Abstract
The description and operation of a novel cyclic electrowinning/precipitation (CEP) system for the simultaneous removal of mixtures of heavy metals from aqueous solutions are presented. CEP combines the advantages of electrowinning in a spouted particulate electrode (SPE) with that of chemical precipitation and redissolution, to remove heavy metals at low concentrations as solid metal deposits on particulate cathode particles without exporting toxic metal precipitate sludges from the process. The overall result is very large volume reduction of the heavy metal contaminants as a solid metal deposit on particles that can either be safely discarded as such, or further processed to recover particular metals. The performance of this system is demonstrated with data on the removal of mixtures of copper, nickel, and cadmium from aqueous solutions.
Keywords: Cyclic electrowinning/chemical precipitation system, spouted particulate electrode, heavy metal co-electrodeposition, environmental chemical engineering
1. Introduction
Chemical precipitation of metal ions is perhaps the simplest and most widely used method of heavy metal removal from aqueous solutions [1]. Traditionally, hydroxide precipitants such as lime and caustic soda have been favored over their sulfide counterparts, due to the higher cost of chemically produced hydrogen sulfide and associated hazards. The formation of sodium hydroxide introduces the least amount of additional material mass to the precipitate sludge, but the volume of sulfide precipitate sludge is generally less. This has significant economic impact on waste management strategies for metal producers, since smaller volumes result in lower disposal and reclamation costs. In addition, the solubilities of metal sulfides are generally less than their corresponding hydroxides or carbonates. Even moderate sulfide addition can effectively reduce dissolved metal levels to below those permitted for discharge [2]. In addition, in certain cases metals can be recovered from sulfide sludges [3]. Metal precipitation with sulfides and hydroxides is well documented in the literature [4, 5, 6, 7].
Electrowinning, electrolytic removal/recovery (ER), or electroextraction, is another technique that has been used to remove heavy metals from contaminated water. This approach involves electrodeposition or reduction of metal ions from an electrolyte. In ER, a current is passed between the electrodes and metal cations diffuse to the surface of the cathode where they form a surface complex, receive electrons from the cathode, and are reduced to the metallic state. Metal removal/recovery rates by ER can be augmented by increasing current density, cathode surface area, and liquid agitation. The major drawback of ER of metals from dilute solutions is low current efficiencies.
Packed beds have been used as ER cathodes [8, 9]. Their operability is limited by agglomeration of the bed particles due to bridging of metal deposits between particles and the accompanying unacceptable increase in pressure drop [10, 11]. Fluidized beds have also been used as ER cathodes [12, 13, 14]. These types of systems offer good liquid-solid contacting and do not generally suffer from particle agglomeration. However, they usually exhibit poor electrical contact between particles, inhomogeneous electrical potentials, and particle segregation effects [15]. The existence of anodic zones in fluidized bed electrodes that were not observed in unexpanded fixed beds of the same particles, has also been reported [16, 17]. In addition, the range of overpotentials tends to be spatially distributed in fluidized bed electrodes [11].
Spouted (or recirculating) particulate electrodes (SPE) utilize conductive particles that are circulated hydrodynamically as a low-density, liquid-solid mixture in an entrained flow mode, and form a denser moving bed in the cathode region. Thus, in a sense, they are hybrids of fixed and fluidized (entrained) bed electrodes, incorporating advantages of both, while minimizing some of their disadvantages. For example, the cathodic particles do not agglomerate due to metal electrodeposition as long as spouting and particle movement are maintained. The removal of copper [18] and nickel [19] separately, as well as the co-electrodeposition of the two metals [20], have been investigated in a SPE of a similar type to that used in the current work. In particular, it was shown that both the quantitative and qualitative behavior of metal co-electrodeposition differs significantly from that of the single metals alone [20]. This is attributed primarily to the metal displacement reaction between Ni0 and Cu2+ that effectively increases the net rate of copper removal, at the expense of increasing the net nickel corrosion rate (initially). It also amplifies the separation of the removal of the two metals in time, such that relatively pure solid deposits of each metal could be obtained, if so desired [20].
Here we report on studies of the removal of mixtures of copper, nickel, and cadmium to low concentrations with a Cyclic Electrowinning/Precipitation (CEP) system that combines the positive characteristics of both chemical precipitation and electrowinning, while avoiding/minimizing some of their individual inherent drawbacks. In a CEP system, chemical precipitation/redissolution is used in two ways: to increase metal concentrations for more efficient electrowinning; and as a finishing step for reducing metal concentrations in the final effluent water. When operated in a cyclic fashion, metal precipitates are formed and redissolved, but no toxic sludge leaves the process. The process effluents are the solid particles with the accumulated electrodeposited metal, and decontaminated effluent water.
2. Experimental
2.1. CEP System Apparatus and Materials
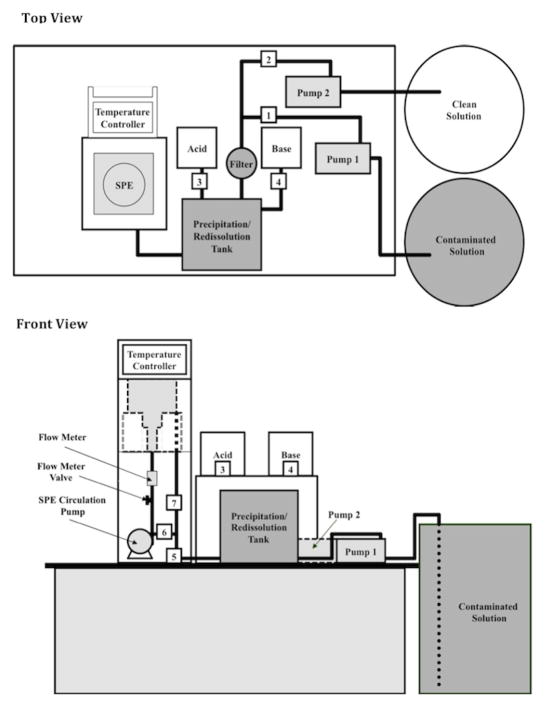
The CEP system is an automated and programmable apparatus for the removal of heavy metals from aqueous solutions using a cyclic combination of electrowinning and in-process precipitation and redissolution steps. As shown in the schematic in Figure 1, it consists of two principal components: the spouted particulate electrode (SPE), and the precipitation/redissolution (P/R) tank. Metal ion-containing water is pumped into the P/R tank from the wastewater reservoir. The solution pH is then increased with 1N NaOH, and the resultant precipitation of the metal ions as hydroxides removes the metals in the supernatant water (on the order 0.3–1.5 ppm). The filtered solution is then drained to the clean solution reservoir. Additional contaminated water is then introduced into the P/R tank, and the entire solution is re-acidified with 1N H2SO4, and remixed with the metal hydroxide sludge, which redissolves and produces a solution with higher metal ion concentrations. Via multiple P/R steps, the metal ion concentrations are increased sufficiently to enable electrowinning (metal ion reduction) onto the solid cathodic particles in the SPE at good current efficiencies.
Figure 1.
Schematic of the Cyclic Electrowinning/Precipitation (CEP) system.
The SPE in the CEP system was constructed by Technic, Inc. (Cranston, RI). Its operation is similar to that of a spouted bed electrochemical reactor used and described in our previous work [18, 19, 20]. Essentially, conductive particles are entrained in the electrolyte jet and convected upwards in the central draft tube. The entrained particles disengage from the liquid flow as the velocity decreases in the freeboard region, and then fall on the inverted conical distributor. The collector/distributor cone channels the particles to the periphery, where they fall onto and become part of the particulate moving bed cathode, which transports them inward and downward on the bottom cone back to the entrainment region. The pumping action of the spout circulates the particles through the vessel in a toroidal fashion - upwards in the spout, and downwards in the moving bed. Electrowinning occurs only on the bottom conical section of the vessel, where the particles form a cathodic moving bed in contact with the current feeder.
A variable 50A DC power supply was used to deliver current to the SPE. The electrolyte solution was circulated with a magnetically coupled centrifugal pump (Iwaki MD-40RT), equipped with a bypass valve for flow rate control. A paddle wheel flow meter (Signet 3–2535) was used to measure the liquid flow rate. A heater (CAL Controls Ltd. CAL3300) located at the bottom of external holding chamber, and a cooler consisting 1/16″ plastic tubing coils circulating cooling water, located on the wall of the reservoir chamber, are used simultaneously to set the solution temperature. An automatic pH controller (Barnant, model HD-PHP) was used to maintain constant pH with potassium hydroxide solution in the SPE reservoir.
Electrically-operated ball valves (ASAHI America 05E01660F) and pumps (Iwaki MD-30RT-115ML) were used to control the solution flows. A mixer (IKA, Eurostar power CV S1) was employed to stir the solution during addition of sulfuric acid or sodium hydroxide solution to the P/R tank. A 20 μm inline filter (ISC, Model No SJC-40-20) was used to retain metal hydroxide precipitate particles. A computer (Dell Optiplex 320), LabView™ software, and electronics interface (National Instruments, NI PCI-6221, M series DAQ; SCB-68 noise rejecting, shielded I/O connector block; and USB-9211A 4 Ch) were used to automatically control all CEP system operations.
The electrolyte solution in the SPE reservoir was sparged with nitrogen to reduce the dissolved oxygen concentration in order to minimize metal corrosion from the cathodic particles. The sparger was constructed from 0.635 cm diameter nylon tubing arranged in a square, 16.5 cm on a side. About 2000 of 0.35 mm diameter were drilled through the sparger tubing. A nitrogen flow rate of 3.5 SLM (standard L min−1) was used. The water volumetric flow rate was 16.2 L min−1, and the volume of conductive bed media used was about 400 cm3.
The granular bed media were 2.0 mm diameter plastic spheres, metallized with a layer of copper (Bead House LLC, CMC02.0/CP).
The initial metal ion concentrations for all the experiments were approximately 20 ppm each in the fresh solutions., consisting of 14.1 g CuSO4·5H2O (>98%, Aldrich), 16.3 g NiSO4·6H2O(>98%, Aldrich), and 8.3 g CdSO4·5H2O (>98%, Aldrich) added to 180 L of water for the ternary mixture. 550 g of Na2SO4 (granular, >99%, Aldrich) and 600 g H3BO3 were added to the precipitation tank just prior electrowinning in the SPE. Metal concentrations in the aqueous samples were determined with an ICP Optical Emission Spectrometer (ICP-OES; Jobin Yvon, JY2000). Sufficient sulfuric acid (1M, Mallinkrodt) and potassium hydroxide (1M, Fisher Scientific) were added automatically to obtain the desired pH values of 4 for redissolution and electrowinning, and 11 for precipitation in the P/R tank.
2.2. CEP System Program
A LabView™ control program was used to automate and control the CEP system so that it could be programmed to accommodate a wide variety of metal removal/remediation/recovery scenarios that may be found in practice. The control program consists of a front panel and block panel modules. The front panel serves as the programmatic interface. Controls and indicators on the front panel allow for inputting and exhibiting data and the status of all the system elements, such as pumps, valves, and pH. The block panel incorporates the code for the control program. The time sequence is the main frame of the program. The precipitation/redissolution tank and SPE processes are executed in the order specified by the program. The “on/off” status of the pumps and the “open/closed” status of the valves are controlled by the program via digital signals. The pH and level sensors provide input to the program via the data acquisition system. The program then sends out the appropriate digital signals to control the corresponding pumps and valves to execute each step in the program.
The CEP System program was run for a specified time, or until the metal ion concentrations reached a desired level, or with programs executing various different CEP cycles. The sequence of fluid handling operations during the precipitation/redissolution cycle and the electrowinning cycle are presented in the Appendix.
3. Results - CEP System Performance and Analysis
During the electrowinning steps in the SPE, electrochemical reduction, corrosion, competing side reactions, and metal displacement reactions occur at the cathode particle surfaces [18,19,20]. The metal displacement reaction between Cu2+ and reduced nickel metal was found to be important during the co-electrodeposition of copper and nickel from solution [20]; i.e.,
| (1) |
In a similar fashion, in Cu/Ni/Cd mixtures, additional metal displacement reactions occur that play an important role in the overall removal of the three metals; viz.:
| (2) |
| (3) |
Reaction (1) has been investigated during electroplating [21,22]. It has a significant effect on the resultant alloy composition, forming compositionally modulated alloys during pulsed plating [23,24]. The metal displacement reactions between copper and cadmium [25], and nickel and cadmium [26] have also been investigated.
The P/R process steps are used to increase the metals concentrations to levels sufficient for efficient electrowinning, and as the final cleanup step for the discharged water. The increase in metal ion concentration with each P/R cycle was linear, as expected. For a cycle time of 8 min., and initial metal concentrations of 20 ppm each, the increase per P/R cycle was about 16 ppm, which is accounted for as follows: The inlet and outlet tubing of the P/R tank was located at about 1/5 of the distance from the bottom of the tank. Thus, for each P/R cycle, about 1/5 of the solution remains in the tank after discharge of the supernatant water. The accumulated concentration in a P/R/redissolution cycle is then (1-1/5) × 20 ppm = 16 ppm. For the current hardware, the precipitation process worked well for total metal ion concentrations less than about 200 ppm. For higher total concentrations, the amount of accumulated hydroxide precipitate sludge would exceed the level of the tank outlet tubing, and some precipitate would be carried over and lost upon draining the tank. Of course, the operating capacity can be increased by using a larger tank and/or relocating the tank outlet.
Metal ion concentrations in the filtered effluent water were also measured for the same runs as 0.23, 0.37, and 1.50 ppm for Cu2+, Ni2+, and Cd2+, respectively, averaged over the 7 cycles. The solubility products of copper, nickel, and cadmium hydroxide are 4.8×10−20, 5.5×10−16, and 7.20×10−15, respectively, at 25°C [27]. Therefore, at equilibrium at pH 11, the Cu2+ ion concentration in solution should be 2.3×10−7 mol L−1 (0.015 ppm), and Ni2+and Cd2+ should be 5.2×10−6 mol L−1 (0.3 ppm), and 1.2×10−5 mol L−1 (1.3 ppm), respectively. These calculated concentrations are slightly less than the experimental values for nickel and cadmium, and much less for copper. These discrepancies are explained by the filter performance. A 20 μm inline filter (ISC, model No SJC-40-20) was used to retain the metal precipitate particles in the P/R tank. Three different filters were tested: 5, 20, and 50 μm. The 5 μm filter was easily plugged, and the 50 μm filter allowed through much more precipitate than the 20 μm filter. Comparison of the calculated concentrations with the data suggests that the 20 μm filter retained 93–99% of the metal hydroxide precipitate particles. The mean size of copper hydroxide precipitate particles has been reported to be on the order of 0.1 – 5 μm [28], which is much less than the 20 – 50 μm for nickel hydroxide [29,30], and 400 μm for cadmium hydroxide [30]. The filter size used is less than that of the average nickel hydroxide and cadmium hydroxide precipitate particles, but larger than that of copper hydroxide particles. The reasonably good agreement between the experimental and calculated nickel and cadmium ion concentrations indicates that the filter retained almost all the nickel hydroxide and cadmium hydroxide precipitate particles. However, the larger difference between the experimental and calculated copper ion concentrations indicates that some copper hydroxide precipitate passed through the filter.
USEPA MCLG (maximum contaminant level goal) values are 1.3 mg L−1 (TT, treatment technique) for copper; 0.1 mg L−1 (remanded 1995; currently under reconsideration) for nickel; and 0.005 mg L−1 for cadmium [31]. The experimental results indicate that the precipitation process reduces the copper ion concentration below its MCLG. However, additional treatment may be necessary to reduce the nickel and cadmium concentrations to below their respective MCLG values to drinking water standards, if so required.
One measure of CEP system performance is the net metal removal rate over the programmed number of P/R steps per electrodeposition step. The removal of a single metal can be expressed as [18, 19, 20]:
| (4) |
where C is the metal ion concentration during electrowinning, te, is the electrowinning time, k is the electrochemical metal reduction rate constant, kL is the interphase mass transfer coefficient, a is the specific interfacial surface area, and kc is the metal corrosion rate. This expression cannot be solved analytically since k varies with overpotential, and thus with time. Consequently, the experimental data were fit to polynomials for the sake of convenience. Some typical Ce vs te data are presented for copper removal in the CEP SPE as a function of applied current in Figure 2(a).
Figure 2.
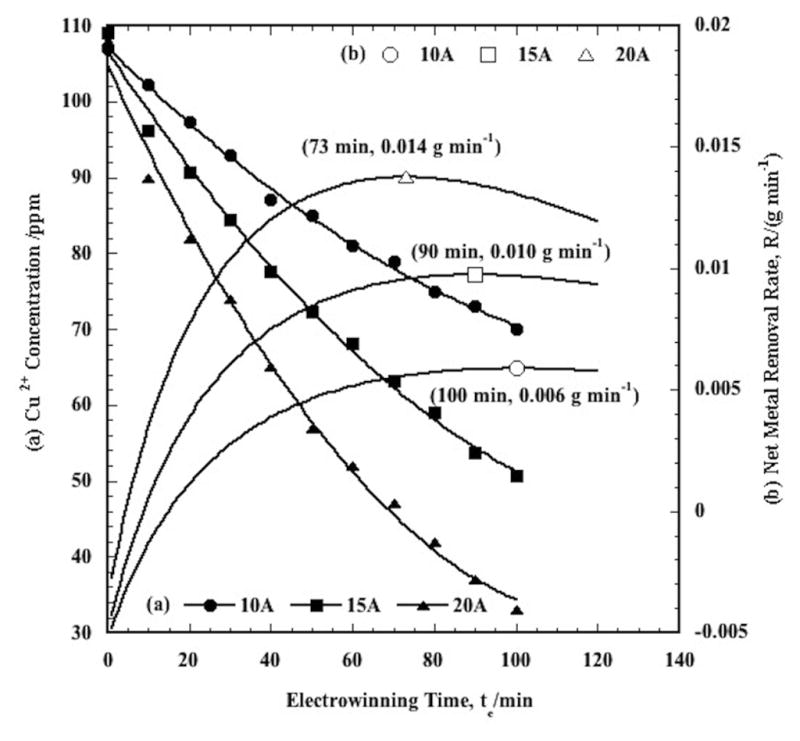
(a) Copper electrowinning in the SPE as a function of applied current. The initial Cu2+ concentration in each case was 99.5 ppm, which was prepared from the 20 ppm stock solution with five P/R cycles. The symbols are the experimental data, and the curves are the polynomial fits for copper electrowinning at 50°C, pH 4, with 3.5 SLM nitrogen sparging. (b) Net CEP copper removal rates, R, as a function of electrowinning time, te, constructed from the electrowinning data in (a) for a fixed value of tp = 40 min., as a function of applied current. The initial Cu2+ concentration in each case was 99.5 ppm. The values in the parentheses are the resultant optimal electrodeposition times, te, and the corresponding copper removal rates at the maxima for the same electrowinning conditions as in (a).
The net rate at which a metal is removed in the CEP system, R (e.g., in g min−1), is given by the expression:
| (5) |
where: C0 is the initial metal concentration prepared for the electrodeposition cycle; Ce is the metal concentration at the end of the electrodeposition cycle, te; tp is the time for P/R process associated with accumulating the metal for electrodeposition; and V is the solution volume in the SPE. The removal rate attains a maximum at dR/dte = 0. Thus, the optimal value of te occurs at the root of the transcendental equation:
| (6) |
For the data presented in Figure 2(a) for copper removal, C0 = 99.5 ppm, which was accumulated from a 20 ppm solution over five P/R cycles. Each P/R cycle was 8 min, so for the five P/R cycles shown, tp = 5 × 8 min = 40 min. The net CEP removal rate, R, for these data as a function of total CEP process time, te + tp, was calculated as a function of applied current from the polynomial curve fits of the data presented in Figure 2(a), and the results are presented in Figure 2(b). As shown, the net CEP removal rate exhibits a maximum. The reason for this behavior is as follows. For a fixed value of tp (i.e., 40 min. in this case) at low CEP process times, or at low values of te, there is little metal removal by electrowinning, so (C0 – Ce) is low. As te increases, however, more metal is removed by electrowinning, such that (C0 – Ce) increases, and the effect of fixed tp decreases in comparison to te. This behavior causes R to initially increase with CEP process time. However, since Ce decreases exponentially with te, the metal ion deposition rate decreases with te. That is, the rate of increase of (C0 – Ce) decreases with te. This causes the net CEP removal rate to exhibit a maximum, which represents the optimum net CEP removal rate achieved at this maximum.
From Figure 2(b) it is also noted that the optimal value of R increases and occurs at earlier times with increasing applied current. The observed net removal rate is the competitive result of metal removal by electrochemical reduction and metal redissolution via corrosion. The electrochemical reduction rate increases with current, while the corrosion rate remains approximately constant for fixed electrowinning conditions, such as temperature and pH. Thus, at a particular time, the removal rate increases with applied current. The higher electrowinning rate requires less time to process the results of P/R cycles at the maximum. Consequently, the optimal time decreases with applied current, while the optimal rate increases.
Using the resultant polynomial curve fits from Figure 2(a), the corresponding optima for the copper data are 73, 90, and 100 min., corresponding to maxima in the copper removal rates of 0.014, 0.010, and 0.006 g min−1, for 20, 15, and 10A, respectively. It is noted that the initial electrowinning concentration obtained with multiple P/R cycles was 99.5 ppm, while in Figure 2(a) the corresponding values are 107, 109, and 108 ppm for 10, 15, and 20A, respectively. The reason for this “discrepancy” is that after the solution is pumped to the SPE, a few minutes are required to heat the solution to the desired temperature (50°C in this case). During that time, some metal corrosion occurs which increases the initial concentration slightly. This also explains the initial “negative” removal rates in Figure 2(b).
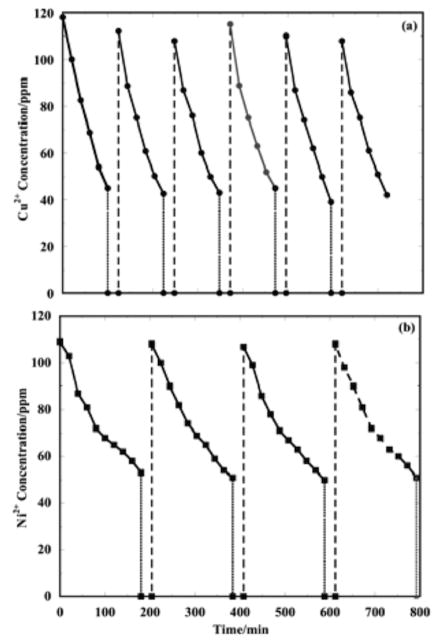
Knowledge of the optimal times is useful for setting the electrowinning time for multiple CEP cycles. From Figure 2(a), at 15A, the net copper removal rate maximum occurs at 90 min. The results for copper removal at 15A over multiple CEP cycles are presented in Figure 3(a). Overall, the feed water copper ion concentration was reduced from 20.1 ppm to 0.23 ppm in the effluent water. The difference between these values is the total amount of copper deposited on the cathodic particles. The copper lost in the process was determined to be on the order of ±1.5%, such that the copper mass balance closes reasonably well.
Figure 3.
(a) Cu2+ concentration in the process water over multiple CEP cycles at 15A. Four P/R steps were used to accumulate the initial Cu2+ concentration for electrowinning. After that, each CEP cycle consisted of one SPE and three P/R steps.
(b) Ni2+ concentration in the process water over multiple CEP cycles at 20A. Four P/R cycles were employed to accumulate the initial Ni2+ concentration for electrowinning. After that, each CEP cycle consisted of one 180 min. SPE and two P/R steps. In both cases: the P/R steps were conducted at pH 11.0 and 4.0, respectively; electrowinning was conducted at 50°C, pH 4, with 3.5 SLM nitrogen sparging. The solid curves are the electrowinning steps; the fine dashed vertical lines are precipitation; and the coarse dashed vertical lines are redissolution.
Nickel and cadmium electrowinning are more sensitive to corrosion rate than for copper. Consequently, nitrogen sparging plays a more important role in reducing dissolved oxygen in the electrolyte solution to reduce the corrosion rate, as discussed in References [18,19,20]. The optimal time for nickel electrowinning was determined in the same manner as for copper. The optimal times and maximum net CEP removal rates for nickel were 112 min, 0.006 g min−1, and 120 min, 0.004 g min−1 for 20A and 15A, respectively, and 173 min. for cadmium at 20A.
Slightly different than for copper, the nickel and cadmium electrowinning times for multiple CEP cycles are determined by both the optimal time and the final concentration at the end of each electrowinning step. Since an integral number of P/R cycles occur for each CEP cycle, the nickel and cadmium ion concentrations are not sufficiently depleted when shorter time intervals than the optimal time are used. Consequently, the initial ion concentration for the second CEP cycle will be greater than that for the first CEP cycle, etc., and the metal ion concentration would accumulate with each successive CEP cycle. Therefore, a time longer than the optimal time was used as the electrowinning time to maintain the initial metal ion concentration at the same level for the subsequent electrowinning step.
Nickel removal data at 20A over multiple CEP cycles are presented Figure 3(b). Each CEP cycle consisted of one electrodeposition step of 180 min. (i.e., greater than the optimal time). With this program, no nickel ion accumulation occurs, while the electrodeposition rate remains close to the maximum rate at the optimal time), and three P/R cycles of 8 min. each for another 24 min. This program was effective for nickel removal. It is noted that the optimal time for nickel removal is greater than that for copper, and also that the maximum nickel removal rate is less than that for copper. The lower nickel electrodeposition rate means that less nickel is deposited over the same time interval, or a lower maximum removal rate for nickel. A lower removal rate requires a greater optimal time for the same initial metal concentration of 100 ppm.
The rate of cadmium removal is less than that of nickel. Cadmium electrowinning rates were found to increase with applied current, pH, and temperature. At 20A, pH 4.0, and 50°C, a 100 ppm cadmium solution was accumulated (from a 20 ppm solution) with five P/R cycles. The resultant optimal removal time and maximum rate were 340 min., 0.003 g min−1, respectively. These values represent a greater time and lower maximum rate than were found for copper and nickel under similar conditions.
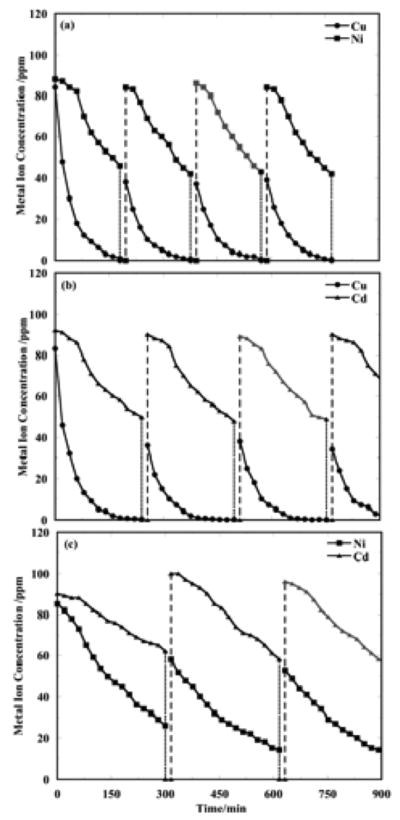
CEP data for co-removal of binary mixtures of the metals over multiple CEP cycles are presented in Figure 4 under similar conditions: (a) Cu2+/Ni2+ co-removal, te = 180 min.; (b) Cu2+/Cd2+ co-removal, te = 240 min.; and (c) Ni2+/Cd2+ co-removal with te = 300 min.
Figure 4.
Binary metal ion concentrations in the process water over multiple CEP cycles. In each case, three P/R steps were employed to accumulate the initial ion concentrations for electrowinning. After that, each CEP cycle consisted of one SPE step for the te values specified below, and two P/R steps (16 min.). The pH values for the P/ER steps were 11.0 and 4.0, respectively, and electrowinning was conducted at 20A, 50°C, pH 4, with 3.5 SLM nitrogen sparging. The solid curves are the electrowinning steps, the fine dashed vertical lines are precipitation, and the coarse dashed vertical lines are redissolution. (a) Cu2+ and Ni2+ for te = 180 min.; (b) Cu2+ and Cd2+ for te = 240 min.; (c) Ni2+ and Cd2+ for te = 300 min.
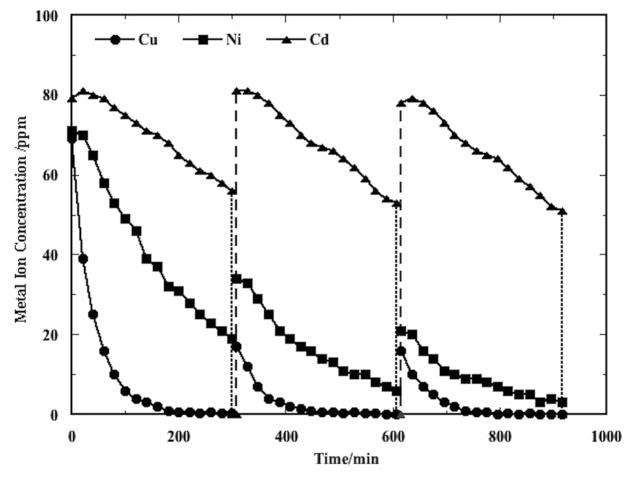
CEP data for co-removal of a ternary Cu2+/Ni2+/Cd2+ mixture over multiple CEP cycles are presented in Figure 5. As shown, all the metal mixture co-removal data over multiple cycles exhibit a characteristic flattened curve at the inception of each electrodeposition step for the last metal to be removed. This characteristic is attributable to the metal displacement reactions [20].
Figure 5.
Cu2+, Ni2+, and Cd2+ concentrations in a ternary mixture over multiple CEP cycles. Three P/R steps were employed to accumulate the initial ion concentrations for electrowinning. After that, each CEP cycle consisted of one 300 min SPE step and one P/R step. The P/R pH values were 11.0 and 4.0, respectively, and electrowinning was conducted at 20A, 50°C, pH 4, with 3.5 SLM nitrogen sparging. The solid curves are the electrowinning steps; the fine dashed vertical lines are precipitation; and the coarse dashed vertical lines are redissolution.
In Figure 6(a, b) are presented Cu2+/Ni2+/Cd2+ co-removal data at 20A, pH 4.0, 50°C. For these data, the initial solution concentration of about 100 ppm of each metal was prepared directly from the reagents, and not via a series of P/R steps, as previously. This was done simply for convenience due to the limited capacity of the P/R tank. As shown in Figure 6(a), the copper electrowinning rate is the greatest, while that of cadmium is the lowest, and that for nickel is intermediate between the two. In Figure 6(b) are presented the corresponding removal rates of Cu2+, Ni2+, and Cd2+ determined from the data in Figure 6(a). The resultant corresponding optimal times and maximum removal rates are 65, 91, and 280 min., and 0.017, 0.006, and 0.002 g min−1, respectively.
Figure 6.
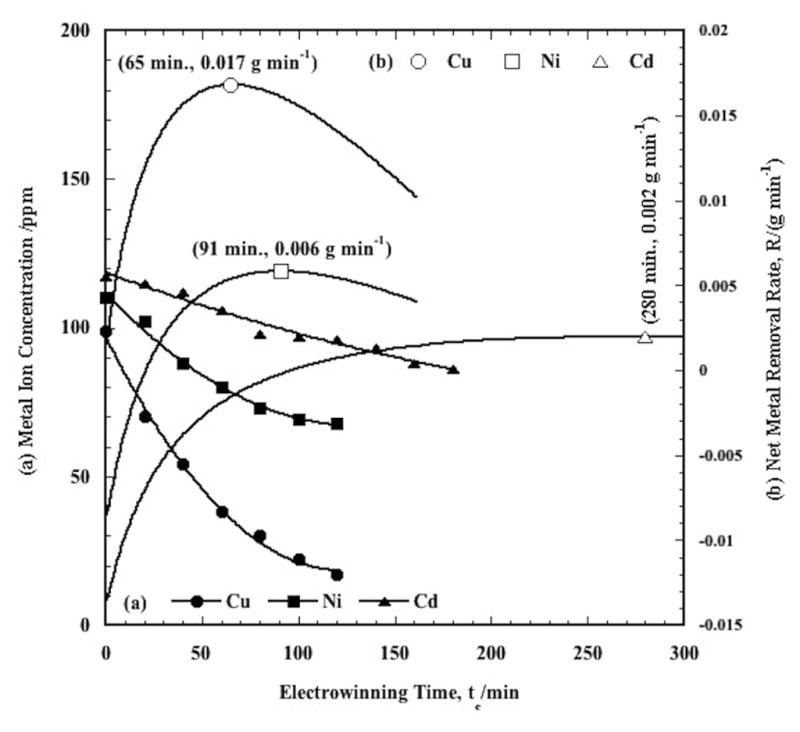
(a) Cu2+, Ni2+, and Cd2+ electrowinning in the CEP SPE at 50°C, pH 4, with 3.5 SLM nitrogen sparging. The initial ion concentrations prior to electrowinning in each case were 100 ppm from a prepared solution. The symbols are the experimental data, and the curves are the polynomial fits.
(b) Net Cu2+, Ni2+, and Cd2+ removal rates, R, calculated with the polynomial fits from (a). The values in parentheses are the electrowinning times, te, and the corresponding metal ion removal rates at the maxima
Although not specifically shown here, it is noted that for the ternary mixture, the copper and nickel removal rates are greater, and the cadmium removal rates are less in comparison to their single metal behavior. This is also reflected in the optimal times that decrease for copper and nickel, and increases for cadmium. This behavior is due to the displacement reactions between copper ion and nickel and cadmium metal on the cathodic particles in a similar fashion as was found for Cu2+/Ni2+ mixtures [20]. In the ternary mixture, Cu2+ can be spontaneously reduced by both nickel and cadmium metal previously deposited on the surface of the cathodic particles, due to the positive standard potentials for the displacement reactions (Eqns. (1, 2)). This enhances the net copper reduction rate, and decreases the optimal time. In a similar fashion, the nickel removal rate is enhanced by the cadmium displacement reaction, Eq. (3). However, since the standard potential difference between nickel ion and metal cadmium is less than that between copper ion and nickel, and copper ion and cadmium, its removal rate is enhanced somewhat less than that for copper. It is also noted that the displacement reactions all act in the same sense to increase the effective cadmium “corrosion” rate, such that its net removal rate decreases and its optimal time increases, in comparison to its single metal behavior.
The effects of the metal displacement reactions are also evident in modifying the initial electrowinning concentrations of the three metal ions. Even though the initial concentration of each metal ion was about 100 ppm, it is noted in Figure 6(a) that by the time the SPE was heated to the 50°C operating temperature and electrowinning was initiated, the metal ion concentrations had changed to 99, 110, 117 ppm for Cu2+, Ni2+, and Cd2+, respectively. This is due to the action of the metal displacement and corrosion reactions over this period. That is, a small amount of copper was net removed, while both the nickel and cadmium ion concentrations increased due to corrosion.
The time interval selected for multiple co-deposition cycles for the Cu2+/Ni2+/Cd2+ mixtures was greater than the optimal times of each of the single metals. Similar to the behavior of multiple CEP cycles with the binary mixtures, both the criteria of avoiding metal ion accumulation with subsequent cycles and maintenance of high electrowinning rates, were used to determine the optimal electrowinning time interval. That is, if a time shorter than the optimum for Cd2+ (but longer than those for Cu2+ and Ni2+) is used, the concentration of Cd2+ (the last metal ion removed in the mixture), will increase with each subsequent electrowinning step, which is counter to the general overall desired effect. Consequently, the time interval for the electrowinning cycle was increased to sufficiently deplete cadmium to maintain decreasing cadmium concentrations with each successive electrowinning cycle. Of course, a number of other program strategies could also be employed as well. For example, the electrowinning time could be programmed so that it starts out as near optimal for Cu2+, which allows Ni2+ and Cd2+ to accumulate with each cycle, while preferentially removing copper, and then operate on the resultant Ni2+ and Cd2+ - concentrated solution with an electrowinning time between the optimal values for Ni2+ and Cd2+ to preferentially remove Ni2+ while accumulating Cd2+, and then finally run at an optimal time for Cd2+ removal on the residual, etc., as well as other permutations thereof. The metal displacement reactions serve to “spread out” the removal of the different metals in time. This characteristic, coupled with the types of programs described above, could be used to allow the preferential accumulation of each metal on a particular set of particles which, since they are entrained, can be removed and introduced hydrodynamically at selected times in the program, so that metal separation and recovery can also be achieved in a continuous fashion.
4. Conclusions
This study demonstrates effective co-removal of the heavy metals copper, nickel, and cadmium from low concentrations in aqueous solutions via Cyclic Electrowinning/Precipitation (CEP). This approach produces very large volume reductions from the original contaminated water by electrochemical reduction of the ions to zero-valent metal on the surfaces of the cathodic particles. (For an initial 10 ppm ion concentration of the metals considered, the volume reduction is on the order of 106.) Although, the CEP approach is certainly not expected to be universally applicable to all heavy metals, there are a number of other heavy metals than the ones specifically investigated here that are also expected to be amenable to removal in an analogous fashion.
Acknowledgments
This work was supported by grant #5 P42 ES013660 from the National Institute of Environmental Health Sciences (NIEHS), NIH. The authors wish to thank Mr. A. Tente from the Chemistry Department for development and wiring of the CEP electrical systems, and Dr. I. Külaots for assistance with the development of the hardware and the LabView™ control program. The analytical assistance provided by Dr. D. Murray and Mr. J.R. Orchardo of the Geological Sciences Department is also gratefully acknowledged.
Appendix: CEP System Sequence of Operations
The precipitation/redissolution process cycle was conducted in the following manner (with reference to Figure 1):
Valve 1 is opened and Pump 1 is activated, with all the other valves closed. The contaminated solution is pumped into the precipitation tank. Valve 1 is closed and Pump 1 is stopped automatically when the solution reaches a preset amount as indicated by a level sensor.
Valve 3 is opened to drain the basic solution into the precipitation tank, with the stirrer on. Valve 3 closes automatically when the pH reaches a preset level of 11 in the present work, and flocculant is added. Approximately 3 min. are required to allow the metal hydroxide precipitate to settle to the bottom of the tank.
Valve 2 is opened and Pump 2 is activated, with all the other valves closed. The clean solution is drained from the precipitation tank. Pump 2 is stopped and Valve 2 is closed.
Valve 4 is opened to add the acid solution to pH 4, and the mixer is activated. Valve 4 is closed automatically when the solution pH attains the preset value. Approximately 4 min. are required to ensure that all the metal precipitate is redissolved.
The electrowinning process cycle was conducted in the following manner:
Na2SO4 and H3BO3 are added to the solution in order to increase its conductivity and suppress hydrogen evolution during electrowinning.
Valve 4 is opened to add acid to the solution, and then closed automatically when the solution pH attains the preset value.
Valves 5 and 6 are opened and Pump 3 is activated, with all other valves closed. The accumulated solution is pumped into the SPE.
With Valve 6 open and Pump 3 running, Valve 5 is closed, and Valve 7 is opened. The solution then circulates within the SPE. The SPE heater/cooler are turned on to achieve the desired preset temperature.
The power is turned on and set to the appropriate current to begin electrowinning.
After the electrowinning cycle is complete, Valve 6 is closed, and Valve 5 is opened, keeping Valve 7 open. The solution is drained into the precipitation tank. Valves 5 and 7 are then closed, and Pump 3 is stopped.
Each of the two types of cycles can be repeated as many times as required to achieve the desired metal removal results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheikholeslami R, Bright J. Silica and metals removal by pretreatment to prevent fouling of reverse osmosis membranes. Desalination. 2002;143(3):255–267. [Google Scholar]
- 2.White C, Sayer JA, Gadd GM. Microbial solubilization and immobilization of toxic metals: key biogeochemical processes for treatment of contamination. FEMS Microbiol Rev. 1997;20:503–516. doi: 10.1111/j.1574-6976.1997.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaksonen AH, Riekkola-Vanhanen ML, Puhakka JA. Optimization of metal sulfide precipitation in sulfate-reducing fluidized-bed treatment of acidic wastewater. Water Res. 2003;37:255–266. doi: 10.1016/s0043-1354(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 4.Machemer SD, Wildeman TR. Adsorption compared with sulfide precipitation as metal removal processes from acid mine drainage in a constructed wetland. J Contam Hydrol. 1992;9(1–2):115–131. [Google Scholar]
- 5.Machemer SD, Reynolds JS, Laudon LS, Wildeman TR. Balance of S in a constructed wetland built to treat acid mine drainage, Idaho Springs, Colorado, U.S.A. Appl Geochem. 1993;8(6):587–603. [Google Scholar]
- 6.Kim BR, Gaines WA, Szafranski MJ, Bernath EF, Miles AM. Removal of heavy metals from automotive wastewater by sulphide precipitation. J Env Eng Div ASCE. 2002;128(7):612–623. [Google Scholar]
- 7.Shinohara R, Katoh N. Dynamics and control of a sulfide precipitation process for waste water purification. Kogaku Kogaku Ronbunshu. 2005;31(3):217–221. [Google Scholar]
- 8.Simonsson D. A flow-by packed bed electrode for removal of metal ions from wastewaters. J Appl Electrochem. 1984;14(5):595–604. [Google Scholar]
- 9.Chu AKP, Fleischmann M, Hills GJ. Packed Bed Electrodes. I. Electrochemical extraction of copper ions form dilute aqueous solutions. J Appl Electrochem. 1974;4(4):323–330. [Google Scholar]
- 10.Houghton RW, Kuhn AT. Mass-transport problems and some design concepts of electrochemical reactors. J Appl Electrochem. 1974;4(3):173–190. [Google Scholar]
- 11.Ferreira BK. Three-dimensional electrodes for the removal of metals from dilute solutions – a review. Mineral Processing & Extractive Metal Rev. 2008;29:330–371. [Google Scholar]
- 12.Sequeira CAC, Marques FDS. Electrochemical Engineering: I. ChemE Symposium Series No. 112. Vol. 112. Hemisphere Pub. Corp; NY: 1989. Effect of current on gold electrowinning with a fluidized bed electrode; pp. 297–306. [Google Scholar]
- 13.Backhurst JR, Goodridge F, Coulson JM, Plimley RE. A preliminary investigation of fluidized bed electrodes. J Electrochem Soc. 1969;116:1600–1607. [Google Scholar]
- 14.Germain S, Goodridge F. Copper deposition in a fluidized bed cell. Electrochim Acta. 1976;21(8):545–550. [Google Scholar]
- 15.Fleischmann M, Oldfield JW, Tennakoon L. Fluidized bed electrodes. IV. Electrodeposition of copper in a fluidized bed of copper-coated spheres. J Appl Electrochem. 1971;1(2):103–112. [Google Scholar]
- 16.Coeuret F. The fluidized bed electrode for the continuous recovery of metals. J Appl Electrochem. 1980;10:687–696. [Google Scholar]
- 17.Hadzismajlovic DzE, Popov KI, Pavlovic MG. The visualization of the electrochemical behaviour of metal particles in spouted, fluidized and packed beds. Powder Tech. 1996;86(2):145–148. [Google Scholar]
- 18.Shirvanian PA, Calo JM. Copper recovery in a particulate spouted bed electrode. J Appl Electrochem. 2005;35(1):101–111. [Google Scholar]
- 19.Grimshaw P, Calo JM, Shirvanian PA, Hradil G., II Electrodeposition/removal of nickel in a spouted electrochemical reactor. Ind Eng Chem Res. 2011;50(16):9525–9531. doi: 10.1021/ie200669b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimshaw JM, Calo P, Hradil G., III Co-electrodeposition/removal of copper and nickel mixtures in a spouted electrochemical reactor. Ind Eng Chem Res. 2011;50(16):9532–9538. doi: 10.1021/ie200670g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley PE, Roy S, Landolt D. Pulse-plating of copper-nickel alloys from sulfamate solution. J Chem Soc, Faraday Trans. 1996;92(20):4015–4019. [Google Scholar]
- 22.Scharfe RR, Sastri VS, Chakrabarti CL. Kinetics of displacement of Ni[II] by Cu[II] in Bis[dithiocarbamato] nickel[II] Can J Chem. 1972;50(20):3384–3386. [Google Scholar]
- 23.Roy S, Matlosz M, Landolt D. Effect of corrosion on the composition of pulse-plated Cu-Ni alloys. J Electrochem Soc. 1994;141(6):1509–1517. [Google Scholar]
- 24.Bradley PE, Landolt D. A surface coverage model for pulse-plating of binary alloys exhibiting a displacement reaction. Electrochim Acta. 1997;42(6):993–1003. [Google Scholar]
- 25.Piontelli R, Poli G. The reactions between metal and solutions of electrolytes. V. The existence of the differential effect in metal-displacement processes. Z Physik Chem. 1942;A190:317–330. [Google Scholar]
- 26.Berge H, Drescher A, Jeroschewski P. Indirekte inversvoltammetrische Bestimmung von Elementen unter Anwendung von Verdrängungsreaktionen. Fresenius’ Zeitschrift fuer Analytische Chemie. 1969;248(1,2):1–6. [Google Scholar]
- 27.Solubility Product Constants. KTF-Split; Croatia: http://www.ktf-split.hr/periodni/en/abc/kpt.html. [Google Scholar]
- 28.Ploss H, Lehne J. Process of producing copper [II] hydroxide. 4,614,640 US Pat. 1986
- 29.Subbaiah T, Mohapatra R, Mallick S, Misra KG, Singh P, Das RP. Characterisation of nickel hydroxide precipitated from solutions containing Ni2+ complexing agents. Hydrometallurgy. 2003;68(1–3):151–157. [Google Scholar]
- 30.Feitknecht W, Studer H. Electron microscope investigation of the form and size of colloidal particles of metal hydroxides. Kolloid-Zeitschrift. 1949;115:13–16. [Google Scholar]
- 31.National Primary Drinking Water Regulations, EPA 816-F-09-0004. USEPA; Washington, DC: May, 2009. [Google Scholar]