Graphical abstract
Several (2-oxaadamant-1-yl)amines have been synthesized and their antiviral, NMDA receptor antagonist, and trypanocidal activities have been studied.
Keywords: NMDA, Memantine, Amantadine, Adamantane, NGP1-01, Trypanosoma, Cage compounds
Abstract
The synthesis of several (2-oxaadamant-1-yl)amines is reported. They were evaluated as NMDA receptor antagonists and several of them were more active than amantadine, but none was more potent than memantine. None of the tested compounds displayed antiviral activity. Two of the derivatives showed a significant level of trypanocidal activity.
1. Introduction
1-Adamantylamine (amantadine) and its 3,5-dimethyl analogue, memantine, are NMDA receptor antagonists approved for the treatment of Parkinson’s and Alzheimer’s disease, respectively.1 Amantadine also has prophylactic and therapeutic activities in influenza A virus infections2, and related adamantane derivatives show antiviral activity.3 Moreover, amantadine, memantine, and related polycyclic amines possess trypanocidal properties.4
It is well known in medicinal chemistry that substitution of a methylene unit in a bioactive compound for an oxygen atom usually leads to analogues showing similar activity to the parent compound.5 Indeed, NGP1-01, a brain-permeable, oxa-polycyclic cage amine, is a dual action uncompetitive NMDA receptor antagonist and blocker of L-type calcium-channels. In addition, several experiments have shown that NGP1-01 has neuroprotective activity (Fig. 1 ).6
Figure 1.
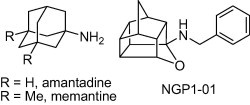
Amantadine, memantine, and NGP1-01 structures.
Based on the interesting and widely observed biological activity of amantadine, memantine, and NGP1-01, we therefore sought to explore the biological profile of (2-oxaadamant-1-yl)amine derivatives.
In this paper, we report the synthesis of several (2-oxaadamant-1-yl)amines and related compounds and their pharmacological evaluation as NMDA receptor antagonists, and antivirals and trypanocidals agents.
2. Results and discussion
2.1. Chemistry
Starting from the known diketone 1,7 we have prepared amines 2−4, 7, 8, and 10−15 using classical methods in amine chemistry (see Scheme 1 ).
Scheme 1.
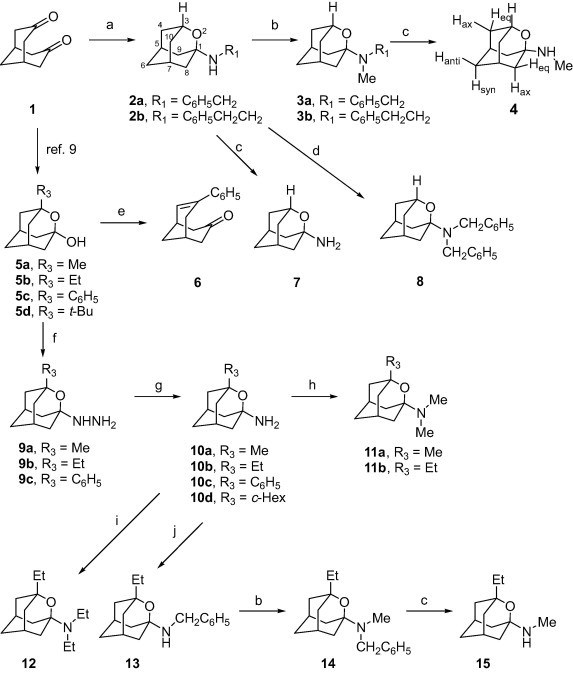
Reagents and conditions: (a) Benzylamine or phenethylamine, anhyd THF, reflux, 30 min; then, LiAlH4, anhyd Et2O, reflux, 6 h; 58% for 2a, 51% for 2b; (b) formaldehyde, NaBH3CN, AcOH, acetonitrile, 4 h; 99% for 3a, 88% for 3b, 86% for 14; (c) H2 (38 atm), 100 °C, Pd/C, EtOH, 24 h; 59% for 4, 70% for 7, 85% for 15; (d) benzyl chloride, K2CO3, NaI, acetonitrile, reflux; 18 h, 85%; (e) acetonitrile, H2SO4, reflux, 18 h; 90%; (f) hydrazine hydrate, concd HCl, reflux, 18 h; 84% for 9a, 45% for 9b, 62% for 9c; (g) H2 (1 atm), PtO2, EtOH, 4 days; 58% for 10a, 48% for 10b, 29% for 10c and 42% for 10d; (h) HCO2H, CH2O 37% aq, 80 °C, 10 h; 51% for 11a, 27% for 11b; (i) acetaldehyde, NaBH3CN, acetic acid, MeOH, 18 h, 73%; (j) benzaldehyde, NaBH3CN, AcOH, acetonitrile, 4 h, 79%.
Secondary amines 2a and 2b were obtained in high yields following a known general procedure that involves reductive amination of diketone 1.8 Thus, reaction of 1 with benzylamine followed by reduction with LiAlH4 led to amine 2a in 58% yield. Similarly, reductive amination of 1 with phenethylamine furnished amine 2b in 51% yield. Reductive alkylation of amines 2a,b with formaldehyde and sodium cyanoborohydride afforded tertiary amines 3a,b in high yields. Hydrogenolysis of the benzyl group of amines 2a and 3a led to 7 and 4, respectively. Finally, alkylation of 2a with benzyl chloride led to 8 in 85% yield.
Alcohols of general structure 5 were prepared using a general method developed some time ago by our group.9 Several attempts to carry out the substitution of the hydroxyl group of 5 by a wide range of amines led to the recovery of the starting material. Moreover, the attempted Ritter reaction of 5c with acetonitrile in acidic medium led to the known enone 6 in high yield.9c However, reaction of 5a–c with aqueous hydrazine led to hydrazines 9a–c in high yields, probably due to the α-effect ensuring a higher nucleophilicity of hydrazine. Even though the reaction failed in the case of 5d.
Catalytic hydrogenation of 9a,b furnished amines 10a,b in high yields. Surprisingly, 9c led to a mixture of the expected amine 10c and the cyclohexyl derivative 10d in the ratio of 3:4. Both products were separated by column chromatography. Reductive methylation of amines 10a and 10b with formaldehyde and sodium cyanoborohydride afforded tertiary amines 11a and 11b, respectively. In a similar way, reductive alkylation of 10b with acetaldehyde or benzaldehyde led to amines 12 and 13, respectively. Finally, secondary amine 15 was synthesized from benzylamine 13 by reductive alkylation followed by catalytic debenzylation in good overall yield.
The structure of all new compounds was confirmed by elemental analysis or by accurate mass measurement, IR, 1H NMR, 13C NMR, and mass spectral data.
2.2. NMDA receptor antagonist activity
In the last years there has been an intensive research on the development of new NMDA receptor antagonists, since this subtype of receptors has been involved in the apoptotic process that develops during neurodegenerative diseases. Memantine is a non-competitive and low affinity blocker that acts selectively on NMDA receptors that is being used in Alzheimer’s disease to slow down its progression.10
The activity of the different (2-oxaadamant-1-yl)amines was assayed on cerebellar granule neurons loaded with the calcium-sensitive probe Fura-2.11 Addition of glutamate or NMDA (100 μM) in the presence of glycine (10 μM) produced a robust and stable increase in intracellular calcium that was challenged with cumulative additions of the compounds to be tested. The data presented in Table 1 indicate that the presence of a substituent in C-3 in the 2-oxaadamantane nucleus is essential for NMDA receptor antagonism. Thus, primary amine 7 and all its N-alkylated and N-dialkylated derivatives (2a,b, 3a,b, 4, and 8) are inactive as it is the case of the methyl derivatives 10a and 11a. Phenyl derivative 10c is only a weak NMDA receptor antagonist while more lipophilic amines 10b and 10d show a similar potency than amantadine. Further enhancement in potency was achieved by alkylation of 10b, the most active derivative being 12, a tertiary amine that is seven times more potent than amantadine, but still ten times less potent than memantine. Contrary to our expectations, all the benzyl derivatives were found to be inactive. This is in striking contrast with NGP1-01 that features a benzyl group essential for NMDA receptor antagonism, and with previous work by our groups that found that benzylated ring-contracted analogues of amantadine showed NMDA receptor antagonism.12
Table 1.
| Compound | Glutamate (100 μM) | NMDA (100 μM) |
|---|---|---|
| 10b | 223 ± 52 | 83 ± 6 |
| 10c | 350 ± 108 | 226 ± 37 |
| 10d | >500 | 94 ± 13 |
| 11b | 106 ± 10 | 55 ± 12 |
| 12 | 22 ± 7 | 14 ± 3 |
| 15 | 108 ± 17 | 32 ± 9 |
| Amantadine | 358 ± 130 | 92 ± 29 |
| Memantine | 55 ± 12 | 1.5 ± 0.1 |
Functional data were obtained from primary cultures of cerebellar granule neurons using the method described in the experimental section by measuring the intracellular calcium concentration. Cells were challenged with glutamate (2nd column) or NMDA (3rd column) as indicated. Data shown are means ± SEM of at least three separate experiments carried out on three different batches of cultured cells.
Compounds 2a, 2b, 3a, 3b, 4, 7, 8, 9a, 10a, 11a, 13, and 14 were found to have low potency as glutamate (IC50 > 500 μM) and NMDA receptor antagonists (IC50 > 200 μM). Hydrazines 9b and 9c were not evaluated.
2.3. Antiviral activity
Primary amines 7, 10c, and 10d and secondary amines 2a, 4, and 15 were tested for antiviral activity. None of the tested compounds displayed activity against the enveloped DNA viruses herpes simplex virus (type 1 or type 2) or vaccinia virus; the enveloped RNA viruses feline coronavirus, parainfluenza-3 virus, respiratory syncytial virus, vesicular stomatitis virus, sindbis virus, or Punta Toro virus; or the non-enveloped RNA viruses Coxsackievirus B4 and Reovirus-1. Also none of the compounds was found to be active against influenza virus A/H1N1, A/H3N2, or B.
2.4. Trypanocidal activity
Protozoan parasites of the Trypanosoma brucei species complex are the causative agents of African trypanosomiasis, a disease that is invariably fatal unless treated. However, current drugs are characterized by toxicity, the need for administration under medical supervision, and in many cases, a lack of efficacy. New drugs to combat this important disease are urgently needed. A range of adamantylamine compounds have trypanocidal activity.4 We therefore investigated whether this property also extended to (2-oxaadamant-1-yl)amine derivatives.
All the new derivatives described in this paper were tested for potency against cultured bloodstream form T. brucei. As was the case with the NMDA receptor antagonism, the presence of a substitution at the C-3 position in the 2-oxadamantane nucleus was found to be essential for trypanocidal activity. Of these compounds, the tertiary amines 11a,b were found to be the most active. Amine 11b had an IC50 = 10.92 ± 0.21 μM and an IC90 = 15.54 ± 1.14 μM, while the trimethyl derivative 11a had an IC50 = 3.97 ± 0.75 μM and an IC90 = 6.82 ± 0.84 μM. Amine 11a was the most potent compound tested, being approximately twice as active as rimantadine (IC50 = 7.04 ± 0.12 μM; IC90 = 13.97 ± 1.68 μM), and at least 30 times more active than amantadine (IC50 > 130 μM). Amines 2a,b, 3a,b, 4, 7, and 8, which lack the C3 substitution, had no activity at concentrations up to 5 mg mL−1. The primary and secondary amines 10a–d, 13, and 15 also displayed no significant trypanocidal properties, even though they do carry a substitution at this position.
Several adamantane derivatives have previously been shown to have trypanocidal properties; however the oxanalogues reported here are the first heteroadamantanes to show significant activity against bloodstream form T. brucei.4
3. Conclusions
In summary, we have synthesized and fully characterized several (2-oxaadamant-1-yl)amines. The presence of a substitution at the C-3 position is essential for NMDA receptor antagonism. Although 11b, 12, and 15 were more potent than amantadine against NMDA-induced calcium increase in cerebellar granule neurons, they were less potent than memantine. In contrast with the model compound NGP1-01, all our benzyl derivatives were devoid of NMDA receptor antagonism activity. None of the tested compounds showed antiviral activity. Two tertiary amines, 11a and 11b, displayed a significant level of trypanocidal activity; 11a was twice as active as rimantadine. The mechanism by which adamantylamine derivatives kill trypanosomes is unknown, but it has been hypothesized by analogy with the known properties of this class of compound that it may involve channel blocking activity.4 The data obtained with the trimethyl amine 11a now provides a basis for exploring if related derivatives have enhanced activity.
The synthesis and pharmacological evaluation of more polycyclic cage amines are in progress.
4. Experimental
4.1. Chemistry
4.1.1. General
Melting points were determined in open capillary tubes. Unless otherwise stated, NMR spectra were recorded in CD3OD in the following spectrometers: 1H NMR (500 MHz), 13C NMR (75.4 MHz). Chemical shifts (δ) are reported in ppm are related to internal tetramethylsilane (TMS). Assignments given for the NMR spectra are based on DEPT, COSY 1H/1H, HETCOR 1H/13C (HSQC and HMBC sequences for one bond and long range 1H/13C heterocorrelations, respectively), and NOESY experiments for selected compounds. For the MS and GC/MS analyses the sample was introduced directly or through a gas chromatograph. For GC/MS analyses, a 30-meter column [5% diphenyl-95% dimethylpolysiloxane, conditions: 10 psi, initial temperature: 35 °C (2 min), then heating at a range of 8 °C/min till 300 °C, then isothermic at 300 °C] was used. The electron impact (70 eV) or chemical ionization (CH4) techniques were used. Only significant ions are given: those with higher relative abundance, except for the ions with higher m/z values. Accurate mass measurements were obtained using ESI technic. Absorption values in the IR spectra (KBr) are given as wave-numbers (cm−1). Only the more intense bands are given. Column chromatography was performed on silica gel 60 Å (35–70 mesh). For the thin layer chromatography (TLC), aluminum-backed sheets with silica gel 60 F254 were used and spots were visualized with UV light and/or 1% aqueous solutions of KMnO4.
4.1.2. N-Benzyl(2-oxaadamant-1-yl)amine hydrochloride, 2a·HCl
To a solution of diketone 1 (6.00 g, 39.4 mmol) in anhyd THF (200 mL), benzylamine (4.29 g, 40.0 mmol) was added and the solution was refluxed for 30 min. After cooling (ice-bath), this solution was added dropwise while stirring vigorously to a suspension of LiAlH4 (3.00 g, 79.0 mmol) in anhyd diethyl ether (80 mL). The mixture was stirred for 6 h at 40 °C and then, while cooling with an ice-bath, 1 N NaOH (19 mL) was added dropwise. The precipitate formed was filtered through Celite®, the clear filtrate was dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give an oily residue (9.29 g). The residue was dissolved in acetone (120 mL), concd HCl (6 mL) was added and the solution was cooled at 4 °C, whereupon 2a precipitated as its hydrochloride. Crystallization from 2-propanol gave pure 2a·HCl (6.39 g, 58% yield), mp 257−259 °C (lit.8b 253−255 °C). IR 2927, 2852, 2712, 2606, 2457, 2408, 2377, 1569, 1456, 1323, 1206, 1194, 1126, 1093, 1008, 994, 754, 739, 695 cm−1. 1H NMR 1.78 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.90 (dquint, J = 14.0 Hz, J′ = 2.5 Hz, 1H, 6-Hsyn), 1.97 (dtt, J = 14.0 Hz, J′ = 2.5 Hz, J″ = 1.5 Hz, 1H, 6-Hanti), 2.01–2.06 [complex signal, 4H, 4(10)-Heq and 8(9)-Hax], 2.14 [dm, J = 11.5 Hz, 2H, 8(9)-Heq], 2.40 [broad s, 2H, 5(7)-H], 4.26 (s, 2H, CH 2–C6H5), 4.39 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H), 7.42–7.49 (complex signal, 3H, Ar-Hmeta and Ar-Hpara) 7.50 (m, 2 H, Ar-Hortho). 13C NMR 29.4 [CH, C5(7)], 35.0 (CH2, C6), 35.1 [CH2, C4(10)], 37.8 [CH2, C8(9)], 45.1 (CH2, CH2–C6H5), 73.6 (CH, C3), 86.6 (C, C1), 130.2 (CH, Cmeta), 130.4 (CH, Cpara), 131.1 (CH, Cortho), 132.9 (C, Cipso). MS (EI), m/z (%): 243 (M• +, 26), 200 (9), 186 (36), 149 (26), 106 (16), 91 (100). Anal. Calcd for C16H21NO·HCl (279.81): C, 68.68; H, 7.92; N, 5.01; Cl, 12.67. Found: C, 68.51; H, 8.10; N, 5.00; Cl, 12.70.
4.1.3. N-(2-Phenylethyl)(2-oxaadamant-1-yl)amine hydrochloride, 2b·HCl
From 1 (3.00 g, 19.7 mmol), phenethylamine (2.55 g, 21.1 mmol) in anhyd THF (100 mL) and following the above procedure, the amine 2b was obtained as its hydrochloride (2.94 g, 51% yield), mp 256–259 °C (2-propanol). IR 2934, 2855, 2721, 2674, 2617, 2419, 1604, 1467, 1455, 1324, 1209, 1192, 1093, 1018, 1001, 784, 725, 698 cm−1. 1H NMR 1.74 [dm, J = 14.0 Hz, 2H, 4(10)-Hax], 1.87 (dquint, J = 13.0 Hz, J′ = 2.5 Hz, 1H, 6-Hsyn), 1.95 (overlapped dm, 1H, 6-Hanti), 1.96–2.03 [complex signal, 4H, 4(10)-Heq and 8(9)-Hax], 2.06 [dm, J = 11.0 Hz, 2H, 8(9)-Heq], 2.38 [broad s, 2H, 5(7)-H], 2.99 (m, 2H, CH 2–C6H5), 3.28 (m, 2H, CH2N), 4.33 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H), 7.27 (tm, J = 7.5 Hz, 1H, Ar-Hpara), 7.29 (dm, J = 7.5 Hz, 2H, Ar-Hortho), 7.35 (tm, J = 7.5 Hz, 2H, Ar-Hmeta). 13C NMR (50.3 MHz) 29.3 [CH, C5(7)], 33.6 (CH2, CH2C6H5), 34.9 (CH2, C6), 35.0 [CH2, C4(10)], 37.7 [CH2, C8(9)], 42.4 (CH2, CH2NH), 73.5 (CH, C3), 86.3 (C, C1), 128.2 (CH, Cpara), 129.7 (CH, Cortho), 130.0 (CH, Cmeta), 137.8 (C, Cipso). MS (EI), m/z (%): 257 (M• +, 1), 200 (10), 167 (12), 166 (100), 137 (54), 105 (22), 104 (27). Anal. Calcd for C17H23NO·HCl (293.84): C, 69.49; H, 8.23; N, 4.77; Cl, 12.07. Found: C, 69.21; H, 8.31; N, 4.71; Cl, 11.98.
4.1.4. N-Benzyl-N-methyl(2-oxaadamant-1-yl)amine, 3a
To a solution of 2a·HCl (838 mg, 3.00 mmol) in acetonitrile (20 mL), formaldehyde (2.36 mL, 37 wt % in water solution, 30 mmol) and NaBH3CN (595 mg, 9.00 mmol) were added. The mixture was stirred for 30 min at room temperature, acetic acid (0.6 mL) was added and the mixture was stirred at room temperature for 2 h. An additional portion of NaBH3CN (595 mg, 9.00 mmol) was added and the mixture was further stirred at room temperature for 2 h. The mixture was concentrated to dryness, 2 N NaOH (30 mL) was added and the suspension was extracted with CH2Cl2 (3 × 45 mL). The combined organic phases were washed with H2O (2 × 30 mL), dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give 3a (765 mg, 99% yield) as a white solid. The analytical sample was obtained by crystallization from CH2Cl2, mp 60−61 °C (dec.). IR 2929, 2897, 2838, 1456, 1442, 1381, 1323, 1190, 994, 972, 957, 856, 747 cm−1. 1H NMR 1.55 [dm, J = 13.5 Hz, 2H, 4(10)-Hax], 1.67 [broad d, J = 12.0 Hz, 2H, 8(9)-Hax], 1.78 (dm, J = 13.5 Hz, 1H, 6-Hsyn), 1.82 (dm, J = 13.5 Hz, 1H, 6-Hanti), 1.90 [dm, J = 13.5 Hz, 2H, 4(10)-Heq], 2.16 [dm, J = 12.0 Hz, 2H, 8(9)-Heq], 2.26 [broad s, 2H, 5(7)-H], 2.29 (s, 3H, CH3–N), 3.81 (s, 2H, CH 2–C6H5), 4.17 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H), 7.19 (tm, J = 7.5 Hz 1H, Ar-Hpara), 7.28 (tm, J = 7.5 Hz, 2H, Ar-Hmeta), 7.32 (dm, J = 7.5 Hz, 2H, Ar-Hortho). 13C NMR (50.3 MHz) 28.5 [CH, C5(7)], 33.8 (CH3, CH3–N), 35.3 (CH2, C6), 35.4 [CH2, C4(10)], 38.4 [CH2, C8(9)], 53.0 (CH2, CH2–C6H5), 70.2 (CH, C3), 85.4 (C, C1), 126.3 (CH, Cpara), 128.07 (CH) and 128.12 (CH) (Cmeta and Cortho), 141.5 (C, Cipso). MS (EI), m/z (%): 257 (M• +, 27), 214 (15), 200 (42), 163 (41), 120 (19), 91 (100). Anal. Calcd for C17H23NO (257.37): C, 79.33; H, 9.01; N, 5.44. Found: C, 79.25; H, 9.11; N, 5.38.
4.1.5. N-Methyl-N-(2-phenylethyl)(2-oxaadamant-1-yl)amine hydrochloride, 3b
To a solution of 2b·HCl (257 mg, 1.00 mmol) in acetonitrile (10 mL), formaldehyde (0.78 mL, 37 wt % in water solution, 10 mmol) and NaBH3CN (188 mg, 3.00 mmol) were added. The mixture was stirred for 30 min at room temperature, acetic acid (0.3 mL) was added and the mixture was stirred at room temperature for 2 h. An additional portion of NaBH3CN (188 mg, 3.00 mmol) was added and the mixture was further stirred at room temperature for 2 h. The mixture was concentrated to dryness, 2 N NaOH (10 mL) was added and the suspension was extracted with CH2Cl2 (3 × 15 mL). The combined organic phases were washed with H2O (2 × 10 mL), dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give 3b (239 mg, 88% yield) as a white solid. Its hydrochloride was obtained by adding an excess of Et2O·HCl to a solution of the amine in EtOAc. The analytical sample of 16·HCl was obtained by crystallization from EtOAc, mp 211−212 °C. IR 2956, 2915, 2855, 2596, 2417, 1481, 1467, 1454, 1411, 1376, 1210, 1086, 1027, 996, 749, 699 cm−1. 1H NMR (3b free base) 1.54 [dm, J = 13.0 Hz, 2H, 4(10)-Hax], 1.59 [dm, J = 12.0 Hz, 2H, 8(9)-Hax], 1.74 (dquint, J = 13.0 Hz, J′ = 2.0 Hz, 2H, 6-Hsyn), 1.80 (dquint, J = 13.0 Hz, J′ = 2.0 Hz, 2H, 6-Hanti), 1.88 [dm, J = 13.0 Hz, 2H, 4(10)-Heq], 2.07 [dm, J = 12.0 Hz, 2H, 8(9)-Heq], 2.23 [broad s, 2H, 5(7)-H], 2.47 (s, 3H, CH3–N), 2.79 (m, 2H, CH 2–C6H5), 2.89 (m, 2H, CH2–N), 4.14 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H), 7.18 (tm, J = 7.5 Hz, 1H, Ar-Hpara), 7.20 (dm, J = 7.5 Hz, 2H, Ar-Hortho), 7.27 (tm, J = 7.5 Hz, 2H, Ar-Hmeta). 13C NMR (50.3 MHz) (3b free base) 28.4 [CH, C5(7)], 34.0 (CH3, CH3–N), 35.3 (CH2, C6), 35.4 [CH2, C4(10)], 36.1 (CH2, CH2C6H5), 38.0 [CH2, C8(9)], 51.6 (CH2, CH2N), 69.9 (CH, C3), 85.4 (C, C1), 125.7 (CH, Ar-Cpara), 128.2 (CH) and 128.7 (CH) (Ar-Cortho and Ar-Cmeta), 140.9 (C, Ar-Cipso). MS (EI), m/z (%): 228 (2), 214 (2), 181 (13), 180 ([M−CH2C6H5]+, 100), 137 (49). Anal. Calcd for C18H25NO·HCl (307.86): C, 70.23; H, 8.51; N, 4.55; Cl, 11.52. Found: C, 70.19; H, 8.59; N, 4.54; Cl, 11.80.
4.1.6. N-Methyl(2-oxaadamant-1-yl)amine hydrochloride, 4·HCl
A mixture of 3a (765 mg, 2.97 mmol) and 10% Pd/C (50% in water, 200 mg) in absolute EtOH (80 mL) was hydrogenated at 38 atm and 100 °C for 24 h. The suspension was filtered, the residue was washed with EtOH, and to the combined organic filtrates an excess of Et2O·HCl was added. Evaporation of the solvents from the filtrate in vacuo followed by crystallization from MeOH/Et2O gave 4·HCl (357 mg, 59% yield), mp 226–230 °C. IR 2928, 2856, 2750, 2694, 2416, 2372, 1467, 1209, 1157, 1097, 1078, 1023, 998 cm−1. 1H NMR 1.75 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.88 (dquint, J = 13.0 Hz, J′ = 2.5 Hz, 1H, 6-Hsyn), 1.95 (overlapped dm, 1H, 6-Hanti), 1.97 [overlapped dm, 4H, 8(9)-Heq and 8(9)-Hax], 1.99 [overlapped dm, 2H, 4(10)-Heq], 2.39 [broad s, 2H, 5(7)-H], 2.64 (s, 3H, CH3–N). 4.33 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H). 13C NMR (50.3 MHz) 25.5 (CH3, CH3–N), 29.2 [CH, C5(7)], 34.9 (CH2, C6), 35.0 [CH2, C4(10)], 37.4 [CH2, C8(9)], 73.4 (CH, C3), 85.6 (C, C1). MS (CI, CH4), m/z (%): 169 (18), 168 ([M+H]+, 51), 167 (20), 125 (30), 112 (21), 111 (100), 110 (44), 75 (23), 74 (79), 73 (44), 72 (48), 59 (32). Anal. Calcd for C10H17NO·1.05HCl·0.25H20 (210.04): C, 57.18; H, 8.90; N, 6.67; Cl, 17.72. Found: C, 57.36; H, 8.77; N, 6.76; Cl, 17.65.
4.1.7. (2-Oxaadamant-1-yl)amine hydrochloride, 7·HCl
A mixture of 2a·HCl (2.20 g, 7.87 mmol) and 10% Pd/C (50% in water, 100 mg) in absolute EtOH (300 mL) was hydrogenated at 38 atm and 100 °C for 24 h. The suspension was filtered, the residue was washed with EtOH, and the combined organic filtrates were concentrated in vacuo to give a solid. 2 N NaOH (25 mL) was added to the residue which was then extracted with EtOAc (3 × 25 mL). The combined organic extracts were dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give a residue that was sublimed at 60 °C/2 Torr to give amine 7. Its hydrochloride (1.05 g, 70% yield) was obtained by adding an excess of a solution of HCl in MeOH to the amine, followed by concentration in vacuo. The analytical sample of 7·HCl was obtained by crystallization from MeOH, mp > 218 °C (dec.). IR 3034, 2945, 2851, 2789, 2744, 2697, 2631, 2563, 1578, 1502, 1384, 1359, 1329, 1304, 1211, 1156, 1016, 996 cm−1. 1H NMR 1.74 [d, J = 13.0 Hz, 2H, 4(10)-Hax], 1.86 (dquint, J = 13.5 Hz, J′ = 2.5 Hz, 1H, 6-Hsyn), 1.95 (overlapped dm, 1H, 6-Hanti), 1.96 [s, 4H, 8(9)-Hax and 8(9)-Heq], 1.98 [overlapped dm, 2H, 4(10)-Heq], 2.35 [broad s, 2H, 5(7)-H], 4.28 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H). 13C NMR 29.2 [CH, C5(7)], 34.7 (CH2, C6), 35.0 [CH2, C4(10)], 39.5 [CH2, C8(9)], 73.0 (CH, C3), 82.3 (C, C1). MS (EI), m/z (%): 153 (M• +, 30), 136 (10), 110 (25), 96 (100), 95 (17), 94 (29), 85 (29), 67 (29), 60 (37), 59 (68), 57 (76). Anal. Calcd for C9H15NO·HCl (189.68): C, 56.99; H, 8.50; N, 7.38; Cl, 18.69. Found: C, 57.08; H, 8.61; N, 7.22; Cl, 18.54.
4.1.8. N,N-Dibenzyl(2-oxaadamant-1-yl)amine, 8
A suspension of 2a·HCl (280 mg, 1.00 mmol), K2CO3 (690 mg, 5.00 mmol), benzyl chloride (0.14 mL, 1.25 mmol), and NaI (50 mg, 0.33 mmol) in acetonitrile (10 mL) was heated under reflux for 18 h. To the cold mixture, CH2Cl2 (20 mL) was added and the solution was washed with water (2 × 20 mL). The organic layer was dried with anhyd Na2SO4, filtered, and concentrated in vacuo. The residue was crystallized from EtOAc to give amine 8 (283 mg, 85% yield), mp 155–157 °C. IR 2932, 2922, 2851, 1600, 1493, 1449, 1382, 1321, 1198, 1158, 1122, 986, 959, 864, 746, 736, 699 cm−1. 1H NMR 1.54 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.59 [dm, J = 12.0 Hz, 2H, 8(9)-Hax], 1.72 (broad d, J = 12.5 Hz, 1H, 6-Hsyn), 1.76 (broad d, J = 12.5 Hz, 1H, 6-Hanti), 1.90 (dm, J = 12.5 Hz, 2H, 4(10)-Heq], 2.14 [dm, J = 12.0 Hz, 2H, 8(9)-Heq], 2.18 [broad s, 2H, 5(7)-H], 4.01 (s, 4H, CH 2–C6H5), 4.21 (broad s, 1H, 3-H), 4.86 (broad signal, mobile H), 7.12 (t, J = 7.5 Hz, 2H, Ar-Hpara), 7.20 (t, J = 7.5 Hz, 4H, Hmeta), 7.30 (d, J = 7.5 Hz, 4H, Hortho). 13C NMR 28.5 [CH, C5(7)], 35.2 (CH2, C6), 35.3 [CH2, C4(10)], 40.2 [CH2, C8(9)], 51.9 (CH2, CH2–C6H5), 70.2 (CH, C3), 86.4 (C, C1), 126.1 (CH, Cpara), 127.84 (CH) and 127.88 (CH) (Cmeta and Cortho), 142.2 (C, Cipso). MS (EI), m/z (%): 333 (M• +, 11), 276 (11), 242 (20), 148 (15), 106 (36), 91 (100). Anal. Calcd for C23H27NO (333.47): C, 82.84; H, 8.16; N, 4.20. Found: C, 82.59; H, 8.19; N, 4.12.
4.1.9. (3-Methyl-2-oxaadamant-1-yl)hydrazine hydrochloride, 9a·HCl
A mixture of alcohol 5a (10.5 g, 62.5 mmol), hydrazine hydrate (68.5 mL, 98% aq solution, 1.38 mol), and concd HCl (2.2 mL) was refluxed for 18 h. The suspension was cooled (ice-bath) and the solid hydrazine was filtered off and dried under reduced pressure. Its hydrochloride (11.5 g, 84% yield) was obtained by adding an excess of Et2O·HCl to a solution of the hydrazine in EtOAc (10 mL). The analytical sample of 9a·HCl was obtained by crystallization from MeOH/Et2O, mp 181−183 °C. IR 3180, 2923, 2681, 1690, 1611, 1528, 1509, 1497, 1383, 1106, 1077, 943, 839 cm−1. 1H NMR 1.16 (s, 3H, CH3–C3), 1.60 [dm, J = 13.5 Hz, 2H, 4(10)-Hax], 1.63 [overlapped dm, 2H, 4(10)-Heq], 1.66 [overlapped dm, J = 12.5 Hz, 2H, 8(9)-Hax], 1.74 [dm, J = 12.5 Hz, 2H, 8(9)-Heq], 1.79 [complex signal, 2H, 6-Hanti and 6-Hsyn], 2.31 [m, 2H, 5(7)-H], 4.86 (s, mobile H). 13C NMR 29.0 (CH3, C3-CH3), 29.7 [CH, C5(7)], 34.9 (CH2, C6), 37.5 [CH2, C8(9)], 41.4 [CH2, C4(10)], 74.8 (C, C3), 84.3 (C, C1). MS (EI), m/z (%): 183 (12), 182 (M• +, 100), 167 (16), 164 (22), 151 (47), 125 (43), 109 (38), 107 (31), 100 (22), 96 (20), 95 (34), 93 (94), 91 (26), 81 (35), 79 (25), 77 (22), 74 (30), 72 (47), 67 (31), 55 (31). Accurate mass measurement (ESI+) calcd for [C10H18N2O+H]+: 183.1491. Found: 183.1493.
4.1.10. (3-Ethyl-2-oxaadamant-1-yl)hydrazine hydrochloride, 9b·HCl
From 5b (5.60 g, 30.7 mmol), hydrazine hydrate (33.5 mL, 98% aq solution, 0.68 mol), and concentrated HCl (1.1 mL) and following the procedure described for 9a, the hydrazine 9b was obtained as its hydrochloride (3.20 g, 45% yield). The analytical sample of 9b·HCl was obtained by crystallization from 2-propanol/hexane, mp 199−200 °C. IR 3176, 2914, 2852, 2682, 1609, 1529, 1515, 1442, 1383, 1206, 1138, 1079, 978, 956, 942, 839 cm−1. 1H NMR 0.91 (t, J = 7.5 Hz, 3H, CH 3–CH2), 1.50 (q, J = 7.5 Hz, 2H, CH3–CH 2), 1.58 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.62 [dm, J = 12.5 Hz, 2H, 4(10)-Heq], 1.67 [dm, J = 11.5 Hz, 2H, 8(9)-Hax], 1.74 [dm, J = 11.5 Hz, 2H, 8(9)-Heq], 1.81 [complex signal, 2H, 6-Hanti and 6-Hsyn], 2.33 [m, 2H, 5(7)-H], 4.86 (s, mobile H). 13C NMR 7.4 (CH3, CH2 CH3), 29.6 [CH, C5(7)], 35.3 (CH2, C6), 35.4 (CH2, CH2CH3), 37.8 [CH2, C8(9)], 38.9 [CH2, C4(10)], 77.0 (C, C3), 84.2 (C, C1). MS (EI), m/z (%): 196 (M• +, 100), 178 (16), 167 (55), 165 (50), 125 (42), 107 (61), 95 (60), 93 (28), 91 (25), 81 (34), 79 (71), 74 (38), 72 (48), 67 (30), 57 (27), 55 (33). Accurate mass measurement (ESI+) calcd for [C11H20N2O+H]+: 197.1648. Found: 197.1644.
4.1.11. (3-Phenyl-2-oxaadamant-1-yl)hydrazine hydrochloride, 9c·HCl
From 5c (1.85 g, 8.04 mmol), hydrazine hydrate (8.8 mL, 98% aq solution, 0.18 mol), and concentrated HCl (0.3 mL) and following the procedure described for 9a, the hydrazine 9c was obtained as its hydrochloride (1.39 g, 62% yield). The analytical sample of 9b·HCl was obtained by crystallization from methanol, mp 203−204 °C. IR 3219, 2948, 2851, 2669, 1573, 1514, 1209, 996, 865, 754, 702 cm−1. 1H NMR 1.78 [dm, J = 12.5 Hz, 2H, 8(9)-Hax], 1.85 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.86–1.92 [complex signal, 3H, 8(9)-Heq and 6-Hanti], 1.98 (dm, J = 13.5 Hz, 1H, 6-Hsyn), 2.07 [dm, J = 12.5 Hz, 2H, 4(10)-Heq], 2.45 [m, 2H, 5(7)-H], 4.86 (s, mobile H), 7.22 (tm, 1H, J = 7.5 Hz, Ar-Hpara), 7.32 (tm, J = 7.5 Hz, 2H, Ar-Hmeta), 7.52 (dm, J = 7.5 Hz, 2H, Ar-Hortho). 13C NMR (100.6 MHz) 30.0 [CH, C5(7)], 34.8 (CH2, C6), 37.6 [CH2, C8(9)], 41.7 [CH2, C4(10)], 78.1 (C, C3), 85.1 (C, C1), 125.3 (CH, Ar-Cortho), 127.9 (CH, Ar-Cpara), 129.1 (CH, Ar-Cmeta), 147.9 (C, Ar-Cipso). MS (EI), m/z (%): 245 (14), 244 (M+, 76), 213 (32), 171 (13), 169 (15), 156 (17), 155 (100), 129 (20), 125 (31), 105 (23), 95 (29), 91 (34), 77 (37), 72 (29). Anal. Calcd for C15H20N2O·HCl (280.80): C, 64.16; H, 7.54; N, 9.98; Cl, 12.63. Found: C, 64.03; H, 7.42; N, 9.86; Cl, 12.51.
4.1.12. (3-Methyl-2-oxaadamant-1-yl)amine hydrochloride, 10a·HCl
A mixture of 9a·HCl (6.70 g, 30.6 mmol) and PtO2 (20 mg) in absolute EtOH (200 mL) was hydrogenated at 1 atm and room temperature for 4 days. The suspension was filtered, the residue was washed with absolute EtOH, and the combined organic filtrates were concentrated in vacuo to dryness. The obtained white residue was taken in water (100 mL), the solution was basified with 2 N NaOH, and was extracted with EtOAc (5 × 80 mL). The combined organic extracts were dried with anhyd Na2SO4, and concentrated in vacuo to dryness. The residue was taken in the minimum amount of AcOEt and the solution was treated with an excess of a solution of HCl in Et2O. The precipitate was filtered and washed with Et2O to give 10a·HCl (3.60 g, 58% yield). The analytical sample of 10a·HCl was obtained by crystallization from MeOH, mp 268−269 °C. IR 2966, 2924, 2852, 1582, 1516, 1379, 1235, 1060, 1038, 1005 cm−1. 1H NMR 1.18 (s, 3H, CH 3–C3), 1.66 [dm, J = 14.0 Hz, 2H, 4(10)-Hax], 1.70 [dm, J = 14.0 Hz, 2H, 4(10)-Heq], 1.81 [complex signal, 2H, 6-Hanti and 6-Hsyn], 1.85 [dm, J = 11.5 Hz, 2H, 8(9)-Heq], 1.90 [dd, J = 11.5 Hz, J′ = 2.5 Hz, 2H, 8(9)-Hax], 2.38 [broad s, 2H, 5(7)-H], 4.86 (s, mobile H). 13C NMR 28.9 (CH3, C3–CH3), 29.8 [CH, C5(7)], 34.0 (CH2, C6), 38.9 [CH2, C8(9)], 40.9 [CH2, C4(10)], 75.8 (C, C3), 83.2 (C, C1). MS (EI), m/z (%): 168 (9), 167 (M• +, 73), 152 (33), 150 (27), 124 (27), 110 (100), 109 (37), 108 (68), 106 (21), 94 (28), 93 (49), 85 (72), 81 (37), 67 (22), 60 (21), 59 (47), 57 (66). Anal. Calcd for C10H17NO·HCl·0.25H2O (208.22): C, 57.69; H, 8.96; N, 6.73; Cl, 17.03. Found: C, 57.78; H, 8.92; N, 7.01; Cl, 17.34.
4.1.13. (3-Ethyl-2-oxaadamant-1-yl)amine hydrochloride, 10b·HCl
From 9b·HCl (2.00 g, 8.60 mmol) and PtO2 (5 mg) in absolute EtOH (200 mL) and following the procedure described for 10a, the amine 10b was obtained as its hydrochloride (900 mg, 48% yield). The analytical sample of 10b·HCl was obtained by crystallization from MeOH, mp 218−219 °C. IR 2934, 2854, 1588, 1507, 1461, 1377, 1345, 1302, 1279, 1057, 1015, 988 cm−1. 1H NMR 0.91 (t, J = 7.8 Hz, 3H, CH 3CH2), 1.48 (q, J = 7.8 Hz, 2H, CH3CH2), 1.62 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.70 [dm, J = 12.5 Hz, 2H, 4(10)-Heq], 1.82 [complex signal, 2H, 6-Hanti and 6-Hsyn], 1.85 [dm, J = 11.5 Hz, 2H, 8(9)-Heq], 1.91 [dd, J = 11.5 Hz, J′ = 2.5 Hz, 2H, 8(9)-Hax], 2.39 [broad s, 2H, 5(7)-H], 4.86 (s, mobile H). 13C NMR 7.2 (CH3, CH3CH2), 29.7 [CH, C5(7)], 34.4 (CH2, C6), 35.3 (CH2, CH3 CH2), 38.5 [CH2, C4(10)], 39.1 [CH2, C8(9)], 77.9 (C, C3), 83.2 (C, C1). MS (EI), m/z (%): 182 (15), 181 (M• +, 76), 166 (11), 164 (31), 152 (79), 124 (39), 123 (37), 122 (69), 120 (20), 110 (100), 95 (35), 94 (55), 93 (63), 85 (81), 81 (33), 59 (43), 57 (82). Anal. Calcd for C11H19NO·HCl (217.74): C, 60.68; H, 9.26; N, 6.43; Cl, 16.28. Found: C, 60.78; H, 9.43; N, 6.44; Cl, 16.26.
4.1.14. (3-Phenyl-2-oxaadamant-1-yl)amine hydrochloride, 10c·HCl and (3-cyclohexyl-2-oxaadamant-1-yl)amine hydrochloride, 10d·HCl
From 9c·HCl (1.30 g, 4.63 mmol) and PtO2 (5 mg) in absolute EtOH (60 mL) and following the procedure described for 10a, a mixture of amines 10c and 10d was obtained as their hydrochlorides. The mixture was diluted with water (25 mL) and then 2 N NaOH was added till basic pH. The suspension was extracted with EtOAc (5 × 10 mL). The combined organic phases were dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give a mixture of 10c and 10d. Column chromatography of this mixture (silica gel, hexane/EtOAc mixtures) gave amine 10d (hexane/EtOAc, 8/2, 454 mg, 43% yield) and amine 10c (hexane/EtOAc, 6/4, 311 mg, 29% yield). Their hydrochlorides were obtained by adding excess of a solution of HCl in Et2O to a solution of the corresponding amine in EtOAc. 10c·HCl, mp 254−260 °C (dec.). IR 2920, 2859, 1502, 1232, 1028, 758, 699 cm−1. 1H NMR 1.86−1.92 [complex signal, 3H, 4(10)-Hax and 6-Hanti], 1.98–2.05 [complex signal, 5H, 8(9)-Hax, 8(9)-Heq and 6-Hsyn], 2.14 [dd, J = 12.7 Hz, J′ = 2.5 Hz, 2H, 4(10)-Hec], 2.51 [m, 2H, 5(7)-H], 4.86 (s, mobile H), 7.24 (tt, J = 7.5 Hz, J′ = 1.5 Hz, 1H, Ar-Hpara), 7.33 (tm, J = 7.5 Hz, 2H, Ar-Hmeta), 7.46 (dm, J = 7.5 Hz, 2H, Ar-Hortho). 13C NMR (100.6 MHz) 30.1 [CH, C5(7)], 33.9 (CH2, C6), 39.0 [CH2, C8(9)], 41.3 [CH2, C4(10)], 79.0 (C, C3), 83.9 (C, C1), 125.0 (CH, Ar-Cortho), 128.1 (CH, Ar-Cpara), 129.2 (CH, Ar-Cmeta), 147.2 (C, Ar-Cipso). MS (EI), m/z (%): 230 (18), 229 (M• +,100), 212 (26), 170 (36), 155 (24), 129 (41), 110 (78), 105 (28), 91 (23), 77 (38), 57 (40). Anal. Calcd for C15H19NO·HCl (265.78): C, 67.79; H, 7.58; N, 5.27; Cl, 13.34. Found: C, 67.60; H, 7.70; N, 5.14; Cl, 13.37; 10d·HCl, mp 255−256 °C. IR 2930, 2914, 2852, 1495, 1227, 1060, 1019 cm−1. 1H NMR 1.04 [dq, J = 2.5 Hz, J′ = 12.5 Hz, 2H, 2′(6′)-Hax], 1.15 [overlapped tq, J = 3.5 Hz, J′ = 13.0 Hz, 1H, 4′-Hax), 1.15–1.25 [overlapped m, 2H, 3′(5′)-Hax], 1.30 [overlapped tt, J = 12.5 Hz, J′ = 3.0 Hz, 1H, 1′-H], 1.65−1.70 [complex signal, 5H, 4′-Heq, 4(10)-Hax and 4(10)-Heq], 1.77−1.92 [complex signal, 10H, 2′(6′)-Heq, 3′(5′)-Heq, 6-Hanti, 6-Hsyn, 8(9)-Hax and 8(9)-Heq], 2.39 [broad s, 2H, 5(7)-H], 4.86 (s, mobile H). 13C NMR (100.6 MHz) 27.3 [CH2, C2′(6′)], 27.7 [CH2, C4′ and C3′(5′)], 29.8 [CH, C5(7)], 34.7 (CH2, C6), 36.5 [CH2, C4(10)], 39.3 [CH2, C8(9)], 49.3 (CH, C1′), 80.0 (C, C3), 83.2 (C, C1). MS (EI), m/z (%): 235 (M• +,28), 218 (11), 177 (12), 176 (60), 153 (18), 152 (100), 110 (56), 94 (38). Anal. Calcd for C15H25NO·HCl (271.83): C, 66.28; H, 9.64; N, 5.15; Cl, 13.04. Found: C, 66.67; H, 9.95; N, 5.01; Cl, 12.63.
4.1.15. N,N-Dimethyl(3-methyl-2-oxaadamant-1-yl)amine hydrochloride, 11a·HCl
To a cold (0 °C) solution of 10a (410 mg, 2.45 mmol) in Et2O (8 mL), formaldehyde (4.85 mL, 37 wt % in water solution, 61 mmol) and formic acid (3.8 mL, 98 mmol) were added dropwise, and the mixture was stirred at 80 °C for 10 h. The mixture was allowed to cool to room temperature, it was diluted with Et2O (15 mL), 5 N NaOH (5 mL) was added dropwise, and the suspension was stirred at room temperature for 15 min. The organic layer was separated and the aqueous phase was extracted with Et2O (4 × 25 mL). The combined organic phases were dried with anhyd Na2SO4, filtered, and an excess of a solution of HCl in Et2O was added. Concentration in vacuo of this solution gave 11a·HCl. The analytical sample of 11a·HCl (300 mg, 51% yield) was obtained by crystallization from MeOH/Et2O, mp 174−175 °C. IR 2963, 2912, 2856, 2654, 2556, 2519, 2458, 1488, 1471, 1450, 1438, 1410, 1378, 1240, 1155, 1033, 1021, 916 cm−1. 1H NMR 1.22 (s, 3H, CH3–C3), 1.69 [overlapped dm, 2H, 4(10)-Hax], 1.71 [overlapped dm, 2H, 4(10)-Heq], 1.82 [complex signal, 2H, 6-Hanti and 6-Hsyn], 1.85 [dm, J = 11.0 Hz, 2H, 8(9)-Heq], 2.05 [dd, J = 11.0 Hz, J′ = 2.0 Hz, 2H, 8(9)-Hax], 2.46 [m, 2H, 5(7)-H], 2.83 [s, 6H, (CH3)2N]. 13C NMR 28.7 (CH3, C3–CH3), 30.2 [CH, C5(7)], 34.1 (CH2, C6), 34.4 [CH2, C8(9)], 36.9 [CH3, (CH3)2N], 40.7 [CH2, C4(10)], 77.2 (C, C3), 91.8 (C, C1). MS (EI), m/z (%): 196 (10), 195 (M• +, 76), 180 (24), 152 (17), 138 (81), 122 (18), 113 (35), 109 (17), 98 (21), 88 (17), 87 (100), 85 (32), 72 (34). Anal. Calcd for C12H21NO·HCl·0.5H2O (240.77): C, 59.86; H, 9.63; N, 5.82; Cl, 14.72. Found: C, 60.04; H, 9.32; N, 5.88; Cl, 14.73.
4.1.16. N,N-Dimethyl(3-ethyl-2-oxaadamant-1-yl)amine hydrochloride, 11b·HCl
To a cold (0 °C) solution of 10b (300 mg, 1.65 mmol) in Et2O (5 mL), formaldehyde (3.5 mL, 37 wt % in water solution, 42.7 mmol) and formic acid (2.85 mL, 74 mmol) were added dropwise, and the mixture was stirred at 80 °C for 10 h. The cold mixture was diluted with Et2O (15 mL), 5 N NaOH (5 mL) was added dropwise, and the suspension was stirred at room temperature for 15 min. The organic layer was separated and the aqueous phase was extracted with Et2O (4 × 15 mL). The combined organic phases were dried with anhyd Na2SO4, filtered, and treated with an excess of Et2O·HCl. Concentration of the above mixture in vacuo gave 11b·HCl. The analytical sample of 11b·HCl (110 mg, 27% yield) was obtained by crystallization from MeOH/Et2O, mp 129−130 °C. IR 2963, 2934, 2859, 2572, 2376, 1467, 1438, 1378, 1297, 1150, 1040, 1022, 972, 938 cm−1. 1H NMR 0.93 (t, J = 7.5 Hz, 3H, CH 3CH2), 1.53 (q, J = 7.5 Hz, 2H, CH3CH 2), 1.64 [ddm, J = 13.0 Hz, J′ = 2.0 Hz, 2H, 4(10)-Hax], 1.73 [broad d, J = 13.0 Hz, 2H, 4(10)-Heq], 1.84 [overlapped signal, 2H, 6-Hanti and 6-Hsyn], 1.85 [overlapped broad d, 2H, 8(9)-Heq], 2.05 [broad dd, J = 11.5 Hz, J′ = 2.5 Hz, 2H, 8(9)-Hax], 2.48 [broad s, 2H, 5(7)-H], 2.83 [s, 6H, (CH3)2N], 4.85 (s, mobile H). 13C NMR 7.2 (CH3, CH3CH2), 30.1 [CH, C5(7)], 34.6 [CH2, C6 and C8(9)], 35.1 (CH2, CH3 CH2), 36.8 [CH3, (CH3)2N], 38.4 [CH2, C4(10)], 79.3 (C, C3), 91.8 (C, C1). MS (EI), m/z (%): 209 (M• +, 60), 180 (59), 152 (25), 138 (39), 122 (27), 113 (33), 88 (16), 87 (100), 72 (25). Accurate mass measurement (ESI+) calcd for [C13H23NO+H]+: 210.1852. Found: 210.1859.
4.1.17. N,N-Diethyl(3-ethyl-2-oxaadamant-1-yl)amine hydrochloride, 12·HCl
To a solution of 10b·HCl (350 mg, 1.60 mmol) in methanol (20 mL), NaBH3CN (95%, 200 mg, 3.20 mmol), AcOH (0.6 mL), and acetaldehyde (0.56 mL, 9.6 mmol) were added and the mixture was stirred at room temperature for 2 h. An additional portion of NaBH3CN (95%, 100 mg, 1.60 mmol) and acetaldehyde (0.26 mL, 4.8 mmol) was added, the mixture was stirred at room temperature for 16 h, and then it was concentrated in vacuo to dryness. Water (30 mL) was added to the residue, the suspension was basified with NaHCO3 (saturated aqueous solution), and was extracted with EtOAc (3 × 15 mL). The combined organic extracts were dried with anhyd Na2SO4, filtered, and concentrated in vacuo. The residue was taken in EtOAc and 12·HCl (320 mg, 73% yield) was precipitated by adding an excess of a solution of HCl in Et2O. The analytical sample of 12·HCl was obtained by crystallization from MeOH/Et2O, mp 195−196 °C. IR 2972, 2933, 2855, 2645, 2579, 2484, 1458, 1446, 1377, 1033, 1014, 975, 949 cm−1. 1H NMR 0.93 (t, J = 7.5 Hz, 3H, CH 3CH2C3), 1.38 (t, J = 7.5 Hz, 6H, (CH 3CH2)2N), 1.53 (q, J = 7.5 Hz, 2H, CH3CH 2C3), 1.64 [dm, J = 13.0 Hz, 2H, 4(10)-Hax], 1.75 [dm, J = 13.0 Hz, 2H, 4(10)-Heq], 1.82 (overlapped dm, 1H, 6-Hanti), 1.85 (overlapped dm, 1H, 6-Hsyn), 1.94 [dm, J = 12.5 Hz, 2H, 8(9)-Heq], 2.09 [dm, J = 12.5 Hz, 2H, 8(9)-Hax], 2.47 [tm, J = 2.5 Hz, 2H, 5(7)-H], 3.06 (broad signal, 2H) and 3.59 (broad signal, 2H) [diastereotopic (CH3CH 2)2N]. 13C NMR (100.6 MHz) 7.2 (CH3, CH3CH2C3), 12.1 [CH3, (CH3CH2)2N], 30.2 [CH, C5(7)], 34.6 (CH2, C6), 35.2 (CH2, CH3 CH2C3), 35.4 [CH2, C8(9)], 38.4 [CH2, C4(10)], 45.4 [CH2, (CH3 CH2)2N], 79.3 (C, C3), 93.9 (C, C1). MS (EI), m/z (%): 238 (16), 237 (M• +, 59), 222 (14), 209 (19), 208 (74), 180 (30), 166 (48), 150 (23), 126 (31), 115 (100), 100 (54). Anal. Calcd for C15H27NO·HCl (273.85): C, 65.79; H, 10.31; N, 5.11; Cl, 12.95. Found: C, 65.64; H, 10.50; N, 5.13; Cl, 13.01.
4.1.18. N-Benzyl(3-ethyl-2-oxaadamant-1-yl)amine hydrochloride, 13·HCl
To a solution of 10b·HCl (400 mg, 1.84 mmol) in MeOH (10 mL), NaBH3CN (95%, 393 mg, 5.93 mmol), AcOH (0.3 mL), and benzaldehyde (0.42 mL, 4.12 mmol) were added, and the mixture was stirred at room temperature for 2 h. An additional portion of NaBH3CN (95%, 190 mg, 2.87 mmol) and benzaldehyde (0.21 mL, 2.06 mmol) was added, the mixture was stirred at room temperature for 16 h, and then it was concentrated in vacuo to dryness. Water (30 mL) was added to the residue, the suspension was basified with 1 N NaOH, and was extracted with EtOAc (4 × 15 mL). The combined organic extracts were washed with brine (2 × 25 mL), dried with anhyd Na2SO4, filtered, and concentrated in vacuo. The residue was taken in EtOAc and 13·HCl (451 mg, 79% yield) was precipitated by adding an excess of a solution of HCl in Et2O. The analytical sample of 13·HCl was obtained by crystallization from MeOH/Et2O, mp 213−214 °C. IR 2922, 2851, 2725, 2656, 2619, 2414, 1566, 1463, 1056, 1042, 1007, 988, 749, 690 cm−1. 1H NMR 0.96 (t, J = 7.5 Hz, 3H, CH 3CH2), 1.56 (q, J = 7.5 Hz, 2H, CH3CH 2), 1.67 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.77 [dm, J = 12.5 Hz, 2H, 4(10)-Heq], 1.87 [broad signal, 2H, 6-Hanti and 6-Hsyn], 1.98 [dm, J = 11.5 Hz, 2H, 8(9)-Hax], 2.04 [dm, J = 11.5 Hz, 2H, 8(9)-Heq], 2.46 [m, 2H, 5(7)-H], 4.25 [s, 2H, NCH2], 4.86 (s, mobile H), 7.42-7.50 (complex signal, 5H, Ar-H). 13C NMR 7.2 (CH3, CH3CH2), 29.9 [CH, C5(7)], 34.7 (CH2, C6), 35.2 (CH2, CH3 CH2), 37.3 [CH2, C8(9)], 38.6 [CH2, C4(10)], 45.1 (CH2, NCH2), 78.7 (C, C3), 87.6 (C, C1), 130.2 (CH, Ar-Cortho), 130.4 (CH, Ar-Cpara), 131.1 (CH, Ar-Cmeta), 133.0 (C, Ar-Cipso). MS (EI), m/z (%): 272 (15), 271 (M• +, 71), 242 (39), 200 (22), 160 (20), 149 (62), 91 (C7H7 +, 100). Anal. Calcd for C18H25NO·1.1HCl (311.51): C, 69.40; H, 8.44; N, 4.50; Cl, 12.52. Found: C, 69.38; H, 8.38; N, 4.43; Cl, 12.29.
4.1.19. N-Benzyl-N-methyl(3-ethyl-2-oxaadamant-1-yl)amine hydrochloride, 14·HCl
To a solution of 13·HCl (90 mg, 0.29 mmol) in acetonitrile (10 mL), formaldehyde (0.23 mL, 37 wt % in water solution, 0.29 mmol) and NaBH3CN (95%, 55 mg, 0.83 mmol) were added. The mixture was stirred at room temperature for 30 min, AcOH (0.2 mL) was added and the mixture was stirred at room temperature for 2 h. An additional portion of NaBH3CN (95%, 55 mg, 0.83 mmol) was added and the mixture was further stirred at room temperature for 16 h. The mixture was concentrated in vacuo to dryness, 1 N NaOH (15 mL) was added, and the suspension was extracted with CH2Cl2 (5 × 10 mL). The combined organic phases were washed with H2O (2 × 10 mL), dried with anhyd Na2SO4, filtered, and concentrated in vacuo to give the amine 14. The amine was taken in EtOAc and was precipitated as its hydrochloride (80 mg, 86% yield) by adding an excess of a solution of HCl in Et2O. The analytical sample of 14·HCl was obtained by crystallization from MeOH/Et2O, mp 165−166 °C. IR 2969, 2921, 2853, 2472, 2353, 1458, 1033, 1024, 972, 938, 750, 702 cm−1. 1H NMR 0.99 (t, J = 7.5 Hz, 3H, CH 3CH2), 1.60 (q, J = 7.5 Hz, 2H, CH3CH2), 1.69 (dm, J = 12.5 Hz, 2H, 4-Hax and 10-Hax,), 1.77−1.84 (broad signal, 2H, 4-Heq and 10-Heq), 1.87 (overlapped dm, 1H, 6-Hanti), 1.89 (overlapped dm, 1H, 6-Hsyn), 1.94−2.08 [broad signal, 2H, 8-Hax and 9-Hax], 2.14−2.25 [broad signal, 2H, 8-Heq and 9-Heq], 2.53 [broad s, 2H, 5(7)-H], 2.71 (s, 3H, NCH3), 3.93 (broad d, 1H, J = 8.0 Hz) and 4.85 (overlapped signal, 1H) (NCH 2Bn), 4.86 (s, mobile H), 7.50 (complex signal, 5H, Ar-H). 13C NMR 7.3 (CH3, CH3CH2), 30.3 [CH, C5(7)], 33.5 (CH3, CH3N), 34.2 (CH2) and 35.8 (CH2) (diastereotopic C8 and C9), 34.7 (CH2, C6), 35.2 (CH2, CH3 CH2), 38.3 (CH2) and 38.5 (CH2) (diastereotopic C4 and C10), 54.7 (CH2, NCH2), 79.8 (C, C3), 93.3 (C, C1), 130.3 (CH, Ar-Cortho), 131.0 (CH, Ar-Cpara), 131.5 (C, Ar-Cipso), 132.4 (CH, Ar-Cmeta). MS (EI), m/z (%): 286 (14), 285 (M• +, 64), 256 (41), 228 (16), 214 (21), 174 (20), 163 (82), 91 (100). Anal. Calcd for C19H27NO·HCl·0.4H2O (332.74): C, 69.34; H, 8.82; N, 4.26; Cl, 10.77. Found: C, 69.39; H, 8.73; N, 4.18; Cl, 11.05.
4.1.20. N-Methyl(3-ethyl-2-oxaadamant-1-yl)amine hydrochloride, 15·HCl
A mixture of 14·HCl (390 mg, 1.21 mmol) and 10% Pd/C (50% in water, 10 mg) in absolute EtOH (80 mL) was hydrogenated at 38 atm and 100 °C for 24 h. The suspension was filtered, the residue was washed with EtOH, and the combined organic filtrates were treated with an excess of a solution of HCl in Et2O. The solution was concentrated in vacuo and the residue was crystallized from MeOH/Et2O to give the analytical sample of 15·HCl (240 mg, 85% yield), mp 155−156 °C. IR 2968, 2931, 2848, 2706, 2592, 1561, 1474, 1118, 1068, 1057, 1028, 991, 972 cm−1. 1H NMR 0.92 (t, J = 7.5 Hz, 3H, CH 3CH2), 1.50 (q, J = 7.5 Hz, 2H, CH3CH 2), 1.63 [dm, J = 12.5 Hz, 2H, 4(10)-Hax], 1.72 [dm, J = 12.5 Hz, 2H, 4(10)-Heq], 1.84 [s, 2H, 6-Hanti and 6-Hsyn], 1.87 [dm, J = 13.0 Hz, 2H, 8(9)-Hax], 1.91 [dm, J = 13.0 Hz, 2H, 8(9)-Heq], 2.43 [broad s, 2H, 5(7)-H], 2.63 (s, 3H, NCH 3). 13C NMR (100.6 MHz) 7.1 (CH3, CH3CH2), 25.5 (CH3, NCH3), 29.8 [CH, C5(7)], 34.6 (CH2, C6), 35.2 (CH2, CH3 CH2), 37.0 [CH2, C8(9)], 38.6 [CH2, C4(10)], 78.5 (C, C3), 86.5 (C, C1). MS (EI, CH4), m/z (%): 196 (11), 195 (M• +, 84), 180 (11), 166 (69), 138 (26), 124 (56), 108 (37), 99 (62), 95 (31), 74 (28), 73 (100), 71 (51). Anal. Calcd for C12H21NO·1.1HCl·0.5H2O (242.60): C, 59.41; H, 9.58; N, 5.77; Cl, 15.34. Found: C, 59.41; H, 9.89; N, 6.11; Cl, 15.61.
4.2. NMDA receptor antagonist activity
The functional assay of antagonist activity at NMDA receptors was performed using primary cultures of cerebellar granule neurons, which were prepared according to established protocols.11 Cells were grown on 10 mm poly-l-lysine-coated glass cover slips, and used for the experiments after 7–14 days in vitro. Cells were loaded with 6 μM Fura-2 AM (Invitrogen-Molecular Probes) for 45 min. Afterwards, the coverslip was mounted on a quartz cuvette containing a Locke-Hepes buffer using a special holder. Measurements were performed using a Perkin–Elmer LS-50B fluorometer equipped with a fast-filter accessory, under mild agitation and at 37 °C. Analysis from each sample was recorded real-time during 1200 s. After stimulation with NMDA or glutamate (100 μM, in the presence of 10 μM glycine), increasing cumulative concentrations of the compound to be tested were added. The percentages of inhibition at every tested concentration were analyzed using a nonlinear regression curve fitting (variable slope) by using the software GraphPad Prism 4.0.
4.3. Antiviral evaluation
The antiviral activity of the compounds was determined in established cell culture assays using a selection of DNA and RNA viruses, including three subtypes of influenza virus [A/Puerto Rico/8/34 (H1N1); A/Hong Kong/7/87 (H3N2); and B/Hong Kong/5/72].13 The compounds′ inhibitory effect on virus replication as well as their cytotoxicity was monitored by microscopical examination, and confirmed by the colorimetric MTS cell viability assay.
4.4. T. brucei culturing and drug test
Cultures of bloodstream form T. brucei (strain 427) were maintained at 37 °C in modified Iscove′s medium (pH 7.4).14 Trypanocidal activity was assessed by growing parasites for 48 h in the presence of various drug concentrations to determine the levels which inhibited growth by 50% (IC50) and 90% (IC90). In the case of untreated cultures (volume 4 mL), cell densities increased from 0.5 × 104 to 1 × 106 cells mL−1 over this period. Experiments were performed in triplicate. Cell densities at each drug concentration were determined using a hemocytometer (Weber Scientific International Ltd), and drug sensitivity was expressed as a percentage of growth of control cells.
Acknowledgments
P.C., F.X.S. and S.V. gratefully acknowledge financial support from Ministerio de Educación y Ciencia (P.C. and S.V.: Project CTQ2005-02192; F.X.S.: Project SAF 2006-13092-C02-01) and Comissionat per a Universitats i Recerca (P.C. and S.V.: Project 2005-SGR-00180, F. X. S.: Project 2005-SGR-00893). M.D.D. thanks the Ministerio de Educación y Ciencia (FPU Program). L.P. thanks the European Commision for a Marie Curie Fellowship. S.R.P. and J.M.K. acknowledge Wellcome Trust for support.
References and notes
- 1.(a) Danysz W., Parsons C.G., Kornhuber J., Schmidt W.J., Quack G. Neurosci. Biobehav. Rev. 1997;21:455. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]; (b) Palmer G.C. Curr. Drug Targets. 2001;2:241. doi: 10.2174/1389450013348335. [DOI] [PubMed] [Google Scholar]
- 2.(a) Aoki F. In: Textbook of Influenza. Nicholson K.G., Webster R.G., Hay A.J., editors. Blackwell Science; Oxford: 1998. pp. 457–476. [Google Scholar]; (b) De Clercq E. Nat. Rev. Drug Disc. 2006;5:1015. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Geluk H.W., Schut J., Schlatmann J.L.M.A. J. Med. Chem. 1969;12:712. doi: 10.1021/jm00304a045. [DOI] [PubMed] [Google Scholar]; (b) Aldrich P.E., Hermann E.C., Meier W.E., Paulshock M., Prichard W.W., Snyder J.A., Watts J.C. J. Med. Chem. 1971;14:535. doi: 10.1021/jm00288a019. [DOI] [PubMed] [Google Scholar]; (c) Tilley T.W., Levitan P., Kramer M.J. J. Med. Chem. 1979;22:1009. doi: 10.1021/jm00194a025. [DOI] [PubMed] [Google Scholar]; (d) Kolocouris N., Foscolos G.B., Kolocouris A., Marakos P., Pouli N., Fytas G., Ikeda S., De Clercq E. J. Med. Chem. 1994;37:2896. doi: 10.1021/jm00044a010. [DOI] [PubMed] [Google Scholar]; (e) Kolocouris N., Kolocouris A., Foscolos G.B., Fytas G., Neyts J., Padalko E., Balzarini J., Snoeck R., Andrei G., De Clercq E. J. Med. Chem. 1996;39:3307. doi: 10.1021/jm950891z. [DOI] [PubMed] [Google Scholar]; (f) Van Derpoorten K., Balzarini J., De Clercq E., Poupaert J.H. Biomed. Pharmacother. 1997;51:464. doi: 10.1016/s0753-3322(97)82327-x. [DOI] [PubMed] [Google Scholar]; (g) Scholtissek C., Quack G., Klenk H.D., Webster R.G. Antiviral Res. 1998;37:83. doi: 10.1016/s0166-3542(97)00061-2. [DOI] [PubMed] [Google Scholar]; (h) Burstein M.E., Serbin A.V., Khakhulina T.V., Alymova I.V., Stotskaya L.L., Bogdan O.P., Manukchina E.E., Jdanov V.V., Sharova N.K. Antiviral Res. 1999;41:135. doi: 10.1016/s0166-3542(99)00006-6. [DOI] [PubMed] [Google Scholar]; (i) Stamatiou G., Kolocouris A., Kolocouris N., Fytas G., Foscolos G.B., Neyts J., De Clercq E. Bioorg. Med. Chem. Lett. 2001;11:2137. doi: 10.1016/s0960-894x(01)00388-2. [DOI] [PubMed] [Google Scholar]; (j) Zoidis G., Kolocouris N., Foscolos G.B., Kolocouris A., Fytas G., Karayannis P., Padalko E., Neyts J., De Clercq E. Antiviral Chem. Chemother. 2003;14:153. doi: 10.1177/095632020301400305. [DOI] [PubMed] [Google Scholar]; (k) Stylianakis I., Kolocouris A., Kolocouris N., Fytas G., Foscolos G.B., Padalko E., Neyts J., De Clercq E. Bioorg. Med. Chem. Lett. 2003;13:1699. doi: 10.1016/s0960-894x(03)00231-2. [DOI] [PubMed] [Google Scholar]; (l) Stamatiou G., Foscolos G.B., Fytas G., Kolocouris A., Kolocouris N., Pannecouque C., Wytvrouw M., Padalko E., Neyts J., De Clercq E. Bioorg. Med. Chem. 2003;11:5485. doi: 10.1016/j.bmc.2003.09.024. [DOI] [PubMed] [Google Scholar]; (m) El-Emam A.A., Al-Deeb O.A., Al-Omar M., Lehmann J. Bioorg. Med. Chem. 2004;12:5107. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]; (n) Zoidis G., Fytas C., Papanastasiou I., Foscolos G.B., Fytas G., Padalko E., De Clercq E., Naesens L., Neyts J., Kolocouris N. Bioorg. Med. Chem. 2006;14:3341. doi: 10.1016/j.bmc.2005.12.056. [DOI] [PubMed] [Google Scholar]; (o) Setaki D., Tataridis D., Stamatiou G., Kolocouris N., Foscolos G.B., Fytas G., Kolocouris N., Padalko E., Neyts J., De Clercq E. Bioorg. Chem. 2006;34:248;. doi: 10.1016/j.bioorg.2006.05.004. [DOI] [PubMed] [Google Scholar]; (p) Tataridis D., Fytas G., Kolocouris N., Fytas C., Kolocouris N., Foscolos G.B., Padalko E., Neyts J., De Clercq E. Bioorg. Med. Chem. Lett. 2007;17:692. doi: 10.1016/j.bmcl.2006.10.092. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kelly J.M., Miles M.A., Skinner A.C. Antimicrob. Agents Chemother. 1999;43:985. doi: 10.1128/aac.43.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kelly J.M., Quack G., Miles M.A. Antimicrob. Agents Chemother. 2001;45:1360. doi: 10.1128/AAC.45.5.1360-1366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kolocouris N., Zoidis G., Foscolos G.B., Fytas G., Prathalingam S.R., Kelly J.M., Naesens L., De Clercq E. Bioorg. Med. Chem. Lett. 2007;17:4358. doi: 10.1016/j.bmcl.2007.04.108. [DOI] [PubMed] [Google Scholar]; (d) Papanastasiou I., Tsotinis A., Kolocouris N., Prathalingam S.R., Kelly J.M. J. Med. Chem. 2008;51:1496. doi: 10.1021/jm7014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick G.L. Oxford University Press; Oxford: 2005. An Introduction to Medicinal Chemistry. pp 204–205. [Google Scholar]
- 6.(a) Geldenhuys W.J., Malan S.F., Bloomquist J.R., Marchand A.P., van der Schyf C.J. Med. Res. Rev. 2005;25:21. doi: 10.1002/med.20013. [DOI] [PubMed] [Google Scholar]; (b) Mdzinarishvili A., Geldenhuys W.J., Abbruscato T.J., Bickel U., Klein J., van der Schyf C.J. Neurosci. Lett. 2005;383:49. doi: 10.1016/j.neulet.2005.03.042. [DOI] [PubMed] [Google Scholar]; (c) Grobler E., Grobler A., van der Schyf C.J., Malan S.F. Bioorg. Med. Chem. 2006;14:1176. doi: 10.1016/j.bmc.2005.09.042. [DOI] [PubMed] [Google Scholar]; (d) Kiewert C., Hartmann J., Stoll J., Thekkumkara T.J., Geldenhuys W.J., Klein J. Neurochem. Res. 2006;31:395. doi: 10.1007/s11064-005-9036-0. [DOI] [PubMed] [Google Scholar]; (e) Geldenhuys W.J., Malan S.F., Bloomquist J.R., van der Schyf C.J. Bioorg. Med. Chem. 2007;15:1525. doi: 10.1016/j.bmc.2006.09.060. [DOI] [PubMed] [Google Scholar]; (f) Hao J., Mdzinarishvili A., Abbruscato T.J., Klein J., Geldenhuys W.J., van der Schyf C.J., Bickel U. Brain Res. 2008;1196:113. doi: 10.1016/j.brainres.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 7.(a) Stetter H., Tacke P. Chem. Ber. 1963;96:694. [Google Scholar]; (b) Bertz S.H. J. Org. Chem. 1985;50:3585. [Google Scholar]
- 8.(a) Geigy A. G. Patent GB 1123609, 1968.; (b) Gagneux A.R., Meier R. Tetrahedron Lett. 1969:1365. doi: 10.1016/s0040-4039(01)87887-4. [DOI] [PubMed] [Google Scholar]
- 9.(a) Stetter H., Gaertner J., Tacke P. Chem. Ber. 1966;99:1435. [Google Scholar]; (b) Camps P., El Achab R., Font-Bardia M., Görbig D.M., Morral J., Muñoz-Torrero D., Solans X., Simon M. Tetrahedron. 1996;52:5867. [Google Scholar]; (c) Camps P., El Achab R., Görbig D.M., Morral J., Muñoz-Torrero D., Badia A., Baños J.E., Vivas N.M., Barril X., Orozco M., Luque F.J. J. Med. Chem. 1999;42:3227. doi: 10.1021/jm980620z. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D.M., Keating G.M. Drugs. 2006;66:1515. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- 11.Canudas A.M., Pubill D., Sureda F.X., Verdaguer E., Camps P., Muñoz-Torrero D., Jiménez A., Camins A., Pallàs M. Exp. Neurol. 2003;180:123. doi: 10.1016/s0014-4886(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 12.Camps P., Duque M.D., Vázquez S., Naesens L., De Clercq E., Sureda F.S., López-Querol M., Camins A., Pallàs M., Prathalingam S.R., Kelly J.M., Romero V., Ivorra D., Cortés D. Bioorg. Med. Chem. 2008;16:9925. doi: 10.1016/j.bmc.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) De Clercq E., Cools M., Balzarini J., Márquez V.E., Borcherding D.R., Borchardt R.T., Drach J.C., Kitaoka S., Konno T. Antimicrob. Agents Chemother. 1989;33:1291. doi: 10.1128/aac.33.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Setaki D., Tataridis D., Stamatiou G., Kolocouris A., Foscolos G.B., Fytas G., Kolocouris N., Padalko E., Neyts J., De Clercq E. Bioorg. Chem. 2006;34:248. doi: 10.1016/j.bioorg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Hirumi H., Hirumi K. J. Parasitol. 1989;75:985. [PubMed] [Google Scholar]


