Introduction
The endocytosis of immunity-related receptors has emerged as a critical control step in the signal transduction process. The first receptors activated during any host-pathogen interaction are members of the Toll-like Receptor (TLR) family, which are responsible for detecting microbial products and inducing innate and adaptive immunity (Akira and Takeda, 2004). Different TLR family members are found in different subcellular compartments, ranging from the plasma membrane to early, late and recycling endosomes (Barton and Kagan, 2009). While it was believed originally that the endocytosis of plasma membrane-localized TLRs downregulates their signaling functions after a microbial encounter (Husebye et al., 2006; Latz et al., 2003), new evidence indicates that receptor delivery to endosomes also activates specific signal transduction pathways (Kagan et al., 2008; Tanimura et al., 2008).
Virtually all of our knowledge of TLR transport is limited to regulators that promote the folding or transport of newly synthesized receptors (e.g. GP96, PRAT4a, UNC93B1) (Kim et al., 2008; Takahashi et al., 2007; Yang et al., 2007). In the absence of each of these regulators, specific sets of TLRs cannot exit the endoplasmic reticulum after translation and, consequently, TLR ligands present in the extracellular and endosomal spaces are not detected. Unlike the emerging knowledge on the trafficking of newly synthesized receptors, almost nothing is known about the regulators that control TLR endocytosis or transport after microbial detection. Filling this gap in our knowledge is of fundamental importance, as microbe-induced receptor transport is a critical control step in the TLR-mediated signal transduction.
The first-described example of microbe-induced TLR transport came from studies of the LPS receptor TLR4, which induces distinct signaling pathways from two different organelles (Kagan et al., 2008; Tanimura et al., 2008). The first signaling pathway is activated from the plasma membrane after TLR4 encounters LPS (Latz et al., 2003). This pathway is mediated by a pair of sorting and signaling adaptor proteins called TIRAP and MyD88, respectively (Kagan and Medzhitov, 2006). These adaptors induce pro-inflammatory cytokine expression by linking TLR4 to downstream enzymes that activate NF-κB and AP-1 (Akira and Takeda, 2004). TLR4 is then internalized into the endosomal network where the second signaling pathway is triggered through the adaptors TRAM and TRIF (Kagan et al., 2008; Tanimura et al., 2008). These adaptors mediate the activation of the transcription factor Interferon Regulatory Factor-3 (IRF3), which regulates Type I Interferon (IFN) expression (Akira and Takeda, 2004). Thus, in the case of TLR4, the LPS-induced endocytosis of the receptor is essential for its signaling functions. While the general endocytic machinery is undoubtedly involved in internalization of plasma membrane-localized TLRs, there are no known membrane proteins that regulate TLR endocytosis specifically upon microbial recognition.
In considering this problem, we reasoned that since TRIF-mediated IFN expression requires TLR4 endocytosis, cell surface proteins that control endosomal signaling may do so by regulating TLR4 entry into the cell. One such regulator is CD14. CD14 is a GPI-linked protein that is found on the surface of many (but not all) TLR4 expressing cells (Wright et al., 1990). CD14 was the first identified Pattern Recognition Receptor (PRR) that binds directly to LPS (Wright et al., 1990), and is known to chaperone LPS molecules to the TLR4-MD-2 signaling complex (da Silva Correia et al., 2001; Gioannini et al., 2004; Moore et al., 2000). Notably, while CD14 is marginally important for MyD88-dependent TNFα expression, it is essential for TRIF-mediated IFN expression (Jiang et al., 2005). Thus, we hypothesized that CD14 specifically regulates TRIF-mediated IFN expression because it regulates TLR4 endocytosis.
Results
CD14 is required for LPS-induced TLR4 endocytosis
To identify proteins that specifically regulate the LPS-induced endocytosis of TLR4, we used a highly sensitive assay to detect endogenous TLR4 by flow cytometry. Using the loss of cell surface expression as a readout for TLR4 endocytosis, we showed previously that LPS induces the TLR4 internalization in mouse bone marrow derived macrophages (BMDM) (Kagan et al., 2008). The loss of TLR4 surface staining was a bona fide endocytic event as it was inhibited by dynasore (Kagan et al., 2008), an inhibitor of dynamin GTPases that control most endocytic processes in mammalian cells. Using this assay, we determined if CD14 regulates TLR4 transport as a means of controlling IFN production.
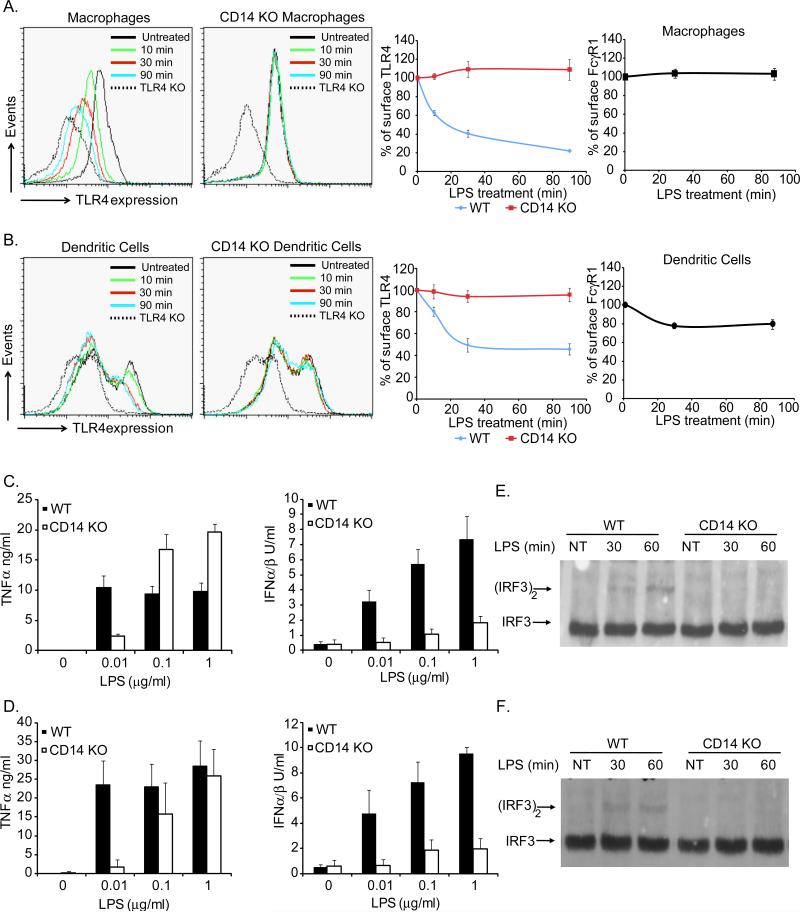
LPS-induced TLR4 endocytosis was examined in BMDM and immature dendritic cells (DCs) from wild type (WT) and CD14-deficient mice. LPS induced the rapid endocytosis of TLR4 in WT BMDM and DCs, but not in cells from CD14-deficient mice (Figure 1A, B). The endocytosis of TLR4 was a specific response, as levels of a different endocytic receptor (FcγR1) were largely unaffected by LPS treatment (Figure 1A, B). To complement this FACS-based assay, TRIF-mediated signaling events from endosomes were examined (Kagan et al., 2008). CD14-deficient BMDM and DCs were defective for TRIF-mediated IFN production but were not defective for MyD88-mediated TNFα production (Figure 1C, D). Of note, at low LPS concentrations, CD14 was needed for TNFα production, which likely reflects its role in delivering LPS to TLR4 (da Silva Correia et al., 2001; Gioannini et al., 2004).
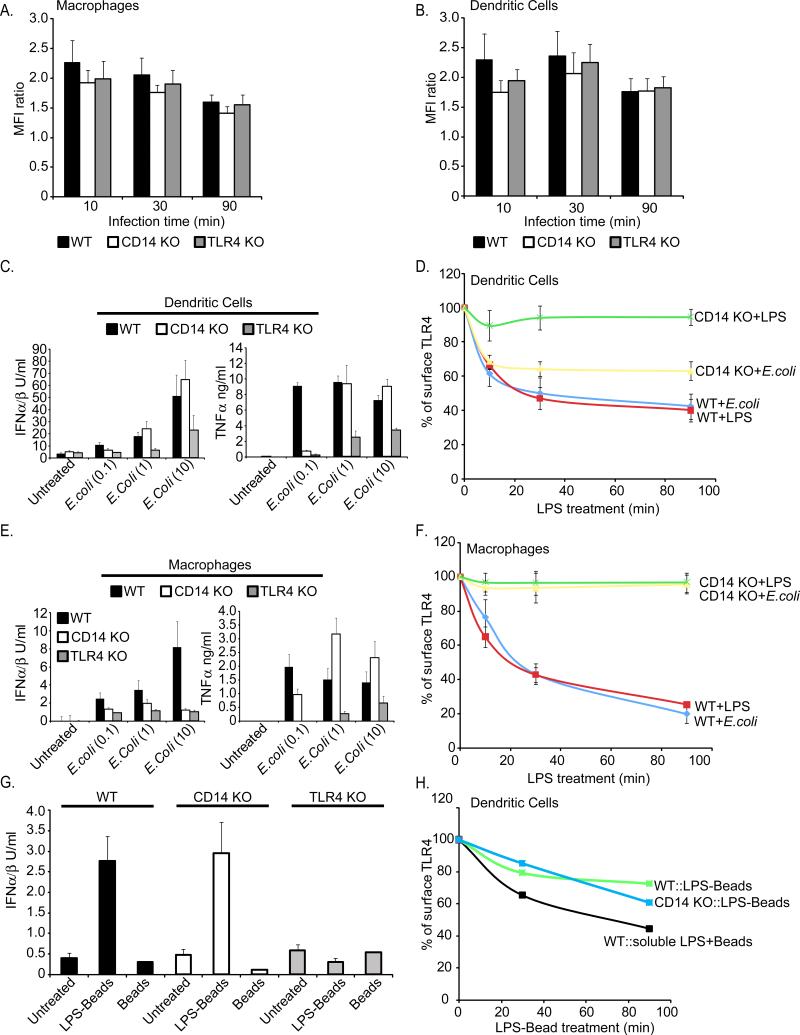
Figure 1. CD14 is required for LPS-induced TLR4 endocytosis.
WT or CD14-deficient (KO) mouse BMDM(A) or DCs (B) were untreated or treated with LPS (1μg/ml) for the times indicated. Flow cytometry was then used to examine receptor endocytosis by determining the surface levels of the endogenous proteins indicated. The third and forth panels in A and B represent the mean fluorescence intensity (MFI) of specific receptor staining at each time point. C, D; BMDM (C) or DCs (D) were treated with the concentrations of LPS indicated for 18 hours and the amounts of secreted cytokines were determined. E, F; BMDM (E) or DCs (F) were treated with LPS (1μg/ml) for the times indicated and the presence of active (dimerized) IRF3 in cell extracts was determined by native PAGE. See also Figure S1.
To more specifically address the role of CD14 in signaling from the plasma membrane or endosomes, we examined protein complexes that define each pathway. TLR4 signaling from the plasma membrane induces the formation of the Myddosome, a complex containing MyD88 and IRAK4 that activates NF-κB (Motshwene et al., 2009). TLR4 signaling from endosomes induces the dimerization of the transcription factor IRF3 (Kagan et al., 2008). We monitored the formation of the Myddosome by co-immunoprecipitations of MyD88 and IRAK4 in WT and CD14-deficient immature DCs. LPS treated WT and CD14-deficient DCs induced Myddosome formation (Figure S1A), suggesting that TLR4 signaling from the plasma membrane does not absolutely require CD14. In addition, CD14-deficient cells retained the ability to activate the MAP kinases p38 and ERK1/2 and induce IκBα degradation in response to LPS treatment (Figure S1B, C). In contrast to the signaling events occurring at the plasma membrane, LPS-induced dimerization of IRF3 was not detected in either CD14–deficient BMDM or DCs (Figure 1E, F). Collectively, using both direct assays of TLR4 endocytosis and assays of signaling events that occur only after endocytosis, these data establish that CD14 is required for LPS-induced endocytosis of TLR4.
In addition to TLR4, TLR2 signaling in inflammatory monocytes and BMDM can induce IFN expression (Barbalat et al., 2009; Dietrich et al., 2010). Under these conditions, TLR2 endocytosis is necessary for IFN expression. To determine the specificity of the role of CD14 for TLR4-mediated IFN expression, we examined the expression of cytokines and IFN-regulated genes in WT and CD14-deficient cells stimulated with TLR2 ligands. Neither inflammatory monocytes nor BMDM from CD14-deficient mice displayed any defects in their responses to their diverse TLR2 ligands (Figure S1D, E), indicating that the role of CD14 in promoting IFN expression is specific to TLR4.
Cell type-specific coexpression of TLR4 and CD14 confers cell type-specific responses to LPS
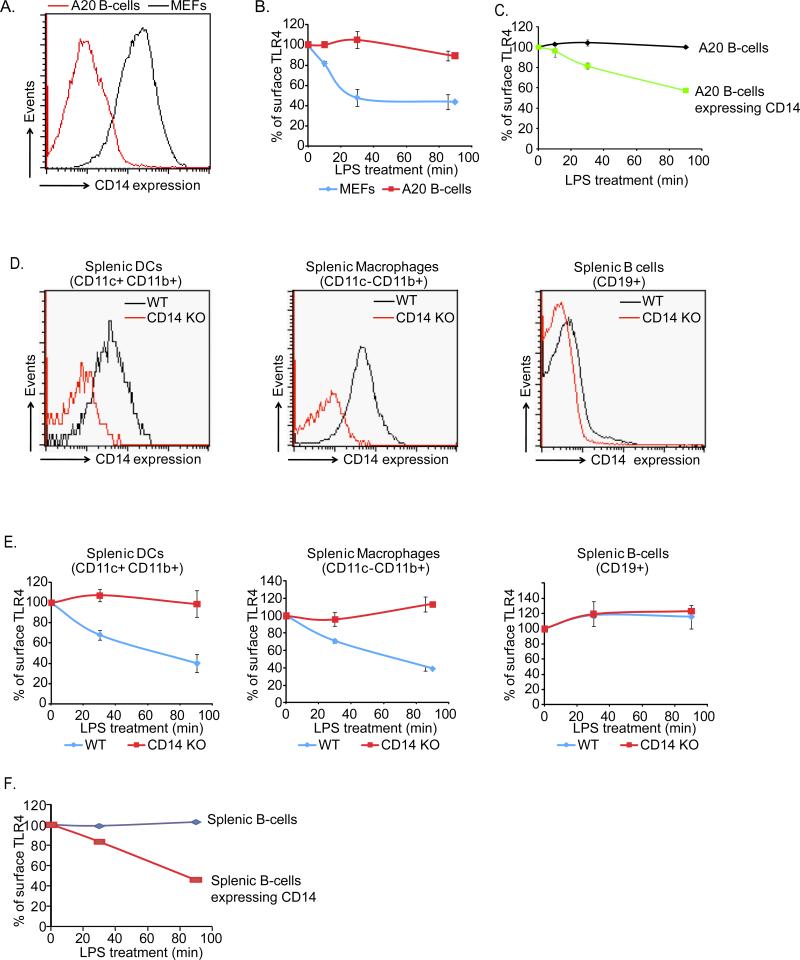
While diverse cell types are LPS responsive, not all LPS-responsive cells respond in the same manner. The molecules that dictate cell type-specific responses to LPS are unknown. We hypothesized that CD14 expression would naturally endow TLR4-bearing cells with the ability to promote TLR4 endocytosis, thus providing a mechanism of cell type-specific control over LPS-induced activities. This hypothesis was addressed by examining the expression of CD14 in cell types that are known to express TLR4 (Hoshino et al., 1999). BMDM, immature DCs and mouse embryonic fibroblasts (MEFs) expressed CD14, but the A20 B-cell line did not (Figure 2A, S2A). Analysis of TLR4-deficient cells indicated that the surface expression of CD14 did not require TLR4 (Figure S2A). Like BMDM and immature DCs, MEFs permitted TLR4 endocytosis in response to LPS (Figure 2B). In contrast, TLR4 surface levels were unchanged in A20 B-cells, although these cells responded to LPS by upregulating MHC-II and CD69 (Figure 2B and S2B). To address directly if the inability of A20 B-cells to internalize TLR4 resulted from deficient CD14 expression, we transfected these cells with a plasmid encoding CD14. A20 B-cells expressing CD14 gained the ability to internalize TLR4 (Figure 2C). These latter results are consistent with previous work showing that the B-cell line Ba/F3 displays enhanced TLR4 endocytosis when CD14 is overexpressed (Tanimura et al., 2008).
Figure 2. The natural expression profile of CD14 determines which cells undergo LPS-induced TLR4 endocytosis.
A, The cells indicated were examined for the expression of surface levels of endogenous CD14 by flow cytometry. B, MEFs and A20 B-cells were treated with LPS (1μg/ml) for the times indicated and TLR4 endocytosis was monitored by flow cytometry. Shown are the MFI of specific TLR4 surface staining at each time point indicated. C, A20 B-cells were transiently transfected with a plasmid encoding CD14 and treated with LPS (1μg/ml) for the times indicated before TLR4 endocytosis was examined by flow cytometry. D, DCs, BMDM or B-cells were isolated from the spleen of mice and examined for the expression of surface levels of CD14 by flow cytometry. E, Mice were injected with LPS (50μg) and at the times indicated spleens were isolated and BMDM, DCs and B-cells were examined for surface levels of TLR4 by flow cytometry. F, Splenic B-cells were transfected with a plasmid encoding CD14 and treated for the indicated times with LPS (1μg/ml) before TLR4 endocytosis was examined by flow cytometry. See also Figure S2.
To determine if our results apply to conditions in vivo, WT and CD14-deficient mice were injected intravenously with LPS and at various times after injection, CD11c+/CD11b+ cells (DCs) or CD11c-/CD11b+, Ly6G- cells (BMDM) or CD19+ cells (B-cells) were harvested from the spleen and examined for CD14 and TLR4 surface levels as a readout for receptor endocytosis. Prior to LPS injection, only DCs and BMDM (not B-cells) expressed CD14 (Figure 2D). Splenic BMDM and DCs internalized TLR4 in response to LPS injection (Figure 2E), and this process was dependent on CD14. In contrast, splenic B-cells were not capable of internalizing TLR4 in response to LPS injection (Figure 2E). Notably, transfection of ex vivo cultured splenic B-cells with plasmids encoding CD14 endowed these cells with the ability to internalize TLR4 (Figure 2F). Collectively, these data establish that the natural coexpression of CD14 and TLR4 determines which cell types internalize TLR4 in response to LPS.
CD14 mediates the LPS-induced internalization of extracellular macromolecules
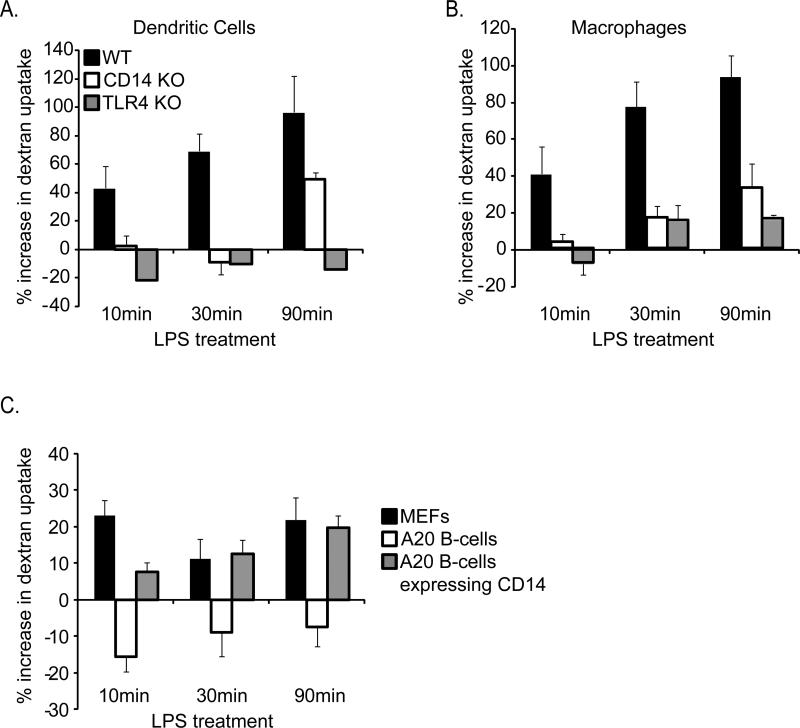
In addition to promoting TLR4 endocytosis, LPS induces DCs to undergo rapid macropinocytosis (West et al., 2004). To determine if CD14 regulates this process, we examined the capture of fluorescent dextrans by immature DCs, BMDM, MEFs and A20 B-cells by FACS. LPS induced rapid macropinocytosis of dextrans only in cells that naturally express CD14 (BMDM, DCs and MEFs, but not A20 B-cells) (Figure 3A-C). Moreover, CD14 was required for LPS-induced macropinocytosis in BMDM and DCs (Figure 3A-C). As expected from previous work implicating the TLR4-activated kinase Rsk in macropinocytosis (Zaru et al., 2007), TLR4 was required for this response (Figure 3A-C).
Figure 3. CD14 is required for LPS-induced macropinocytosis.
Macropinocytosis was measured in DCs (A) or BMDM (B) by flow cytometry. Cells were untreated or treated for the times indicated with fluorescent dextrans (1mg/ml) in the presence or absence of LPS (1μg/ml). Cell associated fluorescence intensity was then compared between LPS-treated and untreated cell populations to determine changes from the steady state levels of macropinocytosis. Note that both CD14 and TLR4 are required for LPS-induced macropinocytosis. C, MEFs or A20 B-cells were untreated or treated for the times indicated with fluorescent dextrans (1mg/ml) in the presence or absence of LPS (1μg/ml). Cell associated fluorescence intensity was then compared between LPS-treated and untreated populations of cells to determine changes from the steady state levels of macropinocytosis.
To address directly if the macropinocytosis defects of A20 B-cells results from deficient CD14 expression, we transiently transfected these cells with plasmids encoding CD14. A20 B-cells expressing CD14 gained the ability to induce macropinocytosis in response to LPS (Figure 3C). These data suggest that the CD14-dependent endocytosis pathway functions to internalize both TLR4 and extracellular macromolecules.
The CD14-dependent endocytosis pathway is upregulated upon DC maturation
Perhaps more than any other antigen presenting cell, the functions of DCs are tied to their endocytic capacity (Mellman and Steinman, 2001). Recent work has demonstrated that mature DCs retain the capacity to capture and present antigens on MHC-II (Drutman and Trombetta, 2010; Platt et al., 2010). We reasoned that if mature DCs retain the ability to capture antigens, then they should also retain the ability to respond to microbial products. We therefore determined if CD14-dependent activities are retained after DC maturation.
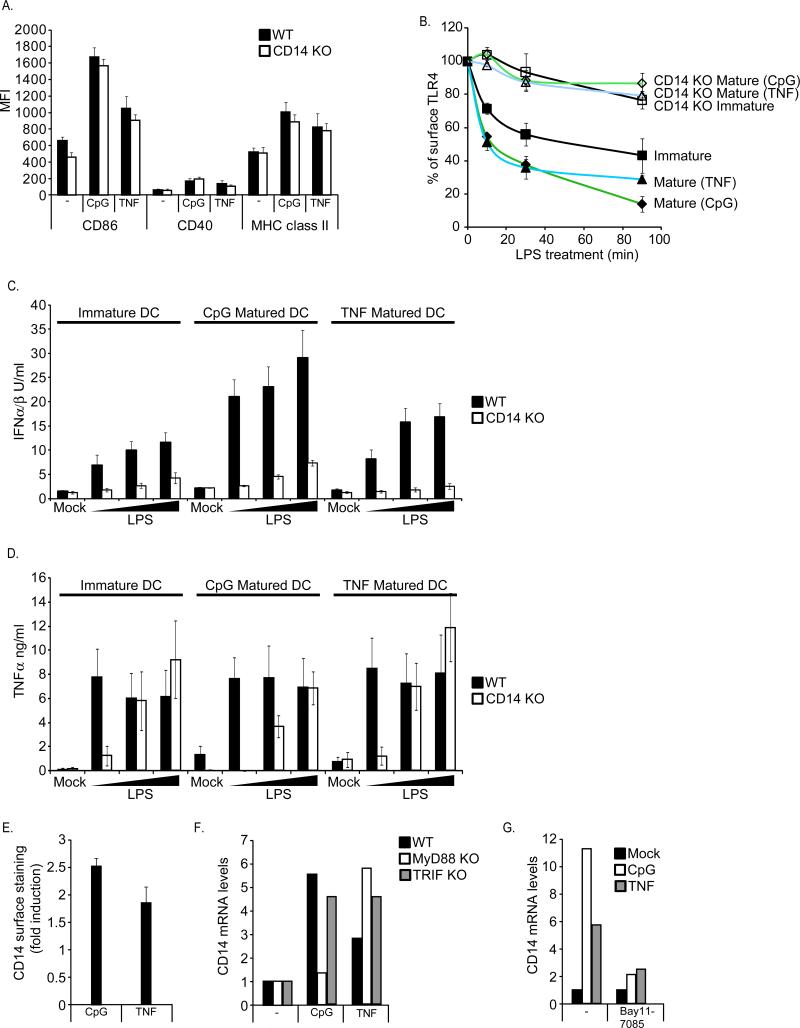
Immature DCs were treated with either TNFα or the TLR9 ligand CpG DNA. Each of these ligands induced the upregulation of costimulatory molecules and MHC-II in WT and CD14-deficient DCs, indicating that DC maturation does not require CD14 (Figure 4A). The resulting mature cells were then stimulated with LPS to examine TLR4 endocytosis and cytokine expression. Interestingly, as compared to immature DCs, LPS-induced TLR4 endocytosis was enhanced (Figure 4B). The enhancement of TLR4 endocytosis was impressive—mature DCs exhibited a faster rate of TLR4 endocytosis, and these cells internalized a larger percent of the total receptors (Figure 4B). This increase in TLR4 entry coincided with an increased production of TRIF-dependent IFNs (Figure 4C), but not MyD88-dependent TNFα (Figure 4D). A potential explanation for the maturation-induced enhancement of TLR4 endocytosis and signaling would be if the maturation stimuli triggered an increase in the expression of CD14. Consistent with this idea, we found that TNFα or CpG DNA treatment resulted in higher surface levels of CD14, compared to immature DCs (Figure 4E). This increase in CD14 surface levels correlated with an increase in CD14 gene expression (Figure 4F). The signaling pathways triggered by CpG DNA and TNFα activate NF-κB (Akira and Takeda, 2004; Muppidi et al., 2004). Thus, the NF-κB-specific inhibitor Bay11-7085 was used to examine the role of this transcription factor in CD14 gene expression. Bay11-7085 prevented CD14 upregulation by either CpG DNA or TNFα (Figure 4G), suggesting that NF-κB mediates CD14 expression. Moreover, MyD88-deficient cells, which are defective for CpG DNA-induced NF-κB expression (Hemmi et al., 2000), were incapable of upregulating CD14 upon CpG DNA treatment (Figure 4F). MyD88-deficient cells retained the ability to induce CD14 expression in response to TNFα (Figure 4F). Collectively, these data suggest that during DC maturation, the functions of the TLR4 signaling pathways are not just retained, but enhanced, and that CD14 expression is rate limiting in the control of TLR4 endocytosis and TRIF signaling.
Figure 4. The CD14-dependent endocytosis pathway is upregulated during DC maturation.
A, DCs were untreated or treated for 18 hours with CpG DNA (1μM) or TNFα (100pg/ml). DC maturation was then assessed by flow cytometry by determining the increase in surface staining of CD86, CD40 or MHC class II. B, Immature DCs or DCs matured for 18 hours with CpG DNA (1μM) or TNFα (100pg/ml) were treated with LPS (1μg/ml) and TLR4 endocytosis was measured by flow cytometry at the times indicated. Displayed are the MFIs of specific surface TLR4 staining at each time point. C, D; Immature DCs or DCs matured for 18 hours with CpG DNA (1μM) or TNFα (100pg/ml) were treated with LPS at the concentrations indicated and the production of the indicated cytokines were measured after 18 hours. E, DCs were either untreated or treated for 18 hours with CpG DNA (1μM) or TNFα (100pg/ml) and the affect of these stimuli on the cell surface levels of CD14 was assessed by flow cytometry. F, DCs of the genotypes indicated were processed as described in (E). After 18 hours, CD14 mRNA levels were determined by qPCR. G, DCs were stimulated with the ligands indicated as described in (E) in the presence of absence of the NF-κB inhibitor Bay11-7085 and then processed to measure CD14 expression by qPCR.
The primary function of CD14 in TRIF signaling is to deliver TLR4 to endosomes
We reasoned that if the primary function of CD14 in mediating TRIF signaling is to promote TLR4 endocytosis, then delivering TLR4 to endosomes independently of CD14 should restore IFN expression in CD14-deficient cells. In contrast, if CD14 must physically change the conformation of TLR4 to permit TRIF signaling (Jiang et al., 2005), then altering TLR4 localization would not restore IFN expression to CD14-deficient cells. This prediction was tested by taking advantage of the fact that some TLRs will be non-specifically internalized into phagosomes (Underhill et al., 1999). Thus, whole E.coli bacteria was used as an alternative source of LPS. BMDM and DCs derived from WT, CD14-deficient and TLR4-deficient mice all internalized E.coli to similar extents (Figure 5A, B), and the expression of MyD88-dependent TNFα was similar in WT and CD14-deficient cells (Figure 5C, E). TLR4-deficient cells produced no cytokines or IFNs in response to E.coli (Figure 5C, E). Interestingly, E.coli treatment induced TLR4 endocytosis and IFN expression in both WT and CD14-deficient DCs (Figure 5C, D). Similarly, LPS-coated latex beads induced the endocytosis of TLR4 in WT and CD14-deficient DCs (Figure 5G, H). Although the total percent of TLR4 internalized using LPS-coated beadswas low when compared to soluble LPS, these particles restored IFN expression to CD14-deficient DCs to a level comparable to WT cells (Figure 5G, H). These data indicate that in DCs, the primary function of CD14 in IFN expression is to deliver TLR4 to endosomes where TRIF-dependent signaling can occur.
Figure 5. The requirement of CD14 for TLR4 endocytosis can be bypassed with phagocytic cargo.
A, B; The cells indicated were treated for the times indicated with fluorescent bacteria at a multiplicity of infection (MOI) of 10. Phagocytosis was then measured at each time point by determining the mean fluorescence intensity (MFI) of the cell populations by flow cytometry. C, DCs were treated with E.coli at the MOIs indicated on the x-axis, and the production of the indicated cytokines were measured after 18 hours. D, DCs were treated with E.coli at an MOI of 10 or LPS (1μg/ml) and TLR4 endocytosis was measured by flow cytometry at the times indicated. Displayed are the MFIs of specific surface TLR4 staining at each time point. E, BMDM were treated with E.coli at the MOIs indicated on the x-axis, and the production of the cytokines indicated were measured. F, BMDM were treated with E.coli at an MOI of 10 or LPS (1μg/ml) and TLR4 endocytosis was measured by flow cytometry at the times indicated. Displayed are the MFIs of specific surface TLR4 staining at each time point. G, DCs were treated with either LPS-coated latex beads or uncoated beads and the production of the cytokines indicated were measured. H, DCs were treated with either LPS-coated latex beads or uncoated beads in the presence of soluble LPS and TLR4 endocytosis was measured by flow cytometry at the times indicated. Displayed are the MFIs of specific surface TLR4 staining at each time point. See also Figure S3.
The ability of phagocytic cargo to bypass the need for CD14 in promoting IFN expression was not universal, however. In contrast to DCs, phagocytic particles were unable to induce IFN expression in CD14-deficient BMDM (Figure 5E, F). To explain these cell type specific differences, we determined if TLR4 was internalized by CD14-deficient BMDM upon exposure to beads or E.coli. Unlike DCs, TLR4 surface levels remained constant in CD14-deficient BMDM treated with either E.coli or LPS-coated beads (Figure 5F and data not shown). Interestingly, the difference between BMDM and DCs in their ability to internalize TLR4 during phagocytosis was not unique to this receptor. Under similar experimental conditions, FcγR1 was also internalized by DCs, but not BMDM, even though no opsonin was used in these studies (Figure S3A). These data suggest that DCs are intrinsically more “permissive” than BMDM in terms of allowing receptors to enter the cell during phagocytosis. While the molecular basis for this distinction remains unknown, these observations provide a plausible explanation for why LPS-coated particles can rescue the transport and signaling defects of CD14-deficient DCs but not BMDM.
CD14 induces a Syk/PLCγ2-dependent endocytosis pathway that promotes the internalization of TLR4
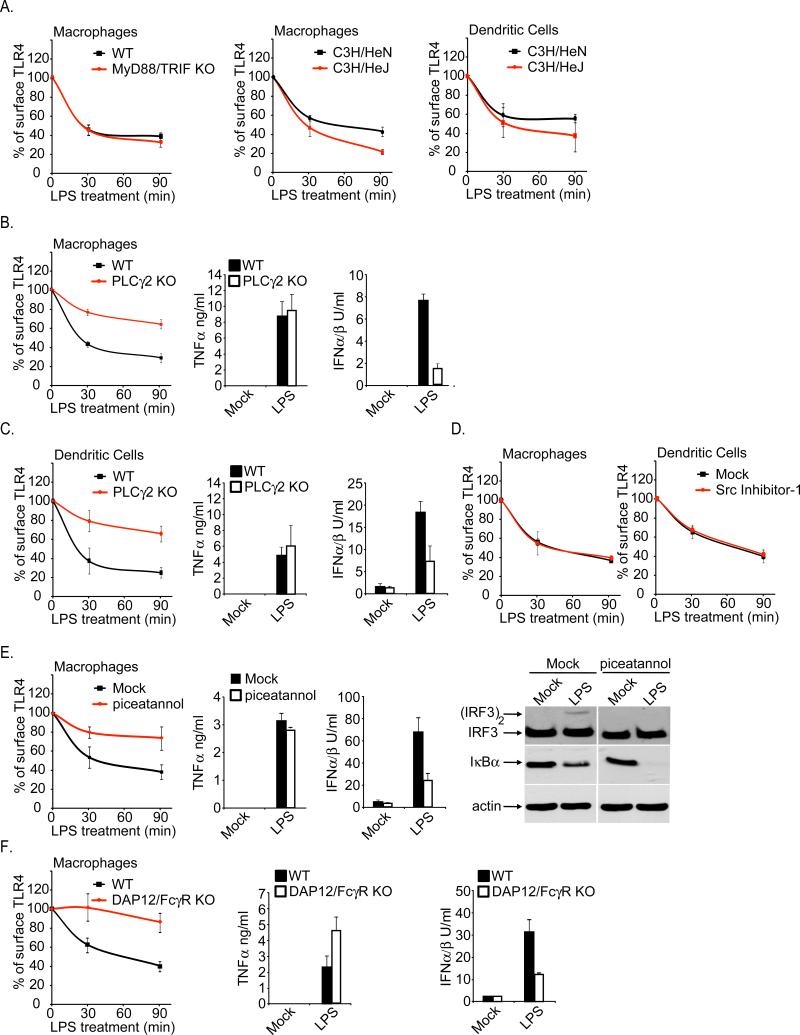
We sought to identify regulators of CD14-dependent endocytosis. To determine if TLR4 signaling was required for its own transport, TLR4 endocytosis was examined in cells lacking individual TIR domain containing adaptors (Akira and Takeda, 2004). The endocytosis of TLR4 was unaffected by the lack of MyD88, TRIF, TIRAP or TRAM in DCs or BMDM (Figure S4A and data not shown). In addition, cells lacking MyD88 and TRIF, which are defective for all TLR4-dependent activities (Hirotani et al., 2005), retained the ability to internalize TLR4 in response to LPS (Figure 6A). Similar results were obtained when examining TLR4 endocytosis in DCs or BMDM derived from the C3H/HeJ mouse (Figure 6A), which contain a point mutation in the TLR4 TIR domain that abolishes its signaling potential (Poltorak et al., 1998). Thus, neither a signaling competent TIR nor the signaling adaptor proteins are necessary for LPS-induced TLR4 endocytosis.
Figure 6. Role of Syk and PLCγ2 in regulating the CD14-dependent endocytosis of TLR4.
A, Immortal BMDM or primary and DCs of the genotypes indicated were either untreated or treated with LPS (1μg/ml) for the times indicated. Flow cytometry was then used to examine receptor endocytosis by determining the surface levels of the endogenous TLR4. Shown are the MFIs of specific TLR4 surface staining at each time point indicated. B, C, BMDM (B) or DCs (C) of the genotypes indicated were treated with 10ng/ml LPS for the times indicated and the rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. D, The cells indicated were treated with the Src inhibitor in the presence or absence of LPS (10ng/ml) and the rate of TLR4 endocytosis was monitored by flow cytometry. E, BMDM were treated with the Syk inhibitor piceatannol (75μM) and were then stimulated with 10ng/ml LPS. At the times indicated, the rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated in the presence or absence of piceatannol with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. Fourth panel, BMDM were treated with LPS (1μg/ml) for 30 min in the presence or absence of piceatannol (75μM). The presence of dimerized IRF3 and IκBα was assessed by western analysis. F, BMDM of the genotypes indicated were treated with 10ng/ml LPS for either 30 or 90 minutes. The rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. See also Figures S4.
These data suggest the existence of an LPS-inducible endocytic process that is CD14-dependent but TLR4-signaling independent. Upon LPS treatment, CD14 can act independently of TLR4 to induce the influx of Ca2+ from the extracellular environment (Zanoni et al., 2009). This process is mediated by the enzyme PLCγ2. PLCγ2 is a regulator of endocytosis (Botelho et al., 2000; Depoil et al., 2009) and is thus a candidate regulator of TLR4 endocytosis. To determine if PLCγ2 is involved in TLR4 endocytosis, we examined the transport of the receptor in cells lacking PLCγ2. When compared to WT cells, PLCγ2-deficient BMDM and DCs were defective for LPS-induced TLR4 endocytosis (Figure 6B, C), although the defect was not as profound as that observed in CD14-deficient cells. Similar results were obtained in WT cells treated with the PLC-specific inhibitor U73122 (Figure S4B, C). This defect in TLR4 transport was most impressive at early time points after LPS treatment, suggesting that PLCγ2 regulates the primary pathway of TLR4 endocytosis and may therefore be important for signaling events that occur from endosomes. Consistent with this prediction, PLCγ2-deficient cells, or WT cells treated with the PLC inhibitor, were deficient in IFN expression (Figure 6B, C and Figure S4B, C). TNFα production was unaffected by the lack of PLCγ2. Overall these results largely phenocopy those obtained with CD14-deficient cells and thus implicate PLCγ2 in CD14-dependent endocytosis.
As Src family members provide a molecular link between CD14 and PLCγ2 for LPS-induced Ca2 mobilization (Zanoni et al., 2009), we determined if TLR4 endocytosis was sensitive to chemical inhibitors of this kinase family. Src inhibitors did not affect TLR4 endocytosis (Figure 6D). We also ruled out a role for extracellular Ca2, as experiments performed in the presence of the Ca2 chelator EGTA did not prevent TLR4 endocytosis (data not shown). Thus, the CD14-dependent/TLR4-independent responses described previously (Zanoni et al., 2009) are distinct from those that lead to TLR4 endocytosis.
In addition to the Src family, the tyrosine kinase Syk is upstream of PLCγ2 (Crowley et al., 1997; Depoil et al., 2009). To determine if Syk is involved in CD14-dependent endocytosis, WT cells were treated with two distinct Syk-specific inhibitors, piceatannol and Bay61-3606. Similar to the results obtained with PLCγ2-deficient cells, treatment with either inhibitor diminished TLR4 endocytosis and IFN production, whereas TNFα expression remained intact (Figure 6E and Figure S4D, E). Moreover, in both BMDM and DCs, Syk inhibition prevented the LPS-induced activation of IRF3, but not the degradation of IκB (Figure 6E and Figure S5A). Thus, like CD14, Syk and PLCγ2 are required for LPS-induced TLR4 endocytosis and signaling from endosomes, but not MyD88-dependent signaling from the plasma membrane.
Activation of Syk-dependent pathways usually involves a membrane protein that contains an immunoreceptor tyrosine-based activation motif (ITAM) (Lowell). The ITAM containing transmembrane adaptors DAP12 and FcεRIγ are involved in TLR4 signaling (Chu et al., 2008; Hamerman et al., 2005). Since CD14 contains neither an ITAM, nor a transmembrane domain, we considered the possibility that ITAM-containing adaptors would be necessary for CD14-dependent TLR4 endocytosis. We examined the transport and signaling of TLR4 in cells lacking both DAP12 and FcεRIγ (Chu et al., 2008). Compared to WT cells, BMDM and DCs derived from mice doubly deficient in DAP12 and FcεRIγ displayed delayed TLR4 endocytosis and expressed low levels of TRIF-dependent IFN expression (Figure 6F and Figure S5B). MyD88-dependent TNFα expression was unaffected by the lack of ITAM-containing adaptors (Figure 6F and Figure S5B). These data support the idea that CD14 activates an ITAM-mediated event that triggers the Syk-PLCγ2 mediated endocytosis of TLR4.
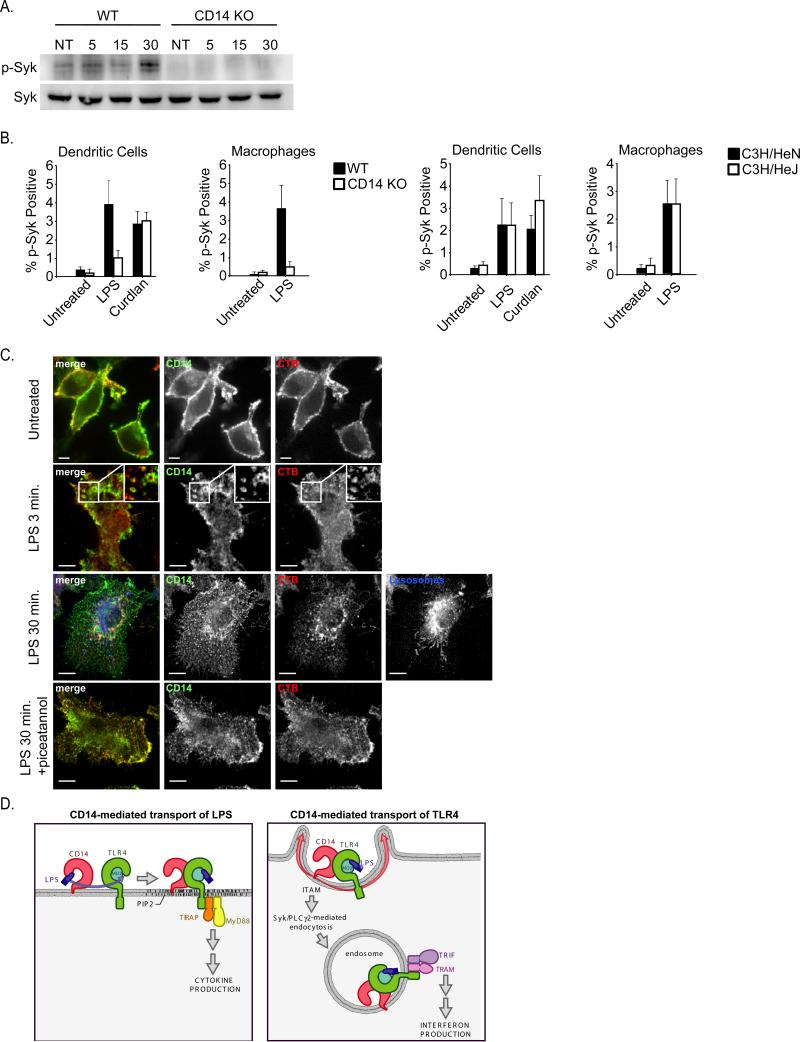
If Syk was truly involved in CD14-dependent endocytosis of TLR4, then this kinase should be activated by LPS in a manner dependent on CD14. This was first addressed using western analysis for the presence of phosphorylated Syk. BMDM stimulated with LPS induced the appearance of phospho-Syk, by a process dependent on CD14 (Figure 7A). To complement this approach, we utilized flow cytometry to detect phospho-Syk after stimulation of cells with either LPS or the known Syk activator curdlan (Rogers et al., 2005; Underhill et al., 2005). LPS and curdlan both induced comparable amounts of phospho-Syk (Figure 7B). Interestingly, Syk-phosphorylation did not occur in CD14-deficient BMDM or DCs stimulated with LPS, whereas curdlan-induced Syk-phosphorylation was unaffected by the lack of CD14 (Figure 7B). Moreover, cells derived from the C3H/HeJ mouse that harbor a signaling defective TLR4 protein retained the ability to induce Syk activation (Figure 7B). Collectively, these data demonstrate that Syk is activated by a CD14-dependent process that does not require TLR4.
Figure 7. Syk is activated by LPS by a process dependent on CD14 but independent of TLR4.
A, BMDM of the genotypes indicated were stimulated with LPS (100ng/ml). At the times indicated, the presence of phospho-Syk was examined by western blot. B, BMDM and DCs of the genotypes indicated were stimulated with either LPS or curdlan and the presence of phospho-Syk was detected by flow cytometry. C, BMDM were stimulated with LPS (100ng/ml) for the times indicated and processed for confocal microscopy to detect the presence of CD14, Cholera Toxin B (CTB) or dextran loaded lysosomes. All images for all panels are representative of at least three independent experiments where over 500 cells were examined per condition and >95% of the cells displayed similar staining. D, Model depicting a cascade of transport events that is mediated by CD14 in order to promote TLR4 signaling. CD14 first captures and transports LPS to the plasma membrane localized complex of TLR4 and MD2, which signals through the TIRAP-MyD88 adaptors to activate inflammatory cytokine expression. CD14 then transports TLR4 to endosomes by a process mediated by Syk and PLCγ2, where TRAM-TRIF signaling can lead to the expression of IFNs. See also Figure S5.
Finally, a microscopic approach was used to examine the role of Syk in CD14-dependent endocytosis. Unfortunately, the antibody that used to detect TLR4 is not suitable for biochemical or microscopic techniques. In fact, there are few (if any) antibodies available that can detect TLR4 reliably. CD14-specific antibodies are reliable and have been useful tools in many applications. To determine if CD14 would serve as a marker for Syk-dependent endocytosis, we examined the requirements for CD14 internalization in response to LPS. Using FACS as a preliminary readout for CD14 endocytosis, we found that LPS induced CD14 endocytosis by a process independent of TLR4 or its signaling adaptors, but was inhibited by Syk or PLC-specific inhibitors (Figure S5C-E). Thus, CD14 and TLR4 are internalized by a similar process and validate the utility of using CD14 as a marker of Syk-dependent endocytosis. By confocal microscopy, CD14 labeled the surface of BMDM with a concentration in membrane ruffles (Figure 7C). Within 3 minutes of LPS treatment, CD14 appeared in macropinosomes that co-stained with cholera toxin B (CTB), which marks GPI rich regions of the plasma membrane and endosomes (Figure 7C). CD14 and CTB costaining persisted for up to 30 minutes, but these endosomes never colocalized extensively with tubular dextran-loaded late endocytic vesicles (Figure 7C). LPS-induced macropinosomes stained positive for the early endosomal marker EEA1 (Figure S5F). When cells were treated with the Syk inhibitor piceatannol, CD14 remained at the plasma membrane and was poorly internalized (Figure 7C), and no enlarged EEA1 positive macropinosomes were observed (Figure S6). These data are very similar to that obtained by FACS, and thus, further validate the existence of a Syk-dependent endocytic pathway that is triggered by LPS.
Discussion
The role of endocytosis in TLR signaling has attracted much attention in recent years. Despite its importance, the only known regulators of TLR endocytosis are dynamins, clathrin and their associated proteins (Husebye et al., 2006; Kagan et al., 2008). While these regulators control many general endocytic processes, they cannot provide insight into the specific means by which microbial products can induce TLRs to enter mammalian cells. Our discovery that the LPS-binding protein CD14 is a regulator of TLR4 endocytosis therefore fills an important gap in our knowledge of how microbial binding can be coordinated with TLR endocytosis.
CD14 was identified originally as being important for MyD88-dependent signal transduction (Moore et al., 2000; Wright et al., 1990). As many others have reported, we have found that CD14 is only necessary for MyD88-dependent signaling at low concentrations of LPS, an observation that is likely explained by the function of this PRR in transporting LPS to the TLR4-MD-2 complex (da Silva Correia et al., 2001; Gioannini et al., 2004). The LPS-transport and TLR4-transport functions of CD14 can be distinguished by the means by which we can bypass their requirement in TLR4 signaling. For example, while the defects in MyD88-dependent TNFα expression can be rescued by increasing the concentration of LPS, the defects in TLR4 endocytosis and TRIF signaling cannot. These data clearly distinguish the two roles of CD14, one in transporting LPS to the receptor (which can be overcome by increasing LPS dose) and one in transporting the receptor into the cell (which can be overcome by forcing the receptor into phagosomes of DCs). Taken together these data suggest that CD14 initiates a cascade of trafficking events (Figure 7D). This cascade begins with CD14 transporting LPS to TLR4, and culminates with CD14 delivering TLR4 to the endosomal signaling machinery that is needed to induce IFN expression (Figure 7D). This “innate immune trafficking cascade” may also apply to other non-signaling PRRs that function to deliver microbial ligands to downstream PRRs with signaling capabilities. Since CD36 and the Mannose Binding Lectin are thought to serve analogous functions to CD14 for TLR2 (Hoebe et al., 2005; Ip et al., 2009), this suggestion may explain why CD14 is not required for TLR2-mediated IFN expression.
CD14 is not only required for TLR4 endocytosis in naïve antigen presenting cells, but also in mature DCs. When compared to immature DCs, mature DCs exhibited an enhanced capacity to internalize TLR4 and induce the expression of TRIF-dependent genes. All of these activities were dependent on CD14, and were observed in DCs matured with either self-encoded or microbial ligands. These data suggest that CD14-dependent endocytosis is broadly important for the functions of both immature and mature DCs. In addition, our data reveal that mature DCs retain the ability to sense and respond to microbial encounters. CD14 has recently been implicated in regulating the differentiation of monocyte-derived DCs (Mo-DCs) upon encountering LPS (Cheong et al.). Once differentiated, Mo-DCs express high levels of CD14 and are potent antigen presenting cells. Thus, a trend is emerging whereby mature DCs exhibit higher levels of CD14 expression than their immature counterparts, and we suspect that the function of this activity is to enhance the ability of Mo-DCs to induce of T-cell differentiation.
For many years, TLR4 has been considered the sole mediator of CD14-dependent responses because all well-studied activities of LPS were dependent on TLR4 and its signaling adaptors. Thus, after delivery of LPS to TLR4, it is thought that the function of CD14 is complete and TLR4 can then act independently of any extracellular factors to promote MyD88 and TRIF signaling. Our discovery that TLR4 actually depends on CD14 for its own endocytosis represents a significant shift in our understanding of the hierarchy of PRRs that regulate responses to endotoxin. Since TLR4 is cargo for a CD14-dependent endocytic pathway, we propose that TLR4 is not the “king” of all LPS responses. Rather TLR4 is subservient to CD14 and must rely on this upstream PRR to not only receive its ligand (LPS) but also to be delivered to endosomes where TRIF-mediated signaling can occur. Thus, CD14 sits at the apex of all cellular responses to LPS and functions to induce an innate immune trafficking cascade that involves the transport of both TLR4 and its ligand LPS.
The endocytic pathway activated by CD14 appears to be ITAM-mediated, and requires Syk and its downstream effector PLCγ2. Evidence in support of this claim comes from observations that inhibition of either Syk or PLCγ2 largely phenocopy CD14-deficiency. Moreover, our finding that CD14 is necessary for Syk-activation in response to LPS provides a direct biochemical link between CD14 and this kinase. It is possible that conditions exist whereby alternative pathways would play a role in promoting the delivery of TLR4 to endosomes, where TRIF signaling can occur. In this regard, we would like to emphasize that our use of the term endosomes does not exclude a possibility that TLR4 signaling may also occur from macropinosomes, which can be formed upon LPS treatment and are likely important in promoting the capture of microbial products. However, since TRIF signaling can occur from at least two distinct endosomal vesicles (endosomes and phagosomes), it is unlikely that TRIF signaling would be restricted to macropinosomes or any specific type of endosome. Rather, we suggest that the TLR4 network is designed such that any means of generating a TLR4 containing endosomal vesicle is sufficient to induce TRIF signaling. The possible number of different means by which TLR4 can be delivered into endosomes is difficult to predict, as evidenced by our unexpected finding that even when using a common means of internalization (phagocytosis), BMDM and DCs differ in their ability allow TLR4 and FcγR1 to enter phagosomes. Despite this possibility of endocytic heterogeneity, the fact that the CD14-PLCγ2-Syk pathway is activated specifically by LPS suggests that this pathway represents the primary means of promoting TLR4 internalization and signaling in cell types as diverse a phagocytes and fibroblasts.
From an evolutionary perspective, our identification of Syk and PLCγ2 as regulators of LPS-induced endocytosis draws similarities to other immunity-related endocytosis receptors that also depend on these factors, such as Dectin-1 and FcγR1 (Crowley et al., 1997; Rogers et al., 2005; Underhill et al., 2005). A unifying theme that could link these observations is the idea of an “inflammatory endocytosis pathway” that is mediated by Syk/PLCγ2 and can be engaged by diverse upstream receptors. Our work provides a mandate to consider the role of this potential inflammatory endocytosis pathway in diverse pathogenic interactions.
Methods
Mice, cells and transfection
C57BL/6 mice were from Harlan-Italy. Cd14-/- mice were from CNRS d'Orléans (Orléans Cedex 2, France). OT-II mice were from Charles River. Tlr4-/- C57BL/6 mice were provided by S. Akira (Osaka University, Japan). PLCγ2 deficient mice were provided by A. Mocsai (Semmelweis University, Hungary). Bone marrow cells from mice lacking DAP12 and FcεRIγ was kindly provided by M. Colonna (Washington University, St. Louis). All experiments were carried out in accordance with the relevant laws and institutional guidelines. E. coli O111 was from ATCC-LGC. DCs and BMDM were derived from bone marrow of C57BL/6 or mutant mice. DCs were collected after 7-8 days of culture in 10% B16-GM-CSF conditioned medium when CD11c expression, assessed by flow cytometry, was higher than 90%. BMDM were used after 6 days of culture in 30% M-CSF conditioned medium. A20 cells and freshly isolated splenic B cells were transfected with pCDNA3-based vector encoding CD14 by nucleofection (AMAXA) using the Nucleofector Buffer V and program L-013.
Antibodies and chemicals
Anti-TLR4 antibody (Sa15-21) was labeled with biotin by BioLegend (San Diego, California). Anti-mouse CD14 was from eBioscience (San Diego, California) and anti-CD14 was from Miltenyi Biotec. Anti-Phospho-Syk (C87C1) antibody was from Cell Signaling Technology (Beverly, MA). All the other antibodies and streptavidin-conjugates used for FACS analysis were purchased from BioLegend (San Diego, California). TLR4-grade LPS (E.Coli, 055:B5) was from Alexis Biochemicals-Enzo Life Science (Farmingdale, New York). Recombinant (r)TNF was from R&D Systems (Minneapolis, USA). CpG DNA (tccatgacgttcctgatgct) was from Primm (Milan, Italy). Curdlan was from WAKO Pure Chemicals (Osaka, Japan). U73122, Piceatannol, BAY 61-3606 and BAY 11-7085 were from Sigma Aldrich (St. Louis, MO). Fluoresbrite Carboxy YG 4.5mm latex beads were from Polysciences (Warrington, PA). For adsorption of LPS onto latex beads, microspheres were resuspended in LPS (1μg/ml) and incubated overnight at 4°C. Latex beads were then washed 15 times in large volumes of sterile endotoxin-free PBS.
Western blotting
2.5*106 cells were subjected to the indicated treatment and lysed with a buffer containing 1% NP-40, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10% glycerol and protease/phosphatase inhibitors (Roche). Cleared lysates were electrophoresed and immunoblotted with the indicated antibodies using standard conditions. IRF3 dimerization assay by native page was performed as described with minor modifications (Iwamura et al., 2001). Briefly, 2.5×106 cells were lysed for 15’ at 4 °C with a buffer containing 1% NP-40, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, protease/phosphatase inhibitors (Roche) and lysates were cleared by centrifugation at 13000 × g for 15’ at 4 °C. Before sample loading, 7% polyacrylamide gels were pre-run for 30’ at 40mA with 25 mM Tris, 192 mM glycine (pH 8.4) with and without 1% deoxycholate (DOC) in the cathode and anode chamber, respectively. 15μg of samples in a native sample buffer (10 μg protein, 62.5mM Tris-Cl, pH 6.8, 15% glycerol and 1% DOC) were applied to the gel and electrophoresed for 2-3 hours at 10-15 mA. Immunoblotting was performed using standard conditions.
Co-immunoprecipitation
Approximately 2*107 cells were lysed with a buffer containing 1% NP-40, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10% glycerol and protease/phosphatase inhibitors (Roche). After clearing by centrifugation at 16000 × g for 15’ at 4 °C, whole cell extracts (INPUT) were quantified with a BCA kit (ThermoScientific) and 1.5 mg of this fraction were immunoprecipated overnight using Protein A Sepharose 4 Fast Flow (GE Healthcare) and 1 μg of rabbit anti-MyD88 (R&D). Immunoprecipated and non-immunoprecipated material were referred to as IP and FT (flowthrough), respectively. All fractions were then subjected to SDS-PAGE and immunoblotting was performed with anti-MyD88 and anti-IRAK4 antibodies according to standard procedures.
Cell purification, activation and FACS staining
B cells were freshly isolated from the spleen of WT animals by positive selection of CD19+ cells using MACS beads (Miltenyi Biotec). For in vitro stimulations, DCs (unstimulated or matured for 18 hours with 1μM CpG or 100pg/ml rTNFα), BMDM, A20 B cells, freshly isolated splenic B cells and MEFs were treated with the various concentrations of LPS, E. coli or latex beads at the appropriate MOI for the times indicated at 37°C. Cells were then washed with ice-cold PBS and stained with the appropriate primary antibodies for 30 min on ice, followed by streptavidin-APC. Staining was assessed with a FACSCalibur (Becton Dickinson). Syk phosphorylation in DCs and BMDM treated with LPS (1μg/ml) or Curdlan (100μg/ml) was assessed by FACS as previously described (Underhill et al., 2005). For in vivo stimulations, WT, CD14- or TLR4-deficient mice were i.v. injected with 50μg of LPS and, at the indicated times, spleens were collected, red-blood cells lysed and unicellular suspensions stained as described above.
TNFα and IFN measurements
ELISA for TNFα was performed using the DuoSet kit (R & D, Minneapolis, MN). IFN activity was measured as described (Dixit et al., 2010).
Real-time quantitative PCR
Total RNA was extracted from 3×106 cells using the TRIZOL reagent according to the recommended procedure (Gibco-BRL). Single-strand cDNA was synthesized using High-capacity cDNA Reverse Transcription Kits (Perkin-Elmer, Applied Biosystem Division, Foster City, CA). The NanoDrop (TermoScientific) was used to titer mRNA and amplification was performed using the Power Sybr Green PCR Master Mix (Perkin Elmer).
Statistical analysis
Means were compared by paired or unpaired t- tests. Data are expressed and plotted as means ± ES values. Sample sizes for each experimental condition are provided in the methods and the figure legends.
Supplementary Material
Figure S1. CD14 is not essential for TLR4 signaling from the plasma membrane.
A, DCs were left untreated or treated with LPS (1μg/ml) for 30 minutes and the complex of MyD88 and IRAK4 was examined by western blot. DCs (B) or BMDM (C) were treated with LPS (1μg/ml). At the times indicated, MyD88-dependent changes in IκB levels, p-p38, and p-ERK1/2 were examined by western blot. D, Immortalized BMDMwere stimulated with the ligands indicated. At either 3 or 6 hr post-treatment, the expression of IL-6 or Viperin was examined by qPCR. E, Inflammatory monocytes were stimulated with either CpG DNA or Vaccinia Virus at the multiplicity of infection (MOI) indicated. The induction of TLR9 or TLR2 dependent IFN was then assessed using a bioassay as described (Barbalat et al., 2009).
Figure S2. CD14 expression levels in DCs and BMDM.
A. CD14 surface staining was determined by flow cytometry in BMDM and DCs derived from WT and TLR4-deficient (KO) mice. B, A20 B-cells were treated with LPS (1μg/ml) for 24 hours and the increase in MHC class II and CD69 was examined by flow cytometry.
Figure S3. DCs, but not BMDM, permit the non-specific entry of FcγR1 into cells during phagocytosis.
A, DCs or BMDM of the genotypes indicated were treated with uncoated latex beads and at the times indicated, the surface staining of FcγR1 was assessed by flow cytometry. Displayed are the MFIs of specific surface FcγR1 staining at each time point.
Figure S4. Chemical inhibitors of PLC and Syk disrupt TLR4 endocytosis and signaling.
A, BMDM and DCs of the genotypes indicated were untreated or treated with LPS (10ng/ml) for the times indicated. Flow cytometry was then used to examine receptor endocytosis by determining the surface levels of the endogenous TLR4. Shown are the MFIs of specific TLR4 surface staining at each time indicated. Note, similar results were obtained with LPS doses as high as 1μg/ml. B, C, BMDM (B) or DCs (C) were treated with 10ng/ml LPS for the times indicated in the presence or absence of the PLC inhibitor U73122. The rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. D, E, Exactly as described for (B, C) except the cells with treated with the Syk inhibitor Bay61-3606.
Figure S5. LPS-induced CD14 endocytosis proceeds by a pathway similar to that taken by TLR4.
A, DCs were treated with the Syk inhibitor piceatannol (75μM) and were then stimulated with 10ng/ml LPS. At the times indicated, the rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated in the presence or absence of piceatannol with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. Fourth panel, DCs were treated with LPS (1μg/ml) for 30 min in the presence or absence of piceatannol (75μM). The presence of dimerized IRF3 and IκBα was assessed by western analysis. B, DCs of the genotypes indicated were treated with 10ng/ml LPS for either 30 or 90 minutes. The rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. C, Mice were injected with LPS (50μg) and at the times indicated spleens were isolated and BMDM and DCs were examined for surface levels of TLR4 by flow cytometry. Shown are the MFIs of CD14 surface staining at each time point indicated. D, E, DCs (D) or BMDM (E) of the genotypes indicated were treated with 10ng/ml LPS for the times indicated in the presence or absence of the Syk inhibitor piceatannol (75μM). The rate of CD14 endocytosis was assessed by flow cytometry. F, BMDM were stimulated with LPS (100ng/ml) for the times indicated and processed for confocal microscopy to detect the presence of CD14 or EEA1. All images for all panels are representative of at least three independent experiments where over 500 cells were examined per condition and >95% of the cells displayed similar staining.
Acknowledgements
We would like to thank the members of our lab for helpful discussions. This work was supported by the Harvard Digestive Diseases Center P30 DK34854 (J.K.), and grants from NIAID 5R00AI072955 (J.K.), European Union FP7 Program TOLERAGE: HEALTH-F4-2008-202156 and ENCITE: HEALTH-F4-2008-201842 (F.G.), Associazione Italiana per la Ricerca sul Cancro and Italian Ministry of Education and Research (F.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38:166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- Depoil D, Weber M, Treanor B, Fleire SJ, Carrasco YR, Harwood NE, Batista FD. Early events of B cell activation by antigen. Sci Signal. 2009;2:pt1. doi: 10.1126/scisignal.263pt1. [DOI] [PubMed] [Google Scholar]
- Dietrich N, Lienenklaus S, Weiss S, Gekara NO. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One. 5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutman SB, Trombetta ES. Dendritic cells continue to capture and present antigens after maturation in vivo. J Immunol. 185:2140–2146. doi: 10.4049/jimmunol.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, Akira S. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem Biophys Res Commun. 2005;328:383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. Embo J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K, Okabe Y, Namiki H, Fujita T. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells. 2001;6:375–388. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- Latz E, Visintin A, Lien E, Fitzgerald KA, Espevik T, Golenbock DT. The LPS receptor generates inflammatory signals from the cell surface. J Endotoxin Res. 2003;9:375–380. doi: 10.1179/096805103225003303. [DOI] [PubMed] [Google Scholar]
- Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 3 doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Andersson LP, Ingalls RR, Monks BG, Li R, Arnaout MA, Golenbock DT, Freeman MW. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J Immunol. 2000;165:4272–4280. doi: 10.4049/jimmunol.165.8.4272. [DOI] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284:25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–465. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CD14 is not essential for TLR4 signaling from the plasma membrane.
A, DCs were left untreated or treated with LPS (1μg/ml) for 30 minutes and the complex of MyD88 and IRAK4 was examined by western blot. DCs (B) or BMDM (C) were treated with LPS (1μg/ml). At the times indicated, MyD88-dependent changes in IκB levels, p-p38, and p-ERK1/2 were examined by western blot. D, Immortalized BMDMwere stimulated with the ligands indicated. At either 3 or 6 hr post-treatment, the expression of IL-6 or Viperin was examined by qPCR. E, Inflammatory monocytes were stimulated with either CpG DNA or Vaccinia Virus at the multiplicity of infection (MOI) indicated. The induction of TLR9 or TLR2 dependent IFN was then assessed using a bioassay as described (Barbalat et al., 2009).
Figure S2. CD14 expression levels in DCs and BMDM.
A. CD14 surface staining was determined by flow cytometry in BMDM and DCs derived from WT and TLR4-deficient (KO) mice. B, A20 B-cells were treated with LPS (1μg/ml) for 24 hours and the increase in MHC class II and CD69 was examined by flow cytometry.
Figure S3. DCs, but not BMDM, permit the non-specific entry of FcγR1 into cells during phagocytosis.
A, DCs or BMDM of the genotypes indicated were treated with uncoated latex beads and at the times indicated, the surface staining of FcγR1 was assessed by flow cytometry. Displayed are the MFIs of specific surface FcγR1 staining at each time point.
Figure S4. Chemical inhibitors of PLC and Syk disrupt TLR4 endocytosis and signaling.
A, BMDM and DCs of the genotypes indicated were untreated or treated with LPS (10ng/ml) for the times indicated. Flow cytometry was then used to examine receptor endocytosis by determining the surface levels of the endogenous TLR4. Shown are the MFIs of specific TLR4 surface staining at each time indicated. Note, similar results were obtained with LPS doses as high as 1μg/ml. B, C, BMDM (B) or DCs (C) were treated with 10ng/ml LPS for the times indicated in the presence or absence of the PLC inhibitor U73122. The rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. D, E, Exactly as described for (B, C) except the cells with treated with the Syk inhibitor Bay61-3606.
Figure S5. LPS-induced CD14 endocytosis proceeds by a pathway similar to that taken by TLR4.
A, DCs were treated with the Syk inhibitor piceatannol (75μM) and were then stimulated with 10ng/ml LPS. At the times indicated, the rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated in the presence or absence of piceatannol with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. Fourth panel, DCs were treated with LPS (1μg/ml) for 30 min in the presence or absence of piceatannol (75μM). The presence of dimerized IRF3 and IκBα was assessed by western analysis. B, DCs of the genotypes indicated were treated with 10ng/ml LPS for either 30 or 90 minutes. The rate of TLR4 endocytosis was assessed by flow cytometry (first panel). Second and third panels, cells were stimulated with 10ng/ml LPS for 18 hours and the amounts of secreted cytokines were determined. C, Mice were injected with LPS (50μg) and at the times indicated spleens were isolated and BMDM and DCs were examined for surface levels of TLR4 by flow cytometry. Shown are the MFIs of CD14 surface staining at each time point indicated. D, E, DCs (D) or BMDM (E) of the genotypes indicated were treated with 10ng/ml LPS for the times indicated in the presence or absence of the Syk inhibitor piceatannol (75μM). The rate of CD14 endocytosis was assessed by flow cytometry. F, BMDM were stimulated with LPS (100ng/ml) for the times indicated and processed for confocal microscopy to detect the presence of CD14 or EEA1. All images for all panels are representative of at least three independent experiments where over 500 cells were examined per condition and >95% of the cells displayed similar staining.