Abstract
The ability of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a water-soluble vitamin E analogue, to prevent oxidative damages is well characterized, but the mechanisms underlying it remain unclear. The protective effect of Trolox pre-treatment on H2O2-induced toxicity might be attributed to the decreased cellular permeability to H2O2 or in vitro scavenging activity of Trolox, induction of antioxidant enzymes or the direct scavenging activity of Trolox. The results obtained rule out the first and second possibilities and intracellular scavenging activity was found to be the mechanism whereby Trolox confers protection. This was confirmed by measuring protein oxidation (levels), and the observed decrease in proteasomal activity indicated that the decrease in protein carbonyls was due to Trolox scavenging activity rather than proteasome activation. In conclusion, the intracellular scavenging activity of Trolox is a key protective mechanism against H2O2. These findings obtained in Schizosaccharomyces pombe, a good model organism for eukaryotic cells, can be used as standard protocols for investigating the antioxidant activity of pure or complex potential antioxidants.
Keywords: Fission yeast, hydrogen peroxide, oxidative stress, protein oxidation, Schizosaccharomyces pombe, Trolox
INTRODUCTION
Reactive oxygen species (ROS) are produced as normal by-products of cellular metabolism. These species are also derived from external environmental factors such as redox active drugs, radiation and heavy metals. The major source of ROS is the mitochondrial respiratory chain, which accounts for 85–90% of the oxygen consumed by the cells.[1] ROS can cause damage to proteins, lipids and nucleic acids and thereby compromise cell viability. Under normal physiological conditions, cellular damages are prevented by antioxidant defenses that neutralize the ROS.[2] These include ROS-scavenging molecules (e.g., superoxide dismutase, catalase), oxidative damage-repair enzymes (e.g., methionine sulfoxide reductase) and mechanisms such as the S-thiolation of oxidation-susceptible proteins, which prevents oxidation by forming reversible mixed-disulfide bonds with glutathione/thiol.[3] However, under specific stress conditions, the levels of ROS exceed the antioxidant capacity of the cells and the cells face an oxidative stress. This unbalanced situation can result from: (i) a decrease in antioxidants, due to depletion of such defenses (e.g., by xenobiotics that are metabolized by conjugation to glutathione or due to mutations that affect antioxidant defenses), (ii) an increased production of ROS (e.g., by exposure to hyperoxia, compounds that generate ROS or due to excessive activation of systems that produce ROS) or (iii) both.[4]
Such “oxidative stress” is associated with several human pathologies, including cancer, cardiovascular diseases, Down's syndrome, Friedreich's ataxia, rheumatoid arthritis, autoimmune diseases and acquired immunodeficiency syndrome. Oxidative damage is also emerging as an important factor in mutagenesis, tumorigenesis, ageing and age-related disorders such as Parkinson's and Alzheimer's diseases.[5]
Protein oxidation is defined as the covalent modification of protein induction, either directly by ROS or indirectly by a reaction with secondary by-products of oxidative stress. Oxidative damages to proteins can lead to diverse functional consequences, such as inhibition of enzymatic and binding activities, protein aggregation and enhanced susceptibility to proteolysis.[6] Protein oxidation serves as a useful marker for assessing oxidative stress. The most commonly used marker of protein oxidation is the protein carbonyls. The use of protein carbonyl groups as biomarkers of oxidative stress has some advantages in comparison with the measurement of other oxidation products because of the relative early formation and the relative stability of carbonylated proteins. Most of the assays for detection of protein carbonyl groups involve derivatization of the carbonyl group with 2,4-dinitrophenylhydrazine (DNPH), which leads to the formation of a stable dinitrophenyl (DNP) hydrazone product. This then can be detected by various means, such as spectrophotometric assay, enzyme-linked immunosorbent assay (ELISA) and one- or two-dimensional electrophoresis followed by Western blot immunoassay.[7]
Proteasomes are large protein complexes inside all eukaryotes and archaea as well as some bacteria. In eukaryotes, they are located in the nucleus and in the cytoplasm. The proteasome is present in two major forms, the 20S and the 26S proteasomes. The former is a multimeric proteolytic enzyme in a cylinder-like shape, whereas the latter is a complex consisting of two 19S regulatory complexes and one 20S proteasome unit. The 26S proteasome degrades various kinds of excess proteins that have been ubiquitinated with the expense of ATP. This proteasome plays essential roles in the regulation of the cell cycle by specific ubiquitin-mediated proteolysis.[8]
Besides targeted degradation of regulatory proteins, an important function of the proteasome is the degradation of oxidized and aberrant proteins.[9] Increased accumulation of highly oxidized and cross-linked protein aggregates within the cell observed during aging has been attributed to decreased proteasome function.[10]
Natural antioxidants like vitamin C and E, carotenoids and polyphenols are claimed to protect against cancer and cardiovascular diseases, and there is an increasing interest in the use of natural and synthetic antioxidants as functional food ingredients or as food supplements. However, on the other hand, at high doses, toxic pro-oxidant action may become important.[11]
Vitamin E is the name for a group of biologically active substances including tocopherols and tocotrienols.[1] α-tocopherol shows the highest biological activity and is the most common form found in the human body. Tocopherols inhibit lipid peroxidation by scavenging lipid peroxyl radicals much faster than these radicals can react with adjacent fatty acid side chains or membrane proteins. In addition, both tocopherols and tocotrienols quench and react with singlet oxygen and slowly react with superoxide anions.[12]
Trolox (6-hydroxy- 2, 5, 7, 8-tetramethylchromane-2-carboxylic acid) is a water-soluble analogue of the free radical scavenger α-tocopherol. Trolox has advantages over α-tocopherol, which is lipid soluble because it can be incorporated in both the water and the lipid compartments of cells. Satoh et al. have claimed that the antioxidant property of Trolox surpasses that of α-tocopherol.[13] Many studies investigated the protective effect of Trolox,[14–16] but the mechanisms underlying these effects remain unclear.
The fission yeast, Schizosaccharomyces pombe, is well known for its contribution to the understanding of molecular mechanisms of cell cycle in eukaryotes. It is probably closer to higher eukaryotes than Saccharomyces cerevisiae, and represents a good model organism for mammalians.[17] Here, it was used as a model system to evaluate the mechanism of Trolox-mediated protective effects against ROS.
We showed that Trolox was able to prevent or reduce some of the H2O2-induced toxic effects in S. pombe. In conclusion, the intracellular scavenging activity of Trolox was found to be the key protective mechanism against H2O2.
These findings obtained in S. pombe can be used as standard protocols for investigating the antioxidant activity of pure or complex potential antioxidants.
MATERIALS AND METHODS
Organisms and growth conditions
The strain used in this work was wild type S. pombe (972h-). Yeast cells were grown in YE medium (0.5% yeast extract, 3% glucose) at 30°C on a rotary shaker at 180 rpm under aerobic conditions. Yeast cells were pre-treated with 1 mM Trolox for 2 (short-term) and 14 h (long-term) in YE medium. Later, they were collected by centrifugation at 1,200 g, washed with sterile distilled water and re-suspended in YE medium. Exponentially growing cells at 1 × 107cells/ml were treated with 1 mM hydrogen peroxide for 30 min.
Viability determinations
To calculate cell viability, appropriate dilutions of the cultures were spread on plates with solid YEA medium (0.5% yeast extract, 3% glucose, 2% agar). These plates were incubated at 30°C and the colony forming units were counted at the end of the third day.
Estimation of hydrogen peroxide in culture media
The H202 assay was adapted from the methods of Pick and Keisari.[18] Immediately prior to the assay, phenol red and horseradish peroxidase were added to 1 ml of assay buffer (10 mM potassium phosphate, pH 7 and 40 mM NaCl) at a final concentration of 0.1 mg/ml and 8.5 U/ml, respectively. A 500 μl aliquot of the culture media was then added and the solution was mixed and incubated at 25°C for 5 min. After the reaction was stopped by adding 10 μl of 1 N NaOH, optical density was measured at 610 nm using a Biotek μQuant Microplate Spectrophotometer (Winooski, VT, USA). H202 concentrations were calculated from a standard curve evaluated for each assay from dilutions of 30% H202.
Measurement of ROS generation
Intracellular oxidation level was measured as described previously.[19] After pre-incubation of the yeast cells (107 cells/ml) in YE medium with 40 μM DCFH-DA at 30°C for 60 min, the cells were treated with 1 mM H2O2 for 30 min and then washed and re-suspended in 100 μl phosphate-buffered saline (PBS). Fluorescent intensity of the cell suspension was measured using a Bio-Tek FL800 fluorescence microplate reader (Winooski, VT, USA). with excitation at 480 nm and emission at 530 nm. The relative fluorescent intensity was expressed as arbitrary units/107 cells.
Preparation of crude cell-free extract
Cells were collected by centrifugation and re-suspended in 200 μl lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1 mM PMSF, 1 mM DTT). Cells were disrupted by vortexing with acid-washed glass beads (diameter 425–600 μm) for 10 min at 60 s intervals, interspersed with periods of cooling in an ice bath. Cellular debris was removed by centrifugation at 15,000 g at 4°C for 20 min.[20] The supernatant was collected and the protein concentration was determined according to Waterborg (2002) using bovine serum albumin as a standard.[21]
Catalase assay
Catalase activity was determined spectrophotometrically by monitoring the disappearance of H2O2 at 240 nm as described previously.[22] The mixture containing 680 μl of 50 mM potassium phosphate buffer (pH 7.2) and 480 μl of 40 mM H2O2 was incubated at 30°C for 2.5 min. Enzymatic reaction was initiated by adding 40 μl of the cell extract. The decrease in absorbance at 240 nm was monitored. Catalase activity was expressed as ∆A240/min/mg protein.
Immuno-detection of protein carbonyls
As a result of protein oxidation, carbonyl groups are introduced into protein side chains by a site-specific mechanism. We used an OxyBlot kit (Millipore, Billerica, MA, USA) to immunodetect these carbonyl groups in oxidatively modified proteins as described by the manufacturer. Briefly, DNPH derivatization was carried out for 15 min on 15 μg of protein. Five micrograms of protein was separated on 10% SDS polyacrylamide gels, transferred to nitrocellulose membrane and stained by Red Ponceau to check for equal transfer. The membrane was probed with first antibody, specific to the DNP moiety of the proteins. The next step was incubation with horseradish peroxidase–antibody conjugate directed against the primary antibody. Immunoblots were visualized using the ECL-Plus Western Blotting Detection system supplied by (GE healthcare, Piscataway, NJ, USA), with exposure times between 30 s and 2 min. A second gel containing duplicate samples was run and stained with the silver staining technique.[23]
Measurement of 20S proteasome activity
Quantitative in vitro analysis of 20S proteasome activity was performed by measuring the hydrolysis of the fluorogenic peptidyl substrate Suc-Leu-Leu-Val-Tyr-AMC.[24] Cells were disrupted by vortexing with acid-washed glass beads into buffer containing 0.25 M sucrose, 25 mM HEPES, pH 7.8, 10 mM MgCl2, 1 mM EDTA and 1 mM DTT. Lysates were centrifuged at 14,000 g for 30 min at 4°C. Cell lysates were diluted with proteolysis buffer (50 mM Tris, pH 7.8, 5 mM magnesium acetate, 20 mM KCl, 0.5 mM DTT) to a protein concentration of 50 μg/ml. The peptidase activity was measured by the addition of 90 μl of proteolysis buffer and 10 μl of Suc-Leu-Leu-Val-Tyr-AMC (4 mM stock solution in DMSO) to 100 μl of the diluted cell lysate. The mixture was incubated at 37°C for 1 h. The reaction was stopped by addition of an equal volume of ice-cold ethanol and 1.6 ml of 0.125 M sodium borate (pH 9.0). The fluorescence determination was performed at 380 nm excitation and 440 nm emission using free AMC as a standard. Results were presented as nM AMC/mg protein/min.
Statistical analysis
All data were represented as mean ± SD. The statistical significance of the difference between the control and the treated sample was assessed by one-way ANOVA and Dunnett's multiple comparison test. Results were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
The objective of the current study was to evaluate the mechanism of Trolox-mediated protective effect against H2O2-induced oxidative damages in the fission yeast S. pombe.
Determination of sub-lethal hydrogen peroxide and non-toxic trolox concentration
To assess the consequences of H2O2-induced oxidative stress and the protective effect of Trolox in S. pombe, we first determined the sub-lethal concentration of H2O2 and non-toxic dose of Trolox. Cells were treated with H2O2 in the concentration range of 0.2–5 mM. The sub-lethal H2O2 concentration was found to be 1 mM, which resulted in 29% increased cell mortality following 30 min of incubation. To establish the maximum non-toxic concentration of Trolox, cells were treated with 0.005–1 mM Trolox. 1 mM Trolox was used throughout the experiments as it increased cell survival significantly (data not shown).
The impact of 1 mM H2O2 and 1 mM Trolox on cell growth in the mid-exponential growth phase was additionally tested. As indicated in Table 1, pre-treatment of S. pombe cells with 1 mM Trolox for 14 h followed by exposure to 1 mM H2O2 for 30 min showed the strongest protective effect compared with cells treated with H2O2 alone.
Table 1.
Effects of Trolox pre-treatment on the viability, H2O2 uptake, generation of ROS, catalase activity and proteasome activity in the fission yeast, S. pombe
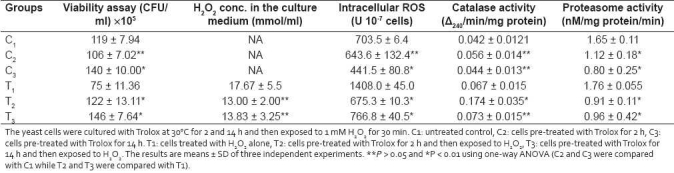
Our results with Trolox are in agreement with those obtained by Raspor et al.,[15] demonstrating that Trolox treatment increased cell viability, decreased intracellular ROS formation and suppressed DNA oxidation in Saccharomyces cerevisiae. Additionally, it has been reported that vitamin E in the concentration range of 50 μM to 100 mM did not have any genotoxic effect on the yeast cells.[25]
Once the non-genotoxic potential of Trolox was confirmed, its protective effect on H2O2-induced toxicity was further investigated.
Effect of trolox on H2O2 uptake, ROS generation and catalase activity
The protective effect of Trolox pre-treatment on H2O2-induced toxicity might be attributed to (i) change in cellular uptake of H2O2 or in vitro scavenging activity, (ii) induction of antioxidant enzymes (glutathione peroxidase, catalase) or (iii) direct scavenging activity of Trolox. For these reasons, extracellular H2O2 concentration, intracellular oxidation level and catalase activity were determined.
H2O2 level in the medium revealed that protection against H2O2 could not be attributed to reduced H2O2 uptake and/or to the in vitro scavenging activity of Trolox, because no significant difference between the H2O2 concentrations of Trolox pre-treated and of non-treated cultures were observed [Table 1].
We analyzed whether the Trolox-protective effect was due to scavenging of intracellular ROS in S. pombe using a fluorescent dye, DCFH-DA [Table 1]. Trolox pre-treatment suppressed ROS generation and a significant decrease in peroxide production was detected when the cells were treated with Trolox for 14 h. These results were consistent with the findings of Peus et al, on human keratinocytes, in which Trolox was found to decrease the intracellular H2O2 generation in a dose-dependent manner.[26]
A significant increase in catalase activity was detected in cells pre-treated with Trolox for 2 h but not in the cells pre-treated for 14 h [Table 1]. Susa et al, also found that a 20 h pre-treatment with 0.5 mM vitamin E did not affect the activities of antioxidant enzymes, including superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione level in rat hepatocytes.[27] According to Raspor et al, after prolonged exposure, such enzymatic activities tend to go to normal level.[15]
It was concluded that the low level of H2O2 is due to Trolox-mediated increased intracellular scavenging ability rather than alteration in H2O2 transport, in vitro scavenging activity or induction of antioxidant defense system.
Effect of trolox on protein oxidative modification and proteasome activity
If Trolox decreases the level of intracellular ROS, it would be expected to decrease damage to biomolecules. We used protein carbonyls as a marker for protein oxidation. Protein carbonyls are generated by a variety of mechanisms[7] and are sensitive indices of oxidative injury. The results obtained from protein carbonyl assays [Figure 1] indicated that pre-treatment of the S. pombe cells with Trolox for 2 and 14 h decreased the oxidative damage to proteins induced by 1 mM H2O2 for 30 min.
Figure 1.
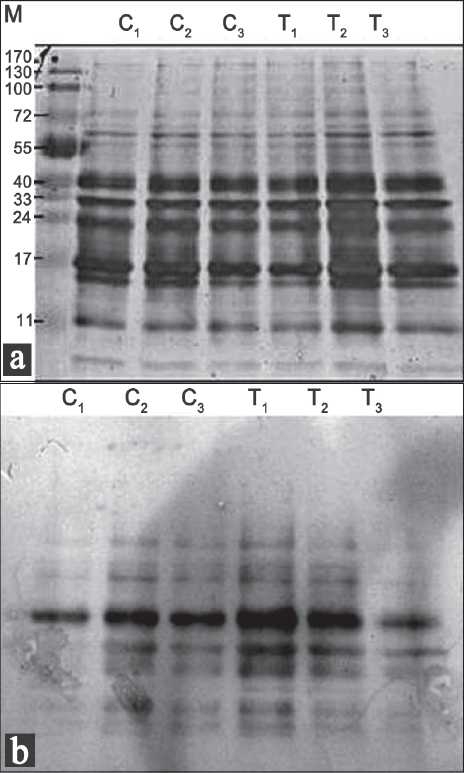
Pat tern of oxidatively damaged proteins of Schizosaccharomyces pombe pre-treated with Trolox and stressed with H2O2. Samples were prepared as described under Materials and Methods. The protein stain was shown in (a). Major oxidatively damaged proteins were indicated in (b). C1: untreated control, C2: cells pre-treated with Trolox for 2 h, C3: cells pre-treated with Trolox for 14 h. T1: cells treated with H2O2 alone, T2: cells pre-treated with Trolox for 2 h and then exposed to H2O2, T3: cells pre-treated with Trolox for 14 h and then exposed to H2O2.
It has been amply documented that the 20S proteasome degrades oxidatively damaged proteins.[28,29] The protective effect of Trolox against H2O2-induced protein damage was further confirmed by measuring the 20S proteasome activity. The results obtained here indicated that the protective effect of Trolox pre-treatment on protein carbonyls was due to the increased intracellular scavenging ability rather than Trolox-mediated activation of the proteasome [Table 1].
This study provides proof that intracellular scavenging activity is the mechanism by which Trolox treatment provides prevention against oxidative damages induced by H2O2 in the fission yeast, which represents a good model for mammalian cells. The methods presented can be used as standard protocols for investigating the antioxidant activity of pure or complex potential antioxidants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Halliwell B, Gutteridge CM. 3rd rev. ed. London: Oxford University Press; 2004. Free radicals in biology and medicine; p. 936. [Google Scholar]
- 2.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 3.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: History and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–12. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Costa V, Moradas-Ferreira P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: Insights into ageing, apoptosis and diseases. Mol Aspects Med. 2001;22:217–46. doi: 10.1016/s0098-2997(01)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Antioxidants and human disease: A general introduction. Nutr Rev. 1997;55:S44–9. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 6.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–26. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 7.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 8.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–10. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 9.Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 10.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 11.Rietjens IM, Boersma MG, de Haan L, Spenkelink B, Awad HM, Cnubben NH, et al. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol. 2002;11:321–33. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Birgelius-Flohe R, Traber MG. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 13.Satoh K, Kadofuku T, Sakagami H. Effect of Trolox, a synthetic analog of alpha-tocopherol, on cytotoxicity induced by UV irradiation and antioxidants. Anticancer Res. 1997;17:2459–63. [PubMed] [Google Scholar]
- 14.Distelmaier F, Visch HJ, Smeitink JA, Mayatepek E, Koopman WJ, Willems PH. The antioxidant Trolox restores mitochondrial membrane potential and Ca2+-stimulated ATP production in human complex I deficiency. J Mol Med. 2009;87:515–22. doi: 10.1007/s00109-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raspor P, Plesnicar S, Gazdag Z, Pesti M, Miklavcic M, Lah B, et al. Prevention of intracellular oxidation in yeast: The role of vitamin E analogue, Trolox (6-hydroxy-2,5,7,8-tetramethylkroman-2-carboxyl acid. Cell Biol Int. 2005;29:57–63. doi: 10.1016/j.cellbi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Poljsak B, Gazdag Z, Pesti M, Filipic M, Fujs S, Farkas N, et al. Role of the vitamin E model compound Trolox in the prevention of Cr(VI)-induced cellular damage. Toxicol Environ Chem. 2006;88:141–57. [Google Scholar]
- 17.Pekmez M, Arda N, Hamad I, Kig C, Temizkan G. Hydrogen peroxide-induced oxidative damages in Schizosaccharomyces pombe. Biologia. 2008;63:151–5. [Google Scholar]
- 18.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–70. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 19.Okai Y, Higashi-Okai K, Machida K, Nakamura H, Nakayama K, Fujita K, et al. Protective effect of antioxidants against para-nonylphenol-induced inhibition of cell growth in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2000;185:65–70. doi: 10.1111/j.1574-6968.2000.tb09041.x. [DOI] [PubMed] [Google Scholar]
- 20.Forsburg LS, Rhind N. Basic methods for fission yeast. Yeast. 2006;23:173–83. doi: 10.1002/yea.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterborg JH. 2nd ed. New Jersey: Humana Press; 2002. Protein protocols handbook. Chapter 2, The Lowry method for protein quantitation; pp. 7–9. [Google Scholar]
- 22.Cho YW, Park EH, Lim CJ. Catalase, glutathione S-transferase and thioltransferase respond differently to oxidative stress in Schizosaccharomyces pombe. J Biochem Mol Biol. 2000;33:344–8. [Google Scholar]
- 23.Dunn MJ. 2nd ed. New Jersey: Humana Press; 2002. The protein protocols handbook. Chapter 33 Detection of proteins in polyacrylamide gels by silver staining; pp. 265–72. [Google Scholar]
- 24.Reinheckel T, Grune T, Davies KJ. Vol. 99. New Jersey: Humana Press; 2000. Stress response: Methods and protocols. Methods in Molecular Biology. Chapter 5, The measurement of protein degradation in response to oxidative stress; pp. 49–60. [DOI] [PubMed] [Google Scholar]
- 25.Bronzetti G, Cini M, Andreoli E, Caltavuturo L, Panunzio M, Croce CD. Protective effects of vitamins and selenium compounds in yeast. Mutat Res. 2001;496:105–15. doi: 10.1016/s1383-5718(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 26.Peus D, Meves A, Pott M, Beyerle A, Pittelkow MR. Vitamin E analog modulates UVB-induced signaling pathway activation and enhances cell survival. Free Radic Biol Med. 2001;30:425–32. doi: 10.1016/s0891-5849(00)00488-3. [DOI] [PubMed] [Google Scholar]
- 27.Susa N, Ueno S, Furukawa Y, Sugiyama M. Protective effects of vitamin E on chromium (VI)-induced cytotoxicity and lipid peroxidation in primary cultures of rat hepatocytes. Arch Toxicol. 1996;71:20–4. doi: 10.1007/s002040050353. [DOI] [PubMed] [Google Scholar]
- 28.Inai Y, Nishikimi M. Increased degradation of oxidized proteins in yeast defective in 26S proteasome assembly. Arch Biochem Biophys. 2002;404:279–84. doi: 10.1016/s0003-9861(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 29.Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–8. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]


