Abstract
In addition to well-documented vascular growth-promoting effects, ANG II exerts proapoptotic effects that are poorly understood. IGF-1 is a potent survival factor for human vascular smooth muscle cells (hVSMC), and its antiapoptotic effects are mediated via the IGF-1 receptor (IGF-1R) through a signaling pathway involving phosphatidylinositol 3-kinase and Akt. We hypothesized that there would be cross talk between ANG II proapoptotic effects and IGF-1 survival effects in hVSMC. To investigate ANG II-induced apoptosis and the potential involvement of IGF-1, we exposed quiescent and nonquiescent hVSMC to ANG II. ANG II induced apoptosis only in nonquiescent cells but stimulated hypertrophy in quiescent cells. ANG II-induced apoptosis was characterized by marked inhibition of Akt phosphorylation and stimulation of membrane Fas ligand (FasL) expression, caspase-8 activation, and a reduction in soluble FasL expression. Adenovirally mediated overexpression of Akt rescued hVSMC from ANG II-induced apoptosis. IGF-1R activation increased Akt phosphorylation and soluble FasL expression, and these effects were completely blocked by coincubating hVSMC with ANG II. In conclusion, ANG II-induced apoptosis of hVSMC is characterized by marked inhibition of Akt phosphorylation and stimulation of an extrinsic cell death signaling pathway via upregulation of membrane FasL expression, caspase-8 activation, and a reduction in soluble FasL expression. Furthermore, ANG II antagonizes the antiapoptotic effect of IGF-1 by blocking its ability to increase Akt phosphorylation and soluble FasL. These findings provide novel insights into ANG II-induced apoptotic signaling and have significant implication for understanding ANG II-induced remodeling in hypertension and atherosclerosis.
Keywords: cell signaling, growth factors, insulin-like growth factor 1
ANG II IS an important growth factor for vascular, cardiac, and renal cells (47). ANG II-mediated growth modulation underlies various pathophysiological processes, including atherosclerosis, vascular and cardiac remodeling, and progression of chronic renal disease (10). ANG II causes hypertrophy of quiescent rat vascular smooth muscle cells (rVSMC) in serum-free media (6, 21, 23) and has also been reported to stimulate proliferation of rVSMC in the presence of serum (44, 48). Although it has been reported that ANG II caused apoptosis in the media of rat blood vessels (13), the mechanisms underlying the proapoptotic effects of ANG II are not clear, and the effect of ANG II on human vascular smooth muscle cells (hVSMC) has not been explored. Furthermore, the potential relation between hVSMC differentiation and ANG II-induced apoptosis is unknown.
Apoptosis is initiated by two principal pathways (43). The intrinsic pathway initiates from mitochondria, whereas the extrinsic pathway is initiated by the activation of death receptors (43). Fas, also called Apo-1 and CD95, is one of several major death receptors that activate the extrinsic pathway (36). The activation of Fas after the binding of Fas ligand (FasL) leads to caspase-8-dependent apoptosis (28). VSMC normally express Fas but not FasL, and upregulation of FasL expression may lead to apoptosis of VSMC (20, 22, 38). Anti-FasL antibody has been reported to inhibit ANG II-induced tubular cell apoptosis (5).
In addition to its direct ability to regulate growth, migration, and gene expression in vascular cells (17, 46), ANG II may exhibit additional actions by modulating the signaling of other growth factors (12). Thus it has been shown that there is cross talk between the insulin and ANG-signaling systems in heart (42), and ANG II acutely inhibits IGF-1-induced phosphatidylinositol 3-kinase (PI3-kinase) activity in smooth muscle cells (18). However, the potential sustained effects of ANG II on IGF-1 signaling in hVSMC have not been addressed.
It has been reported that the activation of Akt inhibits apoptosis in a variety of cell types in vitro (9). The survival effects of Akt are mediated by phosphorylation and inhibition of several proapoptotic proteins, including members of the Forkhead transcription factors (FKHR) (7, 50). Thus decreased Akt phosphorylation leads to the translocation of FKHR from the cytosol to nucleus and an increased transcription of FasL (7). The potential role of Akt phosphorylation in ANG II-induced apoptosis has not been explored.
In this study, we hypothesized that there would be cross talk between ANG II proapoptotic effects and IGF-1 survival effects in hVSMC, and we investigated molecular mechanisms of ANG II-induced apoptosis and the ability of IGF-1 receptor (IGF-1R) activation and Akt phosphorylation to rescue cells from ANG II-induced apoptosis.
MATERIALS AND METHODS
Reagents
Human recombinant IGF-1 was obtained from Genentech; des-IGF-1 from GroPep; anti-total Akt, anti-phospho-Akt, anti-total FKHR, and anti-phosphor-FKHR antibodies from Cell Signaling; anti-IGF-1R antibody from Santa Cruz Biotechnology; ANG II from Sigma; and candesartan from AstraZeneca.
Cell culture
Cultured hVSMC (Clonetics) were grown in SMGM-2 medium with growth factors, 5% fetal bovine serum, glutamine, penicillin, and streptomycin. Studies were conducted on nonquiescent or quiescent hVSMC (i.e., preexposed to serum-free medium alone for 24 h). The human aortic smooth muscle cells were used at passages 4 to 10. To study the effects of IGF-1 and des-IGF-1, cells were treated with 0–50 ng/ml IGF-1 or des-IGF-1 with or without ANG II (100 nM). We chose this concentration of ANG II because lower concentrations of ANG II (20–80 nM) caused minimal apoptosis of nonquiescent hVSMC. This concentration of ANG II has been used extensively in cell culture studies. It is of note that high local concentrations of ANG II are thought to be important in disease states and specifically in the pathogenesis of vascular disease (16).
Cell viability assay
Cell viability was evaluated by intracellular calcein fluorescence, as described previously (31). After cells were incubated in six-well plates with 1 μM calcein AM in culture medium for 30 min, the cells were washed, transferred to microplates, and calcein fluorescence (excitation wavelength, 485 nm) was measured at 538 nm with the use of a Fusion Universal Microplate Analyzer. The data were expressed as the mean fluorescence peak height of samples normalized to a percentage of the control value.
Mitochondrial membrane potential
Δψ assay. After the experimental treatment, the cells in six-well plates were washed with PBS, then incubated with 10 μM rhodamine 123 (Molecular Probes) (31). After 30 min, the cells were washed, removed, and the indicator fluorescence was measured (excitation/emission wavelengths, 480/530 nm). The emission values were expressed as the mean fluorescence intensity of samples normalized to a percentage of the control value.
Western blot analysis
Cells were washed with PBS buffer and lysed in buffer containing 150 mM NaCl, 20 mM Tris·Cl (pH 7.2), 1 mM EDTA, 1% NP40, 5 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 0.1 M okadaic acid, 0.1 μM aprotinin, 10 μg/ml leupeptin, and 10 mM NaF. Lysates were subjected to 10% SDS-PAGE and Western blot analysis with polyclonal anti-Akt or anti-phospho-Akt (1:1,000) or phospho-FKHR antibody (1:1,000). Immunopositive bands were visualized by enhanced chemiluminescence. Blots were stripped and reprobed with monoclonal anti-β-actin antibody as a control for equal loading.
Adenovirus constructs and infection
The IGF-1R adenovirus (AdIGF-1R) was prepared as previously described (29). The constitutively active Akt adenovirus (AdAktmyr) was kindly provided by Dr. Kenneth Walsh, Boston University (33, 40).
Cell culture and infection hVSMC were replicate-plated into six-well plates and grown to 60% confluence. These cells were infected with CsCl gradient-purified recombinant AdIGF-1R, AdAktmyr, or control adenovirus expressing enhanced green fluorescence protein (AdGFP) at a multiplicity of infection (MOI) of 50.
Real-time PCR
To determine the expression of Fas, FasL, caspase-8, and ANG II type 1 (AT1) receptor, 5 μg of total RNA from treated hVSMC were converted into cDNA with the First Strand cDNA Synthesis kit (Amersham) and used for the 40 cycle two-step PCR in the Bio-Rad iCycler apparatus. PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 30 s and 55°C for 10 s. Fluorescence changes were monitored with SYBR Green PCR Super-mix (Bio-Rad) after every cycle, and melting curve analysis was performed at the end of 40 cycles to verify PCR product identity. Amplicon size and reaction specificity were confirmed by 1.5% agarose gel electrophoresis. Each PCR reaction was repeated three times, and the average median threshold cycle values were used for analysis. Results were evaluated with the iCycler IQ Real-Time Detection System Software (Bio-Rad).
Death ELISA
DNA fragmentation analysis was performed with a Cell Death Detection ELISA kit (Boehringer-Mannheim) according to the manufacturer's protocol. Briefly, cells were infected with AdGFP or AdIGF-1R (MOI of 40) or AdAktmyr (MOI of 50) in 24-well plates overnight in serum-containing medium. Cells were then exposed to ANG II for 24 h in serum-free medium, lysed in 100 μl of lysis buffer, and centrifuged for 10 min at 200 g, and triplicate 20 μl samples of supernatant were placed into the streptavidin-coated microtest plates for analysis. DNA fragmentation was quantified by measuring absorbance at 405 nm with a reference wavelength at 492 nm. Data presented are representative of three or more independent experiments.
Analysis of cellular protein-to-DNA ratio
Cellular hypertrophy was determined as described previously by Haider et al. (24). Briefly, cellular DNA and proteins were stained with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI; 1 μg/ml) and sulforhodamine 101 (20 μg/ml), respectively. The ratio of intensity of the red fluorescence of sulforhodamine 101 (>590 nm) to that of the blue fluorescence of DAPI (490 ± 20 nm) was calculated to represent the protein-to-DNA ratio (24).
Cell proliferation assay
Cell proliferation was performed with a BrdU Proliferation Assay (Oncogene). Briefly, the cells were treated with or without ANG II for 24 h. During the final 8 h of incubation, 5-bromo-2′-deoxyuridine (BrdU) was added into culture. The cells were fixed and permeabilized, the DNA was denatured, and the cells were exposed to anti-BrdU monoclonal antibody followed by horseradish peroxidase-conjugated goat anti-mouse and fluorogenic substrate. The blue fluorescent product was measured at excitation 325 nm and emission 420 nm. Data presented are representative of three or more independent experiments.
Caspase-8 activity
The protease activity of caspase-8 was assayed according to the manufacturer's instructions (Calbiochem). Briefly, the cells were washed with PBS buffer; lysed in buffer containing 150 mM NaCl, 20 mM Tris·Cl (pH 7.2), 1 mM EDTA, 1% NP40, 5 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 0.1 M okadaic acid, 0.1 μM aprotinin, 10 μg/ml leupeptin, and 10 mM NaF; lysates were centrifuged at 10,000 g for 10 min; and the supernatants were used for caspase-8 assay. The principle of the assay is that activated caspase-8 cleaves the COOH-terminal peptide bond from colorimetric substrate IETD with pnitroaniline (pNA), then releases pNA, which absorbs maximally at 405 nm. Assays were performed four times in triplicate using 96-well plates.
FasL ELISA
Soluble FasL was assayed with a Fas Ligand ELISA kit (Oncogene). Briefly, 100 μl culture media were placed into the FasL antibody-coated microtest plates. After 3 h, unbound material was washed away, and horseradish peroxidase-conjugated streptavidin and substrate tetramethylbenzidine were then added. The concentration of FasL was quantified by measuring absorbance at 450 nm with a reference wavelength at 595 nm. Data presented were representative of three or more independent experiments.
Cell cycle analysis by flow cytometry
Cells were trypsinized, rinsed once, resuspended in PBS, and fixed with ice-cold 70% ethanol for at least 20 min. Fixed cells were rinsed, resuspended in 50 μg/ml propidium iodide, and analysed on a Becton Dickinson FACScan flow cytometer. The percentage of cells in each phase was calculated using ModFit software (Verify Software House, Topsham, ME) (32). Four independent experiments were performed.
Statistical analysis
Data were presented as means ± SE. Statistical analysis was performed with ANOVA or Student's t-test when appropriate. Significance was established when P < 0.05. All experiments were performed a minimum of three times.
RESULTS
Differential effects of ANG II on hVSMC
With the use of flow cytometry, we found that 75.3 ± 4.1% of nonquiescent hVSMC were in S phase, and only 11.1 ± 3.4% of quiescent hVSMC were in S phase (Fig. 1A). ANG II did not alter the cell cycle distribution compared with control in quiescent hVSMC after 24 h exposure [ANG II + serum free (SF) vs. SF as control = 10.2 ± 1.6% vs. 9.5 ± 1.3% in S phase]. Additionally, ANG II did not alter the cell cycle distribution in nonquiescent cells, including ANG II in normal serum (NS) (ANG II + NS vs. NS as control = 73.7 ± 3.2% vs. 72.8 ± 2.9% in S phase) and ANG II in SF (ANG II + SF vs. SF as control = 12.7 ± 4.2% vs. 11.9 ± 3.9% in S phase) after 24-h exposure.
Fig. 1.
A: cell cycle distribution. Cells grown in 5% serum (nonquiescent) or serum-deprived for 24 h (quiescent) were fixed and permeabilized with ethanol and stained with propidium iodide. Fluorescence intensity was measured by flow cytometry. Representative histograms show DNA contents. G, gap; S, synthesis; FL3 Lin, fluorescence signal linearity. B: differential effects of ANG II on hypertrophy, proliferation, and cell viability. Quiescent and nonquiescent human vascular smooth muscle cells (hVSMC) were stimulated with 100 nM ANG II for 24 h. Proliferation was measured by 5-bromo-2’deoxyuridine (BrdU), cell viability was determined by calcein fluorescence, and hypertrophy was determined by cellular protein-to-DNA ratio. Results are expressed as percentage of control (means ± SE); n = 6 experiments. *P < 0.001 and **P < 0.002, compared with control.
Because ANG II could induce hypertrophy, proliferation, or apoptosis, we assessed the protein-to-DNA ratio to determine ANG II-induced hypertrophic responses, assessed DNA synthesis to determine ANG II-induced proliferation, and assessed cell viability to measure ANG II-induced cell death. Nonquiescent hVSMC had a marked reduction in viability when stimulated with ANG II in the absence of serum and growth factors (Fig. 1B). Quiescent hVSMC underwent hypertrophy when stimulated with ANG II, and ANG II did not significantly alter proliferation of both quiescent and nonquiescent hVSMC (Fig. 1B).
To determine the principal components of ANG II-induced cell death, we measured apoptosis by death ELISA. ANG II caused a marked increase in anti-histone and anti-DNA antibody binding (Fig. 2A), and ANG II-induced apoptosis was blocked by the AT1 receptor antagonist candesartan (Fig. 2A).
Fig. 2.
A: death ELISA. Nonquiescent hVSMC were exposed to serum-free medium alone (control) or with 10 nM candesartan [ANG II Type 2 receptor (AT1) blocker] with or without 100 nM ANG II for 24 h and analyzed for histone-associated DNA fragments as described in materials AND METHODS. Results are expressed as percentage of control (means ± SE); n = 4 experiments. *P < 0.001, compared with control. B: effects of ANG II on mitochondrial membrane potential. hVSMC were exposed to 100 nM ANG II for 0–24 h and washed with PBS three times, and mitochondrial membrane potential was measured by using rhodamine 123 as described in materials and methods. The results were expressed as percentage of control (means ± SE); n = 6 experiments. P = not significant (NS).
Effects of ANG II on intrinsic and extrinsic apoptotic pathways
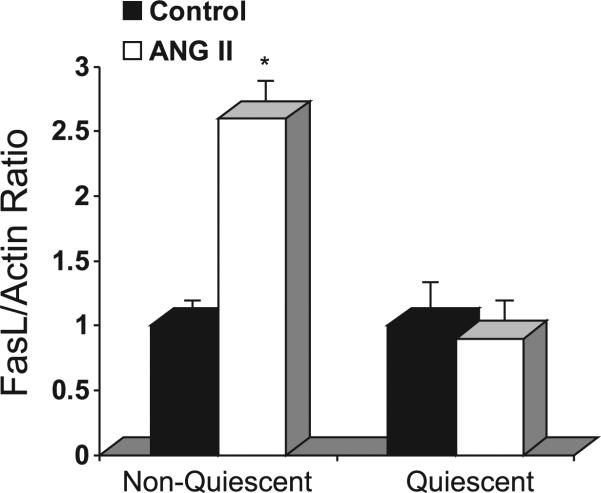
ANG II (100 nM, 0–24 h) did not affect mitochondrial membrane potential measured by rhodamine 123 fluorescence (Fig. 2B), indicating that ANG II did not regulate the intrinsic apoptotic pathway. However, ANG II increased FasL mRNA levels by 2.6 ± 0.2-fold compared with control in nonquiescent hVSMC after 16-h exposure (Fig. 3) but did not alter FasL mRNA levels (0.9 ± 0.1-fold vs. control) in quiescent hVSMC (Fig. 3). The expression of Fas was not significantly altered as measured by real-time PCR (data not shown). Moreover, ANG II increased caspase-8 mRNA levels by 3.2 ± 0.3-fold after 20-h exposure as assessed by real-time PCR (Fig. 4A) and also increased the protease activity of caspase-8 after 20-h exposure (Fig. 4B). These findings were consistent with ANG II stimulation of the extrinsic apoptotic pathway.
Fig. 3.
ANG II increases Fas ligand (FasL) mRNA expression. Quantitative real-time PCR was performed with primers specific for FasL and β-actin after exposure of nonquiescent or quiescent cells with or without ANG II for 16 h. Results were expressed as percentage of control (means ± SE); n = 5 experiments. *P < 0.005, compared with control.
Fig. 4.
A: real-time PCR analysis of caspase-8 mRNA expression in response to exposure of nonquiescent hVSMC with or without 100 nM ANG II for 24 h. Values are means ± SE; n = 6 experiments. *P < 0.01, compared with control. B: ANG II increases caspase-8 activity. Nonquiescent hVSMC were exposed to serum-free medium alone (control), 100 nM ANG II alone, 50 ng/ml IGF-1 alone, or to IGF-1 and ANG II for 20 h; lysates were centrifuged at 10,000 g for 10 min; and the supernatants were assayed for caspase-8 activity. Values are means ± SE; n = 6 experiments. *P < 0.01, compared with control; **P = NS, compared with ANG II.
Effects of des-IGF-1 and overexpression of IGF-1R or Akt on ANG II-induced apoptosis
To determine the potential ability of IGF-1 to prevent ANG II-induced apoptosis, we incubated hVSMC with various concentrations of des-IGF-1 or overexpressed IGF-1R using a specific adenovirus. Neither des-IGF-1 nor overexpression of AdIGF-1R attenuated ANG II-induced cell death, as measured by calcein fluorescence intensity (Fig. 5A), and apoptosis, as assessed by death ELISA (Fig. 5B). The rationale for using des-IGF-1 is that it has markedly lower affinity for IGF binding proteins (IGFBPs), and the effect of IGF-1 independent of IGFBPs can be assessed.
Fig. 5.
Effects of ANG II, des-IGF-1, and overexpression of IGF-1 receptor (IGF-1R) or Akt on cell viability and apoptosis. hVSMC were exposed to serum-free medium alone (control), with 100 nM ANG II alone, with 50 ng/ml des-IGF-1 and ANG II, with IGF-1R adenovirus (AdIGF-1R) and ANG II, adenovirus expressing green fluorescent protein (AdGFP) and ANG II, or with Akt adenovirus (AdAkt) and ANG II for 24 h. A: cell viability was determined by calcein fluorescence. Values are means ± SE; n = 7 experiments. *P < 0.001, compared with control. B and C: death ELISA was performed as described in materials and methods. Values are means ± SE; n = 5 experiments. *P < 0.01, compared with control.
To determine the potential ability of activated Akt to prevent ANG II-induced apoptosis, we incubated hVSMC with ANG II in the presence of a control adenovirus (AdGFP) or an adenovirus overexpressing phospho-(p)Akt. Overexpression of constitutively active Akt completely protected hVSMC against ANG II-induced apoptosis (Fig. 5C).
Effects of ANG II on IGF-1 signaling pathway
To delineate the signal transduction pathways involved in the inability of IGF-1 to rescue cells from ANG II-induced apoptosis of nonquiescent hVSMC, we investigated the effect of ANG II on the IGF-1 signaling pathway. We found that ANG II increased Akt phosphorylation at an early time point (5–30 min) (data not shown) but markedly decreased Akt and FKHR phosphorylation without altering the expression of total Akt or total FKHR at 24 h. IGF-1 failed to block ANG II-induced decrease in pAkt and pFKHR in nonquiescent hVSMC (Fig. 6A). The increase in pAkt by overexpression of IGF-1R using an adenovirus was completely blocked by coincubating cells with ANG II (Fig. 6B). We also found that ANG II only significantly altered Akt phosphorylation in nonquiescent hVSMC rather than in quiescent hVSMC after 24-h exposure (Fig. 6C). Because we found that ANG II alters the Fas signaling pathway, we examined whether ANG II could modulate the effect of IGF-1 on the Fas signaling pathway. Although we found that ANG II increased the expression of FasL mRNA, surprisingly, we found that ANG II decreased soluble FasL protein levels after 24-h exposure as assessed by FasL ELISA (Fig. 7). Furthermore, IGF-1 (50 ng/ml, 24 h) increased soluble FasL, and this IGF-1-induced increase was completely blocked by coincubating VSMC with ANG II (Fig. 7). IGF-1 also failed to block the ANG II increase in caspase-8 activity (Fig. 4B).
Fig. 6.
A and B: effects of ANG II, IGF-1, and overexpression of IGF-1R on expression of phospho-(p)Akt (representative experiment). hVSMC were exposed to serum-free medium alone (control) or for 24 h as indicated, and cell lysates were subjected to 10% SDS-PAGE and Western blot analysis with anti-β-actin, anti-total Akt, anti-pAkt, anti-Forkhead rhabdomyosarcoma transcription factor (anti-FKHR), and anti-pFKHR antibodies. C: effect of ANG II on expression of phospho-Akt in quiescent and nonquiescent hVSMC (representative experiment). hVSMC were exposed to serum-free medium alone (control) or with 100 nM ANG II for 24 h, and cell lysates subjected to 10% SDS-PAGE and Western blot analysis.
Fig. 7.
Effects of ANG II and IGF-1 on FasL levels. hVSMC were exposed to serum-free medium alone (control) or with 100 nM ANG II alone or with 50 ng/ml IGF-1 for 24 h, and soluble FasL in conditioned media was measured by ELISA. Results were expressed as percentage of control (means ± SE); n = 4 experiments. *P < 0.01, **P < 0.02, and #P < 0.01, compared with control.
Effects of serum deprivation on ANG II AT1 receptor expression
In view of the fact that ANG II induced apoptosis only in nonquiescent cells, we measured ANG II AT1 receptor mRNA expression in quiescent and nonquiescent cells. ANG II AT1 receptor mRNA levels were 4.3 ± 0.6-fold higher (means ± SE, n = 3, P < 0.01) in nonquiescent cells. Radioligand binding assays indicated that ANG II AT1 receptor density was increased by 3.2-fold (n = 4, P < 0.01) in nonquiescent hVSMC compared with quiescent hVSMC.
DISCUSSION
For the first time the present study shows that ANG II-induced apoptosis of nonquiescent hVSMC is characterized by marked inhibition of Akt and FKHR phosphorylation, by stimulation of an extrinsic cell death signaling pathway via upregulation of membrane FasL expression and by a reduction in soluble FasL levels (Fig. 8). These findings support the hypothesis that decreased Akt phosphorylation leads to activation of the transcription factor FKHR, which causes the translocation of FKHR from cytosol to nucleus and then increases the transcription of FasL (7). Although the present study did not examine the mechanisms underlying the antagonist function of soluble FasL, recent reports have suggested that the trimeric structure of FasL is necessary for signal transduction. Thus soluble FasL lacks the intracellular and transmembrane parts of the FasL molecule and is unable to trimerize and, consequently, to activate the apoptosis signal when bound to the receptor (35, 39, 41). Our finding that ANG II decreases soluble FasL levels and blocks the ability of IGF-1 to increase soluble FasL supports a role for soluble FasL in the apoptotic signaling pathway induced by ANG II. Of note, it has been reported that a soluble form of FasL has antagonist function and inhibits T-lymphocyte cell death (1).
Fig. 8.
Model of ANG II-induced apoptosis. ANG II-induced apoptosis of VSMC is characterized by marked inhibition of pAkt and pFKHR, and stimulation of extrinsic cell death signaling pathway via an increase in total FasL expression and the activity of caspase-8 and a reduction in soluble FasL expression.
The finding that ANG II did not alter mitochondrial membrane potential but stimulated membrane FasL expression and increased the activity of caspase-8 indicated that ANG II-induced apoptosis of VSMC proceeded via stimulation of an extrinsic rather than an intrinsic pathway. This finding extends the previous report (13) that ANG II induces apoptosis in blood vessels via activation of caspase-3, because caspase-8 is upstream of caspase-3. Our finding that ANG II induced apoptosis of nonquiescent hVSMC via the AT1 receptor is consistent with the finding that ANG II induced apoptosis of rVSMCs through activation of the AT1 receptor (3). It is important to note that there are several prior reports (26, 49) demonstrating that the ANG II type 2 (AT2) receptor mediates programmed cell death. Our findings support the hypothesis that AT1 receptor activation may control hVSMC growth through either proliferation or delayed apoptosis (3) and suggest that AT1-mediated apoptosis is particularly important in the synthetic VSMC phenotype. Although quiescent and nonquiescent hVSMC in our study did not show apparent differences in their morphology (data not shown), the expression of smooth muscle α-actin was markedly lower in nonquiescent hVSMC (data not shown), consistent with a dedifferentiated state and a synthetic rather than a contractile phenotype. It is of note that ANG II has been shown to induce apoptosis of epithelioid rVSMC, which express a lower level of smooth muscle α-actin, rather than spindle VSMC (3). Numerous observations suggest that smooth muscle cells in atherosclerotic lesions have changed from a contractile to synthetic phenotype, which could have profound effects on the capacity of the lesion to respond to various agents (37). It has also been reported that smooth muscle cells in the arteries may be heterogeneous, and this heterogeneity of smooth muscle in different parts of the arterial tree may respond differently to the stimuli that generate atherosclerotic lesions at each of these sites (2, 8, 19, 37). Thus our findings raise the intriguing possibility that ANG II could act differentially on VSMC in vivo, stimulating apoptosis of synthetic VSMC but inducing hypertrophy of contractile quiescent VSMC. Furthermore, our data showing that nonquies-cent hVSMC have significantly higher levels of ANG II AT1 receptor mRNA suggest that ANG II AT1 receptor density may play a critical role in the differential effect of ANG II on contractile vs. synthetic vascular smooth muscle.
The present study demonstrates that ANG II-induced apoptosis of nonquiescent hVSMC is mediated by reduced Akt phosphorylation based on the following lines of evidence: 1) ANG II markedly decreased Akt phosphorylation in nonquiescent hVSMC after 24-h exposure, whereas ANG II did not significantly alter Akt phosphorylation in quiescent hVSMC after 24-h exposure; and 2) overexpression of constitutively active Akt prevented ANG II-induced apoptosis. This finding is consistent with the antiapoptotic effects of Akt in a variety of cell types in vitro (9).
The IGF-1 system has been shown to have a potent survival function for a variety of cell types in vitro (15, 27, 30). Recently, Delafontaine's laboratory (30) has shown that IGF-1R activation inhibits oxidized LDL-induced cyto-chrome-c release and apoptosis of hVSMC. Surprisingly, in this study, des-IGF-1, IGF-1, and overexpression of IGF-1R by adenovirus did not attenuate ANG II-induced cell death. Interestingly, we found that ANG II markedly decreased basal Akt phosphorylation, and the increase in Akt phosphorylation by overexpression of IGF-1R using an adenovirus was also completely blocked by coincubating with ANG II. Furthermore, ANG II antagonized the antiapoptotic effect of IGF-1 by blocking its ability to increase Akt and FKHR phosphorylation and soluble FasL levels. These findings suggest that ANG II inhibits the antiapoptotic effect of IGF-1 through an Akt/ FKHR/FasL signaling pathway. Our finding is consistent with previous reports that ANG II inhibited insulin and IGF-1 stimulated PI3-kinase activity in rat aortic smooth muscle cells (18) and that ANG II injection into rats inhibited both basal and insulin-stimulated PI3-kinase activity in the heart (42). Importantly, our study is the first to demonstrate that ANG II inhibits IGF-1 antiapoptotic effects via an Akt/FKHR/FasL signaling pathway.
VSMC apoptosis occurs in all cardiovascular diseases, including hypertension, restenosis, and atherosclerosis (25). Transgenic rats harboring human renin and angiotensinogen reveal an increased rate of apoptosis in the heart and kidney (34). With respect to atherosclerosis, human plaques contain VSMC with apoptotic-prone behavior (4) and have increased angiotensin-converting enzyme activity (14). It is also reported that ANG II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice (10) and that hypercholesterolemia stimulates angiotensin synthesis and contributes to atherosclerosis through the AT1 receptor (11, 45). Although it has been reported that ANG II infusion enhanced apoptosis in blood vessels, potentially contributing to vascular remodeling in hypertension (13), molecular mechanisms responsible for ANG II-induced VSMC apoptosis have remained largely unknown. Our finding provides a molecular mechanism underlying ANG II-induced apoptosis of hVSMC and suggests that ANG II could contribute to atherosclerosis and vascular disease not only via its role in proliferation/hypertrophy but also via its apoptotic effects.
In summary, ANG II-induced apoptosis of VSMC is characterized by marked inhibition of Akt phosphorylation and stimulation of an extrinsic cell death signaling pathway via stimulation of membrane FasL expression, a reduction in soluble FasL expression, and increased expression and activity of caspase-8 (Fig. 8). Furthermore, ANG II antagonizes the antiapoptotic effect of IGF-1 by blocking its ability to increase Akt phosphorylation and soluble FasL expression. These findings have major implications for devising a strategy to limit ANG II-induced vascular remodeling in hypertension and atherosclerosis.
Acknowledgments
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant 1-RO1-HL-70241 (to P. Delafontaine).
REFERENCES
- 1.Ayroldi E, D'Adamio F, Zollo O, Agostini M, Moraca R, Cannarile L, Migliorati G, Delfino DV, Riccardi C. Cloning and expression of a short Fas ligand: a new alternatively spliced product of the mouse Fas ligand gene. Blood. 1999;94:3456–3467. [PubMed] [Google Scholar]
- 2.Babaev VR, Bobryshev YV, Stenina OV, Tararak EM, Gabbiani G. Heterogeneity of smooth muscle cells in atheromatous plaque of human aorta. Am J Pathol. 1990;136:1031–1042. [PMC free article] [PubMed] [Google Scholar]
- 3.Bascands JL, Girolami JP, Troly M, Escargueil-Blanc I, Nazzal D, Salvayre R, Blaes N. Angiotensin II induces phenotype-dependent apoptosis in vascular smooth muscle cells. Hypertension. 2001;38:1294–1299. doi: 10.1161/hy1201.096540. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MR, Littlewood TD, Schwartz SM, Weissberg PL. Increased sensitivity of human vascular smooth muscle cells from athero-sclerotic plaques to p53-mediated apoptosis. Circ Res. 1997;81:591–599. doi: 10.1161/01.res.81.4.591. [DOI] [PubMed] [Google Scholar]
- 5.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 6.Braun-Dullaeus RC, Mann MJ, Ziegler A, von der Leyen HE, Dzau VJ. A novel role for the cyclin-dependent kinase inhibitor p27(Kip1) in angiotensin II-stimulated vascular smooth muscle cell hypertrophy. J Clin Invest. 1999;104:815–823. doi: 10.1172/JCI5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 8.Chamley-Campbell JH, Campbell GR, Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981;89:379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 12.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 13.Diep QN, Li JS, Schiffrin EL. In vivo study of AT1 and AT2 angiotensin receptors in apoptosis in rat blood vessels. Hypertension. 1999;34:617–624. doi: 10.1161/01.hyp.34.4.617. [DOI] [PubMed] [Google Scholar]
- 14.Diet F, Pratt RE, Berry GJ, Momose N, Gibbons GH, Dzau VJ. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–2767. doi: 10.1161/01.cir.94.11.2756. [DOI] [PubMed] [Google Scholar]
- 15.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci USA. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzau VJ. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis (Theodore Cooper Lecture). Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 17.Feener EP, Northrup JM, Aiello LP, King GL. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995;95:1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 20.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, Morimoto S, Ogihara T. Nitric oxide induces upregulation of Fas and apoptosis in vascular smooth muscle. Hypertension. 1996;27:823–826. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 21.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 22.Geng YJ, Henderson LE, Levesque EB, Muszynski M, Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:2200–2208. doi: 10.1161/01.atv.17.10.2200. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haider A, Lee I, Grabarek J, Darzynkiewicz Z, Ferreri NR. Dual functionality of cyclooxygenase-2 as a regulator of tumor necrosis factor-mediated G1 shortening and nitric oxide-mediated inhibition of vascular smooth muscle cell proliferation. Circulation. 2003;108:1015–1021. doi: 10.1161/01.CIR.0000085211.97972.2C. [DOI] [PubMed] [Google Scholar]
- 25.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi M, Yamada T, Hayashida W, Dzau VJ. Interferon regulatory factor-1 up-regulates angiotensin II type 2 receptor and induces apoptosis. J Biol Chem. 1997;272:11952–11958. doi: 10.1074/jbc.272.18.11952. [DOI] [PubMed] [Google Scholar]
- 27.Kiely PA, Sant A, O'Connor R. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J Biol Chem. 2002;277:22581–22589. doi: 10.1074/jbc.M201758200. [DOI] [PubMed] [Google Scholar]
- 28.Li JH, Kluger MS, Madge LA, Zheng L, Bothwell AL, Pober JS. Interferon-gamma augments CD95(APO-1/Fas) and pro-caspase-8 expression and sensitizes human vascular endothelial cells to CD95-mediated apoptosis. Am J Pathol. 2002;161:1485–1495. doi: 10.1016/s0002-9440(10)64424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome-c release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 2003;23:2178–2184. doi: 10.1161/01.ATV.0000099788.31333.DB. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome-c release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 2003;23:2178–2184. doi: 10.1161/01.ATV.0000099788.31333.DB. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Meyer EM, Walker DW, Millard WJ, He YJ, King MA. Alpha7 nicotinic receptor activation inhibits ethanol-induced mitochondrial dysfunction, cytochrome-c release and neurotoxicity in primary rat hippocampal neuronal cultures. J Neurochem. 2002;81:853–858. doi: 10.1046/j.1471-4159.2002.00891.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu B, Mu Y, Cao C, Zeng F, Schneider S, Tan J, Price J, Chen J, Freeman M, Hallahan DE. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004;64:2840–2845. doi: 10.1158/0008-5472.can-03-3547. [DOI] [PubMed] [Google Scholar]
- 33.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3’-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 34.Muller DN, Heissmeyer V, Dechend R, Hampich F, Park JK, Fiebeler A, Shagdarsuren E, Theuer J, Elger M, Pilz B, Breu V, Schroer K, Ganten D, Dietz R, Haller H, Scheidereit C, Luft FC. Aspirin inhibits NF-kappaB and protects from angiotensin II-induced organ damage. FASEB J. 2001;15:1822–1824. doi: 10.1096/fj.00-0843fje. [DOI] [PubMed] [Google Scholar]
- 35.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 36.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 37.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 38.Sata M, Suhara T, Walsh K. Vascular endothelial cells and smooth muscle cells differ in expression of Fas and Fas ligand and in sensitivity to Fas ligand-induced cell death: implications for vascular disease and therapy. Arterioscler Thromb Vasc Biol. 2000;20:309–316. doi: 10.1161/01.atv.20.2.309. [DOI] [PubMed] [Google Scholar]
- 39.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suhara T, Kim HS, Kirshenbaum LA, Walsh K. Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation in Akt-mediated Fas ligand regulation. Mol Cell Biol. 2002;22:680–691. doi: 10.1128/MCB.22.2.680-691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 42.Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Rao PJ, Shillcutt SD, Newman WH. Angiotensin II induces proliferation of human cerebral artery smooth muscle cells through a basic fibroblast growth factor (bFGF) dependent mechanism. Neurosci Lett. 2005;373:38–41. doi: 10.1016/j.neulet.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 45.Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- 46.Weber H, Taylor DS, Molloy CJ. Angiotensin II induces delayed mitogenesis and cellular proliferation in rat aortic smooth muscle cells. Correlation with the expression of specific endogenous growth factors and reversal by suramin. J Clin Invest. 1994;93:788–798. doi: 10.1172/JCI117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf G, Wenzel UO. Angiotensin II and cell cycle regulation. Hypertension. 2004;43:693–698. doi: 10.1161/01.HYP.0000120963.09029.ca. [DOI] [PubMed] [Google Scholar]
- 48.Xiao F, Puddefoot JR, Barker S, Vinson GP. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension. 2004;44:340–345. doi: 10.1161/01.HYP.0000140771.21243.ed. [DOI] [PubMed] [Google Scholar]
- 49.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]