Abstract
Background
Up to 5% of the population may have a brain aneurysm, and if the brain aneurysm ruptures, there is greater than 50% mortality, and more than onethird of survivors are dependent. Brain aneurysms detected before rupture can be treated to prevent rupture, or ruptured aneurysms can be treated to prevent re-rupture. Endovascular coiling of brain aneurysms is the treatment of choice for some aneurysms; however, up to one quarter of aneurysms may recur. The coiled aneurysms that do not recur are characterized by inflammatory intra-aneurysmal tissue healing; therefore, we studied the biology of this process, specifically, the role of monocyte chemotactic protein-1 (MCP-1), a cytokine known for tissue healing.
Methods and Results
We created coils with a PLGA coating that released MCP-1 at 3 different doses (100 μg/mL; 1 mg/mL; and 10 mg/mL) and performed a dose-response study for effect on intra-aneurysmal tissue healing in a murine carotid aneurysm model. We then demonstrated MCP-1(100 μg/mL)-releasing coils promote significantly greater aneurysm tissue ingrowth than bare platinum or PLGA-only coils. We show that MCP-1 recruits the migration of fibroblasts, macrophages, smooth muscle cells, and endothelial cells in vitro, in cell migration assays; and in vivo, in murine carotid aneurysms. Using gfp bone marrow transplant chimeric mice, we demonstrate that the MCP-1-recruited fibroblasts and macrophages are derived from the bone marrow. We demonstrate that this MCP-1-mediated vascular inflammatory repair occurs via amacrophage inflammatory protein-1α (MIP-1α) and macrophage inflammatory protein-2 (MIP-2)-dependent pathway. MCP-1 released from coiled murine aneurysms causes significant upregulation of MIP-1α and MIP-2 expression by cytokine array assay. Blocking MIP-1α and MIP-2 with antagonist antibody causes significant decrease in MCP-1-mediated intra-aneurysmal tissue healing.
Conclusions
Our findings suggest that MCP-1 has a critical role in promoting inflammatory intra-aneurysmal tissue healing in a MIP-1α and MIP-2-dependent pathway.
Keywords: aneurysm, cerebrovascular disorders, inflammation
INTRODUCTION
Up to 5% of the population may have a brain aneurysm1. If a brain aneurysm ruptures, there is an associated greater than 50% mortality rate, and more than one third of survivors end up dependent2. Brain aneurysms that are detected before they rupture, can be treated to prevent rupture, and ruptured brain aneurysms can be treated to prevent re-rupture. Endovascular coiling is the treatment of choice for someruptured and unruptured brain aneurysms3–5. A significant drawback of endovascular coiling is aneurysm recurrence and need for retreatment which may occur in up to one quarter of aneurysms6.
Surgically-harvested and autopsy-derived specimens of human brain aneurysms demonstrate that durable aneurysm cure is achieved by intra-aneurysmal wound healing. Successful healing is characterized by the presence of connective tissue proliferation, fibroblasts, collagen, smooth muscle cells, capillary ingrowth, and macrophages7. The aim of brain aneurysm therapies should therefore be achievement of this type of intra-aneurysmal wound healing.
We studied the biology of intra-aneurysmal tissue healing, in particular, the role of monocyte chemotactic protein-1 (MCP-1), which is a cytokine known to be important in tissue healing8, 9, for inflammatory vascular tissue repair in a murine carotid aneurysm model.
MATERIALS AND METHODS
Animals
All animal experimentation was performed in accordance with a protocol approved by our institution’s Institutional Animal Care and Use Committee.
Murine Carotid Aneurysm Model
Murine carotid aneurysms were created in C57BL/6 female mice (Charles River) using a method previously described10. Briefly, the right common carotid artery is microsurgically exposed, porcine pancreatic elastase solution (Worthington Biochemical Corp, Lakewood, NJ) 10 U diluted in 1 mL of 1 × phosphate buffered solution (PBS) (Invitrogen, Carlsbad, CA) is applied extravascularly for 20 minutes, then the vessel is occluded distally to create a stump. Three weeks later, a saccular aneurysm has formed at the right common carotid artery stump.
Creation of MCP-1-Releasing Coils
Monocyte chemotactic protein-1 (MCP-1) releasing coils were created by dipping standard platinum aneurysm coils into an acqueous protein suspension consisting of MCP-1 at three different doses of 100 μg/mL, 1 mg/mL, and 10 mg/mL (Sigma-Aldrich, St. Louis, MO), in 50:50 poly(lactic-co-glycolic acid) (PLGA)(Sigma-Aldrich) and dichloromethane anhydrous (Sigma-Aldrich) neutralized to a pH of 7.0 with Mg(0H)2, and drying for 24 hours at 4°C. Control PLGA-only coils were created by dipping standard platinum aneurysm coils into an acqueous suspension of PBS in 50:50 PLGA and dichloromethane anhydrous without protein. Control bare platinum coils were not dipped.
The pharmacokinetic release of MCP-1from the coils was measured by Quickstart Bradford Protein Assay (Bio-Rad Laboratories Inc., Hercules, CA) using Infinite 200 PRO Nanoquant microplate reader (Tecan Group Ltd., Mannedorf, Switzerland).
Dose-Response Study of MCP-1-Releasing Coils in Murine Carotid Aneurysm Model
The three different doses of MCP-1-releasing coils (100 μg/mL, 1 mg/mL, and 10 mg/mL)were microsurgically implanted into 3-week fully developed murine carotid aneurysms (n=10 for each). Control PLGA-only coils (n=10)were microsurgically implanted into control murine carotid aneurysms. Coil implantation was performed by microsurgically inserting coils via a distal portion of the aneurysm and advancing into a proximal saccular part of the aneurysm. Implanted aneurysms were harvested three weeks later, sectioned by cryostat into 5 μM sections, and hematoxylin and eosin staining were performed. Quantitative measurements for tissue ingrowth were performed by blinded observers who measured intraluminal tissue ingrowth under microscopy using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
Effect of MCP-1-Releasing Coil on Intra-Aneurysmal Tissue Ingrowth in Murine Carotid Aneurysm Model
MCP-1-releasing coils (100 μg/mL); control PLGA-only coils; and control bare platinum coils were microsurgically implanted into 3-week fully developed murine carotid aneurysms (n=20 for each). Implanted aneurysms were harvested three weeks later, sectioned, stained, and measured in the same manner described above for the MCP-1 dose response study. Immunohistochemical staining for fibroblasts (anti-FSP-1, Abcam), smooth muscle cells (anti-aSMA, Sigma), endothelial cells (anti-MECA-32, BD Pharmingen), and macrophages (anti-CD11b, BD Pharmingen) was performed.
Cell Migration Assay
The recruitment effect of MCP-1 on fibroblasts, smooth muscle cells, endothelial cells, and macrophages was studied in vitro by cell migration assay. Briefly, 3T3 fibroblasts (ATCC), mouse MOVAS smooth muscle (ATCC) cells, human umbilical vein endothelial cells, and mouse J774 macrophages (ATCC) were grown to 80% confluence followed by serum starvation for 12 hours for fibroblasts, smooth muscle cells, and endothelial cells, and for 24 hours for macrophages; then seeded onto the upper chamber of Transwell Permeable Supports (6.5 mm diameter wells, 8 μm pore size) (Corning Life Sciences, Lowell, MA) at a concentration of 20,000 cells/well for fibroblasts and smooth muscle cells, 30,000 cells/well for endothelial cells, and 200,000 cells/well for macrophages. The bottom chambers were filled with 0.6 mL of serum-free medium with MCP-1 (10 ng/mL). Controls contained serum-free medium without MCP-1 in bottom chambers. Fibroblasts and smooth muscle cells were allowed to migrate for 6 hours, while macrophages and endothelial cells were allowed to migrate for 24 hours at 37°C 5% CO2.
Creation of Chimeras
Identification of bone marrow-derived cells in the intra-aneurysmal tissue ingrowth was achieved by experiments with bone marrow transplant chimeras that were created using a method previously described10. Briefly, C57BL/6 mice harboring fully-developed three-week carotid aneurysms were depleted of bone marrow by irradiation with 950 rads, then were injected via retro-orbital sinus with total gfp+ bone marrow cell populations (5×106) harvested from the femurs of UBC-gfp mice (The Jackson Laboratory), to create chimeras designated as C57BL/6anr. gfpbm. C57BL/6anr. gfpbm chimeras are intended to have aneurysms exhibiting wild-type characteristics, but circulating bone marrow-derived cells which are gfp+. Whole bone marrow engraftment was confirmed three months later by flow cytometry analysis of peripheral blood mononuclear cells for cell surface markers CD3, B220, and CD11b.
Cytokine Array
At two different time points, one day and one week after implantation of MCP-1-releasing coils (100 μg/mL); control PLGA-only coils, and control bare platinum coils; aneurysms were harvested and proteins extracted using radio-immunoprecipitation assay (RIPA) buffer (Sigma-Aldrich), and cytokine array performed using RayBio Mouse Cytokine Antibody Array Kit (RayBiotech, Norcross, GA) which screens for the following cytokines: Axl, CD30 L, CD30 T, CD40, CRG-2, CTACK, CXCL16, Eotaxin, Eotaxin-2, Fas L, Fractalkine, GCSF, GM-CSF, INFγ, IGFBP-3, IGFBP-5, IGFBP-6, IL-1a, IL-1b, IL-2, IL-3, IL-3Rb, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p40/p70, IL-12 p70, IL-13, IL-17, KC, Leptin R, Leptin R, CXCL5, L-Selectin, Lymphotactin, MCP-1, MCP-5, M-CSF, MIG, MIP-1α, MIP-1γ, MIP-2, MIP-3b, CXCL4, P-selectin, RANTES, SCF, SDF-1α, TARC, TCA-3, TECK, TIMP1, TNFα, sTNF-RI, sTNF-RII, TPO, VCAM1.
MIP-1αand MIP-2 Blockade
C57BL/6 mice harboring fully-developed three-week carotid aneurysms were given an antibody antagonist of MIP-1α (15 μg in 100 mL PBS) (R&D Systems) subcutaneous (n=10); an antibody antagonist of MIP-2 (8 μg in 100 mL PBS) (R&D Systems) intravenous (n=10); or vehicle (100 mL PBS) subcutaneous and intravenous (n=10) every 48 hours starting 48 hours prior to implantation of MCP-1 releasing coils(100 μg/mL) and until aneurysms were harvested 3 weeks later.
Statistical Analysis
For the MCP-1-releasing coil dose response study, the comparison of MCP-1-releasing coil with PLGA-alone and bare platinum coils, and the MIP-1α and MIP-2 blockade study, we fit mixed linear models to the data using SAS PROC MIXED (Version 9.1). For all experiments, our response variable was the individual reading taken by one of two observers on one of five slides for each mouse (we did no averaging of measurements). Fixed factors were group membership, observer, and the group-by-observer interaction. We modeled mouse and the mouse-by-observer interaction as random effects, and we assumed a variance components covariance-matrix structure. We verified this covariance choice by using SAS PROC GENMOD to fit general estimating equations models to the data, which produced almost identical results. It was decided a priori that in post hoc tests for all experiments, we would make pairwise comparisons against only one group (vs. PLGA in the dose-response study, vs. MCP-1 in the study with both PLGA-alone and bare platinum control groups, and vs. the control in the MIP-1α and MIP-2 blockade study). Hence, we used Dunnett’s method (two-sided) for multiple comparisons against a single group to control the Type I error rate at 0.05 when performing these tests.
For the test of control for MIP-1α and MIP-2 in the cytokine array study, we used analysis of variance with the proportion of positive control as the response and treatment group as the independent variable. We used Bonferroni’s method to make two-sided post-hoc pairwise comparisons. Finally, for the cell migration assay, we used negative-binomial regression models to determine whether counts in the control and MCP-1 groups differed significantly for each cell type. For each cell type, we used the number of cells observed as the response variable and group membership as the independent variable. We also included test specimen (1, 2 or 3) as a covariate in the analyses.
RESULTS
MCP-1 Promotes Tissue Ingrowth and Healing in Murine Carotid Aneurysms
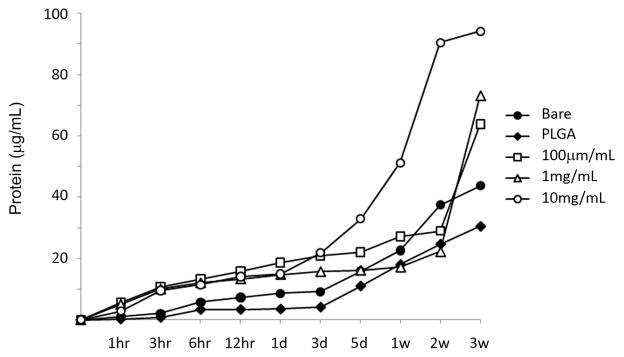
In order to study the role of MCP-1 in the inflammatory vascular repair of aneurysms, we created coated coils which slowly release MCP-1 over 21 days and implanted them in our murine carotid aneurysm model10. We chose to use a 50/50 PLGA (poly-DL-lactic glycolic acid) coating to slowly release protein for several reasons. First, this material is available in various compositions so that the rate of release can be varied as needed. It has been used in other implantable devices that have been cleared by the FDA for human use in several applications, and a significant background literature is available on expected behavior in vivo. Additionally, we, and other groups, have used this material in the water-in-oil-in-water (W/O/W) solvent extraction/evaporation, “double emulsion” manner as well as the more direct “water-in-oil” emulsion method used here to encapsulate and slowly release water soluble biologically active proteins11. This coating is well known to degrade by a simple hydrolysis reaction to yield the known metabolites: lactic acid and glycolic acid. An aqueous solution of the protein and 3w/v % magnesium hydroxide (to neutralize acid released) was emulsified in methylene chloride, a volatile organic solvent. Such degradation products are easily removed by the body, and do not impair the biological activity of the proteins. This emulsion, which resembled milk in appearance, was applied by dip-coating the metal stents into the emulsion, and air dried. The uniformity of the coating was examined by scanning electron microscopy (Figure 1A). We tested the pharmocokinetic release of MCP-1 at three different doses of 100 μg/mL, 1 mg/mL, and 10 mg/mL from the coils by Bradford protein assay which demonstrated slow consistent release over 21 days which would produce the desire effect of sustained continual inflammatory healing over three weeks (Figure 1B).
Figure 1.
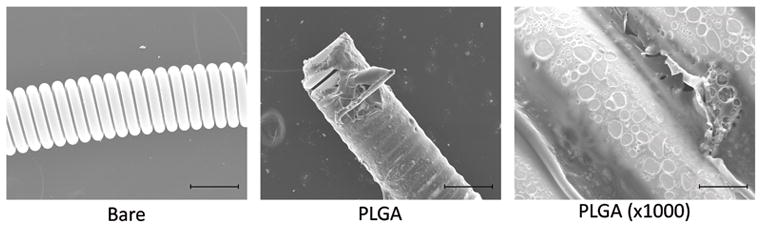
Creation and testing of protein-releasing coils. A) Scanning electron microscopy demonstrates uniformity of coating of the platinum coil with PLGA using our method. Scale bars are 200μm in the left and middle panel, and 20μm in the right panel. B) Sustained delivery of MCP-1 by MCP-1 releasing coils.
MCP-1 releasing coils at three doses (100 μg/mL, 1 mg/mL, and 10 mg/mL), PLGA-only coils, and bare platinum coils (5mm of each) were placed in 100 mL of PBS, shaken in a 37°C incubator, then PBS was collected at each time point and Bradford assay performed.
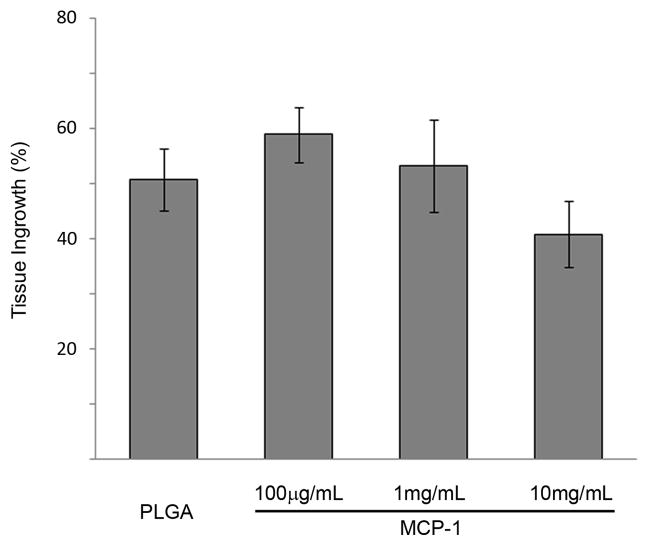
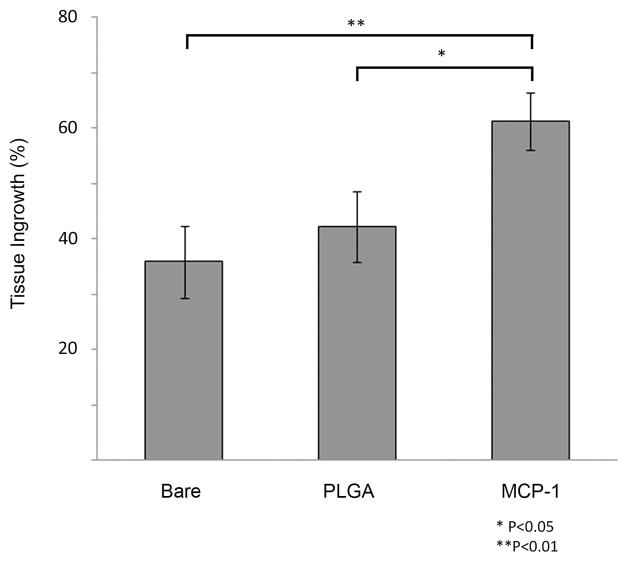
We performed a dose-response study in which murine carotid aneurysms were implanted with MCP-1 releasing coils at three different doses of 100 μg/mL, 1 mg/mL, and 10 mg/mL (n=10 each), and three weeks later, aneurysms were harvested, sectioned, stained, and measured for intra-aneurysmal tissue ingrowth (Figure 2A). We then studied the effect of MCP-1 on intra-aneurysmal tissue ingrowth by comparing MCP-1-releasing coils (100 μg/mL) to control PLGA-only, and control bare platinum coils (n=20 each) implanted into murine carotid aneurysms, and three weeks later, MCP-1-releasing coils were associated with significantly more intra-aneurysmal tissue ingrowth and healing than the aneurysms implanted with control PLGA-only coils (P<0.05)or control bare platinum coils (P<0.01)(Figure 2B).
Figure 2.
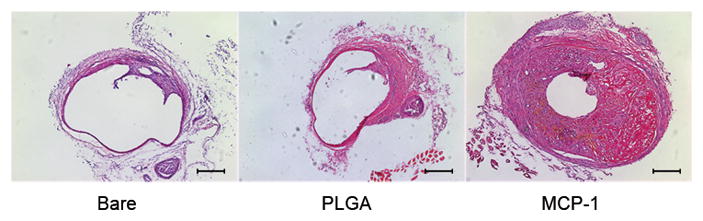
MCP-1 induces in vivo intra-aneurysmal tissue healing.
A) A dose response study was performed by implanting murine carotid aneurysms with MCP-1 releasing coils at three different doses (100 μg/mL, 1 mg/mL, and 10 mg/m L; n=10 each), and three weeks later, examined for intra-aneurysmal tissue ingrowth. Percentage of lumen area occupied by tissue ingrowth as measured by blinded observers using imaging measurement software. B) The effect of MCP-1 on intra-aneurysmal tissue healing was studied by implanting MCP-1-releasing coils (100 μg/mL); control PLGA-only coils; and control bare platinum coils into murine carotid aneurysms (n=20 for each). Aneurysms implanted with MCP-1-releasing coils demonstrated significantly more intra-aneurysmal tissue ingrowth and healing than aneurysms implanted with control PLGA-only coils or control bare platinum coils. Percentage of lumen area occupied by tissue ingrowth as measured by blinded observers using imaging measurement software, means ± s.e.m, *P<0.05, **P<0.01. C) H&E stain of representative sections of aneurysms implanted with bare platinum coil, PLGA_only coil, or MCP-1 releasing coil. Scale bar, 200 mm.
MCP-1 Causes Migration of Fibroblasts, Endothelial Cells, Smooth Muscle Cells, and Macrophages
In harvested human cerebral aneurysms, durable tissue healing is characterized by fibroblasts, smooth muscle cells, capillary ingrowth, and macrophages7. In our murine carotid aneurysm model, we demonstrate MCP-1 promotes greater intra-aneurysmal tissue ingrowth than control PLGA-only and control bare platinum coils. MCP-1 is a potent chemokine of macrophages, fibroblasts, smooth muscle cells, and endothelial cells, and has been shown to be critical in inflammatory healing in extravascular tissues such as cutaneous wounds12 and myocardial infarction13. In intravascular environments, however, MCP-1-induced inflammatory healing produces undesired effects such as neointimal hyperplasia14 and atherosclerosis15. In the specific instance of aneurysms, however, intravascular tissue proliferation is a desired result, and therefore, MCP-1 may have a specific role for this specific disease.
We tested in vitro MCP-1 chemotaxis of the desired cell types: macrophages for positive paracrine production to initiate inflammatory healing, fibroblasts for connective tissue proliferation, endothelial cells for capillary ingrowth, and smooth muscle cells for vascular integrity. Cell migrations assays with MCP-1 and control serum-free medium were performed, and demonstrated MCP-1 has a pronounced recruitment effect on the migration of fibroblasts, endothelial cells, smooth muscle cells, and macrophages (Figure 3). Thus, MCP-1, can recruit the appropriate players to carry out inflammatory tissue healing of aneurysms.
Figure 3.
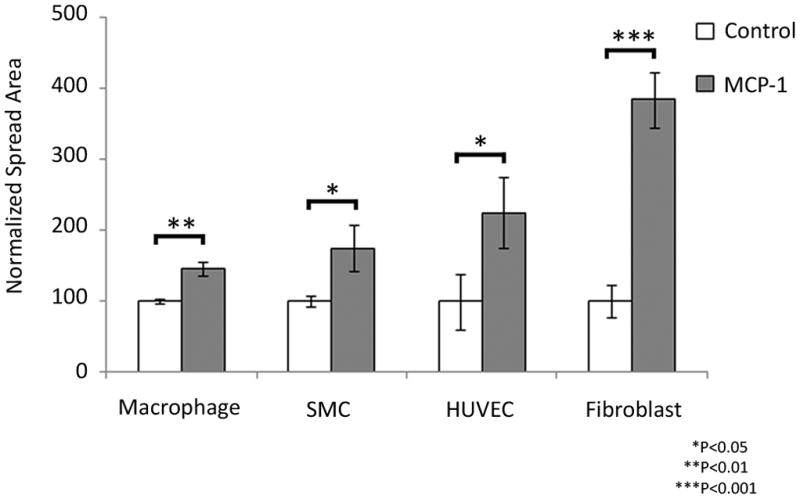
MCP-1 induces in vitro migration of fibroblasts, endothelial cells, smooth muscle cells, and macrophages.
MCP-1 chemotaxis for each specific cell-type was studied using Transwell Permeable Supports with 10 ng/mL of MCP-1 protein (experimental group), or without protein (control group) in the bottom chamber. All cells were seeded on the upper chamber. After the assay was performed, the migratory cells on the bottom side of the membrane were stained and counted. Error bars are means ± s.e.m, *P<0.05, **P<0.01, ***P<0.001.
In vivo, in our murine carotid aneurysm model, the tissue ingrowth and healing promoted by MCP-1 is characterized by significant infiltration of smooth muscle cells, macrophages, fibroblasts, and endothelial cells (Figure 4), replicating what was seen in the cell migration assay. C57BL/6anr. gfpbm aneurysms three weeks after implantation with MCP-1 releasing coils (n=8) are infiltrated with gfp+ cells that are FSP-1+ and CD11b+, demonstrating that fibroblasts and macrophages recruited to intra-aneurysmal tissue ingrowth are derived from the bone marrow (Figure 5). From this, we can conclude that MCP-1 releasing coils induce significantly better intra-aneurysmal tissue healing than PLGA-only coils or bare platinum coils, and that the MCP-1-induced tissue healing is an inflammatory process carried out by fibroblasts, macrophages, smooth muscle cells, and endothelial cells that are derived from the bone marrow and migrate to the site of aneurysm healing via the circulation.
Figure 4.
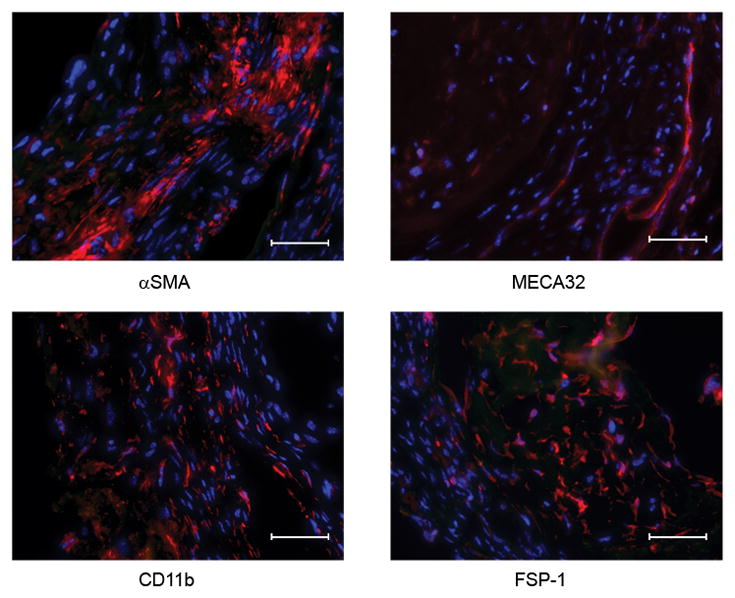
MCP-1-induced intra-aneurysmal tissue healing is infiltrated by macrophages, fibroblasts, smooth muscle cells, and endothelial cells.
Murine carotid aneurysms implanted with MCP-1-releasing coils demonstrate robust intra-aneurysmal tissue ingrowth and immunohistochemical staining demonstrates infiltration with fibroblasts (anti-FSP-1), smooth muscle cells (anti-aSMA), endothelial cells (anti-MECA-32), and macrophages (anti-CD11b). Scale bar, 50 mm.
Figure 5.
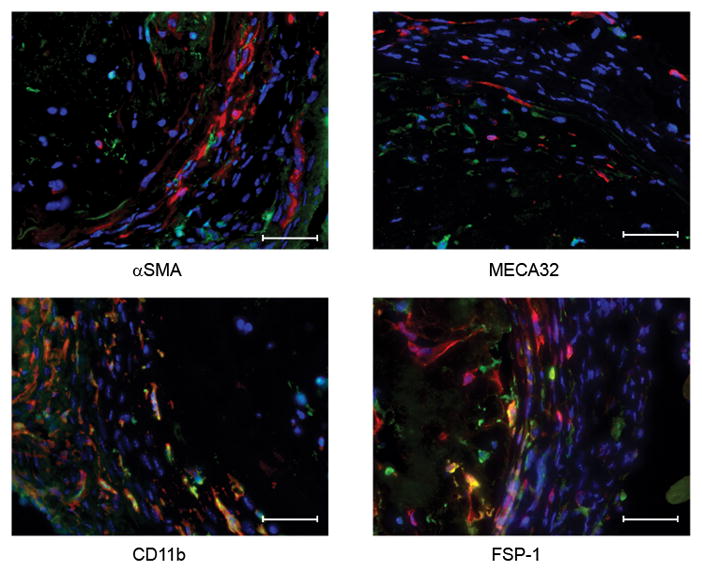
Fibroblasts and macrophages derived from bone marrow infiltrate MCP-1-induced intra-aneurysmal tissue healing.
C57BL/6anr. gfpbm aneurysms three weeks after implantation with MCP-1 releasing coils (n=8) are infiltrated with gfp+ cells that are FSP-1+ and CD11b+, demonstrating that fibroblasts and macrophages recruited to intra-aneurysmal tissue ingrowth are derived from the bone marrow. We did not see gfp+ cells that were SMA+ or MECA-32+. Scale bar, 50 mm.
MCP-1-Mediated Tissue Ingrowth Occurs Via a MIP-1alpha and MIP-2-Regulated Pathway
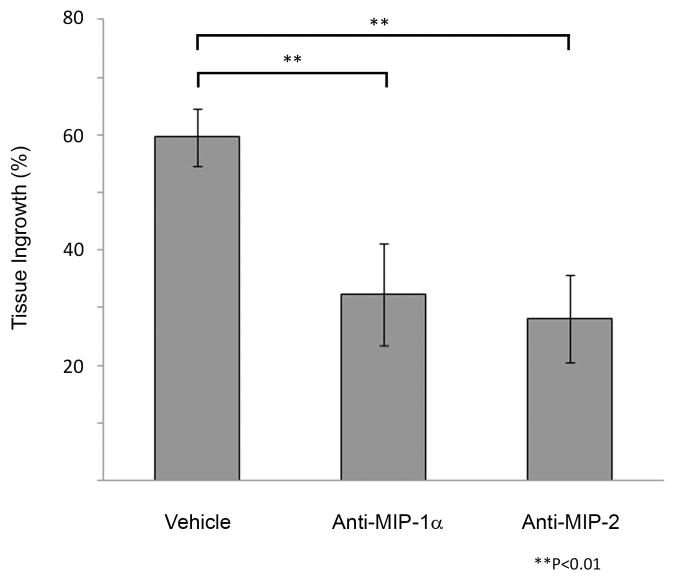
We suspected that other cytokines are activated by MCP-1 in carrying out tissue healing of aneurysms, and to better study the pathway by which MCP-1 initiates this inflammatory repair process, we performed a cytokine array to screen for cytokines upregulated by MCP-1 in our murine carotid aneurysm model. Cytokine array of 59 different cytokines (see Methods for list) performed on aneurysms one day and one week after implantation with MCP-1 releasing coils demonstrates significantly elevated expression of MIP-1α and MIP-2 compared to similar aneurysms after implantation with control PLGA-only coils and control bare platinum coils (Figure 6). To further elucidate the roles of MIP-1α and MIP-2 in MCP-1-mediated intra-aneurysmal tissue ingrowth, mice harboring aneurysms implanted with MCP-1 releasing coils were administered an antibody antagonist of MIP-1α(n=10), antibody antagonist of MIP-2 (n=10), or vehicle (n=10). MIP-1α blocked and MIP-2 blocked aneurysms had significantly reduced tissue ingrowth compared to controls (Figure 7A and 7B). We concluded from these results that MCP-1 upregulates MIP-1α and MIP-2 in carrying out inflammatory tissue healing of aneurysms, and MCP-1-induced aneurysm healing is dependent on MIP-1α and MIP-2.
Figure 6.
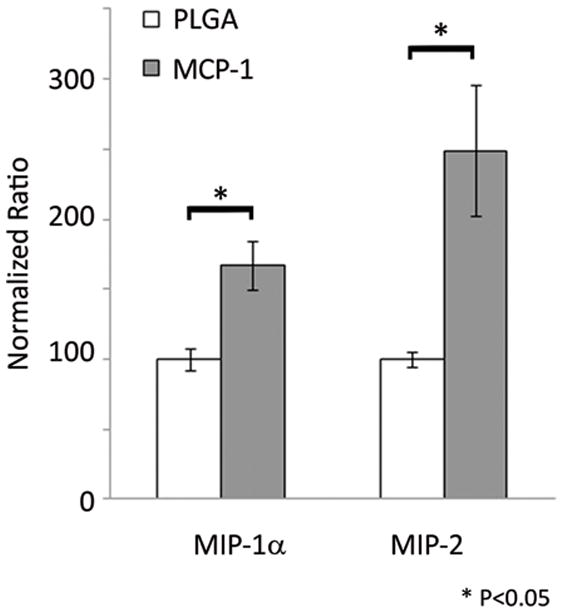
MCP-1 upregulates MIP-1α and MIP-2 expression in inflammatory aneurysm healing.
A cytokine array to screen for cytokines upregulated by MCP-1 in our murine carotid aneurysm model was performed one day and one week after implantation with MCP-1 releasing coils and demonstrated significantly elevated expression of MIP-1α and MIP-2 compared to similar aneurysms after implantation with control PLGA-only coils and control bare platinum coils. Bar graph depicting one day MCP-1 releasing coil vs PLGA-only coil, means ± s.e.m, *P<0.05.
Figure 7.
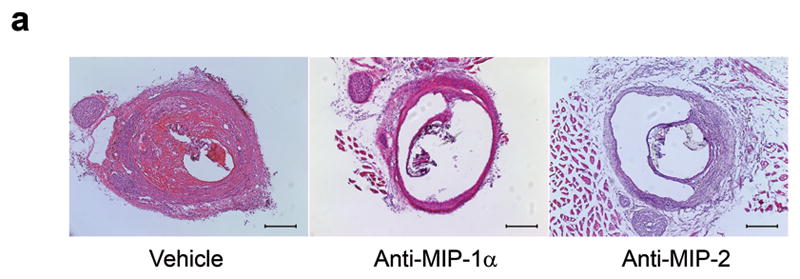
MCP-1-mediated tissue ingrowth is MIP-1a and MIP-2 dependent C57BL/6 mice harboring aneurysms implanted with MCP-1 releasing coils were administered an antibody antagonist of MIP-1α (n=10), antibody antagonist of MIP-2 (n=10), or vehicle (n=10). MIP-1α blocked and MIP-2 blocked aneurysms had significantly reduced tissue ingrowth compared to controls. A) H&E stain of representative aneurysm sections of vehicle, MIP-1α antagonist, and MIP-2 antagonist treated animals. Scale bar, 200 mm. B) Percentage of lumen area occupied by tissue ingrowth as measured by three blinded observers using imaging measurement software, means ± s.e.m, *P<0.05, **P<0.01.
DISCUSSION
Cerebral Aneurysms
Rupture of a cerebral aneurysm can be a devastating event resulting in death in 50% of patients, and permanent disability in more than a third2. Sudden death occurs in 12% who never reach medical attention16. While 24,000 people suffer from a cerebral aneurysm rupture each year in the United States17, up to 5% of the population have a brain aneurysm that is not yet ruptured18. Ruptured cerebral aneurysms are treated with urgent craniotomy and clipping, or endovascular coiling to prevent rebleeding from the aneurysm, but are associated with 30.9% death and disability for clipping, and 23.5% for coiling19. Unruptured cerebral aneurysms are treated with prophylactic clipping or coiling to prevent them from rupturing, but are associated with 10.1–12.6% death and disability for clipping, and 7.1–9.8% for coiling20.
National trends demonstrate that endovascular coiling is the treatment of choice for someruptured and unruptured cerebral aneurysms3, 4. Coiling, however, is associated with recurrence which may occur in up to one quarter of aneurysms21. Clearly, there is a need for a better treatment for ruptured and unruptured cerebral aneurysms.
The aim of cerebral aneurysm treatment should be a safe, effective, durable permanent cure. Histologic analysis of surgically-harvested and autopsy-derived specimens of coiled human cerebral aneurysms demonstrate that durable permanent cure is achieved when there is intra-aneurysmal wound healing characterized by connective tissue proliferation, fibroblasts, collagen, smooth muscle cells, capillary ingrowth, and macrophages7. These are characteristics found in inflammatory-mediated wound healing: 1) macrophage migration and infiltration; 2) fibroblast proliferation; 3) connective tissue proliferation; and 4) capillary arteriogenesis.
MCP-1 in Inflammatory-Mediated Wound Healing
Monocyte chemotactic protein-1 (MCP-1) was originally described as a potent chemoattractant for monocytes, T-cells, NK cells22. It has a critical role in wound healing8, 9, as well as the healing and remodeling response in myocardial infarction13, 23. MCP-1-mediated tissue healing is likely an inflammatory process23, which has different implications in extravascular tissue compared to intravascular healing at the endoluminal wall. At the intravascular endoluminal wall, inflammatory healing of a vascular injury could result in undesired neointimal hyperplasia, and MCP-1 has been implicated in contributing to neointimal hyperplasia14, 24–28.
Intra-aneurysmal healing of cerebral aneurysms is a specific situation where intravascular tissue ingrowth and tissue proliferation is desired. We studied the role of MCP-1, its mechanism, and the potential to harness its actions in intravascular wound healing as a novel therapy to achieve intra-aneurysmal healing in a murine carotid aneurysm model.
If inflammatory-mediated wound healing is the process in which coiled aneurysms progress to a durable permanent cure, then it would seem that MCP-1 would have a critical role in that process. MCP-1 has been shown to mediate all four elements of inflammatory-mediated wound healing (macrophage migration and infiltration; fibroblast proliferation; connective tissue proliferation; and capillary arteriogenesis).
MCP-1 is a potent chemokine for monocytes, macrophages, memory T lymphocytes, and natural killer cells22, 29. MCP-1 expression is significantly expressed in human cutaneous wound healing and coincides with the timing of macrophage migration and infiltration12. MCP-1 has been demonstrated to be critical and necessary for wound healing in murine cutaneous wound models{Low QE, 2001 #1067}.
The role of MCP-1 in inflammatory-mediated wound healing has been studied in models of myocardial infarction. MCP-1 induces macrophage infiltration, neovascularization, and accumulation of cardiac myofibroblasts in a murine model of myocardial infarction13. MCP-1 deficiency results in decreased and delayed macrophage infiltration in healing myocardial infarcts and delayed replacement of injured cardiomyocytes with granulation tissue, and attenuated post-infarction left ventricular remodeling23. In the case of myocardial infarction, inflammatory healing leading to left ventricular remodeling can result in undesired left ventricular dysfunction, and blocking MCP-1 has been shown to reduce left ventricular dysfunction after myocardial infarction30. The receptor for MCP-1 is CCR2, and deficiency of the CCR2 receptor reduces left ventricular dysfunction after myocardial infarction31.
MCP-1 mediates fibroblast proliferation and stimulates fibroblasts to produce collagen32, both key elements in inflammatory-mediated wound healing. MCP-1 is also critical in capillary arteriogenesis, another key finding in inflammatory wound healing. MCP-1 has been shown to induce arteriogenesis in hindlimb ischemia33, 34 and deficiency in CCR2 results in reduced macrophage infiltration and impaired arteriogenesis in hindlimb ischemia35. Endothelial cells express CCR2, the receptor for MCP-1, and MCP-1 promotes endothelial cell migration and inflammatory-activated endothelial wound repair9 and angiogenesis in tumor36 by recruiting macrophages37. Human vascular smooth muscle cells express CCR2, the receptor for MCP-138, and there is evidence that MCP-1 induces smooth muscle cell proliferation39, 40. MCP-1 has been shown to recruit vascular smooth muscle cells and mesenchymal cells towards endothelial cells and is critical for TGF-β-induced angiogenesis in wound healing41.
MCP-1 in Intravascular Inflammatory Healing
Wound healing of cutaneous wounds, myocardial infarction, and hindlimb ischemia are examples of extravascular tissue healing. Endoluminal intravascular wound healing however presents different problems, and the effects of MCP-1-mediated inflammatory healing intravascularly have been found to be deletrious. MCP-1 has been implicated in atherosclerotic disease. MCP-1 has been found in human atherosclerotic lesions and is thought to mediatemacrophage infiltration in atherosclerosis15, 38, 42, 43. MCP-1 recruits monocytes to atheroma and has a critical role in atherosclerosis44–46. Mice deficient in CCR2 have reduced macrophage accumulation in atheroma and reduced atherosclerotic lesion formation47, 48.
A classic model of intravascular inflammatory wound healing is neointimal hyperplasia after arterial injury. MCP-1 has a critical role in neointimal hyperplasia by recruiting inflammatory monocytes to the site of vascular injury14. Blocking MCP-1 has been shown multiple times to inhibit neointimal hyperplasia26, 28, 49; and likewise, deficiency in CCR2 results in reduced neointimal hyperplasia after arterial injury50.
MCP-1 in Intra-aneurysmal Tissue Healing
The deleterious effects of neointimal hyperplasia are due to stenosis of the vessel lumen by the tissue proliferation induced by the inflammatory healing cascade. However, intra-aneurysmal tissue healing represents a unique example in which intravascular tissue proliferation is a desired outcome. The aim of brain aneurysm therapies should be intra-aneurysmal tissue healing characterized by inflammatory elements consisting of connective tissue proliferation, fibroblasts, collagen, smooth muscle cells, capillary ingrowth, and macrophages.
Our study demonstrates that MCP-1 has a recruitment effect on the migration of fibroblasts, smooth muscle cells, endothelial cells, and macrophages; all key players in the inflammatory tissue healing response. Endothelial cells play a critical role in the repair of the endoluminal surface of the aneurysm ostium at the aneurysm-parent artery interface. Fully healed coiled human cerebral aneurysms commonly demonstrate a neo-endothelium at the aneurysm ostium7, and are likely important for preventing thromboembolism as well as providing structural repair of the blood vessel. We have demonstrated that MCP-1 recruits the migration of endothelial cells both in an in vitro cell migration assay, as well as in vivo in an aneurysm repair model.
By creating a coil which releases MCP-1, we were able to demonstrate that MCP-1 promotes intra-aneurysmal tissue ingrowth and healing in murine carotid aneurysms, by the recruitment of fibroblasts, smooth muscle cells, endothelial cells, and macrophages that originate from the bone marrow and migrate to the aneurysm site, and that that pathway is regulated by MIP-1α and MIP-2.
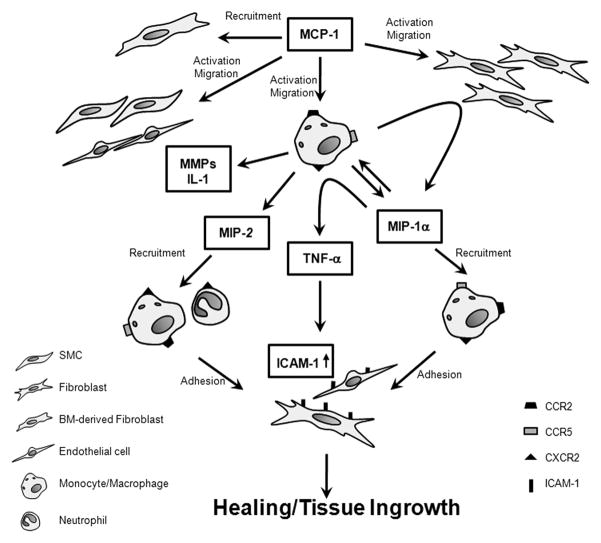
We demonstrate that intravascular MCP-1-mediated inflammatory tissue healing is dependent on MIP-1α and MIP-2. When MIP-1α or MIP-2 are blocked, the tissue ingrowth seen with MCP-1 release in our murine carotid aneurysm model was significantly inhibited. The pathway by which MCP-1 upregulates and is dependent on MIP-1α and MIP-2 has not been elucidated. Figure 8 illustrates the postulated pathway by which MCP-1, MIP-1α and MIP-2 interact in the inflammatory tissue healing cascade.
Figure 8.
Postulated pathway by which MCP-1, MIP-1α and MIP-2 interact in the inflammatory tissue healing cascade.
MCP-1 activates fibroblasts, smooth muscle cells, and monocytes and macrophages. Recruited bone marrow derived monocytes and macrophages release a variety of cytokines including MIP-1α, MIP-2, matrix metalloproteinases, IL-1, and others, which induce further monocyte and macrophage infiltration via autocrine activation. MIP-1α is upregulated by the interaction between macrophages and fibroblasts, and in turn, induces secretion of TNFα which upregulates ICAM-1 expression by fibroblasts, endothelial cells, and macrophages. MIP-1α and MIP-2, which are secreted by activated macrophages, directly recruit the migration of neutrophils, macrophages, and other leukocytes to the site of injury and repair.
Our study and others have demonstrated that MCP-1 activates fibroblasts32, smooth muscle cells39, 40, and monocytes and macrophages22, 29. Recruited bone marrow derived monocytes and macrophages release a variety of cytokines including MIP-1α, MIP-2, matrix metalloproteinases, IL-1, and others, which induce further monocyte and macrophage infiltration via autocrine activation51. MIP-1αis upregulated by the interaction between macrophages and fibroblasts52, and in turn, induces secretion of TNFα which upregulates ICAM-1 expression by fibroblasts53, endothelial cells54, and macrophages55. MIP-1α and MIP-2, which are secreted by activated macrophages, directly recruit the migration of neutrophils, macrophages, and other leukocytes to the site of injury and repair56. Further studies are needed to further define the interaction between MIP-1α and MIP-2 with MCP-1, and the steps in the MIP-1α and MIP-2-regulated pathway upon which MCP-1-mediated inflammatory tissue healing is dependent.
Coils or other endovascular devices designed to release MCP-1, such as the MCP-1 releasing coils in this animal study, could be translated to treatment for human cerebral aneurysms, or may be the first step in developing better biologic therapies as our understanding of the biologic processes of aneurysm healing advance forward. Further studies can examine time of release and amount of MCP-1 or other chemokines to be delivered, other carriers for carrying and releasing chemokines, and possible cocktail combinations of chemokines. There are clear limitation of our murine carotid aneurysm model, such asit is extracranial, and therefore differing in some characteristics of intracranial vessels such as not being surrounded by arachnoid and cerebrospinal fluid; it is created by the application of elastase which clearly does not happen naturally in human cerebral aneurysms; and rodents differ in many anatomic and physiologic ways than humans. Nevertheless, it is a model that can serve as a rapid preclinical screening tool for the biologic study of aneurysm healing, with the hope of eventually translating to therapy for humans..
Acknowledgments
We would like to acknowledge Alex Podlaski, Tristan Tanner, Natalia Cardona, and Marcel Lindsey who were the blinded students who performed measurements of the aneurysm sections. We would like to acknowledge Dan Neal, Ph.D., who performed the statistical analysis.
FUNDING SOURCES
The National Institute of Health (1K08NS067058-01), the Brain Aneurysm Foundation, and the Thomas Maren Foundation.
Footnotes
DISCLOSURES
Brian Hoh: Codman Neurovascular (honorarium)
Koji Hosaka, Daniel P. Downes, Kamil W. Nowicki, Cristina E. Fernandez, Christopher D. Batich, Edward W. Scott: none
References
- 1.Wardlaw JMWP. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn JRG. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain. 2001;124:249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 3.Andaluz NZM. Recent trends in the treatment of cerebral aneurysms: Analysis of a nationwide inpatient database. J Neurosurg. 2008;108:1163–1169. doi: 10.3171/JNS/2008/108/6/1163. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AIVG, Tariq N, Suri MF, Lakshminarayan K, Lanzino G. Impact of international subarachnoid aneurysm trial results on treatment of ruptured intracranial aneurysms in the united states. J Neurosurg. 2010;114:834–841. doi: 10.3171/2010.6.JNS091486. [DOI] [PubMed] [Google Scholar]
- 5.Andaluz N, Zuccarello M. Recent trends in the treatment of cerebral aneurysms: Analysis of a nationwide inpatient database. J Neurosurg. 2008;108:1163–1169. doi: 10.3171/JNS/2008/108/6/1163. [DOI] [PubMed] [Google Scholar]
- 6.Campi ARN, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (isat) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 7.Bavinzski G, Talazoglu V, Killer M, Richling B, Gruber A, Gross CE, Plenk H., Jr Gross and microscopic histopathologic findings in aneurysms of the human brain treated with guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 8.DiPietro LABM, Low QE, Kunkel SL, Strieter RM. Mip-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber KSNP, Gröne HJ, Weber C. Expression of ccr2 by endothelial cells: Implications for mcp-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 10.Hoh BLVG, Wilmer EN, Hosaka K, Fisher RC, Scott EW. A novel murine elastase saccular aneurysm model for studying bone marrow progenitor-derived cell-mediated processes in aneurysm formation. Neurosurgery. 2010;66:544–550. doi: 10.1227/01.NEU.0000365616.46414.2B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thula TTSG, Tran-Son-Tay R, Batich C. Effects of egf and bfgf on irradiated parotid glands. Ann Biomed Eng. 2005;33:685–695. doi: 10.1007/s10956-005-1853-z. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt ETA, Goebeler M, Debus S, Bröcker EB, Gillitzer R. Chemokines il-8, groalpha, mcp-1, ip-10, and mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto HTM, Izawa A, Ise H, Hongo M, Kolattukudy PE, Ikeda U. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res. 2006;99:891–899. doi: 10.1161/01.RES.0000246113.82111.2d. [DOI] [PubMed] [Google Scholar]
- 14.Liehn EAPA, Koenen RR, Soehnlein O, Adage T, Fatu R, Curaj A, Popescu A, Zernecke A, Kungl AJ, Weber C. A new monocyte chemotactic protein-1/chemokine cc motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–1857. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 15.Nelken NACS, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schievink WI, Wijdicks EF, Parisi JE, Piepgras DG, Whisnant JP. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology. 1995;45:871–874. doi: 10.1212/wnl.45.5.871. [DOI] [PubMed] [Google Scholar]
- 17.Group AW. Aha statistical update: Heart disease and stroke statistics—2010 update. A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 19.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 20.ISUIA Investigators. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 21.Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (isat) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 22.Gu LTS, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- 23.Dewald OZP, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. Ccl2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 24.Tatewaki HEK, Kimura S, Nishida T, Morita S, Tominaga R. Blockade of monocyte chemoattractant protein-1 by adenoviral gene transfer inhibits experimental vein graft neointimal formation. J Vasc Surg. 2007;45:1236–1243. doi: 10.1016/j.jvs.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Saiura ASM, Hiasa K, Kitamoto S, Washida M, Egashira K, Nagai R, Makuuchi M. Antimonocyte chemoattractant protein-1 gene therapy attenuates graft vasculopathy. Arterioscler Thromb Vasc Biol. 2004;24:1886–1890. doi: 10.1161/01.ATV.0000141045.49616.6f. [DOI] [PubMed] [Google Scholar]
- 26.Mori EKK, Yamaoka T, Tanii M, Kataoka C, Takeshita A, Usui M, Egashira K, Sugimachi K. Essential role of monocyte chemoattractant protein-1 in development of restenotic changes (neointimal hyperplasia and constrictive remodeling) after balloon angioplasty in hypercholesterolemic rabbits. Circulation. 2002;105:2905–2910. doi: 10.1161/01.cir.0000018603.67989.71. [DOI] [PubMed] [Google Scholar]
- 27.Egashira KZQ, Kataoka C, Ohtani K, Usui M, Charo IF, Nishida K, Inoue S, Katoh M, Ichiki T, Takeshita A. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa YMA, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 29.Charo IFTM. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 30.Hayashidani STH, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–2140. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 31.Kaikita KHT, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of cc chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharaee-Kermani MDE, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 33.van Royen NHI, Buschmann I, Kostin S, Voskuil M, Bode Ch, Schaper W, Piek JJ. Effects of local mcp-1 protein therapy on the development of the collateral circulation and atherosclerosis in watanabe hyperlipidemic rabbits. Cardiovasc Res. 2003;57:178–185. doi: 10.1016/s0008-6363(02)00615-6. [DOI] [PubMed] [Google Scholar]
- 34.van Royen NHI, Böttinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein e-deficient mice but induces systemic monocytic cd11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218–225. doi: 10.1161/01.res.0000052313.23087.3f. [DOI] [PubMed] [Google Scholar]
- 35.Heil MZT, Wagner S, Fernández B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 36.Salcedo RPM, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express ccr2 and respond to mcp-1: Direct role of mcp-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 37.Niiyama HKH, Yamamoto T, Shimada T, Sasaki K, Murohara T, Egashira K, Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol. 2004;44:661–666. doi: 10.1016/j.jacc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Hayes IMJN, Towers S, Smith G, Paterson JR, Earnshaw JJ, Roach AG, Westwick J, Williams RJ. Human vascular smooth muscle cells express receptors for cc chemokines. Arterioscler Thromb Vasc Biol. 1998;18:3970–3403. doi: 10.1161/01.atv.18.3.397. [DOI] [PubMed] [Google Scholar]
- 39.Viedt CVJ, Athanasiou T, Shen W, Orth SR, Kübler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappab and activator protein-1. Arterioscler Thromb Vasc Biol. 2002;22:914–920. doi: 10.1161/01.atv.0000019009.73586.7f. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe TPR, Katagiri T, Benedict CR. Monocyte chemotactic protein 1 amplifies serotonin-induced vascular smooth muscle cell proliferation. J Vasc Res. 2001;38:341–349. doi: 10.1159/000051065. [DOI] [PubMed] [Google Scholar]
- 41.Ma JWQ, Fei T, Han JD, Chen YG. Mcp-1 mediates tgf-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109:987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- 42.Ylä-Herttuala SLB, Rosenfeld ME, Särkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeya MYT, Leonard EJ, Takahashi K. Etection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 44.Aiello RJBP, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 45.Gu LOY, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 46.Gosling JSS, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. Mcp-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein b. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boring LGJ, Cleary M, Charo IF. Decreased lesion formation in ccr2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 48.Dawson TCKW, Osahar TA, Maeda N. Absence of cc chemokine receptor-2 reduces atherosclerosis in apolipoprotein e-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 49.Egashira KZQ, Kataoka C, Ohtani K, Usui M, Charo IF, Nishida K, Inoue S, Katoh M, Ichiki T, Takeshita A. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- 50.Roque MKW, Gazdoin M, Malik A, Reis ED, Fallon JT, Badimon JJ, Charo IF, Taubman MB. Ccr2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554–559. doi: 10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 51.Lambert JMLE, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinhauser MLKS, Hogaboam CM, Evanoff H, Strieter RM, Lukacs NW. Macrophage/fibroblast coculture induces macrophage inflammatory protein-1alpha production mediated by intercellular adhesion molecule-1 and oxygen radicals. J Leukoc Biol. 1998;64:636–641. doi: 10.1002/jlb.64.5.636. [DOI] [PubMed] [Google Scholar]
- 53.Okada NFK, Takano Y, Dogru M, Tsubota K, Fujishima H, Matsumoto K, Nakajima T, Saito H. The implications of the upregulation of icam-1/vcam-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci. 2005;46:4512–4518. doi: 10.1167/iovs.04-1494. [DOI] [PubMed] [Google Scholar]
- 54.Mackay FLH, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (tnf-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one tnf receptor type, tnf-r55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahey TJTK, 3rd, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 56.Wang LLY, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. Mcp-1, mip-1, il-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]