Abstract
OBJECTIVE
Injury or removal of the knee meniscus leads to progressive joint degeneration, and current surgical therapies for meniscal tears seek to maximally preserve meniscal structure and function. However, the factors that influence intrinsic repair of the meniscus are not well understood. The goal of this study was to investigate the capacity of meniscus tissue to repair a simulated defect in vitro and to examine the effect of pro-inflammatory cytokines on this process.
METHODS
Cylindrical explants were harvested from the outer one-third of medial porcine menisci. To simulate a full-thickness defect, a central core was removed and reinserted immediately into the defect. Explants were cultured for 2, 4, or 6 weeks in serum-containing media in the presence or absence of interleukin-1 (IL-1) or tumor necrosis factor alpha (TNF-alpha), and meniscal repair was investigated using mechanical testing and fluorescence confocal microscopy.
RESULTS
Meniscal lesions in untreated samples showed a significant capacity for intrinsic repair in vitro, with increasing cell accumulation and repair strength over time in culture. In the presence of IL-1 or TNF-alpha, no repair was observed despite the presence of abundant viable cells.
CONCLUSIONS
This study demonstrates that the meniscus exhibits an intrinsic repair response in vitro. However, the presence of pro-inflammatory cytokines completely inhibited repair. These findings suggest that increased levels of pro-inflammatory cytokines post-injury or under arthritic conditions may inhibit meniscal repair. Therefore, inhibition of these cytokines may provide a means of accelerating repair of damaged or injured menisci in vivo.
Keywords: Meniscus, Injury, Cartilage, Integrative repair, IL-1, TNF-α, Osteoarthritis
Introduction
The menisci are intra-articular fibrocartilaginous structures that play essential roles in the biomechanical function of the knee joint, including load transmission, shock absorption, stability, and lubrication1–4. Meniscal tissue is composed of approximately 70% water, 20% collagen (predominantly type I and smaller amounts of types II, III, V, VI), with smaller quantities of proteoglycans and non-collagenous proteins, lipids, and cells5. The meniscus is a highly inhomogeneous tissue, exhibiting regional differences in composition, structure, and cell phenotype6,7. The extracellular matrix is synthesized and maintained by several subpopulations of cells that have a range of fibroblastic or chondrocytic phenotypes. For example, cells of the inner radial two-thirds of the meniscus exhibit a rounded morphology and high mRNA levels for chondrocytic markers such as type II collagen and aggrecan, while the outer one-third of the meniscus is populated with elongated cells that exhibit characteristics of a more fibroblast-like phenotype, such as extensive cellular processes and expression of collagen type I mRNA8–10. Similar to other cartilaginous tissues, the metabolic activity of meniscal cells is strongly influenced by factors in the microenvironment, including soluble mediators such as growth factors or cytokines11–15, or physical factors such as mechanical compression or stretch9,16–22.
There is strong evidence that even partial removal of the menisci leads to joint instability23 and eventually joint degeneration24–28. Thus, in current surgical practice, efforts are made to repair meniscal tears instead of removing damaged meniscal tissue whenever. Good clinical outcomes are generally obtained with reconstruction of tears, especially in the outer, vascularized region of the meniscus29–31. However, a number of factors may contribute to the success of a meniscal repair procedure, including the degenerative state of the joint, the site of the injury, and the presence of additional joint injuries, such as rupture of the anterior cruciate ligament (ACL). Meniscal tears that occur in ACL-intact knees are more degenerative in nature and less likely to heal compared to acute, traumatic tears, which more often occur in ACL-deficient knees32–34. It is notable that surgical repair of meniscus is generally very effective in patients who do not have severe cartilage damage or degenerative joint changes29,35,36. However, patients with more severe cartilage lesions exhibit a significantly higher number of degenerative meniscus tears, which generally exhibit poor outcomes with surgical repair37.
Joint injury, as well as degenerative joint diseases such as osteoarthritis or rheumatoid arthritis, are characterized by increased activity of the pro-inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α)38–40. Furthermore, direct injury of the meniscus during surgery can induce prolonged upregulation of IL-141. The inflammatory activities of these cytokines in the joint are partially due to increased production of pro-inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2)42. Furthermore, meniscal cells themselves exhibit pro-inflammatory capabilities and can synthesize significant levels of NO or PGE2 when exposed to cytokines12,14,22 or biomechanical stress9,16,17,19,21,22. This upregulation of pro-inflammatory mediators in injured or arthritic joints, when combined with cytokines produced endogenously by meniscal cells, may act to suppress matrix biosynthesis and increase enzymatic degradation. However, the influence of these factors on the repair capacity of the meniscus is not known.
A number of in vitro studies have examined the repair properties of articular cartilage43–46 or meniscus47–52. In one study, for example, an organ culture model was used to examine the repair of full-thickness defects in human menisci that were filled with autologous synovium, where it was shown that cells could migrate along the synovium and bridge the defect47. Fibrochondrocytes derived from New Zealand white rabbit menisci were shown to invade defects filled with fibrinogen and human thrombin48. In canine medial menisci, devitalized meniscal cores were replaced in vivo and repopulated with cells that expressed alpha smooth muscle actin, suggesting that the presence of these cells may be essential for repopulation and integration of meniscal tissues50. In other studies, it has been shown that superficial zone cells in the meniscus can migrate into such a defect and repopulate an acellular meniscus plug, assisting in wound healing of the meniscus in vitro51. In other studies, intrinsic repair of meniscal tears in a whole meniscus organ culture model was demonstrated by histologic analysis49. These studies have provided valuable insights on the potential for meniscus repair in explant culture models, but there have been no direct quantitative measures of repair strength or investigations of the impact of different cytokines on healing of meniscus lesions.
The goal of this study was to investigate the repair capacity of meniscus tissue in vitro and to examine the effect of pro-inflammatory cytokines on meniscus healing. In a recent study, we reported a novel model to study the meniscal repair, which showed similar intrinsic repair responses between the inner and outer porcine meniscus in vitro52. In this study, cylindrical explants were harvested from the femoral surface of the outer one-third of medial porcine menisci. To simulate a full-thickness defect, a central core was removed and reinserted immediately. Explants were cultured for 2, 4, and 6 weeks in the presence or absence of IL-1 or TNF-α, and meniscal repair was investigated using mechanical testing and fluorescence confocal microscopy to measure cellular accumulation in the defect site.
Materials and Methods
Meniscus specimens
Medial menisci were harvested from knee joints of 2–3 year-old skeletally mature female pigs obtained from a local slaughterhouse within 4 hours of death. Under aseptic conditions, cylindrical explants (8 mm in diameter) were harvested from the peripheral, outer one-third of the femoral surface of the menisci using a biopsy punch (Miltex Instrument Company Inc, Lake Success, NY), which was inserted perpendicular to the femoral surface. A central 4 mm core was punched from the middle of each 8 mm explant, again perpendicular to the femoral surface, taking care to completely separate the two parts. The inner core was replaced into the outer ring immediately (Figure 1). To obtain specimens of uniform height, explants were cut along the bottom surface to a thickness of 2 mm parallel to the femoral surface, taking care to maintain the collagen fiber alignment of the two concentric explants. Adjacent samples from each meniscus were paired at harvest, and one specimen was assigned to the treatment group (IL-1 or TNF-α), while the other specimen was used as its paired control. Only menisci without tears or visible degenerative changes were used for the study.
Figure 1.
Medial meniscus showing (a) location of explants harvested from the outer one-third of the femoral surface of the menisci. (b) side view of the explant in situ. (c) A central 4 mm core was punched from the middle of each 8 mm explant and immediately replaced. Square shows the site and orientation of specimens taken for confocal microscopy. Adjacent explants were site-matched and paired at harvest.
After harvest, specimens were incubated in 4 ml of base culture medium (Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (FBS, HyClone, Logan, UT), 0.1 mM non-essential amino acids (Gibco), 10 mM HEPES Buffer solution (Gibco)) with 1000 U/ml penicillin/streptomycin (Gibco) at 37°C, 5% CO2, and 95% air. After one hour, culture medium was changed to medium with 100 U/ml penicillin/streptomycin, and samples were cultured for 72 hours to allow equilibration of cell metabolic activity in culture21,22. Samples were subsequently cultured in the presence or absence of 10 ng/ml recombinant porcine IL-1α or 10 ng/ml recombinant porcine TNF-α (R&D Systems), and media was changed to 4 ml of base culture medium (described above) with 100 U/ml penicillin/streptomycin, and 37.5μg/ml ascorbate-2-phosphate (Sigma-Aldrich, St Louis, MO) every three days. Samples were incubated at 37°C, 5% CO2, and 95% air for 2, 4, and 6 weeks with media changes every 3 days. Samples originated from 42 different animals, and a total of n=108 explants were used for mechanical testing and n=12 samples for fluorescence cell viability analysis.
Gross appearance
The macroscopic appearance of meniscus explants was recorded when samples were investigated at 2, 4, and 6 weeks. Samples were inspected as to whether openings had occurred between the outer ring and the inner core. The inner core was examined for changes in position or movement relative to the outer ring.
Mechanical testing of repair strength
Interfacial shear strength of the repair between the surfaces of the inner core and the outer ring of the explants was determined using a push-out test on an electromechanical materials testing system (ELF 3200 Series, EnduraTEC Systems Corp., Minnetonka, MN). The specimens, consisting of an 8 mm diameter outer ring and 4 mm inner core, were placed in a custom-built testing fixture that consisted of a suspended cup with a central five mm thru-hole in its base, as described previously52. Explants were aligned with the inner core centered over the thru-hole, allowing it to be freely pushed from the outer annulus by a three mm diameter rod. A tare load of 0.2 grams-force was applied to the central core to ensure contact between the sample and the pushing rod. After equilibration of the tare load, the rod was displaced at a rate of 0.0833 mm per second until the inner core was completely separated from the outer ring. Force measurements were recorded throughout the duration of the test using a load cell (Sensotec Model 31/6775-06) mounted in-line between the pushing rod and the electromechanical actuator.
Specimen thickness was measured using a custom-built vision system consisting of a digital video camera (Model XDC-X700, Sony Electronics, Park Ridge, NJ) with a 44 mm video lens (Infinity, Boulder, CO). Images were acquired and analyzed using LabVIEW Vision Builder AI for Windows (National Instruments Corp., Austin, TX). Shear stress (τ) was then calculated from the F core thickness and force according to where, F is the peak force required to push the inner core completely through the outer annulus, d is the core diameter, and h is the core thickness. Repair strength was reported as the peak shear stress during the push-out test.
Cell viability and structure of healing tissue
Cell viability and accumulation were investigated at various timepoints (2, 4, and 6 weeks) using a fluorescent live/dead assay (Molecular Probes, Eugene, OR). Explants were washed in Dulbecco’s phosphate buffered saline (PBS) (Gibco, Gaithersburg, MD) and incubated for 20 min in PBS containing 1 μg/ml calcein AM as a label of viable cells and 6 μg/ml ethidium homodimer-1 as a label of dead cells (Live-dead Assay, Invitrogen, Eugene OR). Explants were rinsed in PBS to remove unincorporated stain and placed with the femoral surface of the meniscus down in a Lab-Tek chambered coverglass containing PBS (see Figure 1 for orientation). Images were obtained using a confocal laser scanning microscope (LSM 510, Zeiss, Thornwood, NY).
Statistical Analysis
A 2-factor ANOVA with repeated measures and a Newman-Keuls post hoc test was used to determine differences in the repair strength of control samples and explants treated with IL-1 or TNF-α at various time points using the push-out test. Significance is reported at the 95% confidence level.
Results
Gross appearance
At the 2, 4, and 6 weeks harvest times, both control and treated samples showed no apparent differences in macroscopic appearance. There were no visible openings along the interface between the outer ring and the inner core, and no samples had overt signs of infection at any time-point.
Mechanical testing of repair strength
Mechanical testing revealed a significant increase in the strength of repair over time in control specimens. Control samples showed a statistically significant increase in strength from 2 weeks to 6 weeks (p<0.001) and 4 weeks to 6 weeks (p<0.001).
The presence of IL-1 significantly reduced the shear strength at all time points compared to controls (p<0.001), and no significant changes in repair strength were observed in the treated groups over the 6 week culture period (Figure 2a).
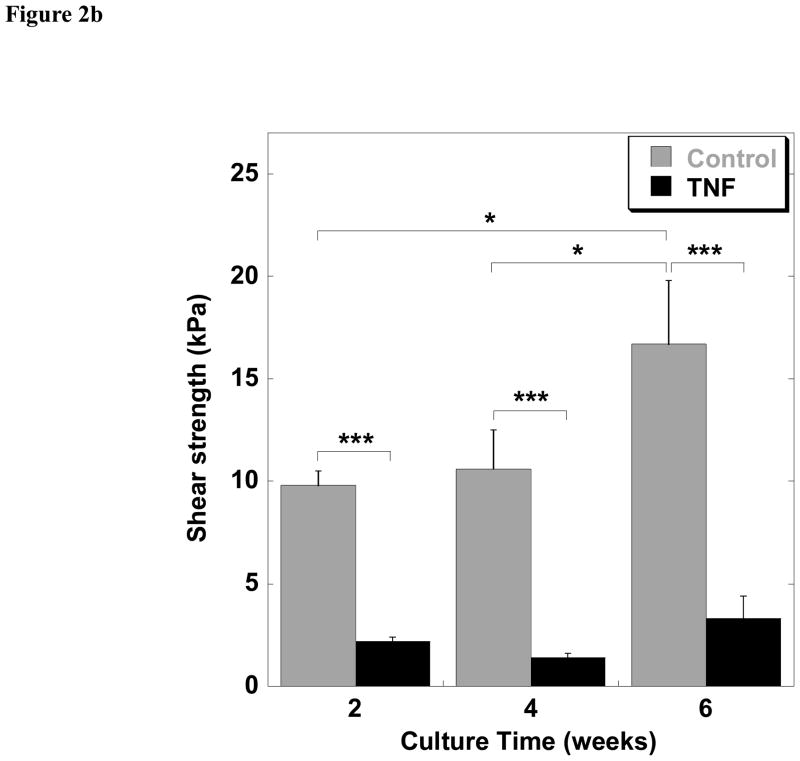
Figure 2.
Interfacial shear strength of repair over time in culture. (a) Interfacial shear strength of control (untreated) explants and explants treated with 10 ng/ml IL-1 at 2, 4, and 6 weeks of culture. (b) Interfacial shear strength of control (untreated) explants and explants treated with 10 ng/ml TNF-α at 2, 4, and 6 weeks of culture. Data are presented as mean ± SEM (n = 9 per treatment and time point, *p<0.05, ***p<0.001).
TNF-α significantly reduced the shear strength of repair at all times points as compared to control specimens (p<0.001). In the TNF-α treated group, no significant changes in repair strength were observed with time of the 6 week culture period (Figure 2b). Samples treated with TNF-α showed a trend of higher shear strengths as compared to samples exposed to IL-1, although this comparison was not analyzed statistically.
Cell viability and structure of healing tissue
In control groups, the fluorescence viability assay revealed viable cells within the defect and the presence of cell clusters in numerous sites by 2 weeks. Cells appeared connected via cellular processes and bridged the artificially created defect (Figure 3a). In samples treated with IL-1, no cellular proliferation was evident, and only a few cells covered the adjacent surfaces of both the inner core and the outer ring of each specimen (Figure 3b). In samples exposed to TNF-α, only one layer of cells covered the surfaces of the defect at a few points, and no bridging of the gap was observed (Figure 3c). In comparison to IL-1 treatment groups, explants treated with TNF-α showed more cellular accumulation and invasion into the tissue gap by 2 weeks. At 4 weeks of culture, further cellular accumulation in the defect interface was evident in the control group, with an increased number of cells present in the repair site and further connections between these cells (Figure 3d). In the IL-1 group, more viable cells were present as compared to the 2 week timepoint, but no cells were observed in the gap (Figure 3e). In samples treated with TNF-α, cells covered the surface of the defect with several cell layers, but there was no network extending across the gap (Figure 3f). At 6 weeks of culture, the gap in control explants was entirely filled with meniscal cells, which completely bridged the tissue interface (Figure 3g). In samples treated with IL-1, the defect surfaces were covered with several cell layers, but there was no accumulation of cells into the gap (Figure 3h). In samples cultured with TNF-α, cells covered the surfaces of both the inner core and outer ring of each specimen, but bridged the gap in only a few locations (Figure 3i).
Figure 3.
Cell viability and migration investigated by a fluorescent live/dead assay using a confocal laser scanning microscope at the tissue interface. At 2 weeks, (a) in the control group, partial bridging of the defect was observed; (b) in samples treated with IL-1, no cellular accumulation was evident; (c) in samples treated with TNF-α, one layer of cells had covered the defect on some locations, but no bridging of the gap was visible. At 4 weeks of culture, (d) in the control group, further cellular migration and accumulation into the gap was observed. In contrast, no cellular migration was seen in samples treated with IL-1 (e) and TNF-α (f). After 6 weeks of culture, (g) in the control group, the gap was completely filled by cells; (h) in samples treated with IL-1, the defect was covered with up to two cell layers, but no cells were observed bridging the tissue gap; (i) in samples treated with TNF-α, cells had bridged the gap in a few areas. Scale bar = 100 Sm.
Discussion
Our studies show that meniscal tissue has the ability in vitro to partially repopulate a defect with cells and tissue, exhibiting the initial characteristics of tissue repair. Under control conditions, cells accumulated in the defect gap over several days, increasingly spanning the gap over the culture period. However, in the presence of either IL-1 or TNF-α, cell accumulation and intrinsic repair were abolished. These inhibitory effects were not the result of meniscal cell death since both control and cytokine-treated explants showed high cell viability throughout the culture period. These findings suggest that the intrinsic ability for meniscal tissue to repair a lesion may be inhibited or delayed in vivo due to local increases in pro-inflammatory cytokines such as IL-1 or TNF-α.
Joint injury, as well as acute or chronic arthritis, are characterized by increased concentrations of IL-1 and TNF-α in the joint as well as the meniscus41,53,54. These mediators can suppress matrix biosynthesis and increase enzymatic degradation of various joint tissues39,55, including the meniscus14,19,22,38. Although the mechanisms involved in meniscal repair are not fully understood, our in vitro findings suggest that meniscus repair in vivo may be inhibited by joint inflammation associated with injury or arthritis. In control samples, the repair process was characterized by the accumulation of cells within the tissue gap, presumably through a combination of cell migration and proliferation, followed by formation of tissue within the defect site. In specimens treated with IL-1 or TNF-α, neither cellular accumulation nor tissue formation was observed, suggesting that these cytokines inhibited integrative repair by influencing cell migration and/or proliferation in the tissue gap, or by altering the balance of biosynthetic and degradative activity of the meniscal cells.
In previous studies, in vitro models of integrative repair of cartilaginous tissues have proven useful for the study of specific factors related to the repair processes43–46,56,57. For example, previous studies have shown that collagen synthesis, deposition, and maturation are critical factors in determining the strength of articular cartilage integration in vitro44,45,56. While these factors specifically have not been investigated in the meniscus, our findings are consistent with previous studies that have demonstrated that cell migration is required for in vitro meniscal repair.47–52 In related work, we have shown similar repair capacity in vitro of the inner, avascular region of the meniscus as compared to the outer vascularized region52, suggesting that the presence of a vascular supply may contribute to the improved repair capabilities of the outer zone observed in vivo. The present study extends the work of others by documenting that IL-1 and TNF-α potently inhibit meniscus repair. We also showed with fluorescence microscopy that cell viability was not decreased in cytokine-treated samples, suggesting that inhibition of repair results from changes in cell function rather than cell death. Recent studies have shown increases in metalloproteinases in the meniscus in response to IL-158. Increased enzymatic activity of the meniscal cells, coupled with suppression of biosynthesis of structural components such as collagen might contribute to the cytokine-mediated decreases in the repair processes.
In summary, we describe a novel model for the assessment of the effects of various factors on meniscal repair in vitro. The primary advantages of this approach are the ability to examine specific mechanisms involved in promoting or suppressing repair processes, and to perform quantitative measures of repair strength. Using this model, we observed that IL-1 and TNF-α potently inhibit meniscal repair, suggesting that treatment with anti-cytokine agents (e.g., antibodies, soluble receptors, or receptor antagonists) might provide a means of accelerating repair of damaged or injured menisci in vivo. The findings of this study should be interpreted in light of other factors within the synovial environment that may affect meniscal physiology and repair, such as biomechanical stress9,17,19,21,22,59, growth factors13,15, or other joint pathologies24,37.
Acknowledgments
We thank Robert Nielsen and Steve Johnson for excellent technical assistance, and Drs. Amy McNulty and Beverley Fermor for insights on this work. This study was supported by the Rehabilitation Research and Development Service of the Department of Veterans Affairs, NIH grants AR48182, AR50245, and AG15768. Dr. Alfred Hennerbichler was the recipient of an Erwin-Schroedinger-Fellowship of the Austrian Science Fund (project no. J2294) at Duke University Medical Center.
Source of support: This study was supported by the Rehabilitation Research and Development Service of the Department of Veterans Affairs, NIH grants AR48182, AR50245, and AG15768. Dr. Alfred Hennerbichler was the recipient of an Erwin-Schroedinger-Fellowship of the Austrian Science Fund (project no. J2294) at Duke University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wojtys EM, Chan DB. Meniscus structure and function. Instr Course Lect. 2005;54:323–330. [PubMed] [Google Scholar]
- 2.Levy IM, Torzilli PA, Fisch ID. The contribution of the menisci to the stability of the knee. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee Meniscus Basic and Clinical Foundations. New York: Raven Press; 1992. pp. 107–115. [Google Scholar]
- 3.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--Part I: Tibial surface of the knee. J Biomech Eng. 1983;105:216–225. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 4.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 5.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990:8–18. [PubMed] [Google Scholar]
- 6.Ghadially FN, Thomas I, Yong N, Lalonde JM. Ultrastructure of rabbit semilunar cartilages. Journal of Anatomy. 1978;125:499–517. [PMC free article] [PubMed] [Google Scholar]
- 7.McDevitt CA, Miller RR, Spindler KP. The cells and cell matrix interactions of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee Meniscus Basic and Clinical Foundations. New York: Raven Press; 1992. pp. 29–36. [Google Scholar]
- 8.Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, et al. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. Journal of Anatomy. 2001;198:525–535. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 10.Upton ML, Chen J, Setton LA. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562–1570. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 12.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11:1141–1148. doi: 10.1089/ten.2005.11.1141. [DOI] [PubMed] [Google Scholar]
- 14.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. JOrthop Res. 1998;16:104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 15.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB) J Orthop Res. 1995;13:201–207. doi: 10.1002/jor.1100130208. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290:C1610–1615. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deschner J, Wypasek E, Ferretti M, Rath B, Anghelina M, Agarwal S. Regulation of RANKL by biomechanical loading in fibrochondrocytes of meniscus. J Biomech. 2006;39:1796–1803. doi: 10.1016/j.jbiomech.2005.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton ML, Hennerbichler A, Fermor B, Guilak F, Weinberg JB, Setton LA. Biaxial strain effects on cells from the inner and outer regions of the meniscus. Connect Tissue Res. 2006;47:207–214. doi: 10.1080/03008200600846663. [DOI] [PubMed] [Google Scholar]
- 19.Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis Cartilage. 2004;12:956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage. 2001;9:481–487. doi: 10.1053/joca.2001.0415. [DOI] [PubMed] [Google Scholar]
- 22.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 23.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883–888. [PubMed] [Google Scholar]
- 24.Casscells SW. The torn or degenerated meniscus and its relationship to degeneration of the weight-bearing areas of the femur and tibia. Clin Orthop Relat Res. 1978:196–200. [PubMed] [Google Scholar]
- 25.DeHaven KE. Meniscectomy versus repair: Clinical experience. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee Meniscus Basic and Clinical Foundations. New York: Raven Press; 1992. pp. 131–139. [Google Scholar]
- 26.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30–B:664–670. [PubMed]
- 27.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Wyland DJ, Guilak F, Elliott DM, Setton LA, Vail TP. Chondropathy after meniscal tear or partial meniscectomy in a canine model. J Orthop Res. 2002;20:996–1002. doi: 10.1016/S0736-0266(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 29.Asahina S, Muneta T, Yamamoto H. Arthroscopic meniscal repair in conjunction with anterior cruciate ligament reconstruction: factors affecting the healing rate. Arthroscopy. 1996;12:541–545. doi: 10.1016/s0749-8063(96)90191-7. [DOI] [PubMed] [Google Scholar]
- 30.Miller DB., Jr Arthroscopic meniscus repair. Am J Sports Med. 1988;16:315–320. doi: 10.1177/036354658801600401. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg TD, Scott SM, Coward DB, Dunbar WH, Ewing JW, Johnson CL, et al. Arthroscopic meniscal repair evaluated with repeat arthroscopy. Arthroscopy. 1986;2:14–20. doi: 10.1016/s0749-8063(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 32.Barber FA. Meniscus repair: results of an arthroscopic technique. Arthroscopy. 1987;3:25–30. doi: 10.1016/s0749-8063(87)80006-3. [DOI] [PubMed] [Google Scholar]
- 33.Cannon WD, Jr, Vittori JM. The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am J Sports Med. 1992;20:176–181. doi: 10.1177/036354659202000214. [DOI] [PubMed] [Google Scholar]
- 34.Hanks GA, Gause TM, Sebastianelli WJ, O'Donnell CS, Kalenak A. Repair of peripheral meniscal tears: open versus arthroscopic technique. Arthroscopy. 1991;7:72–77. doi: 10.1016/0749-8063(91)90082-9. [DOI] [PubMed] [Google Scholar]
- 35.Rubman MH, Noyes FR, Barber-Westin SD. Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med. 1998;26:87–95. doi: 10.1177/03635465980260013301. [DOI] [PubMed] [Google Scholar]
- 36.van Trommel MF, Simonian PT, Potter HG, Wickiewicz TL. Arthroscopic meniscal repair with fibrin clot of complete radial tears of the lateral meniscus in the avascular zone. Arthroscopy. 1998;14:360–365. doi: 10.1016/s0749-8063(98)70002-7. [DOI] [PubMed] [Google Scholar]
- 37.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21:1366–1369. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis Rheum. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Maini RN, Elliott M, Brennan FM, Williams RO, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. APMIS. 1997;105:257–263. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 40.Van den Berg WB, Joosten LA, Van de Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17:S105–S114. [PubMed] [Google Scholar]
- 41.Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17:724–731. doi: 10.1053/jars.2001.23583. [DOI] [PubMed] [Google Scholar]
- 42.Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998;10:263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Reindel ES, Ayroso AM, Chen AC, Chun DM, Schinagl RM, Sah RL. Integrative repair of articular cartilage in vitro: adhesive strength of the interface region. J Orthop Res. 1995;13:751–760. doi: 10.1002/jor.1100130515. [DOI] [PubMed] [Google Scholar]
- 44.Ahsan T, Lottman LM, Harwood F, Amiel D, Sah RL. Integrative cartilage repair: inhibition by beta-aminopropionitrile. J Orthop Res. 1999;17:850–857. doi: 10.1002/jor.1100170610. [DOI] [PubMed] [Google Scholar]
- 45.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19:1105–1112. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 46.Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091–1099. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 47.Ochi M, Mochizuki Y, Deie M, Ikuta Y. Augmented meniscal healing with free synovial autografts: an organ culture model. Arch Orthop Trauma Surg. 1996;115:123–126. doi: 10.1007/BF00434537. [DOI] [PubMed] [Google Scholar]
- 48.Webber RJ, York JL, Vanderschilden JL, Hough AJ., Jr An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M. Regional differences in the healing potential of the meniscus-an organ culture model to eliminate the influence of microvasculature and the synovium. Knee. 2004;11:271–278. doi: 10.1016/j.knee.2002.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Kambic HE, Futani H, McDevitt CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–561. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee S, McDevitt CA. The superficial zone cells initiate a wound healing process in canine meniscus in vitro. Trans Orthop Res Soc; 49th Annual Meeting of the Orthopaedic Research Society, 2.-5. 2; 2003; New Orleans, LA. 2003. p. 49. [Google Scholar]
- 52.Hennerbichler A, Moutos F, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. American Journal of Sports Medicine. 2006 doi: 10.1177/0363546506296416. in press. [DOI] [PubMed] [Google Scholar]
- 53.Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 54.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004:S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 55.Van den Berg WB, van de LF, Joosten LA, Arntz OJ. Animal models of arthritis in NOS2-deficient mice. Osteoarthritis Cartilage. 1999;7:413–415. doi: 10.1053/joca.1999.0228. [DOI] [PubMed] [Google Scholar]
- 56.Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J Biomech. 2005;38:1846–1854. doi: 10.1016/j.jbiomech.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Tognana E, Chen F, Padera RF, Leddy HA, Christensen SE, Guilak F, et al. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthritis Cartilage. 2005;13:129–138. doi: 10.1016/j.joca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Wilson C, Zuo F, Sandy J, Levenston M. Inhibition of MMPs, but no of ADAMTS-4 and -5, reduces IL-1 stimulated fibrocartilage degradation. Trans Orthop Res Soc. 2006;31:31. [Google Scholar]
- 59.Setton LA, Guilak F, Hsu EW, Vail TP. Biomechanical factors in tissue engineered meniscal repair. Clin Orthop Relat Res. 1999:S254–272. doi: 10.1097/00003086-199910001-00025. [DOI] [PubMed] [Google Scholar]