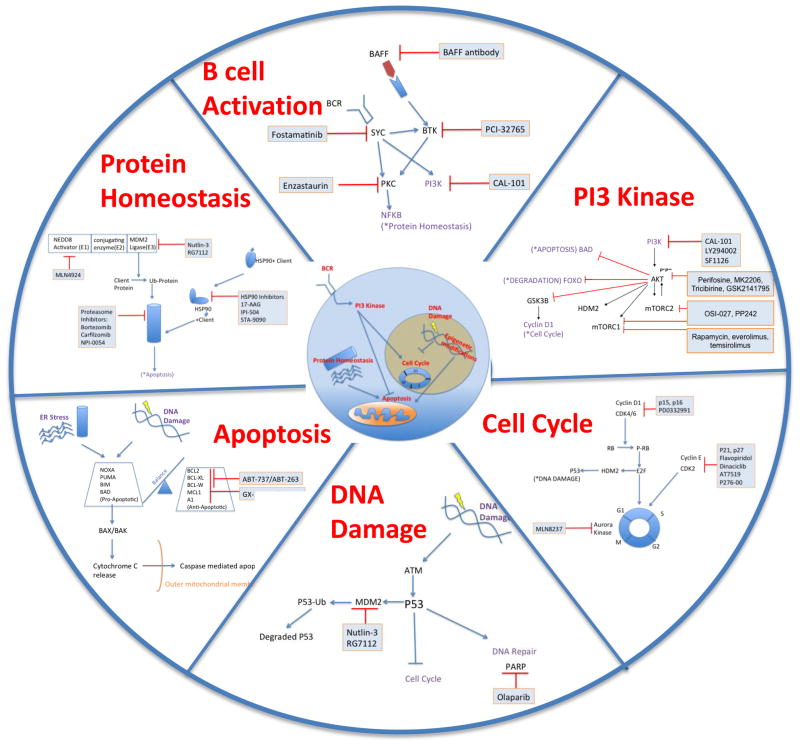
The translocation t(11;14) (q13;q32) leading to overexpression of cyclin D1 in the majority of cases is the diagnostic hallmark that led to the delineation of MCL as a separate entity 1. Early studies of MCL have emphasized cell cycle regulation as the key oncogenic event in this disease. More recently, genomic, epigenomic, and proteomic profiling of MCLs have demonstrated lesions in additional pathways likely contributing to its pathogenesis. We give a brief overview of disease relevant pathways and pathogenic mechanisms in Figure 1. Proteomic analyses of MCL cell lines indicated aberrant B-cell receptor (BCR) signaling 2,3, and studies have suggested a role for BAFF-dependent activation of MCL cells4,5. Alterations in PI3K, WNT and TGFβ signaling have been shown by gene expression profiling of primary MCL cells 6. Cell cycle regulation is disturbed on many levels; in addition to overexpression of cyclin D1, upregulation of CDK4/6 and loss of inhibitory molecules such as p16 are common 7,8. Mutations in tumor suppressors p53 and ATM attenuate DNA damage response 9. Disordered protein homeostasis and imbalances in pro- and anti-apoptotic proteins have been demonstrated in MCL (summarized in 1). Epigenomic changes in DNA methylation and histone modifications can cause genomic instability, resulting in the aberrant expression of oncogenes or repression of tumor suppressor genes, concurrently contributing to the pathogenesis of MCL10,11.
Fig. 1.
Major cellular pathways for targeted therapy of MCL. Intracellular location of aberrant pathways is shown in the center. Around this, individual pathways are highlighted. Pathway-specific inhibitors (mostly limited to clinical grade inhibitors) are boxed. Activating connections are indicated by arrows, inhibitory effects are depicted by lines.
Targeting B-cell activation
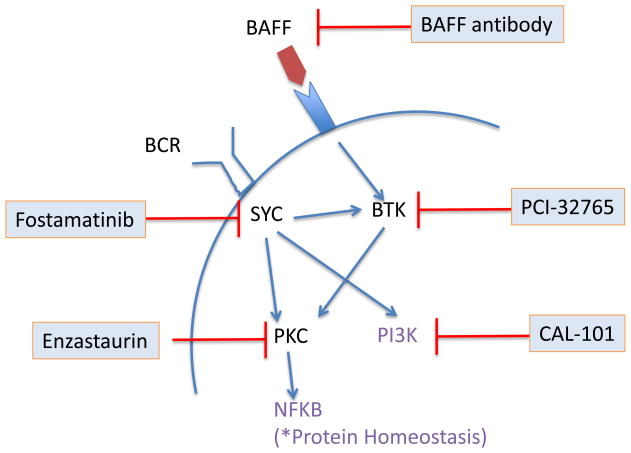
B-cell receptor (BCR) activation is emerging as a key pathway in some B-cell malignancies. BCR oligomerization initiates signaling through the phosphorylation of tyrosine residues in the Immunoglobulin family Tyrosine-based Activation Motifs (ITAMs) of immunoglobulin (Ig) α and β in a concerted action involving LYN, spleen tyrosine kinase (SYK) and Bruton’s tyrosine kinase (BTK) (Fig. 2) 12. Some MCL cell lines express constitutively active forms of the BCR signaling intermediates SYK, BTK, and PKCβ and are sensitive to the SYK inhibitor piceatannol 3. However, inhibition of SYK with fostamatinib and PKCβ with enzastaurin induced rare or no objective responses in MCL patients. In contrast, a phase I study of the BTK inhibitor PCI-32765 reported an overall response rate (ORR) of 43% across lymphoma subtypes with partial responses (PRs) in 3 of 4 MCL patients.13 The B-cell activating factor (BAFF) is a member of the TNF family that potently induces proliferation and survival of B cells via PKC- and NFκB-dependent pathways upon binding to the cognate BAFF receptor. In MCL cells autocrine secretion of BAFF appears to mediate a pro-survival effect that can be blocked with a BAFF-neutralizing antibody in vitro 4,5. The BAFF-neutralizing antibody LY2127399 in combination with bortezomib induced PRs in 11 of 20 patients with relapsed myeloma 14 and may be worth studying in MCL.
Fig. 2.
Targeting B-cell activation. The B-cell receptor signaling pathway is initiated through phosphorylation of co-receptors Igα (CD79a) and Igβ (CD79b), recruiting the tyrosine kinase SYK. In turn, SYK phosphorylates several downstream kinases including BTK and PI3Kδ. BAFF receptor signaling involves cross-talk with BCR and also results in activation of NFκB. Targeted inhibitors used in clinical trials are shown.
BCR activation can also induce activation of the Janus kinase (JAK)-STAT (signal transducer and activator of transcription) pathway that regulates growth, proliferation, differentiation and survival 15. In MCL, 47% of nodal cases 16 and 70% of leukemic cases were found to express the active, phosphorylated form of STAT-3 17. The treatment of primary MCL cells ex vivo with the JAK/STAT inhibitors AG490 or degrasyn reduced levels of phospho-STAT3 and induced apoptosis 17. Moreover, degrasyn in combination with bortezomib synergistically killed tumor cells in an MCL mouse model 18. The oral JAK-2 inhibitor SB1518 is completing phase I testing. At the end of the dose escalation phase the ORR was 12% across multiple lymphoma subtypes but remarkably 2 of 3 MCL patients achieved PRs.19
Targeting the PI3K/AKT/mTOR pathway
PI3 kinase inhibitors
The lipid PI3 kinase is essential for the survival of B cells. Constitutively active PI3K, but not NFκB or MEK rescued BCR-deficient B cells from apoptosis in genetic complementation studies20. PI3Ks generate second messenger phosphatidyl-inositol-3,4,5-phosphate to recruit and activate PDK and AKT kinases (Figure 3). Constitutive activation of PI3Ks is a key survival pathway in many cancers, including MCL (reviewed in 21,1). MCL tumors frequently express the inactive, phosphorylated form of PTEN, the cellular PI3K antagonist, thereby contributing to constitutive PI3K signaling. PI3Ks fall into different classes based on structure, composition and substrate specificity. Members of the class IA form heterodimers that include catalytic subunits p110α (PIK3CA), p110β (PIK3CB) and p110δ (PIK3CD) and smaller regulatory subunits. The expression of the p110δ is restricted to hematopoietic cells, and its ectopic expression transforms cells in vitro 22. Recently, the PI3Kδ selective inhibitor CAL-101 has been shown to exert potent antitumor effects across a range of B-cell malignancies 23. In a phase I study of single-agent CAL-101 the ORR was 62% (10/16) in relapsed or refractory MCL 24. SF1126 is a prodrug of LY294002, a first-generation pan-PI3K inhibitor that is not suitable for in vivo application but has been used extensively in vitro. SF1126 has improved pharmacokinetic properties and inhibits serine 473 phosphorylation of AKT in CLL cells from patients undergoing treatment. SF1126 is currently in early development for CLL and NHL 25.
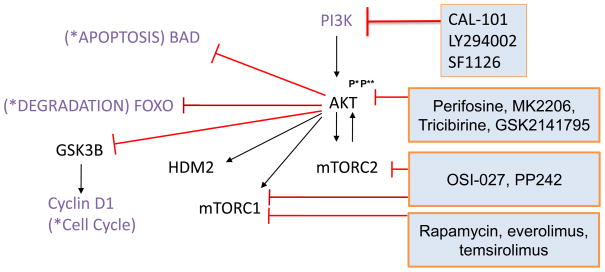
Fig. 3.
Targeting the PI3K/AKT/mTOR pathway. PI3 kinases, heterodimers composed of p85 regulatory and p110 catalytic subunits, activate AKT. AKT activation requires both phosphorylation at threonine 308(P*) and at serine 473(P**) in order to activate mTOR-containing complexes mTORC1 and mTORC2 and HDM2 or inhibit FOXO, GSK3β, and BAD. Several small molecules targeting the PI3K/AKT/mTOR pathway at different levels are indicated in blue boxes.
AKT kinase inhibitors
AKT is recruited by PIP3 to the plasma membrane and phosphorylated at threonine 308 by PDK1. AKT however requires also phosphorylation at serine 473 by mTORC2 (Fig. 3). The AKT inhibitor perifosine targets the pleckstrin homology domain, which prevents AKT from binding to PIP3 and its translocation to the plasma membrane 26. The treatment of solid tumors and hematological malignancies with single-agent perifosine resulted in few and modest responses 27,28. MK2206 is an allosteric inhibitor of AKT kinase that in combination with rapamycin synergistically killed diffuse large B-cell lymphoma cell lines in vitro 29. Phase II trials with single-agent MK2206 are ongoing in patients with relapsed and refractory lymphoma. Other AKT inhibitors in clinical development include tricibirine and GSK2141795 30. Notably, the protease inhibitor nelfinavir used in HIV therapy, also prominently inhibits AKT signaling, and was similarly able to synergize with rapamycin 29.
mTOR kinase inhibitors
The mTOR kinase is the catalytic component of the complexes mTORC1 and mTORC2. Their different composition accounts for not only distinct cellular functions but also for differential sensitivity to pharmacological intervention. Biologically, mTORC1 regulates protein synthesis by phosphorylating proteins of the translation machinery such as 4E binding protein and S6 kinase. The main substrates of mTORC2 are AKT and related kinases (Fig. 3) 31. First discovered as a bacterial product with immunosuppressive function, rapamycin (sirolimus) and analogs such as temsirolimus (CCI-779) and everolimus (RAD001) allosterically inhibit only mTORC1 but not mTORC2. Given that mTORC2 activates AKT and thereby antagonizes some of the antitumor effects, this pharmacological difference may explain some limitations in clinical activity of rapalogs. Nevertheless, single-agent temsirolimus induced responses in up to 40% of patients with relapsed or refractory MCL, with <5% CRs 32,33. A phase III study of single-agent temsirolimus compared with physician s-choice monotherapies found a superior ORR of 22% in the group with the highest temsirolimus dose as compared to 2% for alternative agents 34. These results led to the approval of temsirolimus by the European Medicines Agency for the treatment of relapsed and refractory MCL. Furthermore, the PILLAR-1 study reported PRs with Everolimus in 12% of patients refractory or intolerant to bortezomib (n=26) 35, and the SAKK 36/06 study reported two CRs (6%) and five PRs (14%; n=35) 36. The median progression free survival (PFS) and duration of response (DOR) were between 4–7 months 32–34. Temsirolimus has also been combined with rituximab in relapsed or refractory patients with an ORR of 59% (41 of 69 patients)-13 (19%) patients had CRs and 28 (41%) had PRs. The ORR was 63% (30 of 48; 95% CI 47–76) for rituximab-sensitive patients, and 52% (11 of 21; 30–74) for rituximab-refractory patients 37.
mTOR kinase inhibitors that target both mTORC1 and mTORC2 complexes are being developed since combined inhibition is thought to increase efficacy 38. PP242 reversibly targets the ATP-binding site of mTOR and has preclinical activity in acute leukemia 39,40. Another dual mTOR inhibitor is OSI-027 that inhibits the phosphorylation of AKT targets such as FOXO3A and BAD 41. OSI-027 induced apoptosis in MCL cell lines and primary MCL cells ex vivo that were resistant to rapamycin. OSI-027 is currently being tested in a phase I study in patients with solid tumors or lymphoma.
Targeting the cell cycle
Cyclins are periodically expressed during the cell cycle to regulate the activity of holoenzymes formed with cyclin-dependent kinases (CDKs). The majority of CDKs promote cell cycle progression such as CDK4 and CDK6, whereas CDK7 and CDK9 primarily function to regulate transcription by phosphorylating the C-terminal domain of RNA polymerase II 42. Cyclin D1 primarily binds to CDK4 and CDK6. Inactivation of endogenous cyclin-dependent kinase inhibitors such as p16/ARF by genomic loss 8 or epigenetic modification 7 contributes to the dysregulated cell cycle and increased proliferation seen in MCL (Figure 4). The knockdown of cyclin D1 using small hairpin RNA barely affected the viability of MCL cells in vitro due to induction and compensatory effect of other D-type cyclins D2 and D3 43. Likewise, pharmacologic inhibition of CDK4 and CDK6 using the pyridopyrimidine PD0332991 had no effect on cell viability but potently inhibited proliferation of MCL cells in vitro 44. PD0332991-mediated synchronization of MCL cells in S phase markedly sensitized for killing by other drugs at reduced doses, including the proteasome inhibitor bortezomib and cytosine arabinoside45. In a single-agent phase I trial of PD0332991, 1/17 relapsed MCL patients achieved a CR lasting >2 years; two subjects achieved PRs lasting >2 years and 7/17 demonstrated SD. The degree of reduction of phospho-Rb was correlated with that of Ki67, as well as with percent change in SUVmax on FLT Scans 46.
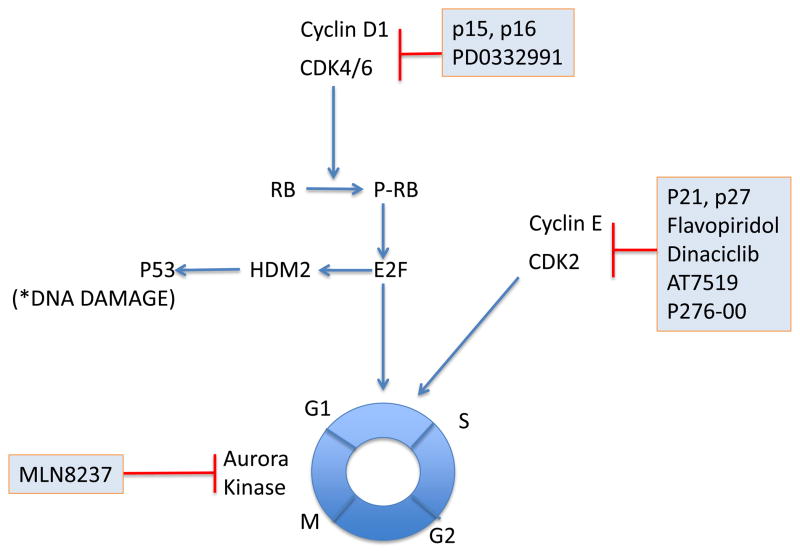
Fig. 4.
Targeting the cell cycle. The Cyclin D1-CDK4/6 complex phosphorylates Rb and promotes G1/S phase transition of the cell cycle. Endogenous proteins p15/16 suppress this complex as do the pharmacologic inhibitors shown in blue boxes. Pan-CDK inhibitors regulate transcription through CDK7, 9 and 10 in addition to cell cycle inhibition.
One of the first pan-CDK inhibitors to be tested in the clinic is flavopiridol (alvocidib, HMR-1275). Flavopiridol not only inhibited cell cycle progression but in addition inhibits RNA synthesis by interfering with RNA polymerase II 42. However, early clinical trials in untreated or relapsed MCL have shown unsatisfactory results with minimal antitumor activity 47. This may be because flavopiridol is highly protein bound in human plasma. A modified dosing schedule consisting of a 30-minute bolus dose followed by a 4-hour infusion provided sustained concentrations of free drug and induced responses in 45% of patients with CLL. Infusional flavopiridol in combination with two highly active drugs fludarabine and rituximab had an ORR of 80% (7 CRs, 1 PR) in relapsed MCL 48. Other broad CDK inhibitors include P276-00, a second generation synthetic flavone, SCH 727965 (dinaciclib), and AT7519. These compounds also primarily appear to exert their antitumor effects through additional mechanisms beyond cell cycle inhibition 49–51.
Aurora kinases are serine/threonine kinases that are essential for cell proliferation52. They play a crucial role during the prophase of mitosis by controlling chromatid segregation. A tissue microarray (TMA) composed of 20 MCL patients demonstrated >75% of patients had high levels of Aurora Kinase A and B expression. MLN8237, an Aurora A/B kinase inhibitor induced G2/M arrest with polyploidy, inhibited cell proliferation at an IC50 of 10–50 nM and synergistically induced apoptosis with docetaxel in MCL cell lines and xenograft models53. Clinical trials of MLN8237 in relapsed refractory MCL patients are currently ongoing.
Targeting DNA damage response pathways
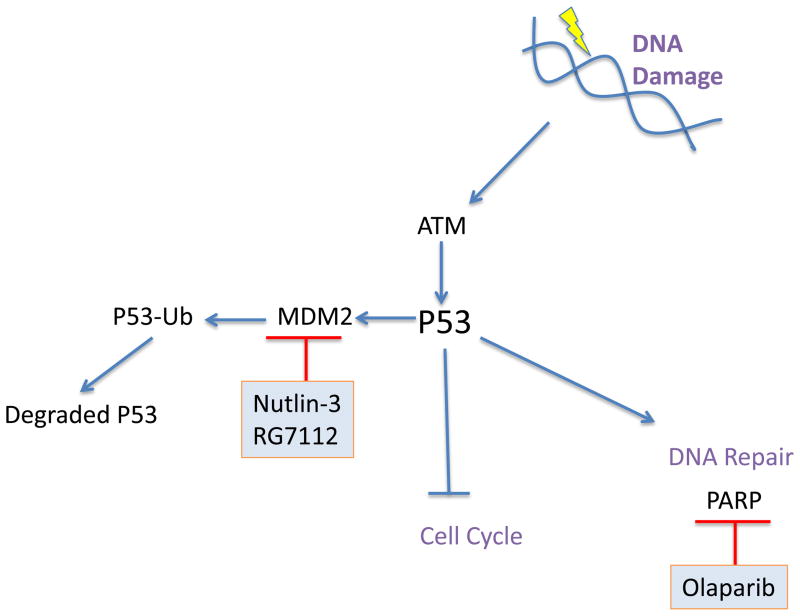
Exogenous (ionizing radiations, chemotherapeutics, chemicals) and endogenous (oxidative stress, replication) factors can induce DNA double-strand breaks. The phosphoprotein ATM signals the presence of double-strand breaks to the cell cycle checkpoints preventing the cell from further divisions in the presence of DNA damage54. While CHK1 and CHK2 mediate the arrest at the G2/M checkpoint, p53 links ATM to the G1/S checkpoint (Fig. 5). ATM is deleted in approximately 20% to 40% of MCL patients 55. Downstream of ATM, p53 can stop the cell cycle, through p21 activation allowing DNA repair. p53 inactivation, as a consequence of deletion or mutations, is more frequent in the blastic variant than in classic type 9. HDM2, an E3 ubiquitin ligase targets p53 for proteasomal degradation. A common alternative mechanism to p53 inactivation, is the overexpression of HDM2, which correlates with inferior survival.1 The treatment of MCL cells with the HDM2 inhibitor nutlin-3 restored p53 activity and induced cell cycle arrest and apoptosis in vitro.56,57 RG7112 is a clinical grade inhibitor of HDM2 that has improved potency and pharmacological properties compared to nutlin-3. RG7112 treatment stabilizes p53 protein and induces p53 target genes including p21, BAX, NOXA, PUMA and FAS. A phase I study of 47 patients with relapsed or refractory leukemia has reported one CR in a patient with acute leukemia and tumor regression in patients with CLL/SLL.58
Fig. 5.
Targeting DNA Damage Response proteins. Exogenous and endogenous stress can activate the DNA damage sensor ATM, which is frequently mutated or deleted in primary MCL. ATM in turn activates p53, which stops cell cycle progression and activates DNA repair mechanisms. Pharmacologic inhibitors of MDM2, which degrades p53; and PARP, which aids in DNA repair, are shown in blue.
Poly(ADP-ribose) polymerase 1 (PARP) is best known for its function in DNA damage response and repair, and is required to maintain genomic integrity. Pharmacological inhibition of PARP activity has become an interesting therapeutic strategy as it can achieve “synthetic lethality” in tumors with dysfunctional DNA repair mechanisms.59 Notably, MCL tumors display a high degree of genomic instability as well as molecular lesions in DNA repair proteins, for example ATM, suggesting that PARP inhibition is an attractive therapeutic approach for the treatment of MCL. Different PARP inhibitors are in advanced clinical development mostly for solid tumors but some studies include lymphoma as well 59. The PARP inhibitor olaparib (AZD-2281/KU-0059436) has significant in vitro and in vivo activity against MCL cells with dysfunctional ATM 60. PARP inhibitor AG014699 in combination with CDK1 inhibitor AG024322 reduced tumor growth and prolonged survival in a mouse model of lung adenocarcinoma 61. AG014699 is currently in clinical testing.
Targeting regulatory proteins of cell death
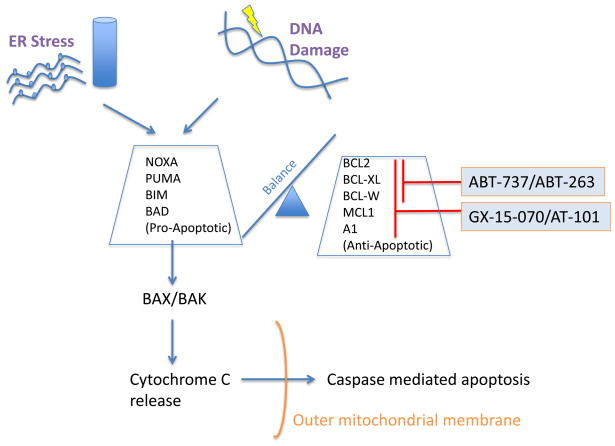
B lymphocytes are prone to apoptosis during their maturation in the lymph nodes. Escaping programmed cell death is therefore a key event in the development of B-cell malignancies. Members of the BCL-2 family can either inhibit or promote apoptotic cell death 62 (Fig. 6). Structurally, anti-apoptotic members including BCL-2, BCL-XL, BCL-W, A1 and MCL-1 harbor two or more of four different types of so-called BCL-2 homology (BH) domains. The pro-apoptotic molecules BIM, BID, PUMA, BAD, and NOXA only contain a BH3 domain. These BH3-only proteins bind to anti-apoptotic proteins and cause the release of BAX and BAK that are then free to trigger apoptosis. Differences in substrate specificity of these BH3-only proteins lead to selective targeting of pro-survival BCL-2 family members. For example, whereas BIM, BID and PUMA inhibit all five anti-apoptotic family members, BAD selectively inhibits BCL-2, BCL-XL, BCL-W, and NOXA selectively targets A1 and MCL-1 (Fig. 6) 63. MCL cells have increased expression of anti-apoptotic proteins BCL-2, MCL-1, and BCL-XL, and loss of BIM is common (reviewed in 1,21).
Fig. 6.
Targeting BCL-2 family proteins. The BCL-2 proteins BCL-2, BCL-XL, BCL-W, MCL-1, and A1 are anti-apoptotic proteins that sequester the apoptotic effectors BAX and BAK. Pro-apoptotic proteins BIM, BID, PUMA antagonize the function of all BCL-2 proteins. BAD specifically antagonizes BCL-2, BCL-XL and BCL-W whereas NOXA antagonizes MCL-1 and A1. The small molecule BH-3 mimetics GX15-070 and AT-101 are pan-BCL-2 inhibitors, in contrast to ABT-737/ABT-263, which specifically inhibit BCL-2, BCL-XL and BCL-W but not MCL-1 and A1.
Targeting anti-apoptotic molecules that mimic the activity of BH3-only proteins is emerging as a promising therapeutic strategy in MCL 64. Since the mode of action of these so-called BH3 mimetics is similar to that of BH3-only proteins, they display different substrate specificity towards BCL-2 family members (Fig. 6). Obatoclax (GX15-070) and AT-101 are pan-BCL-2 inhibitor that mimics both BAD- and NOXA-like activities. In contrast, ABT-737 and its analog ABT-263 (navitoclax) have BAD-like specificity with high affinity for BCL-2 and BCL-XL but cannot target MCL-1 and A1. The restricted substrate activity therefore may somewhat limit their antitumor activity. For instance, MCL cell lines and primary tumor cells with high expression of BCL-2 relative to MCL-1 were sensitive to ABT-737, in contrast to cells with high MCL-1 expression that were resistant 65. Consistently, upregulation of MCL-1 or A1 has been found to confer resistance to ABT-737 in lymphoma cell lines 66.
In clinical studies, ABT-263 has been well tolerated in relapsed NHL and CLL and achieving an ORR of 22% 67. An objective reduction in lymphadenopathy was noted in 46% of patients. However, no responses were seen in the 4 MCL patients. In contrast, GX15-070, a pan-BCL-2 inhibitor, had only modest single-agent activity in patients with CLL 68. AT-101 has recently completed a phase II study as a single agent in patients with relapsed or refractory B-cell malignancies including MCL. Given their ability to sensitize cells to the effect of chemotherapy, radiation, and possibly immunotherapy the best use of these agents may be in combination therapies and many such trials are ongoing. Moreover, BH3 mimetics that antagonize MCL-1 may be particularly useful to overcome chemoresistance mediated by tumor-microenvironment interactions 69,70.
Targeting cellular response to stress
Cell homeostasis has emerged as a key system that can be disrupted on several levels to selectively kill cancer cells. Many oncoproteins increase cell proliferation and metabolic activity causing increased cellular stress in transformed cells. While tumor cells adapt to these conditions in order to ensure cell survival, many homeostatic pathways are utilized to capacity71. Thus, tumor cells are often more susceptible to pharmacologic disruption of homeostatic processes than normal cells (Fig. 7).
Fig. 7.
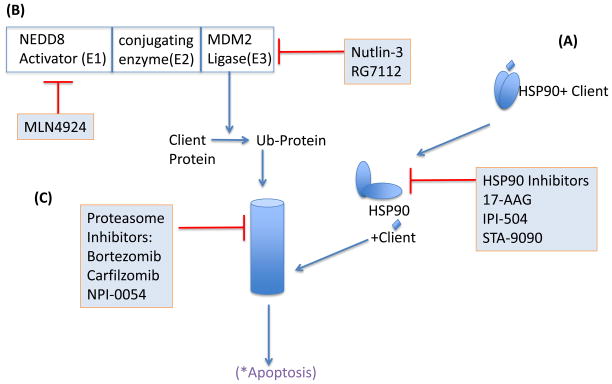
Targeting regulators of protein homeostasis. A) Small ubiquitin-like proteins are sequentially conjugated to protein substrates and affect their localization, function and degradation. E1 enzymes such as the NEDD8-activating enzyme activate small NEDD8 proteins, which are then conjugated and ligated to acceptor proteins by E2 and E3 enzymes, respectively. NEDD8ylation of cullin proteins is essential for function of multi-protein cullin-RING (E3 ubiquitin) ligase complexes. Inhibition of the E1 NEDD8-activating enzyme leads to inhibition of the SCF complex. Inhibition of the E3 ubiquitin ligase HDM2, leads to stabilization of select proteins, e.g., tumor suppressor protein p53. B) HSP90 requires dimerization, mediated through the C-terminal domain, for full chaperone function. Blocking ATP-binding at the N-terminal domain, interferes with HSP90 dimerization and prevents chaperoning of client proteins resulting in their proteasomal degradation. C) Proteins are marked with Lys48-linked ubiquitin chains for proteasomal degradation. Pharmacological inhibition of the enzymatic activity of catalytic proteasome subunits causes a cellular stress response that leads to apoptosis.
The heat shock protein 90kDa (HSP90) is a ubiquitously expressed chaperone that forms complexes with multiple proteins including co-chaperones and substrate proteins, which are also referred to as clients 72. HSP90 proteins have an N-terminal ATP-binding site that mediates dimerization, which is required for complex formation with client proteins. Most pharmacological inhibitors compete with the ATP-binding site that finally leads to the release and degradation of client proteins (Fig. 7). Several HSP90 clients that play important roles in the biology of MCL include cyclin D1, CDK4, AKT, and p53. The ansamycin 17-allylamino-17-demethoxy-geldanamycin (17-AAG, tanespimycin) entered the clinic more than 10 years ago. Treatment of MCL cell in vitro with 17-AAG reduced the levels of cyclin D1, AKT, BID and caspase 9 73, but 17-AAG and first-generation derivatives have generally demonstrated only moderate clinical antitumor activity 74. IPI-504 (retaspimycin) a novel ansamycin, kills MCL cells in vitro and induced tumor regression in a human xenograft mouse model 75. In a phase I study in patients with relapsed or refractory MM, IPI-504 induced SD in 50% of patients 76. The ansamycin class of HSP90 antagonists may require further optimization of dosing schedules and formulation 72. Recently, structurally different synthetic inhibitors of HSP90 have been developed. STA-9090 (ganetespib) demonstrated promising preclinical results in myeloma and lymphoma xenograft models 77. SNX-5422, an orally bioavailable prodrug that is converted to SNX-2112 induced depletion of HSP90 clients more potently than 17-AAG in MM cells 78. Clinical studies testing STA-9090 and SNX-5422 in refractory hematologic malignancies are ongoing.
Targeting the ubiquitin-proteasome pathway
The ubiquitin-proteasome pathway is the major pathway for the non-lysosomal degradation of intracellular proteins and maintains protein homeostasis (Fig. 7). This pathway involves the labeling of proteasome substrates with multiple ubiquitin proteins through an elaborate cascade of enzymes (see below). To date, bortezomib (Velcade) is the only proteasome inhibitor of this class approved by the FDA for the treatment of MCL and MM. Bortezomib is a peptide boronic acid derivative that specifically and reversibly targets the chymotrypsin-like activity of the proteasome. We and others showed that bortezomib triggers oxidative and endoplasmic reticulum (ER) stress that converges on the upregulation of the pro-apoptotic protein NOXA to induce cell death71,79–81. The induction of NOXA involves the concerted action of decreased ubiquitination of histone 2A and transcriptional activation by ATF3 and ATF4 82. CDK5 phosphorylates NOXA suppressing its pro-apoptotic function by promoting its cytosolic sequestration 83. Inhibition of the proteasome impacts many other pathways and this may be particularly important when considering combination therapies 84.
Single-agent bortezomib induces responses in up to 50% of patients with relapsed or refractory disease, but CR rates are low 85. Notably, bortezomib was equally effective in patients that were responsive or refractory to prior treatment. Predictors of treatment response to bortezomib have started to emerge. Recently, tumor cells from MCL patients with late or no response to bortezomib were shown to have features of partial plasma cell differentiation characterized by upregulation of IRF4 and CD3886. In the absence of increased protein synthesis, plasmacytic differentiation may increase the ability of cells to withstand the stress of proteasome inhibition. Bortezomib is increasingly combined with standard chemotherapy. As first-line treatment, the combination of bortezomib with R-CHOP chemoimmunotherapy induced an ORR of 91% with 72% CR/CRu in MCL (n=32) 87. HyperCVAD with bortezomib induced on ORR of 96% with 75% CRs in 76 previously untreated patients 88. Interestingly, bortezomib in combination with EPOCH-R may increase disease free survival in a subset of patients compared to historic controls 89. Interestingly, the addition of bortezomib to standard chemotherapy has in particular benefited patients with the activated B-cell like subtype of diffuse large B-cell lymphoma, that is characterized by constitutive activation of NFκB 87,90. It remains to be seen whether bortezomib combination therapies improves outcome for a distinct subset of MCL patients.
Novel proteasome inhibitors with improved pharmacology and reduced toxicity have entered clinical testing. MLN9708, a second-generation reversible boronic acid proteasome inhibitor, hydrolyzes to the pharmacologically active MLN2238 and has demonstrated improved pharmacologic and antitumor activities in lymphoma xenograft models 91. Carfilzomib (PR171), an epoxyketone irreversible inhibitor of the chymotrypsin-like activity appears to have fewer side effects than bortezomib, in particular less peripheral neuropathy 92,93. Carfilzomib monotherapy achieved an overall response rate of 24% (1 CR, 12 VGPRs and 48 PRs; n=257) in patients with relapsed and refractory MM 94. A phase I study in patients with hematologic malignancies reported a CRu in one MCL patient. ONX0912 (PR047) another epoxyketone, is an irreversible inhibitor of chymotrypsin-like activity that can be given orally 95. ONX0912 is in phase I studies recruiting patients with solid tumors. NPI-0052 (salinosporamide A, marizomib) is an irreversible inhibitor of the proteasome that is structurally related to lactacystein and can be given orally 96,97. NPI-0052 inhibits all three catalytic subunits of the proteasome, possibly accounting for its higher potency and activity against bortezomib-resistant cells 98.
Many combinations of bortezomib with other targeted agents have been tested in preclinical models and increasingly make their way into the clinic 84. HSP90 inhibitors specifically in combinations with proteasome inhibitors result in increased intracellular accumulation of ubiquitinated proteins and enhanced ER stress 84. A direct mechanistic explanation for this effect has come from the observation that IPI-504 in combination with bortezomib could overcome resistance to proteasome inhibition in MCL by destabilizing the interaction of HSP90 with ER-resident chaperone BIP/GRP78 75. A phase II study of this combination in refractory MM has reported an ORR of 14% with 2 PRs 99.
Proteins are marked for proteasomal degradation by covalent addition of multiple ubiquitin molecules in a cascade 100 that involves three distinct sequential steps performed by enzymes that fall into three distinct classes: activation (E1-family of enzymes), conjugation (E2-family) and 3) ligation (E3-family). Post-translational protein modifications through ubiquitination, or neddylation can fulfill regulatory functions. The E1 NEDD8-activating enzyme is the first enzyme in the neddylation cascade that modifies cullin proteins (Fig. 7B). Cullin proteins are essential components of the E3 cullin-RING ubiquitin ligase (CRL) complex. Thus, neddylation is required for CRL activity 100. MLN4924 selectively inhibits the E1 NEDD8-activating enzyme and thereby prevents the ubiquitination and subsequent proteasomal degradation of CRL targets 101. CRL substrate proteins such as CDT1 and p27 accumulate in response to MLN4924 treatment. The resulting DNA damage may ultimately lead to cell death 102. Neddylation also induces activation of the NFκB pathway by promoting degradation of IκBa. In human xenograft mouse models inhibition of E1 NEDD8-activating enzyme was able to inhibit NFκB signaling resulting in tumor regression 103. Clinical testing has only just begun but a PR in a patient with Hodgkin’s lymphoma has been reported 104.
Targeting epigenetic modifications
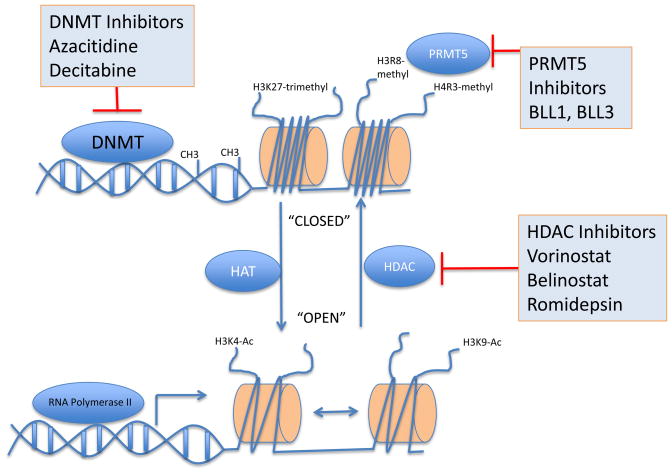
Alterations in the gene-regulatory information encoded in chemical modifications in DNA and DNA-associated proteins such as histones, have been demonstrated to contribute to the pathogenesis of solid tumors and hematological malignancies11. Aberrant DNA methylation has been demonstrated in promoter CpG islands of cancer cells with downstream consequences on gene expression. More recently, methylation changes within genomic areas beyond the promoter, such as in CpG shores and in the body of genes have also been shown to have regulatory consequences 105,106. DNA methylation is carried out enzymatically by DNA methyltransferases DNMT1, DNMT3A and DNMT3B. DNMTs in turn recruit Methyl Binding Proteins and Histone Deacetylases (HDAC) or histone methyltransferases (HMTs) that further repress gene expression (Fig. 8). More recently, a 5-hydroxymethyl cytosine modification intermediate between methylated and unmethylated states has been linked to mutations in TET2 in AML 107.
Figure 8. Targeting epigenetic modifications.
DNA is maintained in a coiled “inactive” state around histones and needs to undergo physical conformational changes to allow access for gene transcription. The biochemical modification of DNA and histones regulates this process and in turn is regulated by opposing groups of enzymes thatcan be inhibited for therapeutic benefit. Inhibitors of enzymes effecting epigenetic changes are shown in blue boxes.
High-throughput analyses demonstrated genome-wide aberrances in methylation in MCL. Primary MCLs demonstrated predominantly hypo-methylation with locus-specific hyper-methylation involving tumor suppressor genes10. Aberrant methylation in MCL may contribute to lymphomagenesis through genomic instability, activation of oncogenes or repression of tumor suppressor genes. Azacitidine and decitabine are DNA methytransferase inhibitors approved for use in MDS. Azacitidine incorporates into DNA whereas decitabine incorporates into both DNA and RNA, resulting in significant differences in the number and type of genes affected by the respective epigenetic de-repression 108. In vitro studies showed that DNMT inhibitors have potent anti-MCL activity and can synergize with HDAC inhibitors 10.
Protein arginine methyltransferase PRMT5 interacts with human SWI/SNF complexes and methylates histones H3R8 and H4R3109. PRMT5 is overexpressed in primary MCL and acts concertedly with histone deacetylase 2 (HDAC2), methyl CpG-binding domain protein 2 (MBD2) and DNA methyltransferase 3a (DNMT3a) to silence genes with anti cancer and immune modulatory activities 110. siRNA-mediated knockdown of PRMT5 in MCL cell lines led to growth arrest and apoptosis109. A small molecule screen using the PRMT5 crystal structure yielded two drugs, BLL1 and BLL3 that inhibit methylation of H4R3, and induced cell cycle arrest. These agents also promoted caspase-independent apoptosis in MCL cell lines 111.
Acetylation of lysine residues on histones leads to decreased binding between DNA and histones and thereby facilitates transcription. The acetylation status of histone lysine residues is maintained by histone acetyltransferases (HATs) and HDACs. HDACs fall into different classes: class I includes HDACs 1, 2, 3, and 8, class IIa includes HDACs 4, 5, 7 and 9, class IIb includes HDACs 6 and 10, class III includes non-classical sirtuins unrelated to HDACs, and class IV includes HDAC11. Acetylation regulates the function of important non-histone proteins including cyclin D1, p53, HSP90, HIF-1α and c-MYC. Thus, the effects of HDAC inhibitors extend well beyond mere changes in gene expression.112 Their precise mechanisms of action on tumor cells are still under investigation, but include modulation of transcription, induction of oxidative stress, cell cycle arrest, and apoptosis.112 The class I and II HDAC inhibitors vorinostat (suberoylanilide hydroxamic acid, Zolinza) and romidepsin (FR901228) are approved for treatment of primary cutaneous T-cell lymphoma.113 Treatment of MCL cell lines with vorinostat inhibited translation of cyclin D1, possibly as a consequence of inhibition of the PI3K/AKT/mTOR pathway.114 In clinical studies vorinostat monotherapy achieved a CRu in one of 11 MCL patients.115,116 There is good preclinical evidence for the combination of HDAC inhibitors with proteasome inhibitors117,118 and studies investigating this combination have been completed in MM and are ongoing in MCL. The potential mechanisms that could result in synergistic antitumor activity include enhanced ER-stress responses, disruption of aggresome formation, and inhibition of NFκB.84 Intriguingly, a recent study found that treatment of breast cancer cell lines with the HDAC inhibitor panobinostat increased acetylation of BIP/GRP78, causing it to dissociate from PERK.119 PERK then triggered the ER-stress response inducing cell death. This synergistic activity is not limited to bortezomib but appears to be shared with other proteasome inhibitors.120 The major dose limiting toxicity of HDAC inhibitors is thrombocytopenia due to defective platelet budding, which may be ameliorated with thrombopoietin agonists121.
Conclusions and Future Directions
The majority of the therapeutics described in this article are in Phase I or II trials. Given the relatively small numbers of MCL patients seen at most centers, innovative trial designs are necessary to rapidly test this multitude of drugs and determine their role in MCL therapy. One such example is the ISPY-2 122 study in locally advanced breast carcinoma patients, which has an adaptive trial design, where study arms are added or dropped dynamically as results are obtained in real-time. Randomized “pick the winner” phase II trial designs by local consortiums may be another option, with only the most promising candidates going on to larger confirmatory trials. The use of appropriate pharmacodynamic endpoints to test for inhibition of intended targets at an early stage of development may also speed up the development of new agents. Easier access to genomic and epigenomic profiling may allow better stratification of therapy, and combined with well chosen pharmacodynamic endpoints may help monitor for on target effects and assist in the formulation of synergistic drug combinations (Table 1). Improvements in our understanding of MCL lymphomagenesis and drug resistance, along with appropriate use of new targeted drugs are likely to improve the outlook for MCL patients.
Table 1.
Critical pathways and novel drugs in MCL
| Pathway targeted | Drug | Mechanism (Target) | Clinical Pharmacology |
|---|---|---|---|
| B-Cell Receptor | PCI-32765 | BTK inhibitor | ORR 75% in relapsed MCL 123. Side effects include nausea, vomiting and diarrhea. |
| Fostamatinib | SYK inhibitor | 11% response rate in relapsed MCL 124. Side effects include neutropenia, diarrhea and thrombocytopenia. | |
| Enzastaurin | PKC inhibitor | 37% disease stabilization in relapsed MCL patients125. Side effects included anemia, nausea, diarrhea, vomiting and syncope. | |
| CAL-101 | PI3K-delta inhibitor | Discussed in PI3K section | |
|
| |||
| SB1518 | small molecule JAK-2 inhibitor | 2/3 MCL patients achieved PR 126. Side effects include constipation, neutropenia and fever. | |
|
| |||
| PI3K/AKT/mTOR | CAL-101 | PI3K-delta inhibitor | ORR 62% in relapsed MCL 127. Dose limiting toxicity is elevation in LFTs. |
|
| |||
| MK2206 | AKT inhibitor | Phase II ongoing in DLBCL. Side effects include skin rash, mucositis and glucose intolerance. Preclinical synergy with rapamycin | |
|
| |||
| CCI-779 | mTOR inhibitor (predominantly TORC1) | ORR 38% as single agent 128 and 59% in combination with Rituximab37. Side effects include hyperglycemia, fatigue, thrombocytopenia and neutropenia. | |
|
| |||
| Cell Cycle | PD0332991 | CDK4/6 inhibitor | Cytostatic as single agent 46 but has preclinical synergy with bortezomib and cytarabine. Side effects include thrombocytopenia and neutropenia. |
|
| |||
| Flavopiridol (alvocidib) | Pan-CDK inhibitor | Limited single agent activity 129. Side effects include secretary diarrhea, fatigue and cytopenias. | |
|
| |||
| DNA Damage Response | RG7112 | Clinical grade HDM2 inhibitor | One AML patient in remission >9 months, responses in CLL/NHL 130. Side effects include moderate to severe nausea and vomiting. |
|
| |||
| Olaparib | PARP-1 inhibitor | May have higher specificity for ATM mutant or deficient MCLs. Side effects include nausea, vomiting and cytopenias. | |
|
| |||
| Protein Homeostasis | 17-AAG | HSP-90 inhibitor | Poor solubility, limited clinical activity but shows preclinical synergy with bortezomib in MM. Side effects include diarrhea, hyperglycemia and hepatotoxicity. |
|
| |||
| Carfilzomib | Irreversible Proteasome Inhibitor | ORR 24% in bortezomib refractory MM 131. and preclinical synergy with HPS90 inhibitors and HDAC inhibitors. Side effects include fatigue and nausea. | |
|
| |||
| Cell Survival/Apoptosis | GX15-070MS (Obatoclax) | small molecule pan-BCL-2 inhibitor | 4% responses in CLL patients in Phase I 132. More likely to be useful as chemo/radio sensitizer. Side effects incude somnolence/ataxia and myelosuppression. |
|
| |||
| ABT-263 (Navitoclax) | BCL-2/BCL-XL inhibitor | 10/46 relapsed NHL patients had a PR 133. Side effects include myelosuppression and diarrhea. | |
|
| |||
| Epigenetic modifications | Vorinostat (SAHA, Zolinza) | Class I & II HDAC inhibitor | 1CRu/11 relapsed MCL patients 134. Has preclinical synergy with bortezomib and DNA hypomethylating agents. Side effects include myelosuppression (especially thrombocytopenia), nausea and rash |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinaldi A, et al. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. British journal of haematology. 2006;132:303–316. doi: 10.1111/j.1365-2141.2005.05883.x. [DOI] [PubMed] [Google Scholar]
- 3.Pighi C, et al. Phospho-proteomic analysis of mantle cell lymphoma cells suggests a pro-survival role of B-cell receptor signaling. Cell Oncol (Dordr) 2011;34:141–153. doi: 10.1007/s13402-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu L, et al. Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood. 2006;107:4540–4548. doi: 10.1182/blood-2005-10-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu L, et al. BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009;113:4627–4636. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzatti EG, et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. British journal of haematology. 2005;130:516–526. doi: 10.1111/j.1365-2141.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- 7.Chim CS, Wong KY, Loong F, Lam WW, Srivastava G. Frequent epigenetic inactivation of Rb1 in addition to p15 and p16 in mantle cell and follicular lymphoma. Hum Pathol. 2007;38:1849–1857. doi: 10.1016/j.humpath.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.de Leeuw RJ, et al. Comprehensive whole genome array CGH profiling of mantle cell lymphoma model genomes. Hum Mol Genet. 2004;13:1827–1837. doi: 10.1093/hmg/ddh195. [DOI] [PubMed] [Google Scholar]
- 9.Greiner TC, et al. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood. 1996;87:4302–4310. [PubMed] [Google Scholar]
- 10.Leshchenko VV, et al. Genomewide DNA methylation analysis reveals novel targets for drug development in mantle cell lymphoma. Blood. 2010;116:1025–1034. doi: 10.1182/blood-2009-12-257485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce SK, Liu W. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat Rev Immunol. 2010;10:767–777. doi: 10.1038/nri2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler N, et al. The Btk Inhibitor, PCI-32765, Induces Durable Responses with Minimal Toxicity In Patients with Relapsed/Refractory B-Cell Malignancies: Results From a Phase I Study. Blood (ASH Annual Meeting Abstracts) 2010;116:964. [Google Scholar]
- 14.Raje NS, Faber RJHEA, Richardson PG, Forero-Torres A, Schiller GJ, Cohen AD, Carpenter SP, Cronier D, Pashkevich M, Wooldridge J, Anderson KC. Phase I study of LY2127399, a human anti-BAFF antibody, and bortezomib in patients with previously treated multiple myeloma. Journal of Clinical Oncology. 2011;29(suppl):abstr 8012. [Google Scholar]
- 15.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122–131. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Yared MA, Khoury JD, Medeiros LJ, Rassidakis GZ, Lai R. Activation status of the JAK/STAT3 pathway in mantle cell lymphoma. Arch Pathol Lab Med. 2005;129:990–996. doi: 10.5858/2005-129-990-ASOTSP. [DOI] [PubMed] [Google Scholar]
- 17.Baran-Marszak F, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. 2010;95:1865–1872. doi: 10.3324/haematol.2009.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham LV, et al. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications. Mol Cancer Ther. 2010;9:2026–2036. doi: 10.1158/1535-7163.MCT-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younes A, et al. Phase I Study of a Novel Oral JAK-2 Inhibitor SB1518 In Patients with Relapsed Lymphoma: Evidence of Clinical and Biologic Activity In Multiple Lymphoma Subtypes. Blood (ASH Annual Meeting Abstracts) 2010;116:2830. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 22.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lannutti BJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahl B, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2010;116:1777. [Google Scholar]
- 25.Garlich JR, et al. Phase I Study of Novel Prodrug Dual PI3K/mTOR Inhibitor SF1126 In B-Cell Malignancies. ASH Annual Meeting Abstracts. 2010;116:1783. [Google Scholar]
- 26.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DR, et al. Pre-Clinical and Interim Results of a Phase II Trial of Perifosine In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) ASH Annual Meeting Abstracts. 2010;116:1842. [Google Scholar]
- 29.Petrich AM, et al. Genomic and Pathway Connectivity Analyses Identify Novel Strategies to Overcome mTOR Inhibitor Resistance In DLBCL. ASH Annual Meeting Abstracts. 2010;116:436. [Google Scholar]
- 30.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janes MR, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010 doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witzig TE, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 33.Ansell SM, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess G, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor OA, et al. PILLAR-1: Preliminary Results of a Phase II Study of mTOR Inhibitor Everolimus In Patients with Mantle Cell Lymphoma (MCL) Who Are Refractory or Intolerant to Bortezomib. Blood (ASH Annual Meeting Abstracts) 2010;116:3963. [Google Scholar]
- 36.Renner C, et al. A Multi-Center Phase II Study (SAKK 36/06) of Single Agent Everolimus (RAD001) In Patients with Relapsed or Refractory Mantle Cell Lymphoma. Blood (ASH Annual Meeting Abstracts) 2010;116:2803. [Google Scholar]
- 37.Ansell SM, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. The lancet oncology. 2011;12:361–368. doi: 10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 39.Evangelisti C, et al. Targeted Inhibition of mTORC1 and mTORC2 by the Active-Site mTOR Inhibitors, PP-242 and OSI-027, Has Cytotoxic Effects In T-Cell Acute Lymphoblastic Leukemia. ASH Annual Meeting Abstracts. 2010;116:3242. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 40.Zeng Z, et al. Targeting mTORC1/2 by a mTOR Kinase Inhibitor (PP242) induces Apoptosis In AML Cells Under Conditions Mimicking Bone Marrow Microenvironment. ASH Annual Meeting Abstracts. 2010;116:778. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta M, et al. Dual Inhibition of mTORC1/mTORC2 Induces Apoptosis of Mantle Cell Lymphoma by Preventing Rictor Mediated AKTS473 Phosphorylation by Potentiating AKT2-PHLPP1 Association. Blood (ASH Annual Meeting Abstracts) 2010;116:772. [Google Scholar]
- 42.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 43.Fu K, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–4321. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marzec M, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–1750. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen-Kiang S. Mechanism- based Targeting of CDK4/CDK6 in Mantle Cell Lymphoma. Mantle Cell Lymphoma Consortium Meeting Proceedings. 2011 [Google Scholar]
- 46.Shapiro Geoffrey. Pilot study of the cdk4/6 Inhibitor PD0332991 in Mantle Cell Lymphoma: Final Results. Mantle Cell Lymphoma Consortium Meeting Proceedings. 2011 [Google Scholar]
- 47.Kouroukis CT, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–1745. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 48.Lin TS, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manohar SM, Rathos MJ, Sonawane V, Rao SV, Joshi KS. Cyclin-dependent kinase inhibitor, P276-00 induces apoptosis in multiple myeloma cells by inhibition of Cdk9-T1 and RNA polymerase II-dependent transcription. Leuk Res. 2011 doi: 10.1016/j.leukres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Santo L, et al. AT7519, A novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3beta activation and RNA polymerase II inhibition. Oncogene. 2010;29:2325–2336. doi: 10.1038/onc.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squires MS, et al. AT7519, a cyclin-dependent kinase inhibitor, exerts its effects by transcriptional inhibition in leukemia cell lines and patient samples. Mol Cancer Ther. 2010;9:920–928. doi: 10.1158/1535-7163.MCT-09-1071. [DOI] [PubMed] [Google Scholar]
- 52.Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112(Pt 21):3591–3601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- 53.Qi W, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81:881–890. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavin MF. ATM: the product of the gene mutated in ataxia-telangiectasia. Int J Biochem Cell Biol. 1999;31:735–740. doi: 10.1016/s1357-2725(99)00028-x. [DOI] [PubMed] [Google Scholar]
- 55.Monni O, et al. Gain of 3q and deletion of 11q22 are frequent aberrations in mantle cell lymphoma. Genes Chromosomes Cancer. 1998;21:298–307. doi: 10.1002/(sici)1098-2264(199804)21:4<298::aid-gcc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 56.Jin L, et al. MDM2 antagonist Nutlin-3 enhances bortezomib-mediated mitochondrial apoptosis in TP53-mutated mantle cell lymphoma. Cancer Lett. 2010;299:161–170. doi: 10.1016/j.canlet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Tabe Y, et al. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009;15:933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreeff M, et al. A Multi-Center, Open-Label, Phase I Study of Single Agent RG7112, A First In Class p53-MDM2 Antagonist, In Patients with Relapsed/Refractory Acute Myeloid and Lymphoid Leukemias (AML/ALL) and Refractory Chronic Lymphocytic Leukemia/Small Cell Lymphocytic Lymphomas (CLL/SCLL) ASH Annual Meeting Abstracts. 2010;116:657. [Google Scholar]
- 59.Annunziata CM, O’Shaughnessy J. Poly (ADP-ribose) polymerase as a novel therapeutic target in cancer. Clin Cancer Res. 2010;16:4517–4526. doi: 10.1158/1078-0432.CCR-10-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weston VJ, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–4587. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 61.Johnson N, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nature medicine. 2011;17:875–882. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 63.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 64.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 65.Touzeau C, et al. Rational for the Use of a Targeted-Therapy Using ABT-737 In Mantle-Cell Lymphoma. Blood (ASH Annual Meeting Abstracts) 2010;116:770. [Google Scholar]
- 66.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson WH, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien SM, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–153. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herishanu Y, et al. Activation of CD44, a receptor for extracellular matrix components, protects CLL cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.569962. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weniger MA, et al. Treatment-Induced Oxidative Stress and Cellular Antioxidant Capacity Determine Response to Bortezomib in Mantle Cell Lymphoma. Clinical Cancer Research. 2011;17:5101–5112. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Georgakis GV, Li Y, Younes A. The heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9. Br J Haematol. 2006;135:68–71. doi: 10.1111/j.1365-2141.2006.06247.x. [DOI] [PubMed] [Google Scholar]
- 74.Kim YS, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roue G, et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2010;117:1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- 76.Richardson PG, et al. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. Br J Haematol. 2010;150:438–445. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs. 2010;11:1466–1476. [PubMed] [Google Scholar]
- 78.Okawa Y, et al. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113:846–855. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Obeng EA, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rizzatti EG, et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leukemia & lymphoma. 2008;49:798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Galan P, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 82.Wang Q, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lowman XH, et al. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Molecular cell. 2010;40:823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 84.Wright JJ. Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy. Clin Cancer Res. 2010;16:4094–4104. doi: 10.1158/1078-0432.CCR-09-2882. [DOI] [PubMed] [Google Scholar]
- 85.Goy A, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Galan P, et al. Bortezomib resistance in mantle cell lymphoma is associated with plasmacytic differentiation. Blood. 2011;117:542–552. doi: 10.1182/blood-2010-02-269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruan J, et al. Bortezomib Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 88.Kahl BS, et al. The VcR-CVAD Regimen Produces a High Complete Response Rate in Untreated Mantle Cell Lymphoma (MCL): First Analysis of E1405 - A Phase II Study of VcR-CVAD with Maintenance Rituximab for MCL. ASH Annual Meeting Abstracts. 2009;114:1661. [Google Scholar]
- 89.Grant CDK, Tweito M, Steinberg SM, Pittaluga S, Jaffe ES, Wiestner A, Wilson WH. Bortezomib plus DA-EPOCH-rituximab followed by bortezomib maintenance versus observation in previously untreated mantle cell lymphoma (MCL) Journal of Clinical Oncology. 2011;29 [Google Scholar]
- 90.Dunleavy K, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kupperman E, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 92.Parlati F, et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 93.Arastu-Kapur S, Shenk K, Parlati F, Bennett MK. Non-Proteasomal Targets of Proteasome Inhibitors Bortezomib and Carfilzomib. ASH Annual Meeting Abstracts. 2008;112:2657. [Google Scholar]
- 94.di Capua Siegel DS, et al. Results of PX-171-003-A1, An Open-Label, Single-Arm, Phase 2 (Ph 2) Study of Carfilzomib (CFZ) In Patients (pts) with Relapsed and Refractory Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2010;116:985. [Google Scholar]
- 95.Zhou HJ, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J Med Chem. 2009;52:3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 96.Potts BC, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macherla VR, et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- 98.Chauhan D, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Richardson PG, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–437. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- 102.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–10320. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milhollen MA, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 104.Shah JJ, et al. Phase 1 Dose-Escalation Study of Multiple Dosing Schedules of the Investigational Drug MLN4924, a Nedd8-Activating Enzyme Inhibitor, In Patients with Relapsed and/or Refractory Multiple Myeloma or Lymphoma. ASH Annual Meeting Abstracts. 2010;116:2801. [Google Scholar]
- 105.Doi A, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ball MP, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ko M, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hollenbach PW, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5:e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pal S, et al. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. Embo J. 2007;26:3558–3569. doi: 10.1038/sj.emboj.7601794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pal S, et al. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol Cell Biol. 2003;23:7475–7487. doi: 10.1128/MCB.23.21.7475-7487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baiocchi RA. Developing Novel Drugs to Target Protein Arginine Methyltransferase Enzyme 5 (PRMT5) Overexpression in Mantle Cell Lymphoma. Mantle Cell Lymphoma Consortium Meeting Proceedings. 2011 [Google Scholar]
- 112.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 113.Copeland A, Buglio D, Younes A. Histone deacetylase inhibitors in lymphoma. Curr Opin Oncol. 2010;22:431–436. doi: 10.1097/CCO.0b013e32833d5954. [DOI] [PubMed] [Google Scholar]
- 114.Kawamata N, Chen J, Koeffler HP. Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood. 2007;110:2667–2673. doi: 10.1182/blood-2005-11-026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watanabe T, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kirschbaum M, et al. Phase II Study of Vorinostat for Treatment of Relapsed or Refractory Indolent Non-Hodgkin’s Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heider U, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 118.Paoluzzi L, et al. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16:554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 119.Rao R, et al. Treatment with panobinostat induces glucose-regulated protein 78 acetylation and endoplasmic reticulum stress in breast cancer cells. Mol Cancer Ther. 2010;9:942–952. doi: 10.1158/1535-7163.MCT-09-0988. [DOI] [PubMed] [Google Scholar]
- 120.Rao R, et al. Treatment with Histone Deacetylase 6-Specific Inhibitor WT-161 Disrupts hsp90 Function, Abrogates Aggresome Formation and Sensitizes Human Mantle Cell Lymphoma Cells to Lethal ER Stress Induced by Proteasome Inhibitor Carfilzomib. Blood (ASH Annual Meeting Abstracts) 2010;116:2856. [Google Scholar]
- 121.Bishton MJ, et al. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood. 2011;117:3658–3668. doi: 10.1182/blood-2010-11-318055. [DOI] [PubMed] [Google Scholar]
- 122.Barker AD, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 123.Fowler NSJ, Smith SM. The Btk Inhibitor, PCI-32765, Induces Durable Responses with Minimal Toxicity In Patients with Relapsed/Refractory B-Cell Malignancies: Results From a Phase I Study. Blood (ASH Annual Meeting Abstracts) 2010;116:964. [Google Scholar]
- 124.Friedberg JW, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morschhauser F, et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008;19:247–253. doi: 10.1093/annonc/mdm463. [DOI] [PubMed] [Google Scholar]
- 126.Younes AFM, McLaughlin P, Copeland A, Zhu J, de Castro Faria S. Phase I Study of a Novel Oral JAK-2 Inhibitor SB1518 In Patients with Relapsed Lymphoma: Evidence of Clinical and Biologic Activity In Multiple Lymphoma Subtypes. Blood (ASH Annual Meeting Abstracts) 2010;116:2830. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kahl BBJ, Flinn IW. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2010;116:1777. [Google Scholar]
- 128.Witzig TE, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 129.Kouroukis CT, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–1745. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 130.Andreeff MKK, Padmanabhan S. A Multi-Center, Open-Label, Phase I Study of Single Agent RG7112, A First In Class p53-MDM2 Antagonist, In Patients with Relapsed/Refractory Acute Myeloid and Lymphoid Leukemias (AML/ALL) and Refractory Chronic Lymphocytic Leukemia/Small Cell Lymphocytic Lymphomas (CLL/SCLL) ASH Annual Meeting Abstracts. 2010;116:657. [Google Scholar]
- 131.di Capua Siegel DSMT, Wang M. Results of PX-171-003-A1, An Open-Label, Single-Arm, Phase 2 (Ph 2) Study of Carfilzomib (CFZ) In Patients (pts) with Relapsed and Refractory Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2010;116:985. [Google Scholar]
- 132.O’Brien SM, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson WH, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watanabe T, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]