Abstract
Objective
Fetal cartilage anlage provides a framework for endochondral ossification and organization into articular cartilage. We previously reported differences between mechanical properties of talar cartilage anlagen and adult articular cartilage. However, the underlying development-associated changes remain to be established. Delineation of the normal evolvement of mechanical properties and its associated compositional basis provides insight into the natural mechanisms of cartilage maturation. Our goal was to address this issue.
Materials and methods
Human fetal cartilage anlagen were harvested from the tali of normal stillborn fetuses from 20 to 36 weeks of gestational age. Data obtained from stress relaxation experiments conducted under confined and unconfined compression configurations were processed to derive the compressive mechanical properties. The compressive mechanical properties were extracted from a linear fit to the equilibrium response in unconfined compression, and by using the nonlinear biphasic theory to fit to the experimental data from the confined compression experiment, both in stress-relaxation. The molecular composition was obtained using FTIR, and spatial maps of tissue contents per dry weight were created using FTIR imaging. Correlative and regression analyses were performed to identify relationships between the mechanical properties and age, compositional properties and age, and mechanical versus compositional parameters.
Results
All of the compositional quantities and the mechanical properties excluding the Poisson’s ratio changed with maturation. Stiffness increased by a factor of ~2.5 and permeability decreased by 20% over the period studied. Collagen content and degree of collagen integrity increased with age by ~3-fold, while the proteoglycan content decreased by 18%. Significant relations were found between the mechanical and compositional properties.
Conclusion
The mechanics of fetal talar cartilage is related to its composition, where the collagen and proteoglycan network play a prominent role. An understanding of the mechanisms of early cartilage maturation could provide a framework to guide tissue-engineering strategies.
Keywords: cartilage anlage, fetal development, Fourier transform infrared spectroscopy, mechanical properties, collagen, proteoglycan
Introduction
The primitive material of which the early cartilaginous precursors (cartilage anlagen) to skeletal system are made undergoes specific and unique changes in composition, structural organization, and mechanical properties as it develops and evolves into fully functional bone, hyaline cartilage, and fibrocartilage.
The biomechanical properties describing the compressive behavior of cartilage are the confined compression aggregate modulus (HA), and the hydraulic permeability (k0) with a factor M describing its dependence on compaction. The equilibrium response in an unconfined compression configuration is captured by the Young’s modulus (Es) of the solid matrix and the Poisson’s ratio (νs). The biomechanical properties of cartilage have been mainly associated with the collagen (COL) network and the glycosaminoglycan (GAG) constituents (negatively charged chains attached to the core protein of proteoglycans (PGs)) 1–5. The electrostatic swelling caused by the negative charges of GAG molecules provides resistance to compression. The collagen network partitions the intra- and extrafibrillar fluid content, which also affects effective osmotic pressures 6,7. Non-electrostatic contribution of the PGs to the equilibrium compressive stiffness has also been identified 3. The collagen network and the cross linking of its fibrils determine the tensile and shear stiffness of cartilage 8,9, as well as its compressive properties 1,2,5–7,10–12. The hydraulic permeability, a measure of the ease of fluid transport through the solid matrix, is dependent physically on the porous structure of the matrix, and chemically on proteoglycans through various mechanisms 3.
Fourier transform infrared (FTIR) spectroscopy is a powerful tool for simultaneous assessment of composition and microstructure of biological tissues such as bone and cartilage 13. The frequency at which a molecule absorbs infrared radiation is sensitive to conformation and can be used to obtain information on protein structure and when polarized, on orientation. FTIR spectroscopy has been used to characterize the spectral signatures of collagen and proteoglycans as well as structural parameters of the collagen network including collagen orientation, integrity (COLInt), and level of collagen cross-linking 13. The coupling of an FTIR spectrometer with an optical microscope with an array detector, FTIR imaging spectroscopy (FT-IRIS), is a technique that provides a unique opportunity to study the relative amount, molecular nature, and distribution of the components of connective tissues and essentially allows for “infrared imaging” of the sample at micron spatial resolutions.
Many studies describe the mechanics, composition and structure of adult articular cartilage 4,14,15, but only few studies focus on prenatal articular cartilage 5,16–18, early postnatal articular cartilage19,20, and growth plate cartilage 21. In more recent studies of postnatal development of the collagen network in articular cartilage, differences between immature and mature collagen network have been revealed 22–25. In other work, the developmental changes in PG concentration, monomer and aggregate size, and side chains in articular cartilage and different zones of growth plate cartilage of long bones, as well as the changes associated with calcification have been investigated 26–30. Few studies have addressed the relationship between structure/composition and function of fetal or early postnatal articular cartilage 5,17,18,31–33; however, such reports are more abundant for adult tissue 2,3,34–36. Overall, most of our knowledge is derived from mature and late fetal stages with the subject being predominantly “articular” cartilage. Additionally, prior studies have only focused on a single fetal stage in comparison to young postnatal and adult specimens, without evaluation of different gestational stages to focus on prenatal development.
While the general development of anlagen and their ossification are well documented, much remains to be elucidated on the temporal and spatial sequences for replacing primitive (i.e. poorly cross-linked) collagen and proteoglycan with mature collagen fibril networks and highly structured aggrecans in the articular cartilage, and the changes associated with bone formation in ossifying cartilage. Knowledge of the changes that anlagen undergo during development may provide a paradigm for guidance constructs tissue engineered from primitive mesenchymal stem cells, particularly those intended for cartilage repair or replacement. Furthermore, relationships between cartilage compositional and mechanical properties may provide for helpful markers for assessment of mechanical quality of engineered cartilage. An improved understanding of such relationships might also aid in development of more realistic theoretical models of developing cartilage. Spatial mapping of tissue contents at various ages at high resolution achievable by FT-IRIS techniques provides easily interpretable visual clues into development.
Our objectives were thus to (1) characterize the mechanical properties of human talar cartilage anlagen in the middle to late fetal period, (2) assess the molecular composition of the same tissue specimen using FTIR analysis and obtain high resolution spatial mappings of these parameters, and (3) determine whether relations existed between the molecular composition and mechanical properties, as well as assess the variation of compositional and mechanical properties with age and with respect to one another.
Materials and Methods
Fresh talus anlagen were obtained from seven still-born fetuses with gestational ages of 20, 25, 28, 30, 31, 33 and 36 weeks as determined from crown-to-rump length measurements (Fig. 1a). Each talus was prepared for two purposes: one half was used for FT-IRIS while the other half was used for mechanical testing followed by FTIR spectroscopy.
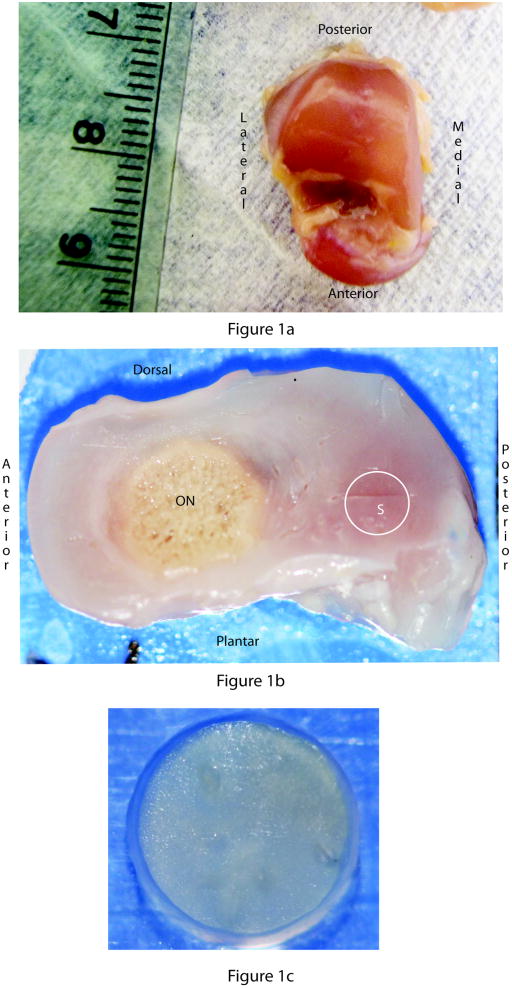
Fig. 1.
The three stages involved in preparation of the samples for testing: isolation of the talus, 1.5mm thick sagittal slices through the talus, and obtaining cylindrical samples from the slices. (A) Dorsal view of an isolated fetal talus. The scale units are millimeters. (B) 1.5mm thick sagittal slice through a fetal talus showing the ossific nucleus (ON) and the general region (S) form which samples were obtained (the circle does not represent specimen size). (C) A cylindrical sample of fetal anlage used for testing.
Mechanical testing
One half of the talus was sliced into 1.5 mm thick sagittal slices (Fig. 1b) using a manual tissue slicer with a micrometer controlled stage. In the younger specimens (20, 25 and 28 weeks of gestation) no ossific nucleus was detected, while in the older specimen a well developed ossific nucleus was present (Fig. 1b). Two samples were obtained from slices of each age (14 total) using a 3.00 mm biopsy punch (Fig. 1c). The plugs were submerged in phosphate-buffered saline (PBS) and frozen at −80 °C until the day of testing. On the day of testing, samples were thawed at room temperature and allowed 1.5 hours in PBS to equilibrate. Each sample was first tested in confined compression, allowed to recover for 1.5 hours at room temperature, and then tested in unconfined compression, both in a custom-designed apparatus37. Samples were submerged in normal saline solution containing enzyme inhibitors during testing and recovery time between the tests. Prior to data collection, a preconditioning load of 3.3 N was applied for 60 seconds, removed, and was followed by a tare load of 0.06 N for 900 seconds. The stress relaxation experiment was then performed by applying a displacement history to the top surface of samples, consisting of four ramps (5% strain each, 1 μm/second), followed by a stress relaxation period of 500 seconds. The loading protocol was the same for both configurations.
The Young’s modulus (Es) and aggregate modulus (HA, a measure of the compressive resistance in a laterally confined geometry) were determined from linear fits to the equilibrium stress-strain data of unconfined and confined compression tests respectively. In a custom-written code in MATLAB, the permeability parameters (k0 and M) were determined by finding the best fit to the stress-time data of the confined compression tests using the nonlinear biphasic theory 38, and the permeability equation proposed by Lai and Mow 39. The Poisson’s ratio was determined indirectly by solving the following equation for νs:
Further detail can be found in our previous report 37.
FTIR Analyses
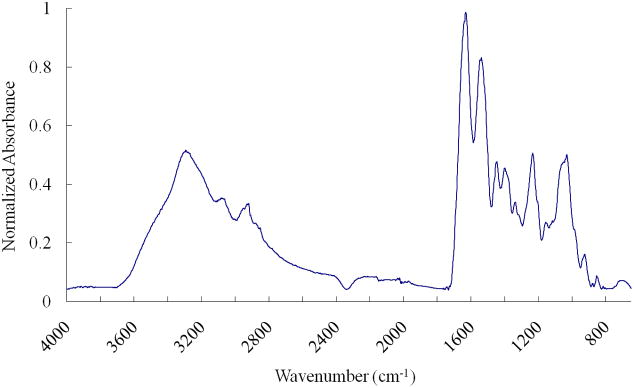
Following mechanical testing, the specimens were kept frozen. On the day of FTIR analysis, specimens were thawed to room temperature and air dried at 20 °C for one hour. Cartilage samples and model compounds containing mixtures of pure collagen type II and pure chondroitin sulfate (CS) - an identifying glycosaminoglycan of the aggrecan PG – in varying proportions (100% collagen/0% CS, 75% collagen/25% CS, 50% collagen/50% CS, 25% collagen/75% CS, 0% collagen/100% CS) were analyzed by FTIR on a Perkin Elmer Spectrum One IR Spectrometer (PerkinElmer, Waltham, MA, USA) with an ATR attachment in the 650 – 4000 cm−1 spectral region with a 4 cm−1 resolution. The FTIR results obtained from the model compounds provided the basis for a semi-quantitative measure of collagen and PG content 40 (% dry weight) of the tarsal anlagen samples. The primary mid-IR vibrations of collagen and PG in the tarsal anlagen samples were monitored simultaneously. The amide A, I, II and III infrared absorbances from collagen result from vibrations of the peptide bonds. The IR spectra of proteoglycans display absorbances from amide bonds, sulfate stretching modes, vibrations of the sugar ring moiety (C-O-C, C-C), and C-O-S modes. Integrated areas of mid infrared absorbance bands are proportional to the quantity of a specific component present. Thus, the areas of the collagen amide I absorbance (1590 – 1720 cm−1) and the PG sugar ring absorbances (900–1200 cm−1) were calculated and used to obtain a semi-quantitative measure of collagen and PG content per dry weight respectively using the area-to-composition relationships developed with constituent model compounds. The ratio of the area under the infrared absorbance centered at 1338 cm−1 (1300–1356 cm−1) to the area of the amide II band (1492–1598 cm−1) was used to measure the integrity of the collagen fibrils 41–43. A graph of a typical spectrum is displayed in Fig. 2. The region of interest of the spectra of the youngest and oldest specimens is shown in Fig. 3.
Fig. 2.
A typical FTIR spectrum obtained from a talus cartilage anlage.
Fig. 3.
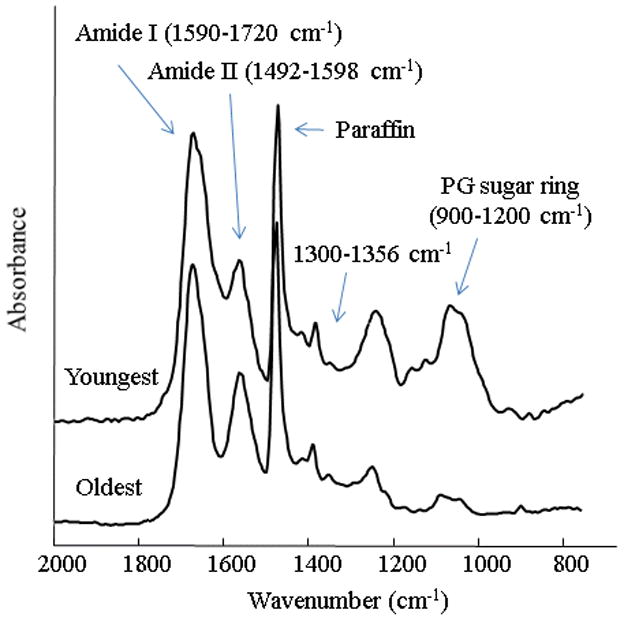
The region of interest in the FTIR spectra obtained from talus cartilage anlagen of the youngest (a) and oldest (b) specimens. The absorbance bands utilized to calculate collagen, proteoglycan and collagen integrity are labeled. The absorbance just above 1400 cm−1 is from paraffin as noted in the figure.
From the remaining halves of the tali we acquired FTIR imaging spectroscopy (FT-IRIS) data from paraffin-embedded tissue sections. Tissues were processed for paraffin embedding through a series of steps that involved decalcification, dehydration and proteoglycan containment. The tissue was fixed with 80% ethanol and 1% cetylpiridiniumcholoride (CPC), decalcified in EDTA, and embedded in paraffin. Sagittal sections were cut at seven micron thickness and mounted onto KBr IR windows. Two co-added scans at a 25 μm pixel resolution were collected in transmission mode from histological tissue sections at 8 cm−1 spectral resolution using a Perkin Elmer Spotlight 300 system in the 400 – 4000 cm−1 spectral region. This system is comprised of an FTIR spectrometer coupled with a light microscope and an 8×2 staggered linear array detector, and allows data collection over a user-defined rectangular region up to 5×5 mm. FT-IRIS images were created based on the specific vibrational absorbances for each component of interest using ISys software v3.1 (Spectral Dimensions, Olney, MD). The absorbance bands were baseline-corrected and then integrated. Information on distribution and dry weight content of collagen and proteoglycan, and spatial mapping of collagen integrity was obtained based on integrated areas or area ratios of infrared absorbance bands, as described in the previous paragraph.
Transmission light microscopy (TLM) images of histologically stained slices from around the talar dome region (articulation with the tibia) in the 20 week (youngest) and 36 week (oldest) specimens were also obtained.
Statistical Analysis
Data are presented as mean ± standard deviation. Mean values of mechanical and compositional quantities measured at each developmental stage were analyzed by bivariate correlations for detecting a linear association with age. Pearson correlation coefficients (r) and significance levels of effects (p) were calculated. Univariate linear regression was utilized to obtain models of mechanical and compositional properties as a function of age, with the exception of HA for which a second order polynomial was used. Linear mixed model analysis based on a maximum likelihood method was performed to study relationships between each of the mechanical properties and each of the compositional parameters. The intercept and regression coefficients were treated as fixed effects while age was used for subject grouping (total of seven groups) and considered a random effect. This allowed for the specimenss belonging to the same fetus (two per fetus) to be considered dependent while measurements from different fetuses are independent. There were no repeated measures. The regression coefficients and significance levels of effects (p) were calculated. Effects were considered significant when p < 0.05. All statistical analyses were performed with PASW Statistics 18.0.0 (IBM Corp., Somers, NY).
Results
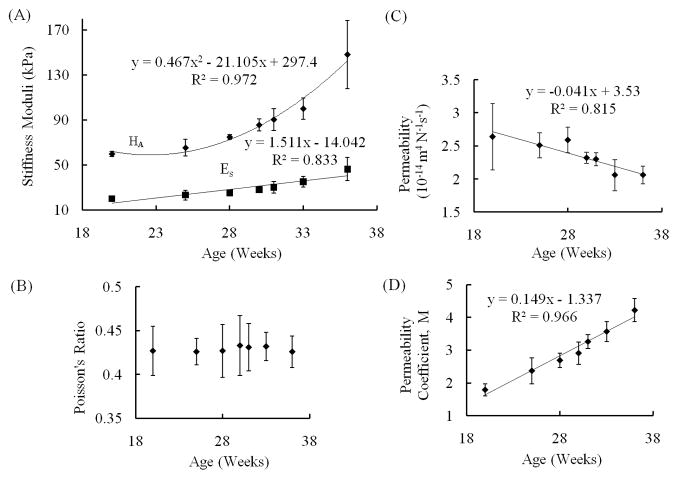
The aggregate modulus, HA, increased with age (r = 0.883, p = 0.008) from 59.9 ± 2.11 kPa in 20 weeks of gestation to 148 ± 30.3 kPa in the 36th week (Fig. 4). ES increased with age (r = 0.913, p = 0.004) from 2.01 ± 0.991 kPa to 46.3 ± 10.3 kPa (Fig. 4). The hydraulic permeability, k0, decreased with age (r = −0.903, p = 0.005) from 2.64 ± 0.45 ×10−14 m4N−1s−1 to 2.06 ± 0.13 ×10−14 m4N−1s−1 (Fig. 4). The strain-dependence coefficient of permeability, M, increased with age (r = 0.983, p < 0.001) from 1.79 ± 0.18 to 4.22 ± 0.33 (Fig. 4). There was no significant correlation between the Poisson’s ratio and age (r = 0.323, p = 0.480) during the period under study (0.43 ± 0.03 to 0.43 ± 0.02). Composition of the talar cartilage as determined by FTIR ATR spectroscopy varied markedly with gestational age (Fig. 5). The collagen content per dry weight and the degree of collagen integrity increased with age (p < 0.001, with r = 0.973 and 0.967 respectively) by a factor of 3.62 and 3.43 respectively. The PG content per dry weight decreased with respect to age (r = −0.931, p = 0.002) by a factor of 1.23.
Fig. 4.
Changes in mechanical properties with age. (A) Young’s and aggregate moduli (Es and HA) as determined by unconfined and confined compression respectively. (B) Poisson’s ratio obtained from solving for . (C) Permeability and (D) strain dependence coefficient of permeability as determined from confined compression. Age is the number of weeks from conception as estimated from the crown-rump length. Tissues became less compliant and less permeable with maturation, and permeability became more compaction dependent. Two specimens were harvested from each fetus; each was tested only once. Data points and error bars represent average values at each age ± standard deviation.
Fig. 5.
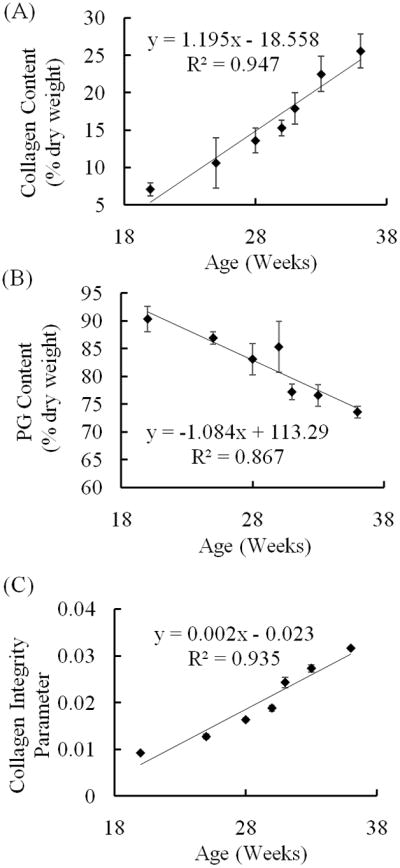
Changes in compositional properties with age. Compositional parameters were determined via FTIR analysis by calculating areas of corresponding absorbance bands: (A) Collagen dry weight content (Amide I band). (B) PG dry weight content (900–1200 cm−1 band). (C) Collagen integrity parameter (area ratio of 1338 cm−1/Amide II bands). Age is the number of weeks from conception as estimated from the crown-rump length. With maturation collagen dry weight content and integrity increased while PG dry weight content decreased. Two specimens were harvested from each fetus; each was tested only once. Data points and error bars represent average values at each age ± standard deviation.
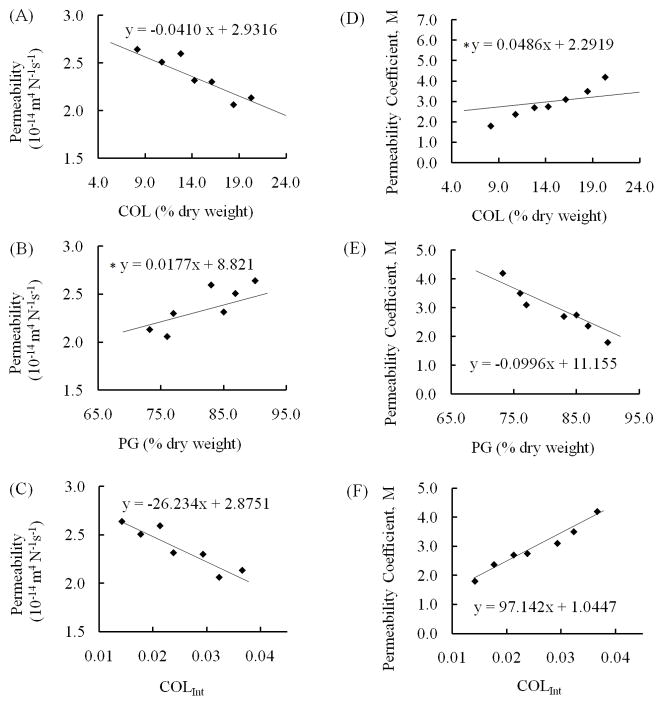
Models of mechanical properties as functions of the measured compositional quantities were obtained (Figs. 6, 7). Significant relations were detected between HA and the three parameters COL (p = 0.03), PG (p = 0.042), and COLInt (p = 0.004); and between ES and COLInt (p = 0.024) but not between ES and COL (p = 0.561) and PG (p = 0.42). Significant relations were found between k0 and COL (p = 0.006) and COLInt (p = 0.005) but not PG (p = 0.157); as well as between the permeability parameter, M, and PG (p = 0.001) and COLInt (p < 0.001) but not COL (p = 0.308). No effects were detected between the Poisson’s ratio and the compositional parameters.
Fig. 6.
Relationships between stiffness moduli and tissue composition. Compositional parameters were determined via FTIR analysis by calculating areas of corresponding absorbance bands as specified in the following parentheses. Figure shows aggregate modulus as obtained from confined compression versus (A) collagen dry weight content (Amide I), (B) PG dry weight content (900–1200 cm−1), and (C) collagen integrity parameter (ratio of 1338 cm−1/Amide II); and Young’s modulus as determined from unconfined compression versus (D) collagen dry weight content, (E) PG dry weight content, and (F) collagen integrity parameter. Regression lines were obtained using a linear mixed model approach to include the effect of dependence of observations within each fetal age group (2 specimens from each of the 7 age groups – each specimen tested once mechanically followed by FTIR analysis). Asterisks mark cases where no significant relationship existed between the two quantities (Es and COL, Es and PG).
Fig. 7.
Relationships between permeability parameters and tissue composition. Compositional parameters were determined via FTIR analysis by calculating areas of corresponding absorbance bands as specified in the following parentheses. Figure shows permeability as obtained from confined compression versus (A) collagen dry weight content (Amide I), (B) PG dry weight content (900–1200 cm−1), and (C) collagen integrity parameter (1338 cm−1/Amide II ratio); and strain dependence coefficient of permeability as determined from confined compression versus (D) collagen dry weight content, (E) PG dry weight content, and (F) collagen integrity parameter. Regression lines were obtained using a linear mixed model approach to include the effect of dependence of observations within each fetal age group (2 specimens from each of the 7 age groups – each specimen tested once mechanically followed by FTIR analysis). Asterisks mark the cases where no significant relationship existed between the two quantities (permeability and PG, M and COL).
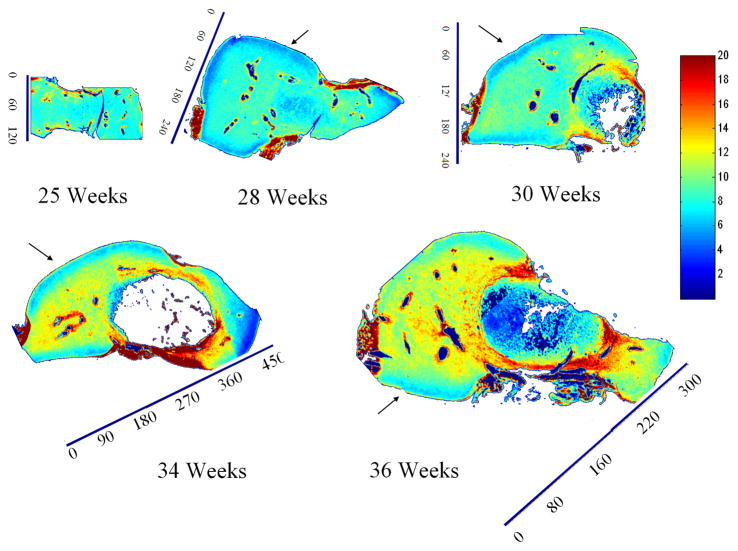
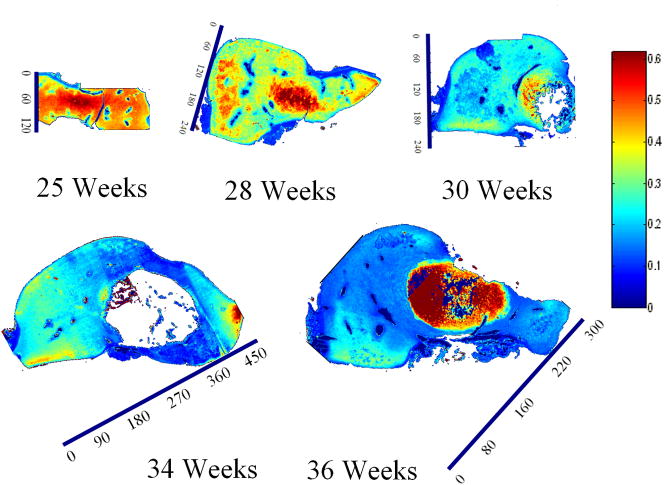
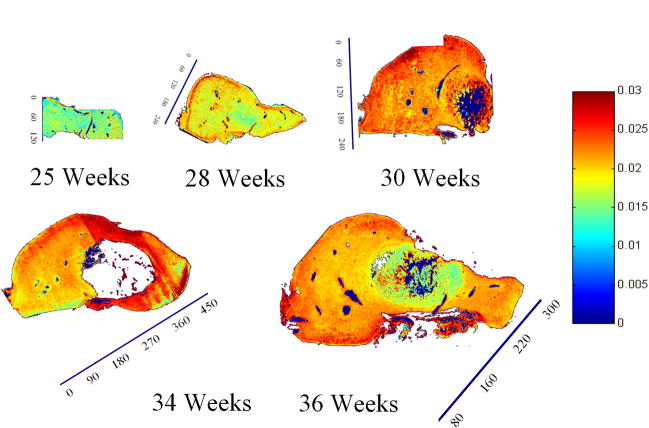
FT-IRIS images of sagittal slices of anlagen showed a continuous increase in the collagen content per dry weight with gestational age in all regions (Fig. 8). Consistently across ages, the spatial maps also showed a very thin layer of higher collagen dry weight content on the surfaces followed by a region of lower collagen dry weight content right beneath the surface, continued by a higher and rather uniform distribution throughout the rest of the cartilage. This band decreased in width and almost entirely disappeared by full term. PG dry weight content decreased with development (Fig. 9), and PG distribution became more uniform with aging. In the older samples (30, 33, and 36 weeks) regions of higher PG concentration seemed to form near the articulation surfaces. The collagen integrity parameter increased with age (Fig. 10). In the 28 week specimen COLInt was distinctively higher on the outer surface than on the interior regions of the cartilage. This difference lessened with development; however, the slightly higher values for this parameter were still observed on most of the outer surface as compared to the inside. Growth of the ossific nucleus can be appreciated in all images as the sample age increases.
Fig. 8.
Spatial mapping of collagen dry weight content (area of Amide I band) in sagittal slices of talus anlage obtained with FT-IRIS (arbitrary units). Tissue sections were obtained from five different human anlage samples, ages 25, 28, 30, 34 and 36 weeks, respectively. Red and blue on the color scale represent the highest and lowest contents respectively. Collagen dry weight content increased with development. An area of lower collagen concentration right below the articulation regions is noted by arrows. This feature disappeared by the 36th week. The ossific nucleus was partially masked in the 30 and 34 week samples to facilitate image comparison. Scale bar units are pixels (1 pixel = 25 μm).
Fig. 9.
Spatial mapping of PG dry weight content (area of 900–1200 cm−1 band) in sagittal slices of talus anlage obtained with FT-IRIS (arbitrary units). Tissue sections were obtained from five different human anlage samples, ages 25, 28, 30, 34 and 36 weeks, respectively. Red and blue on the color scale represent the highest and lowest values respectively. Decrease in PG dry weight content is observed with maturation (the dark red region in the 36 week sample at the location of the ossific nucleus is a saturation artifact). The ossific nucleus was partially masked in the 30 and 34 week samples to facilitate image comparison. Scale bar units are pixels (1 pixel = 25 μm).
Fig. 10.
Spatial mapping of collagen integrity parameter (1338 cm area/Amide II area) in sagittal slices of talus anlage across different gestational ages obtained with FT-IRIS (arbitrary units). Tissue sections were obtained from five different anlage samples, ages 25, 28, 30, 34 and 36 weeks, respectively. Red and blue on the color scale represent the highest and lowest values respectively. The collagen integrity increases with development and is higher on the surfaces. The ossific nucleus was partially masked in the 30 and 34 week samples to facilitate image comparison. Scale bar units are pixels (1 pixel = 25 μm).
TLM images showed enlargement of chondrocytes from the youngest to the oldest specimen (Fig. 11). A band of notably lower cell density was observed near the surface in both images. The width of this band was larger in the more mature sample.
Fig. 11.
Transmission light microscopy image of human cartilage anlage near and including talar dome, obtained from the 20 and 36 week fetuses. The images show an area of low chondrocyte density near the surface. Cell enlargement seen with maturation can be a sign of hypertrophy as the tissue eventually calcifies and forms bone.
Discussion
We previously showed marked differences between the mechanical properties of talar cartilage anlage and mature articular cartilage 37. In the present study, changes in mechanical properties and molecular composition of human talar anlagen during the mid to late fetal stages and the relationships between the two were determined.
Only a small fraction of the talar cartilage anlage located at the articulating surfaces evolves into articular cartilage while most of it (used here for testing and FTIR) undergoes hypertrophy and calcification, and is eventually replaced by bone. It has been demonstrated that PGs have an inhibitory effect on matrix calcification and apparently need to be removed prior to the onset of calcification 44. Several studies report decreased PG content and reduced PG synthesis in the zones of cartilage hypertrophy and calcification 27,30,44 supporting our results showing a decrease in PG content per dry weight with development (p = 0.01).
The increase in the collagen content indicated by our results has been previously reported 5,17. Paralleled with this growth in the collagen network we found an increase in both stiffness moduli HA and Es. Although the collagen network has been commonly believed to primarily contribute to the tensile properties of cartilage, its role in defining the compressive behavior has been highlighted more in recent studies 2,3,34,35.
Positive correlations between HA and GAG content have been previously reported 3,35. In this study we have found an inverse relationship between HA and PG content per dry weight (p = 0.042). Several different factors might explain this observation. It is known that the swelling pressure of the GAG molecules depends on their fixed charge density (FCD - amount of negative charges per free fluid volume) which depends on the volume of the extrafibrillar space where they reside. In this context, it is the amount of the negative charges that contribute to the compressive stiffness and not the PG mass content itself. Due to shrinking of the extrafibrillar space with increase in the collagen content, it is possible that despite the decrease in dry weight percentage values of PG content, the “concentration” of PGs in the extrafibrillar space and thus the FCD may have decreased to a lesser degree than represented by the dry weight measurements, remained constant, or even increased. Consequently, it is not surprising that the developmental changes in the collagen network in general, and the increase in the collagen content in particular can cause an increase in the tissue’s compressive stiffness albeit the reduction in the PG amount. Additionally, another way in which the PGs enhance the structural rigidity of the matrix is by their bulk mass and immobilization of their large aggregates by the collagen network 3. It is expected that this form of contribution of PGs to stiffness is lessened by the decline in PG amounts as tissue develops. These results suggest a more prominent role for the collagen network than has been suggested by many articles, with key functional consequences. Finally, there is a possibility of overestimation of PG content with FTIR in samples with an inherently low PG content due to lack of specificity of this technique 33,45. A similar issue has been reported for estimation of collagen content 15. Nevertheless, in addition to the ATR data showing decreased PG content per dry weight, the FT-IRIS data also showed an approximately two fold decrease in PG dry weight content from the 25 to 36 week samples. With these data together, we can be confident that there is indeed a real decrease in PG content per dry weight during anlagen development, as expected. The current analysis did not attempt to distinguish between different types of collagen. However, more than 90% of collagen in both immature and articular cartilage has been shown to be type II 46,47 and it is not expected that this limitation greatly influenced our results.
Noting that hydraulic permeability is related to the matrix pore structure, and the pore size and connectivity, the decrease with age in this parameter can be explained by augmentation of the collagen network size as development progresses. Permeability is also related to the amount of compression, which causes a decrease in the pore size and an increase in FCD. This effect is captured by the coefficient M. We observed an increase in M with age, in line with findings of Williamson et al. 5 for developing bovine articular cartilage. This suggests that as the tissue matures, the permeability becomes more sensitive to the degree of compaction it experiences. The decrease in porosity with maturation may also explain the inverse relationship found here between permeability and the collagen dry weight content.
The 1338 cm−1 absorbance band used in calculation of the collagen integrity parameter (measured as the 1338 cm−1/amide II ratio) is a feature that arises from collagen CH2 side chain vibrations, and decreases in intensity as collagen denatures 41. The integrated area of the amide II collagen band divided by the area of 1338 cm−1 band (i.e., inverse of the ratio used in our study) has been used as a measure of integrity of the collagen structure in osteoarthritic and collagenase treated articular cartilage, and is higher in OA tissues compared to normal 41. This suggests an active tissue repair response manifested by heightened levels of collagen synthesis early in the development of osteoarthritis. Although this parameter has been used previously to examine normal versus degenerative articular cartilage, the finding of our study is consistent with those reports in that the integrity parameter exhibits a rise with age, possibly attributable to collagen synthesis.
There is a large variation in the literature in the values of Poisson’s ratio from compression tests (0.0–0.42) 4 stemming from - to name a few - the use of non-standardized methods, specimen sources, and varying test configurations. The Poisson’s ratio we observed is at the high range of reported values, among a few other studies 18,37,48–50. One reason is that the Poisson’s ratio obtained here is the intrinsic value. As fluid drains from the lateral surfaces, the total volume reduces and therefore the apparent Poisson’s ratio is lower than the intrinsic Poisson’s ratio of the matrix. Second, the collagen network primarily controls the Poisson’s ratio 2. The tested fetal cartilage anlage (mostly precursor to bone, not articular cartilage) is soft and its collagen network lacks the organized fibril arrangement and high cross-linking (governing the tensile behavior) present in adult articular cartilage, and thus has greater tendency for lateral expansion (e.g., in unconfined compression) which gives rise to higher Poisson’s ratios. Supporting this notion is the inverse relationship between stiffness and Poisson’s ratio 2.
The Poisson’s ratio remained approximately the same across different ages of specimens. Increase of this parameter was previously reported over a wider age range spanning fetal to adult humeral head articular cartilage 18. Lack of detectable changes in the Poisson’s ratio might suggest that the structural organization of the collagen network did not substantially change in our specimens over the period studied. Further investigation focusing on collagen orientation and the degree of cross-linking will help explain these results. Similarly, we found no significant effects between the Poisson’s ratio and the compositional properties. Previous studies reported absence of such correlations between this parameter and GAG/PG content 2,3 which is in agreement with our work; however, an inverse relationship between the Poisson’s ratio and collagen content of adult bovine articular cartilage has also been shown to exist 2.
The valley observed in the spatial mapping of collagen content per dry weight right below the surface corresponds well and can be explained with the TLM images. The state of hypertrophy in the chondrocytes can be inferred from the enlargement of cells. It seems that less collagen synthesis is occurring due to lower concentration of chondrocytes underneath the surface as compared to the rest of the tissue, explaining the observed valley in the collagen content per dry weight in those areas. Understanding the origin and composition-function relationships in this valley is of great value, and remains to be elucidated.
An important limitation of our study is the limited number of samples (standard deviations based on n = 2) to test due to the difficulty in obtaining the human fetal cadaveric material. Despite this factor, the resulting standard deviations were comparable to literature values. Albeit possible to infer wet weight values by assuming tissue’s dry mass content and cartilage density, measurement of collagen and PG content as percentage per dry weight is another limitation of our method.
Our observations have potential applications in functional tissue engineering. Mechanical and compositional properties are currently used mainly to assess the viability of tissue engineered cartilage end-product. However, one might envision inspecting intermediate states of tissue-engineered cartilage to ensure its development is along the path taken by native tissue. The use of FTIR imaging of native and tissue-engineered cartilage has been reviewed 13 and parameters such as those utilized in our study have been highlighted as appropriate measures of the development of engineered cartilage 1,13. The type of data we report may also present a potential in the future for elucidating the mechanisms underlying normal cartilage maintenance as well as the etiology of diseases such as osteoarthritis and congenital deformities.
Acknowledgments
This study was supported by the NIH grant AR053255 (SS), EB000744 (NP) and the ISB dissertation award (RM). The authors would like to gratefully acknowledge Dr. R. Brand, the Editor-in-Chief of Clinical Orthopaedics and Related Research for his kind help with conception and design of the study, and revision of the article; and Dr. C. Schneck of the Department of Anatomy and Cell Biology at Temple University, Philadelphia, PA, for providing the cadaveric material. FT-IRIS data was collected at The Musculoskeletal Repair and Regeneration Core Center at HSS, supported by NIH AR046121.
Footnotes
Conflict of Interest
There were no conflicts of interest for any of the authors.
Contributions
Roza Mahmoodian: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, statistical expertise, collection and assembly of data.
Jeremi Leasure: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, collection and assembly of data.
Phitha Philip: drafting of the article, final approval of the article, collection and assembly of data.
Nancy Pleshko: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data.
Franco Capaldi: conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article.
Sorin Siegler: conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the article, provision of study materials or patients, statistical expertise, obtaining of funding. Sorin Siegler (ssiegler@coe.drexel.edu) takes responsibility for the integrity of the work as a whole, from inception to finished article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roza Mahmoodian, Department of Materials Science and Engineering, Massachusetts Institute of Technology
Jeremi Leasure, Department of Mechanical Engineering and Mechanics, Drexel University
Phitha Philip, Department of Mechanical Engineering and Mechanics, Drexel University
Nancy Pleshko, Department of Mechanical Engineering, Temple University.
Franco Capaldi, Department of Mechanical Engineering and Mechanics, Drexel University.
Sorin Siegler, Department of Mechanical Engineering and Mechanics, Drexel University.
References
- 1.Kelly DJ, Crawford A, Dickinson SC, Sims TJ, Mundy J, Hollander AP, et al. Biochemical markers of the mechanical quality of engineered hyaline cartilage. Journal of Materials Science: Materials in Medicine. 2007;18(2):273–281. doi: 10.1007/s10856-006-0689-2. [DOI] [PubMed] [Google Scholar]
- 2.Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Toyras J, Jurvelin JS. Collagen network primarily controls Poisson’s ratio of bovine articular cartilage in compression. Journal of Orthopaedic Research. 2006;24(4):690 – 699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lu XL, Sun DDN, Guo XE, Chen FH, Lai WM, Mow VC. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Annals of Biomedical Engineering. 2004;32(3):370–379. doi: 10.1023/b:abme.0000017534.06921.24. [DOI] [PubMed] [Google Scholar]
- 4.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Journal of Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 5.Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. Journal of Orthopaedic Research. 2001;19(6):1113 – 1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 6.Maroudas A, Wachtel E, Grushko G, Katz EP, Weinberg P. The effect of osmotic and mechanical pressures on water partitioning in articular cartilage. Biochimica et Biophysica Acta. 1991;1073(2):285–294. doi: 10.1016/0304-4165(91)90133-2. [DOI] [PubMed] [Google Scholar]
- 7.Wilson W, Huyghe JM, van Donkelaar CC. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomechanics and Modeling in Mechanobiology. 2007;6(1–2):43–53. doi: 10.1007/s10237-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 8.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. The Biochemical Journal. 1998;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeRoux MA, Arokoski J, Vail TP, Guilak F, Hyttinen MM, Kiviranta I, et al. Simultaneous changes in the mechanical properties, quantitative collagen organization, and proteoglycan concentration of articular cartilage following canine meniscectomy. Journal of Orthopaedic Research. 2000;18(3):383–392. doi: 10.1002/jor.1100180309. [DOI] [PubMed] [Google Scholar]
- 10.Ficklin T, Thomas G, Barthel JC, Asanbaeva A, Thonar EJ, Masuda K, et al. Articular cartilage mechanical and biochemical property relations before and after in vitro growth. Journal of Biomechanics. 2007;40(16):3607–3014. doi: 10.1016/j.jbiomech.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalsa PS, Eisenberg SR. Compressive behavior of articular cartilage is not completely explained by proteoglycan osmotic pressure. Journal of Biomechanics. 1997;30(6):589–594. doi: 10.1016/s0021-9290(97)84508-3. [DOI] [PubMed] [Google Scholar]
- 12.van Turnhout MC, Kranenbarg S, van Leeuwen JL. Contribution of postnatal collagen reorientation to depth-dependent mechanical properties of articular cartilage. Biomechanics and Modeling in Mechanobiology. 2010 June 6; doi: 10.1007/s10237-010-0233-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Boskey A, Camacho NP. FT-IR imaging of native and tissue-engineered bone and cartilage. Journal of Biomaterials. 2007;28(15):2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin I, Obradovic B, Freed LE, Vunjak-Novakovic G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Annals of Biomedical Engineering. 1999;27(5):656–662. doi: 10.1114/1.205. [DOI] [PubMed] [Google Scholar]
- 15.Potter K, Kidder LH, Levin IW, Lewis EN, Spencer RGS. Imaging of collagen and proteoglycan in cartilage sections using Fourier transform infrared spectral imaging. Arthritis & Rheumatism. 2001;44(4):846–855. doi: 10.1002/1529-0131(200104)44:4<846::AID-ANR141>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Brown TD, Singerman RJ. Experimental determination of the linear biphasic constitutive coefficients of human fetal proximal femoral chondroepiphysis. Journal of Biomechanics. 1986;19(8):597–605. doi: 10.1016/0021-9290(86)90165-x. [DOI] [PubMed] [Google Scholar]
- 17.Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. Journal of Biomechanics. 2007;40(1):182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. Journal of Biomechanics. 2000;33:1049–1054. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 19.Buckley MR, Bergou AJ, Fouchard J, Bonassar LJ, Cohen I. High-resolution spatial mapping of shear properties in cartilage. Journal of Biomechanics. 2010;43(4):796–800. doi: 10.1016/j.jbiomech.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CCB, Guo XE, Sun DN, Mow VC, Ateshian GA, Hung CT. The functional environment of chondrocytes within cartilage subjected to compressive loading: A theoretical and experimental approach. Biorheology. 2002;39(1–2):11–25. [PubMed] [Google Scholar]
- 21.Cohen B, Chorney GS, Phillips DP, Dick HM, Buckwalter JA, Ratcliffe A, et al. The microstructural tensile properties and biochemical composition of the bovine distal femoral growth plate. Journal of Orthopaedic Research. 1992;10(2):263–275. doi: 10.1002/jor.1100100214. [DOI] [PubMed] [Google Scholar]
- 22.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis and Cartilage. 15(9):1099–1099. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Julkunen P, Iivarinen J, Brama PA, Arokoski J, Jurvelin JS, Helminen HJ. Maturation of collagen fibril network structure in tibial and femoral cartilage of rabbits. Osteoarthritis and Cartilage. 2010;18(3):406–415. doi: 10.1016/j.joca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Rieppo J, Hyttinen MM, Halmesmaki E, Ruotsalainen H, Vasara A, Kiviranta I, et al. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthritis and Cartilage. 2009;17(4):448–455. doi: 10.1016/j.joca.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.van Turnhout MC, Haazelager MB, Gijsen MAL, Schipper H, Kranenbarg S, van Leeuwen JL. Quantitative description of collagen structure in the articular cartilage of the young and adult equine distal metacarpus. Animal Biology. 2008;58(4):353–370. [Google Scholar]
- 26.Byers S, Rooden JCv, Foster BK. Structural changes in the large proteoglycan, aggrecan, in different zones of the ovine growth plate. Calcified Tissue International. 1997;60(1):71–78. doi: 10.1007/s002239900188. [DOI] [PubMed] [Google Scholar]
- 27.Campo RD, Romano JE. Changes in cartilage proteoglycans associated with calcification. Calcified Tissue International. 1986;39(3):175–184. doi: 10.1007/BF02555115. [DOI] [PubMed] [Google Scholar]
- 28.Farquharson C, Whitehead CC, Loveridge N. Alterations in glycosaminoglycan concentration and sulfation during chondrocyte maturation. Calcified Tissue International. 1994;54(4):296–303. doi: 10.1007/BF00295954. [DOI] [PubMed] [Google Scholar]
- 29.Nakano T, Sim JS, Imai S, Koga T. Lack of chondroitin sulphate epitope in the proliferating zone of the growth plate of chicken tibia. Histochemical Journal. 1996;28(12):867–873. doi: 10.1007/BF02331390. [DOI] [PubMed] [Google Scholar]
- 30.Poole AR, Pidoux I, Rosenberg L. Role of proteoglycans in endochondral ossification: immunofluorescent localization of link protein and proteoglycan monomer in bovine fetal epiphyseal growth plate. The Journal of Cell Biology. 1982;92(2):249–260. doi: 10.1083/jcb.92.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brama PAJ, Tekoppele JM, Bank RA, Barneveld A, van Weeren PR. Functional adaptation of equine articular cartilage: the formation of regional biochemical characteristics up to age one year. Equine Veterinary Journal. 2000;32(3):217–221. doi: 10.2746/042516400776563626. [DOI] [PubMed] [Google Scholar]
- 32.Brommer J, Brama PAJ, Laasanen MS, Helminen HJ, van Weeren RR, Jurvelin JS. Functional adaptation of articular cartilage from birth to maturity under the influence of loading: a biomechanical analysis. Equine Veterinary Journal. 2005;37(2):148–154. doi: 10.2746/0425164054223769. [DOI] [PubMed] [Google Scholar]
- 33.Rieppo L, Saarakkala S, Narhi T, Holopainen J, Lammi M, Helminen HJ, et al. Quantitative analysis of spatial proteoglycan content in articular cartilage with Fourier transform infrared imaging spectroscopy: Critical evaluation of analysis methods and specificity of the parameters. Microscopy Research and Technique. 2009;73(5):503–512. doi: 10.1002/jemt.20789. [DOI] [PubMed] [Google Scholar]
- 34.Roberts S, Weightman B, Urban J, Chappell D. Mechanical and biochemical properties of human articular cartilage in osteoarthritic femoral heads and in autopsy specimens. The Journal of Bone and Joint Surgery. 1986;68(2):278–88. doi: 10.1302/0301-620X.68B2.3958016. [DOI] [PubMed] [Google Scholar]
- 35.Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Gordinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. Journal of Orthopaedic Research. 2000;18(5):739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 36.Jurvelin JS, Arokoski JPA, Hunziker EB, Helminen HJ. Topographical variation of the elastic properties of articular cartilage in the canine knee. Journal of Biomechanics. 2000;33(6):669–675. doi: 10.1016/s0021-9290(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoodian R, Leasure J, Gadikota H, Capaldi F, Siegler S. Mechanical properties of human fetal talus. Clinical Orthopaedics and Related Research. 2009;467(5):1186–94. doi: 10.1007/s11999-008-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. Journal of Biomechanics. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 39.Lai WM, Mow VC. Drag-induced compression of articular cartilage during a permeation experiment. Biorheology. 1980;17(2-2):111–23. doi: 10.3233/bir-1980-171-213. [DOI] [PubMed] [Google Scholar]
- 40.Camacho NP, West P, Torzilli PA, Mendelsohn R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62(1):1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.West PA, Torzilli PA, Chen C, Lin P, Camacho NP. Fourier transform infrared imaging spectroscopy analysis of collagenase-induced cartilage degradation. Journal of Biomedical Optics. 2005;10(1) doi: 10.1117/1.1854131. [DOI] [PubMed] [Google Scholar]
- 42.Bi X, Yang X, Bostrom MPG, Bartusik D, Ramaswamy S, Fishbein KW, et al. Fourier transform infrared imaging and MR microscopy studies detect compositional and structural changes in cartilage in a rabbit model of osteoarthritis. Analytical and Bioanalytical Chemistry. 2007;387(5):1601–1612. doi: 10.1007/s00216-006-0910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West PA, Bostrom MP, Torzilli PA, Camacho NP. Fourier transform infrared spectral analysis of degenerative cartilage: an infrared fiber optic probe and imaging study. Applied Spectroscopy. 2004;58(4):376–381. doi: 10.1366/000370204773580194. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein SL, Buckwalter JA. Turek’s Orthopaedics: Principles and Their Application. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 45.Saarakkala S, Julkunen P. Specificity of Fourier Transform Infrared (FTIR) microspectroscopy to estimate depth-wise proteoglycan content in normal and osteoarthritic human articular cartilage. Cartilage. 2010 doi: 10.1177/1947603510368689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayne R, Burgeson RE. Structure and function of collagen types. Orlando: Academic Press; 1987. p. 311. [Google Scholar]
- 47.Ballock RT, O’Keefe RJ. The biology of the growth plate. The Journal of Bone and Joint Surgery (American) 2003;85(4):715–726. [PubMed] [Google Scholar]
- 48.Hayes WC, Mockros LF. Viscoelastic properties of human articular cartilage. Journal of Applied Physiology. 1971;31(4):562–8. doi: 10.1152/jappl.1971.31.4.562. [DOI] [PubMed] [Google Scholar]
- 49.Hori RY, Mockros LF. Indentation tests of human articular cartilage. Journal of Biomechanics. 1976;9(4):259–68. doi: 10.1016/0021-9290(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 50.Sergerie K, Lacoursiere M-O, Levesque M, Villemure I. Mechanical properties of the porcine growth plate and its three zones from unconfined compression tests. Journal of Biomechanics. 2009;42(4):510–516. doi: 10.1016/j.jbiomech.2008.11.026. [DOI] [PubMed] [Google Scholar]