Abstract
CRES is the defining member of a reproductive subgroup within the family 2 cystatins of the cystatin superfamily of cysteine protease inhibitors. CRES is synthesized and secreted by the initial segment of the epididymis and is present in the sperm acrosome suggesting roles in sperm maturation and fertilization. We have previously demonstrated that CRES is present within the epididymal lumen as monomeric (14 and N-glycosylated 19 kDa forms) as well as SDS-sensitive and SDS-resistant high molecular mass complexes. We have also shown that recombinant CRES protein will self-aggregate and form amyloid structures in vitro raising the possibility that CRES might also form amyloid in vivo. Amyloid is a large protein aggregate with a specific cross β sheet structure and its presence is usually associated with disease. This review discusses protein aggregation in the epididymis as well as provides a brief overview of amyloid formation including recent studies in other organ systems identifying examples of amyloid that are nonpathological and carry out biological functions, i.e. functional amyloid. Studies that were carried out to determine if amyloid is present in the epididymal lumen and if CRES is associated with these structures are also described. The presence of CRES amyloid in the mouse epididymal lumen yet the absence of pathology suggest either the presence of mechanisms to neutralize the cytotoxicity associated with pathological amyloid or that CRES is a new example of a functional amyloid with roles in epididymal function.
Keywords: cystatins, epididymis, quality control
Epididymal luminal environment
It is well recognized that the primary function of the epididymis is to mature spermatozoa, allowing them to transition from nonfunctional immature cells entering from the testis to functionally mature cells in the cauda epididymis. During epididymal transit spermatozoa acquire progressive motility and the ability to fertilize an oocyte. Because spermatozoa are synthetically inactive the maturation process involves the interaction of spermatozoa with proteins that are synthesized and secreted in a region-dependent manner from the epididymal epithelium. Much emphasis has been placed on identifying and studying these epididymal secretory proteins to establish whether they interact with spermatozoa and/or to determine their roles in sperm maturation.
Perhaps equally important is understanding the complex epididymal luminal environment as a whole, since perturbations in this environment can result in abnormal sperm function. Indeed in addition to sperm maturation, a critical function of the epididymal lumen is to protect the spermatozoa while they undergo maturation. Thus many luminal proteins may not play direct roles in sperm maturation but rather help create the appropriate environment that is conducive for this process to occur. This can include proteins involved in the regulation of luminal pH, osmolality, regulation of oxidative stress, and regulation of protein folding/misfolding. In particular, the epididymal lumen is rich in chaperones with clusterin alone contributing 41% of the total protein secreted into the rat caput epididymidis suggesting an important role for this protein in maintaining protein solubility within the lumen (Dacheux and Dacheux 2002). Mechanisms also exist to remove secreted proteins from the lumen, presumably once their functions have been carried out or if misfolding occurs. While a number of proteins secreted by the initial segment, the most proximal region of the epididymis, are detected in the lumen throughout the rest of the epididymis and into the cauda region, other epididymal secretory proteins are removed from the lumen suggesting that a prolonged stay could be detrimental to epididymal function. While the uptake of luminal proteins is not well understood, the epididymal epithelium carries out fluid phase, adsorptive and receptor-mediated endocytosis (Andonian S and Hermo L, 1999). Components of a functional ubiquitin- proteosome pathway including ubiquitin activating enzyme E1, ubiquitin carrier enzyme E2, and the ubiquitin C-terminal hydrolase PGP9.5/UCHLI have also been detected in the epididymal lumen suggesting an alternative pathway for turnover of luminal proteins and/or removal of defective sperm (Baska et al., 2008). The highly regulated and regionalized secretion and removal of proteins from the lumen is indicative of a unique microenvironment that likely utilizes a variety of quality control mechanisms to maintain the appropriate conditions for sperm maturation.
Finally, it is important to realize that the epididymal luminal compartment is not a dilute sea of soluble proteins and spermatozoa but rather a dense and crowded milieu due to the loss of greater than 90% of the water from the luminal fluid as it moves through the efferent ducts and into the initial segment of the epididymis. This loss of water and the concentration of spermatozoa create a close association of luminal components with spermatozoa which is thought to be critical for normal sperm maturation. The loss of water can also create a situation described in other cellular systems as macromolecular crowding which can result in protein misfolding and aggregation (Rialdi and Barrisel 2007; Minton 2005). Indeed, examination of the luminal compartment by electron microscopy shows that, in addition to spermatozoa, the lumen is highly particulate in nature (Figure 1). Some of these structures are small membrane bound vesicles termed epididymosomes which are released from the epithelium by apocrine secretion and are proposed to interact with spermatozoa as part of the maturation process (Sullivan et al., 2007). Various other aggregate or fibrillar type structures have also been detected in the epididymal lumen; however, how they are formed and what their functions are is not known. These structures include large electron dense bodies which contain heat shock protein 1 (HSPD1, HSP60) and tumor rejection antigen (TRA1), a member of the heat shock protein 90 family (Asquith et al., 2005). Because heat shock proteins have proposed roles in the folding of denatured proteins, these structures may function as a site of protein refolding or sequestration of proteins targeted for clearance from the lumen or may facilitate protein interactions with spermatozoa. Thus, soluble as well as insoluble components are an integral part of the epididymal lumen.
Figure 1. Transmission electron micrograph of the initial segment region of the mouse epididymis showing particulate material within the lumen.
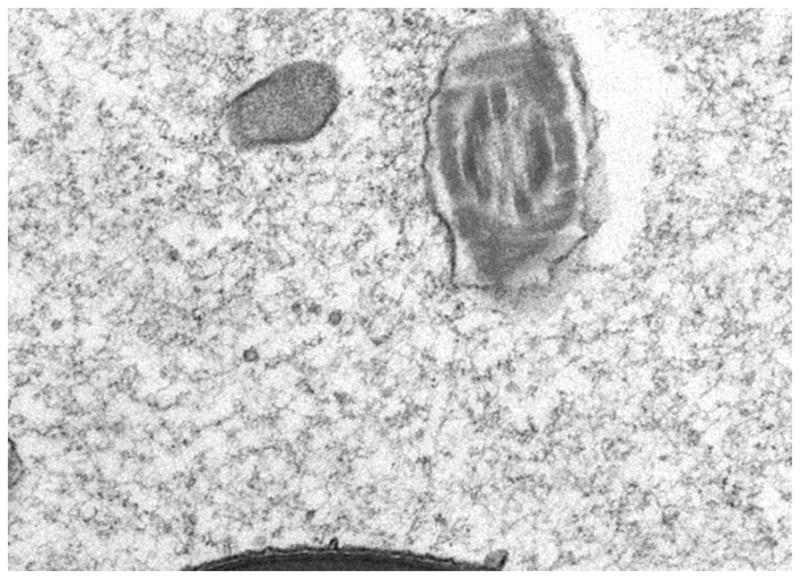
16500X magnification. Micrograph kindly provided by A. Parent and L. Hermo, McGill University, Montreal, Quebec Canada.
Amyloid
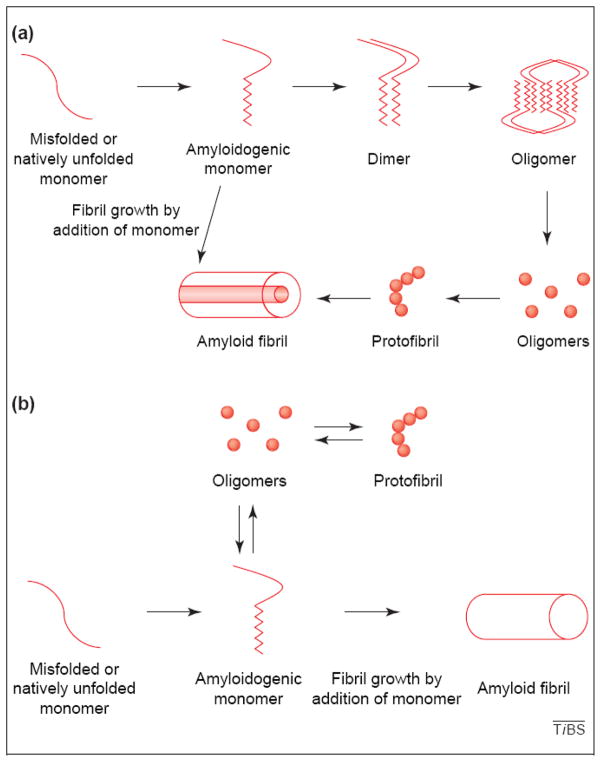
Proteins that misfold and form self aggregates with a specific cross beta sheet structure are called amyloid. In most cases, the formation of these protein aggregates are associated with incurable neurodegenerative diseases such as Creutzfeldt-Jakob, Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral schlerosis, and many others. While the protein responsible for the formation of the initial aggregate/amyloid differs among the diseases, the structures formed by these unrelated proteins are remarkably similar. Indeed proteins that form amyloid exhibit similar characteristics including a cross beta sheet structure, some SDS and/or protease resistance and often the requirement for harsh treatments such as formic acid to solubilze, and can be intra or extracellular. The self aggregated protein can be up to a million Daltons in molecular weight which make it difficult to detect by standard SDS-PAGE. Typically, proteins that form amyloid appear to follow a defined aggregation pathway with monomeric forms of proteins first forming small soluble oligomers that can be detected by electron microscopy as small ball-like structures. (Glabe, 2004; Figure 2). These structures then self-associate to form bead- on- a-string type structure termed a protofibril which can then self-organize into fibrils. While it was previously thought that in diseases such as Alzheimer’s the fibrillar forms of amyloid were causing the pathology, the current view is that the intermediate amyloid structures, precursors to the fibrils, such as the oligomers and protofibrils are the cytotoxic forms of amyloid. Studies suggest that these soluble forms of amyloid may be able to elicit their cytotoxic effects by increasing membrane conductance or creating toxic ion channels in cell membranes resulting in altered ionic homeostasis and ultimately cell death (Kayed et al., 2004; Jang et al., 2010).
Figure 2. Proposed pathways for amyloid formation.
Reprinted from Trends in Biochemical Sciences, vol 29, Charles G. Glabe, “Conformation dependent antibodies target diseases of protein misfolding”, p542–546, 2004, with permission from Elsevier.
Thus far only prion protein has been shown to be infectious. However, recent studies of several other amyloid forming proteins including tau, α-synuclein, and polyglutamine show these aggregates can be released from donor cells and taken up by adjacent acceptor cells in culture (Desplats et al., 2009;). Furthermore, intraperitoneal injection of brain lysates containing β-amyloid aggregates resulted in Alzheimer’s disease-type pathologies in the brains of recipient mice (Eisele et al., 2010). Thus the ability of amyloid to be transmitted between cells, either by direct interactions of the protein aggregate with cell membranes or in combination with other proteins/lipids/may also be a common characteristic between amyloid forming proteins.
Functional amyloid
While it is well established that bacteria and yeast utilize amyloid for biological functions, it has only been within the past few years that examples of functional amyloid in mammals have been identified. The Pmel17 protein in melanosomes forms an amyloid structure that is thought to function as a template for the synthesis of melanin while in the pituitary gland several hormones are stored in secretory granules as highly stable amyloid structures that are then reversed upon secretion (Fowler et al., 2006; Maji et al., 2009). The mechanisms adopted by the organism to avoid any cytotoxicity associated with amyloid, in particular the soluble intermediate forms, are varied. Bacteria control the when and where of amyloid formation of the curl proteins by virtue of the expression of several curli protein family members, one of which facilitates the formation of amyloid in the other family members (Wang et al 2008). Control of Pmel17 amyloid is thought to involve several mechanisms including the rapid transition of monomeric to fibrillar forms such that cytotoxic intermediates are avoided, the formation of Pmel17 amyloid in melanosomes so that, if formed, cytotoxic amyloid is sequestered from the cytosol, and finally the requirement of proteolytic cleavage of Pmel17 prior to amyloid formation (Fowler et al., 2006, Berson et al., 2001).
In addition to its structural advantages, in some cases organisms such as yeast have used amyloid as a means for a protein to adopt a new function. When Sup 35p, a yeast translational termination factor, aggregates and forms amyloid, this allows translational read-through allowing the propagation of a new phenotype and ability to adapt to different growth conditions (Fowler et al., 2007). Recently, in a tantalizing perspective Halfmann and Lindquist, 2010 proposed that prions are in fact an unusual form of epigenetics. Under specific conditions such as environmental stress, some proteins will form a prion (amyloid) conformation allowing diversity in protein function and adaptation to a new environment. This prion structure can then self-propagate its structure resulting in a phenotype that is heritable. Considering that the epididymal lumen must not only mature but protect spermatozoa to ultimately allow propagation of the species, the presence of prion-like proteins in the lumen surrounding the spermatozoa could provide a unique mechanism for the epididymis to rapidly adjust to stresses that could negatively impact sperm function. Taken together, it is clear that amyloid has been maintained throughout evolution and that nature has put to good use this highly stable structure. However, when amyloid formation is no longer controlled such as in pathological states, the outcome is severe ultimately resulting in the death of the organism. Thus understanding mechanisms that an organism utilizes to regulate the formation of functional amyloid to avoid cytotoxicity, and to disaggregate amyloid are of critical importance for ultimately developing new treatments for diseases due to amyloidogenesis.
CRES
CRES is the defining member of a new subgroup within the family 2 of the cystatin superfamily of cysteine protease inhibitors. CRES is distinct from the typical family 2 cystatins, such as cystatin C, by its reproductive specific expression and its lack of consensus sites for cysteine protease inhibition (Cornwall et al., 1994). Specifically CRES is synthesized and secreted by the initial segment region of the mouse epididymis suggesting roles in sperm maturation (Cornwall et al., 1994; Cornwall and Hann, 1995). We have previously demonstrated that in vitro CRES has the propensity to form amyloid and will transition from a monomer to fibril characteristic of amyloid associated with pathological conditions (von Horsten et al., 2007) (Figure 3). This is not unlike cystatin C which also has been shown to form amyloid in vitro. An L68Q mutant form of human cystatin C is highly aggregation prone resulting in cerebral amyloid angiopathy and resulting death due to cerebral hemorrhage in affected patients (Ghiso et al., 1986). Within the epididymal lumen, CRES is synthesized and secreted as a monomeric form in the initial segment region of the mouse epididymis and then appears to transition to a highly stable high molecular mass form in the luminal fluid from more distal epididymal regions (Figure 4a). Based on our studies in vitro, we propose that the high molecular mass forms in the epididymal lumen represent the self-aggregated amyloidogenic form of CRES. In support that CRES is present as a high molecular mass structure are our recent immunofluorescence studies showing that in the cauda lumen CRES is present only as punctuate structures and that some CRES is also detected in the clear cells suggesting the possibility of CRES turnover by endocytosis (Parent et al., 2011) (Figure 4b). Thus along the epididymal tubule there is a transition of CRES from soluble monomeric forms to insoluble aggregates possibly amyloid.
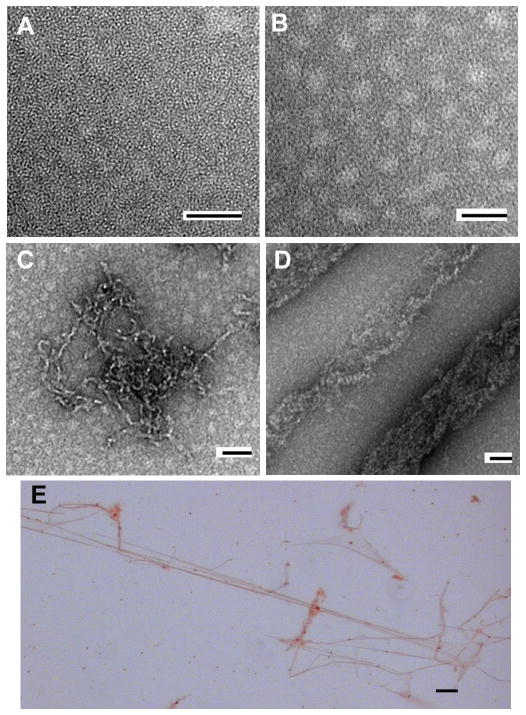
Figure 3. Negative stain electron microscopy and Congo Red staining of CRES amyloid structures.
A–F, electron micrographs of CRES allowed to oligomerize at 37 °C and then negatively stained with 2% uranyl acetate. Monomeric (A) forms of CRES progressed to oligomeric forms (B and C) and eventually formed fibrillar structures (D). Bar in all micrographs, 50 nm. (E), light microscopic analysis of CRES fibrils stained with Congo Red. Bar, 5 μm. This research was originally published in von Horsten HH, Johnson SS, SanFransisco SK, Hastert MC, Whelly SM, Cornwall GA. “Oligomerization and transglutaminase crosslinking of the cystatin CRES in the mouse epididymal lumen: possible mechanism of extracellular quality control”. Journal of Biological Chemistry, 2007; 282:32912–32923. The American Society for Biochemistry and Molecular Biology.
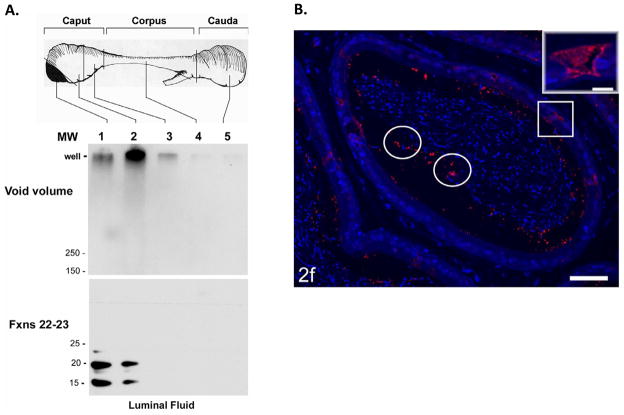
Figure 4.
A) Region-specific detection of SDS-resistant high molecular mass CRES complexes in the epididymal lumen Luminal fluid was isolated from each epididymal region and fractionated by size exclusion chromatography. The void volume fractions (fractions 13 and 14) were examined by SDS-agarose gel electrophoresis (top), whereas the fractions (fractions 19–21) containing the monomeric forms were examined by SDS-PAGE (bottom), followed by Western blot analysis with CRES antibody. This research was originally published in von Horsten HH, Johnson SS, SanFransisco SK, Hastert MC, Whelly SM, Cornwall GA. “Oligomerization and transglutaminase crosslinking of the cystatin CRES in the mouse epididymal lumen: possible mechanism of extracellular quality control”. Journal of Biological Chemistry, 2007; 282:32912–32923. The American Society for Biochemistry and Molecular Biology. B) Immunofluorescence analysis of CRES protein in the mouse cauda epididymidis. An adult mouse epididymis was fixed with zinc fixative, incubated with anti-CRES antibody followed by an Alexafluor 594 labeled secondary antibody and visualized by fluorescence microscopy. The epithelium in the cauda showed no obvious immunoreactivity for CRES; however, a few focal aggregates of intense reactivity were randomly distributed in the lumen (circles). Some highly endocytic clear cells (squares) show strong immunostaining for CRES. Inset: a high magnification of a reactive clear cell. Bar =50μm, inset = 10 μm. This research was originally published in Parent AD, Cornwall GA, Liu LY, Smith CE, Hermo L. “Alterations in the Testis and Epididymis Associated with Loss of Function of the Cystatin Related Epididymal Spermatogenic (CRES) Protein”. Journal of Andrology, in press.
We have recently focused our efforts on establishing the presence of amyloid in the epididymal lumen, and then carrying out experiments to specifically determine if CRES amyloid is present in the epididymal lumen. Based on the use of a variety of reagents including conformation dependent dyes such as Congo Red and thioflavin S and conformation-dependent antibodies including A11 and OC, reagents classically used to identify amyloid, our preliminary studies suggest that amyloid is indeed present in the epididymal lumen and that a proportion of CRES is associated with these amyloid structures. These studies were performed using epididymal samples from a normal mouse of proven fertility with no reproductive defects or any associated pathologies. These studies raise the possibility that either: 1) CRES amyloid formation is potentially pathological yet control mechanisms within the lumen are in place to protect against any cytotoxicity associated with these structures and/or 2) CRES amyloid is functional and may carry out yet to be determined roles in the epididymal lumen;
Epididymal amyloid: Controlled pathology or biological functions?
While studies are ongoing to address a role for CRES amyloid in epididymal function, we have demonstrated that within the epididymal lumen there is abundant transglutaminase 2 activity, especially high within the lumen of the initial segment region (von Horsten et al., 2007). Transglutaminases (TGases) are a family of calcium dependent enzymes that catalyzes the formation of isopeptide bonds between glutamine and lysine residues either within a protein or between two proteins. Transglutaminases are known to directly modulate protein aggregation and the formation of large molecular weight complexes of both soluble and particulate proteins and thus might contribute to protein quality control. The role of the TGase2 in the pathogenesis of amyloidogenic diseases is paradoxical in that some studies indicate a role for TGase2 in enhancing protein aggregation, thus contributing to the disease, such as in Alzheimer’s disease (Caccamo et al., 2010), whereas other reports suggest a protective effect of TGase2 by virtue of its forming protein cross-links, thus preventing the self-assembly of an amyloidogenic protein (Karpu et al., 1999; Konno et al. 2005). While in many cases, TGase substrates contain either reactive glutamines or lysines allowing these proteins to be crosslinked to other proteins, CRES is more unusual in that it is a complete TG substrate and has both reactive glutamine and lysine residues that are substrates for crosslinking resulting in SDS stable high molecular mass self-aggregates both in vitro and in vivo (von Horsten et al., 2007). Thus it is possible that TGase crosslinking may function as a mechanism of extracellular quality control, either stabilizing mature, noncytotoxic amyloid CRES structures, or converting cytotoxic intermediate CRES amyloid structures into inert nonamyloid aggregates.
It is possible that the transglutaminase crosslinked structures may carry out biological roles within the lumen and then may be targeted for removal by endocytosis as seems to occur for CRES in the cauda epididymidis. Possible functions for CRES amyloid within the epididymal lumen may be to form an extracellular matrix-like structure for cell-cell or cell-sperm communication, mediate protein-protein interactions, deliver proteins to the sperm during maturation (prion-like infection), act as a scaffold/organizational center with the lumen, and/or participate in removal of secreted proteins from the lumen.
Taken together, our studies suggest that amyloid is an integral part of normal epididymal function. Further studies of CRES amyloid formation, its modifications within the lumen along the tubule, and ultimately removal from the lumen will provide important insight not only for reproductive function but for regulation of amyloid in general possibly leading to new therapies for amyloidogenic diseases.
Footnotes
Supported by NIH HD56182 (GAC).
References
- Andonian S, Hermo L. Cell- and region-specific localization of lysosomal and secretory proteins and endocytic receptors in epithelial cells of the cauda epididymidis and vas deferens of the adult rat. J Androl. 1999;20:415–429. [PubMed] [Google Scholar]
- Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72:328–337. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baska KM, Madamdhar G, Feng D, Agca Y, Tengowski MW, Sutovsky M, Yi Y-J, Sutovsky P. Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol. 2007:684–696. doi: 10.1002/jcp.21349. [DOI] [PubMed] [Google Scholar]
- Caccamo D, Curro M, Ferlazzo N, Ientile R. Critical role of transglutaminase and other stress proteins during neurodegenerative processes. Amino Acids. 2010;38:653–658. doi: 10.1007/s00726-009-0428-3. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Orgebin-Crist MC, Hann SR. The CRES gene: a unique testis-regulated gene related to the cystatin family is highly restricted in its expression to the proximal region of the mouse epididymis. Mol Endocrinol. 1992;6:1653–64. doi: 10.1210/mend.6.10.1280328. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Hann SR. Transient appearance of CRES protein during spermatogenesis and caput epididymal sperm maturation. Mol Reprod Dev. 1995;41:37–46. doi: 10.1002/mrd.1080410107. [DOI] [PubMed] [Google Scholar]
- Dacheux JL, Dacheux F. Protein secretion in the epididymis. In: Robaire B, Hinton B, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 151–168. [Google Scholar]
- Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee S-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilgronner G, Baumann F, Kaesar SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Aβ-containing incoculates induce cerebral β-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid-from bacteria to humans. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Jensson O, Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C) Proc Natl Acad Sci U S A. 1986;83:2974–8. doi: 10.1073/pnas.83.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe CG. Conformation-dependent antibodies target diseases of protein misfolding. Trends Bio Chemical Sci. 2004;29:542–547. doi: 10.1016/j.tibs.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Halfmann R, Lindquist S. Epigenetics in the extreme: prions and the inheritance of environmentally acquired traits. Science. 2010;330:629–632. doi: 10.1126/science.1191081. [DOI] [PubMed] [Google Scholar]
- Jang H, Arce FT, Ramachandran S, Capone R, Lal R, Nussinov R. β-Barrel topology of Alzheimer’s β-amyloid ion channels. J Mol Biol. 2010;404:917–934. doi: 10.1016/j.jmb.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpu MV, Garren H, Slunt H, Price DL, Gusella J, Becher MW, et al. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers and its enzymatic activity increases in Huntington’s disease brain nuclei. Proc Natl Acad Sci USA. 1999;96:7388–7393. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Sokoloy Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- Konno T, Morii T, Hirata A, Sato SI, Oiki S, Ikura K. Covalent blocking of fibril formation and aggregation of intracellular amyloidogenic proteins by transglutaminase-catalyzed intramolecular crosslinking. Biochemistry. 2005;44:2072–2079. doi: 10.1021/bi047722d. [DOI] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, Eisenberg D, Rivier J, Sawchenko P, Vale W, Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;25:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton AP. Influences of macromolecular crowding upon the stability and state of association of proteins:predictions and observations. J Pharm Sci. 2005:1668–1675. doi: 10.1002/jps.20417. [DOI] [PubMed] [Google Scholar]
- Parent AD, Cornwall GA, Liu LY, Smith CE, Hermo L. Alterations in the testis and epididymis associated with loss of function of the cystatin related epididymal spermatogenic (CRES) protein. J Androl. 2011;32:679–685. doi: 10.2164/jandrol.110.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rialdi G, Battistel E. thermodynamics of proteins in unusual environments. Biophys Chem. 2007:64–79. doi: 10.1016/j.bpc.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–491. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- von Horsten HH, Johnson SS, SanFrancisco SK, Hastert MC, Whelly SM, Cornwall GA. Oligomerization and transglutaminase cross-linking of the cystatin CRES in the mouse epididymal lumen: potential mechanism of extracellular quality control. J Biol Chem. 2007;282:32912–32923. doi: 10.1074/jbc.M703956200. [DOI] [PubMed] [Google Scholar]
- Wang X, Hammer ND, Chapman MR. The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]