Abstract
Here we describe chemical innovations that enable the preparation of fully synthetic tetracyclines containing an all-carbon quaternary, stereogenic center at position C5a, a structurally novel class of compounds in this important family of therapeutic agents. In the key transformation and an important extension of the powerful Michael–Claisen cyclization (AB plus D) approach to the construction of fully synthetic tetracyclines, we show that the six-membered C ring comprising a C5a quaternary carbon center can be assembled by highly stereocontrolled coupling reactions of β-substituted AB enones and o-toluate ester anion D-ring precursors. Novel and versatile β-functionalization reaction sequences employing tris(methylthio)methyllithium and 2-lithio-1,3-dithiane have been developed to transform the AB enone 1 (the key precursor to fully synthetic tetracyclines) into a diverse range of β-substituted AB enone products, including a highly efficient, single-operation method for the synthesis of a β-methyl ester-substituted AB enone. A C5a-C11a-bridged cyclopropane tetracycline precursor was found to undergo efficient and regioselective ring-opening reactions with a range of nucleophiles in the presence of magnesium bromide, thus providing another avenue for the preparation of fully synthetic tetracyclines containing an all-carbon quaternary center at position C5a. Two compounds prepared from the bridged cyclopropane intermediate served as (further) diversifiable branch-points, allowing maximally expedient synthesis of C5a-substituted tetracyclines by final-step diversification.
Keywords: Tetracycline, Enone Synthesis, Michael–Claisen Cyclization, Quaternary Center, Cyclopropane Ring-Opening
1. Introduction
To date more than 3,000 fully synthetic molecules of the tetracycline class, broadly defined, have been prepared by a general and convergent process that involves a Michael-Claisen coupling of the AB enone 1 with structurally diverse D-ring precursors followed by deprotection, a route of typically 3–4 steps (Figure 1).1,2 Most of the candidate antibiotics prepared in this way would have been difficult if not impossible to obtain by chemical transformations of natural tetracyclines (“semisynthetic” processes). A key enablement that permitted the development of this streamlined route was the introduction by Stork and Hagedorn of the 3-benzyloxyisoxazole function as a masked form of the vinylogous carbamic acid of the A ring of tetracyclines, a protective group that is readily cleaved by hydrogenolysis.3 It is therefore appropriate that the present report, in which we build upon our earlier work through methodological advances that permit for the first time modification of position C5a of the tetracycline scaffold, appear in this special publication dedicated to Professor Gilbert Stork, a pioneering investigator in synthetic organic chemistry of extraordinary accomplishment.
Figure 1.
Structurally diverse, fully synthetic tetracyclines, now incorporating angular substitution of position C5a.
Previous work has demonstrated that our fully synthetic approach to tetracyclines allows modifications at positions C6, C7, C8, C9 and C10 that are not feasible by semisynthesis.1,2 One area of focus in our current research is the development of chemical pathways that enable modifications at other positions, such as C5a, that have not previously been viable. Position C5a is one of two points of fusion of the B and C rings of tetracyclines and, as such, substitution of this position gives rise to a quaternary carbon center. Inspection of X-ray crystallographic data of tetracycline bound to the 30S subunit of the ribosome of Thermus Thermophilus suggests that substitution of position C5a would not obviously impede ribosome binding and thus could present an interesting and unexplored avenue for the discovery of potential new antibiotics to address problems such as bacterial resistance.4,5 To apply our general route for tetracycline synthesis to C5a-substituted analogs it was first necessary to develop methodology to prepare AB enones containing different β-substituents, and then to determine if these modified AB enones would successfully undergo Michael–Claisen cyclization reactions with D-ring precursors, transformations that would give rise to a quaternary, stereogenic center at position C5a. The most rapid and straightforward approach to the synthesis of β-substituted AB enones appeared to be the direct functionalization of the AB enone 1. While introduction of simple alkyl groups such as β-methyl proved to be relatively straightforward (though low-yielding), introduction of more highly oxidized β-substituents was less so, and provided us with the opportunity to pursue a number of chemical innovations, which we detail herein. The subsequent stereocontrolled construction of the C ring, comprising an all-carbon quaternary center at position C5a, was viewed to be a challenging transformation, and its successful implementation we view to be a significant advance. During the course of our investigations we also serendipitously discovered a C5a–C11a bridged cyclopropane-containing tetracycline intermediate that has proven to be an extraordinarily versatile precursor to a diverse array of C5a-substituted tetracyclines, providing another avenue for the synthesis of this novel class of substituted tetracycline antibiotics.
2. Results and discussion
2.1. New methods for β-functionalization of enones
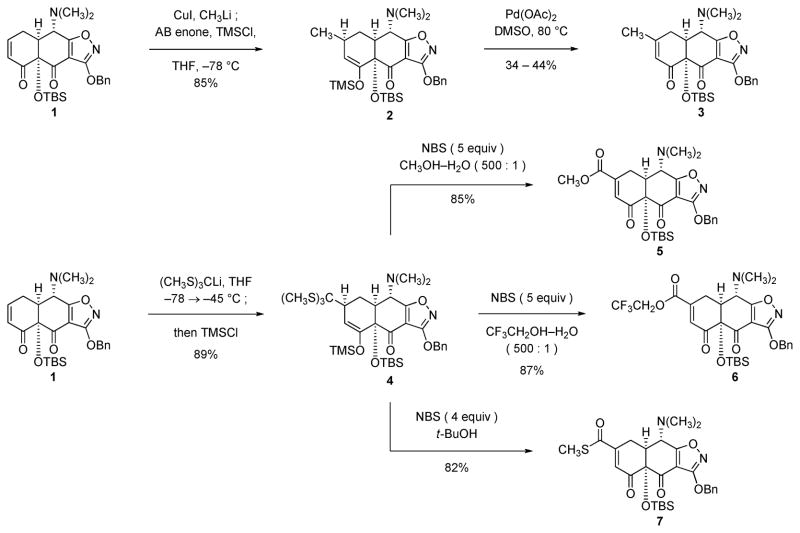
As a first step toward the stereocontrolled construction of all-carbon quaternary, C5a-substituted tetracyclines we first sought to develop methods to transform the AB enone 1 into β-substituted AB enones as novel cyclization substrates (Schemes 1–3 below). A conventional reaction sequence served for the synthesis of the simple β-methyl-substituted AB enone 3 (Scheme 1). Specifically, conjugate addition of lithium dimethylcuprate to the AB enone 1 in the presence of trimethylsilylchloride6 afforded the corresponding β-methyl-substituted trimethylsilyl enol ether 2 in 85% yield (1D-NOESY experiments support the 5a-S-stereochemistry depicted, in accord with all precedent in this system);1 oxidation of this intermediate with palladium acetate in dimethyl sulfoxide (DMSO) at 80 °C then afforded the β-methyl-substituted AB enone 3 in modest yield (44% yield on 160-mg scale, and 34% yield on 2.8-g scale).7,8 Attempted oxidation of the intermediate trimethylsilyl enol ether 2 with the alternative oxidant o-iodoxybenzoic acid (IBX) was not successful.9
Scheme 1.
Two-step syntheses of modified AB enones with methyl, methyl ester, trifluoroethyl ester and thioester groups at the β-position, starting from the β-unsubstituted AB enone 1.
Scheme 3.
Two protocols for the synthesis of the β-methoxymethoxymethyl AB enone 10 from the AB enone 1; the first, and more efficient, proceeds via the β-carbaldehyde AB enone 9.
To transform the AB enone 1 into β-substituted AB enones with more highly oxidized β-substituents we were led to explore novel chemistry, as precedented methods were deemed to be too indirect or were impracticable in the present application.10–12 First, we developed a versatile sequence for β-functionalization of the AB enone 1 initiated by conjugate addition of the Seebach reagent tris(methylthio)methyllithium13 (THF, −78 → −45 °C) followed by trapping of the resulting enolate at −45 °C with chlorotrimethylsilane.14 The corresponding β-tris(methylthio)methyl trimethylsilyl enol ether (4) was isolated as a single stereoisomer (stereochemistry not determined) in 89% yield after purification by flash-column chromatography. We found that oxidation of the trimethylsilyl enol ether function and transformation of the tris(methylthio)methyl group occurred simultaneously upon treatment of a solution of 4 in the solvent mixture 500:1 methanol–water with an excess of N-bromosuccinimide (NBS, 5 equiv) at 23 °C, affording the AB enone containing a β-methyl ester substituent (5) in 85% yield. The detailed mechanism of the transformation of the intermediate 4 into the β-substituted AB enone 5 is not known, but presumably involves some variation of a sequence involving α-bromination of the trimethylsilyl enol ether, bromonium-induced conversion of the tris(methylthio)methyl substituent into the corresponding methyl ester (with incorporation of one molar equivalent each of methanol and water), and elimination of hydrogen bromide. By modification of the reaction solvent we found that different esters could be synthesized, including active esters. For example, addition of NBS (5 equiv) to a solution of the trimethylsilyl enol ether 4 in 2,2,2-trifluoroethanol and water (500:1 mixture) at 23 °C afforded the corresponding β-trifluoroethyl ester-substituted AB enone 6 in 87% yield. Alternatively, treatment of a solution of substrate 4 in tert-butanol with NBS (4 equiv) at 23 °C afforded the AB enone 7 bearing an S-methyl thioester at the β-position in 82% yield.15 The transformations of Schemes 1–3 represent novel, direct and highly efficient β-functionalization reactions of an enone substrate. Important foundational precedents include the conversion of a β-cyano-substituted trimethylsilyl enol ether to the corresponding β-cyano enone upon sequential treatment with NBS and triethylamine10a as well as the original discovery that dithianes undergo oxidative hydrolysis in the presence of N-halosuccinimides.16
In a further optimization, we found that transformation of the β-unsubstituted AB enone 1 to the β-methyl ester-substituted AB enone 5 could be achieved directly, and most efficiently (90% yield), in a single operation (Scheme 2).
Scheme 2.
Synthesis of the β-methyl ester-substituted AB enone 5 from the β-unsubstituted AB enone 1 in a single operation.
A related strategy was effective for the synthesis of the AB enone 9 bearing a β-carbaldehyde substituent, which in turn provided an expedient route to AB enones with β-alkoxymethyl substituents (Scheme 3). Thus, conjugate addition of the Corey–Seebach reagent 2-lithio-1,3-dithiane17 to the AB enone 1 in the presence of hexamethylphosphoramide (HMPA)18 at −78 °C followed by quenching of the resulting enolate intermediate with chlorotrimethylsilane provided the β-(1,3-dithian-2-yl) trimethylsilyl enol ether 8 as a single stereoisomer (90% yield; stereochemistry not determined). Treatment of the latter product with NBS (6 equiv) in the solvent mixture 100:1 tert-butanol–water at 23 °C afforded the β-carbaldehyde AB enone 9 in 90% yield. Selective reduction of the aldehyde group occurred upon warming a solution of 9 and sodium triacetoxyborohydride in benzene at 40 °C. The resulting primary alcohol was then protected as a methoxymethyl ether in the presence of chloromethyl methyl ether and N,N-diisopropylethylamine in benzene at 50 °C to afford the β-methoxymethoxymethyl AB enone 10 (85% yield over two steps). AB enone 10 could also be prepared in fewer steps but much lower (and highly variable) yield by the reaction of the AB enone 1 with (methoxymethoxy)methyllithium (prepared in situ from tri-n-butyl[(methoxymethoxy)methyl]stannane19 and n-butyllithium) in the presence of HMPA at −78 °C, trapping of the resulting enolate with chlorotrimethylsilane (affording the β-methoxymethoxymethyl trimethylsilyl enol ether 11 in 40–55% yield), and then oxidation of the intermediate 11 with palladium acetate in DMSO at 50 °C (providing AB enone 10 in 25–52% yield).
2.2. Stereocontrolled construction of all-carbon quaternary centers by Michael–Claisen cyclization reactions of β-substituted AB enones
Having established versatile methodology for the synthesis of β-substituted AB enone substrates, we next investigated the feasibility of constructing the C ring of tetracyclines with an all-carbon quaternary C5a stereocenter by a Michael–Claisen cyclization reaction (Scheme 4). The D-ring precursors 12 (with OBoc protection at “C10”) and 13 (with OBn protection at “C10”) were chosen for initial cyclization experiments. These precursors comprise the D-ring functionality of minocycline (Scheme 4) and are known from prior research to be highly effective substrates in Michael–Claisen cyclization reactions.1b Addition of the β-methyl-substituted AB enone 3 (1 equiv) to a bright red solution of the o-toluate ester anion formed by deprotonation of the minocycline D-ring precursor 12 (3 equiv) with lithium diisopropylamide (LDA, 3 equiv) in the presence of N,N,N′,N′-tetramethylethylenediamine (TMEDA, 6 equiv) at −78 °C, followed by warming to −10 °C, provided the Michael–Claisen cyclization product 14 in 80% yield as a single stereoisomer after purification by flash-column chromatography. A minor by-product (<5%), thought to be the product of 1,2-addition-cyclization (by lactonization), was isolated separately. The stereochemical assignment of the Michael–Claisen cyclization product (14), with C5a-R configuration, is supported by nOe studies; this stereochemistry is homologous with that of Michael–Claisen cyclization products derived from the non-substituted AB enone 1.1,2 In both cases, addition appears to occur from a single diastereoface of the enone, that opposite the C12a tert-butyldimethylsilyloxy substituent.1b There are two examples in the literature of Michael–Claisen cyclization reactions of achiral β-methyl cyclohexenones with o-toluate ester anions,20 but we are unaware of any examples beyond those described here of the stereocontrolled construction of a six-membered ring containing a quaternary center by a Michael–Claisen cyclization reaction.
Scheme 4.
Stereocontrolled formation of an all-carbon quaternary center by Michael–Claisen cyclization reactions of o-toluate ester anions with β-substituted AB enones.
Michael–Claisen reaction of the β-methoxymethoxymethyl AB enone 10 and the minocycline D-ring precursor 13 (chosen so as to allow later deprotection of the methoxymethyl ether at C5a without concomitant cleavage of the C10 phenoxy protective group) was also efficient, affording the cyclization product 15, with a protected C5a-hydroxymethyl-substituted quaternary center, in 72% yield (Scheme 4). Reaction of the β-methyl ester AB enone 5 with the anion formed from the minocycline D-ring precursor 12 afforded a complex mixture of products containing the desired Michael–Claisen cyclization product 16 as one component. Cycloadduct 16 was isolated in 23% yield after purification by sequential flash-column chromatography and reverse-phase high-performance liquid chromatography (rp-HPLC). Two-step deprotection of cyclization products 14 and 16 under typical conditions1,3 provided C5a-methylminocycline (17, 100% yield, Scheme 5) and C5a-carbomethoxyminocycline (18, 89% yield), respectively, after purification by rp-HPLC. Thus, hydrogenolytic deprotection of the 3-benzyloxyisoxazole function, as reported by Stork and Hagedorn,3 reveals the vinylogous carbamic acid function of the A ring of tetracyclines in the final step of the synthetic sequence.
Scheme 5.
Synthesis of C5a-methylminocycline (17) and C5a-carbomethoxyminocycline (18) by two-step deprotection of cyclization products 14 and 16, respectively.
2.3. Discovery and diversification of a C5a-C11a bridged cyclopropane-containing tetracycline intermediate
In a search for a versatile branch point for the synthesis of various C5a-substituted tetracyclines we were led to a serendipitous but highly effective solution (Scheme 6). Removal of the methoxymethyl ether protective group within the Michael–Claisen cyclization product 15 was achieved by treatment with perchloric acid (CAUTION!),21 providing the substituted neopentyl alcohol 19 (73% yield). Addition of phosgene to a solution of 19 in dichloromethane-pyridine (10:1) at 0 °C unexpectedly afforded the C5a-C11a-bridged cyclopropane tetracycline precursor 20 in 81% yield. After the fact, the formation of 20 is easily rationalized. In this context it is interesting to note that Barton and co-workers had previously reported that the (C11–C12) 1,3-diketone can participate in an internal nucleophilic addition, albeit in that case with addition to an electrophilic carbonyl group that had been introduced at position C4.22
Scheme 6.
Discovery of a C5a-C11a-bridged cyclopropane tetracycline precursor (20).
Cyclopropanes with geminal electron-withdrawing substituents are known to undergo nucleophilic ring-opening,23 a transformation often enhanced in the presence of Lewis acids.24 We were led to explore the use of magnesium salts as Lewis acid activators in this system in view of the well documented affinity of Mg2+ for binding to the (C11-C12) 1,3-diketone function of tetracyclines, complexation which is critical for inhibition of the ribosome.4 We observed that the bridged cyclopropane intermediate 20 underwent regioselective ring-opening at the bridging carbon atom in the presence of various nucleophiles and magnesium bromide as Lewis acid. The ring-opened products were readily deprotected in the typical two-step sequence to furnish the corresponding C5a-substituted tetracyclines (Table 1). For example, reaction of the C5a-C11a-bridged cyclopropane tetracycline precursor 20 with pyrrolidine (10 equiv) in the presence of a stoichiometric amount of anhydrous magnesium bromide in THF at 23 °C, followed by direct deprotection of the crude ring-opened product (21), provided C5a-pyrrolidinomethylminocycline (22) in 74% yield over the three steps after purification by rp-HPLC. A number of different amines and alcohols were found to function effectively as nucleophiles in the magnesium bromide-promoted cyclopropane ring-opening reaction (Table 1). Ring-opening reactions were typically performed with a large excess of nucleophile (≥ 7 equiv) and stoichiometric or superstoichiometric quantities of anhydrous magnesium bromide in THF (in the cases of low molecular weight alcohols as nucleophiles, the alcohol was used as solvent) at a range of temperatures (23–75 °C, see experimental procedures for details). Partial (and inconsequential) loss of the benzyl ether phenolic protective group was observed to occur in the cyclopropane ring-opening reaction in some instances.
Table 1.
Fully synthetic tetracyclines prepared from the C5a-C11a bridged cyclopropane intermediate 20 by magnesium bromide-promoted ring-opening followed by two-step deprotection

|
The C5a-C11a-bridged cyclopropane intermediate 20 also served as a precursor to tetracyclines with aminomethyl (32) and piperazinylmethyl (34) substituents at position C5a (Scheme 7), highly versatile compounds which functioned as further branch-points for the synthesis of C5a-substituted tetracyclines (Figures 2 and 3). Thus, treatment of cyclopropane 20 with sodium azide in dimethylformamide at 23 °C afforded the azido-substituted ring-opened product 31 in 78% yield after purification by flash-column chromatography.25 Addition of trimethylphosphine (2 equiv) to a solution of the azide 31 and 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile (Boc-ON, 2 equiv) in THF at −10 °C followed by warming to 23 °C afforded the corresponding tert-butyl carbamate (51% yield).26 Two-step deprotection of the tert-butyl carbamate intermediate was best achieved by an inverted deprotection sequence (hydrogenolysis followed by treatment with hydrofluoric acid),27 providing C5a-aminomethylminocycline 32 after purification by rp-HPLC (69% yield over two steps). In addition, magnesium bromide-promoted ring-opening of cyclopropane 20 with N-tert-butoxycarbamyl-piperazine followed by deprotection of the ring-opened product 33 provided C5a-piperazinylmethylminocycline (34, 58% yield over three steps). Final-step diversification of 32 and 34 was readily achieved, affording a range of novel tetracyclines with C5a substituents incorporating amides, sulfonamides and amines (Figures 2 and 3). In this manner the C5a-C11a-bridged cyclopropane 20 served as a common precursor for the synthesis of more than 25 structural variants of minocycline with a diverse range of substituents at C5a.
Scheme 7.
Synthesis of substrates for final-step diversification from cyclopropane 20.
Figure 2.
Fully synthetic tetracyclines prepared by final-step diversification of C5a-aminomethylminocycline (32).
Figure 3.
Fully synthetic tetracyclines prepared by final-step diversification of C5a-piperazinylmethylminocycline (34).
3. Conclusion
Synthetic methodological advances have permitted efficient and stereocontrolled construction of diverse fully synthetic tetracyclines containing an all-carbon quaternary center at position C5a. These examples serve to further demonstrate the broad applicability of the Michael–Claisen cyclization reaction as a powerful method for the assembly of stereochemically complex six-membered rings.28 New strategies presented herein for the introduction of ester, thioester and aldehyde substituents at the β-position of cyclohexenones are anticipated to be of value in many contexts. The discovery of a highly diversifiable bridged cyclopropane-containing tetracycline intermediate enabled efficient synthesis of numerous C5a-substituted tetracyclines, a structurally novel class of compounds in this important family of therapeutic agents. Although it is conceivable that many of these structures could also have been accessed by Michael–Claisen cyclization reactions of individually prepared β-substituted AB enones, the discovery of a diversifiable late-stage intermediate greatly expedited the process of synthesis, in that a single Michael–Claisen cycloadduct served as a precursor to large numbers of C5a-substituted tetracyclines. The success of the C-ring-forming cyclization reactions of β-substituted AB enones described herein, combined with the extraordinary number and diversity of D-ring precursors known to be effective nucleophiles in this key coupling reaction, implies that a multiplicative expansion of the pool of fully synthetic tetracyclines is achievable, in the sense that each modified AB enone could in theory be coupled with the hundreds of different D-ring precursors that have been found to be effective coupling partners with the AB enone 1.
4. Experimental
4.1. General Experimental Procedures
All reactions were performed in round-bottom flasks fitted with rubber septa under a positive pressure of argon, unless otherwise noted. Air- and moisture-sensitive liquids were transferred via syringe or stainless steel cannula. Organic solutions were concentrated by rotary evaporation (house vacuum, ca. 25–40 Torr) at ambient temperature, unless otherwise noted. Analytical thin-layer chromatography (TLC) was performed using glass plates pre-coated with silica gel (0.25 mm, 60 Å pore-size, 230–400 mesh, Merck KGA) impregnated with a fluorescent indicator (254 nm). TLC plates were visualized by exposure to ultraviolet light, then were stained with aqueous potassium permanganate solution. Flash-column chromatography was performed as described by Still et al.,29 employing silica gel (60 Å, 32–63 μM, standard grade, Dynamic Adsorbents, Inc.).
Materials
Commercial solvents and reagents were used as received with the following exceptions. Diisopropylamine, TMEDA, chlorotrimethylsilane, and hexamethylphosphoramide were distilled from calcium hydride under an atmosphere of argon or dinitrogen. Tetrahydrofuran was purified by the method of Pangborn et al.30 The molarity of n-butyllithium solutions was determined by titration against a standard solution of diphenylacetic acid in tetrahydrofuran (average of three determinations).31
Instrumentation
Proton magnetic resonance (1H NMR) spectra were recorded on Varian INOVA 400 (400 MHz), 500 (500 MHz) or 600 (600 MHz) NMR spectrometers at 23 °C. Proton chemical shifts are expressed in parts per million (ppm, δ scale) and are referenced to residual protium in the NMR solvent (CHCl3, δ 7.26). Data are represented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and/or multiple resonances, br = broad), integration, and coupling constant (J) in Hertz. Carbon nuclear magnetic resonance spectra (13C NMR) were recorded on Varian INOVA 500 (125 MHz) NMR spectrometers at 23 °C. Carbon chemical shifts are expressed in parts per million (ppm, δ scale) and are referenced to the carbon resonance of the NMR solvent (CDCl3, δ 77.0). Infrared (IR) spectra were obtained using a Shimadzu 8400S FT-IR spectrometer and were referenced to a polystyrene standard. Data are represented as follows: frequency of absorption (cm−1), intensity of absorption (s = strong, m = medium, w = weak, br = broad). High-resolution mass spectra were obtained at the Harvard University Mass Spectrometry Facility.
4.2. Experimental procedures
4.2.1. β-Methyl-substituted trimethylsilyl enol ether 2
A round-bottomed flask charged with copper (I) iodide (1.89 g, 9.95 mmol, 1.6 equiv) was flame-dried under high vacuum. After cooling to room temperature, the flask was blanketed with dry argon. Tetrahydrofuran (50 mL) was added and the resulting suspension was cooled to 0 °C. A solution of methyllithium in ethyl ether (1.6 M, 12.2 mL, 19.6 mmol, 3.15 equiv) was added dropwise via syringe over 5 min. The resulting solution was stirred at 0 °C for 20 min, then was cooled to −78 °C. A solution of the AB enone 1 (3.0 g, 6.22 mmol, 1 equiv) and chlorotrimethylsilane (1.25 mL, 9.95 mmol, 1.6 equiv) in tetrahydrofuran (10 mL) was added to the cuprate solution dropwise via syringe at −78 °C. After stirring at −78 °C for 90 min, the cooling bath was removed and the product solution was diluted with ethyl acetate (100 mL) and hexanes (100 mL). A mixture of saturated aqueous ammonium chloride solution and saturated aqueous ammonium hydroxide solution (19:1, 100 mL) was then added carefully. The phases were separated and the organic phase was washed sequentially with saturated aqueous ammonium chloride solution (100 mL) and saturated aqueous sodium chloride solution (2 × 100 mL). The organic phase was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (7% ethyl acetate-hexanes), providing β-methylsubstituted trimethylsilyl enol ether 2 as a white solid (3.01 g, 85%). Rf = 0.57 (15% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.48 (dd, 2H, J = 8.0, 1.5 Hz), 7.36-7.31 (m, 3H), 5.36 (AB quartet, 2H), 4.69 (d, 1H, J = 3.0 Hz), 3.76 (d, 1H, J = 10.0 Hz), 2.55-2.52 (m, 1H), 2.44 (s, 6H), 2.32-2.25 (m, 2H), 1.84 (d, 1H, J = 13.5 Hz), 1.18 (d, 3H, J = 7.5 Hz), 0.87 (s, 9H), 0.22 (s, 3H), 0.11 (s, 3H), −0.04 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 189.7, 181.5, 167.4, 147.2, 135.2, 128.6, 128.5, 128.4, 110.7, 108.3, 80.7, 72.2, 61.1, 46.3, 41.9, 26.2, 26.0, 25.1, 24.0, 18.8, −0.5, −2.8, −3.6; FTIR (neat film), 2955 (w), 1721 (m), 1653 (w), 1614 (w), 1510 (m), 1471 (w), 1250 (m), 1198 (m), 1148 (m), 936 (m), 833 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C30H47N2O5Si2, 571.3018; found, 571.3041.
4.2.2. β-Methyl-substituted AB enone 3.8
Palladium (II) acetate (75 mg, 0.329 mmol, 1.15 equiv) was added to a solution of β-methyl-substituted trimethylsilyl enol ether 2 (163 mg, 0.286 mmol, 1 equiv) in anhydrous dimethyl sulfoxide (3.0 mL) at 23 °C. The resulting mixture was heated to 80 °C. After stirring at this temperature for 16 h, the reaction mixture was allowed to cool to 23 °C. The cooled suspension was diluted with ethyl acetate (20 mL) and the whole was filtered through a pad of Celite. Hexanes (20 mL) were added to the filtrate and the resulting solution was washed sequentially with saturated aqueous sodium bicarbonate solution (20 mL) and saturated aqueous sodium chloride solution (2 × 20 ml). The organic phase was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (10% ethyl acetate-hexanes), affording the β-methyl-substituted AB enone 3 as a pale yellow solid (63 mg, 44%). Rf = 0.26 (15% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.50 (d, 2H, J = 7.0 Hz), 7.40-7.32 (m, 3H), 5.93 (s, 1H), 5.35 (AB quartet, 2H), 3.74 (d, 1H, J = 11.0 Hz), 2.81-2.70 (m, 3H), 2.46 (s, 6H), 2.01 (s, 3H), 0.82 (s, 9H), 0.26 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.1, 188.1, 181.2, 167.5, 161.2, 135.0, 128.5, 128.5, 124.7, 108.4, 82.5, 72.5, 59.8, 47.5, 42.0, 30.4, 25.9, 24.4, 19.0, −2.5, −4.2; FTIR (neat film), 2930 (w), 1719 (s), 1672 (m), 1510 (m), 1472 (w), 1175 (m), 1045 (m), 936 (s), 829 (m) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C27H37N2O5Si, 497.2466; found, 497.2494.
4.2.3. β-Tris(methylthio)methyl trimethylsilyl enol ether 4
A solution of n-butyllithium in hexanes (2.5 M, 518 μL, 1.30 mmol, 1.25 equiv) was added dropwise via syringe to a solution of tris(methylthio)methane (176 μL, 1.30 mmol, 1.25 equiv) in tetrahydrofuran (11 mL) at −78 °C. The resulting colorless solution was stirred at this temperature for 20 min, whereupon a solution of the AB enone 1 (500 mg, 1.04 equiv, 1 equiv) in tetrahydrofuran (3.0 mL) was added dropwise via syringe, forming a bright orange-yellow solution. The reaction solution was allowed to warm slowly to −45 °C over 60 min, then chlorotrimethylsilane (199 μL, 1.55 mmol, 1.5 equiv) was added. The (yellow) reaction mixture was stirred at −45 °C for 30 min, then was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 20 mL) and dichloromethane (30 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange-yellow oil. The crude product was purified by flash-column chromatography (6% ethyl acetate-hexanes), providing β-tris(methylthio)methyl trimethylsilyl enol ether 4 as a pale yellow foam (654 mg, 89%). Rf = 0.69 (20% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.49-7.47 (m, 2H), 7.37-7.32 (m, 3H), 5.37 (AB quartet, 2H), 5.36 (d, 1H, J = 2.0 Hz), 4.00 (d, 1H, J = 9.3 Hz), 3.15-3.12 (m, 1H), 2.52 (dd, 1H, J = 14.4, 4.2 Hz), 2.45 (s, 6H), 2.42-2.39 (m, 1H), 2.18 (s, 9H), 2.14-2.06 (m, 1H), 0.86 (s, 9H), 0.22 (s, 3H), 0.12 (s, 3H), −0.01 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 189.6, 181.6, 167.2, 150.5, 135.2, 128.6, 128.5, 128.4, 108.7, 103.8, 81.6, 75.1, 72.2, 61.7, 46.6, 42.1, 41.7, 26.1, 21.2, 19.0, 14.1, −0.3, −2.6, −3.3; FTIR (neat film), 1722 (m), 1651 (w), 1614 (w), 1510 (m), 1472 (w), 1254 (m), 1206 (w), 903 (m), 839 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C33H53N2O5S3Si2, 709.2650; found, 709.2617.
4.2.4. β-Methyl ester-substituted AB enone 5
N-Bromosuccinimide (151 mg, 0.846 mmol, 5.0 equiv) was added in one portion to a stirring solution of β-tris(methylthio)methyl trimethylsilyl enol ether 4 (120 mg, 0.169 mmol, 1 equiv) in methanol (4.5 mL) and water (9.0 μL, 3.0 equiv, 500:1 mixture of methanol and water) at 23 °C. The pale yellow reaction solution was allowed to stir at 23 °C for 30 min, then was concentrated. The resulting yellow oil was dissolved in dichloromethane (20 mL) and the resulting solution was washed with saturated aqueous sodium bicarbonate solution (20 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude product was purified by flash-column chromatography (11% ethyl acetate-hexanes, grading to 15%), providing the β-methyl ester-substituted AB enone 5 as a yellow solid (78 mg, 85%). Rf = 0.26 (15% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.50 (d, 2H, J = 6.9 Hz), 7.40-7.33 (m, 3H), 6.82 (d, 1H, J = 1.8 Hz), 5.36 (AB quartet, 2H), 3.87 (s, 3H), 3.65 (d, 1H, J = 10.5 Hz), 3.21 (d, 1H, J = 18.8 Hz), 2.91-2.84 (m, 2H), 2.46 (s, 6H), 0.80 (s, 9H), 0.26 (s, 3H), 0.03 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.2, 187.0, 181.1, 167.4, 166.1, 147.1, 134.9, 131.4, 128.6, 128.5, 128.5, 108.3, 82.5, 72.6, 59.3, 52.9, 47.2, 41.9, 25.9, 24.6, 19.0, −2.5, −4.1; FTIR (neat film), 1721 (s), 1684 (m), 1609 (w), 1510 (m), 1252 (m), 1173 (m), 1030 (m), 831 (m), 737 (m) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C28H37N2O7Si, 541.2365; found, 541.2368.
4.2.5. β-Methyl ester-substituted AB enone 5 (single-operation method)
A solution of n-butyllithium in hexanes (2.5 M, 104 μL, 0.259 mmol, 1.25 equiv) was added dropwise via syringe to a solution of tris(methylthio)methane (35.2 μL, 0.259 mmol, 1.25 equiv) in tetrahydrofuran (2.0 mL) at −78 °C. The resulting colorless solution was stirred at this temperature for 20 min, whereupon a solution of the AB enone 1 (100 mg, 0.207 mmol, 1 equiv) in tetrahydrofuran (0.4 mL) was added dropwise via syringe, forming a bright orange-yellow solution. The reaction solution was allowed to warm slowly to −45 °C over 30 min, then chlorotrimethylsilane (39.7 μL, 0.311 mmol, 1.5 equiv) was added. The resulting (yellow) mixture was allowed to warm to 23 °C over 30 min, whereupon methanol (4.0 mL), water (8.0 μL, 2.1 equiv, 500:1 mixture of methanol and water) and N-bromosuccinimide (221 mg, 1.24 mmol, 6.0 equiv) were added in sequence. The reaction mixture was allowed to stir at 23 °C for 30 min, then was concentrated. The resulting oily (yellow) suspension was dissolved in dichloromethane (15 mL) and the resulting solution was washed with saturated aqueous sodium bicarbonate solution (15 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (15 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude product was purified by flash-column chromatography (7% acetone-hexanes), providing the β-methyl ester-substituted AB enone 5 as a yellow solid (101 mg, 90%).
4.2.6. β-Trifluoroethyl ester-substituted AB enone 6
N-Bromosuccinimide (100 mg, 0.564 mmol, 5.0 equiv) was added in one portion to a stirring solution of β-tris(methylthio)methyl trimethylsilyl enol ether 4 (80 mg, 0.113 mmol, 1 equiv) in 2,2,2-trifluoroethanol (3.0 mL) and water (6.0 μL, 500:1 mixture of 2,2,2-trifluoroethanol and water) at 23 °C. The bright orange reaction solution was allowed to stir at 23 °C for 45 min, then was concentrated. The resulting yellow oil was dissolved in dichloromethane (20 mL) and the resulting solution was washed with saturated aqueous sodium bicarbonate solution (20 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude product was purified by flash-column chromatography (10% ethyl acetate-hexanes), providing the β-trifluoroethyl estersubstituted AB enone 6 as a yellow solid (60 mg, 87%). Rf = 0.33 (15% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.50 (d, 2H, J = 6.9 Hz), 7.41-7.35 (m, 3H), 6.90 (d, 1H, J = 2.3 Hz), 5.36 (AB quartet, 2H), 4.68-4.62 (m, 2H), 3.65 (d, 1H, J = 11.0 Hz), 3.22 (d, 1H, J = 18.8 Hz), 2.95-2.87 (m, 2H), 2.47 (s, 6H), 0.80 (s, 9H), 0.27 (s, 3H), 0.04 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.0, 186.7, 181.0, 167.4, 164.1, 145.2, 134.9, 132.7, 128.6, 128.5, 128.5, 122.5 (q, J = 276.5 Hz), 108.3, 82.4, 72.7, 61.3 (q, J = 37.5 Hz), 59.3, 47.0, 41.9, 25.9, 24.5, 18.9, −2.5, −4.1; FTIR (neat film), 1742 (m), 1721 (s), 1688 (m), 1607 (w), 1510 (m), 1167 (s), 936 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C29H36F3N2O7Si, 609.2238; found, 609.2299.
4.2.7. β-S-Methyl thioester-substituted AB enone 7
N-Bromosuccinimide (48 mg, 0.271 mmol, 4.0 equiv) was added in one portion to a stirring solution of β-tris(methylthio)methyl trimethylsilyl enol ether 4 (48 mg, 0.068 mmol, 1 equiv) in tert-butanol (3.0 mL) at 23 °C. The resulting bright yellow suspension was allowed to stir at 23 °C for 45 min (slowly clearing to give a yellow solution). The reaction mixture was diluted with dichloromethane (20 mL) and the resulting solution was washed with saturated aqueous sodium bicarbonate solution (20 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude product was purified by flash-column chromatography (8% ethyl acetate-hexanes), providing the β-S- methyl thioester-substituted AB enone 7 as a pale yellow solid (31 mg, 82%). Rf = 0.51 (20% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.51-7.50 (m, 2H), 7.41-7.35 (m, 3H), 6.72 (d, 1H, J = 2.4 Hz), 5.36 (AB quartet, 2H), 3.70 (d, 1H, J = 10.7 Hz), 3.30 (d, 1H, J = 19.5 Hz), 2.98-2.93 (m, 1H), 2.89 (dd, 1H, J = 10.5, 3.7 Hz), 2.49 (s, 6H), 2.45 (s, 3H), 0.82 (s, 9H), 0.26 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.2, 192.9, 186.8, 181.0, 167.4, 153.2, 134.9, 128.6, 128.5, 128.5, 127.8, 108.3, 82.6, 72.6, 59.3, 47.3, 41.9, 25.9, 25.9, 24.4, 19.0, 11.9, −2.5, −4.0; FTIR (neat film), 1721 (m), 1684 (w), 1661 (w), 1510 (m), 1136 (w), 1040 (m), 937 (s), 735 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C28H37N2O6SSi, 557.2136; found, 557.2109.
4.2.8. β-(1,3-Dithian-2-yl) trimethylsilyl enol ether 8
A solution of n-butyllithium in hexanes (2.5 M, 2.91 mL, 7.27 mmol, 1.15 equiv) was added to a solution of 1,3-dithiane (862 mg, 6.95 mmol, 1.1 equiv) in tetrahydrofuran (60 mL) at −78 °C. The resulting solution was stirred at this temperature for 30 min, at which point hexamethylphosphoramide (2.44 mL, 13.9 mmol, 2.2 equiv) was added dropwise. After stirring at −78 °C for a further 2 min, a solution of the AB enone 1 (3.05 g, 6.32 mmol, 1 equiv) in tetrahydrofuran (15 mL) was added to the reaction solution dropwise via syringe. The brownish-yellow reaction mixture was stirred at −78 °C for 40 min whereupon chlorotrimethylsilane (1.20 mL, 9.48 mmol, 1.5 equiv) was added. After stirring at −78 °C for 40 min, aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 100 mL) was added to the reaction solution. The resulting mixture was allowed to warm to 23 °C, then was extracted with dichloromethane (3 × 100 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (8% ethyl acetate-hexanes), affording β-(1,3-dithian-2-yl) trimethylsilyl enol ether 8 as a white foam (3.85 g, 90%). Rf = 0.53 (30% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.48 (d, 2H, J = 7.0 Hz), 7.38-7.31 (m, 3H), 5.36 (AB quartet, 2H), 4.98 (d, 1H, J = 3.0 Hz), 4.12 (d, 1H, J = 5.0 Hz), 3.89 (d, 1H, J = 9.5 Hz), 2.96-2.82 (m, 5H), 2.46 (s, 6H), 2.34-2.29 (m, 1H), 2.28-2.23 (m, 2H), 2.15-2.09 (m, 1H), 1.90-1.80 (m, 1H), 0.86 (s, 9H), 0.21 (s, 3H), 0.10 (s, 3H), −0.01 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 189.4, 181.5, 167.3, 149.5, 135.1, 128.7, 128.5, 128.4, 108.5, 104.8, 81.0, 72.3, 61.3, 54.9, 46.1, 41.9, 37.1, 31.1, 30.8, 26.1, 25.7, 21.8, 18.9, −0.4, −2.7, −3.6; FTIR (neat film), cm−1 2953 (w), 1721 (s), 1653 (w), 1614 (w), 1510 (s), 1472 (w), 1454 (w), 1254 (s), 1204 (w), 1150 (w), 1024 (w), 934 (s), 901 (s), 835 (s); HRMS–ESI (m/z): [M+H]+ calcd for C33H51N2O5S2Si2, 675.2772; found, 675.2783.
4.2.9. β-Carbaldehyde AB enone 9
N-Bromosuccinimide (158 mg, 0.889 mmol, 6.0 equiv) was added in one portion to a stirring solution of β-(1,3-dithian-2-yl) trimethylsilyl enol ether 8 (100 mg, 0.148 mmol, 1 equiv) in tert-butanol (4.0 mL) and water (40 μL) at 23 °C. The reaction mixture was stirred at this temperature for 1 h, then was partitioned between dichloromethane (20 mL) and saturated aqueous sodium bicarbonate solution (20 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude product was purified by flash-column chromatography (12% ethyl acetate-hexanes), affording the β-carbaldehyde AB enone 9 as a yellow solid (68 mg, 90%). Rf = 0.18 (15% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 9.82, (s, 1H), 7.50 (d, 2H, J = 6.9 Hz), 7.41-7.35 (m, 3H), 6.66 (d, 1H, J = 2.7 Hz), 5.36 (AB quartet, 2H), 3.58 (d, 1H, J = 11.0 Hz), 3.17 (d, 1H, J = 19.7 Hz), 2.90 (dd, 1H, J = 10.8, 4.8 Hz), 2.77-2.71 (m, 1H), 2.44 (s, 6H), 0.78 (s, 9H), 0.27 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.6, 193.4, 186.5, 181.2, 167.4, 152.5, 137.2, 134.9, 128.6, 128.5, 128.5, 108.3, 83.2, 72.7, 59.4, 47.0, 41.8, 25.9, 21.5, 19.0, −2.5, −4.0; FTIR (neat film), 1721 (m), 1694 (m), 1607 (w), 1510 (m), 1173 (w), 1036 (m), 937 (s), 737 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C27H35N2O6Si, 511.2259; found, 511.2286.
4.2.10. β-Methoxymethoxymethyl AB enone 10
Sodium triacetoxyborohydride (205 mg, 0.918 mmol, 3.5 equiv) was added in one portion to a solution of the β-carbaldehyde AB enone 9 (134 mg, 0.262 mmol, 1 equiv) in benzene (2.0 mL) at 23 °C. The resulting solution was heated to 40 °C. After stirring at 40 °C for 5 ½ h, the reaction mixture was allowed to cool to 23 °C. The cooled solution was diluted with dichloromethane (30 mL), and the resulting solution was added slowly and carefully to saturated aqueous sodium bicarbonate solution (30 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (30 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. N,N-Diisopropylethylamine (229 μL, 1.31 mmol, 5.0 equiv) and chloromethyl methyl ether (59.8 μL, 0.787 mmol, 3.0 equiv) were added sequentially to a solution of the crude reduction product in benzene (1.5 mL) at 23 °C. The reaction flask was sealed and the solution was heated to 50 °C. After stirring at 50 °C for 24 h, the reaction mixture was allowed to cool to 23 °C. The cooled solution was partitioned between dichloromethane (30 mL) and saturated aqueous sodium bicarbonate solution (30 mL). The layers were separated and the aqueous phase was extracted with dichloromethane (30 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange oil. The product was purified by flash-column chromatography (15% ethyl acetate-hexanes, grading to 17% ethyl acetate-hexanes), providing the β-methoxymethoxymethyl AB enone 10 as a pale yellow solid (124 mg, 85% yield, two steps). Rf = 0.40 (30% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.50 (d, 2H, J = 7.0 Hz), 7.40-7.32 (m, 3H), 6.21 (s, 1H), 5.35 (AB quartet, 2H), 4.67 (AB quartet, 2H), 4.16 (m, 2H), 3.74 (d, 1H, J = 10.0 Hz), 3.38 (s, 3H), 2.80-2.74 (m, 3H), 2.45 (s, 6H), 0.82 (s, 9H), 0.26 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.1, 187.7, 181.1, 167.5, 159.7, 135.0, 128.5, 128.5, 128.5, 122.4, 108.4, 96.0, 83.0, 72.6, 68.4, 59.6, 55.5, 47.5, 41.9, 25.9, 25.8, 19.0, −2.5, −4.1; FTIR (neat film), cm−1 2951 (w), 2930 (w), 1719 (s), 1674 (m), 1510 (s), 1175 (m), 1152 (m), 1038 (s), 934 (s), 829 (s), 735 (s); HRMS–ESI (m/z): [M+H]+ calcd for C29H41N2O7Si, 557.2678; found, 557.2690.
4.2.11. β-Methoxymethoxymethyl trimethylsilyl enol ether 11
A solution of n-butyllithium in hexanes (2.5 M, 374 μL, 0.936 mmol, 2.1 equiv) was added to a solution of tri-n-butyl[(methoxymethoxy)methyl]stannane19 (326 mg, 0.893 mmol, 2.0 equiv) in tetrahydrofuran (4.0 mL) at −78 °C. The resulting solution was stirred at this temperature for 15 min, at which point hexamethylphosphoramide (313 μL, 1.78 mmol, 4.0 equiv) was added dropwise. After stirring at −78 °C for a further 1 min, a solution of the AB enone 1 (215 mg, 0.446 mmol, 1 equiv) in tetrahydrofuran (1.0 mL) was added to the reaction solution dropwise via syringe. The reaction mixture was stirred at −78 °C for 30 min whereupon chlorotrimethylsilane (170 μL, 1.34 mmol, 3.0 equiv) was added. After stirring at −78 °C for 30 min, aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 10 mL) was added to the reaction solution. The resulting mixture was allowed to warm to 23 °C, then was extracted with ethyl acetate (3 × 20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (10% ethyl acetate-hexanes), affording β-methoxymethoxymethyl trimethylsilyl enol ether 11 as a pale yellow solid (156 mg, 55%). Rf = 0.43 (20% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.49-7.46 (m, 2H), 7.36-7.30 (m, 3H), 5.36 (AB quartet, 2H), 4.78 (d, 1H, J = 2.4 Hz), 4.67 (s, 2H), 3.82 (d, 1H, J = 9.8 Hz), 3.54-3.45 (m, 2H), 3.38 (s, 3H), 2.71-2.66 (m, 1H), 2.42 (s, 6H), 2.30-2.22 (m, 2H), 2.01 (d, 1H, J = 13.7 Hz), 0.86 (s, 9H), 0.23 (s, 3H), 0.12 (s, 3H), −0.05 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 189.6, 181.6, 167.4, 148.9, 135.1, 128.7, 128.5, 128.4, 108.3, 105.8, 96.5, 81.1, 73.0, 72.2, 61.0, 55.2, 46.1, 41.9, 32.4, 26.0, 20.1, 18.9, −0.5, −2.8, −3.6; HRMS–ESI (m/z): [M+Na]+ calcd for C32H50N2O7Si2Na, 653.3049; found, 653.3056.
4.2.12. β-Methoxymethoxymethyl AB enone 10 (alternative preparation)
Palladium (II) acetate (242 mg, 1.06 mmol, 1.05 equiv) was added to a solution of β-methoxymethoxymethyl trimethylsilyl enol ether 11 (635 mg, 1.01 mmol, 1 equiv) in anhydrous dimethyl sulfoxide (8.0 mL) at 23 °C. The resulting mixture was heated to 50 °C. After stirring at this temperature for 14 ½ h, the reaction mixture was allowed to cool to 23 °C. The cooled suspension was diluted with ethyl acetate (50 mL) and the whole was filtered through a pad of Celite. Hexanes (50 mL) were added to the filtrate and the resulting solution was washed sequentially with saturated aqueous sodium bicarbonate solution (50 mL) and saturated aqueous sodium chloride solution (50 ml). The aqueous phases were combined and the combined solution was extracted with ethyl acetate-hexanes (1:1, 100 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (17% ethyl acetate-hexanes), affording the β-methoxymethoxymethyl AB enone 10 as a pale yellow solid (290 mg, 52%).
4.2.13. C5a-Methylminocycline (17)
A freshly prepared solution of lithium diisopropylamide in tetrahydrofuran (1.0 M, 1.21 mL, 1.21 mmol, 3.0 equiv) was added dropwise via syringe to a solution of phenyl ester 12 (449 mg, 1.21 mmol, 3.0 equiv) and TMEDA (365 μL, 2.42 mmol, 6.0 equiv) in tetrahydrofuran (15 mL) at −78 °C, forming a bright red solution. After stirring at −78 °C for 40 min, a solution of the β-methyl-substituted AB enone 3 (200 mg, 0.403 mmol, 1 equiv) in tetrahydrofuran (3.0 ml) was added to the reaction solution dropwise via syringe. The resulting mixture was allowed to warm slowly to −10 °C over 80 min, then was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 60 mL) and dichloromethane (60 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (2 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange-yellow oil. The product was purified by flash-column chromatography (15% ethyl acetate-hexanes, grading to 20%), providing the Michael–Claisen cyclization product 14 as a yellow solid (249 mg, 80%). Rf = 0.28 (20% ethyl acetate-hexanes); 1H NMR (600 MHz, CDCl3) δ 15.96 (s, 1H), 7.49 (d, 2H, J = 7.8 Hz), 7.39-7.33 (m, 3H), 7.26-7.24 (m, 1H), 7.04 (d, 1H, J = 8.5 Hz), 5.36 (s, 2H), 4.16 (d, 1H, J = 10.0 Hz), 3.20 (d, 1H, J = 16.1 Hz), 2.75 (d, 1H, J = 16.1 Hz), 2.66 (s, 6H), 2.54-2.50 (m, 7H), 2.37 (d, 1H, J = 14.4 Hz), 2.16 (dd, 1H, J = 14.8, 4.5 Hz), 1.56 (s, 9H), 1.12 (s, 3H), 0.90 (s, 9H), 0.25 (s, 3H), 0.21 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 186.8, 185.6, 181.7, 178.3, 167.6, 152.3, 150.4, 145.4, 136.4, 135.1, 128.5, 128.4, 128.3, 124.2, 123.9, 122.3, 112.0, 108.1, 83.8, 81.7, 72.4, 60.7, 47.0, 44.2, 41.9, 40.6, 32.4, 32.1, 29.8, 27.7, 26.4, 19.2, −1.9, −2.3; FTIR (neat film), 1759 (w), 1721 (m), 1613 (w), 1510 (m), 1456 (w), 1265 (m), 1152 (m), 737 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C42H55N3O9Si, 774.3780; found, 774.3796.
Concentrated aqueous hydrofluoric acid solution (48 wt%, 2.0 mL) was added to a solution of the Michael–Claisen cyclization product 14 (249 mg, 0.322 mmol, 1 equiv) in acetonitrile (3.0 mL) in a polypropylene reaction vessel at 23 °C. The reaction solution was stirred vigorously at 23 °C for 17 h, then was poured into water (100 mL) containing dipotassium hydrogenphosphate trihydrate (20.0 g). The resulting mixture was extracted with ethyl acetate (100 mL, then 2 × 50 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange-brown solid. Methanol (3.0 mL) and dioxane (3.0 mL) were added to the crude product, forming an orange-brown solution. Palladium black (13.7 mg, 0.129 mmol, 0.4 equiv) was added in one portion at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 h, then was filtered through a plug of Celite. The filtrate was concentrated, affording a brownish yellow solid. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, 2 batches, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 24–31 min were collected and concentrated, affording C5a-methylminocycline trifluoroacetate 17 as a yellow solid (188 mg, 100%, two steps). 1H NMR (600 MHz, CD3OD, trifluoroacetate) δ 7.90 (d, 1H, J = 9.4 Hz), 7.07 (d, 1H, J = 9.4 Hz), 4.13 (d, 1H, J = 1.0 Hz), 3.25 (s, 6H), 3.17 (d, 1H, J = 15.8 Hz), 3.07-3.02 (m, 1H), 3.04 (s, 6H), 2.80 (d, 1H, J = 15.7 Hz), 2.05 (dd, 1H, J = 13.8, 2.9 Hz), 1.93 (dd, 1H, J = 14.1, 13.9 Hz), 1.26 (s, 3H); HRMS–ESI (m/z): [M+H]+ calcd for C24H29N3O7, 472.2078; found, 472.2087.
4.2.14. Michael–Claisen cyclization product 15
A freshly prepared solution of lithium diisopropylamide (1.0 M, 7.86 mL, 7.86 mmol, 3.6 equiv) was added dropwise via syringe to a solution of phenyl ester 13 (2.84 g, 7.86 mmol, 3.6 equiv) and TMEDA (2.27 mL, 15.1 mmol, 7.0 equiv) in tetrahydrofuran (60 mL) at −78 °C, forming a bright red solution. After stirring at −78 °C for 40 min, a solution of the β-methoxymethoxymethyl AB enone 10 (1.20 g, 2.16 mmol, 1 equiv) in tetrahydrofuran (15 mL) was added to the reaction solution dropwise via syringe. The resulting mixture was allowed to warm slowly to −10 °C over 80 min, then was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 100 mL) and dichloromethane (100 mL). The phases were separated and the aqueous phase was further extracted with dichloromethane (2 × 75 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange-yellow oil. The product was purified by flash-column chromatography (3.5% ethyl acetate-dichloromethane), providing the Michael–Claisen cyclization product 15 as a yellow solid (1.29 g, 72%). Rf = 0.31 (30% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 16.77 (s, 1H), 7.51 (d, 4H, J = 8.0 Hz), 7.41-7.28 (m, 6H), 7.21 (d, 1H, J = 9.0 Hz), 6.90 (d, 1H, J = 9.0 Hz), 5.38 (s, 2H), 5.17 (AB quartet, 2H), 4.47 (d, 1H, J = 6.5 Hz), 4.34 (d, 1H, J = 6.5 Hz), 4.15 (d, 1H, J = 9.5 Hz), 3.78 (d, 1H, J = 16.5 Hz), 3.38 (d, 1H, J = 9.0 Hz), 3.27 (d, 1H, J = 9.5 Hz), 3.12 (s, 3H), 2.63 (s, 6H), 2.65-2.58 (m, 1H), 2.51 (s, 6H), 2.51-2.41 (m, 2H), 2.32 (dd, 1H, J = 14.5, 2.0 Hz), 0.93 (s, 9H), 0.29 (s, 3H), 0.20 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 186.9, 184.6, 183.0, 181.6, 167.7, 154.7, 145.7, 136.8, 136.1, 135.1, 128.5, 128.4, 128.4, 128.3, 127.7, 127.0, 125.0, 120.7, 113.7, 108.1, 107.2, 96.1, 82.3, 72.9, 72.4, 71.4, 61.1, 54.6, 46.4, 44.4, 41.9, 35.8, 34.7, 28.4, 26.5, 19.3, −2.0, −2.1; FTIR (neat film), 2932 (w), 1721 (s), 1611 (w), 1510 (m), 1472 (m), 1452 (m), 1269 (w), 1148 (w), 1107 (w), 1040 (s), 1020 (s), 922 (w), 831 (s), 733 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C46H58N3O9Si, 824.3937; found, 824.3885.
4.2.15. C5a-Carbomethoxyminocycline (18)
A freshly prepared solution of lithium diisopropylamide in tetrahydrofuran (1.0 M, 0.416 mL, 0.416 mmol, 3.0 equiv) was added dropwise via syringe to a solution of phenyl ester 12 (155 mg, 0.416 mmol, 3.0 equiv) and TMEDA (126 μL, 0.832 mmol, 6.0 equiv) in tetrahydrofuran (6 mL) at −78 °C, forming a bright red solution. After stirring at −78 °C for 40 min, a solution of the β-methyl ester-substituted AB enone 5 (75 mg, 0.139 mmol, 1 equiv) in tetrahydrofuran (1.5 ml) was added to the reaction solution dropwise via syringe. The resulting mixture was allowed to warm slowly to −10 °C over 75 min, then was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 25 mL) and dichloromethane (25 mL). The phases were separated and the aqueous phase was further extracted with dichloromethane (2 × 20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording a yellow solid. The crude product was purified first by flash-column chromatography (15% acetone-hexanes), then by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: water, Solvent B: methanol, injection volume: 6.0 mL (5.0 mL methanol, 1.0 mL water), gradient elution with 85→100% B over 40 min, flow rate: 15 mL/min]. Fractions eluting at 25–28 min were collected and concentrated, providing the Michael–Claisen cyclization product 16 as a yellow solid (26 mg, 23%). Rf = 0.34 (25% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 15.97 (s, 1H), 7.49 (d, 2H, J = 7.3 Hz), 7.39-7.33 (m, 3H), 7.24 (d, 1H, J = 8.3 Hz), 7.03 (d, 1H, J = 8.3 Hz), 5.37 (s, 2H), 4.11 (d, 1H, J = 9.3 Hz), 3.84 (d, 1H, J = 16.1 Hz), 3.36 (s, 3H), 2.80 (d, 1H, J = 16.1 Hz), 2.67-2.64 (m, 1H), 2.64 (s, 6H), 2.57-2.53 (m, 2H), 2.51 (s, 6H), 1.57 (s, 9H), 0.82 (s, 9H), 0.23 (s, 3H), 0.17 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 187.6, 186.2, 181.5, 176.3, 175.2, 167.6, 152.4, 150.0, 145.4, 135.0, 134.2, 128.5, 128.5, 128.3, 124.6, 124.4, 123.1, 108.0, 106.8, 83.8, 80.9, 72.5, 60.6, 52.4, 46.6, 44.2, 44.1, 41.9, 38.2, 27.7, 26.2, 19.1, −2.1, −2.9; FTIR (neat film), 1761 (w), 1722 (m), 1512 (m), 1234 (s), 1150 (s), 833 (m), 733 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C43H56N3O11Si, 818.3679; found, 818.3760.
Concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added to a solution of the HPLC-purified product 16 from the cyclization step above (25.0 mg, 0.031 mmol, 1 equiv) in acetonitrile (1.2 mL) in a polypropylene reaction vessel at 23 °C. The reaction solution was stirred vigorously at 23 °C for 17 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate trihydrate (10.0 g). The resulting mixture was extracted with ethyl acetate (30 mL, then 2 × 20 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, affording an orange solid. Palladium black (4.9 mg, 0.046 mmol, 1.5 equiv) was added in one portion to a solution of the crude product in methanol (1.5 mL) and dioxane (1.5 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 30 min, then was filtered through a plug of Celite. The filtrate was concentrated, affording a yellow solid. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 27–32 min were collected and concentrated, affording C5a-carbomethoxyminocycline trifluoroacetate 18 as a yellow solid (17.1 mg, 89%). 1H NMR (600 MHz, CD3OD) δ 7.81 (d, 1H, J = 9.2 Hz), 7.03 (d, 1H, J = 9.2 Hz), 4.14 (s, 1H), 3.66 (d, 1H, J = 15.7 Hz), 3.61 (s, 3H), 3.14 (s, 6H), 2.97 (s, 6H), 2.87-2.83 (m, 2H), 2.53 (dd, 1H, J = 14.3, 2.6 Hz), 2.04 (dd, 1H, J = 14.2, 14.1 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C26H30N3O9, 516.1977; found, 516.2011.
4.2.16. Substituted neopentyl alcohol 19
Perchloric acid (CAUTION!)21 (13.0 mL, 70% solution) was added dropwise over 5 min to a solution of the Michael–Claisen cyclization product 15 (1.04 g, 1.26 mmol, 1 equiv) in tetrahydrofuran (130 mL) at 23 °C. After stirring at this temperature for 10 min, the reaction solution was slowly and carefully poured into ice-cold saturated aqueous sodium bicarbonate solution (300 mL). The resulting mixture was extracted with dichloromethane (2 × 250 mL, then 50 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, providing an orange-yellow oil. The product was purified by flash-column chromatography (55% ethyl acetate-hexanes, grading to 75% ethyl acetate-hexanes), affording the substituted neopentyl alcohol 19 as a yellow solid (720 mg, 73%). Rf = 0.26 (65% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 16.76 (s, 1H), 7.53-7.49 (m, 4H), 7.41-7.28 (m, 6H), 7.22 (d, 1H, J = 9.0 Hz), 6.90 (d, 1H, J = 9.0 Hz), 5.38 (s, 2H), 5.17 (AB quartet, 2H), 4.11 (d, 1H, J = 9.5 Hz), 3.66 (d, 1H, J = 16.0 Hz), 3.48 (d, 1H, J = 11.0 Hz), 3.32 (d, 1H, J = 11.0 Hz), 2.64 (s, 6H), 2.68-2.59 (m, 1H), 2.56-2.48 (m, 1H), 2.51 (s, 6H), 2.38 (dd, 1H, J = 14.5, 4.5 Hz), 2.23 (brd, 1H, J = 14.0 Hz), 0.92 (s, 9H), 0.25 (s, 3H), 0.18 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 186.7, 184.7, 182.7, 181.4, 167.7, 154.9, 145.7, 136.8, 135.9, 135.1, 128.5, 128.5, 128.5, 128.3, 127.8, 126.9, 125.3, 120.7, 113.7, 108.2, 107.3, 82.3, 72.4, 71.4, 68.2, 61.3, 46.2, 44.7, 42.0, 36.8, 34.5, 28.2, 26.5, 19.3, −1.8, −2.0; FTIR (neat film), 2938 (w), 1719 (m), 1609 (w), 1510 (s), 1452 (s), 1265 (m), 1020 (m), 829 (s), 733 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C44H54N3O8Si, 780.3675; found,780.3654.
4.2.17. C5a-C11a-Bridged cyclopropane tetracycline precursor 20
4Å molecular sieves (2.4 g, small chunks) were added to a solution of the substituted neopentyl alcohol 19 (720 mg, 0.923 mmol, 1 equiv) in dichloromethane (72 mL) and pyridine (7.2 mL) at 23 °C. The resulting mixture was stirred at 23 °C for 1 h, then was cooled to 0 °C. A solution of phosgene in toluene (20 wt%, 537 μL, 1.02 mmol, 1.1 equiv) was added dropwise to the cooled mixture. The resulting solution was stirred at 0 °C for 1 h, whereupon aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 20 mL) was added. The resulting mixture was allowed to warm to 23 °C, then was filtered to remove the molecular sieves. Dichloromethane (60 mL) and aqueous potassium phosphate buffer solution (pH 7.0, 0.2M, 60 mL) were added and the phases were separated. The aqueous phase was further extracted with dichloromethane (2 × 60 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, providing an orange-yellow oil. The product was purified by flash-column chromatography (20% ethyl acetate-hexanes, grading to 30% ethyl acetate-hexanes), affording the C5a-C11a-bridged cyclopropane tetracycline precursor 20 as a white solid (572 mg, 81%). Rf = 0.25 (30% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 7.52 (d, 2H, J = 7.3 Hz), 7.44-7.24 (m, 8H), 7.13 (d, 1H, J = 9.0 Hz), 6.86 (d, 1H, J = 9.0 Hz), 5.35 (s, 2H), 5.05 (AB quartet, 2H), 4.01 (d, 1H, J = 10.5 Hz), 3.85 (d, 1H, J = 17.4 Hz), 2.77 (d, 1H, J = 17.4 Hz), 2.68-2.57 (m, 3H), 2.62 (s, 6H), 2.49 (s, 6H), 2.25 (d, 1H, J = 5.0 Hz), 1.71 (d, 1H, J = 5.0 Hz), 0.89 (s. 9H), 0.28, (s, 3H), 0.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.4, 191.8, 185.3, 180.9, 167.6, 152.6, 144.8, 136.7, 135.0, 132.2, 128.6, 128.5, 128.5, 128.4, 127.6, 127.1, 123.3, 123.2, 113.5, 107.9, 84.0, 72.6, 71.2, 58.8, 49.0, 44.8, 43.1, 41.8, 32.1, 31.1, 30.9, 26.6, 26.3, 19.5, −2.0, −2.6; FTIR (neat film), 2938 (w), 1728 (s), 1711 (m), 1670 (w), 1510 (m), 1474 (m), 1452 (m), 1362 (w), 1258 (m), 916 (m), 827 (s), 733 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C44H52N3O7Si, 762.3569; found,762.3569.
4.2.18. C5a-Pyrrolidinomethylminocycline (22)
Anhydrous magnesium bromide (8.2 mg, 0.045 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (17.0 mg, 0.022 mmol, 1 equiv) and pyrrolidine (18 μL, 0.223 mmol, 10 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction mixture was stirred at 23 °C for 16 h, then was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution (10 mL each). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product (21) was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 20 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ¼ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→35% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 39–43 min were collected and concentrated, affording C5a-pyrrolidinomethylminocycline bistrifluoroacetate 22 as a yellow solid (12.5 mg, 74%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.55 (d, 1H, J = 9.0 Hz), 6.95 (d, 1H, J = 9.0 Hz), 4.05 (s, 1H), 3.84 (brs, 1H), 3.75-3.71 (brs, 1H), 3.73 (d, 1H, J = 14.4 Hz), 3.62 (d, 1H, J = 16.8 Hz), 3.20 (d, 1H, J = 13.2 Hz), 3.14 (d, 1H, J = 15.0 Hz), 3.13-3.03 (brs, 1H), 3.10 (s, 6H), 2.70 (s, 6H), 2.67 (d, 1H, J = 16.8 Hz), 2.59 (dd, 1H, J = 15.0, 3.0 Hz), 2.50 (brs, 1H), 2.02-1.90 (m, 5H); HRMS–ESI (m/z): [M+H]+ calcd for C28H37N4O7, 541.2657; found, 541.2684.
4.2.19. C5a-Piperidinylmethylminocycline (23)
Anhydrous magnesium bromide (8.2 mg, 0.045 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (17.0 mg, 0.022 mmol, 1 equiv) and piperidine (22 μL, 0.223 mmol, 10 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction mixture was stirred at 23 °C for 21 h, then was heated to 45 °C. After stirring at this temperature for 14 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between dichloromethane (15 mL) and saturated aqueous sodium bicarbonate solution (10 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 13 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ½ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→35% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 40–44 min were collected and concentrated, affording C5a-piperidinylmethylminocycline bistrifluoroacetate 23 as a yellow solid (12.0 mg, 70%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.56 (d, 1H, J = 9.0 Hz), 6.96 (d, 1H, J = 9.0 Hz), 4.10 (d, 1H, J = 2.4 Hz), 3.66 (d, 1H, J = 16.8 Hz), 3.46 (d, 1H, J = 14.4 Hz), 3.40-3.35 (brm, 1H), 3.27-3.23 (m, 1H), 3.23-3.18 (m, 1H), 3.10 (s, 6H), 3.10-3.00 (m, 2H), 2.71 (s, 6H), 2.71-2.62 (m, 2H), 2.59 (dd, 1H, J = 15.0, 3.0 Hz), 2.02 (dd, 1H, J = 15.1, 12.7 Hz), 1.95-1.88 (brm, 1H), 1.85-1.78 (brm, 2H), 1.72-1.61 (brm, 2H), 1.43-1.36 (brm, 1H); HRMS–ESI (m/z): [M+H]+ calcd for C29H39N4O7, 555.2813; found, 555.2788.
4.2.20. C5a-Morpholinomethylminocycline (24)
Anhydrous magnesium bromide (6.3 mg, 0.034 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (13.0 mg, 0.017 mmol, 1 equiv) and morpholine (15 μL, 0.17 mmol, 10 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction flask was sealed and the reaction mixture was heated to 55 °C. After stirring at 55 °C for 14 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution (10 mL each). The phases were separated and the aqueous phase was further extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 16 ½ h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ¾ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→25% B over 50 min, then 25→100% B over 20 min, flow rate: 7.5 mL/min]. Fractions eluting at 49–52 min were collected and concentrated, affording C5a-morpholinomethylminocycline bistrifluoroacetate 24 as an orange-yellow solid (7.5 mg, 56%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.56 (d, 1H, J = 9.0 Hz), 6.95 (d, 1H, J = 9.0 Hz), 4.04 (s, 1H), 3.77-3.69 (m, 4H), 3.53 (d, 1H, J = 16.8 Hz), 3.24 (d, 1H, J = 13.2 Hz), 3.18-3.14 (m, 1H), 3.08 (s, 6H), 2.99-2.94 (m, 2H), 2.88 (d, 1H, J = 15.0 Hz), 2.81-2.72 (m, 2H), 2.74 (s, 6H), 2.62 (d, 1H, J = 16.8 Hz), 2.53 (brd, 1H, J = 14.4 Hz), 1.92 (dd, 1H, J = 14.2, 14.0 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C28H37N4O8, 557.2606; found, 557.2611.
4.2.21. C5a-Diethylaminomethylminocycline (25)
Anhydrous magnesium bromide (7.2 mg, 0.039 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (15.0 mg, 0.020 mmol, 1 equiv) and diethylamine (102 μL, 0.987 mmol, 50 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction flask was sealed and the reaction mixture was heated to 45 °C. After stirring at this temperature for 20 h, the reaction mixture was allowed to cool to room temperature. The reaction flask was opened briefly and a second portion of diethylamine (204 μL, 1.97 mmol, 100 equiv) was added. The flask was re-sealed and the reaction mixture was heated to 45 °C. After stirring at this temperature for a further 55 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution (10 mL each). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 10 ½ h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ¾ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 36–39 min were collected and concentrated, affording C5a-diethylaminomethylminocycline bistrifluoroacetate 25 as a yellow solid (12.0 mg, 79%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.54 (d, 1H, J = 9.0 Hz), 6.95 (d, 1H, J = 9.0 Hz), 4.10 (d, 1H, J = 3.0 Hz), 3.61 (d, 1H, J = 16.8 Hz), 3.42 (d, 1H, J = 15.0 Hz), 3.25-3.20 (m, 2H), 3.16-3.02 (brm, 3H), 3.10 (s, 6H), 2.93 (brs, 1H), 2.73-2.69 (m, 1H), 2.70 (s, 6H), 2.52 (dd, 1H, J = 15.0, 3.0 Hz), 2.07 (dd, 1H, J = 14.1, 13.3 Hz), 1.28 (brs, 3H), 1.04 (brs, 3H); HRMS–ESI (m/z): [M+H]+ calcd for C28H39N4O7, 543.2813; found, 543.2821.
4.2.22. C5a-N-Imidazolylmethylminocycline (26)
Anhydrous magnesium bromide (7.2 mg, 0.039 mmol, 3.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (10.0 mg, 0.013 mmol, 1 equiv) and imidazole (6.2 mg, 0.091 mmol, 7.0 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction flask was sealed and the reaction mixture was heated to 60 °C. After stirring at this temperature for 60 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution (15 mL each). The phases were separated and the aqueous phase was extracted with dichloromethane (15 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 18 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 3.6 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ¼ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 25–27 min were collected and concentrated, affording C5a-imidazolylmethylminocycline bistrifluoroacetate 26 as a yellow solid (7.3 mg, 73%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 8.42 (t, 1H, J = 1.3 Hz), 7.51 (d, 1H, J = 9.0 Hz), 7.40 (dd, 1H, J = 1.8, 1.5 Hz), 7.23 (dd, 1H, J = 1.8, 1.6 Hz), 6.86 (d, 1H, J = 9.0 Hz), 4.48 (AB quartet, 2H), 4.13 (s, 1H), 3.42 (d, 1H, J = 16.8 Hz), 3.25 (dd, 1H, J = 13.8, 1.2 Hz), 3.11 (s, 6H), 2.73 (s, 6H), 2.69 (d, 1H, J = 16.2 Hz), 2.14 (dd, 1H, J = 15.0, 3.0 Hz), 1.98 (dd, 1H, J = 14.5, 14.2 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C27H32N5O7, 538.2296; found, 538.2285.
4.2.23. C5a-Cyclopropylaminomethylminocycline (27)
Anhydrous magnesium bromide (8.2 mg, 0.045 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (17.0 mg, 0.022 mmol, 1 equiv) and cyclopropylamine (15 μL, 0.223 mmol, 10 equiv) in tetrahydrofuran (0.5 mL) at 23 °C. The reaction mixture was stirred at 23 °C for 16 h, then was heated to 40 °C. After stirring at this temperature for 22 h, the reaction mixture was allowed to cool to room temperature. The reaction flask was opened briefly and a second portion of cyclopropylamine (15 μL, 0.223 mmol, 10 equiv) was added. The flask was sealed and the reaction mixture was heated to 40 °C. After stirring at this temperature for a further 13 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between dichloromethane (15 mL) and saturated aqueous sodium bicarbonate solution (10 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 12 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 3 ½ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→35% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 35–36 min were collected and concentrated, affording C5a-cyclopropylaminomethylminocycline bistrifluoroacetate 27 as a yellow solid (3.0 mg, 18%, three steps).271H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.58 (d, 1H, J = 9.0 Hz), 6.96 (d, 1H, J = 9.0 Hz), 4.00 (d, 1H, J = 2.4 Hz), 3.60 (d, 1H, J = 14.4 Hz), 3.55 (d, 1H, J = 16.8 Hz), 3.19-3.10 (m, 1H), 3.13 (s, 6H), 3.03 (d, 1H, J = 14.4 Hz), 2.76 (s, 6H), 2.76-2.70 (m, 1H), 2.62-2.58 (m, 1H), 2.25 (dd, 1H, J = 14.8, 2.8 Hz), 1.98 (dd, 1H, J = 14.2, 13.8 Hz), 0.89-0.82 (m, 1H), 0.77-0.69 (m, 3H); HRMS–ESI (m/z): [M+H]+ calcd for C27H35N4O7, 527.2500; found, 527.2502.
4.2.24. C5a-Methoxymethylminocycline (28)
Anhydrous magnesium bromide (9.1 mg, 0.049 mmol, 2.5 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (15.0 mg, 0.020 mmol, 1 equiv) in methanol (1.5 mL) at 23 °C. The reaction flask was sealed and the reaction mixture was heated to 65 °C. After stirring at 65 °C for 24 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 10 mL) and dichloromethane (10 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 15 h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate trihydrate (10.0 g). The resulting mixture was extracted with ethyl acetate (3 × 30 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (6.0 mg, 0.057 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 2 ½ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 7.0 mL (6.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 29–33 min were collected and concentrated, affording C5a-methoxymethylminocycline trifluoroacetate 28 as a yellow solid (7.1 mg, 58%, three steps). 1H NMR (600 MHz, CD3OD, hydrochloride) δ 7.82 (d, 1H, J = 9.4 Hz), 7.05 (d, 1H, J = 9.2 Hz), 4.15 (s, 1H), 3.49 (d, 1H, J = 10.3 Hz), 3.36 (d, 1H, J = 15.6 Hz), 3.25 (s, 3H), 3.14 (s, 6H), 3.12 (d, 1H, J = 10.3 Hz), 3.08-3.04 (m, 1H), 2.99 (s, 6H), 2.56 (d, 1H, J = 15.6 Hz), 2.41 (dd, 1H, J = 14.1, 2.9 Hz), 1.69 (dd, 1H, J = 14.1, 13.9 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C25H32N3O8, 502.2184; found, 502.2186.
4.2.25. C5a-n-Butoxymethylminocycline (29)
Anhydrous magnesium bromide (7.2 mg, 0.039 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (15.0 mg, 0.020 mmol, 1 equiv) in n-butanol (1.0 mL) at 23 °C. The resulting mixture was heated to 75 °C. After stirring at 75 °C for 14 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 10 mL) and dichloromethane (10 mL). The phases were separated and the aqueous phase was extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 13 ½ h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.4 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 3 ¾ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 41–44 min were collected and concentrated, affording C5a-n-butoxymethylminocycline trifluoroacetate 29 as a yellow solid (7.3 mg, 56%, three steps). 1H NMR (600 MHz, CD3OD, trifluoroacetate) δ 7.81 (d, 1H, J = 9.0 Hz), 7.04 (d, 1H, J = 9.6 Hz), 4.16 (s, 1H), 3.50 (d, 1H, J = 10.2 Hz), 3.38-3.30 (m, 3H), 3.21 (d, 1H, J = 9.6 Hz), 3.13 (s, 6H), 3.11-3.06 (m, 1H), 2.98 (s, 6H), 2.58 (d, 1H, J = 15.6 Hz), 2.42 (dd, 1H, J = 13.8, 3.0 Hz), 1.71 (dd, 1H, J = 13.9, 13.8 Hz), 1.50-1.39 (m, 2H), 1.34-1.24 (m, 2H), 0.87 (t, 3H, J = 7.2 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C28H38N3O8, 544.2653; found, 544.2655.
4.2.26. C5a-Methoxyethoxymethylminocycline (30)
Anhydrous magnesium bromide (6.2 mg, 0.034 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (13.0 mg, 0.017 mmol, 1 equiv) in 2-methoxyethanol (0.5 mL) at 23 °C. The resulting mixture was heated to 60 °C. After stirring at 60 °C for 26 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture was partitioned between aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 10 mL) and dichloromethane (10 mL). The phases were separated and the aqueous phase was further extracted with dichloromethane (10 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The crude ring-opened product was dissolved in acetonitrile (1.2 mL). The resulting solution was transferred to a polypropylene reaction vessel and concentrated aqueous hydrofluoric acid solution (48 wt%, 0.8 mL) was added. The reaction mixture was stirred vigorously at 23 °C for 10 ½ h, then was poured into water (30 mL) containing dipotassium hydrogenphosphate (8.0 g). The resulting mixture was extracted with ethyl acetate (3 × 40 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. Palladium black (5.0 mg, 0.047 mmol, 2.8 equiv) was added in one portion to a solution of the crude product in methanol (1.0 mL) and dioxane (1.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 ¾ h, then was filtered through a plug of Celite. The filtrate was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 32–34 min were collected and concentrated, affording C5a-methoxyethoxymethylminocycline trifluoroacetate 30 as a yellow solid (5.8 mg, 52%, three steps). 1H NMR (600 MHz, CD3OD, trifluoroacetate) δ 7.83 (d, 1h, J = 9.0 Hz), 7.05 (d, 1H, J = 9.0 Hz), 4.13 (s, 1H), 3.54-3.45 (m, 4H), 3.44-3.40 (m, 1H), 3.34 (d, 1H, J = 15.6 Hz), 3.27-3.25 (m, 1H), 3.26 (s, 3H), 3.18 (s, 6H), 3.11 (brd, 1H, J = 14.4. Hz), 2.99 (s, 6H), 2.61 (d, 1H, J = 16.2 Hz), 2.45 (dd, 1H, J = 14.4, 3.0 Hz), 1.71 (t, 1H, J = 14.4 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C27H36N3O9, 546.2446; found, 546.2459.
4.2.27. Azido-substituted ring-opened product (31)
Sodium azide (50.0 mg, 0.763 mmol, 3.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (194 mg, 0.255 mmol, 1 equiv) in dimethylformamide (7.5 mL) at 23 °C. The resulting solution was stirred at this temperature for 14 h, then was partitioned between saturated aqueous sodium chloride solution and diethyl ether (60 mL each). The phases were separated and the aqueous phase was further extracted with diethyl ether (60 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (12% ethyl acetate-hexanes), providing the azido-substituted ring-opened product 31 as a yellow solid (160 mg, 78%). Rf = 0.41 (30% ethyl acetate-hexanes); 1H NMR (500 MHz, CDCl3) δ 16.72 (s, 1H), 7.51 (brd, 4H, J = 8.0 Hz), 7.41-7.26 (m, 7H), 6.95 (d, 1H, 8.5 Hz), 5.38 (s, 2H), 5.18 (AB quartet, 2H), 4.11 (d, 1H, J = 10.5 Hz), 3.72 (d, 1H, J = 16.5 Hz), 3.30 (d, 1H, J = 11.5 Hz), 3.13 (d, 1H, J = 11.5 Hz), 2.65 (s, 6H), 2.65-2.45 (m, 2H), 2.51 (s, 6H), 2.35-2.25 (m, 2H), 0.92 (s, 9H), 0.27 (s, 3H), 0.19 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 186.5, 184.0, 183.6, 181.5, 167.7, 154.9, 145.9, 136.7, 135.3, 135.0, 128.5, 128.5, 128.5, 128.4, 127.9, 127.0, 125.8, 120.2, 114.0, 108.3, 107.3, 82.3, 72.5, 71.5, 61.1, 59.1, 46.6, 44.6, 41.9, 36.4, 35.2, 28.3, 26.5, 19.3, −1.9, −2.1; FTIR (neat film), 2099 (s), 1721 (s), 1609 (m), 1510 (s), 1258 (m), 829 (s) cm−1; HRMS–ESI (m/z): [M+H]+ calcd for C44H53N6O7Si, 805.3740; found, 805.3722.
4.2.28. C5a-Aminomethylminocycline (32).26,27
A solution of trimethylphospine in tetrahydrofuran (1.0 M, 398 μL, 0.398 mmol, 2.0 equiv) was added dropwise via syringe to a solution of the azido-substituted ring-opened product 31 (160 mg, 0.199 mmol, 1 equiv) and 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile (98.0 mg, 0.398 mmol, 2.0 equiv) in tetrahydrofuran at −10 °C. The reaction mixture was allowed to warm to 23 °C over 15 min. After stirring at this temperature for 15 h, the product solution was partitioned between dichloromethane and water (60 mL each). The phases were separated and the organic phase was washed sequentially with water (60 mL) and saturated aqueous sodium chloride solution (2 × 60 mL). The organic solution was then dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product was purified by flash-column chromatography (25% ethyl acetate-hexanes), providing the desired tert-butyl carbamate as a yellow solid (90 mg, 51%). Methanol (2.5 mL) and dioxane (2.5 mL) were added to this product, forming a yellow solution. Palladium black (25 mg, 0.235 mmol, 2.3 equiv) was added in one portion at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 1 h, whereupon more palladium black (25 mg) was added. The resulting mixture was stirred at 23 °C for a further 2 h, then was filtered through a plug of Celite. The filtrate was concentrated, providing an orange solid. Concentrated aqueous hydrofluoric acid (48 wt%, 1.4 mL) was added to a solution of the crude product in acetonitrile (2.0 mL) in a polypropylene reaction vessel at 23 °C. The reaction mixture was stirred vigorously at 23 °C for 15 h. Excess hydrofluoric acid was quenched by the careful addition of methoxytrimethylsilane (9.0 mL). The resulting mixture was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 22–27 min were collected and concentrated, affording C5a-aminomethylminocycline bistrifluoroacetate 32 as a yellow solid (50 mg, 69%, two steps). 1H NMR (500 MHz, CD3OD, bistrifluoroacetate) δ 7.88 (d, 1H, J = 9.0 Hz), 7.10 (d, 1H, J = 9.0 Hz), 4.09 (d, 1H, J = 2.5 Hz), 3.60 (d, 1H, J = 17.0 Hz), 3.34 (d, 1H, J = 14.5 Hz), 3.18 (s, 6H), 3.16 (s, 6H), 3.20-3.14 (m, 1H), 3.02 (d, 1H, J = 14.5 Hz), 2.95 (d, 1H, J = 17.0 Hz), 2.33 (dd, 1H, J = 15.0, 3.0 Hz), 1.96 (dd, 1H, J = 14.6, 13.7 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C24H31N4O7, 487.2187; found 487.2181.
4.2.29. C5a-Piperazinylmethylminocycline (34).27
Anhydrous magnesium bromide (51.0 mg, 0.276 mmol, 2.0 equiv) was added to a solution of the C5a-C11a-bridged cyclopropane 20 (105 mg, 0.138 mmol, 1 equiv) and tert-butyl 1-piperazine carboxylate (186 mg, 1.00 mmol, 7.2 equiv) in tetrahydrofuran (2.0 mL) at 23 °C. The reaction flask was sealed and the reaction mixture was heated to 45°C. After stirring at 45 °C for 36 h, the reaction mixture was allowed to cool to 23 °C. The cooled mixture waspartitioned between dichloromethane and saturated aqueous sodium bicarbonate solution (25 mL each). The phases were separated and the aqueous phase was extracted with dichloromethane (25 mL). The organic extracts were combined and the combined solution was dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated. The product mixture was filtered through a short pad of silica gel (eluting with 40% ethyl acetate-hexanes) and the filtrate was concentrated, affording an orange-yellow oil. Methanol (2.5 mL) and dioxane (2.5 mL) were added to the crude ring-opened product (33), forming an orange-yellow solution. Palladium black (25 mg, 0.235 mmol, 1.7 equiv) was added in one portion at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The reaction mixture was stirred at 23 °C for 2 h, then was filtered through a plug of Celite. The filtrate was concentrated, providing an orange solid. Concentrated aqueous hydrofluoric acid (48 wt%, 1.5 mL) was added to a solution of the crude product in acetonitrile (2.0 mL) in a polypropylene reaction vessel at 23 °C. The reaction mixture was stirred vigorously at 23 °C for 14 h. Excess hydrofluoric acid was quenched by the careful addition of methoxytrimethylsilane (10.0 mL). The resulting mixture was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, 2 batches, injection volume (for each batch): 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→35% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 22–29 min were collected and concentrated, affording C5a-piperazinylmethylminocycline bistrifluoroacetate 34 as a yellow solid (63 mg, 58%, three steps). 1H NMR (600 MHz, CD3OD, bistrifluoroacetate) δ 7.58 (d, 1H, J = 9.6 Hz), 6.93 (d, 1H, J = 9.0 Hz), 4.06 (s, 1H), 3.44 (d, 1H, J = 16.2 Hz), 3.11 (brd, 1H, J = 12.6 Hz), 3.08-3.02 (m, 2H), 3.05 (s, 6H), 3.01-2.95 (m, 2H), 2.82 (s, 6H), 2.61-2.56 (m, 3H), 2.50 (d, 1H, J = 16.2 Hz), 2.48-2.42 (m, 3H), 2.20 (dd, 1H, J = 13.8, 3.0 Hz), 1.74 (dd, 1H, J = 14.1, 13.9 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C28H38N5O7, 556.2766; found, 556.2771.
4.2.30. General procedure for final-step diversification of C5a-aminomethylminocycline (32) and C5a-piperazinylmethylminocycline (34)
C5a-N-acetylaminomethylminocycline (35)
Acetyl chloride (0.9 μL, 0.013 mmol, 2.3 equiv) was added to a solution of C5a-aminomethylminocycline bistrifluoroacetate 32 (4.0 mg, 0.0056 mmol, 1 equiv) and N,N-diisopropylethylamine (4.6 μL, 0.027 mmol, 4.8 equiv) in methanol (200 μL) at 0 °C. The resulting solution was allowed to warm to 23 °C over 5 min. The reaction mixture was stirred at this temperature for 1 h, then was concentrated. The product was purified by preparative HPLC on an Agilent Prep C18 column [10 μm, 250 × 21.2 mm, UV detection at 350 nm, Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: acetonitrile, injection volume: 5.0 mL (4.0 mL 0.1% trifluoroacetic acid in water, 1.0 mL acetonitrile), gradient elution with 5→40% B over 50 min, flow rate: 7.5 mL/min]. Fractions eluting at 25–28 min were collected and concentrated, affording C5a-N-acetylaminomethylminocycline trifluoroacetate 35 as a yellow solid (3.2 mg, 89%). 1H NMR (600 MHz, CD3OD, trifluoroacetate) δ 7.80 (d, 1H, J = 9.0 Hz), 7.03 (d, 1H, J = 9.0 Hz), 3.89 (s, 1H), 3.46 (d, 1H, J = 14.4 Hz), 3.27 (d, 1H, J = 13.8 Hz), 3.23 (d, 1H, J = 16.8 Hz), 3.15-3.08 (m, 13H), 2.63 (d, 1H, J = 16.2 Hz), 2.11 (dd, 1H, J = 14.4, 2.4 Hz), 1.91 (s, 3H), 1.69 (dd, 1H, J = 13.9, 13.8 Hz); HRMS–ESI (m/z): [M+H]+ calcd for C26H33N4O8, 529.2293; found 529.2299.
Supplementary Material
Acknowledgments
This work was generously supported by the National Institutes of Health (AI048825). We thank AstraZeneca for a Graduate Student Fellowship (P.M.W.).
Footnotes
1H and 13C NMR spectroscopic data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Charest MG, Lerner CD, Brubaker JD, Siegel DR, Myers AG. Science. 2005;308:395–398. doi: 10.1126/science.1109755. [DOI] [PubMed] [Google Scholar]; (b) Sun C, Wang Q, Brubaker JD, Wright PM, Lerner CD, Noson K, Charest MG, Siegel DR, Wang YM, Myers AG. J Am Chem Soc. 2008;130:17913–17927. doi: 10.1021/ja806629e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Clark RB, He M, Fyfe C, Lofland D, O’Brien WJ, Plamondon L, Sutcliffe JA, Xiao XY. J Med Chem. 2011;54:1511–1528. doi: 10.1021/jm1015389. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun C, Hunt DK, Clark RB, Lofland D, O’Brien WJ, Plamondon L, Xiao XY. J Med Chem. 2011;54:3704–3731. doi: 10.1021/jm1015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stork G, Hagedorn AA., III J Am Chem Soc. 1978;100:3609–3611. [Google Scholar]
- 4.(a) Brodersen DE, Clemons WM, Jr, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]; (b) Pioletti M, Schlünzen F, Harms J, Zarivach R, Glühmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F. EMBO J. 2001;20:1829–1839. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.While the introduction of new substituents at C5a would not be expected to significantly affect affinity for the bacterial ribosome (based on analysis of crystal structures), such modifications could certainly influence processes such as cell penetration and susceptibility to resistance mechanisms: O’Shea R, Moser HE. J Med Chem. 2008;51:2871–2878. doi: 10.1021/jm700967e.
- 6.Corey EJ, Boaz NW. Tetrahedron Lett. 1985;26:6019–6022. [Google Scholar]
- 7.Ito Y, Hirao T, Saegusa T. J Org Chem. 1978;43:1011–1013. [Google Scholar]
- 8.The highest yield of the β-methyl-substituted enone 3 was obtained when the oxidation was performed on a small scale (160 mg, 1.15 equiv Pd(OAc)2, DMSO, 80 °C, 16 h) and the work-up was carried out before the starting material had been completely consumed. Mass recovery from this reaction was consistently poor.
- 9.Nicolaou KC, Gray DLF, Montagnon T, Harrison ST. Angew Chem Int Ed. 2002;41:996–1000. doi: 10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Enones with ester, aldehyde and alkoxymethyl substituents at the β-position can be prepared indirectly from unsubstituted enone starting materials via β-cyano-substituted enones: Fukuta Y, Mita T, Fukuda N, Kanai M, Shibasaki M. J Am Chem Soc. 2006;128:6312–6313. doi: 10.1021/ja061696k.Nicolaou KC, Li A. Angew Chem Int Ed. 2008;47:6579–6582. doi: 10.1002/anie.200802632.Isobe M, Nishikawa T, Pikul S, Goto T. Tetrahedron Lett. 1987;28:6485–6488.
- 11.Though not successful in the current application (using the AB enone 1 as substrate), phosphoniosilylation methodology has been used to introduce β-alkoxycarbonyl substituents in enone substrates: Kim S, Lee PH, Kim SS. Bull Korean Chem Soc. 1989;10:218–219.Kim JH, Jung HK. Bull Korean Chem Soc. 2004;25:1729–1732.
- 12.Alternative synthetic sequences for the preparation of enones with β-alkoxymethyl and β-aldehyde substituents from unsubstituted enone starting materials: Kienzle F, Minder RE. Helv Chim Acta. 1976;59:439–452. doi: 10.1002/hlca.19760590211.Heguaburu V, Schapiro V, Pandolfi E. Tetrahedron Lett. 2010;51:6921–6923.
- 13.(a) Seebach D. Angew Chem Int Ed. 1967;6:442–443. [Google Scholar]; (b) Bürstinghaus R, Seebach D. Chem Ber. 1977;110:841–851. [Google Scholar]; (c) Gröbel BT, Seebach D. Synthesis. 1977:357–402. [Google Scholar]; (d) “Tris(methylthio)methane” Electronic Encyclopedia of Reagents for Organic Synthesis. 2007 [Google Scholar]
- 14.Other examples of conjugate addition–enolate trapping reactions of tris(methylthio)methyllithium with a,β-unsaturated carbonyl compounds: Damon RE, Schlessinger RH. Tetrahedron Lett. 1976;19:1561–1564.Mikolajczyk M, Kielbasiński P, Wieczorek MW, Blaszczyk J, Kolbe A. J Org Chem. 1990;55:1198–1203.
- 15.Degani I, Dughera S, Fochi R, Gatti A. Synthesis. 1996;4:467–469. [Google Scholar]
- 16.Corey EJ, Erickson BW. J Org Chem. 1971;36:3553–3560. [Google Scholar]
- 17.(a) Corey EJ, Seebach D. Angew Chem Int Ed. 1965;4:1075–1077. [Google Scholar]; (b) Seebach D, Corey EJ. J Org Chem. 1975;40:231–237. [Google Scholar]; (c) Page PCB, van Niel MB, Prodger JC. Tetrahedron. 1989;45:7643–7677. [Google Scholar]
- 18.(a) Brown CA, Yamaichi A. J Chem Soc, Chem Commun. 1979:100–101. [Google Scholar]; (b) Lucchetti J, Dumont W, Krief A. Tetrahedron Lett. 1979;20:2695–2696. [Google Scholar]; (c) Mukhopadhyay T, Seebach D. Helv Chim Acta. 1982;65:385–391. [Google Scholar]
- 19.For preparation of tri-n-butyl[(methoxymethoxy)methyl]stannane, see: Danheiser RL, Romines KR, Koyama H, Gee SK, Johnson CR, Medich JR. Org Synth. 1993;71:133.Org Synth Coll. 1998;9:704.
- 20.Hill B, Rodrigo R. Org Lett. 2005;7:5223–5225. doi: 10.1021/ol052058u. [DOI] [PubMed] [Google Scholar]
- 21.Perchloric acid is a strong oxidizing agent and can form explosive mixtures. For safety information, see: Wolsey WC. J Chem Educ. 1973;50:A335–A337.Muse LA. J Chem Educ. 1972;49:A463–A466.
- 22.Barton DHR, Ley SV, Meguro K, Williams DJ. J Chem Soc, Chem Commun. 1977:790–791. [Google Scholar]
- 23.For an overview, see: Danishefsky S. Electrophilic Cyclopropanes in Organic Synthesis. Acc Chem Res. 1979;12:66–72.
- 24.Examples of Lewis acid-promoted ring-opening of activated cyclopropanes: Swain NA, Brown RCD, Bruton G. J Org Chem. 2004;69:122–129. doi: 10.1021/jo035365r.Tanimori S, He M, Nakayama M. Synth Commun. 1993;23:2861–2868.Lifchits O, Charette AB. Org Lett. 2008;10:2809–2812. doi: 10.1021/ol8009286.
- 25.Examples of ring-opening of activated cyclopropanes by azide: Ok T, Jeon A, Lee J, Lim JH, Hong CS, Lee HS. J Org Chem. 2007;72:7390–7393. doi: 10.1021/jo0709605.Izquierdo ML, Arenal I, Bernabé M, Fernández Alvarez E. Tetrahedron. 1985;41:215–220.
- 26.Ariza X, Urpí F, Viladomat C, Vilarrasa J. Tetrahedron Lett. 1998;39:9101–9102. [Google Scholar]
- 27.The hydrogenolysis deprotection step (typically the final step) was slow and low-yielding in the presence of primary and secondary amines. To synthesize the amines 32 and 34 most efficiently the usual order of deprotection steps was reversed and the hydrogenolysis reaction was performed on substrates in which primary or secondary amines were protected as tert-butyl carbamates. For discussion of the inhibitory effects of amines on Pd-catalyzed hydrogenolysis, see: Sajiki H, Hirota K. Tetrahedron. 1998;54:13981–13996.
- 28.Kummer DA, Li D, Dion A, Myers AG. Chem Sci. 2011;2:1710–1718. doi: 10.1039/C1SC00303H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Still WC, Khan M, Mitra A. J Org Chem. 1978;43:2923–2925. [Google Scholar]
- 30.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518–1520. [Google Scholar]
- 31.Kofron WG, Baclawski LM. J Org Chem. 1976;41:1879–1880. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.