Geriatric adult emergency department (ED) visits increased by 34% between 1993 and 2003, a trend that will double annual volumes among those aged 65 to 74 years from 6.4 million to 11.7 million by 2013.1 The fastest growing segment of the population is the old-old (>85 years) who also happen to be using the ED at the highest rate.2 Geriatric patients already consume more ED time and resources than younger populations3 and orthopedic injuries represent a substantial proportion of their emergency care issues. After age 50 years, the lifetime risks for fractures in women are hip 17.5%, vertebrate 16%, and Colles 16%. In men aged 50 years and older, the lifetime risks of fracture are hip 6%, vertebrate 5%, and Colles 2.5%.4 In the United States, the National Hospital Ambulatory Care Survey reported 21 million injury-related ED visits among adults more than 65 years of age from 2000 to 2004, including 22% with fractures.5 Geriatric trauma is not unique to North American emergency medicine. In the United Kingdom, injuries represent 33% of older adult complaints presenting to EDs.2 Currently, more than 250,000 hip fractures present to EDs in the United States each year, but this number is projected to double by 2040.6 Despite the evolving epidemiologic imperative in the Institute of Medicine report, Hospital-Based Emergency Care: At the Breaking Point, geriatric issues that will shape twenty-first century acute care were widely underemphasized.7,8
Aging is associated with a variety of physiologic changes that affect emergency orthopedic care.9,10 Hormonal changes and malnutrition result in osteoporosis, which increases the likelihood and severity of fractures and concomitantly affects orthopedic surgical management.4,11–14 In distinction, frailty is poorly defined and difficult to quantify but prevalent and associated with suboptimal recovery.15 Furthermore, diminished gastrointestinal (GI) absorption of medications and impaired renal function impede effective pain management.16 Functionally, balance and gait problems diminish independence and increase the risk of falls; 27% of community-dwelling older adults suffer a fall each year.17
Therapeutically, older adult orthopedic injury management offers unique challenges. In patients with hip fracture, preoperative delirium is reported in 34% to 92% of cases.18 Not surprisingly, delirium is independently associated with poor functional recovery.19,20 Previously undiagnosed dementia, usually unrecognized by ED physicians,21 can be present in 40% of patients.22,23 Dementia is an independent risk factor for delirium.24 In addition, cognitive dysfunction can impede timely analgesia,25 impair full informed consent, and delay prompt diagnosis.9 Delayed diagnosis and surgical management can adversely affect fracture recovery and increase mortality.26
Unfortunately, the traditional emergency care model is not geriatric friendly.27 For example, standing level falls are a leading cause of older adult fractures and traumatic mortality,28 but patients who have fallen rarely receive guideline-directed care in today's ED.29,30 For prevention, previously described fall risk factors lack ED validation so identifying high-risk subsets can be challenging.17,31 Although one trial reported success with an ED-initiated multidisciplinary intervention to prevent falls,32 others have not reported reduced fall rates or fall injuries with different models.33,34 In addition, emergency medicine clinical decision rules for orthopedic injuries often lack validation in older patients.35,36 This review summarize some of the unique therapeutic options and models in caring for geriatric ED patients with skeletal injuries.
GERIATRIC PHYSIOLOGY
Physiologic changes associated with aging are universal and affect every organ system, generally resulting in a decline in functional reserve capacity. However, these expected changes do not represent disease processes.37,38 An age-related loss of both reserve and the ability to maintain homeostatic mechanisms, especially under conditions of physiologic stress, results in an increased risk of injury and disease. The resulting trauma or illness is often a complex and synergistic interplay between coexisting disease and the normal processes of aging.39 Falls in the elderly and the traumatic orthopedic injuries that result are one example.
The musculoskeletal system undergoes several important changes with aging. As a percentage of total body weight, lean body mass decreases, whereas total body fat increases. Loss of muscle mass resulting from a decrease in the number of muscle fibers causes a reduction in muscle strength. After the age of 60 years, muscle strength decreases by approximately 33%, contributing to difficulty in maintaining balance and predisposing the elderly to subsequent falls.40 Other intrinsic factors related to aging compound the risks of falls and injury, including impaired coordination, peripheral neuromuscular dysfunction, and deficits in vision, equilibrium, gait, proprioception, and cognition.31,41,42 Physical activity can improve or slow the progression of some of these age-related deficits and therefore has been found to reduce the risk of falling.34,43–46 Exercise has been shown to increase muscle strength, with specific resistance training actually increasing muscle mass and improving neural coordination and strength.47–49
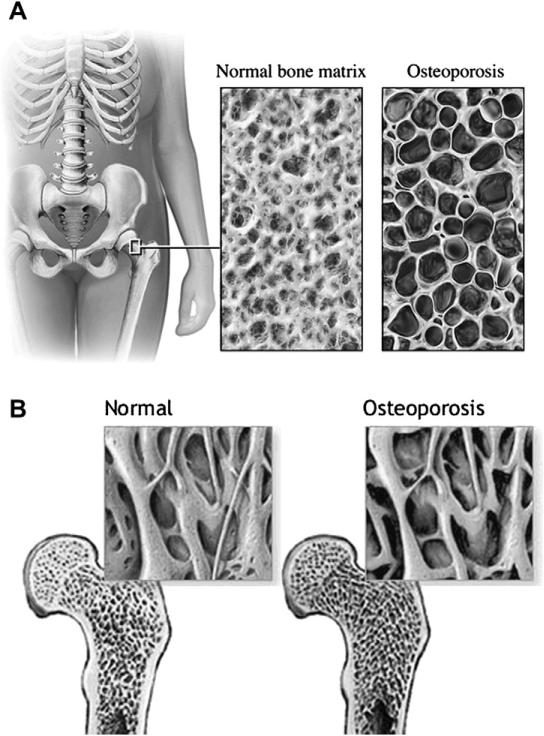
The loss of skeletal bone mass and density in the elderly is another important physiologic change associated with the risk of orthopedic injury (Fig. 1). Bone loss occurs at different rates for women (greatly accelerated during the postmenopausal period) and men, but by age 60 years they have equal rates of bone loss, with increased loss of total bone mass for both at age 80 years. In addition, there are age-related changes to bone quality. This decline in bone integrity combined with the loss of bone mass and changes in its distribution all result in loss of bone strength.14 Skeletal fragility occurs as the bones become more vulnerable to the mechanical forces of trauma, causing injuries to elderly patients to occur with less transmitted kinetic energy compared with younger populations.
Fig. 1.
(A, B) Osteoporosis. This artistic rendition of normal and osteoporotic bone demonstrates the striking difference in bone density and bone microstructure. (From Nucleus Medical Media, Inc, Kennesaw, Georgia; with permission; MedlinePlus Medical Encyclopedia. Available at: http://www.nlm.nih.gov/medlineplus/ency/imagepages/17156.htm. Accessed June 18, 2010.)
GERIATRIC PHARMACOLOGY
Poor pain management in the elderly is unfortunately a common problem.50–52 Several barriers to timely and effective analgesia exist, including inadequate knowledge about pain assessment and management, failure to assess for pain, physician misperception that pain is a natural and expected consequence of aging, concerns about the use of analgesics in patients with cognitive dysfunction or other comorbid illnesses, or in postoperative patients a dogma that pain should be expected after surgery.53–55 Oligoanalgesia, the undertreatment of pain, has many deleterious consequences including56–58 delirium,59,60 or other impaired cognitive function,61,62 decreased functional independence,54,63 depression,64,65 poorer clinical outcomes,60 as well as increased hospital length of stay, health care use and overall costs.66,67 The American Pain Society, the American Geriatrics Society, and the Agency for Health Care Policy and Research (AHCPR) have created evidence-based clinical practice and quality assurance pain management guidelines for clinicians.16
Effective and safe pain management in the older adult must incorporate knowledge of age-related changes that affect both pharmacokinetics and pharmacodynamics: drug absorption, distribution, metabolism, excretion, and the physiologic response to drugs.68 The 3 most commonly used geriatric analgesics are nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen, and narcotic analgesics.16,69,70 Each drug class has specific pharmacologic considerations in the geriatric orthopedic patient.
NSAIDs are among the most commonly used pain medications in the elderly because they provide effective rapid and sustained relief for mild to moderate pain, and they can decrease the swelling and tenderness associated with both acute and postoperative inflammation.70 However, NSAIDs are associated with significant adverse effects, especially GI and renal toxicity, which are particularly prevalent in the elderly. An age-related decrease in gastric bicarbonate secretion, blood flow, and mucosal function, as well as delayed gastric emptying time, all contribute to a loss of stomach protection and an increased risk of gastritis, ulcer formation, and GI bleeding.68 Bleeding complications from NSAIDs also occur in the esophagus, duodenum, and small and large intestine.71 Misoprostol, an oral cytoprotective prostaglandin E1 analogue, acts by replacing GI mucosal prostaglandins that have been reduced by NSAIDs. Cotherapy for NSAIDs with misoprostol has been shown to decrease the incidence of adverse GI events such as perforations and bleeds by 40%.16,72
An age-related decline in renal blood flow, functional renal mass, and tubular efficiency causes a decrease in glomerular filtration rate and creatinine clearance, thus affecting drug elimination by the kidneys.71 Therefore, the elderly depend more on prostacyclin-mediated renal afferent arteriolar vasodilatation to maintain glomerular blood flow. Because NSAIDs impair this compensatory mechanism, a further decrease in renal elimination of drugs occurs.71 Because creatinine production decreases with a decline in lean body mass that parallels the reduction in creatinine clearance associated with aging, serum creatinine levels are not a reliable marker of renal function in the elderly. Creatinine clearance is a more reliable marker of renal function. As a result of this renal dysfunction, increased drug serum levels and subsequent clinical toxicity can result.68,71 NSAIDs can also directly cause papillary necrosis and interstitial nephritis.73 In addition, because NSAIDs are highly lipid soluble with extensive protein binding, they are distributed widely in increased adipose stores of the elderly. Malnourished elderly patients with reduced plasma protein levels also have increased levels of unbound (active) drug.
Antihypertensive medications the activity of which is mediated via renal prostaglandins (such as β-blockers and angiotensin-converting agents) may well be inhibited by NSAIDs, causing hyperkalemia, fluid retention, hypertension, and frank heart failure.71 Cyclooxygenase-2 (COX-2) inhibitors have an improved GI safety profile with approximately the same analgesic efficacy compared with conventional NSAIDs.74,75 However, they show no decrease in the risk of renal complications and appear to increase the risk of cardiovascular thrombotic events in patients not taking aspirin.76
Based on available data, it is not yet possible to accurately quantify the risk of NSAID use in the elderly, in terms of number needed to harm (NNH) for renal injury and gastropathy, along with the number needed to treat (NNT) for effective analgesia. To date, NSAIDs as a class of medication have not been deemed inappropriate for use in the elderly population because of inadequate evidence, with 2 specific exceptions. Indo-methacin has been labeled as inappropriate because of toxicity to the central nervous system (CNS), as well as phenylbutazone because of its risk of bone marrow suppression.77 Current guidelines call for judicious use of NSAIDs with low doses and short-term therapy, as well as close monitoring of renal and gastrointestinal function, blood pressure, and fluid status during and immediately after therapy in all elderly patients.78
Acetaminophen (alone and in combination with other medications) is the most widely used analgesic in the world and is often used to treat mild to moderate pain in the elderly. Yet, its safe use must incorporate dosage and length-of-therapy adjustments for older adults. Acetaminophen hepatic metabolism in aging adults is multifactorial and can be affected by physiologic changes of aging, lifestyle, genotype, comorbidities, as well as interactions with other medications. As a result, acetaminophen metabolism may be reduced by 50% in this population.71 Decreased hepatic blood flow and an age-related decline in functional hepatocyte number and enzyme activity affects first-pass metabolism and the clearance of certain drugs. Aging also alters the nonsynthetic hepatic biotransformation reactions (eg, oxidative) more readily than synthetic enzymatic reactions (eg, conjugation). In an acute overdose (usually unintentional) or when the maximum daily dose is exceeded over a prolonged period, metabolism by conjugation becomes saturated, and excess acetaminophen undergoes oxidative metabolism by the CYP enzymes to a reactive metabolite, N-acetyl-p-benzoquinone-imine (NAPQI) leading to liver necrosis. Therefore, traditionally therapeutic doses (4 g/24 h) and long-term high-dose (>2 g/24 h) acetaminophen use in older adults can result in liver and even renal injury, as a result of similar enzymatic reactions occurring in extrahepatic organs.79–81 In addition, because acetaminophen has a maximum dose beyond which it has no additional analgesic efficacy (ceiling effect), it has limited use for the moderate to severe pain that often accompanies an orthopedic injury.16
Evidence suggests that physicians’ biases and knowledge deficits are the main culprits for improperly managing pain in the elderly.55 Misconceptions occur most commonly with treatment using opioid analgesics. In addition, older patients themselves have misperceptions about addiction and drug abuse that can contribute to the barrier to proper/improved pain management. Intentional nonadherence (deciding to discontinue or change the dose of a drug) and unintentional nonadherence (misreading the label or forgetting a dose) are common with elderly patients.82 Yet, studies of cancer, medical, and burn patients suggest that the medical treatment of pain with opioids rarely leads to drug abuse or iatrogenic opioid addiction.16
Opioid analgesics are central to proper pain management in elderly patients with orthopedic injuries.83 However, individual agents (synthetic vs nonsynthetic) have different pharmacokinetic and pharmacodynamic profiles, and knowledge of these differences is imperative to provide safe and effective analgesia. Morphine (a nonsynthetic opiate) is the most commonly used.16 It relieves all types of pain with no ceiling effect. Steady state can be achieved within 1 day as a result of an effective half-life (parent drug and its metabolites) of 3 to 4 hours.16 Morphine is eliminated in the liver via conjugation and therefore is not greatly affected by hepatic changes associated with aging. However, its metabolites are excreted by the kidneys. These age-related renal changes and altered pharmacokinetics cause a prolonged half-life, therefore, a reduction in morphine dose or a lengthened time interval between dosing should be used in elderly patients.84 Standing doses of narcotic analgesics should be avoided in older patients with dehydration, acute renal failure, or oliguria pre- or postoperatively.16 Instead, as-needed administration of the opiate should be initiated, as this has the added benefit of requiring the physician to reassess the patient's pain requirements and general condition on a regular basis.
Although morphine may be administered via virtually every conceivable route, site-specific bioavailability exists. Transdermal and transmucosal routes have the lowest bioavailability. Because of alterations in the clearance of opioids, because of the effect that an age-related decrease in hepatic blood flow has on rapid first-pass hepatic metabolism, higher oral or rectal doses of morphine compared with subcutaneous or intravenous administration may not be required for the same analgesic effect.16,71 When using an equianalgesic dosing table for opioid analgesics, this potential age-related change in pharmacokinetics should be taken into consideration.85
Understanding the side effects of opioids and how to manage them is an important aspect of their effective usage. The most common adverse effects are constipation, nausea, vomiting, and sedation; dizziness, hallucinations, confusion, and respiratory depression occur less frequently.16 All of these side effects are treatable, and some are mitigated by the development of tolerance over time.16 Sedation and mild confusion are predictable side effects of opioid dose escalation, but care must be taken to distinguish these symptoms from delirium, which confers significant morbidity and mortality. However, delirium has been shown to occur more commonly as a result of the undertreatment of pain rather than as an adverse effect of opioids.59,60
The updated Beer guidelines clearly state that certain analgesics should be avoided in the elderly, including pentazocine, propoxyphene, and meperidine.77 Pentazocine, a mixed opiate agonist/antagonist, increases the risk of seizures as well as other effects on the CNS compared with other analgesics. Propoxyphene has doubtful efficacy in the elderly and can potentiate the anticoagulant effect of warfarin.71,86 In addition, it has an active metabolite, norpropoxyphene, with a long half-life that increases the risk of CNS toxicity.87 Meperidine lowers the seizure threshold, has poor analgesic efficacy, causes sedation, and has cardiotoxicity, especially in patients with renal insufficiency or hepatic dysfunction, caused by an active metabolite, normeperidine, with a long half-life.71,77
ACUTE FRACTURE ANALGESIC ALTERNATIVES
Aging physiology with concomitant comorbid illnesses including occult cognitive dysfunction and labile blood pressure all complicate acute fracture pain reduction in older adults. In addition, traditional narcotic analgesia can cause delirium and increase the risk of falls. Specific management strategies may augment or replace narcotic analgesia in geriatric orthopedic injury therapy for the 3 most common fractures (hip, vertebral, Colles).88 For example, in osteoporotic vertebral compression fractures, 5 randomized trials with 246 subjects have demonstrated significantly improved pain control at 1 week with salmon calcitonin (daily doses of 100 IU IM or 200 IU intranasal or 200 IU suppository) with reduced concomitant analgesic use.89
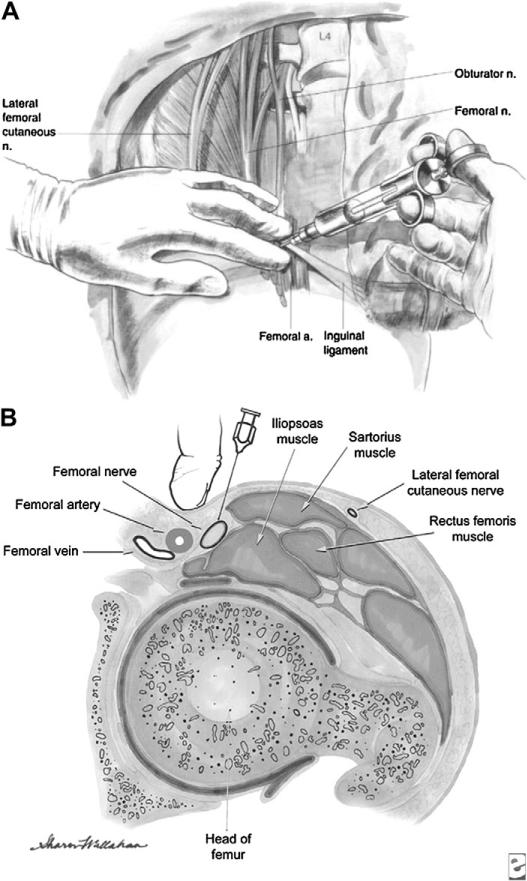
The femoral nerve provides much of the sensory innervation to the femur. Fracture pain originates from the sensitive periosteum and quadriceps muscle spasm. In addition to appropriate splinting, acute ED analgesia for hip and distal femur fracture is most commonly intravenous systemic narcotic agents (hydromorphone, morphine). Femoral nerve blocks (Fig. 2) reduce pre- and postoperative hip fracture pain.90 McGlone and colleagues91 assessed femoral nerve blocks performed by house staff and ED physicians for femoral shaft fractures using lignocaine (mean onset 8.7 minutes, mean duration 3.8 hours) or bupivacaine (mean onset 9.3 minutes, mean duration 11.5 hours) with sufficient analgesia to permit comfortable manipulation of the injured extremity within 15 minutes of injection in most cases. A similar population of geriatric adults with femoral neck fractures randomized to systemic analgesia alone or femoral nerve block performed by an orthopedic surgeon using 0.3 mL/kg of 0.25% bupivacaine demonstrated significantly improved pain scores at 15 minutes and 2 hours in the nerve block patients.92 Others have reported similar successes without any adverse events related to nerve blocks.93,94 The 3-in-1 femoral nerve block infiltrates the femoral nerve sheath then tracks cranially and laterally anesthetizing the femoral and obdurator nerves, lumbar plexus, and lateral cutaneous nerves. In one randomized controlled trial of femoral neck fracture victims, trained ED physicians using the 3-in-1 block provided patients with faster pain relief (2.8 hours vs 5.8 hours) with significantly less morphine.95
Fig. 2.
(A, B) Femoral nerve block. A femoral nerve block can be easily performed in the ED by using anatomic landmarks (ie, palpation of the femoral artery) or by using ultrasound guidance to localize the femoral artery and the high-signal femoral nerve that lies lateral to the artery. (A: From Brown DL, Clifford JA, Wild J. Atlas of regional anesthesia 2006. p. 113–21, Fig. 13-4. Available at: http://polanest.webd.pl/pliki/varia/books/AtRegAn/micro189.lib3.hawaii.edu_3a2127/das/book/body/0/1353/i4-u1.0-b1-4160-2239-2..50017-5–f4.fig.htm. Accessed June 18, 2010; with permission; B: Reprinted from eMedicine.com, 2009. Available at: http://emedicine.medscape.com/article/1143675-overview. Accessed June 18, 2010; with permission.)
Although early femoral nerve block investigations used house staff, ED, or orthopedic physicians to administer the anesthetic locally without imaging or extra equipment, anesthesiologists today use a nerve stimulator to identify the nerve before instilling the medication.96,97 No study has demonstrated that the nerve stimulator-based or anesthesiology-based approach is superior to femoral nerve blocks performed by ED physicians. However, compared with the fascial pop technique, ultrasound-guided femoral nerve blocks provide faster analgesia and permit identification of the adjacent vascular structures.98,99 Nurse-based femoral nerve block teams have also been described.100
For femoral nerve blocks (see Fig. 2), bupivacaine (0.5%) is the analgesic of choice based on duration of action, greater degree of motor blockade, and accumulating trial evidence. The dose is 0.3 mL/kg to a maximum volume of 20 mL to maximize analgesia without significantly increasing the risk of cardiotoxicity.95 With the patient in the supine position, a 7- to 9-MHz linear array ultrasound probe in the transverse orientation is used to identify the femoral artery and high-signal area lateral to the artery where the nerve lies.99,101 Under ultrasound visualization, a 22-guage short beveled needle is inserted at a 45° angle and advanced to the iliopectinal fascia in the immediate proximity of the nerve. After aspirating to ensure that a vessel has not been penetrated, 0.5% bupivicaine (0.3 mg/kg up to 20 mg) is administered with deposition directly visualized sonographically. The 3-in-1 femoral nerve block involves distal compression following administration of the local anesthetic, which then tracks cranially and laterally anesthetizing the femoral and obdurator nerves, lumbar plexus, and lateral cutaneous nerves.99
Colles fracture-related pain can be managed with a systemic analgesia, a hematoma block, or a Bier block. To place a hematoma block, one aspirates directly over the fracture hematoma before injecting 5 to 15 mL of 1% lidocaine.102 Hematoma blocks do not require periprocedural fasting, but they offer inferior analgesia compared with a Bier block.103,104 The risks of hematoma blocks include the introduction of an infectious agent at the fracture site so injection should never occur through nonsterile skin or in an open fracture.102,105 In addition, lidocaine hematoma blocks have been associated with delirium and seizures.106
OSTEOPOROSIS
Osteoporosis is the most common metabolic bone disorder, affecting 200 million people worldwide and more than 10 million people in the United States. Those at risk for developing the disease total another 18 million in the United States alone.14,107 The lifetime risk of osteoporotic fractures in a 50-year-old white woman has been estimated to be 30% to 40% in the United States, including a 15% to 18% risk for hip fractures.108 Yet, because osteoporosis is a clinically silent disease, often only manifesting with a fracture after a fall, it is under-recognized and undertreated.14 However, as physician awareness increases, appropriate medical management is improving.109
Causes of osteoporosis are multifactorial. Several factors influence the development of osteoporosis, including age, gender, race, lifestyle, body weight, and peak bone mass, which occurs during the fourth decade. Primary osteoporosis can be divided into type I (postmenopausal) osteoporosis and type II (age-related) osteoporosis. Type I osteoporosis is linked to menopause as a result of estrogen deficiency. In women, accelerated bone loss occurs in the perimenopausal period, with roughly 3% to 5% per year lost during the first decade after menopause onset.9,110 Subsequently, the loss of bone mass and density proceeds at a rate of 1% per year for women. Type I osteoporotic fractures occur in bones with higher trabecular content: vertebrae, pelvis, distal radius, and proximal femur. Type II osteoporosis affects both genders older than 70 years of age and is characterized by less rapid bone loss (0.5%–3% per year). Hip fractures predominate in this group. Type II osteoporosis is attributable to increases in parathyroid hormone levels, and decreased circulating vitamin D, growth hormone, and insulinlike growth factors.9 Secondary causes include medications, endocrine disorders, chronic renal disease, hematopoietic disorders, immobilization, inflammatory arthropathy, nutrition, gastrointestinal disorders, liver disease, and connective tissue disorders.14 For men in particular, specific factors such as alcohol abuse, glucocorticoid excess, and hypogonadism contribute to 50% of their osteoporosis.111
Osteoporosis is characterized by significant bone loss as a result of a simultaneous reduction in bone mass and deterioration of bone microstructure (see Fig. 1), leading to increased bone fragility and a subsequent increased risk of fracture. The World Health Organization defines osteoporosis as a T-score of greater than 2.5 standard deviations less than the mean of young healthy individuals at their peak bone mass.112 This bone mineral density (BMD) measurement is obtained using a dual energy X-ray absorptiometry (DEXA) scan. Increased fracture risk has been shown to be correlated with a low BMD.113 Additional laboratory studies must be performed in the work-up of osteoporosis: levels of calcium, 25-hydroxy vitamin D, parathyroid, bone alkaline phosphatase, urinary calcium and creatinine, thyroid-stimulating hormone, complete blood count, serum and urine protein electrophoresis, and liver function tests.14
To prevent the devastating sequelae of osteoporosis, appropriate treatment, both pharmacologic and nonpharmacologic, must be initiated after diagnosis. Pharmacologic treatment includes calcium, vitamin D, bisphosphonates, calcitonin, estrogen, and selective estrogen receptor modulators (SERMs). Osteoporosis management extends beyond pills, capsules, and pharmaceutical prescriptions. Although recent trials have cast doubt on their efficacy, minimally invasive treatment options for spinal compression fractures (vertebroplasty and kyphoplasty) continue to be studied.114–116 Other nonpharmaceutical treatments such as hip protectors, posture training supports, as well as balance and exercise training programs should be used as complements to optimize the outcomes for patients with osteoporosis.14
Treatment with a daily requirement of 1200 to 1500 mg of calcium is recommended and is better absorbed in the citrate form, as it dissolves at all pH levels. A daily dose of vitamin D (400–800 IU) is recommended in addition to the calcium, with even higher dosing for elderly patients with little sun exposure. In elderly women, the administration of both calcium and vitamin D has been shown to prevent hip and other nonvertebral fractures.117 In a follow-up study, intention-to-treat analysis showed a 36-month benefit in terms of reduction in both types of fractures, with decreased probability (odds ratios of 0.72 and 0.73, respectively).118
The most potent drugs in the prevention and treatment of osteoporosis are the bisphosphonates, which strongly bind to the hydroxyapatite of bone and inhibit osteoclast activity. Alendronate and risedronate are widely used and decrease the fracture rate for both the spine and hip. The oral route can cause GI side effects, especially for prone, hospitalized patients, therefore remaining upright for at least 30 minutes after administration is recommended.14 In type I osteoporosis, the Fracture Intervention Trial (FIT) demonstrated that alendronate increased BMD and decreased the risk of vertebral and hip fractures.119,120 In addition, alendronate has been shown to be efficacious for men and patients on steroids. The length of bisphosphonate treatment remains unclear, but usually ends after 5 years because of a plateau in bone mass measurements and the potential risk of microfracture accumulation.14
In a 5-year randomized, double-blind, placebo-controlled study, nasal calcitonin was shown to increase BMD and decrease the risk of vertebral fractures by 33% in women. Calcitonin also acted as an effective analgesia for bony pain secondary to fracture.121 However, calcitonin has not been shown to provide any protection against hip fractures.122,123 Estrogen has been used widely in postmenopausal women as hormone replacement therapy (HRT) to alleviate symptoms. Although estrogen has been shown to decrease the incidence of hip and spine fractures by 35%, it is not recommended for treatment of osteoporosis because of its potential health risks (increased risk of breast cancer and thrombotic events).14 Raloxifene, a SERM, may increase BMD, but is not as effective as bisphosphonates at treating osteoporosis, and it confers a risk of venous thromboembolism and causes hot flashes.124
Parathyroid hormone (PTH) has shown promise in treating osteoporosis. PTH works by increasing BMD, bone resorption, and formation, and enhancing bone architecture and integrity.125 Several studies have shown that PTH reduces the risk of fracture by increasing the connectivity of bone,126 thickening trabeculae,127 increasing cortical thickness, and inhibiting osteocyte apoptosis.128 However, compared with bisphosphonates, PTH takes 3 to 6 months longer to provide fracture protection, and more recent studies indicate that its place in treatment algorithms is still unclear.14
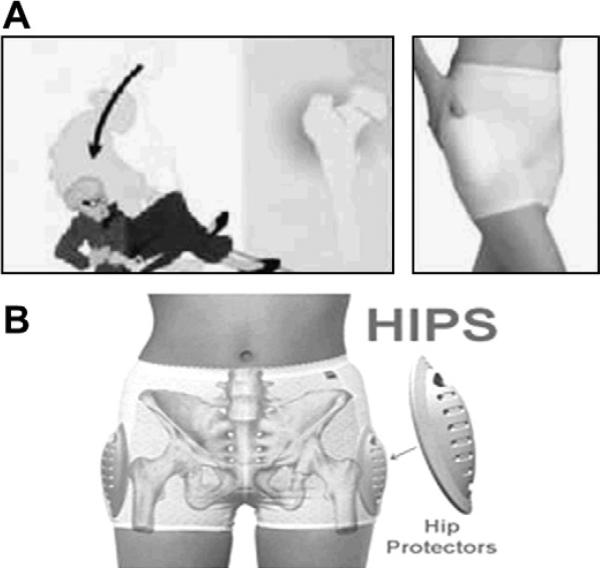
Nonpharmacologic treatment methods are an important part of the comprehensive multidisciplinary approach in the treatment of osteoporosis. Some trials have shown that polypropylene hip protectors (Fig. 3) reduce hip fractures129 and improve self-efficacy in frail older adults, defined as the belief in their own ability to avoid falling. However, other trials have failed to demonstrate a benefit so further research is currently underway.130–133 The major drawback of hip protectors is noncompliance.134 Posture training supports are lightweight orthoses, worn backpack style, that have been shown to provide symptomatic relief and increase extensor muscle strength in patients with thoracic kyphosis, as well as reduce vertebral fractures in estrogen-deficient women.135,136 Tai chi chuan, a Chinese martial art form that involves slow-motion routines, has been shown to improve balance and is associated with a 47.5% reduction in risk of falls.137 Several studies have shown that continued exercise training can increase BMD, yet it has not been shown to reduce fracture rates.14
Fig. 3.
(A, B) Hip protectors. Several trials have shown that polypropylene hip protectors reduce hip fracture, although low compliance rates impede widespread use. (From Kiel D. Hip protectors. Slide presentation at the Surgeon General's Workshop on Osteoporosis and Bone Health. Washington, DC; 12–13 December 2002. Available at: http://www.surgeongeneral.gov/library/bonehealth/chapter_6.html. Accessed June 18, 2010; with permission). (From e-pill, LLC, Wellesley, MA. Available at: http://www.hipprotectors.com. Accessed June 18, 2010; with permisssion.)
CARE MODELS
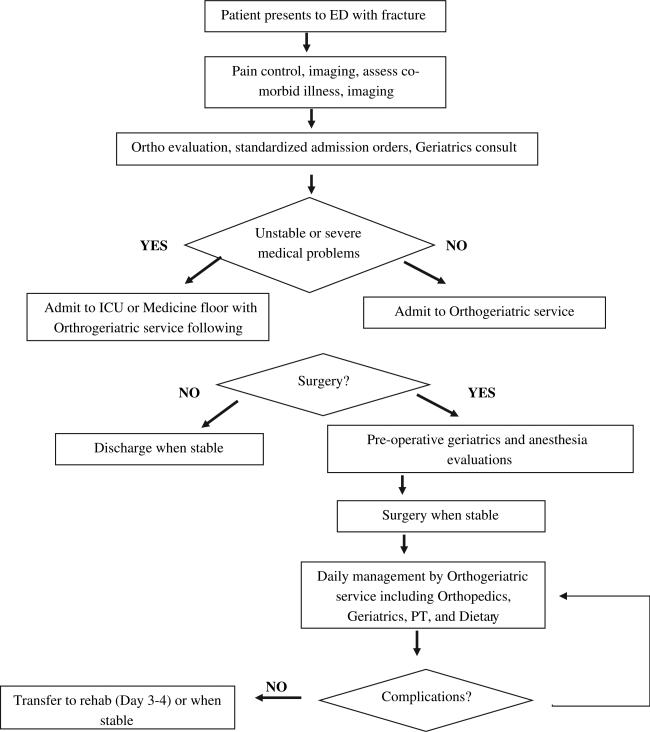
Fracture management can be variable as shown by research demonstrating that age and geography may affect orthopedic management decisions.138 A timely assessment of older adult fracture victim's preexisting functional status and support network is essential to guide effective acute orthopedic management.139 Dementia may impede or inhibit appropriate rehabilitation, whereas medical comorbidities may significantly alter the risk-to-benefit ratio for operative intervention. Most research suggests that for hip fractures, an operative delay beyond 48 hours increases mortality, although older patients with fracture merit careful assessment of surgical risk.140,141 Complications of delayed definitive care and prolonged immobility include pressure ulcers, thromboembolism, and pneumonia. Therefore, prompt preoperative recognition of surgical and nonsurgical injuries along with relevant geriatric syndromes requires a team approach involving orthopedics, anesthesiology, geriatrics, physiotherapy, and dieticians in conjunction with emergency medicine.142 In addition, in the postoperative period following a hip fracture multidisciplinary rehabilitation has demonstrated a trend toward improved functional outcomes with lower caregiver burdens.143
Amatuzzi and colleagues144 noted a sustained in-hospital mortality decrease (5% to 1.4%) in elderly patients with hip fracture for 4 years in Brazil after initiating an orthogeriatric group practice including educational outreach programs and routine joint orthopedic and geriatric evaluation of all ED patients with fractures. Their model also includes weekly meetings to discuss inpatient progress and outpatient therapy issues. Comprehensive geriatric interventions involving uniform older adult orthopedic patient assessment by a geriatrician, rehabilitation specialist, and social worker reduced in-hospital mortality and 3-month functional outcomes, but did not affect 6- or 12-month outcomes.145 Similar management models have been described elsewhere (Fig. 4).146,147 A decade's experience with a hip fracture clinical pathway at the New York University Hospital for Joint Diseases was associated with significant decreases in acute hospital length of stay, in-hospital mortality (5.3% vs 1.5%), and 1-year mortality (14.1% vs 8.8%).148 This model includes a standardized set of orders and consultant protocols beginning preoperatively and extending from the recovery room to postoperative day 1 and discharge. Another fast-track protocol for hip fractures involving local femoral nerve blocks, early anesthesiologist assessment, preoperative assessment of nutritional, fluid, urinary retention, and oxygen status significantly decreased multiple postoperative complications.149 Alternatively, geriatric and orthopedic comanagement of geriatric patients with a fractured femur has significantly reduced hospital length of stay, surgical delays, complication rates, and mortality.150
Fig. 4.
Multidisciplinary geriatric fracture management model. (Data from De Jonge KE, Christmas C, Andersen R, et al. Hip fracture service – an interdisciplinary model of care. J Am Geriatr Soc 2001;49(12):1737–8.)
Pre-and postoperative delirium are common in elderly patients with fractures.18 Traditionally, the Confusion Assessment Method (CAM) has been used to diagnose delirium,151 but recently the CAM-ICU has been validated and may be more appropriate for ED-based screening.152,153 Independent preoperative delirium risk factors include cognitive impairment, indoor injury, fever, and prolonged preoperative waiting time. Risk factors for postoperative delirium include cognitive impairment, indoor injury, and body mass index less than 20 kg/m2.24 Most patients who suffer preoperative delirium remain delirious postoperatively.154
Five studies have evaluated interventions to reduce delirium.155,156 Three involved nursing education and routine screening by specially trained nurses.156–158 The Milisen model also focused on postoperative pain management, but failed to reduce the incidence of delirium.158 The Lundström model, on the other hand, focused on regional anesthesia, avoiding hypoxia, and early rehabilitation cooperation between anesthesia, orthopedics, and geriatrics demonstrating a reduction of delirium from 61% to 31%.157 In Lundström and colleagues's156 subsequent evaluation of postoperative care for femoral neck fractures on a geriatric ward versus a conventional orthopedic floor, staff education emphasized comprehensive geriatric education and routine delirium screening with the duration (5 vs 10 days) and incidence (55% vs 75%) of delirium significantly reduced in conjunction with fewer falls, urinary tract infections, or decubitus ulcers. Marcantonio and colleagues159 implemented proactive geriatric consults for patients with hip fracture noting a 77% adherence rate for geriatrician recommendations by the managing orthopedic services, but no reduction in delirium. Gustafson and colleagues160 established an anesthesia and geriatric early collaborative model for femoral neck fractures emphasizing early and routine pre- and postoperative delirium assessments, oxygen therapy, prompt surgical intervention, avoiding hypotension and falls. They demonstrated a reduction in acute confusional states from 61.3% to 47.6% with their intervention along with a reduction in delirium duration, severity, and postoperative complications.
DISPOSITION CONSIDERATIONS
No level I triage criteria exist for geriatric orthopedic trauma.161 Although not every geriatric fracture patient requires hospital admission or immediate operative intervention, the emergency physician must carefully assess older adults for underlying markers of frailty, baseline functional impairments, socioeconomic constraints, and support system. Although ambulatory assist devices such as canes or walkers may promote functional independence, they can also increase subsequent fall risk. Effective analgesia necessary to facilitate ambulation may precipitate orthostatic hypotension or drug-related cognitive dysfunction. Underlying unrecognized dementia may impair outpatient compliance with orthopedic follow-up, rehabilitation, and pharmacologic pain control.162 All of these factors should be considered before ED discharge.163
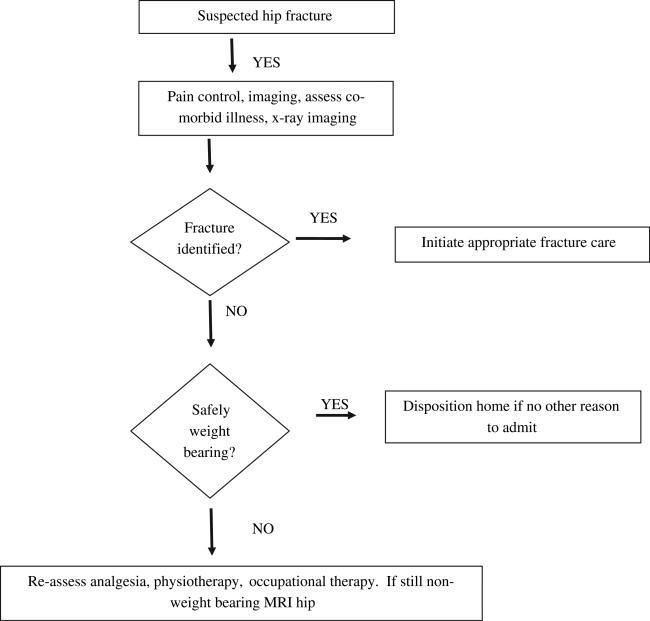
When initial plain film imaging is unremarkable following a standing level fall, the ambulatory patient who cannot bear weight represents a challenge to emergency physicians. In ED and postoperative trauma patients, the prevalence of occult hip fractures (negative radiographs, subsequent fracture diagnosed) has been reported as 2.9% to 4.4%.164–166 In ED settings, the sensitivity of anteroposterior and cross-table lateral projections of the affected hip to identify fractures is 90%, which is lower than in other settings.164,167 These patients are often admitted for pain control, further imaging, and subsequent physiotherapy if no bony injury is identified (Fig. 5).168
Fig. 5.
Pathway for elderly patients with suspected hip fracture. (Data from Smith JE, Jenkin A, Hennessy C. A retrospective chart review of elderly patients who cannot weight bear following a hip injury but whose initial x rays are normal. Emerg Med J 2009;26(1):50–1.)
Several features of the history and physical examination can distinguish older adult trauma patients at increased risk for occult hip fracture. A new inability to bear weight is 73% sensitive for occult fractures in one small series.169 In addition, pain induced with straight leg raise (sensitivity 50%, specificity 45%) and with passive internal and external rotation (sensitivity 61%, specificity 59%) are not adequate to identify patients with occult hip fracture.169
Three imaging modalities are often contemplated when occult hip fracture is suspected. The computerized tomography diagnostic test characteristics for ED patients has not been well described and is generally not supported.167 Bone scan is rarely used by emergency physicians, but has reported sensitivity of 75% to 97.8% and specificity 94% to 95%.170,171 Scintigraphy has several disadvantages including inadequate accessibility and inferior spatial resolution resulting in incomplete fracture identification.167 Magnetic resonance imaging (MRI) is the superior study to identify occult hip fractures with 100% sensitivity, 100% specificity (93% for junior radiologists), and excellent interobserver reproducibility (κ = 0.79).172 MRI also has the advantage of providing alternative diagnoses that may impede weight bearing such as hematoma, muscle tears, degenerative join disease, and osteonecrosis.173 Although MRI is not always readily available, early incorporation of MRI into the ED diagnostic armamentarium for occult hip fracture is cost-effective and can save days in reaching the diagnosis.174,175
SUMMARY
Multidisciplinary orthogeriatric care can enhance prompt ED diagnosis, optimal pre-and postoperative care, and functional recovery in older adults with bony injuries. Emergency care providers should be cognizant of prevalent geriatric syndromes including delirium and standing level falls to minimize fracture-related morbidity. Recognizing the implications of aging physiology, acute care physicians should be aware of effective efficient alternatives to analgesia, procedural sedation, and definitive imaging to promote early surgical management and postoperative recovery.
REFERENCES
- 1.Roberts DC, McKay MP, Shaffer A. Increasing rates of emergency department visits for elderly patients in the United States, 1993 to 2003. Ann Emerg Med. 2008;51:769–74. doi: 10.1016/j.annemergmed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Downing A, Wilson R. Older people's use of accident and emergency services. Age Ageing. 2005;34:24–30. doi: 10.1093/ageing/afh214. [DOI] [PubMed] [Google Scholar]
- 3.McNamara RM, Rousseau E, Sanders AB. Geriatric emergency medicine: a survey of practicing emergency physicians. Ann Emerg Med. 1992;21:796–801. doi: 10.1016/s0196-0644(05)81024-8. [DOI] [PubMed] [Google Scholar]
- 4.Lips P. Epidemiology and predictors of fractures associated with osteoporosis. Am J Med. 1997;103:3S–8S. doi: 10.1016/s0002-9343(97)90021-8. [DOI] [PubMed] [Google Scholar]
- 5.Carter MW, Gupta S. Characteristics and outcomes of injury-related ED visits among older adults. Am J Emerg Med. 2008;26:296–303. doi: 10.1016/j.ajem.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;252:163–6. [PubMed] [Google Scholar]
- 7.Wilber ST, Gerson LW, Terrell KM, et al. Geriatric emergency medicine and the 2006 Institute of Medicine reports from the Committee on the Future of Emergency Care in the U.S. Health System. Acad Emerg Med. 2006;13:1345–51. doi: 10.1197/j.aem.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Committee on the Future of Emergency Care in the U.S. Health System . Hospital-based emergency care: at the breaking point. National Academies Press; Washington, DC: 2006. [Google Scholar]
- 9.Potter JF. The older orthopedic patient. Clin Orthop Relat Res. 2004;425:44–9. doi: 10.1097/01.blo.0000131483.19877.fa. [DOI] [PubMed] [Google Scholar]
- 10.Heyburn G, Beringer T, Elliott J, et al. Orthogeriatric care in patients with fractures of the proximal femur. Clin Orthop Relat Res. 2004;425:35–43. doi: 10.1097/01.blo.0000131492.01669.51. [DOI] [PubMed] [Google Scholar]
- 11.Olofsson B, Stenvall M, Lundstrom M, et al. Malnutrition in hip fracture patients: an intervention study. J Clin Nurs. 2007;16:2027–38. doi: 10.1111/j.1365-2702.2006.01864.x. [DOI] [PubMed] [Google Scholar]
- 12.Duncan DG, Beck SJ, Hood K, et al. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing. 2006;35:148–53. doi: 10.1093/ageing/afj011. [DOI] [PubMed] [Google Scholar]
- 13.Chao EYS, Inoue N, Koo TK, et al. Biomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosis. Clin Orthop Relat Res. 2004;425:12–25. doi: 10.1097/01.blo.0000132263.14046.0c. [DOI] [PubMed] [Google Scholar]
- 14.Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004;425:126–34. [PubMed] [Google Scholar]
- 15.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 16.Karani R, Meier DE. Systemic pharmacologic postoperative pain management in the geriatric orthopaedic patient. Clin Orthop Relat Res. 2004;425:26–34. doi: 10.1097/01.blo.0000132403.53010.6f. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter CR. Evidence based emergency medicine/rational clinical examination abstract: will my patient fall? Ann Emerg Med. 2009;53:398–400. doi: 10.1016/j.annemergmed.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Bruce AJ, Ritchie CW, Blizard R, et al. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr. 2007;19:197–214. doi: 10.1017/S104161020600425X. [DOI] [PubMed] [Google Scholar]
- 19.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 20.Pitkala KH, Laurila JV, Strandberg TE, et al. Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–63. doi: 10.1159/000082888. [DOI] [PubMed] [Google Scholar]
- 21.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39:248–53. doi: 10.1067/mem.2002.122057. [DOI] [PubMed] [Google Scholar]
- 22.Wilber ST, Carpenter CR, Hustey FM. The six-item screener to detect cognitive impairment in older emergency department patients. Acad Emerg Med. 2008;15:613–6. doi: 10.1111/j.1553-2712.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter CR. Does this patient have dementia? Ann Emerg Med. 2008;52:554–6. doi: 10.1016/j.annemergmed.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Juliebo V, Bjoro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354–61. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 25.Feldt KS, Ryden MR, Miles S. Treatment of pain in cognitively impaired compared with cognitively intact older patients with hip-fracture. J Am Geriatr Soc. 1998;46:1079–85. doi: 10.1111/j.1532-5415.1998.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 26.Grimes JP, Gregory PM, Noveck H, et al. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112:702–9. doi: 10.1016/s0002-9343(02)01119-1. [DOI] [PubMed] [Google Scholar]
- 27.Hwang U, Morrison RS. The geriatric emergency department. J Am Geriatr Soc. 2007;55:1873–6. doi: 10.1111/j.1532-5415.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 28.Kocher KE. Public health and aging: nonfatal injuries among older adults treated in hospital emergency departments – United States, 2001. JAMA. 2003;290:2657–8. [PubMed] [Google Scholar]
- 29.Salter AE, Khan KM, Donaldson MG, et al. Community-dwelling seniors who present to the emergency department with a fall do not receive guideline care and their fall risk profile worsens significantly: a 6-month prospective study. Osteoporos Int. 2006;17:672–83. doi: 10.1007/s00198-005-0032-7. [DOI] [PubMed] [Google Scholar]
- 30.Bloch F, Jegou D, Dhainaut JF, et al. Do ED staffs have a role to play in the prevention of repeat falls in elderly patients? Am J Emerg Med. 2009;27:303–7. doi: 10.1016/j.ajem.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter CR, Scheatzle MD, D'Antonio JA, et al. Identification of fall risk factors in older adult emergency department patients. Acad Emerg Med. 2009;16:211–9. doi: 10.1111/j.1553-2712.2009.00351.x. [DOI] [PubMed] [Google Scholar]
- 32.Close J, Ellis M, Hooper R, et al. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–7. doi: 10.1016/S0140-6736(98)06119-4. [DOI] [PubMed] [Google Scholar]
- 33.Baraff LJ, Lee TJ, Kader S, et al. Effect of a practice guideline for emergency department care of falls in elder patients on subsequent falls and hospitalizations for injuries. Acad Emerg Med. 1999;6:1224–31. doi: 10.1111/j.1553-2712.1999.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 34.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;2:CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Barry TB, McNamara RM. Clinical decision rules and cervical spine injury in an elderly patient: a word of caution. J Emerg Med. 2005;29:433–6. doi: 10.1016/j.jemermed.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Bub LD, Blackmore CC, Mann FA, et al. Cervical spine fractures in patients 65 years and older: a clinical prediction rule for blunt trauma. Radiology. 2005;234:143–9. doi: 10.1148/radiol.2341031692. [DOI] [PubMed] [Google Scholar]
- 37.Peddi R, Morley JE. The physiology of aging. In: Meldon SW, Ma OJ, Woolard R, editors. Geriatric emergency medicine. McGraw-Hill; New York: 2004. pp. 4–12. [Google Scholar]
- 38.Evans R. Physiology of aging. In: Sanders AB, editor. Emergency care of the elder person. Beverly Cracom; St. Louis (MO): 1996. pp. 11–28. [Google Scholar]
- 39.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day S, Karpman R. Geriatric orthopedics. In: LoCicero J, Rosenthal RA, Katlic MR, et al., editors. A Supplement to new frontiers in geriatrics research: an agenda for surgical and related medical specialties. American Geriatrics Society; New York: 2007. pp. 289–99. [Google Scholar]
- 41.Ganz DA, Bao Y, Shekelle PG, et al. Will my patient fall? JAMA. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 42.Close JC, Hooper R, Glucksman E, et al. Predictors of falls in a high risk population: results from the prevention of falls in the elderly trial (PROFET). Emerg Med J. 2003;20:421–5. doi: 10.1136/emj.20.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graafmans WC, Ooms ME, Hofstee HM, et al. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–36. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 44.Grisso JA. Prevention of falls in patients with osteoporosis. Rev Rhum Engl Ed. 1997;64:75S–7S. [PubMed] [Google Scholar]
- 45.Sorock GS, Labiner DM. Peripheral neuromuscular dysfunction and falls in an elderly cohort. Am J Epidemiol. 1992;136:584–91. doi: 10.1093/oxfordjournals.aje.a116536. [DOI] [PubMed] [Google Scholar]
- 46.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly person living in the community. N Engl J Med. 1988;319:1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 47.Butler RN, Davis R, Lewis CB, et al. Physical fitness: benefits of exercise for the older patient. 2. Geriatrics. 1998;53:49–52. 61–2. [PubMed] [Google Scholar]
- 48.Evans WJ. Exercise, nutrition, and aging. Clin Geriatr Med. 1995;11:725–34. [PubMed] [Google Scholar]
- 49.Province MA, Hadley EC, Hornbrook MC, et al. The effects of exercise on falls in elderly patients. A preplanned meta-analysis of the FICSIT Trials. Frailty and Injuries: Cooperative Studies of Intervention Techniques. JAMA. 1995;273:1341–7. [PubMed] [Google Scholar]
- 50.Flaherty JH. Who's taking your 5th vital sign? J Gerontol A Biol Sci Med Sci. 2001;56:M397–9. doi: 10.1093/gerona/56.7.m397. [DOI] [PubMed] [Google Scholar]
- 51.Neighbor ML, Honner S, Kohn MA. Factors affecting emergency department opioid administration to severely injured patients. Acad Emerg Med. 2004;11:1290–6. doi: 10.1197/j.aem.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004;43:494–503. doi: 10.1016/j.annemergmed.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Ferrell BA. Pain evaluation and management in the nursing home. Ann Intern Med. 1995;123:681–7. doi: 10.7326/0003-4819-123-9-199511010-00007. [DOI] [PubMed] [Google Scholar]
- 54.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10:591–8. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 55.Herr KA, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med. 2001;17:457–78. doi: 10.1016/s0749-0690(05)70080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sloss EM, Solomon DH, Shekelle PG, et al. Selecting target conditions for quality of care improvement in vulnerable older adults. J Am Geriatr Soc. 2000;48:363–9. doi: 10.1111/j.1532-5415.2000.tb04691.x. [DOI] [PubMed] [Google Scholar]
- 57.Etzioni S, Chodosh J, Ferrell BA, et al. Quality indicators for pain management in vulnerable elders. J Am Geriatr Soc. 2007;55:S403–8. doi: 10.1111/j.1532-5415.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- 58.Terrell KM, Hustey FM, Hwang U, et al. Quality indicators for geriatric emergency care. Acad Emerg Med. 2009;16:441–9. doi: 10.1111/j.1553-2712.2009.00382.x. [DOI] [PubMed] [Google Scholar]
- 59.Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9:19–27. doi: 10.1016/0885-3924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 60.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 61.Duggleby W, Lander J. Patient-controlled analgesia for older adults. Clin Nurs Res. 1992;1:107–13. doi: 10.1177/105477389200100111. [DOI] [PubMed] [Google Scholar]
- 62.Hurley AC, Volicer BJ, Hanrahan PA, et al. Assessment of discomfort in advanced Alzheimer patients. Res Nurs Health. 1992;15:369–77. doi: 10.1002/nur.4770150506. [DOI] [PubMed] [Google Scholar]
- 63.Brochet B, Michel P, Barberger-Gateau P, et al. Population-based study of pain in elderly people: a descriptive study. Age Ageing. 1998;27:279–84. [Google Scholar]
- 64.Magni G, Caldieron C, Rigatti-Luchini S, et al. Chronic musculoskeletal pain and depressive symptoms in the general population. An analysis of the 1st National Health and Nutrition Examination Survey data. Pain. 1990;43:299–307. doi: 10.1016/0304-3959(90)90027-B. [DOI] [PubMed] [Google Scholar]
- 65.Williamson GM, Schulz R. Pain, activity restriction, and symptoms of depression among community-residing elderly adults. J Gerontol. 1992;47:367–72. doi: 10.1093/geronj/47.6.p367. [DOI] [PubMed] [Google Scholar]
- 66.Ferrell BR, Schaffner M. Pharmacoeconomics and medical outcomes in pain management. Semin Anesth. 1997;16:152–9. [Google Scholar]
- 67.Sengstaken EA, King SA. The problems of pain and its detection among geriatric nursing home residents. J Am Geriatr Soc. 1993;41:541–4. doi: 10.1111/j.1532-5415.1993.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 68.Snyder L, Connolly S, Becker B. Pharmacology and adverse drug-related events in elders treated in the emergency department. In: Meldon SW, Ma OJ, Woolard R, editors. Geriatric emergency medicine. McGraw-Hill; New York: 2004. pp. 13–21. Chapter 3. [Google Scholar]
- 69.Philips A, Polisson R, Simon L. NSAIDs and the elderly. Toxicity and economic implications. Drugs Aging. 1997;10:119–30. doi: 10.2165/00002512-199710020-00005. [DOI] [PubMed] [Google Scholar]
- 70.Elseviers M, De Broe M. Analgesic abuse in the elderly. Renal sequelae and management. Drugs Aging. 1998;12:391–400. doi: 10.2165/00002512-199812050-00005. [DOI] [PubMed] [Google Scholar]
- 71.Blanda MP. Pharmacologic issues in geriatric emergency medicine. Emerg Med Clin North Am. 2006;24:449–65. doi: 10.1016/j.emc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241–9. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 73.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310:563–72. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- 74.Deeks DJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ. 2002;325:619. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999;282:1921–8. doi: 10.1001/jama.282.20.1921. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 77.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Arch Intern Med. 1997;152:1531–6. [PubMed] [Google Scholar]
- 78.Bell GM, Schnitzer TJ. Cox-2 inhibitors and other nonsteroidal anti-inflammatory drugs in the treatment of pain in the elderly. Clin Geriatr Med. 2001;17:489–502. doi: 10.1016/s0749-0690(05)70082-3. [DOI] [PubMed] [Google Scholar]
- 79.Rikans LE. Influence of aging on chemically induced hepatotoxicity: role of age-related changes in metabolism. Drug Metab Rev. 1989;20:87–110. doi: 10.3109/03602538908994145. [DOI] [PubMed] [Google Scholar]
- 80.Eriksson LS, Broomé U, Kalin M, et al. Hepatotoxicity due to repeated intake of low doses of paracetamol. J Intern Med. 1992;231:567–70. doi: 10.1111/j.1365-2796.1992.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 81.Kurtovic J, Riordan SM. Paracetamol-induced hepatotoxicity at recommended dosage. J Intern Med. 2003;253:240–3. doi: 10.1046/j.1365-2796.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- 82.Corlett AJ. Aids to compliance with medication. BMJ. 1996;313:926–9. doi: 10.1136/bmj.313.7062.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gloth FM. Principles of perioperative pain management in older adults. Clin Geriatr Med. 2001;17:553–73. doi: 10.1016/s0749-0690(05)70086-0. [DOI] [PubMed] [Google Scholar]
- 84.Ferrell BA. Pain management. In: Hazzard WR, Blass JP, Ettinger WH, editors. Principles of geriatric medicine and gerontology. McGraw-Hill; New York: 1999. pp. 413–33. [Google Scholar]
- 85.Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718–39. [PubMed] [Google Scholar]
- 86.Collins SL, Edwards JE, Moore RA, et al. Single dose dextropropoxyphene, alone and with paracetamol (acetaminophen), for postoperative pain. Cochrane Database Syst Rev. 2000;2:CD001440. doi: 10.1002/14651858.CD001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nickander RC, Emmerson JL, Hynes MD, et al. Pharmacologic and toxic effects in animals of dextropropoxyphene and its major metabolite norpropoxyphene: a review. Hum Toxicol. 1984;3(Suppl):13S–36S. doi: 10.1177/096032718400300103. [DOI] [PubMed] [Google Scholar]
- 88.Ducharme J. Acute pain and pain control: state of the art. Ann Emerg Med. 2000;35:592–603. doi: 10.1016/s0196-0644(00)70033-3. [DOI] [PubMed] [Google Scholar]
- 89.Knopp JA, Diner BM, Blitz M, et al. Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int. 2005;16:1281–90. doi: 10.1007/s00198-004-1798-8. [DOI] [PubMed] [Google Scholar]
- 90.Parker MJ, Griffiths R, Appadu B. Nerve blocks (subcostal, lateral cutaneous, femoral, triple psoas) for hip fractures. Cochrane Database Syst Rev. 2002;1:CD001159. doi: 10.1002/14651858.CD001159. [DOI] [PubMed] [Google Scholar]
- 91.McGlone R, Sadhra K, Hamer DW, et al. Femoral nerve block in the initial management of femoral shaft fractures. Arch Emerg Med. 1987;4:163–8. doi: 10.1136/emj.4.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haddad FS, Williams RL. Femoral nerve block in extracapsular femoral neck fractures. J Bone Joint Surg Br. 1995;77:922–3. [PubMed] [Google Scholar]
- 93.Finlayson BJ, Underhill TJ. Femoral nerve block for analgesia in fractures of the femoral neck. Arch Emerg Med. 1988;5:173–6. doi: 10.1136/emj.5.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mutty CE, Jensen EJ, Manka MA, et al. Femoral nerve block for diaphyseal and distal femoral fractures in the emergency department. J Bone Joint Surg Am. 2007;89:2599–603. doi: 10.2106/JBJS.G.00413. [DOI] [PubMed] [Google Scholar]
- 95.Fletcher AK, Rigby AS, Heyes FL. Three-in-one femoral nerve block as analgesia for fractured neck of femur in the emergency department: a randomized, controlled trial. Ann Emerg Med. 2003;41:227–33. doi: 10.1067/mem.2003.51. [DOI] [PubMed] [Google Scholar]
- 96.Gille J, Gille M, Gahr R, et al. Acute pain management in proximal femoral fractures: femoral nerve block (catheter technique) vs. systemic pain therapy using a clinic internal organisation model. Anaesthesist. 2006;55:414–22. doi: 10.1007/s00101-005-0949-4. [DOI] [PubMed] [Google Scholar]
- 97.Mutty CE, Jensen EJ, Manka MA, et al. Femoral nerve block for diaphyseal and distal femoral fractures in the emergency department. Surgical technique. J Bone Joint Surg Am. 2008;90:218–26. doi: 10.2106/JBJS.H.00314. [DOI] [PubMed] [Google Scholar]
- 98.Marhofer P, Schrögendorfer K, Koinig H, et al. Ultrasonographic guidance improves sensory block and onset time of three-in-one blocks. Anesth Analg. 1997;85:854–7. doi: 10.1097/00000539-199710000-00026. [DOI] [PubMed] [Google Scholar]
- 99.Reid N, Stella J, Ryan M, et al. Use of ultrasound to facilitate accurate femoral nerve block in the emergency department. Emerg Med Australas. 2009;21:124–30. doi: 10.1111/j.1742-6723.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 100.Layzell M. Pain management: setting up a nurse-led femoral nerve block service. Br J Nurs. 2007;16:702–5. doi: 10.12968/bjon.2007.16.12.23717. [DOI] [PubMed] [Google Scholar]
- 101.Vloka JD, Hadzic A, Drobnik L, et al. Anatomical landmarks for femoral nerve block: a comparison of four needle insertion sites. Anesth Analg. 1999;89:1467–70. doi: 10.1097/00000539-199912000-00028. [DOI] [PubMed] [Google Scholar]
- 102.McGee DL. Local and topical anesthesia. In: Roberts JR, Hedges JR, Chanmugam AS, et al., editors. Clinical procedures in emergency medicine. 4th edition Saunders; Philadelphia: 2004. pp. 533–51. [Google Scholar]
- 103.Kendall JM, Allen P, Younge P, et al. Haematoma block or Bier's block for Colles’ fracture reduction in the accident and emergency department – which is best? J Accid Emerg Med. 1997;14:352–6. doi: 10.1136/emj.14.6.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roberts JR. Intravenous regional anesthesia. In: Roberts JR, Hedges JR, Chanmugam AS, et al., editors. Clinical procedures in emergency medicine. 4th edition Saunders; Philadelphia: 2004. pp. 591–5. [Google Scholar]
- 105.Singh GK, Manglik RK, Lakhtakia PK, et al. Analgesia for the reduction of Colles fracture. A comparison of hematoma block and intravenous sedation. Online J Curr Clin Trials. 1992 Doc no. 23. [PubMed] [Google Scholar]
- 106.Dorf E, Kuntz AF, Kelsey J, et al. Lidocaine-induced altered mental status and seizure after hematoma block. J Emerg Med. 2006;31:251–3. doi: 10.1016/j.jemermed.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 107.NIH Consensus Development Panel of Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 108.Dirschl DR, Henderson RC, Oakley WC. Accelerated bone mineral loss following a hip fracture: a prospective longitudinal study. Bone. 1997;21:79–82. doi: 10.1016/s8756-3282(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 109.Gardner MJ, Flik KR, Mooar P, et al. Improvement in the undertreatment of osteoporosis following hip fracture. J Bone Joint Surg Am. 2002;84:1342–8. doi: 10.2106/00004623-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Ross PD. Risk factors for osteoporotic fracture. Endocrinol Metab Clin North Am. 1998;27:289–301. doi: 10.1016/s0889-8529(05)70006-2. [DOI] [PubMed] [Google Scholar]
- 111.Melton LJ, Atkinson EJ, O'Fallon WM, et al. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8:1227–33. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 112.Genant HK, Cooper C, Poor G, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–64. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 113.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 114.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–68. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 115.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016–24. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 117.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 118.Chapuy MC, Arlot ME, Delmas PD, et al. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ. 1994;308:1081–2. doi: 10.1136/bmj.308.6936.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 120.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 121.Chesnut CH, III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 122.Martens MG. Risk of fracture and treatment to prevent osteoporosis-related fracture in postmenopausal women. A review. J Reprod Med. 2003;48:425–34. [PubMed] [Google Scholar]
- 123.Silverman SL. Calcitonin. Endocrinol Metab Clin North Am. 2003;32:273–84. doi: 10.1016/s0889-8529(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 124.Morello KD, Wurz GT, DeGregorio MW. SERMs: current status and future trends. Crit Rev Oncol Hematol. 2002;43:63–76. doi: 10.1016/s1040-8428(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 125.Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 126.Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 127.Reeve J, Meunier PJ, Parsons JA, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. BMJ. 1980;280:1340–4. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jilka RL, Weinstein RS, Bellido T, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506–13. doi: 10.1056/NEJM200011233432101. [DOI] [PubMed] [Google Scholar]
- 130.Kiel DP, Magaziner J, Zimmerman S, et al. Efficacy of a hip protector to prevent hip fracture in nursing home residents: the HIP PRO randomized controlled trial. JAMA. 2007;298:413–22. doi: 10.1001/jama.298.4.413. [DOI] [PubMed] [Google Scholar]
- 131.Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly. Cochrane Database Syst Rev. 2004;3:CD001255. doi: 10.1002/14651858.CD001255.pub2. [DOI] [PubMed] [Google Scholar]
- 132.Barton BA, Birge SJ, Magaziner J, et al. The Hip Impact Protection Project: design and methods. Clin Trials. 2008;5:347–55. doi: 10.1177/1740774508095120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cameron ID, Robinovitch S, Birge SJ, et al. Hip protectors: recommendations for conducting clinical trials – an international consensus statement (part II). Osteoporos Int. 2010;21:1–10. doi: 10.1007/s00198-009-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57–62. doi: 10.1093/ageing/29.1.57. [DOI] [PubMed] [Google Scholar]
- 135.Kaplan RS, Sinaki M, Hameister MD. Effect of back supports on back strength in patients with osteoporosis: a pilot study. Mayo Clin Proc. 1996;71:235–41. doi: 10.4065/71.3.235. [DOI] [PubMed] [Google Scholar]
- 136.Sinaki M, Itoi E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–41. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- 137.Wolf SL, Barnhart HX, Kutner NG, et al. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996;44:489–97. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 138.Fanuele J, Koval KJ, Lurie J, et al. Distal radial fracture treatment: what you get may depend on your age and address. J Bone Joint Surg Am. 2009;91:1313–9. doi: 10.2106/JBJS.H.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koval KJ, Meek R, Schemitsch E, et al. Geriatric trauma: young ideas. J Bone Joint Surg Am. 2003;85:1380–8. [PubMed] [Google Scholar]
- 140.Egol KA, Strauss EJ. Perioperative considerations in geriatric patients with hip fracture: what is the evidence? J Orthop Trauma. 2009;23:386–94. doi: 10.1097/BOT.0b013e3181761502. [DOI] [PubMed] [Google Scholar]
- 141.Radcliff TA, Henderson WG, Stoner TJ, et al. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90:34–42. doi: 10.2106/JBJS.G.00065. [DOI] [PubMed] [Google Scholar]
- 142.Pioli G, Giusti A, Barone A. Orthogeriatric care for the elderly with hip fractures: where are we? Aging Clin Exp Res. 2008;20:113–22. doi: 10.1007/BF03324757. [DOI] [PubMed] [Google Scholar]
- 143.Handoll HH, Cameron ID, Mak JC, et al. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev. 2009;4:CD007125. doi: 10.1002/14651858.CD007125.pub2. [DOI] [PubMed] [Google Scholar]
- 144.Amatuzzi MM, Carelli CD, Leme LEG, et al. Interdisciplinary care in orthogeriatrics: a good cost-benefit model of care. J Am Geriatr Soc. 2003;51:134–6. doi: 10.1034/j.1601-5215.2002.51026.x. [DOI] [PubMed] [Google Scholar]
- 145.Vidan M, Serra JA, Moreno C, et al. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1476–82. doi: 10.1111/j.1532-5415.2005.53466.x. [DOI] [PubMed] [Google Scholar]
- 146.De Jonge KE, Christmas C, Andersen R, et al. Hip fracture service – an interdisciplinary model of care. J Am Geriatr Soc. 2001;49:1737–8. doi: 10.1046/j.1532-5415.2001.49292.x. [DOI] [PubMed] [Google Scholar]
- 147.Gilchrist WJ, Newman RJ, Hamblen DL, et al. Prospective randomised study of an orthopaedic geriatric inpatient service. BMJ. 1988;297:1116–8. doi: 10.1136/bmj.297.6656.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koval KJ, Chen AL, Aharonoff GB, et al. Clinical pathway for hip fractures in the elderly. Clin Orthop Relat Res. 2004;425:72–81. doi: 10.1097/01.blo.0000132266.59787.d2. [DOI] [PubMed] [Google Scholar]
- 149.Pedersen SJ, Borgbjerg FM, Schousboe B, et al. A comprehensive hip fracture program reduces complication rates and mortality. J Am Geriatr Soc. 2008;56:1831–8. doi: 10.1111/j.1532-5415.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 150.Friedman SM, Mendelson DA, Kates SL, et al. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56:1349–56. doi: 10.1111/j.1532-5415.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- 151.Inouye SK, van Dyck CH, Slessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 152.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 153.Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16:193–200. doi: 10.1111/j.1553-2712.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Edlund A, Lundstrom M, Brannstrom B, et al. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49:1335–40. doi: 10.1046/j.1532-5415.2001.49261.x. [DOI] [PubMed] [Google Scholar]
- 155.Bitsch MS, Foss NB, Kristensen BB, et al. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthop Scand. 2004;75:378–89. doi: 10.1080/00016470410001123. [DOI] [PubMed] [Google Scholar]
- 156.Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19:178–86. doi: 10.1007/BF03324687. [DOI] [PubMed] [Google Scholar]
- 157.Lundström M, Edlund A, Lundstrom G, et al. Reorganization of nursing and medical care to reduce the incidence of postoperative delirium and improve rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 1999;13:193–200. [PubMed] [Google Scholar]
- 158.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–32. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 159.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 160.Gustafson Y, Brannstrom B, Berggren D, et al. A geriatric-anesthesiologic program to reduce acute confusion states in elderly patients treated for femoral neck fractures. J Am Geriatr Soc. 1991;39:655–62. doi: 10.1111/j.1532-5415.1991.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 161.Jacobs DG, Plaisier BR, Barie PS, et al. Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma. 2003;54:391–416. doi: 10.1097/01.TA.0000042015.54022.BE. [DOI] [PubMed] [Google Scholar]
- 162.Bryce SN, Han JH. Cognitive impairment and comprehension of emergency department discharge instructions in older patients [abstract #257]. Ann Emerg Med. 2009;54:S80–1. [Google Scholar]
- 163.Siebens H. The domain management model–a tool for teaching and management of older adults in emergency departments. Acad Emerg Med. 2005;12:162–8. doi: 10.1197/j.aem.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 164.Dominguez S, Liu P, Roberts C, et al. Prevalence of traumatic hip and pelvic fractures in patients with suspected hip fracture and negative initial standard radiographs–a study of emergency department patients. Acad Emerg Med. 2005;12:366–9. doi: 10.1197/j.aem.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 165.Pandey R, McNally E, Ali A, et al. The role of MRI in the diagnosis of occult hip fractures. Injury. 1998;29:61–3. doi: 10.1016/s0020-1383(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 166.Lee YP, Griffith JF, Antonio GE, et al. Early magnetic resonance imaging of radiographically occult osteoporotic fractures of the femoral neck. Hong Kong Med J. 2004;10:271–5. [PubMed] [Google Scholar]
- 167.Cannon J, Silverstri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37:144–52. doi: 10.1016/j.jemermed.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 168.Smith JE, Jenkin A, Hennessy C. A retrospective chart review of elderly patients who cannot weight bear following a hip injury but whose initial x rays are normal. Emerg Med J. 2009;26:50–1. doi: 10.1136/emj.2007.056333. [DOI] [PubMed] [Google Scholar]
- 169.Hossain M, Barwick C, Sinha AK, et al. Is magnetic resonance imaging (MRI) necessary to exclude occult hip fracture? Injury. 2007;38:1204–8. doi: 10.1016/j.injury.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 170.Evans PD, Wilson C, Lyons K. Comparison of MRI with bone scanning for suspected hip fracture in elderly patients. J Bone Joint Surg Br. 1994;76:158–9. [PubMed] [Google Scholar]
- 171.Holder LE, Schwarz C, Wernicke PG, et al. Radionuclide bone imaging in the early detection of fractures of the proximal femur (hip): multifactorial analysis. Radiology. 1990;174:509–15. doi: 10.1148/radiology.174.2.2404320. [DOI] [PubMed] [Google Scholar]
- 172.Verbeeten KM, Hermann KL, Hasselqvist M, et al. The advantages of MRI in the detection of occult hip fractures. Eur Radiol. 2005;15:165–9. doi: 10.1007/s00330-004-2421-2. [DOI] [PubMed] [Google Scholar]
- 173.Oka M, Monu JU. Prevalence and patterns of occult hip fractures and mimics revealed by MRI. AJR Am J Roentgenol. 2004;182:283–8. doi: 10.2214/ajr.182.2.1820283. [DOI] [PubMed] [Google Scholar]
- 174.Lubovsky O, Liebergall M, Mattan Y, et al. Early diagnosis of occult hip fractures MRI versus CT scan. Injury. 2005;36:788–92. doi: 10.1016/j.injury.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 175.Rubin SJ, Marquardt JD, Gottlieb RH, et al. Magnetic resonance imaging: a cost-effective alternative to bone scintigraphy in the evaluation of patients with suspected hip fractures. Skeletal Radiol. 1998;27:199–204. doi: 10.1007/s002560050365. [DOI] [PubMed] [Google Scholar]