Abstract
Objective
The Glasgow Coma Scale (GCS) classifies Traumatic Brain Injuries (TBI) as Mild (14–15); Moderate (9–13) or Severe (3–8). The ATLS modified this classification so that a GCS score of 13 is categorized as mild TBI. We investigated the effect of this modification on mortality prediction, comparing patients with a GCS of 13 classified as moderate TBI (Classic Model) to patients with GCS of 13 classified as mild TBI (Modified Model).
Methods
We selected adult TBI patients from the Pennsylvania Outcome Study database (PTOS). Logistic regressions adjusting for age, sex, cause, severity, trauma center level, comorbidities, and isolated TBI were performed. A second evaluation included the time trend of mortality. A third evaluation also included hypothermia, hypotension, mechanical ventilation, screening for drugs, and severity of TBI. Discrimination of the models was evaluated using the area under receiver operating characteristic curve (AUC). Calibration was evaluated using the Hoslmer-Lemershow goodness of fit (GOF) test.
Results
In the first evaluation, the AUCs were 0.922 (95 %CI, 0.917–0.926) and 0.908 (95 %CI, 0.903–0.912) for classic and modified models, respectively. Both models showed poor calibration (p<0.001). In the third evaluation, the AUCs were 0.946 (95 %CI, 0.943 – 0.949) and 0.938 (95 %CI, 0.934 –0.940) for the classic and modified models, respectively, with improvements in calibration (p=0.30 and p=0.02 for the classic and modified models, respectively).
Conclusion
The lack of overlap between ROC curves of both models reveals a statistically significant difference in their ability to predict mortality. The classic model demonstrated better GOF than the modified model. A GCS of 13 classified as moderate TBI in a multivariate logistic regression model performed better than a GCS of 13 classified as mild.
Keywords: Traumatic brain injury, Glasgow Coma Scale, mortality, prediction
1. Introduction
Traumatic brain injury (TBI) is a leading cause of disability, morbidity, and mortality world-wide and is responsible for a significant proportion of all traumatic deaths in the U.S.1 Every year, an estimated 1.5 million people die and hundreds of millions require emergency treatment after a TBI. Fatality rates and disability rates vary depending on the severity and mechanisms of the TBI, but the rates of unfavorable outcomes (death, vegetative state, and severe disability) following TBI can be higher than 20%. 2,3
The Glasgow Coma Scale (GCS) was developed to describe level of consciousness in patients with head injuries. 4 This scale was created primarily to facilitate the assessment and grading of brain dysfunction severity and outcome in a multicenter study of severe brain damage. 5 GCS measures a TBI patient’s best eye, motor, and verbal responses and is a widely used and accepted prognostic indicator for both traumatic and non-traumatic altered consciousness levels. 6 The score has been validated for its inter-observer reliability, which improves with training and experience in different scenarios. 7–10 In addition, the GCS has been recognized as a reliable tool to monitor patients with TBI and to identify when their condition deteriorates. The scale is also an index of injury severity, since the scores are related to either outcomes mortality or disability.11
Traditionally, the GCS classified TBIs as Mild (14–15), Moderate (9–13), or Severe (3–8). Based on the findings of the Canadian Computed Tomography (CT) Head Rule Study, Advanced Trauma Life Support (ATLS) recently modified this classification so that a GCS score of 13 would fall within the mild category of TBI (Mild 13–15). 12–14 Multiple major organizations, including the Eastern Association of the Surgery of Trauma and the Centers for Disease Control and Prevention (CDC) use 13–15 to define mild traumatic brain injury. A recent study showed that a pre-hospital GCS < 14 accurately predicts the need for full trauma team activation and patient hospitalization after motor vehicle collisions; 15 however, there is no standardized approach regarding this classification and there is not enough evidence to support the necessity of this recent modification to the GCS.
Most previous studies have suggested a linear relationship between GCS score and the odds of mortality. 3 Evidence found in two prospective series has shown that patients with a GCS of 13 harbor intracranial lesions at a frequency similar to patients with moderate head injury defined as GCS of 9–12. 16,17
The ability to accurately predict patient outcome after TBI has an important role in clinical practice and research. Prognostic models are statistical models that combine two or more pieces of patient data to predict clinical outcome and are likely to be more accurate than simple clinical predictions. 18 Furthermore, due to limited resources in underserved and austere environments, GCS is relied on as an accurate predictor of outcome and to determine resource allocation after TBI. 19 In this study, we investigated the effect of GCS score on mortality prediction, comparing patients with admission GCS scores of 13 classified as moderate (classic model) to patients with admission GCS scores of 13 classified as mild TBI (modified model).
2. Methods
Population
We performed a secondary analysis on 1998–2007 data from the Pennsylvania Trauma Outcome Study (PTOS) database, a trauma registry that contains trauma patients’ information from 31 accredited trauma centers in Pennsylvania. Patients between 18–65 years 20 of age who sustained TBI were selected to perform the comparative analysis between the two multivariate logistic regression models.
TBI patients admitted to Pennsylvania trauma centers were identified from the PTOS using the Barell Matrix to categorize injury diagnosis. 21 We excluded patients with more than 48 hours between their injury event and admission to trauma centers to avoid diagnoses of late effect or late consequences of the primary injury event. The database uses diagnostic codes from the 9th revision of the International Classification of Diseases (ICD-9), included in the CDC Case Definition for TBI based on case reports from coded death certificates or hospital discharge data: 22 800.0 – 801.9 (fracture of the vault of base of the skull), 803.0 – 804.9 (other and unqualified and multiple fractures of the skull), 850.0 – 854.1 (intracranial injury, including concussion, contusion, laceration, and hemorrhage), and 873.0 – 873.9 (other open wound of head). 23
Outcome
Our dependent variable was discharge status, categorized as deceased or alive. This outcome variable was evaluated to assess the effect of both GCS classifications and prognostic factors in TBI patients.
Prognostic Models
To evaluate the effect of the modified GCS criteria for mild TBI on mortality prediction, we developed three evaluations between the two prognostic models. In the first evaluation, we built models that included the clinical and demographic variables described above. We reviewed patients’ demographics, injury characteristics, and anatomical diagnoses for severity categorization and hospital information. In order to evaluate the change in effect for the prediction of mortality in TBI patients, we constructed two models with dummy variables, using GCS at admission to emergency room. The first model included the dummy variables of GCS scores of 13 classified as moderate TBI (classic model) and the second model included the dummy variables of GCS scores of 13 classified as mild TBI (modified model).
For the classic model, we set the first indicator variable equal to 1 for GCS scores from 14 to 15 (mild TBI) and equal to 0 otherwise, and set the second indicator variable equal to 1 for GCS scores from 9 to 13 (moderate TBI) and equal to 0 otherwise. For the modified model, we set the first indicator variable equal to 1 for GCS scores from 13 to 15 (mild TBI) and equal to 0 otherwise, and set the second indicator variable equal to 1 for GCS scores from 9 to 12 (moderate TBI) and equal to 0 otherwise. The reference level for the dummy variables in both models was GCS scores from 3 to 8 (severe TBI).
The selection of predictors was based on clinical considerations and literature review. We considered age, sex, cause of injury, injury severity score, presence of co-morbidities, trauma center level, and whether the patient had sustained isolated TBI, which is defined as no other injuries or no extra-cranial injuries with a maximum Abbreviated Injury Scale (AIS) score of 1. 24,25
We did not find an adequate goodness of fit for either model; therefore, we hypothesized that the mortality trend was an important confounder of the association between GCS and TBI mortality and could be a good reason for the lack of calibration. Hence, we added year of the injury as a dummy variable to adjust for this trend in both the classic and modified GCS models. 26,27. We still did not find an appropriate goodness of fit in this second evaluation, so we conducted a third comparison between the two models.
In the third evaluation, we added TBI severity according to the Barell Matrix, 21,22,28 the presence of hypothermia and hypotension at ED admission and the need for mechanical ventilation at the time the first set of vital signs were taken. We also included any positive screening for drugs either at the facility or prior to arrival at the trauma center. 29,30
Hypothermia, hypotension, and hypoxia are associated with increased probability of death as secondary insults. 31 Hypothermia was defined as body temperature ≤ 35° degrees Celsius31–33 and hypotension was defined as systolic blood pressure (SBP) <90mmHg.31,34 Unfortunately, hypoxia is not available in the PTOS database.
The need for mechanical ventilation was defined as the need for intubation with or without the use of paralyzing medications at the time of the first set of vital signs assessment.35,36 Intubation with or without the use of paralyzing medications do not necessarily implicate the presence of hypoxia. We do not use these variables as surrogates of hypoxia. There are several studies and reviews that have reported intubation and induced paralysis as clinical confounders of the association between GCS and TBI mortality. Furthermore, the use of intubation with or without paralyzing medications may impair the assessment of the verbal response.10,30,37,38
Analysis
Continuous variables were compared according to the outcome with unpaired t-test or Mann-Whitney test, according to their distribution. Categorical variables were compared by Chi2 test. 26 Proportions of mortality were calculated using denominators of the total number of TBI patients between 18–65 years of age.
The odds of survival were fit into multivariate logistic regressions. Most of the variables included in these evaluations have been previously associated with prognosis in TBI, so we included all of them in the multivariable logistic regression analyses. Each of these predictors was evaluated for its contribution in both the classical and modified models. Findings are presented as odds ratio (OR), with 95% confidence intervals (CI) and P values. 39 We assessed both models’ prediction of mortality by sensitivity, specificity, positive predictive value, negative predictive value, and proportion of correctly classified cases. The models’ performance was evaluated with calibration and discrimination. Discrimination was defined as the ability of the models to accurately predict mortality and was evaluated using the area under receiver operating characteristic curve (AUC). Calibration, defined as the ability of the models to accurately quantify the risk of mortality, was evaluated using the Hosmer-Lemershow goodness of fit (GOF) test. 39,40 After these estimations, we tested different models with and without each of one of the predictors in order to see if there were any changes on the effect of both GCS classifications on the prediction of mortality. 40,41 Analyses were performed in STATA, Version 11 (Stata Corporation College Station TX). 39
3. Results
General Characteristics
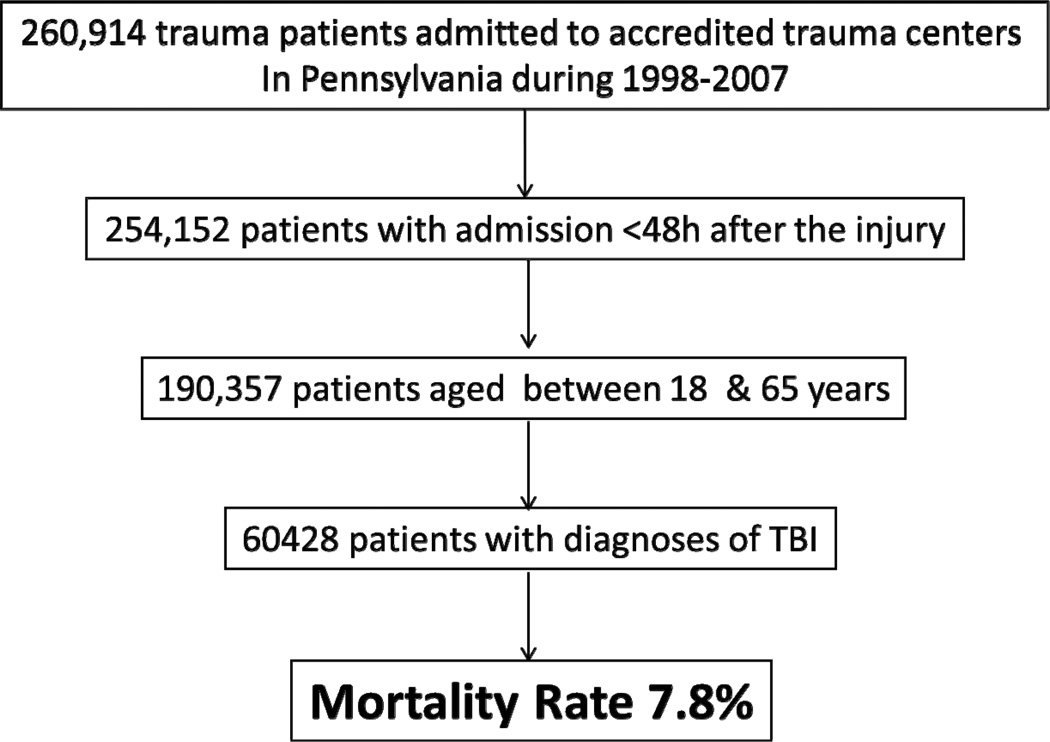
We analyzed 60,428 adult TBI patients out of more than 200,000 patients admitted to trauma centers in Pennsylvania. The mortality rate was 7.8% (figure1). The median age was 37 (inter quartile range [IQR]=25–49) and there was no difference in age between survivors and non survivors (p=0.885). We found a statistical difference in the severity of injury between survivors and patients who died (p<0.001). Most of the patients were male (72.5%). Road traffic crashes were the most common cause of injury (57.3%); the second most common cause was falls (20%). Table 1 shows the general patient characteristics.
Figure 1.
Inclusion and exclusion criteria and final sample size.
Table 1.
General Characteristics of study population.
| Predictor | Total (N=60428) |
Alive | Death | p-value* |
|---|---|---|---|---|
| Age Median (IQR)± | 37 (25–49) | 37 (25–48) | 37 (24–50) | 0.885 |
| ISS Median (IQR)± | 14 (9–19) | 13 (9–17) | 22 (17–29) | <0.001 |
| Sex (%) α | ||||
| Male | 72.4 | 71.9 | 78.2 | <0.001 |
| Cause of injury (%) | ||||
| MotorVehicleTraffic | 57.4 | 58.0 | 49.3 | |
| Fall | 19.9 | 20.1 | 16.5 | |
| Transport/other | 6.1 | 6.3 | 3.5 | |
| Struckby/against | 5.3 | 5.6 | 1.8 | |
| Fire/Burn | 4.2 | 4.5 | 1.4 | |
| Firearms | 3.1 | 1.4 | 23.3 | |
| Other | 5.1 | 5.1 | 5.0 | <0.001 |
| Trauma Center Level (%) | ||||
| I | 62.9 | 62.8 | 64.7 | |
| II | 37.1 | 37.2 | 35.3 | 0.009 |
| Severity of TBI by BM¥ (%) | ||||
| Concussions | 35.4 | 38.2 | 2.8 | |
| Penetrating&Hemorrage | 39.6 | 38.1 | 56.8 | |
| SkullFracture&Other | 25.0 | 23.7 | 40.4 | <0.001 |
| Preexistence conditions (%) | 56.4 | 55.1 | 72.2 | <0.001 |
| Isolated TBI (%) | 8.6 | 8.1 | 14.2 | <0.001 |
| Hypotension (%) | 6.6 | 3.6 | 42.6 | <0.001 |
| Hypothermia (%) | 13.5 | 10.9 | 44.9 | <0.001 |
| Drug Screening (%) | 19.9 | 20.3 | 15.2 | <0.001 |
| Mechanical Ventilation (%) | 18.1 | 13.7 | 70.1 | <0.001 |
P value for comparison between alive/death.
Age and ISS were comparing according to mortality outcome with Wilcoxon - Mann-Whitney test.
Proportions were compared with chi2 test.
Severity of TBI by the Barrel Matrix codification.
Other predictors such as length of stay, need for ICU and need for CT scan were analyzed but they were not significant results.
The dummy variables of GCS scores that were constructed to perform the comparative analyses are depicted in table 2. Most patients had mild brain injuries (67.9% in the classic GCS model vs. 70.8% in the modified GCS model). The percentage of patients with moderate TBI was 10.4% in the classic GCS model vs. 4.8% in the modified GCS model. We found significant statistical differences between survivors and patients who died across the different levels in both the modified and the classic classifications of GCS dummy variables (p<0,001).
Table 2.
Glasgow Coma Scale (GCS) dummy variables for the predictive models.
| GCS Dummy Variables | Total (N=60428) | Alive | Death | p-value* |
|---|---|---|---|---|
| GCS score Classic Model (%) | ||||
| Mild (14–15) | 69.8 | 75.1 | 7.6 | |
| Moderate (9–13) | 7.9 | 8.2 | 4.3 | |
| Severe (3–8) | 22.3 | 16.7 | 88.1 | <0.001 |
| GCS score Modified Model (%) | ||||
| Mild (13–15) | 72.8 | 78.3 | 8.4 | |
| Moderate (9–12) | 4.9 | 5.0 | 3.5 | |
| Severe (3–8) | 22.3 | 16.7 | 88.1 | <0.001 |
P value for comparison between proportions of alive/deceased with chi2 test.
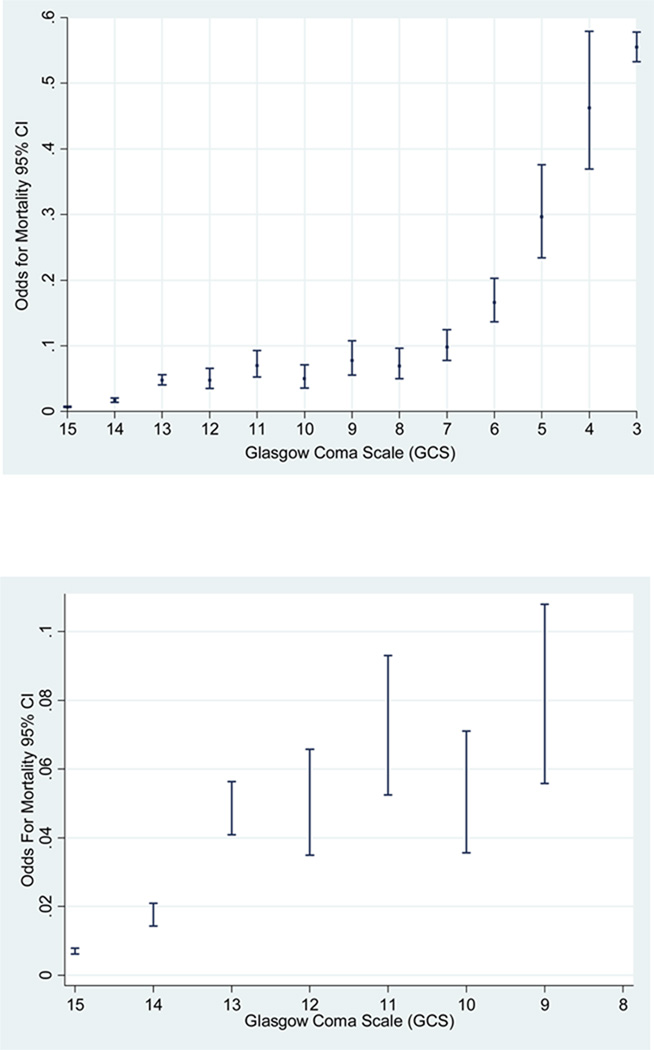
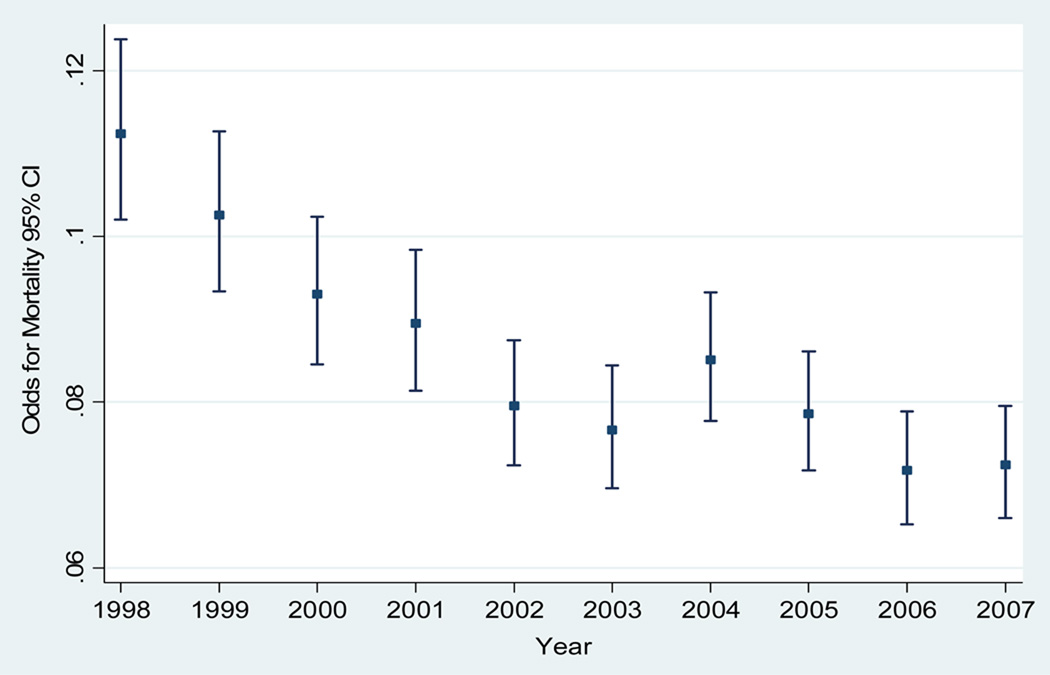
We plotted the odds of mortality and the GCS scores as continuous variable (score 15 to 3). We found that the odds of mortality for GCS score of 13 are closer to a GCS score of 12 rather than a GCS score of 14 (figure 2). In addition, we plotted the trend of mortality by TBI and we also found a significant trend in the odds of mortality across the years of the study (test score for trend of odds: chi2 = 70.21. Pr chi2 = < 0.001) (figure 3). This result leads us to take into account the effect of this variable prediction of TBI mortality in both the classic and modified models.
Figure 2.
Odds of TBI Mortality 95% CI for of Glasgow Coma Scale (GCS) moderate & mild TBI classic model.
Figure 3.
Odds of TBI mortality by year. PTOS, 1998–2007.
Score test for trend of odds: chi2 = 70.21. Pr chi2 = < 0.001.
Compared to the modified classification, the classic GCS model established an important difference in the proportion of mortality in patients with several predictors included in the analyses. Table 3 depicts the mortality proportions in the categories of classic and modified GCS classifications. We observed differences in mortality between patients who sustained traffic injuries, falls, and firearms injuries, between patients with the presence of penetrating and hemorrhage TBI and those with skull fracture, and between patients with hypothermia and hypotension at admission and the need for mechanical ventilation.
Table 3.
Mortality proportions across the three categories of GCS according to 4.3 predictors.
| GCS Classic Model |
GCS Modified Model |
|||||
|---|---|---|---|---|---|---|
| Predictor | Mild (14–15) |
Moderate (9–13) |
Severe (3–8) |
Mild (13–15) |
Moderate (9–12) |
Severe (3–8) |
| Age Median (IQR) |
50 (39.5–58) |
45 (29–56) |
35 (23–48) |
50 (39–59) |
44.5 (30–56) |
35 (24–48) |
| ISS Median (IQR) | 21 (17–29) |
20 (16–29) |
22 (17–29) |
20 (17–29) |
21 (17–34) |
22 (17–29) |
| Sex (%) | ||||||
| Male | 7.2 | 6.7 | 86.0 | 9.4 | 4.5 | 86.0 |
| Cause of injury (%) | ||||||
| Traffic | 7.6 | 5.2 | 87.2 | 9.3 | 3.5 | 87.2 |
| Fall | 14.8 | 11.4 | 73.8 | 18.7 | 7.5 | 73.8 |
| Transport/other | 7.3 | 5.5 | 87.3 | 10.1 | 2.6 | 87.3 |
| Struck by | 10.4 | 12.6 | 77.0 | 13.6 | 9.4 | 77.0 |
| Fire/Burn | 16.2 | 19.1 | 64.7 | 20.7 | 14.6 | 64.7 |
| Firearms | 1.2 | 4.8 | 94.0 | 3.4 | 2.6 | 94.0 |
| Other | 5.8 | 11.0 | 83.2 | 7.3 | 9.5 | 83.2 |
| Trauma Center Level (%) |
||||||
| I | 7.0 | 7.4 | 85.6 | 9.8 | 4.6 | 85.6 |
| II | 8.1 | 5.6 | 86.3 | 9.2 | 4.5 | 86.3 |
| Severity TBI (%) | ||||||
| Concussions | 44.8 | 6.0 | 49.3 | 47.0 | 3.7 | 49.3 |
| Penetrating-Hem | 8.1 | 7.7 | 84.1 | 11.0 | 4.9 | 84.1 |
| Skull-Fracture | 3.7 | 5.4 | 90.8 | 6.4 | 2.8 | 90.8 |
| Preexistence conditions (%) |
8.6 | 4.4 | 87.1 | 9.3 | 3.6 | 87.1 |
| Isolated TBI (%) | 4.6 | 4.3 | 91.1 | 5.2 | 3.7 | 91.1 |
| Hypotension (%) | 1.7 | 5.4 | 92.9 | 3.8 | 3.3 | 92.9 |
| Hypothermia (%) | 3.2 | 6.3 | 90.5 | 5.9 | 3.6 | 90.5 |
| Drug Screen (%) | 7.3 | 6.8 | 85.9 | 8.9 | 5.2 | 85.9 |
| Mechanical Ventilation (%) |
0.2 | 3.4 | 96.3 | 1.4 | 2.3 | 96.3 |
Multivariable Predictive Models
We performed three multivariable predictive models. Table 3 depicts the OR for each GCS category in the three evaluations. Because we gave the baseline value to the category of severe TBI, all the ORs for the moderate and mild TBI are less than 1. We show that the ORs for moderate cases are significantly different in both multivariable models (Table 3).
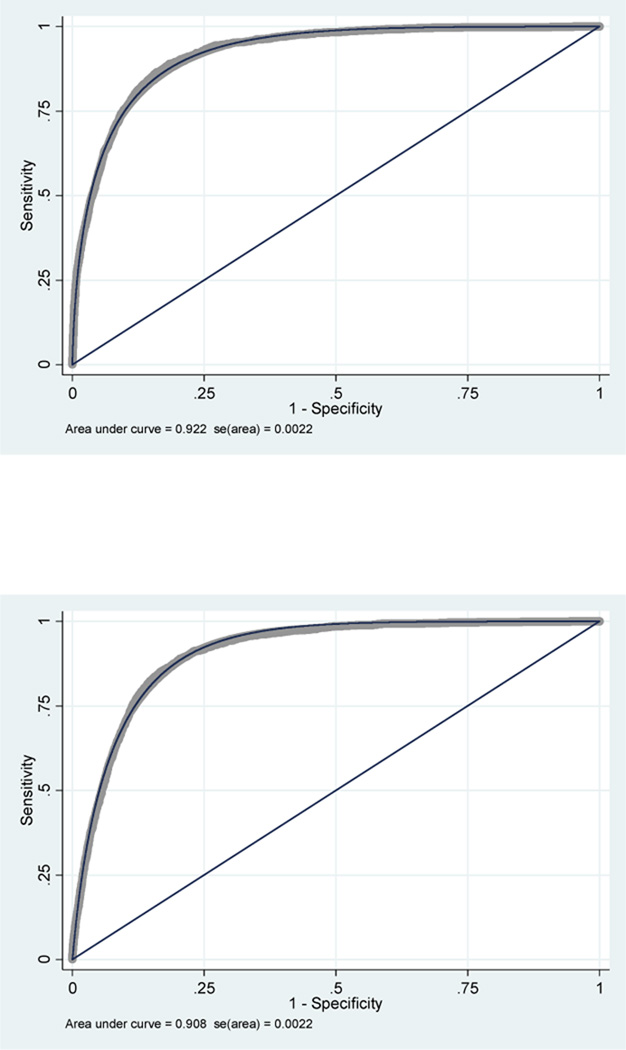
The results of sensitivity, specificity, predictive values, and correctly classified cases are summarized in table 4. These parameters were better in the classic model GCS score of 13 category. The first evaluation of the AUC of the classic model was 0.922 (95 %CI, 0.918 – 0.926); the AUC of the modified model was 0.908 (0.904 – 0.912). The lack of overlap between confidence intervals for the two models reveals a statistically significant difference in their ability to predict mortality (figure 4). However, the two models showed poor calibration (p<0.001 for both models).
Table 4.
Multivariable predictive models for GCS score. Figures are odds ratios (95% confidence intervals)
| GCS dummy Variables |
Unjusted Analysis |
Multivariable Models |
||
|---|---|---|---|---|
| Evaluation 1* | Evaluation2± | Evaluation 3α | ||
| Odds Ratio | Odds Ratio | Odds Ratio | Odds Ratio | |
| Classic Model | 95%CI | 95%CI | 95%CI | 95%CI |
| 36.98 | ||||
| Severe (3–8) | (33.7 – 40.6) | Reference | 1.00 | 1.00 |
| 0.51 | 0.12 | 0.12 | 0.19 | |
| Moderate (9–13) | (0.43 – 0.58) | (0.11 – 0.14) | (0.10 – 0.14) | (0.16 – 0.23) |
| 0.027 | 0.029 | 0.030 | 0.06 | |
| Mild (14–15) | (0.025 – 0.030) | (0.026 – 0.033) | (0.027 – 0.033) | (0.05 – 0.07) |
| Modified Model | ||||
| 32.65 | ||||
| Severe (3–8) | (29.78 – 35.78) | Reference | 1.00 | 1.00 |
| 0.69 | 0.18 | 0.16 | 0.30 | |
| Moderate (9–12) | (0.59 – 0.82) | (0.15 – 0.21) | (0.14 – 0.20) | (0.25 – 0.37) |
| 0.028 | 0.035 | 0.034 | 0.08 | |
| Mild (13–15) | (0.024 – 0.031) | (0.032 – 0.039) | (0.30 – 0.38) | (0.07 – 0.09) |
First evaluation adjustment for age, sex, cause, severity, trauma center level, comorbidities, and isolated TBI.
Second Evaluation adjustment for trend
Third Evaluation adjustment for the presence of hypothermia, hypotension at admission, need for paralyzing drugs and intubation, screening for psychoactive drugs, and final diagnosis at discharge.
Figure 4.
First comparative analysis of classic GCS model vs. modified GCS model.
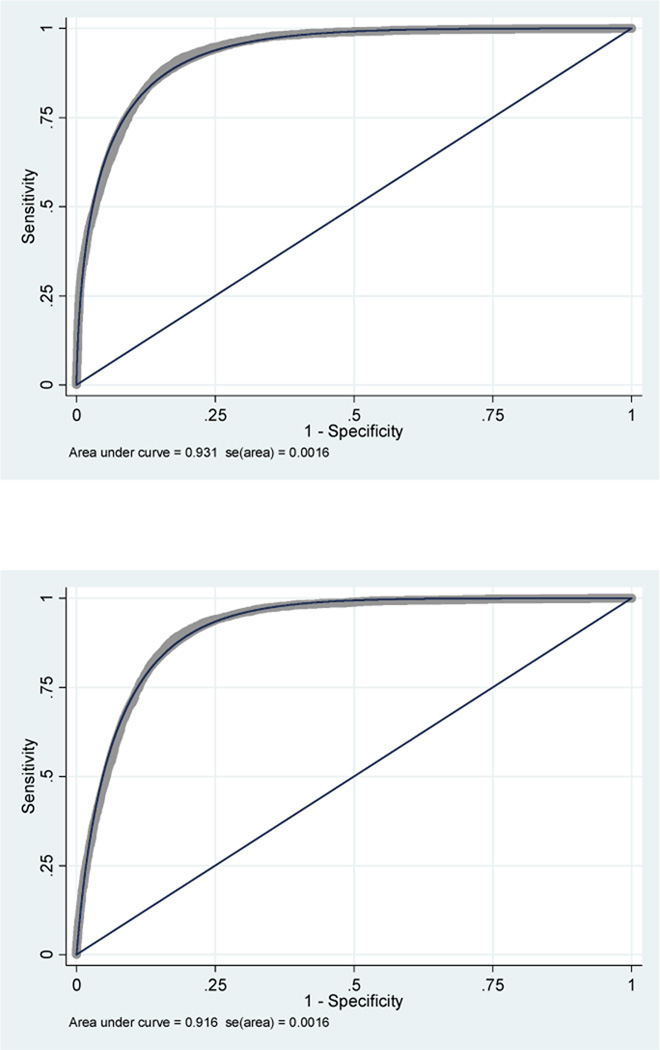
In the second evaluation with a dummy variable of the year of injury, the AUC of the classic model was 0.931 (95 %CI, 0.928 – 0.934) and the AUC of the modified model was 0.916 (95 %CI, 0.913 – 0.919). These AUCs were closer to each other but there was still a lack of overlap between 95% CI for both models, maintaining the statistically significant difference in their ability to predict mortality (figure 5). However, in both models, there was still evidence of poor calibration (p<0.001 for both models).
Figure 5.
Second comparative analysis of classic GCS model vs. modified GCS model.
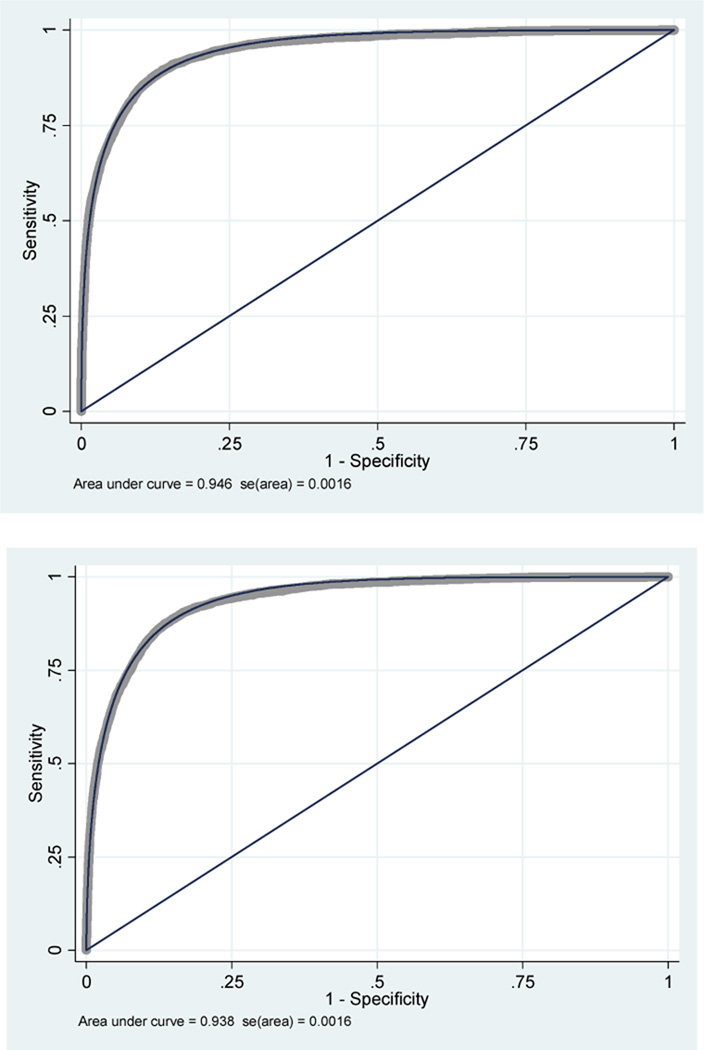
In the third evaluation, the AUC of the classic model was 0.946 (95 %CI, 0.943 – 0.949) and the AUC of the modified model was 0.938 (95 %CI, 0.934 – 0.940). The 95% CIs were closer but still did not overlap each other. In addition, the classic model showed better calibration (p=0.298) than the modified model (p=0.02) (figure 6).
Figure 6.
Third comparative analysis of classic GCS model vs. modified GCS model.
4. Discussion
We have developed a comparative analysis in order to measure the effect of an admission GCS score of 13 classified as moderate TBI (classic model) versus an admission GCS score of 13 classified as mild TBI (modified model) in mortality prediction. We performed three comparisons between those logistic multivariable models with a major outcome using clinical and demographic predictors available in a state trauma registry. Our analyses show that an admission GCS score of 13 classified as moderate predicts mortality better than the modified model in TBI patients.
All the models depicted excellent discrimination and the third evaluation showed better GOF for the classic model. Increased age, the presence of hypothermia, and the need for mechanical ventilation have all been previously identified as predictors for poor outcome in patients with TBI. In addition, variables such as diagnosis at discharge and screening for drugs were important predictors that increased the discrimination and calibration of the models. 24,25,27,29,31–33,37,42
The inclusion of hypotension and hypothermia was based on the results of McHugh et al. from the IMPACT study. The authors of this study performed a meta-analysis in order to establish the association between secondary insults (hypoxia, hypotension, and hypothermia) occurring prior to or on admission to hospital and 6-month outcome after TBI. The results of their analysis demonstrated that an increased pre-enrollment insult of hypoxia, hypotension, or hypothermia were strongly associated with a poorer outcome. 31
The association between hypoxia and hypotension and bad prognosis has been explored for several years. Miller et al. found that hypoxia and hypotension are highly related to a poor outcome following TBI. 43 Recent studies have found a greater detrimental combined effect of hypoxia, hypotension, and hypothermia on adverse outcome following TBI. 32,44 In this study, the inclusion of hypotension and hypothermia with other clinical predictors improved the discrimination and calibration of the logistic regression models. Unfortunately, we were not able to include hypoxia in the models because that variable was not in the dataset.
Our study strengths are the use of a well described cohort of patients of a state wide trauma registry with over 10 years of data, a large sample size in relation to several clinical predictors and adjusting for the trend of mortality by TBI strengthens the internal validity of our study. Some criticism could be raised because of the pseudo scoring in significant numbers of intubated or aphasic patients and the fall of GCS score reliability to undesirable levels. There are several studies and reviews that have considered intubation and induced paralysis as main clinical confounders of GCS;10,30,36–38,45,46 some of them were included in our analysis, some others were not. We hypothesized that intubation and paralysis were likely to confound the association between the different categories of the GCS score and TBI mortality; therefore, we took into account these variables in the analysis as the need of mechanical ventilation. The PTOS belongs to accredited trauma centers only; therefore, TBI patients admitted and managed in other hospitals or in places without trauma systems were not part of the current analysis. However, several guidelines for prehospital TBI care highly recommend that emergency medical personnel transfer the patient to a trauma center when one is available. This is what usually occurs when a trauma system is in place and working appropriately. 47The use of GCS as a discriminative instrument is that the GCS has been shown to possess all the necessary clinimetric properties for head trauma patients if it is used by experienced or trained personnel. Prasad et al. found that the scale has a good sensibility and reliability; it has well-established cross-sectional construct validity and its predictive validity in traumatic coma, and when combined with age and brainstem reflexes, is good in the generating sample. However, the validity of GCS as a predictive and evaluative instrument, which is the primary purpose for which the scale was constructed, has not yet been adequately studied. 48
As a predictive instrument, the GCS works best if each of the subscales (eye, motor, verbal) is used as a separate predictor variable and is combined with other important predictor variables. It remains to be determined how to perform such a combination and which other variables should be combined to give adequate predictive power in terms of the evaluation of the effect of the modified GCS criteria48,49. In this study, we obtained all the predictive parameters of both multivariable models adjusting for important clinical predictors of mortality and adjusting for trend. The final results showed that the classic GCS model predicts mortality better than the modified GCS model.
In conclusion, we have developed a methodologically valid, simple, and accurate comparison that does not support the ATLS recommendation to reclassify a GCS score of 13 as mild TBI. Our data shows that the classification of an admission GCS score of 13 as moderate TBI in a multivariable logistic regression model results in better performance and accuracy (discrimination and calibration). This analysis may complement the clinical decision making process. However, more studies are required in order to evaluate the implications of this new classification in the clinical scenario and to obtain reliable findings related to this important issue. Further prospective cohorts and cost effectiveness analyses are needed to improve the generalizability of the results. 41,46
Table 5.
Comparison of the predictive validity of GCS score and discrimination and calibration of prognostic models.
| Performance of Multivarible Models |
Evaluation 1 * | Evaluation2 ± | Evaluation 3 α | |||
|---|---|---|---|---|---|---|
| Classic Model |
Modified Model |
Classic Model |
Modified Model |
Classic Model |
Modified Model |
|
| Sensitivity | 32.60% | 17.95% | 32.93% | 18.76% | 50.52% | 43.76% |
| Specificity | 98.97% | 98.84% | 98.25% | 98.85% | 98.71% | 98.58% |
| Positive predictive value | 72.86% | 56.60% | 72.70% | 57.86% | 76.75% | 72.31% |
| Negative predictive value | 94.55% | 93.45% | 94.58% | 93.51% | 95.93% | 95.40% |
| Correctly classified | 93.80% | 92.54% | 93.80% | 92.61% | 94.95% | 94.31% |
| 0.922 | 0.908 | 0.931 | 0.916 | 0.946 | 0.938 | |
| Discrimination (AUC) |
(0.917 - 0.926) |
(0.904 - 0.912) |
(0.928 - 0.934) |
(0.913 - 0.919) |
(0.943 - 0.949) |
(0.934 - 0.941) |
| Calibration (GOF) |
106.96 (<0.001) |
117.01 (<0.001) |
98.89 (<0.001) |
120.67 (<0.001) |
9.56 (0.298) |
17.59 (0.025) |
First evaluation adjustment for age, sex, cause, severity, trauma center level, comorbidities, and isolated TBI.
Second Evaluation adjustment for trend
Third Evaluation adjustment for the presence of hypothermia, hypotension at admission, need for paralyzing drugs and intubation, screening for psychoactive drugs, and final diagnosis at discharge.
Acknowledgments
The Pennsylvania Trauma Systems Foundation, Mechanicsburg, PA, provided these data. The foundation specifically disclaims responsibility for any analyses, interpretations, or conclusions. Credit must be given to the Pennsylvania Trauma Outcome Study (PTOS) as the source of data.
Acknowledgements to Dr. Clifton Callaway, MD, PhD. Vice-Chair of the Emergency Department of the University of Pittsburgh Medical Center. Credit must be given for his suggestions to the final edition of the manuscript.
Sources of funding
This work was supported by the Fogarty International Center of the National Institutes of Health, grant 1 D43 TW007560 01.
Role of the funding source
The study sponsors had no role in the study design, in the data collection, data analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Access
The corresponding author had full access to all study data and takes responsibility for the integrity of the data.
Conflict of interest
We declare that we have no conflicts of interest.
References
- 1.Bruns J, Jr., Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44 Suppl 10:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ. 2005 Dec 17;331(7530):1419–1420. doi: 10.1136/bmj.331.7530.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008 Feb 23;336(7641):425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 5.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 6.Jones C. Glasgow coma scale. Am J Nurs. 1979 Sep;79(9):1551–1553. [PubMed] [Google Scholar]
- 7.Rowley G, Fielding K. Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet. 1991 Mar 2;337(8740):535–538. doi: 10.1016/0140-6736(91)91309-i. [DOI] [PubMed] [Google Scholar]
- 8.Menegazzi JJ, Davis EA, Sucov AN, Paris PM. Reliability of the Glasgow Coma Scale when used by emergency physicians and paramedics. J Trauma. 1993 Jan;34(1):46–48. doi: 10.1097/00005373-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Juarez VJ, Lyons M. Interrater reliability of the Glasgow Coma Scale. J Neurosci Nurs. 1995 Oct;27(5):283–286. doi: 10.1097/01376517-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med. 2004 Feb;43(2):215–223. doi: 10.1016/s0196-0644(03)00814-x. [DOI] [PubMed] [Google Scholar]
- 11.Gomez PA, Lobato RD, Ortega JM, De La Cruz J. Mild head injury: differences in prognosis among patients with a Glasgow Coma Scale score of 13 to 15 and analysis of factors associated with abnormal CT findings. Br J Neurosurg. 1996 Oct;10(5):453–460. doi: 10.1080/02688699647078. [DOI] [PubMed] [Google Scholar]
- 12.Kortbeek JB, Al Turki SA, Ali J, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008 Jun;64(6):1638–1650. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 13.Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005 Sep 28;294(12):1519–1525. doi: 10.1001/jama.294.12.1519. [DOI] [PubMed] [Google Scholar]
- 14.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005 Sep 28;294(12):1511–1518. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 15.Norwood SH, McAuley CE, Berne JD, Vallina VL, Creath RG, McLarty J. A prehospital glasgow coma scale score < or = 14 accurately predicts the need for full trauma team activation and patient hospitalization after motor vehicle collisions. J Trauma. 2002 Sep;53(3):503–507. doi: 10.1097/00005373-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Fearnside M, McDougall P. Moderate head injury: a system of neurotrauma care. Aust N Z J Surg. 1998 Jan;68(1):58–64. doi: 10.1111/j.1445-2197.1998.tb04638.x. [DOI] [PubMed] [Google Scholar]
- 17.Stein SC, Ross SE. Moderate head injury: a guide to initial management. J Neurosurg. 1992 Oct;77(4):562–564. doi: 10.3171/jns.1992.77.4.0562. [DOI] [PubMed] [Google Scholar]
- 18.Lee KL, Pryor DB, Harrell FE, Jr., et al. Predicting outcome in coronary disease. Statistical models versus expert clinicians. Am J Med. 1986 Apr;80(4):553–560. doi: 10.1016/0002-9343(86)90807-7. [DOI] [PubMed] [Google Scholar]
- 19.Ting HW, Chen MS, Hsieh YC, Chan CL. Good mortality prediction by Glasgow Coma Scale for neurosurgical patients. J Chin Med Assoc. 2010 Mar;73(3):139–143. doi: 10.1016/S1726-4901(10)70028-9. [DOI] [PubMed] [Google Scholar]
- 20.Patel HC, Bouamra O, Woodford M, King AT, Yates DW, Lecky FE. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: an observational study. Lancet. 2005;366(9496):1538–1544. doi: 10.1016/S0140-6736(05)67626-X. Oct 29–Nov 4. [DOI] [PubMed] [Google Scholar]
- 21.Barell V, Aharonson-Daniel L, Fingerhut LA, et al. An introduction to the Barell body region by nature of injury diagnosis matrix. Inj Prev. 2002 Jun;8(2):91–96. doi: 10.1136/ip.8.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989 Apr;27(4):412–422. doi: 10.1097/00005650-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Santrucek M, Dolejsi V. 9th revision of the International classification of diseases. Cas Lek Cesk. 1978 Feb 24;117(8):225–231. [PubMed] [Google Scholar]
- 24.Demetriades D, Kuncir E, Brown CV, et al. Early prediction of mortality in isolated head injury patients: a new predictive model. J Trauma. 2006 Oct;61(4):868–872. doi: 10.1097/01.ta.0000219135.33398.f3. [DOI] [PubMed] [Google Scholar]
- 25.Mushkudiani NA, Engel DC, Steyerberg EW, et al. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007 Feb;24(2):259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B. Fundamentals of biostatistics. 6th ed. Belmont, CA: 2006. p. Thomson-Brooks/Cole. [Google Scholar]
- 27.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999 Sep 8;282(10):954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 28.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008 Mar–Apr;23(2):123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa Y, Sakamoto T, Okada Y. Recovery from a psychotropic drug overdose tends to depend on the time from ingestion to arrival, the Glasgow Coma Scale, and a sign of circulatory insufficiency on arrival. Am J Emerg Med. 2007 Sep;25(7):757–761. doi: 10.1016/j.ajem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Fischer M, Ruegg S, Czaplinski A, et al. Inter-rater reliability of the Full Outline of UnResponsiveness score and the Glasgow Coma Scale in critically ill patients: a prospective observational study. Crit Care. 2010;14(2):R64. doi: 10.1186/cc8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007 Feb;24(2):287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- 32.Clifton GL, Miller ER, Choi SC, et al. Hypothermia on admission in patients with severe brain injury. J Neurotrauma. 2002 Mar;19(3):293–301. doi: 10.1089/089771502753594864. [DOI] [PubMed] [Google Scholar]
- 33.Wang HE, Callaway CW, Peitzman AB, Tisherman SA. Admission hypothermia and outcome after major trauma. Crit Care Med. 2005 Jun;33(6):1296–1301. doi: 10.1097/01.ccm.0000165965.31895.80. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber MA, Aoki N, Scott BG, Beck JR. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002 Mar;137(3):285–290. doi: 10.1001/archsurg.137.3.285. [DOI] [PubMed] [Google Scholar]
- 35.Rutledge R, Lentz CW, Fakhry S, Hunt J. Appropriate use of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma. 1996 Sep;41(3):514–522. doi: 10.1097/00005373-199609000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Matis G, Birbilis T. The Glasgow Coma Scale--a brief review. Past, present, future. Acta Neurol Belg. 2008 Sep;108(3):75–89. [PubMed] [Google Scholar]
- 37.Zuercher M, Ummenhofer W, Baltussen A, Walder B. The use of Glasgow Coma Scale in injury assessment: a critical review. Brain Inj. 2009 May;23(5):371–384. doi: 10.1080/02699050902926267. [DOI] [PubMed] [Google Scholar]
- 38.Fischer J, Mathieson C. The history of the Glasgow Coma Scale: implications for practice. Crit Care Nurs Q. 2001 Feb;23(4):52–58. doi: 10.1097/00002727-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Vittinghoff E. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models. New York: Springer; 2005. [Google Scholar]
- 40.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999 Mar 16;130(6):515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 42.Servadei F, Teasdale G, Merry G. Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001 Jul;18(7):657–664. doi: 10.1089/089771501750357609. [DOI] [PubMed] [Google Scholar]
- 43.Miller JD, Sweet RC, Narayan R, Becker DP. Early Insults to Injured Brain. Jama-Journal of the American Medical Association. 1978;240(5):439–442. [PubMed] [Google Scholar]
- 44.Strachan RD, Whittle IR, Miller JD. Hypothermia and severe head injury. Brain Inj. 1989 Jan–Mar;3(1):51–55. doi: 10.3109/02699058909008073. [DOI] [PubMed] [Google Scholar]
- 45.Elliott M. Interrater reliability of the Glasgow Coma Scale. J Neurosci Nurs. 1996 Aug;28(4):213–214. [PubMed] [Google Scholar]
- 46.Fabbri A, Servadei F, Marchesini G, Stein SC, Vandelli A. Observational approach to subjects with mild-to-moderate head injury and initial non-neurosurgical lesions. J Neurol Neurosurg Psychiatry. 2008 Oct;79(10):1180–1185. doi: 10.1136/jnnp.2007.135178. [DOI] [PubMed] [Google Scholar]
- 47.Badjatia NCN, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(1):S1–S52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 48.Prasad K. The Glasgow Coma Scale: a critical appraisal of its clinimetric properties. J Clin Epidemiol. 1996 Jul;49(7):755–763. doi: 10.1016/0895-4356(96)00013-3. [DOI] [PubMed] [Google Scholar]
- 49.Franke CL, van Swieten JC, Algra A, van Gijn J. Prognostic factors in patients with intracerebral haematoma. J Neurol Neurosurg Psychiatry. 1992 Aug;55(8):653–657. doi: 10.1136/jnnp.55.8.653. [DOI] [PMC free article] [PubMed] [Google Scholar]