Abstract
Introduction
Recent data has associated improved survival after hemorrhagic shock with the early use of plasma based resuscitation. Our lab has shown that FFP5 has decreased hemostatic potential compared to freshly thawed plasma (FFP0). We hypothesized that FFP5 would increase bleeding and mortality compared to FFP0 in a rodent bioassay model of uncontrolled liver hemorrhage.
Methods
Hemostatic potential of plasma was assessed with the Calibrated Automated Thrombogram (CAT) assay. Rats underwent isovolemic hemodilution by 15% of blood volume with the two human plasma groups (FFP0 and FFP5) and two controls (sham and lactated Ringers). A liver injury was created by excising a portion of liver resulting in uncontrolled hemorrhage. Rats that lived for 30 minutes after liver injury were resuscitated to their baseline blood pressure and followed for 6 hours. Hemostasis was assessed by thromboelastography.
Results
Hemostatic potential of FFP5 decreased significantly in all areas measured in the CAT assay as compared to FFP0 (p<0.01). In the FFP5 group overall survival was 54%, compared to 100% in the FFP0 and sham group (p=0.03). For animals that survived 30 minutes and were resuscitated, there was no difference in bleeding and/or coagulopathy between groups. Irrespective of treatment, animals that died following resuscitation demonstrated increased intraperitoneal fluid volume (14.85 ± 1.9 mL vs. 7.02 ± 0.3 mL, p<0.001).
Conclusion
In this model of mild pre-injury hemodilution with plasma, rats that received FFP5 had decreased survival after uncontrolled hemorrhage from hepatic injury. There were no differences in coagulation function or intraperitoneal fluid volume between the two plasma groups.
Keywords: thawed plasma, resuscitation, blood storage, endothelial permeability
Introduction
The advent of damage control resuscitation (DCR) has altered the way that many trauma patients are resuscitated. The tenants of DCR define a number of important maneuvers during the resuscitation.[1–3] First is permissive, or hypotensive, resuscitation, with a goal systolic blood pressure below normal while allowing for adequate cerebral perfusion and oxygen delivery. Next is prevention and treatment of hypothermia, acidosis, and hypocalcemia, while avoiding hemodilution with crystalloid fluids. Early mechanical and surgical control of bleeding is paramount to this methodology. Lastly, hemostatic resuscitation with high ratios of fresh frozen plasma (FFP) and platelets to red blood cells (RBC) is considered fundamental to treating the severely injured patient.[2, 4–9]
Multiple investigators have demonstrated that increased use of plasma is associated with increased survival in massively transfused patients.[4, 7, 10] An early report from our center argued that plasma should be used earlier in a massive transfusion.[11] Subsequent retrospective trials demonstrated that early transfusions of plasma and platelets in high ratios to RBCs were associated with improved survival over low ratios.[7, 9, 12] On the other hand, other investigators have not found similar benefit and have argued that these positive outcomes are the result of survival bias in the retrospective studies.[13–18]
In order to facilitate early use of plasma in higher ratios, many trauma centers have placed thawed plasma (TP) (stored at 4° C) in the ED.[10] According to the AABB, TP may be stored for up to 5 days (FFP5) before transfusion.[19] Previous experiments, both in our lab and by others, have demonstrated decreased hemostatic potential and clotting factors in stored thawed plasma compared to freshly thawed plasma (FFP0).[20–22] Therefore, in a bioassay animal model, we hypothesized that a 15% isovolemic hemodilution with FFP5 followed by uncontrolled hemorrhage would demonstrate increased blood loss and decreased survival compared to FFP0. We designed an experimental protocol that functioned as a biological in vivo assay to assess the effect of the age of plasma on clot formation and strength, fluid loss, and mortality.
Materials and Methods
Human FFP was purchased from the Gulf Coast Regional Blood Center (Houston, TX). The units were defrosted using established blood banking procedures and 1.5 ml aliquots were made. FFP0 was immediately refrozen at −80°C until use. In order to produce FFP5 individual aliquots were stored for 5 days at 4°C and then frozen at −80°C until its use. There were 9 donors for FFP0 and 7 donors for FFP5. Clotting potential of the plasma were compared by thrombin generation (TG) assessed using the Calibrated Automated Thrombogram (CAT, Thrombinoscope, Maastricht, Netherlands). The CAT assay measures several parameters including the lag time (representing the time until initial thrombin had formed (min), the thrombin peak height (nM thrombin), the time to peak (ttPeak, min), the endogenous thrombin potential (ETP) (reflects the area under the curve). The CAT assay reflects the intravascular hemostatic potential of the plasma. For each animal experiment three donor samples of FFP0 or FFP5 were randomly selected and pooled.
This experiment was performed after approval by the University of Texas Health Science Center at Houston (UTHealth) Animal Welfare Committee (protocol AWC-09-100). Sprague Dawley rats (male, 240–365g) were obtained from Charles River Laboratories (Willmington, MA). Rats were anesthetized with 5% inhaled isoflurane (Vedco, St. Joseph, MO; 1–2.5% isoflurane for maintenance) and polyethylene cannulae (Instech Laboratories, Plymouth Meeting, PA), and were placed in the femoral artery and vein. The animals were maintained on heating blankets at 37°C. Following an equilibration period, a baseline blood sample was drawn for thromboelastograpy (TEG) (Haemoscope Thromboelastography (TEG) analyzer; Niles, IL), blood gas analysis, and hemoglobin content (I-STAT;Abbott Laboratories, Princeton, NJ). The volume of blood removed was replaced with Hextend® (Hospira, Lake Forest, IL). Blood pressure was monitored throughout the experiment. Animals were then assigned to one of four treatment groups: (1) sham (animals received identical treatment throughout, other than hemodilution), or dilution with (2) lactated Ringer’s (LR, 3x amount of blood removed), and (3) FFP0, or (4) FFP5 (at 1x amount of blood removed). The animals then underwent isovolemic hemodilution of 15% of calculated blood (64 mL/kg) volume, according to their assigned group. Following hemodilution, blood was again drawn for TEG analysis. A reproducible liver injury was then created by excising a portion of liver resulting in uncontrolled hemorrhage.[23] Only animals that reached a mean arterial pressure (MAP) of 40 or less were included in the study, in order to replicate hemorrhagic shock parameters. Rats that lived for 30 minutes after liver injury had a blood sample taken and were then resuscitated to their baseline blood pressure with Hextend®, with additional volume provided as needed to maintain blood pressure; they were then followed for 6 hours or until death. Blood pressure was monitored following resuscitation for any evidence of “popping the clot,” with associated drop in blood pressure.[24] Death was defined as the cessation of spontaneous respiration. Blood samples were again taken at 6 hours or at death. Intraperitoneal fluid volume was quantified by soaking pre-weighed gauze sponges in the peritoneal cavity until the cavity was dry and subsequently weighing the sponges again. The difference in weight was considered to be equal (in mL) to the amount of fluid in the abdomen. Fluid resuscitation was performed with Hextend© (Hospira, Lake Forest, IL). We intended that 10 animals per group would survive to the resuscitation arm of the study. Thus, animals that did not survive for 30 minutes were included in the final survival analysis. However, these experiments were repeated as necessary to ensure 10 animals per group were resuscitated.
We defined the primary variables as coagulation status, death prior to resuscitation, intraperitoneal blood volume, and overall survival. Statistical analysis was performed by the Student’s t-test, Fisher’s exact test, log-rank test, generalized estimating equations (GEE), and one-way Analysis of Variance (ANOVA) with Tukey multiple comparison test, where appropriate. Statistical analysis was performed on SAS software (Cary, NC) and STATA (College Station, TX). P-values of ≤0.05 were considered statistically significant.
Results
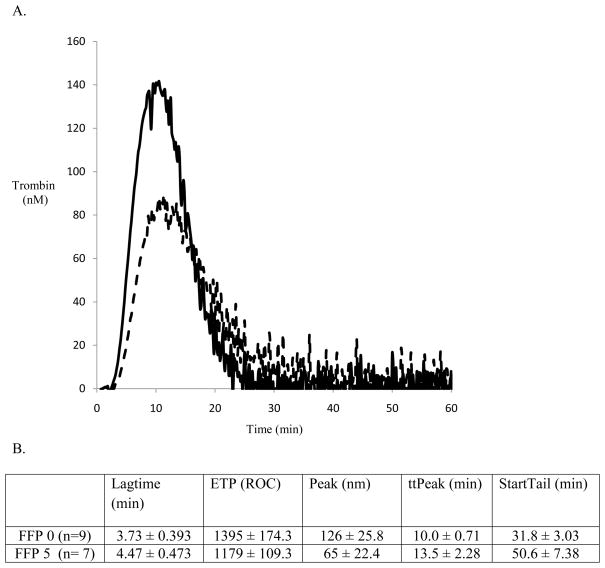
CAT analysis performed on the donor plasma demonstrated a significant reduction in hemostatic potential in FFP5 compared to FFP0. Representative tracings of single FFP0 and FFP5 thrombograms are presented at Figure 1A. Analysis of thrombograms demonstrated significant changes in all parameters between FFP0 and FFP5 (Figure 1B.). The largest decline was observed for the rate of thrombin generation with a reduction of 48.4% in FFP5 compared to FFP0 (p<0.01).
Figure 1.
Figure 1A. Thrombograms generated by CAT of representative tracings of FFP0 vs. FFP5 (the solid line represents FFP0, the dashed line FFP5.)
1B. Summary of thrombin generation as measured by Calibrated Automated Thrombogram. All categories are significantly different with p-values < 0.01.
Overall survival in the sham and FFP0 groups were 100% (n=10). However, overall survival was significantly decreased in the FFP5 group, 54% (7/13), compared to the FFP0 and sham groups (p=0.03, Table 1). Overall survival in the LR group was 83% (10 of n=12) and was not significantly different from sham or FFP0.
Table 1.
Overall survival.
| Control (n=10) | LR (n=12) | FFP0 (n=0) | FFP5 (n=13) | |
|---|---|---|---|---|
| Survival at 30 min. | 100% (10) | 83% (10) | 100% (10) | 77% (10) |
| Survival at 6 hrs. | 100% (10) | 75% (9) | 100% (10) | 54% (7) |
For all animals, baseline physiologic and experimental data are presented in Tables 2 and 3. Animal weights, absolute mass of liver excised, and mass of liver excised as a percent of total body weight (%TBW) were not significantly different between groups. TEG results indicated hypercoagulability, and there was absence of significant changes in TEG variables between groups, as the 15% hemodilution did not significantly alter hemostatic capacity by this measurement technique.
Table 2.
Baseline data for all groups.
| Control (n=10) | LR (n=12) | FFP0 (n=10) | FFP5 (n=13) | |
|---|---|---|---|---|
| Weight (range) | 299.6(254–363) | 307.4 (274–358) | 307.6(254–359) | 290.3(247–330) |
| Baseline pH | 7.32 (7.25–7.43) | 7.33(7.25–7.41) | 7.32(7.25–7.43) | 7.31(7.22–7.39) |
| Percent of liver excised (%TBW) | 0.355(0.3–0.4) | 0.322 (0.3–0.4) | 0.351(0.3–0.46) | 0.349 (0.3–0.45) |
Table 3.
Baseline and experimental data for resuscitated animals. Data is presented as mean with range (unless otherwise noted).
| Control (n=10) | LR (n=10) | FFP0 (n=10) | FFP5 (n=10) | |
|---|---|---|---|---|
| Weight (range) | 299.6(254–363) | 313.1 (278–334) | 307.6(254–359) | 292.5(247–330) |
| Baseline pH | 7.32 (7.25–7.43) | 7.33(7.25–7.41) | 7.32(7.25–7.43) | 7.33(7.28–7.39) |
| Pre-injury MAP | 84.14 (76.1–90.8) | 83.4 (72.3–91.5) | 84.4 (70.7–100.4) | 81.6 (74.5–97.0) |
| Shock nadir MAP | 23.9 (16.3–34.4) | 25.4 (15.6–38.4) | 21.5 (15.5–33.0) | 23.7 (14.5–35.2) |
| Pre-resuscitation MAP | 52.3 (42.5–63.1) | 50.8 (31.3–70.4) | 47.3 (23.5–65.9) | 47.3 (27.5–61.6) |
| Post-resuscitation MAP | 77.8 (70.9–87.2) | 78.7 (70.6–89.3) | 73.9 (50.5–92.5) | 75.3 (54.8–86.0) |
| Time of Death | 6 (6–6) | 5.98 (5.88–6) | 6 (6–6) | 4.9 (0.5–6) |
| Survival at 6 hours n (%) | 10 (100) | 9 (90) | 10 (10) | 7 (70) |
| Percent of liver excised (%TBW) | 0.355 (0.3–0.4) | 0.327 (0.3–0.4) | 0.351 (0.3–0.46) | 0.354 (0.3–0.45) |
| Resuscitation Vol. (mL) | 2.15 (0.5–4) | 2.6 (1–4) | 3.85 (1.5–9) | 2.4 (0.5–6) |
| Intraperitoneal blood (mL) | 6.7 (4.07–9.79) | 8.38 (5.27–15.96) | 7.51 (3.54–10.8) | 8.64 (4.43–19.9) |
| Intraperitoneal blood (% total blood vol.) | 34.7 (25.1–42.6) | 41.9 (24.7–70) | 37.5 (21.7–47.0) | 47.5 (27.2–122.1) |
In the 30 minutes post liver excision all of the sham and FFP0 survived, while 2 (16.7%) of the animals diluted with LR and 3 (23.1%) receiving FFP5 died. Deaths during this period were associated with active bleeding, as BP did not increase following the nadir, indicative of a reduction in clotting capacity and subsequent clot formation.
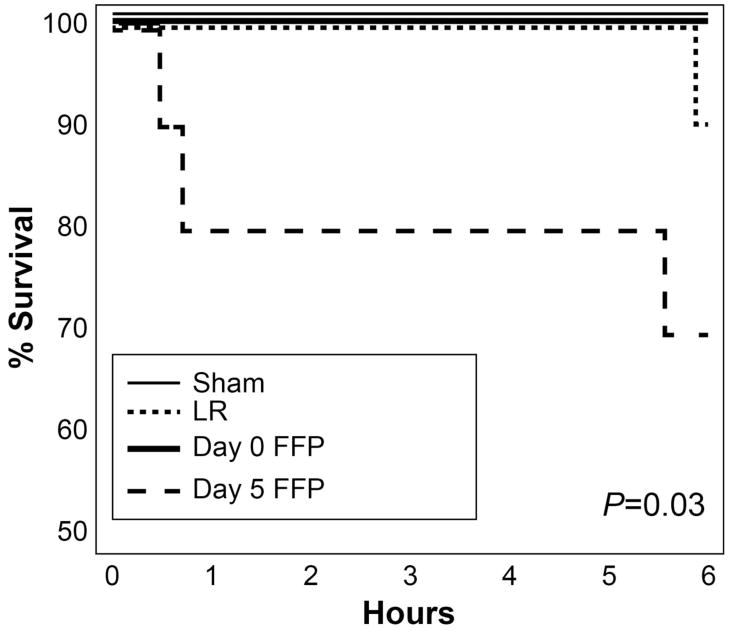
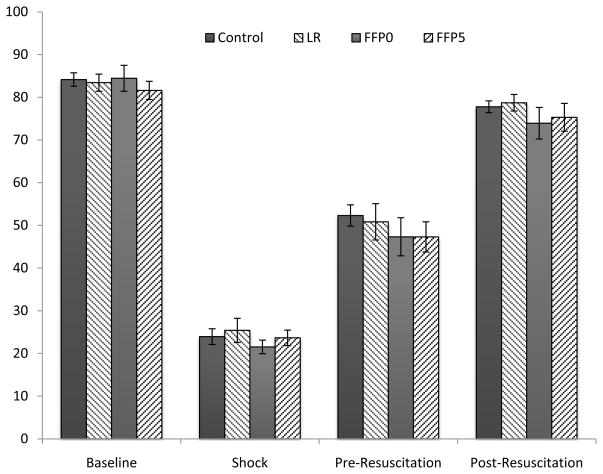
For animals that survived until resuscitated (n=10/group), again FFP5 animals had an increased mortality compared to all groups (p=0.03, log-rank test, Figure 2). Blood pressure analysis demonstrated no differences between groups (Figure 3). Of note, following resuscitation, no animal demonstrated the precipitous decrease in blood pressure associated with rupture of a clot. For animals that survived 30 minutes and were resuscitated, there was no difference in intraperitoneal fluid volume amongst the groups. However, irrespective of treatment, those animals that died following resuscitation had increased intraperitoneal fluid volumes compared to those that survived for 6 hours (14.85 ± 1.9 mL vs. 7.02 ± 0.3 mL, p<0.001, t-test).
Figure 2.
Kaplan-Meier survival curve of resuscitated animals (n=10/group).
Figure 3.
Mean blood pressure of resuscitated animals (n=10/group) at baseline, shock nadir, before resuscitation and post resuscitation. There are no significant differences at any time point between the groups. (Error bars represent SEM)
Discussion
The earlier use of FFP in resuscitation of seriously injured trauma patients who undergo massive transfusion has been associated with improved survival.[4, 7, 9, 25] In response to this trend, many trauma centers have placed thawed plasma in the emergency department.[10] However, while the effects of storage on coagulation factor degradation are described in the transfusion literature, to our knowledge there are no associated clinical outcome data. In fact, we and others have demonstrated that FFP5 has decreased activity of coagulation proteins, including factors V, VII, VIII, VWF, and free Protein S, by up to 30%, and also decreased thrombin generating potential by 40%.[10, 20, 21] Additionally, we have shown improved endothelial repair activity with FFP0 compared to FFP5.[26]
Other studies have suggested deleterious effects associated with the age of RBCs. In both patients and in vitro studies, storage age of RBCs has been associated with increased inflammatory gene expression, infection rates, and decreased survival.[27–29] In contrast, Welsby, et al, found that storage age of platelets was not associated with poorer outcomes or survival in patients undergoing cardiac surgery.[30] Furthermore, three small randomized control trials failed to show an association between RBC age and poorer outcomes, though these were primarily septic medical ICU patients.[31–33] At the current time, there are no clinical data we are aware of comparing FFP0 to FFP5. This present study aimed to explore the effect of FFP5 on mortality, bleeding, and hemostasis in an in vivo model that was designed as a biological assay to assess the function of FFP5 on fluid loss, coagulation, and mortality.
Matsuoka, et al, described a standardized model of uncontrolled hemorrhage from a liver injury. This model was designed to more closely mimic blunt trauma, though this model was used to quantify blood loss associated with fluid resuscitation.[34] We previously employed this model to investigate hemostatic agents.[23] In our current study, the liver is excised, as true blunt liver injuries have been difficult to create in rats experimentally. It is also important to note that our current model does not simulate clinical scenarios, but rather is designed to detect differences in fluid transfusions, and is therefore referred to as a bioassay. A similar study to ours was conducted by Kheirbadi, et al, who performed a 40% hemodilution in rabbits with Hextend®, Dextran 70, and albumin. The investigators subsequently performed a splenic injury that resulted in uncontrolled hemorrhage and reported TEG and thrombin generation assays, with differences observed between Hextend®, Dextran 70 and albumin.[35]
The results of our study show that FFP5 has decreased hemostatic potential (based on CAT data) and represents a different product than FFP0. Furthermore, after hemodilution with these two different plasmas, our in vivo bioassay demonstrated a decreased ability to form a clot in the animals that received FFP5, represented in physiologic data from animals that died before resuscitation, who did not demonstrate a compensatory increase in BP after liver injury. The only other group that this occurred in was the LR group, which does not contain any clotting factors.
A phenomenon that has been widely reported in the literature, but we did not observe, was the occurrence of “popping the clot.” Sondeen, et al, have described that following aortotomy and hemorrhage, once a MAP of 64 was attained in resuscitation, that a clot formed on the aortotomy was dislodged along with re-bleeding.[24] In our study this was not seen, and our TEG data showed no difference in cloth strength amongst the groups. Deaths observed in this study therefore may be attributed to causes other than differences in clot strength.
All of the deaths that were observed were in the FFP5 and LR groups. These deaths were likely related to increased endothelial leak rates, as those animals demonstrated a two-fold increase in intraperitoneal fluid volume compared to animals that survived. Therefore, we believe that although there is no difference in coagulation status as measured by TEG between groups, the animals that died after resuscitation, died because of increased fluid loss. This may be related to increased endothelial permeability that has been shown to be associated with FFP5 compared to FFP0.[26] This increased permeability may result in exaggerated fluid leak from the vascular space leading to an increased intraperitoneal fluid. Increased fluid leak resulting in earlier death was also demonstrated by Solomonov, et al, in a model of uncontrolled hemorrhage as result of splenic injury with large volume saline resuscitation.[36]
Lack of difference in TEG values may be due to the fact that TEG showed the rats to be hypercoagulable, and that TEG is not a sensitive enough test to detect differences, with hemodilution of this degree. There are data from patients that indicates that larger dilutions are necessary to produce changes in TEG. Ng, et al, described a hemodilution of 30% with normal saline produced a hypercoagulable state in patients undergoing hepatobiliary surgery.[37] Another study found that plasma must be diluted by 40% with saline to produce reductions in thrombin generation and clot formation by the CAT and TEG assays.[38] Based on these data it is not surprising that our rats did not appear coagulopathic by TEG.
Our study is the first in vivo study that associates the use of aged plasma with increased mortality. However, it does have limitations. First, we used Hextend® as our resuscitative fluid, and it has been associated with coagulopathy in swine and humans (at a 30% dilution).[39, 40] On the other hand, our animals were not significantly coagulopathic and received resuscitation with Hextend® to less than 20% of their blood volume. Furthermore, our design of this model as a bioassay prohibits it from accurately recreating a clinical scenario. On the other hand, other published models have used similar designs to evaluate fluids used in resuscitation.[35]
In conclusion, our study demonstrates that FFP5 increased overall mortality compared to FFP0. These differences were seen without increased coagulopathy, as measured by TEG. However, those animals that died following initiation of resuscitation did show increased intraperitoneal fluid volume compared to those who survived to 6 hours, indicating increased endothelial leak in those animals. The clinical implications of this study are important, as we have found that the age of plasma may impact clinical outcome. We do not feel that the results of this study should affect current massive transfusion protocols for trauma, but may inform clinicians on the adverse effects associated with the use of plasma and its age. Further studies investigating the interaction between endothelial biology, resuscitative fluids and coagulopathy in uncontrolled hemorrhage are ongoing in our laboratory. Furthermore, we are conducting a prospective observational trial that will examine the correlation of plasma product storage age and outcomes.
Acknowledgments
Sources of Support: NIH Grant T32 GM 0879201, the Texas Emerging Technology Fund, the Memorial Hermann Hospital Fund.
Footnotes
Presented at: the 41st Annual Meeting of the Western Trauma Association, Big Sky, MT, February 28, 2011
Contributor Information
Phillip A. Letourneau, Email: Phillip.A.Letourneau@uth.tmc.edu.
Madonna McManus, Email: Madonna.mcmanus@gmail.com.
Kendell Sowards, Email: Kendell.j.sowards@uth.tmc.edu.
Weiwei Wang, Email: Weiwei.wang@uth.tmc.edu.
Yao-wei Wang, Email: Yao-wei.w.wang@uth.tmc.edu.
Nena Matijevic, Email: nevenka.matijevic-aleksic@uth.tmc.edu.
Shibani Pati, Email: Shibani.pati@uth.tmc.edu.
Charles E. Wade, Email: Charles.e.wade@uth.tmc.edu.
References
- 1.Holcomb JB, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 2.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 Suppl):S267–74. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 3.McMullin NRHJ, Sondeen JL. Hemostatic resuscitation. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin Heidelberg: Springer-Verlag; 2006. pp. 265–278. [Google Scholar]
- 4.Borgman MA, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23(6):231–40. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–8. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 8.Cotton BA, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177–82. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182–3. [DOI] [PubMed] [Google Scholar]
- 9.Duchesne JC, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67(1):33–7. doi: 10.1097/TA.0b013e31819adb8e. discussion 37–9. [DOI] [PubMed] [Google Scholar]
- 10.Duchesne JC, et al. Damage control resuscitation: the new face of damage control. J Trauma. 2010;69(4):976–90. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez EA, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira PG, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66(3):693–7. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- 13.Kashuk JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–1. [DOI] [PubMed] [Google Scholar]
- 14.Stahel PF, Smith WR, Moore EE. Injury. Suppl 4 4. Vol. 40. 2009. Current trends in resuscitation strategy for the multiply injured patient; pp. S27–35. [DOI] [PubMed] [Google Scholar]
- 15.Kor DJ, Gajic O. Blood product transfusion in the critical care setting. Curr Opin Crit Care. 2010;16(4):309–16. doi: 10.1097/MCC.0b013e32833bc4a4. [DOI] [PubMed] [Google Scholar]
- 16.Watson GA, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 228–30. [DOI] [PubMed] [Google Scholar]
- 17.Snyder CW, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–62. doi: 10.1097/TA.0b013e318196c3ac. discussion 362–4. [DOI] [PubMed] [Google Scholar]
- 18.Scalea TM, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–84. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 19.Roback J. Technical Manual. 16. AABB; 2008. [Google Scholar]
- 20.von Heymann C, et al. Activity of clotting factors in fresh-frozen plasma during storage at 4 degrees C over 6 days. Transfusion. 2009;49(5):913–20. doi: 10.1111/j.1537-2995.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 21.Matijevic NKV, Wang Y, Wade CE, Wang W, Letourneau PA, Hartwell E, Kozar R, Ko T, Holcomb JB. Multiple levels of degradation dimish hemostatic potential of thawed plasma. Journal of Trauma. 2010 doi: 10.1097/TA.0b013e318207abec. In Press. [DOI] [PMC free article] [PubMed]
- 22.Yazer MH, Cortese-Hassett A, Triulzi DJ. Coagulation factor levels in plasma frozen within 24 hours of phlebotomy over 5 days of storage at 1 to 6 degrees C. Transfusion. 2008;48(12):2525–30. doi: 10.1111/j.1537-2995.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb JB, et al. Fibrin sealant foam sprayed directly on liver injuries decreases blood loss in resuscitated rats. J Trauma. 2000;49(2):246–50. doi: 10.1097/00005373-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Sondeen JL, V, Coppes G, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110–7. doi: 10.1097/01.TA.0000047220.81795.3D. [DOI] [PubMed] [Google Scholar]
- 25.Sperry JL, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 26.Pati S, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69(Suppl 1 1):S55–63. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offner PJ, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137(6):711–6. doi: 10.1001/archsurg.137.6.711. discussion 716–7. [DOI] [PubMed] [Google Scholar]
- 28.Escobar GA, et al. Stored packed red blood cell transfusion up-regulates inflammatory gene expression in circulating leukocytes. Ann Surg. 2007;246(1):129–34. doi: 10.1097/01.sla.0000264507.79859.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg JA, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69(6):1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsby IJ, et al. Storage age of transfused platelets and outcomes after cardiac surgery. Transfusion. 2010;50(11):2311–7. doi: 10.1111/j.1537-2995.2010.02747.x. [DOI] [PubMed] [Google Scholar]
- 31.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44(12):1256–61. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes CJ, Jr, et al. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care. 2001;5(6):362–7. doi: 10.1186/cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh TS, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32(2):364–71. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka T, Hildreth J, Wisner DH. Liver injury as a model of uncontrolled hemorrhagic shock: resuscitation with different hypertonic regimens. J Trauma. 1995;39(4):674–80. doi: 10.1097/00005373-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Kheirabadi BS, et al. Effects of synthetic versus natural colloid resuscitation on inducing dilutional coagulopathy and increasing hemorrhage in rabbits. J Trauma. 2008;64(5):1218–28. doi: 10.1097/TA.0b013e31816c5c6c. discussion 1228–9. [DOI] [PubMed] [Google Scholar]
- 36.Solomonov E, et al. The effect of vigorous fluid resuscitation in uncontrolled hemorrhagic shock after massive splenic injury. Crit Care Med. 2000;28(3):749–54. doi: 10.1097/00003246-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Ng KF, Lam CC, Chan LC. In vivo effect of haemodilution with saline on coagulation: a randomized controlled trial. Br J Anaesth. 2002;88(4):475–80. doi: 10.1093/bja/88.4.475. [DOI] [PubMed] [Google Scholar]
- 38.Schols SE, et al. Effects of plasma dilution on tissue-factor-induced thrombin generation and thromboelastography: partly compensating role of platelets. Transfusion. 2008;48(11):2384–94. doi: 10.1111/j.1537-2995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 39.Alam HB, et al. Testing of blood products in a polytrauma model: results of a multi-institutional randomized preclinical trial. J Trauma. 2009;67(4):856–64. doi: 10.1097/TA.0b013e3181b5ae75. [DOI] [PubMed] [Google Scholar]
- 40.Fenger-Eriksen C, et al. Mechanisms of hydroxyethyl starch-induced dilutional coagulopathy. J Thromb Haemost. 2009;7(7):1099–105. doi: 10.1111/j.1538-7836.2009.03460.x. [DOI] [PubMed] [Google Scholar]