Abstract
Progress in preventing atherosclerotic coronary artery disease (CAD) has been stalled by the epidemic of type 2 diabetes. Further advances in this area demand a thorough understanding of how two major features of type 2 diabetes, insulin resistance and hyperglycemia, impact atherosclerosis. Insulin resistance is associated with systemic CAD risk factors, but increasing evidence suggests that defective insulin signaling in atherosclerotic lesional cells also plays an important role. The role of hyperglycemia in CAD associated with type 2 diabetes is less clear. Understanding the mechanisms whereby type 2 diabetes exacerbates CAD offers hope for new therapeutic strategies to prevent and treat atherosclerotic vascular disease.
Introduction
Atherothrombotic cardiovascular disease is the leading cause of death world-wide despite significant progress in the management of critical risk factors (Callow, 2006). A major reason for this trend is the ongoing epidemic of obesity-induced insulin resistance and type 2 diabetes (Behn and Ur, 2006). An important goal in preventive medicine, therefore, is to reverse this trend. On the one hand, public health measures that address overnutrition and lack of physical exercise are key. However, achieving success in life-style changes has been extremely challenging, and so complementary approaches that identify potential therapeutic targets relevant to atherosclerosis per se in diabetics are needed. This approach requires a thorough understanding of how insulin resistance and type 2 diabetes promote atherosclerosis. There are fundamental gaps in this area. Most notably, we need to more fully understand the relative importance of (a) insulin resistance vs. hyperglycemia and the concept that “insulin resistance” can mean either defective insulin receptor signaling or, ironically, over-stimulation of insulin receptor pathways caused by hyperinsulinemia (Brown and Goldstein, 2008); and (b) systemic risk factors induced by these syndromes vs. direct processes acting at the level of the arterial wall.
Another important issue is related to the point that the pathophysiological processes involved in the initiation and progression of early lesions are quite different from those that cause the formation of clinically dangerous plaques (Lusis, 2000; Tabas, 2010a), and distinguishing the effects of insulin resistance and hyperglycemia on these processes is critically important. Early-to-mid-stage atherogenesis involves the subendothelial retention of apolipoprotein B (apoB)-containing lipoproteins; activation of endothelial cells; recruitment of monocytes and other inflammatory cells; cholesterol loading of lesional cells; and migration of smooth muscle cells to the intima. In contrast, advanced plaque progression is influenced primarily by processes that promote plaque necrosis and thinning of a collagenous “scar” overlying the lesion called the fibrous cap. The focused objective here is to review current knowledge on how insulin resistance and hyperglycemia may promote atherogenesis and advanced plaque progression by affecting the biology of atherosclerotic lesional cells, emphasizing studies that are mechanistically sound and include evidence of causation in vivo. It should be noted that insulin resistance and hyperglycemia are likely to have additive or synergistic pro-atherogenic effects in the setting of type 2 diabetes. For example, glucotoxicity may contribute to insulin resistance, and treatment of hyperglycemia in type 2 diabetes improves insulin resistance in some tissues (Henry, 1996). For ease of presentation, however, the effects on insulin resistance and hyperglycemia on atherosclerosis are discussed in separate sections in this review.
Insulin Resistance and Atherosclerosis (Figures 1–2)
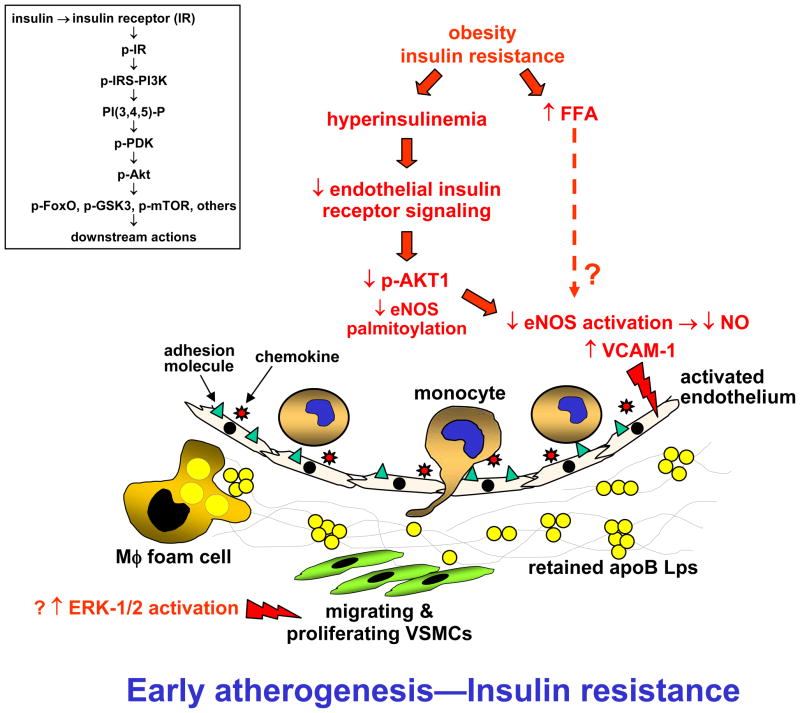
Figure 1. Possible mechanisms through which insulin resistance in ECs, SMCs, and macrophages promotes atherogenesis.
In early-mid-stage atherosclerotic lesions, insulin resistance is associated with a decrease in eNOS activation and NO production and increase in VCAM-1 expression by arterial ECs. Both of these perturbations may be due to down-regulation of the insulin receptor-Akt1 pathway in ECs. The net effect is endothelial dysfunction and activation, leading to defective vasodilation and increased entry of inflammatory cells into the plaque. Inset, summary scheme of canonical insulin receptor signaling pathway; see text for details.
Figure 2. Possible mechanisms through which insulin resistance in ECs, SMCs, and macrophages promotes advanced plaque progression.
In advanced plaques, insulin resistance may promote apoptosis of all three major cell types. Death of SMCs can lead to fibrous cap thinning, while death of macrophages, coupled with defective phagocytic clearance of the cells (efferocytosis), promotes plaque necrosis. Both fibrous cap thinning and plaque necrosis can precipitate plaque rupture and acute thrombotic vascular occlusion. Not shown in this scheme is the possibility that elevated saturated fatty acids associated with obesity and insulin resistance causes defective efferocytosis of apoptotic macrophages.
There is ample clinical evidence that insulin resistance increases the risk for coronary artery disease (CAD) even in the absence of hyperglycemia (DeFronzo, 2010). Insulin resistance syndromes can promote both atherogenesis and advanced plaque progression, and the mechanisms likely involve both systemic factors that promote these processes, particularly dyslipidemia but also hypertension and a pro-inflammatory state, as well as the effect of perturbed insulin signaling at the level of the intimal cells that participate in atherosclerosis, including endothelial cells, vascular smooth muscle cells, and macrophages. All three cell types have insulin receptors and insulin receptor-mediated signaling pathways that are down-regulated markedly, though not completely, in the setting of hyperinsulinemia (Rask-Madsen et al., 2010). To review, insulin receptor (IR) activation by insulin leads to the recruitment of IR substrate-1 and -2 (IRS) and phosphoinositide 3 (PI3)-kinase to the cytoplasmic tail of the IR (Kido et al., 2001) (Figure 1, inset). PI3-kinase forms phosphatidylinositol 3,4,5 trisphosphate [PtdIns(3,4,5)P3] in the plasma membrane, which in turn recruits 3-phosphoinositide-dependent protein kinase 1 (PDK1). PDK1 activates various isoforms of protein kinase B (PKB)/Akt, which mediate many actions of the IR, including phosphorylation/nuclear exclusion of FoxO proteins, which are transcription factors for many metabolic genes; phosphorylation/inactivation of glycogen synthase kinase-3 (GSK3), which promotes glycogen synthesis and protein translation; and activation of mTOR, which promotes protein translation. These and other IR pathways also activate mitogen-activated protein kinases, hepatic lipid synthesis pathways, and glucose transporter translocation to the cell surface. As alluded to above, “insulin resistance” can refer to down-regulated IR signaling or hyperinsulinemia-mediated excessive IR signaling. Most of the experimental work in atherosclerosis has focused on the suppression of IR signaling and has used proof-of-concept models in which IRs, or their adaptors, in these cell types have been genetically eliminated.
Endothelial Cells
In a non-atherosclerosis model in which the endothelial cell (EC) IR was targeted using the Cre-loxP method, ECs and aorta showed reduced levels of endothelial nitric oxide synthetase (eNOS) and endothelin-1 mRNA (Vicent et al., 2003). To address atherosclerosis, the mice were crossed onto the Apoe−/− background (“EIRAKO”) and maintained on a chow diet for 24 or 52 weeks. Several measures of atherosclerosis were increased in the EIRAKO vs. control mice despite no difference in plasma lipids, glucose, insulin, or blood pressure (Rask-Madsen et al., 2010).
Mechanistic studies focused on two key atherogenic properties of ECs, suppression of eNOS activity and adhesion of leukocytes. In wild-type ECs and aorta, insulin was able to induce phosphorylation of eNOS on Ser1177, which is a measure of the enzyme’s activation state, and suppress VCAM-1, which is a key endothelial-leukocyte adhesion molecule in atherogenesis. Both of these effects of insulin, which are predicted to be anti-atherogenic, were decreased in EIRAKO aorta and in lung and aortic ECs isolated from the mice. As predicted from these data, EIRAKO aorta had decreased acetylcholine-induced vasodilation, but no resistance to direct application of NO, and VCAM-1-dependent leukocyte rolling and adhesion were increased in the KO mice. Thus, normal IR signaling in vascular endothelium appears to induce a number of processes that are athero-protective. If so, down-regulation of endothelial IRs may explain one mechanism of atherogenesis in the setting of insulin resistance. However, whether endothelial insulin resistance affects the progression to necrotic plaques, which are the clinically relevant lesions in humans, remains to be explored.
A critical issue in insulin resistance is the identity of the branch(es) of IR pathways that are down-regulated in the setting of hyperinsulinemia. In one study, insulin-induced PI3K and Akt phosphorylation was suppressed in the aorta and microvessels of obese rats, but the ERK-1/2 pathway was not suppressed (Jiang et al., 1999). However, another study investigating endothelial dysfunction and decreased in eNOS Ser1177 phosphorylation in obese, insulin-resistant mice impugned high levels of free fatty acids, not defective IR-Akt1 signaling (Symons et al., 2009). Moreover, another study showed that insulin can activate eNOS in ECs through induction of fatty acid synthase, which promotes eNOS palmitoylation and translocation to the plasma membrane (Wei et al., 2011). These complexities highlight the importance of a study that investigated the effect of genetically targeting Akt1 in Apoe−/− mice fed the high-fat Western-type diet for 14 weeks, which is a model of atherosclerosis and insulin resistance (Fernandez-Hernando et al., 2007). Akt1−/−Apoe−/− mice showed an increase in aortic atherosclerosis compared with Apoe−/− mice; very large coronary arterial lesions, which are rarely seen in non-aged mouse models of atherosclerosis; and increased lesional inflammatory cytokines and decreased p-S1176-eNOS. ECs isolated from the double KO mice showed decreased proliferation and viability, which may compromise vascular repair in response to injury, and bone marrow transplant experiments showed that the effect of Akt1 deficiency on lesion area was due to non-bone marrow-derived cells, not myeloid cells. Thus, in fat-fed Apoe−/− mice, down-regulation of endothelial Akt1 phosphorylation pathway plays a role in the pro-atherogenic effects of insulin resistance. However, the differences in the diet, timing, and endpoints between the EIRAKO and Akt1−/−Apoe−/− studies, and the fact that the latter study was not endothelial-specific, makes it difficult to draw more precise conclusions.
Vascular Smooth Muscle Cells
Vascular smooth muscle cells (VSMCs) express heterodimers of IRs and insulin-like growth factor-1 receptors (IGF1Rs), and in vitro data suggest that the effects of insulin in VSMCs are mediated mostly through IGF1R despite the fact that insulin has a higher affinity for IRs than for IGF1R (Johansson and Arnqvist, 2006). Thus, there is much uncertainty as to the role of VSMCs in mediating the atherogenic effects of insulin resistance. One hypothesis is that hyperinsulinemia, by selectively down-regulating IRs, promotes the formation of “pro-atherogenic” IGF1R homodimers. As a proof-of-concept model, IR-deficient VSMCs were incubated with insulin, and this led to decreased activation of Akt, increased activation of ERK-1/2, and increased proliferation and migration, presumably through IGF1R signaling (Lightell, Jr. et al., 2011). Conversely, IGF1R silencing by siRNA in cultured VSMCs to “force” signaling through IRs enhanced insulin-induced Akt activation (Engberding et al., 2009). These data raise the possibility that an imbalance of IGF1R over IR signaling in insulin resistant states may favor pathways that promote atherosclerosis. The importance of Akt down-regulation was suggested by a study showing that Akt1−/− VSMCs had decreased proliferation and migration, as well as increased susceptibility to apoptosis (Fernandez-Hernando et al., 2009). Increased VSMC proliferation and migration would be expected to promote early/mid stage atherosclerosis by converting a fatty streak to a more irreversible VSMC-rich plaque. Ironically, in advanced plaques, intimal SMCs may lessen the risk of plaque rupture through fibrous cap collagen synthesis, but this beneficial effect may be offset in the setting of insulin resistance by enhanced SMC apoptosis, perhaps via disruption of Akt cell-survival signaling. Intact IR signaling was also shown to suppress TNF-α-induced NF-κB activation in VSMCs with silenced IGF1R (Engberding et al., 2009), suggesting another mechanism whereby loss of intact IR signaling in the setting of hyperinsulinemia may be atherogenic.
Other data, however, question the idea that an increase in IGF1R vs. IR signaling in VSMCs promotes atherosclerosis in the setting of insulin resistance. First, gene expression studies have shown that downstream signaling from IGF1R and IR are very similar (Boucher et al., 2010). Second, a number studies have suggested that IGF1R signaling protects VSMCs from apoptosis (Allard et al., 2008), and when IGF1 was overexpressed in VSMCs in Apoe−/− mice, lesion size was not altered and plaque stability was actually increased, not decreased (Shai et al., 2010). Moreover, there is a possibility that obesity and insulin resistance may be associated with a decrease in signaling originating from both receptors. For example, obesity is associated with higher levels of angiotensin II (Olivares-Reyes et al., 2009), and angiotensin II promotes the degradation of the common IR/IGF1R adaptor IRS-1 in VSMCs (Taniyama et al., 2005). Furthermore, other potential atherogenic effects in VSMCs associated with insulin resistance have not yet been linked to disturbances in IR or IGF1R signaling. As an example, VSMCs from pre-diabetic obese rats demonstrate increased NADPH oxidase-induced oxidative stress through a pathway involving transforming growth factor-β (Tong et al., 2010). These complexities and uncertainties highlight the critical need for studies that address whether insulin resistance alters the biology of lesional SMCs in vivo and, if so, whether these alterations affect atherogenesis and/or advanced plaque progression.
Macrophages
Monocyte-derived macrophages play critical roles in all stages of atherosclerosis (Moore and Tabas, 2011). In early lesions, monocytes are recruited to the intima by activated endothelium overlying areas of apoB-lipoprotein retention and then, after differentiation to macrophages in the intima, ingest these retained lipoproteins to become cholesterol-loaded foam cells. Intimal macrophages participate in a number of pro-atherogenic processes, including inflammation, secretion of proteases and pro-coagulant/thrombotic factors, and formation of the necrotic core of clinically dangerous lesions (below).
Macrophage IRs are markedly down-regulated in the settings of obesity and hyperinsulinemia, and there is evidence that defective IR signaling promotes atherosclerosis (Tabas et al., 2010). In one study, the Cre-loxP strategy was used to target IRs in lysozyme M-expressing myeloid cells (Baumgartl et al., 2006), which includes not only macrophages but also neutrophils, and, to a lesser degree, monocytes. Importantly, the IR-floxed mice were on a mixed genetic background, which can have profound effects on atherosclerosis, and the diet included a high concentration of cholesterol and cholate, which promotes inflammation (Vergnes et al., 2003). When placed on the Apoe−/− background and fed that diet for 4 months, the myeloid IR deficient mice displayed a ~50% reduction in en face aortic lesion area compared with Apoe−/− mice despite no effect on plasma, lipoproteins, glucose, or insulin. To substantiate these findings using a different strategy, the investigators conducted an Apoe−/− bone marrow transplant experiment using donor marrow from control Apoe−/− mice vs. Apoe−/− mice lacking the macrophage IR adaptor IRS-2. Both aortic root en face and cross-sectional area were reduced ~25–30% in the Irs2−/−Apoe−/− mice. Thus, in the setting of a mixed genetic background and a “pro-inflammatory” diet, macrophage IR signaling seems to modestly promote atherogenesis. With regard to inflammation, in vitro studies showed that the IR-deficient macrophages had blunted IL-6 and IL-1β responses to LPS. The molecular mechanism of this anti-inflammatory effect, and whether it can explain the lesional results in vivo, remains to be determined, although the mechanism is likely related to the finding that nuclear FoxO1, a hallmark of insulin resistance, suppresses NF-κB signaling (Senokuchi et al., 2008). As a final note, the IRS-2 bone marrow transplant experiment was accompanied by a holo-Irs2−/− experiment to assess the effect of systemic hyperinsulinemia on atherogenesis. In this case, the lesions were slightly larger in the KO group, suggesting that the pro-atherogenic effects of systemic insulin resistance trumped the putative anti-atherogenic effects of defective macrophage IR signaling.
Another study used a different model, namely, transplantation of IR KO bone marrow into C57BL6 Ldlr−/− mice fed the Western diet, which has a lower cholesterol content without cholate (Han et al., 2006). Most importantly, the primary objective of this study was to address an entirely different question, namely, the effect of myeloid IR deficiency on advanced lesional macrophage apoptosis and plaque necrosis. Recall that most atherosclerotic lesions in humans do not cause acute coronary artery disease, because they undergo outward remodeling of the arterial wall, which preserves lumen patency, and do not undergo plaque rupture or erosion and thus do not trigger acute lumenal thrombosis (Virmani et al., 2002). The small percentage of lesions that do cause acute vascular disease are distinguished by the presence of large areas of necrosis and thin fibrous caps, which promote plaque disruption, acute lumenal thrombosis, and tissue infarction (Tabas, 2011). This concept is particularly important for the topic of this review, because advanced atherosclerotic lesions in diabetic subjects are characterized by large necrotic cores when compared with similarly sized lesions from non-diabetic individuals (Tabas et al., 2010).
The mechanistic basis of the study by Han et al. was a series of in vitro investigations exploring how defective IR signaling in macrophages might promote the type of apoptotic processes that are thought to occur in advanced lesions (Han et al., 2006). By way of background, mechanistic and in vivo data in mice and humans support a role for prolonged endoplasmic reticulum (ER) stress in advanced lesional macrophage apoptosis and plaque necrosis, primarily through the action of the ER stress effector C/EBP-homologous protein (CHOP) (Tabas, 2010b). Macrophages from obese, insulin-resistant mice or mice lacking IRs demonstrate enhanced ER-stress-induced apoptosis, which is mediated through at least three mechanisms: (a) up-regulation of scavenger receptors (Liang et al., 2004), which are activated in atherosclerosis and signal to enhance ER stress-induced macrophage apoptosis (Tabas, 2010b); (b) suppression of Akt and NF-κB cell-survival pathways, the latter of which is mediated by nuclear FoxO1 (Senokuchi et al., 2008); and (c) down-regulation of the ER calcium pump SERCA, which promotes the accumulation of cytoplasmic calcium (Liang et al., 2011). This latter mechanism is likely relevant to the recent finding that CHOP promotes macrophage apoptosis by stimulating the release of calcium from the ER, which subsequently activates an apoptosis execution program coordinated by calcium/calmodulin-dependent protein kinase II (CaMKII) (Tabas and Ron, 2011).
With this background, Western diet-fed Insr−/− → Ldlr−/− chimeric mice showed a significant increase in advanced lesional macrophage apoptosis and plaque necrosis comported with WT → Ldlr−/− mice (Han et al., 2006). Further mechanistic studies will be needed to link the aforementioned mechanisms to this in vivo result, although a subsequent study showed that the lesions of Akt1−/−Apoe−/− mice had more apoptotic macrophages than those of Apoe−/− (Fernandez-Hernando et al., 2007). Finally, in contrast to the result in the cholate-diet study described in the previous section, cross-sectional aortic root lesion area was similar between Western diet-fed Apoe−/− mice with normal or absent myeloid insulin receptors (Han et al., 2006). This apparent inconsistency may reflect differences in genetic background and/or diet. For example, it is possible that the NF-κB-suppressive effect of macrophage insulin resistance (Senokuchi et al., 2008) may play a dominant, athero-protective role when mice are placed on the pro-inflammatory high cholesterol/cholate diet.
Another characteristic of insulin-resistant states is elevated levels of free fatty acids (Boden and Shulman, 2002). It is generally believed that saturated fatty acids (SFAs) are the most detrimental, and SFAs can trigger ER stress-induced apoptosis in macrophages, perhaps by decreasing the fluidity of the ER membrane (Borradaile et al., 2006). Macrophage ER stress and apoptosis induced by SFAs in vitro and in aortic root lesions of fat-fed Apoe−/− mice appear to require an intracellular “lipid chaperone” called macrophage fatty acid-binding protein-4, also known as aP2 (Erbay et al., 2009). The requirement for aP2 may be related to its ability to prevent stearoyl-CoA desaturase-mediated conversion of SFAs to unsaturated fatty acids, which are much less lipotoxic than SFAs. SFAs can also amplify the apoptotic response in macrophages exposed to other ER stressors in vitro and in vivo through a CD36-mediated signaling mechanism that promotes oxidative stress (Seimon et al., 2010). Finally, macrophages from obese mice, including those in advanced atherosclerotic lesions of ob/obLdlr−/− mice, have a decreased ability to ingest apoptotic cells (efferocytosis) (Li et al., 2009). Defective efferocytosis leads to secondary cellular necrosis and inflammation and is thought to be a critical pathological process leading to plaque necrosis (Tabas, 2010a). The efferocytosis defect can be mimicked by SFA, which may hinder phagocytosis by decreasing the fluidity of the plasma membrane (Li et al., 2009). Although this finding will require further investigation, the combined pro-apoptotic effect of macrophage insulin resistance and the anti-efferocytic effect of SFAs may create a “perfect storm” for plaque necrosis.
Hyperglycemia and Atherosclerosis (Figure 3)
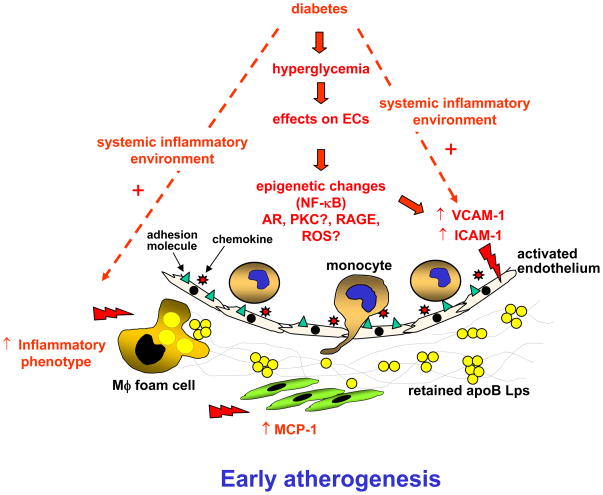
Figure 3. Possible mechanisms through which hyperglycemia in ECs, VSMCs, and macrophages promotes atherogenesis.
Hyperglycemia may accelerate formation of early/mid stage lesions of atherosclerosis by promoting adhesion molecule expression in ECs through epigenetic changes, increased flux through the AR pathway, and maybe through activation of PKC, RAGE, and increased reactive oxygen species (ROS) levels. Increased adhesion molecule expression leads to increased monocyte/macrophage accumulation and atherogenesis. In VSMCs, a principal effect of increased glucose uptake appears to be increased secretion of the chemokine MCP-1, which could act in concert with the EC changes to bring more monocytes into the growing lesion. Hyperglycemia also promotes an inflammatory phenotype in macrophages, which most likely further contributes to early atherogenesis. The effects of hyperglycemia on both ECs and macrophages are most pronounced in the presence of an inflammatory environment.
Data from human and animal studies supporting a direct pro-atherogenic role of hyperglycemia in vascular cells are not as strong as those for insulin resistance, but there is suggestive evidence that high glucose is atherogenic, particularly at the level of the arterial endothelium. In this section, we will summarize the evidence that hyperglycemia can promote atherosclerosis and discuss selected mechanisms that are supported by mechanistic and in vivo causation studies.
Evidence from Human Studies and Animal Models
Several clinical studies demonstrate a correlation between suboptimal glycemic control and cardiovascular events and suggest a CAD benefit of glucose lowering in patients with type 2 diabetes (Brown et al., 2010; Mazzone, 2010). The most compelling evidence comes from long-term follow-up studies in which intensive glucose lowering was initiated soon after diabetes diagnosis or before the onset of cardiovascular events (Brown et al., 2010). For example, the DCCT-EDIC study demonstrated an impressive 57% reduction in the risk of nonfatal MI, stroke, or death from cardiovascular disease in the intensive glucose-lowering group of subjects with type 1 diabetes compared with the conventionally treated group (Nathan et al., 2005). Similar beneficial effects of blood glucose lowering have been reported in newly diagnosed subjects with type 2 diabetes (Holman et al., 2008). At the level of the vascular wall, human postmortem studies show that lesions from patients with diabetes have a higher macrophage content than lesions from subjects without diabetes in a manner that correlates with glycated hemoglobin levels rather than lipid levels (Burke et al., 2004).
However, the relationship between hyperglycemia and CAD is still unclear (Brown et al., 2010; Mazzone, 2010). For example, a recent large study demonstrated that whereas intensive blood glucose control for 3.7 years in patients with advanced type 2 diabetes and a pre-existing high risk of cardiovascular disease reduced 5-year nonfatal MI, it increased 5-year mortality, as compared with patients receiving standard therapy (Gerstein et al., 2011). The increased mortality in the intensive therapy group might have been due to the larger number of glucose-lowering drugs used to achieve glycated hemoglobin levels of <6% or to other unidentified factors.
There are a number of issues that might explain, at least in part, the confusion in this area. First, type 2 diabetes is associated with several cardiovascular risk factors as discussed above, and hyperglycemia may provide a relatively minor contribution to overall CAD risk. Second, elevated glycated hemoglobin A1c (HbA1c), used in clinical studies as a measure of glycemic control, may not always accurately reflect the biological effect of hyperglycemia because transient spikes in glucose do not result in overall changes in HbA1c and/or because HbA1c levels can be influenced by genetic components unrelated to glucose (Soranzo et al., 2010). Third, CAD often occurs before frank type 2 diabetes has developed in subjects with insulin resistance. Fourth, CAD develops over decades, whereas clinical intervention studies to lower blood glucose are usually conducted over a much shorter time span, with the exception of the positive studies cited above.
The use of animal models to study the effects of diabetes on atherosclerosis is often complicated by the co-existence of hyperlipidemia, which overrides the effects of diabetes on atherosclerosis (Reaven et al., 1997; Renard et al., 2004; Kanter et al., 2007). In some models, however, diabetes increases blood glucose levels without associated increases in plasma lipids, and in these models a pro-atherogenic effect of diabetes can be observed (Kunjathoor et al., 1996; Gerrity et al., 2001; Renard et al., 2004; Vikramadithyan et al., 2005). The most commonly used models are ones in which Ldlr−/− or Apoe−/− mice are injected with streptozotocin, a toxin that primarily targets beta-cells. Another model relies on transgenic expression of the lymphocytic choriomeningitis virus (LCMV) glycoprotein gene under control of the rat insulin promoter in Ldlr−/− mice (Renard et al., 2004), where diabetes can be induced at will by a single injection of LCMV, which results in T cell-mediated beta-cell destruction. Studies using either model have shown that diabetes accelerates formation of early, macrophage-rich atherosclerotic lesions at susceptible sites in the arterial wall (Renard et al., 2004; Vikramadithyan et al., 2005) and that this effect can be prevented by insulin treatment (Renard et al., 2004; Schuyler et al., 2011; Johansson et al., 2008). In the LCMV model, diabetes-induced increases in plasma LDL and VLDL were found to be necessary for progression to lesions that exhibit intraplaque hemorrhage and a rupture-prone phenotype (Johansson et al., 2008). These combined data suggest that hyperglycemia in the setting of a non-diabetes-mediated hypercholesterolemic background is sufficient to promote early lesion formation, but that accelerated progression of advanced plaques in diabetic mice, beyond that normally observed in non-diabetic mice, requires diabetes-induced elevation of atherogenic lipoproteins.
Endothelial Cells
While there are many in vitro studies that have examined the direct effect of high glucose on ECs, there is paucity of in vivo studies. However, there is circumstantial evidence from in vivo studies both in animals and humans that the endothelium is particularly sensitive to changes in glucose concentrations. Investigators have evaluated the effects of acute (≤ 12 h) glucose administration on leukocyte adhesion to microvascular ECs in rodents using intravital microscopy and found evidence of increased leukocyte rolling and adhesion to microvessels in the absence (Booth et al., 2002) or presence of co-administered IL-1β or TNF-α (Azcutia et al., 2010a). The effects of elevated glucose in these studies were most likely mediated by increased expression of the adhesion molecules P-selectin, VCAM-1, and ICAM-1, through pathways mediated by PKC and increased oxidative stress and/or activation of the NF-κB pathway (Booth et al., 2002; Azcutia et al., 2010b). Thus, at least acutely, hyperglycemia promotes leukocyte adhesion to endothelial cells, an initial step in atherogenesis, likely through direct effects of glucose on ECs. Consistently, transient hyperglycemia induces long-lasting epigenetic changes in the promoter of the NF-κB subunit p65 in ECs in vitro and in vivo, resulting in increased p65 and VCAM-1 gene expression (El-Osta et al., 2008). Such epigenetic changes in the NF-κB pathway appear to be mediated by increased oxidative stress (Giacco and Brownlee, 2010), and could explain the synergistic effects of hyperglycemia and cytokines, but whether these processes in ECs promote atherosclerosis is not yet known.
Elevated glucose can increase flux through the aldose reductase (AR)/polyol pathway, in which intracellular glucose is converted to sorbitol by AR and then further to fructose and downstream metabolites (Vikramadithyan et al., 2005). Transgenic expression of AR in streptozotocin-diabetic Ldlr−/− mice selectively increased the effect of diabetes on atherosclerosis, whereas overexpression of AR in non-diabetic atherosclerotic mice had no effect (Vikramadithyan et al., 2005). Recent data suggest that the pro-atherosclerotic effect of AR overexpression is due, at least in part, to endothelial changes (Vedantham et al., 2011). Surprisingly, systemic inhibition of the low endogenous levels of murine AR selectively increased atherosclerosis in both non-diabetic and hyperlipidemic diabetic Apoe−/− mice (Srivastava et al., 2009). These data suggest that the effect of AR on atherosclerosis may depend critically on the expression level of AR, the absence or presence of hyperglycemia, and/or effects of specific cell types, but further mechanistic work is needed to clarify these issues. Clinical trials of AR inhibitors have so far yielded mostly unimpressive results on microvascular complications of diabetes, but large human trials using new classes of AR inhibitors with fewer off-target effects will be needed to assess the role of AR in the development of cardiovascular disease in humans with diabetes.
Hyperglycemia has also been proposed to exert vascular effects through de novo synthesis of diacylglycerol and subsequent activation of protein kinase C (PKC) in ECs (Geraldes and King, 2010). Although more work is needed in this area, PKCβ deficiency in Apoe−/− mice leads to diminished atherosclerosis, and this protective effect was attributed to the absence of PKCβ in ECs (Harja et al., 2009). Clinical trials of a PKCβ inhibitor (ruboxistaurin) have so far produced promising or mixed results on microvascular complications of diabetes (Geraldes and King, 2010).
In addition to direct effects of glucose, hyperglycemia-induced advanced glycation end products (AGEs) have been proposed as pro-atherogenic mediators in diabetes. AGEs are formed by the nonenzymatic reaction of glucose and other glycating compounds with proteins and lipids and can occur both extracellularly and intracellularly (Giacco and Brownlee, 2010). AGEs are produced as a result of diabetes, aging, oxidative stress, or hypoxia, or they can be provided by the diet, and so they are not specific to diabetes (Yan et al., 2010). Modification of both intracellular and extracellular molecules by AGEs can result in altered function of these molecules. For example, AGE-modified proteins and lipoproteins can bind to and activate receptors, such as the receptor for AGEs (RAGE). RAGE is expressed in ECs, where it promotes VCAM-1 expression (Harja et al., 2008). Indeed, blocking of RAGE function results in protection against atherosclerosis in hyperlipidemic diabetic mice, but it also has anti-atherogenic effects in non-diabetic mice (Park et al., 1998; Soro-Paavonen et al., 2008; Harja et al., 2008). The deleterious effects of RAGE on early atherosclerosis have been ascribed to changes in ECs. However, a recent study demonstrates that lack of RAGE in bone marrow-derived cells, presumably monocyte-derived macrophages, results in reduced necrotic core formation in advanced lesions (Morris-Rosenfeld et al., 2011). Interestingly, diabetes and RAGE activation regulate mostly different sets of genes in the aorta of Apoe−/− mice before the onset of frank atherosclerosis (Bu et al., 2010). These findings are most likely explained by the ability of RAGE to bind several different ligands, such as S100/calgranulins and high-mobility group box 1, which are elevated in a large number of inflammatory diseases, including diabetes (Yan et al., 2010; Soro-Paavonen et al., 2008). The relative importance of AGEs as RAGE activators in diabetes is therefore still unknown, in part due to the difficulty of specifically blocking AGE-RAGE interactions without blocking RAGE binding to its other ligands in vivo and to issues related to preparing pure and physiologically relevant AGE preparations (Valencia et al., 2004). Thus, while endothelial or possibly macrophage RAGE activation may conspire with diabetes to promote early/mid stage atherosclerosis, it is as yet uncertain whether RAGE activation is an important mediator of hyperglycemia-induced atherosclerosis per se.
Vascular Smooth Muscle Cells
VSMCs take up glucose largely through glucose transporter 1 (GLUT1; SLC2A1). Although elevated glucose concentrations result in acute downregulation of GLUT1 in cultured VSMCs (Kaiser et al., 1993), more prolonged exposure to high glucose does not cause reduced GLUT1 protein levels and hence increases glucose metabolism in VSMCs (Suzuki et al., 2001). Most of the work assessing the effect of hyperglycemia on VSMCs has focused on cell proliferation and response to injury, not atherosclerosis, although hyperglycemia may induce a pro-inflammatory phenotype in VSMCs, which could be relevant to atherosclerosis. As an example, a recent study explored the effect of overexpression of the glucose transporter 1 (GLUT1; SLC2A1) in sm22α-positive smooth muscle cells in mice, resulting in increased glucose uptake in these cells (Adhikari et al., 2011). The mice exhibited increased accumulation of neutrophils in the arterial wall after vascular injury, suggesting that increased glucose uptake enhances the pro-inflammatory phenotype of post-injury VSMCs. These mice also exhibited increased circulating levels of MCP-1, haptoglobin, and reduced glutathione (GSH) after vascular injury, the latter two hypothesized to reflect an increased glucose flux trough the pentose-phosphate pathway. Seven days after injury, neointimal VSMCs in the experimental group showed an increased proliferative index, measured by Ki67 immunoreactivity, but there was no difference in neointimal thickness at later time-points. However, as alluded to above, this was not a hyperlipidemic atherosclerosis model, and so features of atherosclerosis, such as macrophage accumulation, were absent.
Macrophages
When macrophages are exposed to high glucose concentrations in vitro, inflammation is either induced or more often enhanced in the setting of classical inflammatory stimuli such as lipopolysaccharide (LPS). These findings are consistent with studies demonstrating an increased inflammatory phenotype of macrophages from diabetic mice and human subjects (Wen et al., 2006; Bradshaw et al., 2009; Devaraj et al., 2011). A recent study examined the effect of diabetes on lesion regression (Parathath et al., 2011). Atherosclerosis was first induced in Ldlr−/− mice by feeding a Western diet, followed by control or streptozotocin treatment and then induction of rapid and marked lowering of plasma cholesterol to promote regression. Both groups showed a similar reduction in plasma cholesterol, but lesion regression was hindered in the diabetes group, as evidenced by a less effective reduction in lesional cholesterol and macrophages. Macrophages in the diabetic lesions exhibited increased oxidative stress and inflammatory gene expression and a reduced polarization toward an anti-inflammatory phenotype (Parathath et al., 2011). Whether diabetes acts mainly by retarding the egress of macrophages from plaques or by enhancing recruitment of monocytes into these regressing lesions, e.g., by promoting adhesion molecule expression on ECs, in an important area of future research.
Therapeutic Implications
The work presented in this review raises the possibility that mechanism-based therapy that targets arterial wall cells may have a special niche in the treatment and prevention of CAD in subjects with type 2 diabetes. Throughout this review, certain common pathophysiologic themes have emerged, including the importance of inflammation, ER stress, and oxidative stress, particularly in the critical process of advanced plaque progression. With regard to inflammation, liver × receptors (LXRs) dampen the inflammatory response in macrophages and other cells, and oral LXR agonists suppress atherogenesis and plaque progression in mouse models of atherosclerosis, including those that have some degree of obesity and insulin resistance (Bensinger and Tontonoz, 2008). LXR agonists also promote HDL- and apolipoprotein A–I (ApoA-I)-induced cholesterol efflux from macrophages by inducing ABCG1 and ABCA1 transporter proteins, respectively (Bensinger and Tontonoz, 2008). In this regard, a clinical study with subjects with type 2 diabetes showed that their monocytes had deceased ABCG1 and cholesterol efflux potential, both of which were correctable in vitro by treatment with an LXR agonist (Mauldin et al., 2008). Moreover, the beneficial effect of the insulin-sensitizing drug pioglitazone, a thiazolidinedione activator of the transcription factor PPAR-γ and an LXR inducer (Bensinger and Tontonoz, 2008; Ogata et al., 2009), on carotid atherosclerosis in type 2 diabetes was highly correlated with its ability to raise HDL (Davidson et al., 2008). However, systemically administered LXR agonists promote steatosis, and currently available thiazolidinediones have been associated with heart failure and possibly bladder cancer (Bensinger and Tontonoz, 2008). In addition, a statistically significant beneficial effect of PPAR-γ agonists on CAD in the PROactive study was shown only for the secondary composite endpoint of mortality, MI, and stroke, whereas benefit was not seen in the prespecified primary composite endpoint, which also included acute coronary syndrome and peripheral vascular disease (Dormandy et al., 2005).
Another approach to reduce inflammation is by blocking cytokine action. A clinical trial is currently under way to evaluate the effect of an IL-1β neutralizing antibody on cardiovascular events (Libby et al., 2011). Such a strategy might be especially beneficial in diabetes, because IL-1β neutralization might also improve pancreatic islet function. Finally, as summarized in the section on hyperglycemia and endothelial cells, pre-clinical data raise the possibility that a therapeutic strategy that blocks RAGE-induced inflammation may have cardiovascular benefit (Park et al., 1998; Soro-Paavonen et al., 2008; Harja et al., 2008).
Insulin resistance and hyperglycemia have been shown to activate pro-atherogenic ER stress pathways in macrophages and ECs (Tabas, 2010b; McAlpine et al., 2010), and hepatic ER stress likely contributes to hepatic insulin resistance and thus atherogenic diabetic dyslipidemia (Ozcan et al., 2004). When 4-phenyl butyric acid (PBA), an ER stress-relieving “chemical chaperone,” was given to Western diet-fed Apoe−/− mice, vascular ER stress and atherosclerosis were suppressed (Erbay et al., 2009). Fat-fed Apoe−/− mice have a modest level of obesity and insulin resistance, and the mechanism of protection by PBA appears to be particularly relevant to saturated fatty acid-induced ER stress. Although PBA has other effects on cells, the overall concept that ER stress-relieving therapy may be beneficial in diabetes is supported by mechanistic data and the results of causation studies using genetically targeted mice (Tabas et al., 2010).
The adverse effects of insulin resistance and possibly hyperglycemia on oxidative stress in lesional cells raises the possibility that anti-oxidant treatment may be useful in this setting. Although clinical trials using vitamin E have been largely disappointing (Williams and Fisher, 2005), vitamin E treatment was associated with a decreased incidence of myocardial infarction (MI), stroke, and cardiovascular death in a type 2 diabetes subgroup with a common loss-of-function polymorphism in the anti-oxidant protein haptoglobin (Milman et al., 2008). It is therefore possible that further elucidation of the specific oxidative mechanisms promoted by insulin resistance and hyperglycemia in specific lesional cell types will lead to useful anti-atherosclerosis therapy in diabetes. For example, the apparent atherogenic roles of NADPH oxidase in insulin-resistant VSMCS (Tong et al., 2010) and in advanced lesional macrophage death and plaque necrosis (Moore and Tabas, 2011) raise the possibility that drugs that target this oxidase may have promise. Another study found that deletion of the anti-oxidant enzyme glutathione peroxidase-1 promotes atherosclerosis in diabetic Apoe−/− mice but not in non-diabetic controls (Lewis et al., 2007), raising the possibility that therapeutic enhancement of this enzyme may have benefit in type 2 diabetes.
The above examples represent a few of the many opportunities in this area, and new ones will continue to emerge based on human genetic studies examining CAD risk in type 2 diabetes. For example, a genetic variant in the gene encoding ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1), which inhibits IR signaling, is an independent risk factor for CAD with particular potency in obese subjects with type 2 diabetes (Bacci et al., 2011). Moreover, while this review has focused on ECs, VSMCs, and macrophages, insulin resistance and hyperglycemia undoubtedly affect other cell types that affect atherosclerosis. As a prime example, thrombosis is the final arbiter of acute vascular events, and platelet function, which is abnormal in diabetic patients, provides a promising target for drug therapy (Morel et al., 2010).
Finally, two substantial barriers must be overcome in any discussion of strategies that target arterial wall cells. First, one must be able to look beyond orally delivered systemic drugs. Fortunately, new advances in lesion-targeted therapy, such as through the use of nanoparticles (Chan et al., 2010), provide promise in this area. Second, the current use of CAD endpoints to evaluate drugs that directly affect atherosclerosis is extremely expensive and time-consuming, and so progress in this arena must be linked to ongoing efforts to develop, validate, and eventually use lesional imaging and biomarkers as intermediate endpoints to identify the most promising drug candidates (Fryburg and Vassileva, 2011). In the end, however, cardiovascular endpoints with a large number of subjects followed for a sufficient period of time must be used to evaluate new therapies.
Concluding Remarks
We have reviewed how insulin resistance and hyperglycemia may promote atherosclerosis at the level of the arterial wall, with special emphasis on in vivo studies when available. These studies have provided evidence that insulin resistance in macrophages and endothelial cells may play important roles in both atherogenesis and clinically relevant advanced plaque progression. Hyperglycemia, on the other hand, appears to primarily promote early stages of lesion formation, although it is possible that hyperglycemia acts synergistically with other CAD risk factors and even insulin resistance itself in advanced lesions. Moreover, the hyperglycemia studies have to be viewed in the context of currently available clinical data, which have not yet proven definitively the impact of hyperglycemia on CAD. One possibility is that hyperglycemia-induced early atherogenesis leads to an increased probability of CAD later in life. This possibility, perhaps mediated through epigenetic changes, might help explain the finding that improved glycemic control appears most effective when implemented earlier in life or soon after diabetes diagnosis compared with implementation in patients with advanced type 2 diabetes and pre-existing cardiovascular disease or risk factors (Brown et al., 2010; Gerstein et al., 2011). The prediction that the obesity epidemic will continue to accelerate the incidence of type 2 diabetes and its deadly consequence of atherosclerotic vascular disease over the next decades emphasizes the importance of further mechanistic and translational work in this critical area of biomedical research.
Acknowledgments
This work was supported by NIH grants HL087123, HL075662, HL054591, HL062887, HL092969, and HL097365. The authors acknowledge invaluable discussions with members of their laboratories and colleagues in the field.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z, Lehman U, Mullegama S, Weir EK, Hall JL. Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2011;31:86–94. doi: 10.1161/ATVBAHA.110.215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- Azcutia V, Abu-Taha M, Romacho T, Vazquez-Bella M, Matesanz N, Luscinskas FW, Rodriguez-Manas L, Sanz MJ, Sanchez-Ferrer CF, Peiro C. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS ONE. 2010a;5:e10091. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcutia V, Abu-Taha M, Romacho T, Vazquez-Bella M, Matesanz N, Luscinskas FW, Rodriguez-Manas L, Sanz MJ, Sanchez-Ferrer CF, Peiro C. Inflammation determines the pro-adhesive properties of high extracellular d-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS ONE. 2010b;5:e10091. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci S, Rizza S, Prudente S, Spoto B, Powers C, Facciorusso A, Pacilli A, Lauro D, Testa A, Zhang YY, Di SG, Mallamaci F, Tripepi G, Xu R, Mangiacotti D, Aucella F, Lauro R, Gervino EV, Hauser TH, Copetti M, De CS, Pellegrini F, Zoccali C, Federici M, Doria A, Trischitta V. The ENPP1 Q121 variant predicts major cardiovascular events in high-risk individuals: evidence for interaction with obesity in diabetic patients. Diabetes. 2011;60:1000–1007. doi: 10.2337/db10-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behn A, Ur E. The obesity epidemic and its cardiovascular consequences. Curr Opin Cardiol. 2006;21:353–360. doi: 10.1097/01.hco.0000231406.84554.96. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. 14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Booth G, Stalker TJ, Lefer AM, Scalia R. Mechanisms of amelioration of glucose-induced endothelial dysfunction following inhibition of protein kinase C in vivo. Diabetes. 2002;51:1556–1564. doi: 10.2337/diabetes.51.5.1556. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Boucher J, Tseng YH, Kahn CR. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J Biol Chem. 2010;285:17235–17245. doi: 10.1074/jbc.M110.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Bu DX, Rai V, Shen X, Rosario R, Lu Y, D’Agati V, Yan SF, Friedman RA, Nuglozeh E, Schmidt AM. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- Callow AD. Cardiovascular disease 2005--the global picture. Vascul Pharmacol. 2006;45:302–307. doi: 10.1016/j.vph.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee JW, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci U S A. 2010;107:2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Meyer PM, Haffner S, Feinstein S, D’Agostino R, Sr, Kondos GT, Perez A, Chen Z, Mazzone T. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117:2123–2130. doi: 10.1161/CIRCULATIONAHA.107.746610. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009 Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011 doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberding N, San MA, Martin-Garrido A, Koga M, Pounkova L, Lyons E, Lassegue B, Griendling KK. Insulin-like growth factor-1 receptor expression masks the antiinflammatory and glucose uptake capacity of insulin in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009;29:408–415. doi: 10.1161/ATVBAHA.108.181727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Jozsef L, Jenkins D, Di LA, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:2033–2040. doi: 10.1161/ATVBAHA.109.196394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryburg DA, Vassileva MT. Atherosclerosis drug development in jeopardy: the need for predictive biomarkers of treatment response. Sci Transl Med. 2011;3:72cm6. doi: 10.1126/scitranslmed.3002029. [DOI] [PubMed] [Google Scholar]
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50:1654–1665. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metabolism. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCβ and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RR. Glucose control and insulin resistance in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:97–103. [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson GS, Arnqvist HJ. Insulin and IGF-I action on insulin receptors, IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2006;291:E1124–E1130. doi: 10.1152/ajpendo.00565.2005. [DOI] [PubMed] [Google Scholar]
- Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- Kanter JE, Johansson F, LeBoeuf RC, Bornfeldt KE. Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res. 2007;100:769–781. doi: 10.1161/01.RES.0000259589.34348.74. [DOI] [PubMed] [Google Scholar]
- Kido Y, Nakae J, Accili D. Clinical review 125: The insulin receptor and its cellular targets. J Clin Endocrinol Metab. 2001;86:972–979. doi: 10.1210/jcem.86.3.7306. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- Li S, Sun Y, Thorp E, Liang CP, Han S, Jehle A, Viswanathan S, Kanter J, Li R, Welch CL, Hasty AH, Bornfeldt KE, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and its reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CP, Han S, Li G, Senokuchi T, Tabas I, Tall AR. Impaired MEK signaling and SERCA expression promotes ER stress and apoptosis in insulin resistant macrophages and is reversed by exenatide treatment. 2011 doi: 10.2337/db11-1415. Ref Type: Unpublished Work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;19(473):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Lightell DJ, Jr, Moss SC, Woods TC. Loss of canonical insulin signaling accelerates vascular smooth muscle cell proliferation and migration through changes in p27Kip1 regulation. Endocrinology. 2011;152:651–658. doi: 10.1210/en.2010-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin JP, Nagelin MH, Wojcik AJ, Srinivasan S, Skaflen MD, Ayers CR, McNamara CA, Hedrick CC. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation. 2008;117:2785–2792. doi: 10.1161/CIRCULATIONAHA.107.741314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation. 2010;122:2201–2211. doi: 10.1161/CIRCULATIONAHA.109.913350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine CS, Bowes AJ, Werstuck GH. Diabetes, hyperglycemia and accelerated atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc Hematol Disord Drug Targets. 2010;10:151–157. doi: 10.2174/187152910791292529. [DOI] [PubMed] [Google Scholar]
- Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel O, Kessler L, Ohlmann P, Bareiss P. Diabetes and the platelet: toward new therapeutic paradigms for diabetic atherothrombosis. Atherosclerosis. 2010;212:367–376. doi: 10.1016/j.atherosclerosis.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Morris-Rosenfeld S, Blessing E, Preusch MR, Albrecht C, Bierhaus A, Andrassy M, Nawroth PP, Rosenfeld ME, Katus HA, Bea F. Deletion of bone marrow-derived receptor for advanced glycation end products inhibits atherosclerotic plaque progression. Eur J Clin Invest. 2011:10–2362. doi: 10.1111/j.1365-2362.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Tsujita M, Hossain MA, Akita N, Gonzalez FJ, Staels B, Suzuki S, Fukutomi T, Kimura G, Yokoyama S. On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis. 2009;205:413–419. doi: 10.1016/j.atherosclerosis.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol. 2009;302:128–139. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven P, Merat S, Casanada F, Sutphin M, Palinski W. Effect of streptozotocin-induced hyperglycemia on lipid profiles, formation of advanced glycation endproducts in lesions, and extent of atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:2250–2256. doi: 10.1161/01.atv.17.10.2250. [DOI] [PubMed] [Google Scholar]
- Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler CA, Ta NN, Li Y, Lopes-Virella MF, Huang Y. Insulin Treatment Attenuates Diabetes-Increased Atherosclerotic Intimal Lesions and Matrix Metalloproteinase-9 Expression in Apolipoprotein E-Deficient Mice. J Endocrinol. 2011 doi: 10.1530/JOE-10-0420. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Liao X, Magallon J, Moore KJ, Witztum JL, Tsimikas S, Golenbock DT, Webb NR, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metabolism. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, Depinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–2976. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol. 2010;30:1916–1924. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, Bouatia-Naji N, Langenberg C, Prokopenko I, Stolerman E, Sandhu MS, Heeney MM, Devaney JM, Reilly MP, Ricketts SL, Stewart AF, Voight BF, Willenborg C, Wright B, Altshuler D, Arking D, Balkau B, Barnes D, Boerwinkle E, Bohm B, Bonnefond A, Bonnycastle LL, Boomsma DI, Bornstein SR, Bottcher Y, Bumpstead S, Burnett-Miller MS, Campbell H, Cao A, Chambers J, Clark R, Collins FS, Coresh J, de Geus EJ, Dei M, Deloukas P, Doring A, Egan JM, Elosua R, Ferrucci L, Forouhi N, Fox CS, Franklin C, Franzosi MG, Gallina S, Goel A, Graessler J, Grallert H, Greinacher A, Hadley D, Hall A, Hamsten A, Hayward C, Heath S, Herder C, Homuth G, Hottenga JJ, Hunter-Merrill R, Illig T, Jackson AU, Jula A, Kleber M, Knouff CW, Kong A, Kooner J, Kottgen A, Kovacs P, Krohn K, Kuhnel B, Kuusisto J, Laakso M, Lathrop M, Lecoeur C, Li M, Li M, Loos RJ, Luan J, Lyssenko V, Magi R, Magnusson PK, Malarstig A, Mangino M, Martinez-Larrad MT, Marz W, McArdle WL, McPherson R, Meisinger C, Meitinger T, Melander O, Mohlke KL, Mooser VE, Morken MA, Narisu N, Nathan DM, Nauck M, O’Donnell C, Oexle K, Olla N, Pankow JS, Payne F, Peden JF, Pedersen NL, Peltonen L, Perola M, Polasek O, Porcu E, Rader DJ, Rathmann W, Ripatti S, Rocheleau G, Roden M, Rudan I, Salomaa V, Saxena R, Schlessinger D, Schunkert H, Schwarz P, Seedorf U, Selvin E, Serrano-Rios M, Shrader P, Silveira A, Siscovick D, Song K, Spector TD, Stefansson K, Steinthorsdottir V, Strachan DP, Strawbridge R, Stumvoll M, Surakka I, Swift AJ, Tanaka T, Teumer A, Thorleifsson G, Thorsteinsdottir U, Tonjes A, Usala G, Vitart V, Volzke H, Wallaschofski H, Waterworth DM, Watkins H, Wichmann HE, Wild SH, Willemsen G, Williams GH, Wilson JF, Winkelmann J, Wright AF, Zabena C, Zhao JH, Epstein SE, Erdmann J, Hakonarson HH, Kathiresan S, Khaw KT, Roberts R, Samani NJ, Fleming MD, Sladek R, Abecasis G, Boehnke M, Froguel P, Groop L, McCarthy MI, Kao WH, Florez JC, Uda M, Wareham NJ, Barroso I, Meigs JB. Common variants at 10 genomic loci influence hemoglobin A(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: lack of direct growth-promoting effects of high glucose levels. Diabetes. 2001;50:851–860. doi: 10.2337/diabetes.50.4.851. [DOI] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Rev Immunol. 2010a;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010b;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Pulling down the plug on atherosclerosis: Finding the culprit in your heart. Nat Med. 2011;17:791–793. doi: 10.1038/nm0711-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Molecular mechanisms integrating pathways of endoplasmic reticulum stress-induced apoptosis. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Tall AR, Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ Res. 2010;106:58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- Tong X, Hou X, Jourd’heuil D, Weisbrod RM, Cohen RA. Upregulation of Nox4 by TGFβ1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ Res. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE. Binding of receptor for advanced glycation end products (RAGE) ligands is not sufficient to induce inflammatory signals: lack of activity of endotoxin-free albumin-derived advanced glycation end products. Diabetologia. 2004;47:844–852. doi: 10.1007/s00125-004-1392-9. [DOI] [PubMed] [Google Scholar]
- Vedantham S, Noh H, Ananthakrishnan R, Son N, Hallam K, Hu Y, Yu S, Shen X, Rosario R, Lu Y, Ravindranath T, Drosatos K, Huggins LA, Schmidt AM, Goldberg IJ, Ramasamy R. Human aldose deductase expression accelerates atherosclerosis in diabetic apolipoprotein E−/− mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011 Jun 2; doi: 10.1161/ATVBAHA.111.226902. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Wei X, Schneider JG, Shenouda SM, Lee A, Towler DA, Chakravarthy MV, Vita JA, Semenkovich CF. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J Biol Chem. 2011;286:2933–2945. doi: 10.1074/jbc.M110.193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147:2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Fisher EA. Oxidation, lipoproteins, and atherosclerosis: which is wrong, the antioxidants or the theory? Curr Opin Clin Nutr Metab Care. 2005;8:139–146. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;19(106):842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]