Abstract
Background
Coronary artery disease (CAD) is the leading killer in the U.S. Patients with severe CAD and ischemia have worse prognosis. Therefore expansion of biomarker research, to identify at risk individuals and explain the complex biology between cardiovascular growth factors and atherosclerosis is needed. Neuregulin-1β (NRG-1β) is a myocardial stress activated growth and survival factor released from endocardial and endothelial cells. NRG-1β is essential for cardiovascular development and a regulator of angiogenesis. We postulated that plasma and serum levels of NRG-1β would vary in relation to CAD severity and the presence of stress-induced ischemia.
Methods
We measured serum and plasma levels of NRG-1β and vascular endothelial growth factor (VEGF) in 60 patients undergoing cardiac catheterization. CAD severity was calculated from angiographic results using a modified Duke jeopardy score.
Results
Serum NRG-1β (sNRG-1β), plasma NRG-1β (pNRG-1β), serum VEGF (sVEGF), and plasma VEGF (pVEGF) were detectable in the majority of patients. The pNRG-1β levels were approximately two fold higher than sNRG-1β. Both sNRG-1β and pNRG-1β correlated inversely with CAD severity. Plasma NRG-1β levels were statistically higher in patients with stress-induced ischemia denoted by a positive myocardial perfusion imaging study that correlated with angiographic findings (p = 0.02).
Conclusions
Both serum and plasma NRG-1β correlated inversely with angiographic severity of CAD. Plasma NRG-1β levels were two fold higher than serum and were higher in patients with stress-induced ischemia. Therefore we conclude that plasma is the optimal source for the further exploration of the biological significance of NRG-1β as a biomarker of CAD severity and ischemia.
Keywords: biomarkers, growth factors, atherosclerosis, stress-induced ischemia, heregulin
Introduction
The severity of coronary artery disease (CAD) relates to several biological factors such as inflammatory cytokines and lipoproteins that subsequently influence atherosclerosis and angiogenesis. The relationship between vascular growth factors and CAD severity is poorly understood. A validated biomarker that reflects CAD severity and ischemic burden would be an important screening tool to facilitate the management of patients with cardiac risk factors and cardiac symptoms.
Neuregulin (NRG-1β) is a novel stress activated cardiac growth and angiogenic factor, which is activated by ischemia and exercise in animals [1, 2]. NRG-1β acts through ErbB receptors to regulate cell survival, growth, metabolism, as well as angiogenesis [1, 3–5]. We developed an assay to quantify circulating NRG-1β in the serum (sNRG-1β) [6]. Higher sNRG-1β levels were associated with worse symptomatic heart failure (HF) and poor prognosis, particularly in ischemic heart disease [7]. These results suggest that further work is needed to understand NRG-1β’s relationship with ischemia.
Vascular endothelial growth factor (VEGF), a potent initiator of angiogenesis, is rapidly up-regulated in animal models of myocardial ischemia and induced by neuregulin [8, 9]. Systemic and intracardiac VEGF levels in samples from patients with CAD have been measured previously with variable results [10–14].
The primary objective of this study was to determine if plasma/serum NRG-1β levels were associated with CAD severity or differed in the presence of ischemia in a cross-sectional sampling of patients who underwent coronary angiogram. The secondary objective was to further characterize the relationship of NRG-1β and VEGF as paracrine regulator of angiogenesis [15]. We postulated in our null hypothesis that there is no variation in NRG-1β levels by CAD severity and no difference due to ischemia. A rejected null hypothesis suggests that NRG-1β has a biological association with atherosclerosis and might serve as a potential vascular biomarker reflecting CAD severity and ischemia in patients with cardiovascular risk factors.
Methods
Study Patients
The study population consisted of a retrospective cohort of 60 patients who underwent coronary angiography due to either chest pain and/or a positive stress test from September 2004 – May 2005 from a single urban, academic referral center. Patients were selected and divided into 3 groups according to the severity of CAD: 20 consecutive patients with angiographically normal coronary arteries, 20 with >50% stenotic disease in at least one major coronary artery branch and 20 with occluded disease in at least one major coronary artery branch. The CAD severity was further characterized in these subgroups using the modified Duke jeopardy score, which accounts for lesion location, degree of stenosis, and number of vessels involved. Severity score of 0 = no angiographic CAD, 2 = mild CAD, 4–6 = moderate CAD, and 8–12 = severe CAD [16]. Stress-induced ischemia was identified in those patients who underwent a nuclear myocardial stress test with perfusion mismatch or diagnostic EKG changes that correlated with angiographic findings of a hemodynamically significant lesion in the corresponding location. Collateral arteries were noted during angiography when territories were supplied by alternative artery distal to a significant stenosis in a major coronary vessel.
Exclusion criteria were atrial fibrillation, impaired left ventricular function (ejection fraction <45%), significant valvular heart disease, recent (<3 months) ischemic stroke, renal failure, hepatic impairment, chronic obstructive pulmonary disease, neoplasm, connective tissue disease, infections, or steroid use prior to cardiac catheterization. The Institutional Review Board approved the study and all participants signed an informed consent allowing blood samples to be stored and used for future cardiac studies.
Collection of Samples
Arterial blood samples were collected in vacuum tubes containing no additives and in vacuum tubes containing EDTA from a 6F arterial sheath prior to any anticoagulation. Samples were drawn from a femoral arterial sheath to avoid unnecessary peripheral blood draws and to standardize processing of blood samples. After centrifugation at 3,000 rpm for 10 min at room temperature, serum and plasma were separated, stored in aliquots and kept frozen at −80° C until measurement. Plasma samples were centrifuged within 30 minutes of collection, and serum samples were centrifuged after 30 minutes at room temperature. Frozen serum and plasma samples were thawed for VEGF and NRG-1β in a single run.
Description of ELISA’s: VEGF and NRG-1β
Growth factors were measured by enzyme-linked immunoassays (ELISA) as follows: A commercially available Quantikine ELISA kit from R&D Systems, Minneapolis, MN (cat#DVE00) was used to quantify VEGF levels. Both plasma and serum samples were diluted (1:4 in PBS).
We measured NRG-1β levels using DuoSet ELISA development system (R&D cat# DY377) [7]. Briefly a 96 well plate (Pierce cat # 15041) was coated with the capture antibody overnight at room temperature on a plate shaker. Capture antibody was washed and the plate was blocked with blocking buffer as described in the package insert. A standard curve was generated using the lyophilized NRG-1β according to manufacturer’s instructions with 2% normal goat serum (Invitrogen: cat # PCN5000). The standards and diluted samples (1:3 in PBS) were added to each well and incubated for 2 hours. After serial washing, the detection antibody was added, incubated at room temperature and then washed. Streptavidin-HRP was added and incubated on the plate shaker for 30 minutes at room temperature. The plate was washed and then the substrate solution (Thermo Scientific 1-Step Ultra TMB-ELISA cat # 34028) was added for 10 minutes protected from light. Sulfuric acid stop solution was added and the absorbance was read at 450nm using a spectraphotometric plate reader.
Aliquoted samples were run in duplicates and the average of the two values were used in the analysis. The detection limit for VEGF ranged from 31.2 – 2,000 pg/ml. The average intra-assay coefficient of variation for serum VEGF was 9.7% and plasma VEGF was 13.5 % with inter-assay coefficient of variation for all VEGF < 14.0%. Serum and plasma NRG-1β detection limit ranged from 0.3 to 30 ng/ml. Samples below the detectable limit (n = 12 serum and n = 0 plasma) were assigned a value half-way between 0 and lowest limit (0.15 ng/ml). The average intra-assay coefficient of variation for serum NRG-1β was 5.6% and plasma NRG-1β 3.9% with inter-assay coefficient of variation for all NRG1β < 7.0%.
Statistical analysis
Continuous variables were summarized as mean± SD. Kruskal-Wallis test was used to test for a difference between multiple CAD severity groups and Mann-Whitney U test was used to detect a difference between two groups. Categorical variables were summarized as count and percentage and compared by Chi-square test between multiple CAD severity groups. VEGF and NRG-1β values were non-normally distributed based on Shapiro-Wilks test and therefore the median values and ranges were also reported in addition to the standard deviation (± SD). Spearman’s correlation coefficient was calculated and tested between serum and plasma VEGF and NRG-1β and continuous cardiac risk factors. A p value < 0.05 was considered statistically significant. Due to the discovery nature of the study, multiple comparisons are not adjusted and further confirmatory studies are needed. Statistical analysis was performed with statistical software R (www.r-project.org) and SPSS Statistics version 17.0 (SPSS Inc, Chicago Illinois).
Results
Characteristics of study patients
Baseline characteristics showed that aspirin was used more frequently in groups with CAD (mild Duke score = 2, moderate Duke score = 4–6, and severe Duke score 8 –12) (p = 0.01) than patients without CAD (none Duke score = 0). No other clinically significant differences were seen between groups with varying CAD severity (Table 1). Although not statistically different within the Duke severity score groups, more than half of all patients were on a statin medication. The high percentage of statin use reflects a higher risk population that was referred for cardiac catheterization.
Table 1.
Baseline Cardiac Risk Factors and Medication Profile
| No CAD | Mild CAD | Moderate CAD | Severe CAD | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | Duke Score 0 | Duke Score 2 | Duke Score 4 – 6 | Duke Score 8 –12 | |||||
| number (%) | n = 21 | n = 12 | n = 16 | n = 11 | |||||
| Age | 55 | ± 11 | 58 | ± 8 | 62 | ± 10 | 63 | ± 9 | 0.15 |
| Body Mass Index | 36 | ± 13 | 33 | ± 6 | 33 | ± 10 | 41 | ± 18 | 0.35 |
| EF (%) | 63 | ± 8 | 55 | ± 7 | 62 | ± 3 | 56 | ± 14 | 0.06 |
| Males (%) | 9 | 42.9% | 10 | 83.3% | 11 | 68.8% | 7 | 63.6% | 0.12 |
| Hypertension | 19 | 90.5% | 10 | 83.3% | 13 | 81.3% | 8 | 72.7% | 0.63 |
| Hypercholesterolemia | 15 | 71.4% | 9 | 75.0% | 12 | 75.0% | 11 | 100.0% | 0.28 |
| Diabetes | 6 | 28.6% | 5 | 33.3% | 4 | 25.0% | 5 | 41.7% | 0.71 |
| Current Smoker | 7 | 33.3% | 7 | 58.3% | 7 | 43.8% | 7 | 63.6% | 0.32 |
| Family History CAD | 13 | 61.9% | 7 | 58.3% | 7 | 43.8% | 6 | 54.5% | 0.73 |
| Prior angioplasty/stent | 0 | 0.0% | 2 | 16.7% | 3 | 18.8% | 0 | 0.0% | 0.09 |
| Aspirin | 10 | 47.6% | 12 | 100.0% | 13 | 81.3% | 8 | 72.7% | 0.01 |
| Clopidogrel | 3 | 14.3% | 4 | 33.3% | 6 | 37.5% | 3 | 27.3% | 0.41 |
| Beta-Blocker | 9 | 42.9% | 7 | 58.3% | 9 | 56.3% | 5 | 45.5% | 0.78 |
| ACE | 8 | 38.1% | 4 | 33.3% | 6 | 37.5% | 5 | 45.5% | 0.95 |
| ARB | 5 | 23.8% | 3 | 14.3% | 1 | 6.3% | 0 | 0.0% | 0.16 |
| CCB | 6 | 28.6% | 3 | 25.0% | 4 | 25.0% | 2 | 18.2% | 0.94 |
| Diuretics | 9 | 42.9% | 6 | 50.0% | 4 | 25.0% | 5 | 45.5% | 0.53 |
| Statin | 11 | 52.4% | 9 | 75.0% | 10 | 62.5% | 9 | 81.8% | 0.33 |
SD = standard deviation
Serum and Plasma VEGF/NRG-1β and Severity of CAD
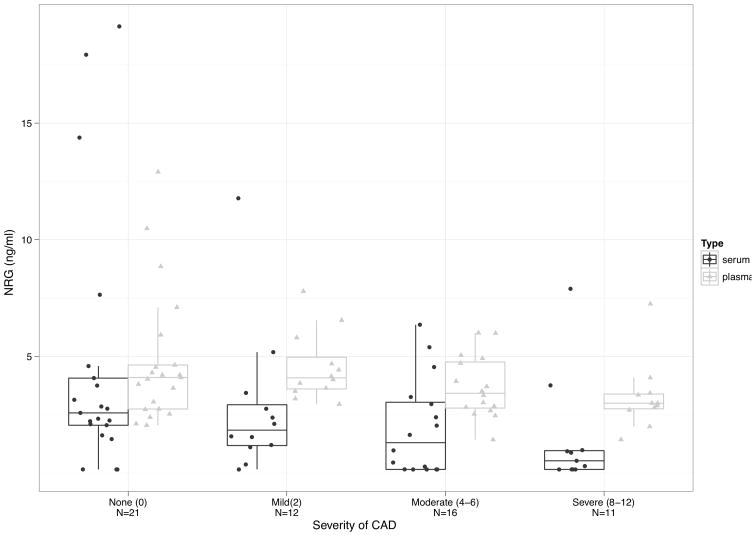
There was a statistically significant correlation between serum and plasma NRG-1β demonstrating an association with lower circulating NRG-1β in the presence of more diffuse coronary artery disease (sNRG-1β rho = 0.363, p = 0.004 and pNRG-1β rho = 0.261, p = 0.044) (Figure 1). There was no statistically significant association with serum or plasma VEGF and CAD severity. Serum and plasma NRG-1β and VEGF were non-normally distributed with a trend toward lower levels in the presence of more severe CAD (Table 2).
Figure 1.
Boxplots with mean and standard deviation of sixty patients who underwent coronary angiogram to assess CAD severity. CAD severity was classified into four groups using the modified Duke jeopardy score (none, mild, moderate, and severe CAD). Both serum and plasma NRG-1β levels were inversely associated with CAD severity (sNRG-1β rho = 0.363, p = 0.004, and pNRG-1β rho = 0.261, p = 0.044, Spearman’s correlation).
Table 2.
Variation in VEGF and NRG-1β Levels by CAD Severity
| median (range) ± SD | No CAD | Mild CAD | Moderate CAD | Severe CAD | ||||
|---|---|---|---|---|---|---|---|---|
| Duke Score 0 | Duke Score 2 | Duke Score 4 – 6 | Duke Score 8 –12 | |||||
| n = 21 | n = 12 | n = 16 | n = 11 | |||||
| serum VEGF (pg/ml) | 176 (16 – 1538) | ± 491 | 260 (16 –813) | ± 294 | 200 (16 –822) | ± 263 | 122 (16 –496) | ± 209 |
| plasma VEGF (pg/ml) | 101 (16 –1399) | ± 293 | 80 (30 –257) | ± 728 | 237 (30 –744) | ± 215 | 256 (55 –370) | ± 120 |
| serum NRG (ng/ml) | 2.6 (0.2 –19.1) | ± 5.5 | 1.8 (0.2 –11.8) | ± 3.1 | 1.3 (0.2 –6.4) | ± 2.1 | 0.5 (0.2 –7.9) | ± 2.4 |
| plasma NRG (ng/ml) | 4.1 (2.0 –12.9) | ± 2.9 | 4.0 (2.9 – 7.8) | ± 1.5 | 3.4 (1.4 –6.0) | ± 1.3 | 3.0 (1.4 –7.2) | ± 1.5 |
Difference between Serum and Plasma VEGF/NRG-1β and Stress-induced Ischemia
Levels of pNRG-1β were higher in the group with ischemia (n = 23) (median 4.1 ng/ml ± 2.2 ng/ml) than in the group without ischemia (pNRG-1β in absence of stress-induced ischemic group n = 37 median 3.3 ng/ml ± 1.9 ng/ml, p = 0.02). No statistical differences were observed in sNRG-1β, sVEGF, or pVEGF in the presence or absence of stress-induced ischemia, however there was a trend toward higher mean sNRG-1β in patients with stress-induced ischemia (Table 3).
Table 3.
Difference in VEGF and NRG-1β by Stress-Induced Ischemia
| Median (range) ± SD | Positive Ischemia n = 23 | Negative Ischemia n = 37 | p value |
|---|---|---|---|
| serum VEGF (pg/ml) | 247 (16 –1148) ± 312 | 163 (16 –1538) ± 384 | 0.40* |
| plasma VEGF (pg/ml) | 131 (16 –1399) ± 298 | 103 (20 –744) ± 150 | 0.84* |
| serum NRG (ng/ml) | 2.4 (0.2 –19.1) ± 4.3 | 1.6 (0.2 –17.9) ± 3.8 | 0.13* |
| plasma NRG (ng/ml) | 4.1 (1.9 –12.9) ± 2.2 | 3.3 (1.4 –10.5) ± 1.9 | 0.02* |
Mann-Whitney U test
Correlations between VEGF, NRG-1β and Collateral Coronary Arteries
No significant correlation was observed between sVEGF and sNRG-1β (rho = 0.121, p = 0.356), or pVEGF and pNRG-1β (rho = 0.058, p = 0.657). No correlation was observed between collateral artery presence and sNRG-1β, pNRG-1β, sVEGF, or pVEGF.
Discussion
The principle finding of this study was that in patients with stable CAD serum and plasma NRG-1β inversely correlated with CAD severity. Plasma NRG-1β levels were detectable in all participants and were two-fold higher than serum, making plasma the optimal source for detecting circulating NRG-1β. Additionally plasma NRG-1β levels were statistically higher in patients with stress-induced ischemia, supporting the notion that circulating NRG-1β is induced by cardiac stress and perfusion mismatch related to atherosclerosis. Similarly we observed in a heart failure cohort of nearly 900 patients that patients with ischemic heart failure (HF) had higher circulating NRG-1β levels than patients with other causes HF [7]. Additionally patients with ischemic HF had increased risk of death or transplantation suggesting that NRG-1β is a potentially useful biomarker corresponding with ongoing cardiovascular ischemic stress that has prognostic significance. The study confirms that in a mixed cohort of patients ranging from relatively healthy patients with few cardiac risk factors to severe CAD, circulating NRG-1β is detectable in measurable amounts. While larger studies are needed to understand the biological significance of circulating NRG-1β in the progression of atherosclerosis, and acute coronary syndromes, there are plausible explanations for higher levels of pNRG-1β due to ischemia and lower levels in more severe CAD.
The association between reduced circulating NRG-1β in the presence of more severe CAD may be an indication of endothelial dysfunction. NRG-1β is released from vascular endothelial cells and exerts paracrine effects on local myocytes and blood vessels [5]. It is well known that preceding angiographically evident CAD, endothelial dysfunction can be demonstrated with altered vasoreactivity and decreased nitric oxide production. Thus, one explanation for our findings is that as CAD progresses endothelial cells have impaired NRG-1β release as a manifestation of this dysfunction. In addition, severe CAD is known to be a state of increased inflammation. Inflammatory cytokines decrease total NRG-1β transcript and expression level, in vitro (unpublished data, Cote and Sawyer), providing another explanation for the relationship between NRG-1β levels and CAD severity. Previous works has established that NRG-1β is expressed in atherosclerotic lesions in human coronaries and carotid arteries [17–19]. Recent studies demonstrated that NRG-1β was atheroprotective by suppressing macrophage foam cell formation in plaques [20]. Additionally they showed that in ApoE knock out mice that a chronic infusion of NRG-1β decreased atherosclerosis in the aorta while anti-NRG-1β antibody (aka Her2+ receptor antibody) accelerated aortic atherosclerosis. The current study supports the concept that endogenous NRG-1β plays a role in modulating severity of atherosclerosis.
There are other explanations for the lower NRG-1β level in patients with more advanced CAD. We have previously found that multiple NRG-1β isoforms are expressed in adult human and rat skeletal muscle. Exercise of skeletal muscle in rodents induces activation of NRG-1β/ErbB signaling [2]. In a cohort of healthy subjects, serum NRG-1β levels are positively correlated with cardio-respiratory fitness represented by maximum oxygen consumption (VO2 max) [6]. Thus, lower NRG-1β levels in patients with significant CAD, especially those with occluded coronary arteries, may be attributable to reduced physical activity and release of skeletal muscle derived NRG-1β. In addition, sarcopenia is associated with advanced cardiac disease including CAD, and this may contribute to the current findings [21]. These observations expand our understanding of the relationship between NRG-1β and cardiovascular disease and set the foundation for future studies.
Our investigation also demonstrated that plasma concentrations of NRG-1β were statistically higher in patients with stress-induced ischemia. Previous work has shown oxidatitve stress, as well as ischemia/reperfusion promotes the proteolytic cleavage of NRG-1β from endothelial cells, which in turn promotes cell survival through paracrine signaling. NRG-1β expression can be up-regulated by cerebral ischemia and provides neuroprotective benefit decreasing the size of cerebral infarction in animals with ischemic stroke [19, 22]. Since NRG-1β is known to induce angiogenic factors, the presence of higher plasma levels in patients with stress-induced ischemia may reflect a cardioproetective mechanism signaling neovascularization and cardioprotection similar to its neuroprotective property. This study is the first to suggest that plasma NRG-1β is a detectable biomarker of stress-induced ischemia in humans and further studies are needed to explore the biological implications and clinical outcomes of these findings.
The biology of NRG-1β is complex and will require larger studies to clarify the effects of cardiac medications (i.e. aspirin, clopidogrel, beta-blockers, angiotensin converting enzyme nhibitor, angiotensin receptor blockers) on the measurement of NRG-1β as a biomarker. In the current study there was no statistically significant difference in the cohort with regards to cardiac medications and severity of CAD. We hypothesize that cardiac medications that improve endothelial function and cardiovascular remodeling may result in increased endothelial cell NRG-1β expression reflecting an improvement in vascular health.
Although in vivo studies suggest that NRG-1β can regulate VEGF expression [15], we did not observe a correlation between sNRG-1β/sVEGF or pNRG-1β/sVEGF. In addition neither serum nor plasma VEGF varied by CAD severity. We did observe a statistically significant inverse correlation between serum and plasma NRG-1β and CAD severity. The correlation coefficient may have been improved with a more rigorous angiographic scoring system such as the SYNTAX or other scoring systems [23, 24]. The Duke jeopardy scoring system was selected for this study due fact it is easily calculated and accounts for major coronary factors that modify clinical outcomes (i.e. number of coronary vessels, location of stenosis, and severity of occlusion). The correlation coefficients may have been strengthened if the Duke jeopardy scoring system was not categorized, but classification system (mild, moderate, severe CAD) improved clinical utility.
In conclusion our results suggest that NRG-1β levels inversely correlates with CAD severity in stable patients and plasma NRG-1β levels are statistically higher in patients with stress-induced ischemia. Recently several biomarkers such as cystatin C and angiogenin demonstrate an association with severity of CAD similar to NRG-1β [25, 26]. NRG-1β also appears to be altered by the stress-induced ischemia, and therefore may have additional utility over other biomakers. The small sample size and baseline confounders prevent us from concluding a cause-effect association. Further studies are needed with larger sample sizes, prospective design, and patients with unstable CAD/acute infarctions to investigate if plasma NRG-1β is a reliable biomarker reflecting severity of CAD and burden of cardiac ischemia. NRG-1β biology is complex and will require more studies to understand its role as a surrogate biomarker of atherosclerosis and ischemia stress response but this study demonstrates that plasma is optimal source for future biomarker development.
Limitations
The convenient sample of 60 subjects with different CAD severity provides a relatively small sample size. The exclusion criteria limits the generalizability of our findings to other cohorts. The study was performed at a single center, and therefore subject to aggregation bias.
Acknowledgments
Support
This project was supported in part by American Heart Association Established Investigator Award, Dallas, Texas, USA (DBS), HL068144, Heart Failure Society of America Research Fellowship Grant, Saint Paul, Minnesota, USA (CAG), and Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH, Nashville, TN, USA (CAG).
References
- 1.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, et al. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004 Dec 3;279(49):51141–7. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 2.Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003 May;284(5):C1149–55. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 3.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007 Aug 21;116(8):954–60. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 4.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006 Jul 14;281(28):19469–77. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 5.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005 Nov 15;311(1):135–46. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Moondra V, Sarma S, Buxton T, Safa R, Cote G, Storer T, et al. Serum Neuregulin-1β as a Biomarker of Cardiovascular Fitness. The Open Biomarkers Journal. 2009;2:1–5. doi: 10.2174/1875318300902010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, et al. Neuregulin-1{beta} Is Associated With Disease Severity and Adverse Outcomes in Chronic Heart Failure. Circulation. 2009 Jul 28;120(4):310–7. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto E, Ogita T, Nakaoka T, Matsuoka R, Takao A, Kira Y. Rapid induction of vascular endothelial growth factor expression by transient ischemia in rat heart. Am J Physiol. 1994 Nov;267(5 Pt 2):H1948–54. doi: 10.1152/ajpheart.1994.267.5.H1948. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga T, Warltier DC, Tessmer J, Weihrauch D, Simons M, Chilian WM. Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ Physiol. 2003 Jul;285(1):H352–8. doi: 10.1152/ajpheart.00621.2002. [DOI] [PubMed] [Google Scholar]
- 10.Kucukardali Y, Aydogdu S, Ozmen N, Yonem A, Solmazgul E, Ozyurt M, et al. The relationship between severity of coronary artery disease and plasma level of vascular endothelial growth factor. Cardiovasc Revasc Med. 2008 Apr-Jun;9(2):66–70. doi: 10.1016/j.carrev.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Alber HF, Frick M, Dulak J, Dorler J, Zwick RH, Dichtl W, et al. Vascular endothelial growth factor (VEGF) plasma concentrations in coronary artery disease. Heart. 2005 Mar;91(3):365–6. doi: 10.1136/hrt.2003.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenihan DJ, Osman A, Sriram V, Aitsebaomo J, Patterson C. Evidence for association of coronary sinus levels of hepatocyte growth factor and collateralization in human coronary disease. Am J Physiol Heart Circ Physiol. 2003 May;284(5):H1507–12. doi: 10.1152/ajpheart.00429.2002. [DOI] [PubMed] [Google Scholar]
- 13.Fleisch M, Billinger M, Eberli FR, Garachemani AR, Meier B, Seiler C. Physiologically assessed coronary collateral flow and intracoronary growth factor concentrations in patients with 1- to 3-vessel coronary artery disease. Circulation. 1999 Nov 9;100(19):1945–50. doi: 10.1161/01.cir.100.19.1945. [DOI] [PubMed] [Google Scholar]
- 14.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin Sci (Lond) 2002 Feb;102(2):187–94. [PubMed] [Google Scholar]
- 15.Yonezawa M, Wada K, Tatsuguchi A, Akamatsu T, Gudis K, Seo T, et al. Heregulin-induced VEGF expression via the ErbB3 signaling pathway in colon cancer. Digestion. 2009;80(4):215–25. doi: 10.1159/000229775. [DOI] [PubMed] [Google Scholar]
- 16.Califf RM, Phillips HR, 3rd, Hindman MC, Mark DB, Lee KL, Behar VS, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985 May;5(5):1055–63. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 17.Panutsopulos D, Arvanitis DL, Tsatsanis C, Papalambros E, Sigala F, Spandidos DA. Expression of heregulin in human coronary atherosclerotic lesions. J Vasc Res. 2005 Nov-Dec;42(6):463–74. doi: 10.1159/000088100. [DOI] [PubMed] [Google Scholar]
- 18.Sigala F, Georgopoulos S, Papalambros E, Chasiotis D, Vourliotakis G, Niforou A, et al. Heregulin, cysteine rich-61 and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with clinical data. Eur J Vasc Endovasc Surg. 2006 Sep;32(3):238–45. doi: 10.1016/j.ejvs.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004 Aug;25(7):935–44. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, Nagashima M, et al. Preventive effects of heregulin-beta1 on macrophage foam cell formation and atherosclerosis. Circ Res. 2009 Aug 28;105(5):500–10. doi: 10.1161/CIRCRESAHA.109.193870. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 22.Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neuregulin expression after focal stroke in the rat. Neurosci Lett. 2002 Dec 16;334(3):169–72. doi: 10.1016/s0304-3940(02)01126-6. [DOI] [PubMed] [Google Scholar]
- 23.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983 Feb;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 24.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. Euro Intervention. 2005 Aug;1(2):219–27. [PubMed] [Google Scholar]
- 25.Kiyosue A, Hirata Y, Ando J, Fujita H, Morita T, Takahashi M, et al. Plasma cystatin C concentration reflects the severity of coronary artery disease in patients without chronic kidney disease. Circ J. Nov;74(11):2441–7. doi: 10.1253/circj.cj-10-0158. [DOI] [PubMed] [Google Scholar]
- 26.Krecki R, Krzeminska-Pakula M, Drozdz J, Szczesniak P, Peruga JZ, Lipiec P, et al. Relationship of serum angiogenin, adiponectin and resistin levels with biochemical risk factors and the angiographic severity of three-vessel coronary disease. Cardiol J. 17(6):599–606. [PubMed] [Google Scholar]