Abstract
Introduction
Anemia in burn patients is due to surgical blood loss and anemia of critical illness. Since the commitment paradigm of common bone marrow progenitors dictates the production of erythroid, myeloid, and lymphoid cells, we hypothesized that skewed bone marrow lineage commitment decreases red cell production and causes anemia after a burn injury.
Methods
After anesthesia, B6D2F1 mice received a 15% TBSA dorsal scald burn. The sham group did not receive scald burn. Femoral bone marrow was harvested on 2, 5, 7, 14 and 21 days post burn (PBD). Total bone marrow cells were labeled with specific antibodies to erythroid (CD71/Ter119), myeloid (CD11b), and lymphoid (CD19) lineages and analyzed by flowcytometry. To test whether erythropoietin (EPO) could increase red blood cell production, EPO was administered to sham and burn animals and their reticulocyte response was measured on PBD#2 and PBD#7.
Results
Burn injury reduced the erythroid cells of the bone marrow from 35% in sham to 17% by PBD#5 and remained at similar level until PBD#21. Myeloid cells however, increased from 42% in sham to 60% on PBD #5 and 77% on PBD#21. Burn injury reduced reticulocyte counts on PBD#2 and PBD#7 indicating that the erythroid compartment is severely depleted. This depleted compartment however responded to EPO but was not sufficient to change red cell production.
Conclusion
Burn injury skews the bone marrow hematopoietic commitment away from erythroid and toward myeloid cells. Shrinkage of the erythroid compartment contributes to resistance to EPO and the anemia of critical illness.
Introduction
Anemia is highly prevalent in the burn population. Due to surgical blood loss and the stress of the critical illness, burn patients have massive transfusion requirements1,2. For patients with greater than 40% total body surface area burn, on average, 20 units of packed red blood cells are transfused2. While the bone marrow production of red blood cells cannot replace massive surgical blood loss, it should be able to maintain adequate production during the recovery phase of the critical illness. However, burn patients continue to require over 50% of their transfusions outside of the operating room 1 and due to the anemia of critical illness 3. In fact, the inability to replenish red blood cells and correct anemia can persist for several months following a critical illness 4. Erythropoiesis enhancing therapies, including the administration of erythropoietin 5 and correction of any nutritional deficiencies, 6 have not been found to augment erythropoiesis in these patients.
One possibility for burn-induced anemia is significant impairment of bone marrow erythropoiesis. Hematopoiesis proceeds in a hierarchical pattern of differentiation with hematopoietic stem cells leading to the production of all blood cells 7. As cells progress through this hierarchy, they commit to one of the three major lineages: erythroid (red blood cells), myeloid (monocyte, neutrophil, macrophages) or lymphoid (B and T lymphocytes). Leukemias and other hematologic disorders result from malignant hematopoiesis in which there are dramatic shifts in the production of a particular lineage of cells 8. While the paradigm that governs altered lineage commitment in malignant hematopoiesis is well delineated, alterations in bone marrow progenitor lineage commitment as a result of acute injury are less well known.
We have previously demonstrated that burn injury augments monocytopoiesis through increased myeloid commitment of bone marrow progenitors 9. As erythrocytes and myeloid leukocytes develop from a common progenitor, it is essential to study the impact of burn injury on erythropoiesis not just in terms of red blood cells but rather in the context of the leukocytes as well. Therefore, we sought to determine quantitative changes in the erythroid, myeloid and lymphoid components of the bone marrow immediately following burn injury and during recovery. To identify changes in these cells, we tracked erythroid, myeloid, and lymphoid bone marrow cells using fluorescence-activated cell sorting (FACS) during a three-week period following burn injury. In addition, we determined the impact of erythropoietin administration on bone marrow erythropoiesis following burn injury.
Methods
Mice
6–8 week old B6D2F1 male mice weighing approximately 25 grams were purchased from Jackson Laboratories (Barr Harbor, ME). Mice were housed in our Comparative Medicine Facility with a 12 h light/dark cycle with controlled temperature (20–22° C). The mice were allowed to acclimate to our facility for 7 days prior to use. Institutional Animal Care and Use Committee at Loyola University Medical Center approved all the experimental protocols.
Burn Injury
B6D2F1 male mice were randomly divided into sham and burn groups. Mice were anesthetized using ketamine and xylazine (100mg/kg, 2.5 mg/kg respectively; i.p) and their dorsal hair was removed by shaving. A 15% total body surface area full thickness scald burn along the dorsum was administered by immersion in a 100°C water bath for 8 seconds 10. Immediately after the injury protocol, the animals were resuscitated with an intraperitoneal injection of normal saline (2mL). Mice were euthanized on post-burn days (PBD) 2, 5, 7, 14 and 21, and both femurs were removed. A sham group of mice were administered anesthesia, shaved and resuscitated but were not subjected to burn injury. Six animals per group were used for each time point. During the three-week post-burn period no mortality was associated with any experimental group. None of the animals showed any evidence of wound infection or sepsis. Burn wounds were not treated with any topical agents. There was no evidence of wound infection or overt morbidity in any of the animals.
Bone Marrow Elution and Total Bone Marrow Counts
Bone marrow was eluted from the femurs by flushing the marrow with McCoy’s medium via a 25gauge needle in the proximal end of the femur. Total bone marrow cells were determined by taking a 10µL aliquot of the cells in a 1mL suspension of PBS and then counting in a Neubauer hemocytometer. Trypan blue staining was used to exclude dead cells. Two different investigators counted the total bone marrow cells independently and the two counts were averaged.
Markers of Erythroid, Myeloid, and Lymphoid Populations
To determine the most common cell surface markers unique to erythroid, myeloid, and lymphoid populations in the bone marrow, 2 µg each of 9 biotinylated monoclonal antibodies (BD Pharmigen, San Jose, CA) conferring lineage specificity were added to 1×106 bone marrow cells. Those antibodies and their corresponding lineage include erythroid (Ter119) 11, myeloid (CD11b, Gr1) and lymphoid (CD19, CD3e, B220, Thy1.1, CD8a)12 cell surface markers. To identify the percentage of bone marrow cells expressing each individual cell surface marker, 10 µg of APC Cy-7 conjugated streptavidin was then added to the solution. Ter119, CD11b, Gr1, CD19 and B220 were the most commonly identified cell surface markers on bone marrow cells. These findings were then used to determine universal cell surface marker combinations to reliably distinguish erythroid, myeloid and lymphoid components of the bone marrow.
Identification of Erythroid, Myeloid, and Lymphoid Cells With Cell Surface Marker Combinations
Ter119 and CD71 are the cell surface markers that were used to identify the erythroid population11. The myeloid population in the bone marrow was identified with the universal myeloid marker CD11b expression.
Lymphoid population in the bone marrow was identified with fluorochrome labeled antibodies to B220, CD3e, CD8a, Thy 1.1, and CD19. Approximately, 85% of all B220, CD3e, CD8a and Thy 1.1 positive cells also express CD19 and therefore CD19 was used as the universal marker for lymphoid cells 12.
To confirm that the cell surface marker corresponded to each particular lineage, bone marrow cells were stained with the PE Cy7-CD11b/myeloid and PerCP Cy 5.5-CD19/lymphoid markers and the distinct populations were separated by sort purification using FACS Aria. The cells were then cytospun, stained with Wright-Giemsa and examined for lineage purity.
FACS Analysis
Based on the cell surface marker combinations described above, 2 × 106 total bone marrow cells were aliquoted and labeled with 4µg each of anti-Ter119 FITC (eBiosciences, San Diego, CA), anti-CD71 PE (BD Pharmingen, San Jose, CA), anti-CD11b PE Cy7 (BD Pharmingen, San Jose, CA), and anti-CD19 PerCP Cy5.5 (eBiosciences, San Diego, CA). Nonspecific Fc receptor binding was neutralized with anti-mouse CD16/CD32 (1µg) (BD Pharmingen, San Jose, CA) by incubating the cell suspension for 5 minutes at 4°. The fluorchrome-conjugated antibodies were then added to the cell suspension for 30 minutes in the dark at room temperature. Cells were washed, resuspended in PBS, and immediately analyzed with a FACS Canto II (BD Biosciences, San Jose, California). Results from all FACS analyses were further analyzed using FlowJo Software (Tree Star, Ashland, OR). To confirm trends found with FACS, bone marrow cell samples were stained with Wright-Giemsa and scored by a hematopathologist. Images were obtained using an Olympus DP12 digital camera attached to an Olympus BX40 microscope.
Reticulocyte Response to Exogenous Administration of Erythropoietin
To assess whether erythropoietin administration could correct impaired erythropoiesis after a burn injury, mice were randomly divided into sham or burn groups. Sham mice were anesthetized but not burned. Burn mice were subjected to a 15% TBSA scald burn as previously described. These mice were then administered either saline (no EPO group) or intraperitoneal recombinant human EPO (Stem Cell Technologies, Vancouver, Canada) daily for two days (12.5 units/day) beginning either on the day of sham treatment, PBD#2 or PBD#7 (n=6 per each group and for each time point). Three days after the last injection, all the experimental groups were euthanized and their femoral bone marrow cells were collected for assessment of reticulocyte numbers.
Statistics
Data are reported as mean ± SE. Comparisons both between burn groups and between burn groups and sham were performed using ANOVA and a Tukey post-hoc test (InStat3, GraphPad Software, Inc, La Jolla, CA.
Results
Total Bone Marrow Cells
The total number of bone marrow cellularity did not significantly differ at any time point between sham and any burn groups or between burn groups. Sham mice averaged 31.4 × 106 ± 1.3 bone marrow cells. The post-burn group total bone marrow cells ranged from 27.9 × 106 ± 1.2 on PBD #2 to 30.6 × 106 ± 3.4 on PBD #7. None of these values were significantly different from each other.
Bone Marrow Changes in Cell Size and Complexity Following Burn Injury
Gating total bone marrow cells on forward and side scatter can reveal global changes in the bone marrow cell populations. Bone marrow cells segregate into three distinct populations of cells based on forward and side scatter, and these three populations were found to be consistent with erythroid, myeloid and lymphoid populations identified by cell surface marker combinations and histologic examination. Figure 1 is the typical distribution of total bone marrow cells that shows three distinct bone marrow populations. The FSC large group includes mostly myeloid cells, the FSC medium group contains predominantly lymphoid cells and the FSC small group contains mostly erythroid cells. To corroborate these results, we separated the myeloid and lymphoid cells by their specific cell surface markers CD11b and CD19 respectively (Figure 2). While this method does not quantify each cell compartment, it helps to demonstrate the changes in bone marrow cell populations following burn injury.
Figure 1.
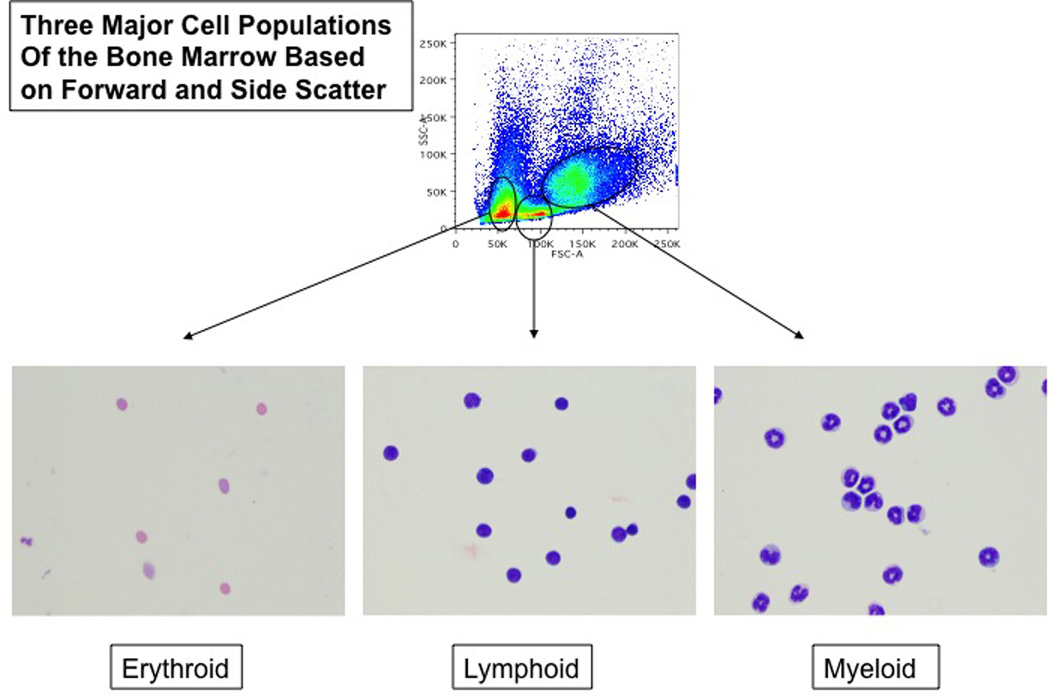
Identification of erythroid, lymphoid, and myeloid populations in the bone marrow by forward and side scatter:
Total bone marrow cells from naive mice were analyzed by their forward and side scatter representing their size and granularity. The identities of these populations were then confirmed by separating them by FACS and staining them with Wright-Giemsa stain (magnification of 200X).
Figure 2.
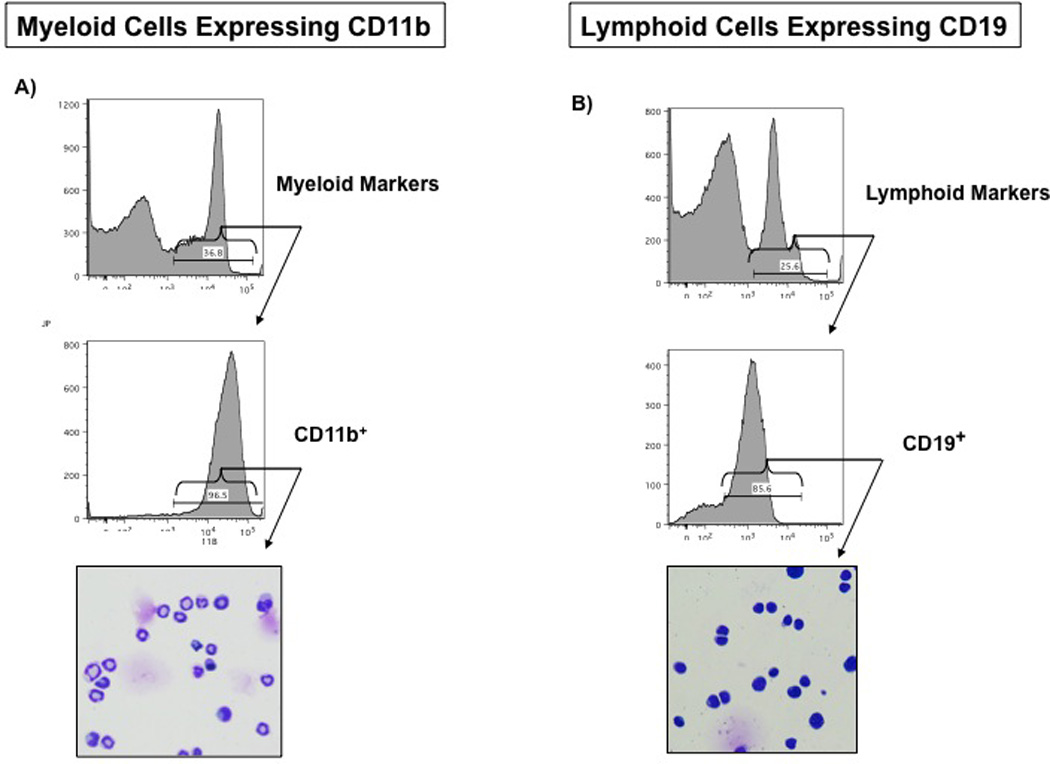
Separation and identification of myeloid and lymphoid cells in the bone marrow through cell surface expression of lineage specific markers:
Total Bone marrow cells (1× 106 cells) were labeled with biotinylated antibodies to cell surface markers CD11b (specific myeloid cells) and CD19 (specific for lymphoid cells) and then incubated with APCy7 conjugated streptavidin and analyzed by flow cytometry. Those cells that express CD11b or CD19 were then separated through FACS sorting and stained with Wright - Giemsa stain to confirm their identity (magnification of 200X).
While figure 1 shows the FACS data depicting burn-induced changes in bone marrow erythroid, myeloid, and lymphoid populations, figures 3 and 4 provide the distribution of these cell populations in bone marrow over a three-week period following burn injury. The percentage of erythroid cells gradually decreases after burn injury over time, reaching a nadir on PBD #14. Sham mice contain 34.8% ± 2.1 erythroid cells. On PBD #2, this percentage is similar at 36.9% ± 2.0 erythroid cells. By PBD #5, the percentage of erythroid cells has significantly decreased to 17.6% ± 2.0 of bone marrow cells. This percentage remains fairly consistent through PBD #21 with a range of 8.9–19.6% of bone marrow cells being erythroid. These differences are statistically significant in comparison to sham on all PBDs except PBD #2 and between PBD#2 and all other burn groups (p<0.05 vs sham).
Figure 3.
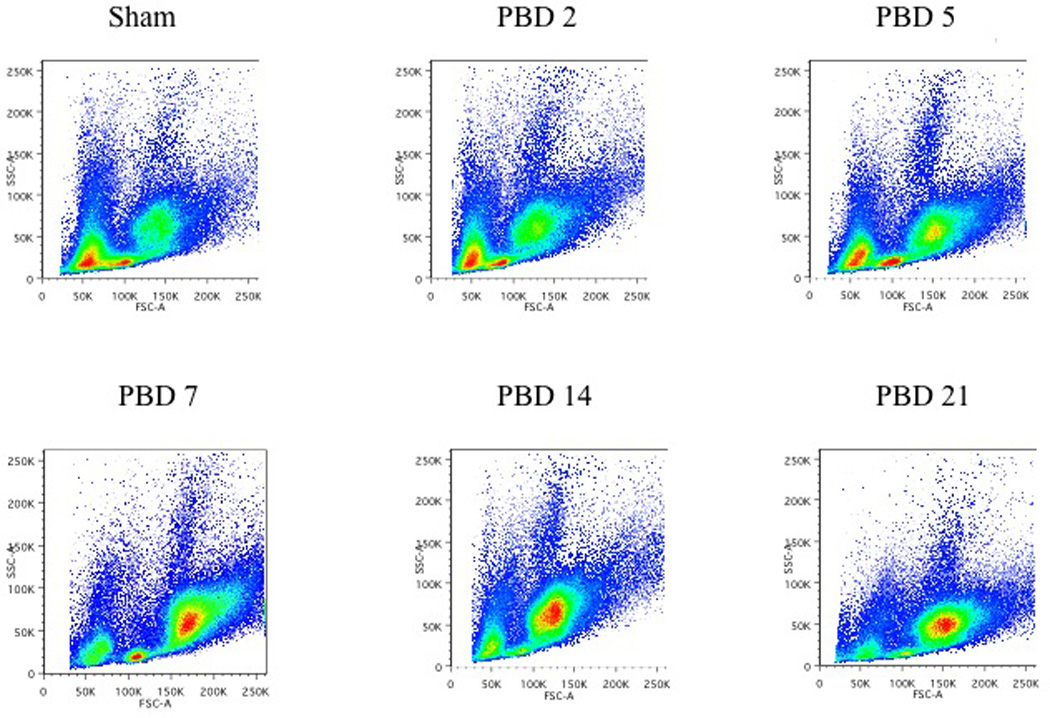
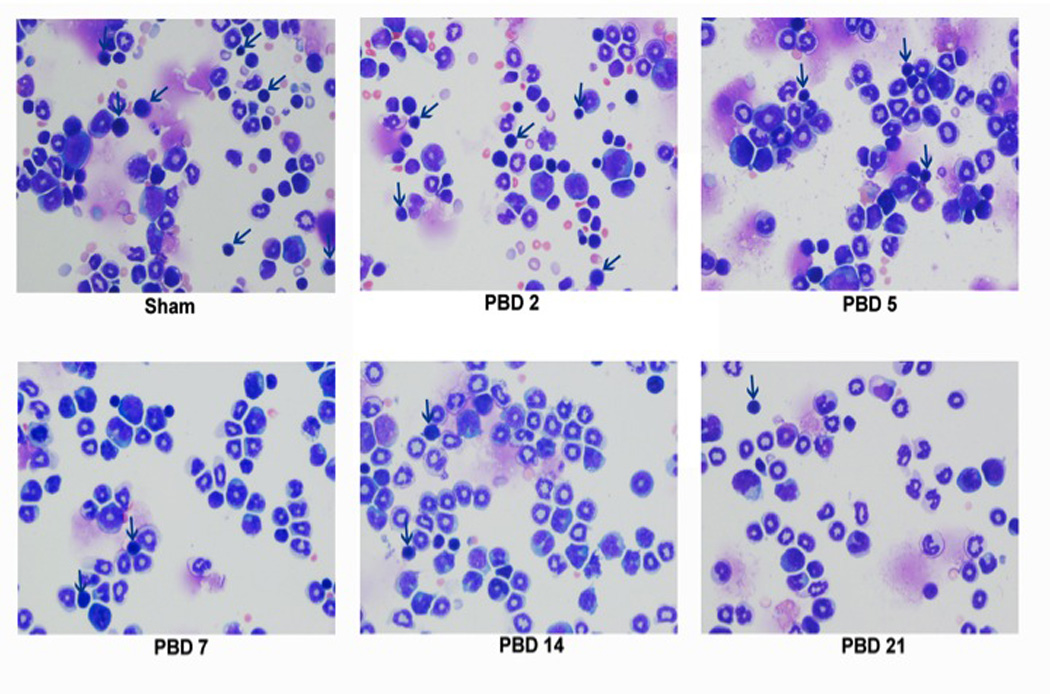
Temporal changes in the erythroid, lymphoid, and myeloid populations following burn injury:
3a) Total bone marrow cells were harvested from sham mice and burn mice on PBD #2, 5, 7, 14, and 21. Flow cytometric analysis of the bone marrow cells on the basis of forward (size) and side (granularity) scatters demonstrated the temporal changes in the erythoid, lymphoid and myeloid populations.
3b) The erythroid, lymphoid, and myeloid populations identified by forward and side scatter were separated by FACS sorting and stained with Wright-Giemsa at various times after burn injury (magnification of 200X). The arrows indicate erythroid progenitors.
Figure 4.
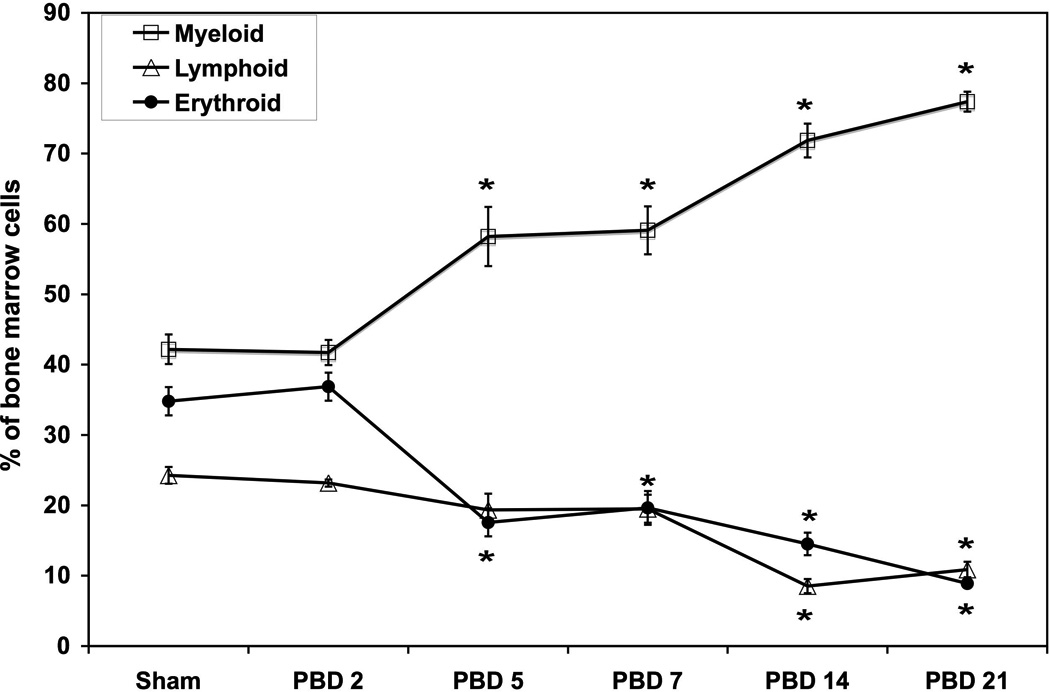
Percentage of erythroid, lymphoid, and myeloid cells in the bone marrow at various times after burn injury.
*=p<0.05 vs sham and PBD#2. N=6 per group /each time point. Data represent mean ± SE.
The gradual shift in the bone marrow hematopoietic paradigm toward myelopoiesis and away from erythropoiesis and lymphopoiesis was confirmed by examining the histology of bone marrow cells with Wright-Giemsa stain. Figure 3b shows the decrease in erythroid progenitors (arrows) in the PBD #5–21 mice in comparison to sham and PBD #2. The increase in myeloid cells during this same time period is demonstrated as well.
While the erythroid component of the bone marrow decreased following burn, the opposite occurred for the myeloid component (Figures 3a, 3b and 4). Sham bone marrow contained 42.2% ± 2.1 myeloid cells. Myeloid cell content was similar to sham on PBD #2 (41.7% ± 1.8). The percentage of myeloid cells in the bone marrow significantly increased after PBD #2 with an average of 60% on PBD#5 and over 70% on PBD#14 and PBD#21 (p<0.05 vs sham and PBD#2).
The lymphoid component of the bone marrow follows a similar pattern to the erythroid component and decreases following burn injury (Figure 4). Sham bone marrow contains 24.3% ± 1.2 lymphoid cells with a similar percentage on PBD #2 (23.2 ± 0.5). The lymphoid component then decreases steadily until PBD #14 and 21 (8.5 ± 1.0 and 10.9 ± 1.1% respectively). The only statistically significant differences with the lymphoid groups were PBD#14 and PBD#21 compared to sham (p<0.05).
Absolute Number of Erythroid, Myeloid, and Lymphoid Bone Marrow Cells
To determine the absolute number of erythroid, myeloid, and lymphoid cells in the bone marrow the percentage of each population was multiplied by the total bone marrow cells. The absolute number of each particular cell type was determined in order to demonstrate that the observed changes after the burn injury were not mainly changes in percentage, without direct correlation to the whole bone marrow cellularity. Our results show the absolute numbers strictly paralleled the percent changes in each of the cell populations. As expected, the absolute number of erythroid cells slightly decreases by PBD #2 and then continues to decline and remains significantly less than sham from PBD #5–21 (Figure 5). Total myeloid cells followed the same trend as with the percentage of myeloid cells with significant differences between sham and all burn groups except PBD#2, between PBD#2 and all other burn groups and between PBD#5 and all other burn groups. Total lymphoid cells declined gradually over the course of the burn injury reaching statistical significance on day PBD#14 and PBD#21 compared to sham (P<0.05).
Figure 5.
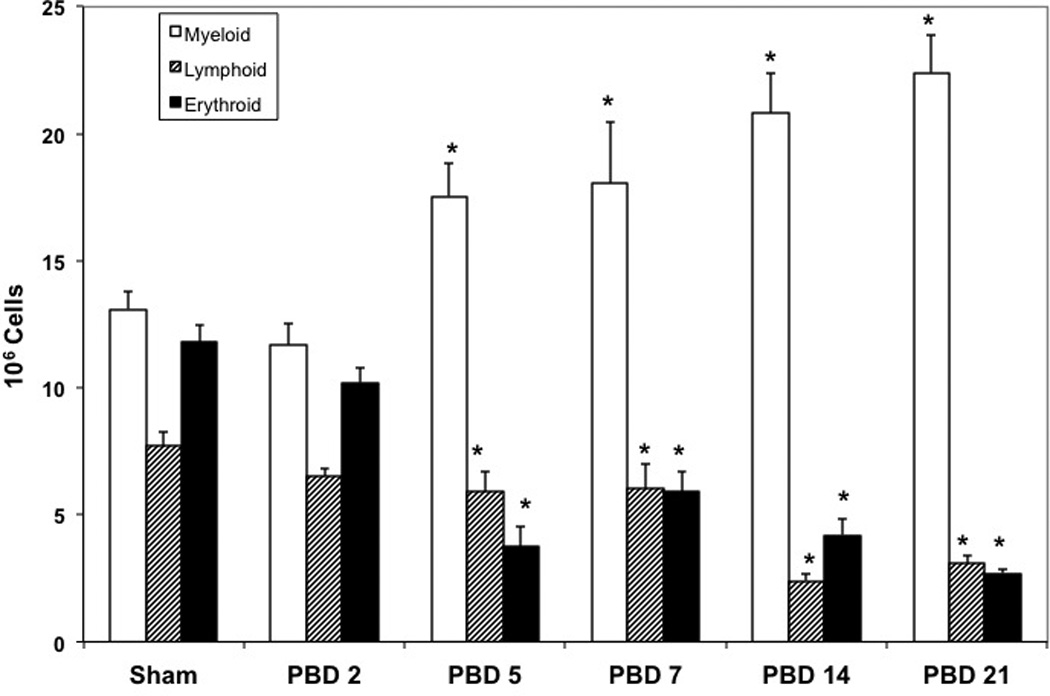
Total number of erythroid, lymphoid, and myeloid cells in the bone marrow at various times after burn injury:
The total number was calculated by multiplying the percentage of each population with the total number of bone marrow cells. *=p,0.05 vs sham and PBD#2. N=6 per group /each time point. Data represent mean ± SE.
Erythroid Precursors, Reticulocytes, and Erythrocyte Populations Post Burn
The erythroid population was divided into three groups (erythroid precursors, reticulocytes, and erythrocytes) based on combined Ter119 and CD71 expression as previously described 13. First, when gated for Ter119 and CD71, erythroid progenitors were considered the Ter119medium, CD71+ population (Figure 6a). Second, Ter119+ cells were gated on forward scatter and with CD71 (Figure 6b). CD71+ cells with high forward scatter are erythroid progenitors (P). Those CD71+ cells with lower forward scatter are reticulocytes (R). Finally, CD71− and low forward scatter cells are erythrocytes (E). This method of FACS analysis technique reliably identifies each erythroid group when sorted (FACS Aria, BD Biosciences, San Jose, California), stained with Wright-Giemsa, and histologically examined by a pathologist (Figure 6b, side panel).
Figure 6.
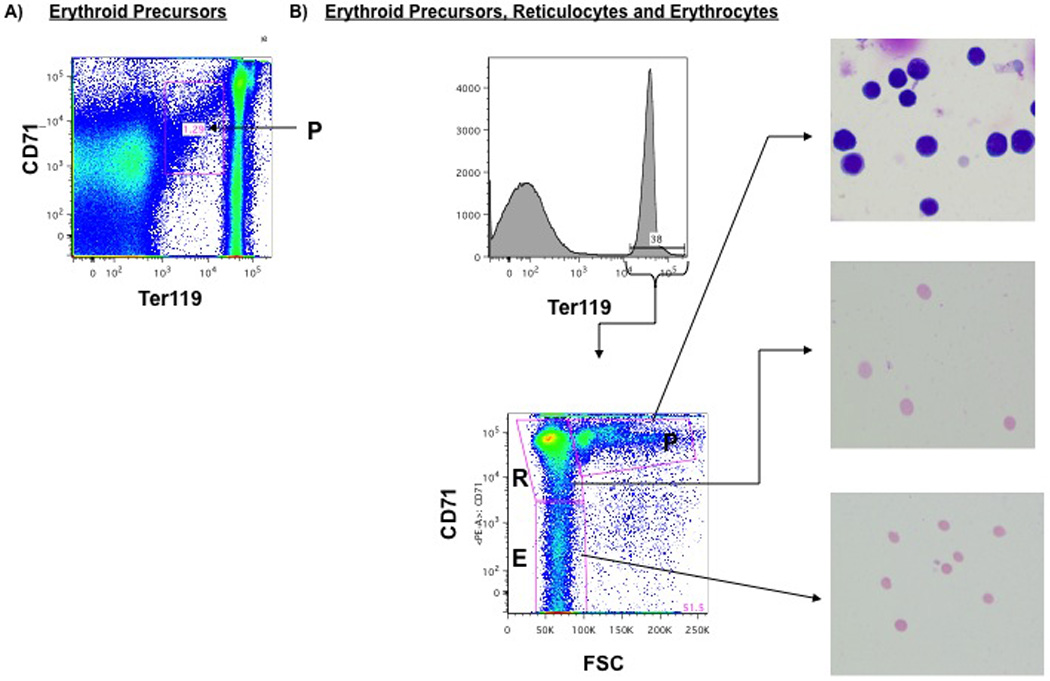
Gating of Ter119+/erythroid cells into erythroid precursor (P), reticulocyte (R) and erythrocyte (E) groups:
Erythroid precursors are identified as both 1) Ter119medium, CD71+ cells and 2) those Ter119+ cells gated on CD71 and forward scatter in the population labeled P. Reticulocytes are identified as Ter119+ cells gated on CD71 and forward scatter in the population labeled R, and erythrocytes are those cells labeled as E. Wright-Giemsa stain of erythroid precursors (P), reticulocytes (R) and erythrocytes (E) sorted by FACS confirm the cell populations (magnification of 200X).
This separation scheme allowed us to quantify changes in the erythroid precursor, reticulocyte, and erythrocyte or red blood cell populations in response to a burn injury over time Figure 7). In all three populations, there is a gradual, steady decline starting on PBD#5. All three erythroid populations remain significantly reduced even on PBD#21.
Figure 7.
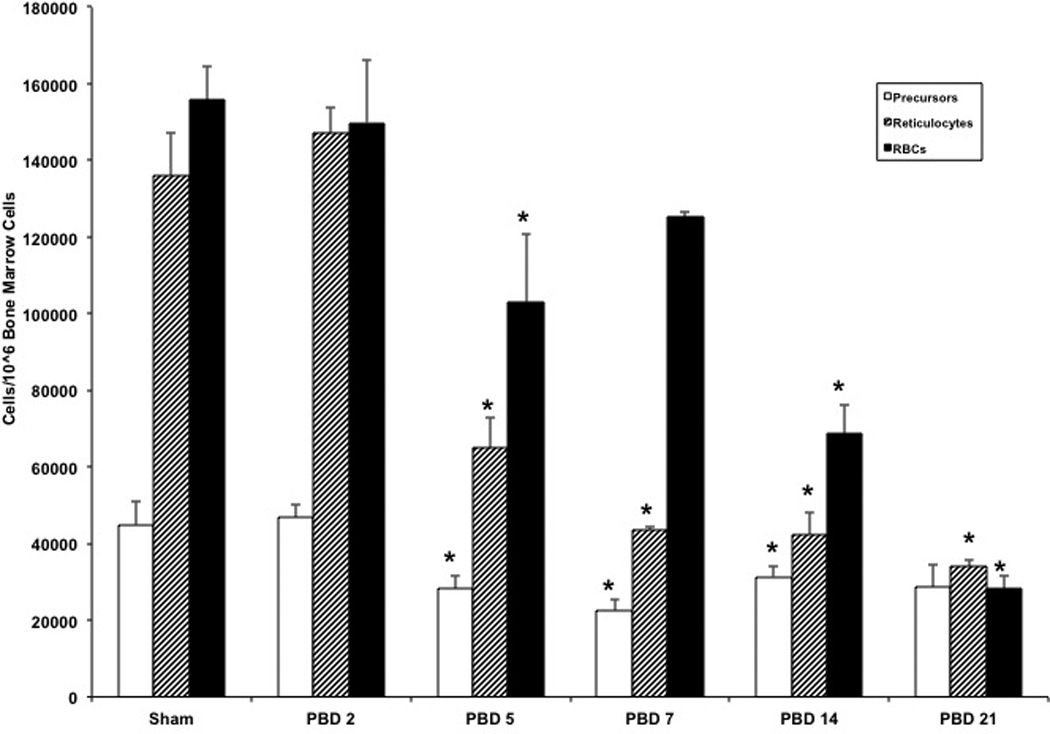
Temporal changes in erythroid precursor, reticulocyte, and erythrocyte populations following burn injury:
Mice were subjected to either a burn or sham injury. Total bone marrow cells from the femurs were harvested on sham, PBD#2, 5, 7, 14 and 21 mice. Total bone marrow cells from these groups were labeled with CD71 and TER119 as described in Figure 6 legend and erythroid precursor, reticulocyte, and erythrocyte populations per 106 bone marrow cells were determined. *=p<0.05 vs Sham. N=6 per group /each time point. Data represent mean ± SE.
Effect of exogenous erythropoietin on burn induced impairment in bone marrow red cell production
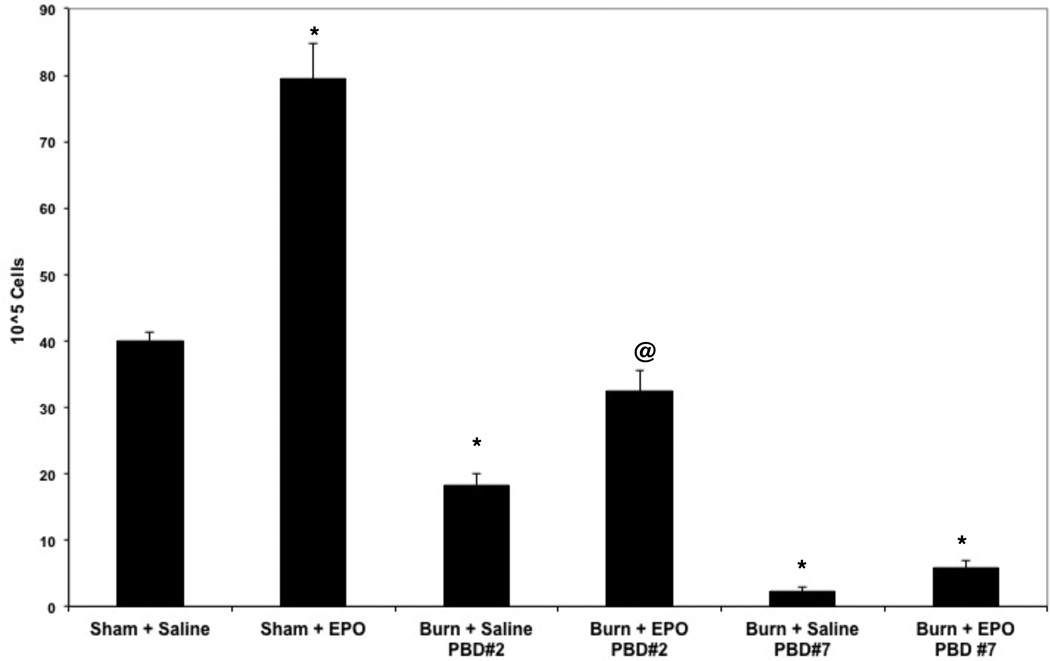
Initial dose response experiments showed that two doses of EPO were sufficient to induce a maximum erythropoietic response in normal mice (data not shown). Reticulocyte count in response to EPO is considered a reliable indicator of its ability to induce erythropoiesis14. Our results showed that in sham animals EPO induced a robust reticulocyte response in the bone marrow (Figure 8). In sham animals EPO administration increased bone marrow reticulocytes from 39.9 ± 1.5 per 105 bone marrow cells to 79.4 ± 5.5 per 105 cells after EPO treatment. However, after burn injury, in animals that received saline, the reticulocyte number dropped to 18.3 ± 1.8 by day 2 and to 2.2 ± 0.6 per 105 cells after day #7. Although the cells in the EPO responsive compartments did respond to exogenous administration of EPO, this effect was significantly diminished in the bone marrow by pBD#7. These results indicate that this relative restricted EPO response following burn injury is likely due to the inability to populate the EPO responsive erythroid compartment of the bone marrow, which in turn may be due to skewing of hematopoiesis in bone marrow commitment away from erythropoiesis and toward myelopoiesis.
Figure 8.
Effect of exogenous administration of EPO on bone marrow reticulocyte numbers after burn injury:
Sham mice or burn mice on PBD#2 and PBD7 were administered either saline or recombinant human EPO (i.p) daily for two days (12.5 units/day) Three days after the last injection, all the experimental groups were euthanized and their femoral bone marrow cells were collected for reticulocyte count analysis. *=p<0.05 vs sham; @ =p<0.05 vs PBD#2. N=6 per group /each time point. Data represent mean ± SE.
Discussion
Burn injury creates significant shifts in bone marrow hematopoiesis. Our results demonstrate that burn injury leads to a significant and gradual decrease in both the percentage and absolute number of erythroid and lymphoid cells within the bone marrow. These changes however, were associated with an increase in myeloid cells. Despite the dramatic shift in each particular lineage, there is no significant difference in total number of bone marrow cells following burn. As these changes are gradual and progressive, spanning a few weeks, burn injury-induced commitment shifts that dampen erythropoiesis and increase myelopoiesis are most likely due to micro-environmental changes within the bone marrow milieu rather than a large efflux of these cell populations.
Overall, dampened erythropoiesis with significant decrease in erythroid precursors, reticulocytes, and erythrocytes may explain the anemia of critical illness often observed in critically injured burn patients and why burn patients require continued transfusions aside from those required to correct surgical blood loss 1,3. The persistent need for transfusions outside of the operating room may be due to the anemia of critical illness 3. The anemia of critical illness most likely results from a combination of blood loss (dressing changes, phlebotomy, and nutritional deficiency) and diminished erythropoiesis leading to the inability to adequately repopulate the blood 15. The anemia of critical illness can persist for several months following hospital discharge without additional iatrogenic blood losses, suggesting that dampened erythropoiesis may play a significant role in the etiology of the anemia of critical illness 4.
Unlike chronic renal failure patients and patients receiving cancer chemotherapy, critically ill trauma patients including burn patients fail to respond adequately to exogenously administered erythropoietin (EPO). Several studies have demonstrated that administering EPO or EPO receptor agonists failed to reduce the transfusion requirements in critically ill patients16. Our study shows that exogenously administered EPO has failed to adequately increase the number of reticulocytes in the bone marrow. EPO receptors are expressed only in progenitors that are committed to the erythroid lineage and EPO acts on these progenitors by augmenting their differentiation to erythrocytes and prolonging their half-lives. Therefore, if the commitment pattern of early bone marrow progenitors is skewed toward myelopoiesis and away from erythropoiesis following burn injury, the number of EPO responsive erythroid committed progenitors will be significantly reduced. This in turn will limit the capacity of exogenously administered EPO to significantly increase reticulocyte numbers. Our results indicated that on PBD# 2, there were still enough erythroid progenitors that responded to EPO. But by PBD#7, the erythroid compartment had been depleted so much that it contained very few cells that could respond to EPO. Another possibility for the lack of adequate EPO responsiveness following burn injury may be due to reduced availability of iron. However, this scenario is unlikely because the expression of CD71 (transferrin receptor), which mediates iron uptake, is significantly downregulated in burn injury. In addition, CD71 is also a transmembrane protein that has been shown to promote cell proliferation17. A significant downregulation of CD71 following burn is likely to impede the proliferation and differentiation of erythroid precursors into erythrocytes independent of both iron levels. Therefore it is reasonable to assume that anemia of chronic illness often seen in burn patients and the inability of critically ill patients to adequately respond to EPO may be in part due to the skewed bone marrow commitment away from erythroid and toward myeloid commitment.
Dampened erythropoiesis in the bone marrow following burn injury is not a new concept. In burn patients, dampened erythropoiesis was identified in an autopsy study in which the bone marrow of burn patients was compared to those who died from sepsis or myocardial infarction18. The bone marrow of burn patients was found to contain significantly less erythroid progenitors but more granulocytes. This study was limited by examining the bone marrow only following the death of these patients rather than at different time points following burn and did not address total bone marrow changes. In the murine model, burn sera has been shown to decrease erythroid colony formation, and burn injury leads to changes in bone marrow and spleen erythropoiesis 19. Our study supports and extends these findings on erythropoiesis by examining the whole bone marrow in the context of the relationship among its major components (erythroid, myeloid, and lymphoid) both immediately following burn and during recovery. By studying the bone marrow components in relation to each other and over the time span of a few weeks, our findings suggest that commitment patterns of bone marrow progenitor cells for these populations change as a result of the burn. In addition, these other studies examined the bone marrow histologically which does not afford the ability to quantify the large population shifts we see by examining 1–2 ×106 cells at a time using FACS. In fact, the ability to reliably sort these cells using FACS will allow for the study of the mechanisms (growth factors, cytokines or transcription factors) responsible for the lineage commitment changes. Furthermore, our study also provides a direct cell biological evidence for erythropoietin resistance.
The cause of this dampened erythropoiesis may lie in the metabolic and inflammatory response to the burn injury. Both catecholamines and inflammatory cytokines are significantly increased following burns. In our model of burn injury, we have demonstrated that inhibition of catecholamines after the burn injury can reverse the increased myelopoietic commitment of bone marrow progenitors and cytokine expression of monocytes20,21. In addition, catecholamines and inflammatory cytokines have been shown to alter erythropoiesis. Catecholamines modulate erythropoiesis in vitro22, and inflammatory cytokines including tumor necrosis factor, IL-6, and interferon gamma have been shown to alter erythropoiesis 23. These studies suggest a potential but not an exclusive role for these bioactive compounds in regulating erythropoiesis after a burn injury.
Neutrophils and macrophages are essential for the inflammatory and proliferative phases of wound healing, but this is necessary only for the first week. Actually, an overabundance of neutrophils may impair wound healing 24. In this study, the increase in myeloid cells was not a result of infection as there was no mortality in any burn group. In fact, given the dysfunctional myeloid cells present following burn, the increase in myeloid production may be at the expense of their function. Our previous studies have documented that burn injury and sepsis not only cause increased monocytopoiesis but also the bone marrow progenitor derived macrophages are hyporeactive in terms of their LPS-stimulated inflammatory cytokine responses 9,25,26. Similarly, other investigators have also demonstrated the presence of hyporesponsive monocytes in both trauma and septic patients27. In addition, reduced lymphocytes and functional qualitative changes such as T cell anergy, IL-2 production, and antigen- responsiveness have been reported following burn injury. These qualitative and quantitative lymphocyte responses are considered to be central to burn induced immune depression28. In the light of these observations our current finding of global changes in both leukocyte and erythroid cells in bone marrow in response to burn injury could provide a plausible explanation for both leukocyte-driven immune dysfunction and erythroid differentiation driven anemia present in severely burned patients. However, our findings on myeloid cells are quantitative and did not include functional analysis. Nor did we differentiate between neutrophils and monocytes. Nonetheless, they form the basis for further studies exploring the functional implications and mechanisms of this shift in lineage commitment.
Our use of Ter119 as a marker for the erythroid component of the bone marrow could be considered a potential limitation because the mature erythrocytes are not necessarily a component of the bone marrow stroma. As erythroid progenitor cells mature, the reticulocyte is released into the periphery at which time it continues to mature into an erythrocyte. Therefore, the only erythrocytes that are present in the bone marrow would be those re-entering the bone marrow via capillaries and sinusoids or from blood spillage during our elution technique and would not reflect bone marrow erythropoiesis. Ideally, removal of the mature erythrocytes with a lysis buffer would adequately address this problem. However, we found that RBC lysis buffer also lysed erythroid precursors and reticulocytes, possibly due to a shared fragility of the erythroid membrane (data not shown). Therefore, we used a combination of Ter119 and CD71 expression to identify erythroid precursors and reticulocytes. In human bone marrow, CD71 is present on erythroid precursors and reticulocytes in combination with Ter119 in a manner in which CD71 expression decreases and Ter119 expression increases as the cells mature 29. In murine bone marrow, the progressive change in CD71 and Ter119 expression was combined with changes in cell size and complexity to derive a gating scheme in which bone marrow erythroid precursors, reticulocytes and erythrocytes could be reliably identified using FACS 13. Therefore, in our study the validity of this gating scheme was corroborated with histologic analysis of FACS sorted cells. As expected, not only does the overall Ter119+ component of the bone marrow decreased progressively following burn injury as did the erythroid precursor and reticulocyte components. These results thus, verify the commitment shift away from erythroid cell formation following burn injury.
Our study tested the efficacy of a single dose of EPO in our burn model. Therefore, it could be argued that a higher dose of EPO could have resulted in increased erythropoiesis leading to improved reticulocyte counts. However, our optimum dose of EPO was chosen based on different doses of EPO in normal animals, and our EPO dose produced a robust response in normal animals. Therefore it is unlikely that the bone marrow eryhroid responses to a higher dose of EPO would be any different after burn injury, especially considering the fact that burn injury progressively reduced the erythroid compartment dramatically by PBD#7. This shrinkage leaves very little capacity for additional EPO to stimulate more red blood cells from this compartment.
In conclusion, using FACS analysis, we have demonstrated a gradual and progressive shift in hematopoiesis with dampened erythropoiesis and lymphopoiesis and enhanced myelopoiesis in the bone marrow following burn injury. This dampened erythropoiesis may contribute to the anemia of critical illness suffered by severely burned patients as their bodies are unable to adequately supply the bloodstream with red blood cells. In addition, the reduced erythroid compartment may provide a plausible explanation for erythropoietin resistance in these patients. Further investigation into the stem and progenitor cell commitment changes and the molecular mechanisms behind them may provide the essential insight into appropriately devising therapeutic modalities to correct anemia of critical illness.
Acknowledgments
We would like to that Pat Simms, director of the LUMC FACS lab, for her assistance with experimental design and data collection and interpretation.
Funding
NIH T32 GM008750 (RLG), Dr. Ralph and Marian C. Falk Medical Research Trust (RL
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmieri TL, Caruso DM, Foster KN, et al. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006 Jun;34(6):1602–1607. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 2.Yogore MG, 3rd, Boral L, Kowal-Vern A, Patel H, Brown S, Latenser BA. Use of blood bank services in a burn unit. J Burn Care Res. 2006 Nov–Dec;27(6):835–841. doi: 10.1097/01.BCR.0000245418.73538.25. [DOI] [PubMed] [Google Scholar]
- 3.Posluszny JA, Jr., Conrad P, Halerz M, Shankar R, Gamelli RL. Classifying Transfusions Related to the Anemia of Critical Illness in Burn Patients. The Journal of trauma. 2010 Dec 2; doi: 10.1097/TA.0b013e3181f2d9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009 Jun;37(6):1906–1912. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 5.Still JM, Jr., Belcher K, Law EJ, et al. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma. 1995 Feb;38(2):233–236. doi: 10.1097/00005373-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Harris RL, Cottam GL, Johnston JM, Baxter CR. The pathogenesis of abnormal erythrocyte morphology in burns. J Trauma. 1981 Jan;21(1):13–21. doi: 10.1097/00005373-198101000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002 May 13;21(21):3295–3313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
- 8.Bonifer C, Bowen DT. Epigenetic mechanisms regulating normal and malignant haematopoiesis: new therapeutic targets for clinical medicine. Expert Rev Mol Med. 2010;12:e6. doi: 10.1017/S1462399410001377. [DOI] [PubMed] [Google Scholar]
- 9.Santangelo S, Gamelli RL, Shankar R. Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Annals of surgery. 2001 Jan;233(1):97–106. doi: 10.1097/00000658-200101000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker HL, Mason AD., Jr. A standard animal burn. J Trauma. 1968;8(6):1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000 May;109(2):280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 12.Lai L, Alaverdi N, Maltais L, Morse H., 3rd Mouse cell surface antigens: nomenclature and immunophenotyping. J Immunol. 1998 Apr 15;160(8):3861–3868. [PubMed] [Google Scholar]
- 13.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charuruks N, Voravud N, Limpanasithikul W. Ratio of baseline erythropoietin (EPO) level and corrected reticulocyte count as an indicator for a favourable response to recombinant human erythropoietin (rhEPO) therapy in anaemic cancer patients. Journal of clinical laboratory analysis. 2001;15(5):260–266. doi: 10.1002/jcla.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallner S, Vautrin R, Murphy J, Anderson S, Peterson V. The haematopoietic response to burning: studies in an animal model. Burns Incl Therm Inj. 1984 Apr;10(4):236–251. doi: 10.1016/0305-4179(84)90002-0. [DOI] [PubMed] [Google Scholar]
- 16.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. The New England journal of medicine. 2007 Sep 6;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 17.Trowbridge IS, Omary MB. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallner SF, Warren GH. The haematopoietic response to burning: an autopsy study. Burns Incl Therm Inj. 1985 Oct;12(1):22–27. doi: 10.1016/0305-4179(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 19.Wallner SF, Vautrin RM, Buerk C, Robinson WA, Peterson VM. The anemia of thermal injury: studies of erythropoiesis in vitro. J Trauma. 1982 Sep;22(9):774–780. doi: 10.1097/00005373-198209000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MJ, Shankar R, Stevenson J, Fernandez R, Gamelli RL, Jones SB. Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis. Annals of surgery. 2004 Jul;240(1):132–141. doi: 10.1097/01.sla.0000130724.84914.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthu K, He LK, Szilagyi A, Stevenson J, Gamelli RL, Shankar R. Propranolol restores the tumor necrosis factor-alpha response of circulating inflammatory monocytes and granulocytes after burn injury and sepsis. J Burn Care Res. 2009 Jan–Feb;30(1):8–18. doi: 10.1097/BCR.0b013e3181921f22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca RB, Mohr AM, Wang L, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004 Winter;5(4):385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 23.Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995 Jan;162(1):134–138. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 24.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thrombosis and haemostasis. 2004 Aug;92(2):275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MJ, Carroll C, He LK, et al. Severity of burn injury and sepsis determines the cytokine responses of bone marrow progenitor-derived macrophages. J Trauma. 2007 Apr;62(4):858–867. doi: 10.1097/01.ta.0000222975.03874.58. [DOI] [PubMed] [Google Scholar]
- 26.Muthu K, He LK, Melstrom K, Szilagyi A, Gamelli RL, Shankar R. Perturbed bone marrow monocyte development following burn injury and sepsis promote hyporesponsive monocytes. Journal of burn care & research : official publication of the American Burn Association. 2008 Jan–Feb;29(1):12–21. doi: 10.1097/BCR.0b013e31815fa499. [DOI] [PubMed] [Google Scholar]
- 27.Wutzler S, Maier M, Lehnert M, et al. Suppression and recovery of LPS-stimulated monocyte activity after trauma is correlated with increasing injury severity: a prospective clinical study. The Journal of trauma. 2009 May;66(5):1273–1280. doi: 10.1097/TA.0b013e3181968054. [DOI] [PubMed] [Google Scholar]
- 28.Munster AM, Eurenius K, Katz RM, Canales L, Foley FD, Mortensen RF. Cell-mediated immunity after thermal injury. Annals of surgery. 1973 Feb;177(2):139–143. doi: 10.1097/00000658-197302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okumura N, Tsuji K, Nakahata T. Changes in cell surface antigen expressions during proliferation and differentiation of human erythroid progenitors. Blood. 1992 Aug 1;80(3):642–650. [PubMed] [Google Scholar]