Abstract
Detection of Mycoplasma genitalium-mediated, chlamydia-negative nongonococcal urethritis and other M. genitalium-linked infectious etiologies has been very challenging. Although M. genitalium is considered a leading cause of genitourinary symptoms in men and women, extreme difficulties in its cultivation due to its highly fastidious nature and the lack of routine and effective diagnostic tests have slowed the generation of clinical data which directly implicate the presence of M. genitalium in disease pathogenesis. In this study, we compared enzyme-linked immunosorbent assays (ELISAs) and immunoblot and PCR assays in M. genitalium culture-positive women over 1 to 3 years of clinical visits to determine the usefulness of independent diagnostic strategies. Furthermore, the value of combinatorial diagnostic assessments is described, which provides insights into the dynamics of M. genitalium-host interactions. Overall, we show that neither ELISA nor PCR, alone or in combination, provides the sensitivity required to confidently predict the existence of viable M. genitalium organisms in cervical and vaginal samples. Additionally, culture-positive women exhibited a range of antibody responsiveness to M. genitalium based upon ELISA and immunoblot assessments, indicating immune diversity among this high-risk population.
Mycoplasma genitalium holds a distinct position in the spectrum of emerging pathogenic bacteria for humans. It is a major cause of nongonococcal urethritis in men; has been directly linked to cervicitis, endometritis, and pelvic inflammatory disease in women; and has been isolated, along with the human pathogen Mycoplasma pneumoniae, from the respiratory tracts and synovial fluid of diseased individuals (3, 5-7, 9, 12-14, 17, 19, 22, 25, 27, 28). M. genitalium is also the smallest known self-replicating cell, with a genome size of 580 kb, which imposes severe biosynthetic limitations. The highly fastidious nature of M. genitalium is reflected in the absence of direct isolations from urethral specimens in cell-free media since it was first reported >20 years ago (26, 28). The most recent successes in M. genitalium isolation from clinical samples were reported in 1988 and 1995 from extragenital sites (3, 27) and from urethral specimens in 1996 (15); the last required the use of Vero cells and “blind passage.” Because of extreme difficulties in cultivating M. genitalium, many researchers have relied on the results of serological and PCR assays to establish links between M. genitalium and human disease. Challenge studies of animals, specifically chimpanzees, have reinforced the pathogenic capabilities of M. genitalium (20, 23, 24, 29).
We have been studying a group of minority women who were recruited from public health clinics in San Antonio, Tex., into a controlled randomized trial of behavioral-cognitive interventions to reduce the recurrence of sexually transmitted diseases (STDs). During a 6-month period in 2000, we were able to establish axenic primary cultures of M. genitalium using vaginal and cervical specimens from 31 women, and these mycoplasmas were subsequently passaged and single-colony cloned. In this report, with follow-up complete through the end of 2002, we describe the relationships among enzyme-linked immunosorbent assay (ELISA) and immunoblot and PCR assays as diagnostic tools in M. genitalium culture-positive women over a 17- to 39-month period of clinical visits.
MATERIALS AND METHODS
Human subjects.
The subjects were low-income minority women enrolled in a randomized controlled trial of behavioral-cognitive interventions to reduce the recurrence of STDs. A subpopulation (n = 31) from whom positive M. genitalium cultures were established were the focus of this investigation, which was approved by the institutional review boards of The University of Texas Health Science Center at San Antonio (UTHSCSA) and the San Antonio Metropolitan Health District.
Collection of clinical specimens.
At each clinic visit, cervical and vaginal specimens were obtained with sterile cotton swabs during physical examination, and individual samples were inoculated into tubes containing 3 ml of SP-4 medium with 10 μl of fungizone (Gibco-BRL)/ml and 500 U of penicillin G/ml. In addition, vaginal secretions were collected from each participant by using a large sterile cotton swab, which was placed in 20 ml of sterile phosphate-buffered saline (PBS). All cervical and vaginal samples were transported to the UTHSCSA for processing on the day of collection. Also, whole blood and serum fractions were collected and stored at −80°C. Baseline values for negative ELISA and immunoblot assays were established by using stored serum samples from 54 pregnant women (primarily Hispanic) with no known history of STDs. The use of anonymous stored samples was approved by the UTHSCSA institutional review board.
Processing of clinical specimens.
Aliquots of cervical and vaginal specimens were processed for culture by first diluting samples 10−1 in SP-4 medium containing 10 μl of fungizone/ml and 0.25 mg of ciprofloxacin/liter; the latter eliminated or limited overgrowth of common microbial flora, especially Mycoplasma hominis, but had no effect on M. genitalium, which is resistant to this level of ciprofloxacin (data not shown). Then, the culture broths were passed through 0.45-μm-pore-size filters (Pall-Gelman) to reduce the additional microbial load and serially diluted to 10−3 in fresh SP-4 broth. All dilutions were placed in 24-well tissue culture plates and incubated at 37°C in an atmosphere of 10% (vol/vol) CO2 for 1 month. The remaining specimen material was stored at −80°C. The cultures were routinely examined for changes in pH and signs of microbial growth.
Identification of M. genitalium-positive cultures from patients.
Initial evidence of M. genitalium growth was determined by acidic pH change in SP-4 broth and microscopic detection of surface-adhering colonies. Broth-grown clinical isolates fulfilling these criteria were further examined by inoculation of SP-4 agar plates and incubation at 37°C for 2 to 3 weeks. Visible colonies were tested for hemadsorption of sheep erythrocytes (BioWhittaker) (10), and hemadsorption-positive colonies were further tested by immunofluorescence using fluorescein isothiocyanate-conjugated M. genitalium antibodies and PCR analysis (see below). Individual colonies that met all of the criteria were picked and grown in SP-4 broth at 37°C and processed through 0.45-μm-pore-size filters to remove clumps, followed by single-colony cloning on SP-4 agar; this procedure of broth-to-agar passage and colony cloning was repeated three more times in order to generate specific single-cell clones of M. genitalium. Each clone was expanded in SP-4 broth and further analyzed using PCR and immunoblot criteria before stock cultures were established, which were then stored at −80°C in 1-ml aliquots.
M. genitalium ELISA.
The ELISA protocol and preparation of lipid-associated membrane proteins (LAMPs) of M. genitalium were described previously (31). Briefly, LAMPs (0.5 μg/ml) were extracted using Triton X-114 and diluted in carbonate-bicarbonate buffer (pH 9.6), and 50 μl was added to each well of microtiter plates (Dynex HBX-4), which were incubated at 4°C overnight. After the wells were blocked with 50% horse serum in PBS for 2 h at room temperature, 50 μl of human sera diluted 10−2 in blocking solution was added to each well in duplicate and incubated for 2 h at room temperature. Subsequent steps at room temperature included the addition of 50 μl of 10−3 goat anti-human immunoglobulin G (IgG) conjugated to alkaline phosphatase (Invitrogen) in blocking reagent for 2 h, followed by 30-min development with 0.2 mg of p-nitrophenyl phosphate/ml in 0.1 M diethanolamine. The plates were washed three times with PBS (pH 7.2) after each step. Appropriate positive serum controls, obtained from STD study group participants and verified by immunoblotting using M. genitalium protein profiles, were included. Negative serum controls were obtained from the group of 54 pregnant women described above.
Immunoblotting of M. genitalium proteins.
For immunoblot analyses, 12 μg of M. genitalium LAMPs was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on 4 to 12% NuPage preparative gels (Invitrogen) under reduced conditions. The proteins were semidry transferred onto Protran BA83 nitrocellulose membranes (Schleicher & Schuell) for 1 h at 4°C in buffer containing 0.1% SDS, followed by overnight blocking at 4°C in Tris-buffered salt solution (TBS; pH 8.0) containing 5% nonfat milk. Individual membranes were washed in TBS with 0.01% Tween 20 (TBS-T), air dried, and cut into 12 strips. The strips were rewetted in TBS-T containing 2% nonfat dry milk plus individual patient serum at a dilution of 10−2. After the strips were incubated for 2 h at room temperature and washed, a dilution of 2 × 10−3 goat anti-human IgG conjugated to alkaline phosphatase (Invitrogen) in TBS-T was added, and incubation continued for 2 h at room temperature. The strips were washed extensively with TBS-T and developed for 5 min with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma). Negative control sera were obtained from within the negative control group of 54 pregnant women. Rabbit antisera generated against the major M. genitalium adherence-associated proteins, P140 and P110, served as positive controls, and in this case, goat anti-rabbit IgG conjugated to alkaline phosphatase (Invitrogen) was used as an indicator.
M. genitalium PCR.
For PCR analysis, aliquots of vaginal and cervical specimens, stored at −80°C, were thawed on ice and centrifuged at 16,000 × g. The supernatants were discarded, and the individual pellets were resuspended in 1 ml of OmniPur sterile water (EM Science) and incubated for 20 min on ice, followed by another centrifugation. The pellets were resuspended in 200 μl of water and boiled for 15 min. M. genitalium-specific P140 adhesin primers and probe (Mg1, Mg2, and MG-1, respectively) were used for detection of M. genitalium (8). Each 50-μl reaction mixture contained 37.25 μl of extracted sample; 150 μM (each) dATP, dCTP, dGTP, and dTTP; 0.1 μM each primer, and 5.0 U of Platinum Taq DNA polymerase (Invitrogen) in 1× PCR buffer containing 5 mM MgCl2. Test samples were placed in a PE 9700 automated DNA thermal cycler (Perkin-Elmer) and held at 95°C for 5 min. Then, samples were denatured at 95°C for 1 min and the primers were annealed at 55°C for 1 min and extended at 72°C for 1 min for a total of 40 cycles. The samples were held at 72°C for 10 min to complete extension of the primers, followed by 4°C until the samples were analyzed. Stringent precautions were taken during extraction and preparation to prevent contamination, which included use of an isolated and dedicated room for M. genitalium PCR assays. Internal water controls were included at each step to monitor foreign DNA contamination. Positive controls, which were composed of M. genitalium genomic DNA at concentrations of 1.0 and 0.1 pg and 10 fg, were utilized to ensure protocol sensitivity and assay integrity.
Positive PCR products were identified by Southern blot analysis. Samples were electrophoresed through 1% agarose gels, and the DNA products were transferred onto nylon membranes (Zeta Probe) for 15 to 20 min at 500 mA using a semidry transfer unit (Bio-Rad). The membranes were subjected to UV irradiation at 120,000 μJ per cm2, followed by prehybridization at 50°C for 2 to 3 h in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.2% SDS-5× Denhardt's solution plus 100 μg of denatured salmon sperm DNA/ml. Hybridization was performed at 50°C overnight by placing the membranes in 5× SSC, 0.2% SDS, 5× Denhardt's solution, 100 μg of denatured salmon sperm DNA/ml, and P32 end-labeled oligonucleotide probe. After hybridization, the membranes were washed with 6× SSC and 0.1% SDS for 3 to 5 min at 50°C, followed by two successive washes with 2× SSC and 0.1% SDS for 20 min each time at 50°C. The membranes were air dried and exposed to XOMAT-AR (Kodak) film with intensifying screens overnight at room temperature. Positive samples were scored by the appearance of a single hybridization band (374 bp) as observed with positive M. genitalium DNA controls, provided that all internal negative controls demonstrated no signal.
RESULTS
M. genitalium culture-positive patients.
Evidence of M. genitalium growth was observed 14 to 20 days after inoculation of SP-4 broth with cervical or vaginal specimens from 31 subjects. Broth cultures that demonstrated pH change and contained surface-adhering microbial colonies were further processed as described in Materials and Methods. Identification of M. genitalium was confirmed by immunoblot and PCR analyses. Twenty-six M. genitalium primary cultures were obtained from cervical specimens, 24 were obtained from vaginal specimens, and 3 were obtained from vaginal secretions diluted in PBS. Among the 31 culture-positive women, 21 yielded M. genitalium cultures from both cervical and vaginal samples.
Comparative ELISA-based reactivities to M. genitalium among negative control and culture-positive women.
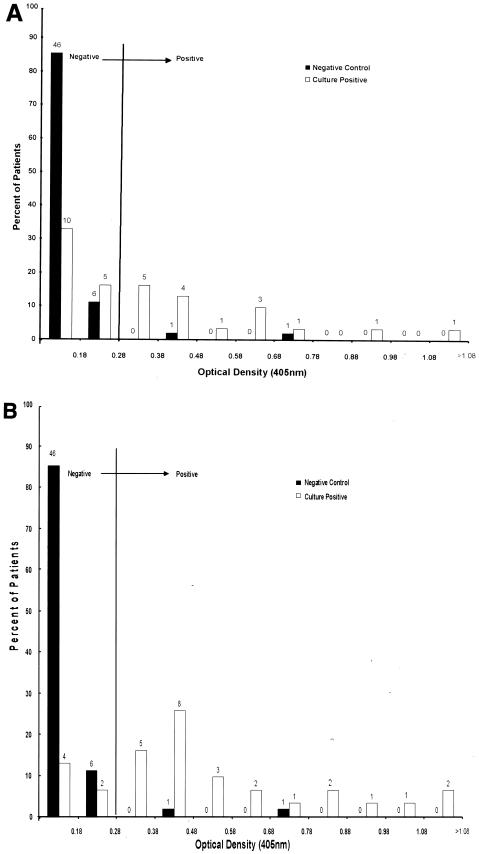
M. genitalium LAMP-coated microtiter plates were used to compare antibody reactivities in single serum samples obtained from 54 asymptomatic pregnant women (the negative control group) and 31 M. genitalium culture-positive women with STD. Figure 1A presents ELISA values for culture-positive individuals at the time of M. genitalium isolation, and Fig. 1B presents ELISA values for serum samples that provided the highest ELISA values (i.e., peak seroconversion) recorded over the duration of clinic visits by each culture-positive participant. We identified two clear outliers in the ELISA distribution for the negative controls. The mean of the sums of optical densities at 405 nm (OD405) for the remaining 52 negative control women plus 3 standard deviations, or 0.28, was defined as the boundary which distinguished negative from positive ELISA categories. In Fig. 1A, which shows ELISA values for all 54 negative control women, 96.3% of the negative control group exhibited immune reactivities of <0.28 OD405 units, and of these individuals, >65% had OD405 values of <0.1. In contrast, 51.6% of M. genitalium culture-positive women had OD405 values of 0.28 or higher, and 22.6% were above 0.5. In the culture-positive category, only 31 (15.5%) out of 200 individual serum samples tested over the 17- to 39-month period of clinic visits had OD405 values of <0.1. Furthermore, as shown in Fig. 1B, the proportion of culture-positive women who seroconverted (OD405 ≥ 0.28) was 80.6%, with 35.5% exhibiting OD405 values of >0.5.
FIG. 1.
(A) Comparative distribution of ELISA values against M. genitalium LAMP antigens among negative control samples (n = 54) and M. genitalium culture-positive serum samples (n = 31) at time of culture. The negative cutoff (OD405 = 0.28) was determined as 3 standard deviations above the negative control mean. The numbers above individual bars represent the number of patients in each group. (B) Same as panel A except that ELISA values for the culture-positive group correspond to the clinic visit at which each woman's peak antibody titer was achieved.
Comparative immunoblotting profiles of M. genitalium LAMPs among negative-control and culture-positive women.
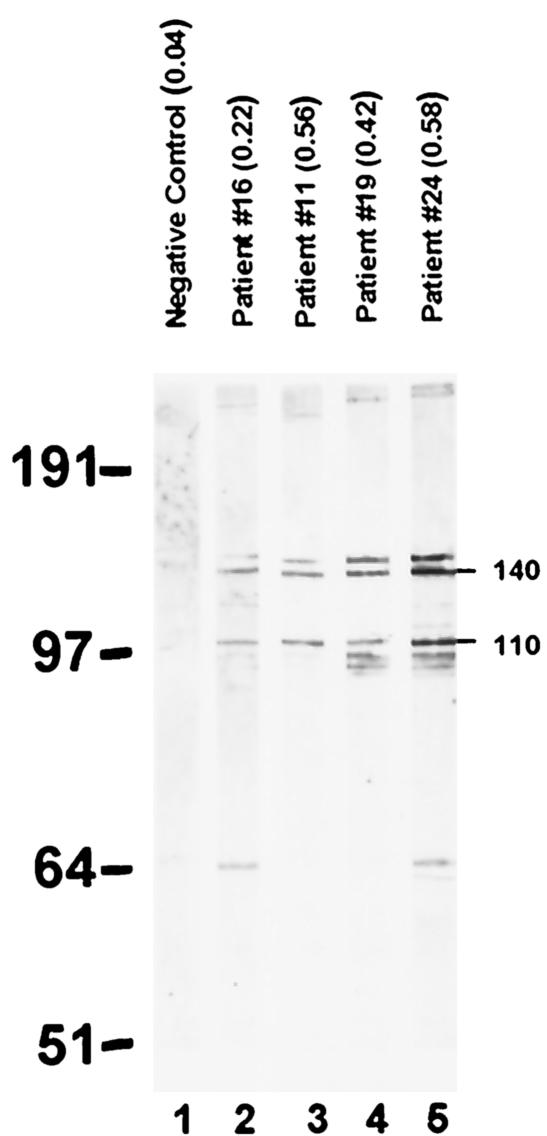
The immunoblot reactivities of M. genitalium LAMP proteins were assessed for negative control and culture-positive sera in order to identify predominant immunogenic M. genitalium LAMPs. All serum samples were read blindly. As indicated in Fig. 2, representative antisera with a range of ELISA values (strips 2 to 5; OD405 values, 0.22 to 0.58) demonstrated various degrees of immunoblot reactivities to 146 (P146)-, 140 (P140; MG191)-, and 110 (P110; MG192)-kDa proteins of M. genitalium. All 31 women at the time of positive M. genitalium culture exhibited positive immunoblots, which scored either 1+ (weak; lanes 2 and 3), 2+ (moderate; lane 4), or 3+ (strong; lane 5), depending upon the protein band intensities. This suggests, using ≥1+ as the definition of test positivity, that the immunoblot assay was 100% sensitive for detection of M. genitalium. The sensitivity of the immunoblot assay using ≥2+ as the definition of test positivity was 41.9% (13 of 31 samples had immunoblot values of 2+ or 3+). In the latter case (i.e., 3+), LAMP antigens with lower molecular masses were occasionally detected. The identities of P140 and P110 as cytadherence-related proteins (2, 11) were confirmed by parallel immunoblots using rabbit monospecific and mouse monoclonal antibodies generated against the proteins and M. genitalium mutants lacking the proteins (data not shown). Interestingly, P146 (the band above P140) reacted to anti-P140 antibodies, suggesting that it may represent the “doublet” P140 reported earlier (18) or preprocessed P140. Representative negative control sera were individually tested, and each produced essentially no immunoblot reactivity (Fig. 2, strip 1 represents pooled negative control sera from five women) except for the two outliers (Fig. 1A), who may have been infected with M. genitalium. In those cases, both sera (ELISA values, 0.41 and 0.69) demonstrated positive immunoblot patterns similar to those presented in Fig. 2, lanes 2 and 4.
FIG. 2.
Immunoblot analysis of serum antibody reactivities to M. genitalium LAMP antigens among negative control and M. genitalium culture-positive women. Lane 1, negative control pooled sera; lanes 2 to 5, representative profiles from culture-positive individuals (corresponding ELISA values are given in parentheses). The band intensities of M. genitalium P146, P140, andP110 were scored as 1+ (lanes 2 and 3), 2+ (lane 4), and 3+ (lane 5) in comparison to the negative control.
In order to determine possible relationships between immunoblot intensities and ELISA values among all culture-positive individuals at the time of culture, immunoblot protein band profiles were compared with OD ELISA readings (Table 1). Among 18 women with immunoblot scores of 1+, the median ELISA value was 0.17 (range, 0.05 to 0.93). It should be noted that 4 of these 18 sera reached or exceeded the ELISA negative cutoff of 0.28, with two having OD405 values of 0.56 and 0.93. Among eight women with immunoblot scores of 2+, the median ELISA value was 0.33. Again, in this group, there was considerable variation among the ELISA values (range, 0.13 to 1.56). Among five women with immunoblot scores of 3+, the median ELISA value was 0.62. In this group, the clustering of ELISA values was much tighter (range, 0.41 to 0.69). We used the Wilcoxon rank sum test to assess group differences in ELISA scores among the three immunoblot groups (i.e., 1+, 2+, and 3+). All comparisons were statistically significant, with exact P values of 0.047 for 1+ versus 2+, 0.030 for 2+ versus 3+, and 0.004 for 1+ versus 3+.
TABLE 1.
M. genitalium-specific serological and PCR assessments at time of positive M. genitalium culture
| STD Patient no. | ELISA valuea | Immunoblot scoreb | PCR resultc |
|---|---|---|---|
| 6 | 0.05 | 1+ | − |
| 23 | 0.06 | 1+ | − |
| 26 | 0.08 | 1+ | + |
| 31 | 0.10 | 1+ | − |
| 13 | 0.12 | 1+ | − |
| 25 | 0.12 | 1+ | + |
| 14 | 0.13 | 1+ | − |
| 15 | 0.13 | 2+ | − |
| 22 | 0.13 | 1+ | − |
| 12 | 0.15 | 1+ | − |
| 4 | 0.18 | 1+ | + |
| 9 | 0.20 | 1+ | − |
| 29 | 0.20 | 1+ | − |
| 16 | 0.22 | 1+ | − |
| 10 | 0.25 | 1+ | − |
| 30 | 0.29 | 2+ | − |
| 2 | 0.30 | 2+ | + |
| 5 | 0.33 | 2+ | − |
| 17 | 0.33 | 2+ | + |
| 21 | 0.34 | 2+ | + |
| 19 | 0.40 | 2+ | + |
| 7 | 0.41 | 3+ | − |
| 1 | 0.42 | 1+ | − |
| 3 | 0.47 | 1+ | − |
| 11 | 0.56 | 1+ | + |
| 24 | 0.58 | 3+ | + |
| 27 | 0.62 | 3+ | + |
| 28 | 0.65 | 3+ | + |
| 8 | 0.69 | 3+ | − |
| 18 | 0.93 | 1+ | − |
| 20 | 1.56 | 2+ | + |
ELISA values are expressed as OD405.
Immunoblot scores are determined by subjective analysis of LAMP band intensities: 3+, strong; 2+, moderate; 1+, weak compared to negative controls (see Fig. 2).
PCR results are based upon presence (+) or absence (−) of predicted amplified product (374-bp fragment) by hybridization.
M. genitalium PCR analyses of vaginal and cervical specimens.
Vaginal and cervical specimens obtained at the time of M. genitalium isolation were analyzed by PCR and Southern blotting and examined in relation to ELISA and immunoblot data (Table 1). Only 12 of the 31 subjects were PCR positive at the time of culture. Interestingly, individual specimens provided diverse PCR patterns, ranging from positive amplification in both cervical and vaginal samples through positive amplification in either sample to negative results in both samples. We routinely detected amplified products from M. genitalium DNA-positive control samples and vaginal and cervical specimens as low as 1 to 10 genome copies per sample. Extrinsic DNA contamination was extremely rare.
We examined the PCR results in more depth, to further characterize the culture-positive women who tested negative by PCR assay. Among all 31 women, the number of visits with PCR data ranged from 3 to 12, with an average of 6.9. In analyses of women with at least six PCR tests (n = 20) versus women with fewer than six (n = 11), we found that women with more tests were more likely to have at least one positive PCR result (95.0 versus 54.6%; P = 0.006) and had more PCR-positive results over the study period (an average of 2.4 versus 0.6; P = 0.0001).
Correspondence between assays and combined sensitivity of assays.
Of the 31 culture-positive individuals, 38.7% were positive by PCR for M. genitalium DNA at the time of culture and 80.6% were PCR positive at some point over the 17- to 39-month period of scheduled visits (Table 2). Most individuals with at least one positive PCR also had at least one positive ELISA during the study period (84.0%). Of the group of STD patients negative by PCR for M. genitalium DNA at positive culture (n = 19), 36.8% were ELISA positive. Of the remaining 12 patients negative by PCR and by ELISA at the time of positive culture, 8 were positive by PCR and 7 were positive by ELISA at some other time over the duration of their clinic visits. Only two women (6.5%) were negative for both PCR and ELISA over the entire study period.
TABLE 2.
ELISA and PCR assessment of M. genitalium culture-positive individuals at time of culture and during 17- to 39-month period of clinic visits
| Patient group | % at time of culture | No. of patients in group at time of positive culture | % at clinic visits during 17- to 39-month period | No. of patients in group throughout 17- to 39-month period |
|---|---|---|---|---|
| PCR+ ELISA+ | 29.0 | 9 | 67.7 | 21 |
| PCR+ ELISA− | 9.7 | 3 | 12.9 | 4 |
| PCR− ELISA+ | 22.6 | 7 | 12.9 | 4 |
| PCR− ELISA− | 38.7 | 12 | 6.5 | 2 |
| Total | 100 | 31 | 100 | 31 |
We examined PCR, ELISA, and immunoblotting to assess their combined sensitivity at the time of positive culture. Using PCR alone, 12 of 31 women (38.7%) were correctly identified as positive. Using ELISA alone, 16 (51.6%) were identified; and with PCR and ELISA together, 19 (61.3%) were identified. When either PCR or ELISA was combined with immunoblot data (the last was considered positive at 2+ or 3+), 17 women (54.8%) were correctly identified as positive; when all three assays were combined, 20 women (64.5%) were identified as positive.
Distinct seroconversion ELISA patterns among M. genitalium culture-positive individuals.
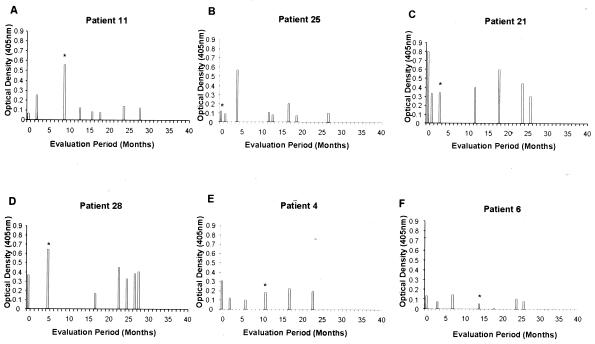
Serum samples from 31 culture-positive individuals were collected at each visit over the 17 to 39 months of follow-up (average number of visits per woman, 7) (Table 3), and M. genitalium LAMP ELISAs were performed. This permitted a longitudinal comparison of ELISA values from each patient in order to identify points at which peak seroconversion occurred and relate the timing of peak seroconversion to the time of positive culture. Two subjects were eliminated from this comparison because of limited serum samples available for evaluation. Six representative samples of ELISA patterns over time appear in Fig. 3 (also refer to Table 3). As can be observed, patient 11 exhibited the highest ELISA value (OD405 = 0.56) at the time of culture, and patient 25 seroconverted 4 months after M. genitalium isolation (OD405 = 0.59). Patient 21, however, exhibited peak seroconversion (OD405 = 0.80) 3 months before isolation, with a second peak 15 months after isolation. Patient 28 exhibited peak antibody levels at the time of culture, with a second peak between 1.5 and 2 years later. Patients 4 and 6 show no clear peak, although patient 4 slightly exceeded the threshold OD405 value of 0.28 11 months before isolation of M. genitalium.
TABLE 3.
Longitudinal evaluation of 31 M. genitalium culture-positive patients over a 17- to 39-month period of clinic visits
| Patient no. | No. of mo enrolled | No. of visits analyzeda | ELISA rangeb | No. of visits positive by PCRc |
|---|---|---|---|---|
| 6 | 26 | 9 | 0.01-0.15 | 1 |
| 23 | 22 | 3 | 0.03-0.10 | 0 |
| 26 | 25 | 6 | 0.02-0.10 | 3 |
| 31 | 25 | 5 | 0.08-0.17 | 0 |
| 13 | 32 | 9 | 0.07-0.27 | 2 |
| 25 | 27 | 8 | 0.07-0.57 | 3 |
| 14 | 29 | 8 | 0.07-0.31 | 2 |
| 15 | 20 | 6 | 0.10-0.19 | 2 |
| 22 | 30 | 9 | 0.07-0.40 | 0 |
| 12 | 25 | 5 | 0.13-0.30 | 0 |
| 4 | 23 | 7 | 0.10-0.31 | 1 |
| 9 | 35 | 12 | 0.10-0.60 | 3 |
| 29 | 24 | 10 | 0.06-0.47 | 2 |
| 16 | 29 | 11 | 0.14-0.29 | 2 |
| 10 | 23 | 8 | 0.09-0.30 | 2 |
| 30 | 24 | 4 | 0.10-0.38 | 1 |
| 2 | 33 | 5 | 0.17-0.46 | 1 |
| 5 | 31 | 9 | 0.31-0.74 | 3 |
| 17 | 21 | 6 | 0.31-0.49 | 3 |
| 21 | 26 | 8 | 0.23-0.80 | 6 |
| 19 | 24 | 4 | 0.23-0.42 | 1 |
| 7 | 22 | 4 | 0.11-0.41 | 0 |
| 1 | 17 | 4 | 0.25-0.42 | 0 |
| 3 | 21 | 9 | 0.12-0.47 | 1 |
| 11 | 28 | 9 | 0.06-0.56 | 3 |
| 24 | 24 | 9 | 0.33-1.02 | 2 |
| 27 | 23 | 5 | 0.34-1.24 | 1 |
| 28 | 28 | 9 | 0.17-0.65 | 6 |
| 8 | 39 | 7 | 0.36-0.87 | 1 |
| 18 | 23 | 4 | 0.53-0.93 | 2 |
| 20 | 27 | 3 | 0.52-1.56 | 1 |
Number of visits analyzed, number of independent clinic visits during the evaluation period during which serum and vaginal and cervical specimens were obtained.
ELISA range, lowest to highest values obtained, expressed as OD405.
Number positive by PCR, number of positive hybridizations from either vaginal or cervical samples, with the maximum number from a single visit equal to 1 (whether or not cervical and vaginal specimens were both positive).
FIG. 3.
Six representative serological patterns from M. genitalium culture-positive patients evaluated over multiple visits. Peak antibody levels appeared at the time of mycoplasma isolation (A), 4 months after mycoplasma isolation (B), 3 months prior to mycoplasma isolation with a second peak at 18 months (C), and at the time of isolation with a second peak between 23 and 28 months (D). Patterns E and F show no obvious peak antibody titer throughout the 40-month period. The asterisks indicate the time of M. genitalium isolation.
Of the 29 individuals evaluated, 13% had seroconverted (i.e., OD405 ≥ 0.28) prior to positive culture (for example, patients 4, 21, and 28), 32% seroconverted at the time of positive culture (for example, patients 11 and 28), and 26% were found to have seroconverted after positive culture (for example, patient 25). No seroconversions were observed among 5 (17%) of the 29 individuals (for example, patient 6). Therefore, no predictable pattern emerges to suggest a direct relationship between peak ELISA values and time of culture positivity.
DISCUSSION
Mycoplasmas play important roles in acute and chronic human diseases and are associated with a spectrum of complicated sequelae (4, 5, 16, 21). The latter can arise many months after the initial infectious process and include arthritides, central and peripheral nervous system involvement, pancreatitis, and respiratory and heart-associated pathologies, including asthma and pericarditis. Among the pathogenic mycoplasmas, M. genitalium has a particularly interesting history. Since its first isolation from male urethral specimens directly onto cell-free SP-4 medium in 1981, only three reports have described the isolation of M. genitalium from humans (3, 15, 27) and none has duplicated the original isolation of M. genitalium from the genitourinary tract in primary axenic cultures until now. The advent of sensitive and specific serological and PCR assays has linked M. genitalium to a range of genitourinary symptoms, indicating a much broader impact of M. genitalium on STD pathogenesis. Additional evidence suggests that M. genitalium infects extragenital sites, which implies hematogenous spread from the initial genitourinary infection and/or the ability to directly colonize and invade multiple sites, such as mucous membranes of the respiratory and gastrointestinal tracts (5). Consistent with this scenario, we have reported that M. genitalium possesses highly specialized mechanisms of parasitism, including the use of its unique tip organelle to colonize and invade human cells. Recently, the preferential binding of M. genitalium to cervical and vaginal mucin was described, mediated by a novel pathway of adherence (1). Therefore, it appears that M. genitalium has compensated for its streamlined genome and lack of biosynthetic and catabolic enzymes by evolving an intimate interplay with human cells and tissues that leads to persistence and replication in vivo over many months (8). This parasitic existence underscores the limitations and challenges of using direct-culture techniques to implicate M. genitalium as an important and emerging infectious agent.
In this study, we accomplished primary isolation and single-colony cloning of M. genitalium from vaginal and cervical samples derived from a subpopulation of women attending a specialized STD clinic in San Antonio. This microbiological achievement occurred over a period of 6 months and has not since been duplicated. We attribute the success partly to a combination of specific commercial lots of serum and fresh yeast extract components that are no longer available, plus the use of low levels of ciprofloxacin to control overgrowth of normal microbial flora. No doubt, other unidentifiable variables were involved. We screened well over 3,670 cervical and vaginal samples from 838 women during the 17- to 39-month period of clinic visits described in this report and isolated M. genitalium by culture from 31 individuals only during this single 6-month interval. In separate reports, we describe genitourinary symptoms associated with M. genitalium in these culture-positive women (J. E. Korte, J. B. Baseman, J. M. Piper, M. P. Eagle, A. E. C. Holden, S. T. Perdue, J. D. Champion, and R. N. Shain, submitted for publication) and infectious patterns of M. genitalium in human vaginal cells from clinical samples (6a). In the present study, using M. genitalium culture-positive status as the “gold standard,” we evaluated ELISA, immunoblot, and PCR tests at the time of M. genitalium isolation and over the entire period of clinic visits in order to determine both independent and combinatorial assessments of diagnostic-test validity. Recent studies have established putative links between M. genitalium and symptomatology by using either serological or PCR assays, i.e., in the absence of successful M. genitalium colony isolation. These diagnostic tests are considered sensitive and specific and have replaced primary cultivation of microorganisms as methods of direct pathogen detection. However, their meaningfulness is limited, especially when assessing the roles of difficult-to-grow and emerging pathogens (such as M. genitalium) in disease etiology. Nonetheless, strong associations between serology and PCR for M. genitalium were described in patients attending STD clinics (30). Other reports used PCR as the single determinant of the linkage between M. genitalium and STDs, with considerable variation in the percentages of M. genitalium PCR positivity associated with chlamydia-negative nongonococcal urethritis (9). In one report, PCR values were coupled with fluorescent anti-M. genitalium rabbit antibody staining of urine-related specimens in men to evaluate the association between M. genitalium and nongonoccocal urethritis (25).
A highly noteworthy finding of the present study, and one deserving careful attention, is that 19 of 31 women tested negative by PCR at the time of M. genitalium isolation in culture. Although such a result would not be surprising for single serological data points, it was somewhat unexpected for PCR assessments. Our record of internal positive and negative controls reflects appropriate and effective PCR technique, as evidenced by consistently negative results for internal water controls and routine amplification of 1 to 10 genome equivalents of M. genitalium in internal positive DNA controls. Many experimental factors can influence the sensitivity and specificity of PCR assays, including the source and quality of individual clinical samples, handling and storage conditions, specific primers and PCR-related reagents, and individual test parameters. Nonetheless, we carefully controlled for methodological and reagent variables and included negative and positive samples with appropriate genomic titrations for each assay set. Our analysis of PCR results over time strongly suggests that a single PCR assay may be much less sensitive than commonly assumed for M. genitalium; i.e., among those women with an average or higher number of laboratory tests during the study period, 95% were positive by PCR at some point versus only 55% of women with fewer than the average number of laboratory tests. Reduced sensitivity of the PCR assay at any single time may be due to an inadequate amount of genetic material in the sample or assay complications related specifically to the nature of cervical and vaginal specimens, such as the presence of PCR-inhibitory factors. In the latter case, we added 10 fg of purified M. genitalium DNA directly to both vaginal and cervical specimens from 17 M. genitalium culture-positive, PCR-negative women (Table 1); these specimens were obtained at the time of positive M. genitalium culture and stored frozen at −80°C. Among 34 specimens (i.e., 1 vaginal and cervical sample from each woman), 3 samples were completely negative by PCR and 2 samples exhibited marked reduction in product amplification. This outcome indicates that high levels of PCR-inhibitory substances exist in these specimens, which would predict substantial false-negative M. genitalium PCR results. We strongly suggest that M. genitalium PCR analyses based on one or a few time points be interpreted with much caution.
Interestingly, of the six patients who remained PCR negative throughout the entire duration of clinic visits, four demonstrated no meaningful seroconversion based upon ELISA values (patients 1, 12, 23, and 31, in contrast to patients 7 and 22) (Table 3). It would appear that PCR and serological determinations are likely to underestimate the presence of M. genitalium; this is further reinforced by the fact that 38.7% of the women at the time of positive culture were ELISA and PCR negative (Table 2). In contrast, all 31 culture-positive women scored at least 1+ in the immunoblot assay at the time of culture. Since we had definitive microbiological evidence for the existence of viable M. genitalium in vaginal and cervical samples and had collected a library of vaginal and cervical specimens and sera over the 17 to 39 months of clinic visits, we compared ELISA values among culture-positive women at the time of M. genitalium isolation and at the time of peak antibody titer. In both analyses, negative control serum ELISA values were heavily skewed toward the nonreactive category, while the majority of culture-positive individuals were defined as ELISA positive (Fig. 1). Potential diagnostic associations of ELISA, immunoblotting, and PCR at the time of M. genitalium culture appear in Table 1. What is apparent is that neither PCR nor ELISA provided the sensitivity needed to confidently confirm the presence of viable M. genitalium. The combined use of two or even all three tests (considering immunoblotting to be positive at ≥2+) failed to achieve a sensitivity of 65% at the time of positive culture. However, the use of a cutoff point of 1+ on immunoblots produced 100% sensitivity for detection of M. genitalium at the time of culture. It should be noted that in several instances, band intensities categorized as 1+ were marginally discernible.
Concerning the relationship between ELISA and immunoblot intensities, predominant immunogenic protein bands included the P140 adhesin (MG191) and P110 (MG192) cytadherence-associated proteins (2, 11). Although there was a strong association between ELISA value and immunoblot intensity, there was obvious discordance in some patients (Table 1). For example, patients 1, 3, 11, and 18 exhibited high ELISA but weak immunoblotting values. Since both assays include identical goat anti-human antibody probes and LAMP preparations, the differences were likely due to the use of nondenatured mycoplasma proteins in the ELISA format, in contrast to reducing conditions associated with SDS-polyacrylamide gel electrophoresis and other assay variables. Therefore, individual women are expected to generate distinct repertoires of antibodies reactive against linear and conformational epitopes, which could be reflected in concordance or discordance between ELISA and immunoblot intensities.
The representative ELISA profiles presented in Fig. 3 further reinforce the diversity of individual responses to M. genitalium infections and emphasize the challenges associated with correlating diagnostic criteria with symptomatology. Obvious seroconversion patterns could be observed (Fig. 3, patients 11 and 25) where >8-fold differences occurred in ELISA readings over the span of clinic visits (Table 3). However, individuals with relatively high baseline ELISA readings (i.e., greater than the negative OD cutoff) and relatively high peak ELISA values (Table 3, patients 5, 8, 18, and 24) exhibited <4-fold changes. Does this indicate a lack of “classical” seroconversion and persistence of M. genitalium with periodic bouts of infection, clinical manifestations (or lack thereof), and limited immune responsiveness? Other culture-positive women (Table 3, patients 6, 15, 23, 26, and 31) remained ELISA negative throughout the duration of clinic visits. Intrinsic variations in the M. genitalium antigenic load in the context of distinct immunological responses, along with genetic, nutritional, and hormonal variables of individual women and unique characteristics of the microbial flora, including coinfections, could dramatically influence the patterns of M. genitalium parasitism and host immunity and the usefulness of diagnostic criteria. Furthermore, delayed or late antibody responses impart additional problems in assessing the role of M. genitalium in human disease. Certainly, the presence of viable M. genitalium in the genitourinary tracts of women, whether these women are defined as serologically and/or PCR positive or negative, indicates that M. genitalium can be readily transmitted though sexual contact. At present, we are using confocal microscopy and real-time PCR to determine the extent of the M. genitalium infectious burden in culture-positive women and have observed wide variations in the levels of parasitism of individual vaginal and cervical cells (submitted for publication).
The hallmarks of mycoplasma infections in humans run the gamut of clinical signs and symptoms associated with acute and chronic diseases. Also, the ability of mycoplasmas to persist for extended periods of time, despite an intact immune response and adequate doses of antibiotics, further typifies mycoplasma infections. These features underscore the need for a more complete understanding of mycoplasma parasitism, including the processes by which mycoplasmas colonize and invade host cells and establish long-term residence. In the case of M. genitalium, the limited availability of basic biological and pathogenic information represents a major barrier in identifying virulence determinants and host factors of susceptibility and resistance. The present study is part of a continuing effort to understand the M. genitalium-host interplay and provide insights related to establishing effective strategies of disease control and prevention.
Acknowledgments
We thank William Peairs of the Avante Health Clinic for sample preparation and delivery.
This work was supported by grants AI45429 and AI41010 from the National Institutes of Health and the Institute of Allergy and Infectious Disease.
REFERENCES
- 1.Alvarez, R. A., M. W. Blaylock, and J. B. Baseman. 2003. Surface localized glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma genitalium binds mucin. Mol. Microbiol. 48:1417-1425. [DOI] [PubMed] [Google Scholar]
- 2.Baseman, J. B. 1993. The cytadhesins of Mycoplasma pneumoniae and Mycoplasma genitalium, p. 243-259. In S. Rottem and I. Kahane (ed.), Subcellular biochemistry: mycoplasma cell membranes. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 3.Baseman, J. B., S. F. Dallo, J. G. Tully, and D. L. Rose. 1988. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J. Clin. Microbiol. 26:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baseman, J. B., P. S. Reddy, and S. F. Dallo. 1996. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am. J. Respir. Crit. Care Med. 154:S137-S144. [DOI] [PubMed] [Google Scholar]
- 5.Baseman, J. B., and J. G. Tully. 1997. Mycoplasmas: sophisticated, reemerging and burdened by their notoriety. Emerg. Infect. Dis. 3:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornelius, E., P. Lidbrink, and J. S. Jensen. 2000. Mycoplasma genitalium in non-gonococcal urethritis—a study in Swedish male STD patients. Int. J. STD AIDS 11:292-296. [DOI] [PubMed] [Google Scholar]
- 6a.Blaylock, M. W., O. Musatova, J. G. Baseman, and J. B. Baseman. J. Clin. Microbiol., in press.
- 7.Cohen, C. R., L. E. Manhart, E. A. Bukusi, S. Astete, R. C. Brunham, K. K. Holmes, S. K. Sinei, J. J. Bwayo, and P. A. Totten. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359:765-766. [DOI] [PubMed] [Google Scholar]
- 8.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29:301-309. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi, T., and S. Maeda. 2002. Mycoplasma genitalium: another important pathogen of nongonococcal urethritis. J. Urol. 167:1210-1217. [DOI] [PubMed] [Google Scholar]
- 10.Dhandayuthapani, S., M. W. Blaylock, C. M. Bebear, W. G. Rasmussen, and J. B. Baseman. 2001. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J. Bacteriol. 183:5645-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhandayuthapani, S., W. G. Rasmussen, and J. B. Baseman. 1999. Disruption of gene mg218 of Mycoplasma genitalium through homologous recombination leads to an adherence-deficient phenotype. Proc. Natl. Acad. Sci. USA 96:5227-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooton, T. M., M. C. Roberts, P. L. Roberts, K. K. Holmes, W. E. Stamm, and G. E. Kenny. 1988. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet i:266-268. [DOI] [PubMed] [Google Scholar]
- 13.Horner, P., B. Thomas, C. B. Gilroy, M. Egger, and D. Taylor-Robinson. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin. Infect. Dis. 32:995-1003. [DOI] [PubMed] [Google Scholar]
- 14.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause, D. C., and D. Taylor-Robinson. 1992. Mycoplasmas which infect humans, p. 417-444. In R. N. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, D.C.
- 17.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 18.Mernaugh, G. R., S. F. Dallo, S. C. Holt, and J. B. Baseman. 1993. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin. Infect. Dis. 17:S69-S78. [DOI] [PubMed] [Google Scholar]
- 19.Moller, B. R., D. Taylor-Robinson, and P. M. Furr. 1984. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet i:1102-1103. [DOI] [PubMed] [Google Scholar]
- 20.Moller, B. R., D. Taylor-Robinson, P. M. Furr, and E. A. Freundt. 1985. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br. J. Exp. Pathol. 66:417-426. [PMC free article] [PubMed] [Google Scholar]
- 21.Talkington, D. F., K. B. Waites, S. B. Schwartz, and R. E. Besser. 2001. Emerging from obscurity: understanding pulmonary and extrapulmonary syndromes, pathogenesis, and epidemiology of human Mycoplasma pneumoniae infections, p. 57-84. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections. ASM Press, Washington, D.C.
- 22.Taylor-Robinson, D. 2002. Mycoplasma genitalium—an update. Int J. STD AIDS 13:145-151. [DOI] [PubMed] [Google Scholar]
- 23.Taylor-Robinson, D., P. M. Furr, J. G. Tully, M. F. Barile, and B. R. Moller. 1987. Animal models of Mycoplasma genitalium urogenital infection. Isr. J. Med. Sci. 23:561-564. [PubMed] [Google Scholar]
- 24.Taylor-Robinson, D., J. G. Tully, and M. F. Barile. 1985. Urethral infection in male chimpanzees produced experimentally by Mycoplasma genitalium. Br. J. Exp. Pathol. 66:95-101. [PMC free article] [PubMed] [Google Scholar]
- 25.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 26.Tully, J., D. Taylor-Robinson, D. L. Rose, R. M. Cole, and J. M. Bove. 1983. Mycoplasma genitalium, a new species from the human urogenital tract. Int. J. Syst. Bacteriol. 33:387-396. [Google Scholar]
- 27.Tully, J. G., D. L. Rose, J. B. Baseman, S. F. Dallo, A. L. Lazzell, and C. P. Davis. 1995. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J. Clin. Microbiol. 33:1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 29.Tully, J. G., D. Taylor-Robinson, D. L. Rose, P. M. Furr, C. E. Graham, and M. F. Barile. 1986. Urogenital challenge of primate species with Mycoplasma genitalium and characteristics of infection induced in chimpanzees. J. Infect. Dis. 153:1046-1054. [DOI] [PubMed] [Google Scholar]
- 30.Wang, R. Y., T. Grandinetti, J. W. Shih, S. H. Weiss, C. L. Haley, M. M. Hayes, and S. C. Lo. 1997. Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol. Med. Microbiol. 19:237-245. [DOI] [PubMed] [Google Scholar]
- 31.Wang, R. Y.-H., and S.-C. Lo. 1996. ELISA in human urogenital infections and AIDS, p. 237-245. In J. Tully and S. Razin (ed.), Molecular and diagnostic procedures in mycoplasmology. Academic Press, New York, N.Y.