Abstract
The purpose of this study was to highlight the role of twin designs in understanding children’s conversational interactions. Specifically, we (a) attempted to replicate the findings of genetic effects on children’s conversational language use reported in DeThorne et al. (2008), and (b) examined whether the language used by examiners in their conversation with twins reflected differences in the children’s genetic similarity. Behavioral genetic analyses included intraclass correlations and model fitting procedures applied to 514 same-sex twins (202 MZ, 294 DZ, 10 unknown zygosity) from the Western Reserve Reading Project (Petrill, Deater-Deckard, Thompson, DeThorne, & Schatschneider, 2006). Analyses focused on child and examiner measures of talkativeness, average utterance length, vocabulary diversity, and grammatical complexity from a fifteen-minute conversational exchange. Substantial genetic effects on children’s conversational language measures replicated results from DeThorne et al. (2008) using an expanded sample. However, no familiality was reflected in the examiner language measures. Modest phenotypic correlations between child and examiner language measures suggested that differences in examiner language use may elicit differences in child language use, but evidence of evocative rGE in which genetic differences across children evoke differences in examiner language use, was not found. The discussion focuses on a comparison of findings to previous studies and implications for future research.
Keywords: twin design, gene-environment correlation, child language, conversational interaction, heritability
In the scientific literature, human twin designs have been used for nearly 85 years beginning with a focus on intellectual development (Merriman, 1924) and expanding to address causal influences on phenotypes ranging from television viewing (e.g., Plomin, Corley, DeFries, & Fulker, 1990) to sexual orientation (e.g.; Kirk, Bailey, & Martin, 2000). Twins provide a natural prism through which to differentiate genetic versus environmental effects due to the consistent difference in genetic similarity between identical (i.e., monozygotic) and fraternal (i.e., dizygotic) twins. Monozygotic (MZ) twins develop from the same zygote and consequently share 100% of their segregating genes (exceptional circumstances not withstanding; see Evans & Martin, 2000 and Stromswold, 2006). In contrast, dizygotic (DZ) twins develop from separate zygotes, two separate eggs fertilized by two distinct sperm, and consequently share on average, 50% of their segregating genetic material. As a result of this consistent difference in genetic similarity between MZ and DZ twins, any trait influenced by genetics is predictably more similar between MZ than DZ twins, which is largely why MZ twins resemble each other more than DZ twins in regard to physical appearance. In short, the extent to which twin zygosity serves as a predictor of twin similarity serves as a proxy for genetic influence on the phenotype, or trait, under study. Specifically, the proportion of phenotypic differences across individuals that can be attributed to genetic variation is referred to as an estimate of heritability (h2). Individual differences that can not be attributed to genetics are attributed to either shared or nonshared environmental influences. Shared environment (c2) is responsible for resemblance within families, such as caregiver interaction style or exposure to toxins within the home environment. In contrast, nonshared environment (e2) contributes to differences within families; potential candidates include accidents, illness, and classroom placement. Estimates of genetic and environmental influence can be derived from intraclass correlations and model fitting procedures.
Intraclass correlations provide an index of similarity between two members within a group. In this case, indices of within-pair twin similarity on the variable of interest are generated separately for MZ and DZ twins. The extent to which MZ twins resemble each other more than DZ twins provides an estimate of heritability; i.e., h2=2(MZr − DZr). Similarly, the extent to which similarity between MZ twins can not be accounted for by genetic effects provides an estimate of shared environmental effects (c2 =MZr − h2), i.e., environmental influences that are common between twins within a pair. Finally, the extent to which MZ twins differ on the trait of interest provides an estimate of nonshared environmental effects, including error (1-MZr; readers are referred to Purcell, 2008 for further detail regarding variance decomposition).
Built upon the same logic as intraclass correlations, ACE model fitting provides estimates of genetic and environmental influences through decomposition of variance into additive genetic (A), shared environmental (C), and nonshared environmental effects (E) with likelihood-based confidence intervals to evaluate statistical significance of h2, c2 and e2 respectively (Purcell, 2008).1 With an alpha of .05, confidence intervals include the true value of the parameter with a probability greater than or equal to (l-[alpha]), or 95%. Specifically, a parameter is considered statistically significant if its corresponding confidence interval does not include zero (see Neale & Miller, 1997 for a detailed discussion of likelihood-based confidence intervals).
Two key points need to be highlighted in regard to the interpretation of model fitting results from behavioral genetic studies. First, the ACE model is intended to examine additive genetic effects rather that the interaction effects associated with dominance and epistasis (Neale & Cardon, 1992, pp. 12–13). Additive genetic effects ‘breed true’ in the sense that the alleles contribute to the child’s phenotype to the same extent they did to the parent’s phenotype regardless of other genotypic variation. In other words, the gene(s) is expected to have a similar effect in the child as it did in the parent. When nonadditive genetic effects are suspected, different models or methodologies should be considered (cf. Purcell, 2008). Second, estimates of both genetic and environmental effects account for individual differences in the phenotype and do not account for near-universals across individuals. As an extreme example, consider the possibility that the ubiquitous development of language across humans may be due entirely to genetic effects, whereas individual differences in the sophistication of vocabulary development could be largely due to environmental influences. In such case, a behavioral genetic analyses of vocabulary would reveal the extent of environmental influence on vocabulary differences rather than the universal impact of genes on one’s ability to develop a vocabulary system at all. On a related note, genetic and environmental estimates will vary as a function of the population sampled because the variation in influential factors differs across samples (cf. Plomin, DeFries, McClearn, & McGuffin, 2008). For example, exposure to environmental toxins can have a deleterious effect on child development (e.g., Jacobson & Jacobson, 1996; Lidsky & Schneider, 2003; Vreugdenhil, Lanting, Mulder, Boersma, & Weisglas-Kuperus, 2002); however if the sample being studied does not include variance in exposure to such toxins, then that influence will not be factored into environmental estimates. In short, the contributions of any genetic or environmental effect cannot be estimated if it does not vary in the sample being studied; as stated succinctly by Plomin et al. (2008, p.307), behavioral genetics serves to document ‘what is’ rather than predicting ‘what could be.’
One critique of the twin design is that its use has focused conceptually on partitioning variance into genetic versus environmental influences rather than illuminating the means by which the two may interrelate. Despite validity in this critique, twin designs are in a unique position to address the complex interplay between genetic and environmental factors characterized as gene-environment interaction and correlation (see Kendler & Eaves, 1986; Plomin & Bergeman, 1991; Plomin, DeFries, & Loehlin, 1977; Rowe, 2003). Gene-environment interaction (GxE) is viewed as genetic control over differences in sensitivity to environmental influences. A recent example from Caspi et al. (2008) found that the positive impact of early breastfeeding on later IQ was dependent on genotypic differences. In other words, early breastfeeding conferred an advantage in later IQ for children with one form of allele but children without this allelic form did not appear to benefit from early breastfeeding, at least in regard to IQ. Instead of interaction effects, we focus in the current paper on the related issue of gene-environment correlation (rGE), which is conceptualized as genetic control of differences in exposure to environmental effects (see Kendler & Eaves, 1986 and Plomin et al., 2008 for further differentiation of gene-environment interaction versus correlation). In other words, the probability of exposure to certain environments is dependent in part on one’s genetic make-up.
Gene-environment correlation (rGE) has been categorized as three types: passive, active, and evocative (Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983; Plomin et al., 2008). All three forms of rGE have the potential to distort estimates of genetic and environmental effects (Plomin et al., 1977; Purcell, 2002). Passive effects arise from the genetic similarity between parents and their biological offspring. For example, children of loquacious parents may be likely to grow up in an environment with more talking. Active g-e correlation refers to the tendency of individuals to seek out environments that are consistent with their genetic proclivities. As an example, children who inherently like to talk may be more likely to seek out activities and relationships that provide the opportunity for them to use and develop this skill. Finally, evocative g-e correlation, also referred to as ‘reactive g-e correlation’ (cf. Plomin et al., 1977), is associated with the differing responses that children evoke from their social and physical environments. For example, evidence suggests that children’s challenging behaviors are likely to elicit higher degrees of maternal negative affect and control (Anderson, Lytton, & Romney, 1986; Deater-Deckard, 2000; Pike, McGuire, Hetherington, Reiss, & Plomin, 1996). Across all three types of rGE, the unifying theme is that the genetic make-up of the child is not independent from the environment to which the child is exposed.
Applying the concept of evocative gene-environment correlation to child language development, it is possible that observed differences in parent-child interactions result in part from adult “adjustments” to pre-existing differences in child language ability. For example, parents might talk more often and in more sophisticated ways to children who show a strong innate predisposition to language learning, which in turn, could help accelerate children’s language learning even more. Or at the other extreme, children with a genetic liability to language difficulties might elicit less frequent and less sophisticated language models from their communicative partners. In this case, the adult’s language use represents the “environmental” factor and the child’s language use the “genetic” factor which evokes differences in caregiver behavior (see Plomin & Bergeman, 1991). Although the possibility of such child effects on adult-child interactions has been acknowledged (e.g., Conti-Ramsden, 1985; Leonard, 1987), attempts to derive empirical support have been slow to emerge and have focused largely on a comparison of discourse variables observed in mothers of children with language impairment versus the mothers of age-matched controls (e.g., Paul & Elwood, 1991).
A clever design by Cramblit and Siegel (1977) examined the language used by three adults when interacting with two 4-year-old children, one with language impairment and the other with typical language skills. The three adults were the mother, father, and aunt of the child with language impairment, and the same-aged child was his cousin. In sum, the study found that all three adults used less complex utterances when interacting with the child whose language was impaired than when conversing with his typically-developing cousin. Although suggestive of evocative effects, many factors remained uncontrolled that limit interpretation. For example, the two children differed in terms of gender as well as language ability.
DeThorne and Channell (2007) took a similar approach to examining evocative effects but focused on the language use of one adult examiner in her interactions with 29 different children with varying levels of language disability. The examiner was naïve to the purpose at the time the study was conducted, and yet the grammatical and vocabulary complexity of her utterances correlated positively with the grammatical complexity of the children’s productions. Although interpretation is limited by the correlational design, results suggested that children with more complex language use elicited more complex language use on the part of the examiner.
To our knowledge, no studies of evocative effects on conversational language have been conducted within a genetically-sensitive design. Because of predictable genetic differences, adult-twin conversational exchanges can directly examine evocative rGE effects by utilizing the adult examiners’ language as the phenotype of interest. If the unrelated conversational partners of MZ twins speak more similarly to each other than the conversational partners of DZ twins, evocative rGE would be implicated.
The purpose of the present study is to highlight the role of the twin design in examining evocative gene-environment correlations by focusing on a conversational context between children and adult examiners. Specifically, we asked whether examiners’ language use in conversation with twins would reflect differences in the children’s genetic similarity. In accordance with a transactional model in which child and adult exert reciprocal influences upon each other during development (see Anderson, Lytton, & Romney, 1986; Sameroff, 1975, Scarr, 1983), we predicted modest but significant heritability estimates for the language used by independent adult examiners in their interaction with twin children, particularly in regard to vocabulary diversity (Cramblit & Siegel, 1977; DeThorne & Channell, 2007; Beals & Tabors, 1995), frequency of talk (Hart & Risley, 1995; Paul & Elwood, 1991; Whitehurst et al., 1988), and utterance length (Cramblit & Siegel, 1977).
Methods
Participants
Participants included 514 twins (202 MZ, 294 DZ, 10 unknown zygosity) from the second annual home visit of the Western Reserve Reading Project (Petrill, Deater-Deckard, Thompson, DeThorne, & Schatschneider, 2006). This longitudinal study of causal influences on the development of reading and related cognitive abilities includes annual visits to the children’s homes between kindergarten entry and fifth grade. In addition to standardized assessment, conversational language samples between child and examiner were collected beginning with the second annual visit. The Western Reserve Reading Project (WRRP) is in a unique position to examine potential evidence for evocative gene-environment correlation related to child language development for two primary reasons. First, the genetically sensitive design provides an opportunity to control for genetic differences across children, as previously discussed. Second, WRRP includes conversational exchanges between each child and a biologically unrelated examiner. Focusing on examiner-child interactions, rather than parent-child interactions, provides a key opportunity to examine evocative gene-environment correlation because the potential confound between shared genes is removed. In other words, observed correlations between child and examiner language cannot be attributed to shared genetic similarity between the conversational partners.
The present study included twins from WRRP with complete language samples containing at least 50 complete and intelligible examiner utterances. The sample from this study encompassed all but six of the twins examined in DeThorne et al. (2008) and expanded that sample by an additional 140 twins due to ongoing data collection and completed zygosity information. In addition, 16 children with less than 50 complete and intelligible utterances were included here who would have been excluded in the previous paper. Given the present focus on examiner measures, samples in this paper were selected based on the number of examiner utterances rather than child.
Zygosity and sex of twins in the present paper was distributed as follows: 206 MZ (41% male),’298 DZ (42% male), and 10 twins of unknown zygosity (20% male). The mean age of the sample was 7.15 years (SD=.66), with approximately half of the students in first grade, a quarter in second grade, and the remaining quarter in kindergarten. The mean child IQ approximated the normative mean on the Stanford Binet Intelligence Scale (Thorndike, Hagen, & Sattler, 1986) at 103 (SD=12). In terms of race/ethnicity, the caregivers who completed the questionnaires identified themselves as follows: 91% White/European American, 5% Black/African American, 1% Asian, 1% Hispanic, and 1% Other. In regard to parental education, approximately 90% of the primary caregivers reported having some college or professional education beyond high school, with 60% reporting receipt of at least one four-year University degree. Although not directly comparable to census data, our sample appears to over represent more highly educated families (U.S. Census Bureau, 2000).
Procedures
Conversational samples were audio-recorded during fifteen minutes of interaction between the child and examiner during play with modeling clay. A review of 85% (435/514) of the samples revealed that the mean sample length was 15.37 minutes (SD=1.03), with a range of 11.85 to 20.53 minutes. Different examiners interacted with each twin within a pair to help ensure that similarity in twin measures was due to similarity between twins rather than factors specific to the examiner interacting with them. Consistent with conventional language sampling methods (Miller et al., 2005), the interaction was unscripted, but examiners were given basic recommendations as a guide. For example, examiners were instructed to limit direct requests and closed-questions (e.g., Do you like soccer?) and to focus instead on commenting and open-ended questions (e.g., What’s your soccer team like?). Given that evocative effects were not the primary focus of WRRP, language samples were collected by a variety of examiners as needed to manage the logistics of a longitudinal study on such a large sample. Specifically, fourteen different examiners collected language samples during the second year home visits, ranging from 4 to 90 total samples collected by each. All examiners held bachelor’s degrees, and many were pursuing graduate degrees in related disciplines such as psychology or genetic counseling. Training included a full review of assessment procedures, observation of two home visits conducted by an experienced examiner, and continued communications with project coordinators. Guidelines provided to the examiners for the conversational interaction are provided in the Appendix. Note that although the examiners were welcome to talk specifically about the modeling clay activity, they were also instructed to introduce at least three other conversational topics, such as school, movies, and family.
All conversational samples were recorded onto audiocassette using a Marantz PMD201 recorder and sent to the first author’s research laboratory for transcription using Systematic Analysis of Language Transcripts (SALT; Version 8.0; Miller, 2004). Both child and examiner were transcribed using traditional SALT conventions for marking mazes and bound morphemes (Miller et al., 2005). In addition, utterances were segmented into conversational units, C-units, which involved separating all independent clauses joined by coordinating conjunctions (Loban, 1976; Nippold, 1998). For example, the sentence “I went to the ballgame and the pitcher was injured” would be segmented into two utterances as follows: “I went to the ballgame/And the pitcher was injured.” All transcriptions were checked independently by a second research assistant before output measures were derived. Inter-examiner agreement for independent transcription of utterance boundaries and individual morphemes reached 90% and 91% respectively based on 43 randomly selected transcripts.
Specific measures of language structure and content were selected for (a) consistency with identified effects in prior literature (Cramblit & Siegel, 1977; DeThorne & Channell, 2007; Beals & Tabors, 1995; Hart & Risley, 1995; Paul & Elwood, 1991; Whitehurst et al., 1988) and (b) ease of automated analyses. All measures were derived through Systematic Analysis of Language Transcripts (Miller, 2004). Total complete and intelligible C-units (TCICU), an index of verbosity, and Mean Length of C-unit (MLU-C), a measure of average utterance length, were both calculated from the entire 15-minute sample. In contrast, the remaining three measures were calculated from the first 100 complete and intelligible C-units within each sample in order to control for differences in sample size: number of total word (NTW), a measure of verbosity; number of different root words (NDW), a measure of expressive vocabulary diversity; and total number of conjunctions (TNC), a general measure of syntactic complexity (Miller et al., 2005). The same measures were derived from both the examiner and child utterances respectively. Support for the validity of these measures with school-age children is summarized in DeThorne et al. (2008).
Analyses
In addition to descriptive and correlational information on the sample as a whole, intraclass correlations (Falconer, 1960) and model fitting procedures were first utilized to estimate genetic and environmental effects on child language measures. Based on the presumption that findings would replicate the genetic effects on children’s conversational language use from DeThorne et al. (2008), we planned to examine potential evocative gene-environment correlation through univariate analyses of examiner conversational measures. Specifically, similarity in examiners’ conversational language was examined as a function of twin zygosity, an approach consistent with Plomin and Bergeman (1991). Evidence that examiners interacted more similarly with MZ than DZ twins would provide support for evocative gene-environment correlation.
Results
Descriptives
Descriptive results for the child and examiner language sample measures, prior to correction and standardization procedures, are provided in Table 1. Note that due to concerns regarding measurement reliability (see Gavin & Giles, 1996), twin pairs were excluded from all analyses if either sample contained fewer than 50 complete and intelligible examiner utterances. In addition, measures of NDW, NTW, and TNC were calculated on a set number of utterances, in this case 100, in order to control for differences in talkativeness. Therefore these measures were only calculated for child or examiner if at least 100 complete and intelligible utterances were produced by that individual within the sample. Consequently, note in Table 1 the larger number of participants for TCICU and MLU than for the other language sample measures.
Table 1.
Descriptive Data on Child and Examiner Language Sample Measures.
| Language Measure | MZ | DZ | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | |
| Child | ||||||
| Total Complete & Intelligible C-Units | 206 | 141 (47) | 31–272 | 298 | 136 (45) | 10–236 |
| Mean Length of C-unit | 206 | 5.60 (1.03) | 2.59–8.43 | 298 | 5.62 (1.31) | 1.81–9.50 |
| Number of Total Words | 167 | 514 (89) | 263–730 | 238 | 528 (110) | 283–887 |
| Number of Different Words | 167 | 188 (27) | 104–275 | 238 | 191 (29) | 105–274 |
| Total Number of Conjunctions | 167 | 35 (15) | 6–85 | 238 | 37 (20) | 5–109 |
| Examiner | ||||||
| Total Complete and Intelligible Units | 208 | 131 (36) | 53–259 | 298 | 127 (36) | 50–261 |
| Mean Length of C-unit | 208 | 5.95 (.75) | 4.09–8.43 | 298 | 5.81 (.78) | 3.33–8.16 |
| Number of Total Words | 162 | 540 (73) | 355–779 | 230 | 528 (80) | 225–748 |
| Number of Different Words | 162 | 176 (17) | 131–216 | 230 | 173 (23) | 99–252 |
| Total Number of Conjunctions | 162 | 26 (10) | 7–56 | 230 | 24(10) | 6–53 |
Correction Procedures
As is standard in twin research, all child and examiner measures were corrected for child age and sex prior to correlation analyses and model-fitting (cf. McGue & Bouchard, 1984). Specifically, each language measure was regressed on child sex, child age, and child age squared, with remaining analyses conducted on the residuals. The purpose of the correction procedure is to adjust for phenotypic differences that might be attributed primarily to these variables. Although behavioral genetic analyses can be fashioned to examine causal differences based on sex or age (cf. Neale & Cardon, 1992), that was not the purpose of the current analyses.
Model Assumptions
To address model assumptions regarding group differences, we examined mean differences between MZ and DZ twins for the corrected child and examiner language measures using independent-samples t-tests. No mean differences emerged; however variance differences were observed in child MLU (F=6.34, p=.012), child TNC (F=6.29, p=.013), and examiner NDW (F=14.13, p=.00). In all cases, greater variance was associated with the DZ twins. This latter point is critical in understanding the potential impact of the variance differences on the model-fitting results. In an unbalanced design, variance differences that favor the smaller group inflates Type I error, whereas larger variance in the larger group decreases the likelihood of Type I error (Maxwell & Delaney, 2004, p.113). Consequently, in the present case the larger variance is associated with the larger group (i.e., DZ) and will thereby lead to more conservative estimates of statistical significance.
Correlation Matrix
Table 2 provides a correlation matrix of child and examiner language measures after correction for child age and sex. Although effect sizes were relatively small in most cases, note that the total number of examiner utterances, TCICU-E, correlated positively with total number of child utterances and negatively with all other child measures. The negative correlations indicate that the more utterances produced by an examiner, the lower the children’s MLU, NTW, NDW, and TNC. Similarly, examiner utterance length and total number of words correlated negatively with total number of child utterances, utterance length, and total number of conjunctions.
Table 2.
Correlation matrix of child (-C) and examiner (-E) language sample measures corrected for age and sex.
| TCICU-C | MLU-C | NTW-C | NDW-C | TNC-C | |
|---|---|---|---|---|---|
| TCICU-E | .27** | −.24** | −.37** | −.29** | −.30** |
| MLU-E | −.27** | −.15** | −.09 | −.07 | −.13* |
| NTW-E | −.20** | −.11* | −.08 | −.07 | −.12* |
| NDW-E | −.09 | .06 | .03 | .07 | −.09 |
| TNC-E | −.01 | .10 | .04 | .05 | −.01 |
Note. TCICU=Total number of complete and intelligible utterances; MLU=mean length of utterance in morphemes; NTW=number of total root words in 100 utterances; NDW=number of different root words in 100 utterances; TNC=total number of conjoining and subordinating conjunctions in 100 utterances.
Significance at an alpha of .001.
Statistical significance at .01.
The consistent significant correlations between examiner and child are suggestive of evocative effects but the direction of causality is ambiguous. Are children evoking language differences in the examiner, as rGE would imply, or are differences in language use by the examiner eliciting differences in children’s productions? Definitive evidence of rGE requires observed differences in examiner language as a function of twin zygosity. It is worth noting that if such correlations were indicative of rGE, the negative coefficients represent an association in the direction opposite to that predicted. Specifically we predicted an increase in examiner language complexity to accompany increased language complexity used by the child, rather than an increase in examiner complexity accompanying decreased child complexity.
Intraclass Correlations
Intraclass correlations for both child and examiner language measures are provided in Table 3. The pattern of significant MZ and DZ correlations across language sample measures is suggestive of both genetic and shared environmental effects with estimated heritability, h2=2(MZr-DZr), ranging from .62 for TCICU-C to .26 for MLU-C. Shared environmental influences are also implicated given that the MZ twin correlations are not greater than two times the DZ twin correlation. In contrast, the intraclass correlations for examiner measures did not reach significance for MZ or DZ pairs, thereby suggesting a lack of any familiality. Familiality refers to similarity across family members and encompasses both heritability and shared environmental effects.
Table 3.
Intraclass Correlations for Child and Examiner Measures across Monozygotic (MZ) and Dizygotic (DZ) Twins.
| Language Variable | MZr | DZr |
|---|---|---|
| Child | ||
| Total Complete and Intelligible C-Units | .57** | .26** |
| Mean Length of C-Unit | .54** | .41** |
| Number of Total Words | .50** | .30** |
| Number of Different Words | .48** | .29** |
| Total Number of Conjunctions | .46** | .20** |
| Examiner | ||
| Total Complete and Intelligible C-units | −.10 | −.11 |
| Mean Length of C-Unit | .08 | .04 |
| Number of Total Words | .15 | −.03 |
| Number of Different Words | .10 | .07 |
| Total Number of Conjunctions | .03 | −.07 |
Note. All measures have been corrected for child age and sex.
Denotes significance at .05.
Denotes significance at .01.
Univariate Estimates
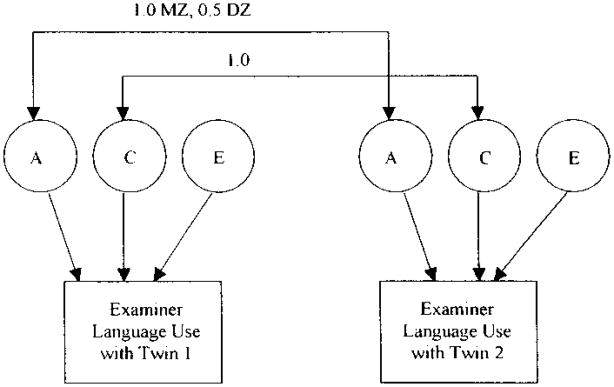
Univariate ACE structural equation models were employed in Mx (Neale, Boker, Xie, & Maes, 2006) using maximum likelihood methods for raw data in order to test the statistical significance of genetic and environmental effects (Neale & Cardon, 1992). To reiterate, ACE modeling partitions the covariance between individuals into variance associated with genetic (h2), shared environmental (c2) and nonshared environmental (plus error; e2) influences. In the case, the covariation between twins, as well as experimenters working with pairs of twins, on various language outcomes was examined. See Figure 1 for an illustration of twin effects working through the examiner language measures. Results for the univariate analyses on both child and examiner measures are presented in Table 4. The heritability estimates for child language measures ranged from .48 for NDW to .62 for NTW. Heritability for all child measures, except NDW, reached statistical significance as indicted by confidence intervals that did not include zero. Shared environmental effects were not statistically significant for any of the child language measures. Note that the univariate estimates differ somewhat from the approximations derived via intraclass correlations due to variance differences across MZ and DZ twins. In regard to the examiner measures, all genetic and shared environmental estimates failed to reach statistical significance as one would predict based on the insignificant intraclass correlations.
Figure 1.
ACE model of genetic (A), shared environmental (C), and nonshared environmental (E) effects on examiner language use by twin zygosity (MZ/DZ).
Table 4.
Univariate estimates for heritability (h2), shared environment (c2) and nonshared environmental (e2) influences for examiner and child conversational language measures (with 95% confidence intervals).
| Variable | h2 | c2 | e2 |
|---|---|---|---|
| Child | |||
| Total Complete & Intelligible C-Units | .56 (.22–.72) | .00 (.00–.28) | .44 (.35–.57) |
| Mean Length of C-unit | .52 (.18–.81) | .12 (.00–.39) | .37 (.28–.50) |
| Number of Total Words | .62 (.22–.82) | .00 (.00–.29) | .40 (.29–.57) |
| Number of Different Words | .48 (.00–.73) | .05 (.00–.41) | .48 (.36–.67) |
| Total Number of Conjunctions | .49 (.17–.70) | .00 (.00–.21) | .48 (.35–.67) |
| Examiner | |||
| Total Complete & Intelligible C-Units | .00 (.00–.08) | .00 (.00–.06) | 1.00 (.88–1.14) |
| Mean Length of C-unit | .09 (.00–.26) | .00 (.00–.19) | .89 (.72–1.09) |
| Number of Total Words | .10 (.00–.33) | .00 (.00–.18) | .88 (.67–1.12) |
| Number of Different Words | .20 (.00–.48) | .00 (.00–.24) | .79 (.55–1.01) |
| Total Number of Conjunctions | .01 (.00–.21) | .00 (.00–.14) | .94 (.73–1.11) |
Discussion
The purpose of the present study was to highlight the role of univariate behavioral genetic analyses in understanding children’s conversational interactions. Results largely replicated the genetic effects on children’s conversational language use reported in DeThorne et al (2008), but failed to find evidence of evocative rGE within this context. Of interest, the phenotypic analyses revealed significant correlations between some child and examiner language sample measures of small to moderate effect size. However, when paired with the behavioral genetic findings, such correlations suggest an influence of examiner language use on the child rather than the other way around. For example, if less talkative children elicited more utterances from the examiners one would have expected evidence of heritability on the total number of examiner utterances because of the genetic effects on the children’s language measures. Taken together, results suggest that as examiners produced more utterances (i.e., took more conversational turns), children produced more utterances in turn but used shorter and potentially less complex sentences. The remainder of the discussion section will address the (a) generalizability of this twin sample, (b) significance of the genetic effects on child language, (c) interpretation of the null rGE evidence, and (d) recommendations for future study.
Generalizability of Sample
The descriptive information on this sample becomes particularly relevant in light of concerns with the generalizability of results from twin studies of language in particular. The primary concern tends to center on whether or not it is reasonable to expect causal influences on language in twins to overlap with causal influences on the language of singletons. In other words, might we expect the genetic and environmental effects on twins to be unique to twins and not prevalent in the population at large? Although it is true that twins as a group tend to lag behind singleton peers in language development, particularly during the early years (e.g., Rutter, Thorpe, Greenwood, Northstone, & Golding, 2003), such differences have been associated with the same environmental factors that impact singletons, albeit less frequently and perhaps less severely—factors such as prematurity, low birth weight, and less individualized adult interaction (e.g., Field, Dempsey, & Shuman, 1981; Luke & Keith, 1992). More relevant to genetic findings from the present study, there is no evidence to suggest that twins possess genetic differences related to language that are not similarly present in the singleton population. The distinction between twins and singletons is blurred further when one considers that one in eight pregnancies may begin as twins, many of whom are reduced to singleton status before identification, thereby leading to the birth of a number of potential ‘unidentified twins’ who are thought of by themselves and others as singletons (Segal, 1999, p. 40–41). In sum, there is currently no reason to assume that results from twin studies of language will not generalize to the singleton population (see also Evans & Martin, 2000).
The second and more valid concern regarding generalization is not specific to twin studies and relates to how well the sample captures typical variation in the population at large. For the present study, representing typical variation in conversational language abilities is an important point. Although conversational language, by definition, does not lend itself easily to standardized comparisons, reference to other studies and existing databases is possible. Specifically, MLU provides more ready comparison than the other measures in this study due to its ability to be compared across transcripts of differing lengths. A comparison of average child MLU from the twins in this study, 5.62 (SD= 1.21), yielded similar results to the mean MLU of 5.43 (SD= 1.07) derived from 128 similar-age children in the WisconsinConCunits database available in SALT (Version 8.0; Miller, 2004).
Although roughly comparable to reference data in regard to conversational language ability, the present sample over-represents highly educated families compared to the population at large (cf., U.S. Census Bureau, 2000). This becomes relevant given the possible interaction between causal factors and home environment. For example, Rowe, Jacobson, and Van den Oord (1999) found lower heritability of verbal IQ in children whose parents had not finished high school compared to children whose parents’ education exceeded high school. He interprets this finding as evidence that some environmental factors associated with lower parental education, such as abuse or lead exposure, may be detrimental to intellectual development (see also Rowe, 2003). Analogously, if an environmental influence, such as lead exposure or poor prenatal care, is not prevalent in the households of more highly-educated parents, then its effects will not be translated into significant environmental effects within the present study. On a related note, Scarr (1992, p.5) suggests that individuals have evolved to adapt to the average expectable environment’ and are consequently able to reach their genetic potential within a wide variety of home situations. Consequently much of the environmental variation present in families with higher education levels may be ‘functionally equivalent’ in regard to the impact on child language development. The relevance to the present study is that causal factors associated primarily with caregivers without a high school degree would be underestimated in the present sample due to limited sampling of this population. Currently we are examining potential differences in the extent of genetic and environment influences using a smaller subgroup of twins who experienced environmental risk factors, such as extreme prematurity and very low birth weight.
Significance of Genetic Effects on Child Language
Based on the population studied, the present work provides a replication of the genetic effects on children’s conversational language ability published in DeThorne et al (2008) with a larger sample (i.e., 514 versus 380 twins). In short, both studies found significant genetic effects on MLU and NTW, without significant shared environmental estimates for any conversational measure. Two differences across studies are worth mentioning here. First, the heritability estimate of .49 for TNC failed to reach significance in DeThorne et al., presumably due to lesser statistical power associated with the smaller sample. Second, the present study added the variable TCICU as a measure of how many complete and intelligible utterances were produced by the child within the 15-minute sample.
One point worth noting in both studies is the potential confound between our measures of linguistic complexity and the construct of child verbosity. Although all frequency measures were calculated on a standardized number of utterances, the utterances themselves vary in length. Consequently it is possible that our findings reflect genetic effects on personality traits instead of or in addition to causal effects on linguistic ability (see Ebstein, Benjamin, & Belmaker, 2003; Segal, 1999 for review of genetic effects on personality). However, it is also highly likely that measures of expressive language skills cannot be distinctly separated from volubility in a conversational context (DeThorne, Coletto, Wendorf, Petrill, & Johnson, 2007), just as scores on standardized tests of language are likely influenced by constructs such as attention, motivation, and frustration tolerance (e.g., Speltz, DeKlyen, Calderon, Greenberg, & Fisher, 1999). It is wise to consider that causal influences on conversational language measures reflect a confluence of traits, language proficiency and personality being two likely factors. To assume that any behavioral measure is a ‘pure’ reflection of the trait under investigation is to grossly underestimate the inherent complexity of human behavior.
Interpretation of Null rGE Evidence
Despite genetic effects on children’s conversational language use, the present study failed to reveal evidence that those genetic effects ‘evoked’ differences in the language used by examiners during interactions with those same children. Consequently it is possible that evocative rGE effects simply are not impacting adult-child conversations in the fashion we anticipated. However, given that rGE effects have been documented within the contexts of adult-child interaction (e.g., Anderson, Lytton, & Romney, 1986; Deater-Deckard, 2000; Pike, McGuire, Hetherington, Reiss, & Plomin, 1996) and evidence from correlation and group designs have suggested its impact on language interactions in particular (e.g., Cramblit & Siegel, 1977; DeThorne & Channell, 2007), two additional possibilities are explored.
First, it is conceivable that evidence of gene-environment correlation was overshadowed by substantial inter-examiner variability. Recall that to produce conservative estimates of twin similarity we made sure that each twin within a pair was examined by a different examiner. Consequently any individual differences between examiners that might impact their conversational language use (e.g., personality, language ability, mood) would be reflected in the nonshared environmental estimates (e2). Note the large estimates of e2, not only for the examiner measures but also for the child measures—a feature likely to be characteristic of measures from ‘messy’ real-world contexts (the reader is referred to Plomin et al., 2008 for extensive discussion of nonshared environmental influences).
Second, it is possible that the conversational interaction within the present study was not naturalistic enough to capture the didactic quality of most everyday interactions. Although examiners were given instructions geared toward making the interaction comfortable and naturalistic (e.g., comment and ask open-ended questions), the interaction itself was embedded within a two-hour protocol of standardized testing administered by the same examiner. It was probably difficult for both child and examiner to switch gears from test mode to ‘let’s hang out and enjoy each other’s company’ for the next fifteen minutes before switching back to test mode. This interpretation may also help explain the unexpected negative phenotypic correlations between examiner and child language use. It is possible that examiners viewed their role as ‘getting the child to talk’, rather than engaging them in a naturalistic conversational exchange. Consequently examiners may have tried to encourage reticent children to talk by asking more directive questions (e.g., “What are you making?”) that tend to elicit frequent but minimally-complex responses (e.g., “A penguin.”) In sum, null findings for rGE may have emerged due in part to inter-examiner variability and the somewhat contrived nature of the conversational exchange.
Recommendations for Future Study
With present findings in mind, future twin studies focused on evocative gene-environment correlation may want to ‘standardize’ children’s conversational partners in some fashion and strive to make the exchange as naturalistic as possible. If one did not want to use the same interactive partner for both twins within a set due to logistical constraints or worry of inflating twin similarity, the same two partners could be used for all twin pairs, MZ and DZ alike. Scripting the partners’ role would standardize the partners’ role further, but it would not provide the flexibility needed to observe evocative effects, by definition. Along those lines, attempts should be made to keep the interaction as naturalistic as possible, perhaps through keeping the conversational interaction separate from standardized test procedures or by including a familiar conversational partner.
Once specific genes which influence child language ability are identified, methodological options for examining all forms of rGE widen substantially. For example, children could be grouped by genotype (i.e., presence or absence of specific allele(s)) and the language used by their communicative partner(s) compared. For now the specific genes associated with child language differences appear isolated to certain families (see Fisher, 2003) or associated with syndromes that impact multiple aspects of a child’s development including their physical appearance (e.g., Down syndrome). Physical differences make pinpointing the phenotypic source of evocative effects challenging. In other words, it could be unclear whether individuals were responding to some children differently because they talked differently or because they looked different. On this latter point, one advantage of the twin designs that we were not able to capitalize on in the present study is the potential of multivariate analyses to help elucidate the nature of any observed rGE effect. For example, if estimates of heritability on examiner measures had been significant in the present study we could have utilized multivariate analyses to examine whether the heritability of child language measures overlapped with the heritability of the examiner language measures, thereby specifying that it was the child’s language that was eliciting the evocative effects rather than other genetically-influenced qualities (cf. Plomin et al., 2008). In sum, we hope that the present study serves to broaden the view of twin designs—highlighting their potential, not only to divvy up variance into ‘nature versus nurture,’ but to expose the intricate causal web of human behavior for exactly what it is: complex and inherently fascinating.
Acknowledgments
The Western Reserve Reading Project is supported by NICHD (HD38075) and NICHD/OSERS (HD46167). In addition, transcription and analyses have been supported by the American Speech-Language-Hearing Foundation New Investigator Award, the UIUC Campus Research Board, and the Children Youth and Families Consortium at the Pennsylvania State University. Interdisciplinary collaborations have been enhanced by the American Speech-Language-Hearing Association Advancing Academic-Research Careers (AARC) Award. Sincere thanks to all participating families and affiliated research staff, with particular thanks to fellow project investigators: Stephen Petrill (PI), Lee Anne Thompson, Kirby Deater-Deckard, and Chris Schatschneider.
Biographies
Laura S. DeThorne is an Assistant Professor of Speech and Hearing Science at the University of Illinois at Urbana-Champaign. Her research focuses on causal influences on speech-language development for the purpose of facilitating successful prevention and intervention efforts in children with communication disabilities.
Sara A. Hart is a doctoral student in the Department of Human Development and Family Science at The Ohio State University, Columbus. Her research interests focuses on the genetic and environmental influences on children’s cognitive development.
Appendix
Language Interaction Collection Procedures (Based on Leadholm & Miller, 1992)
Goal
Collecting a 15-min conversational language sample that is representative of children’s spontaneous expressive language abilities.
Basic Room Set-up
The tester and child should be seated at a table, approximately an arm’s length of each other. Recording equipment should be easily accessible.
Materials
Clay will be provided as “something to do” while conversing. Talking about the clay (e.g., what you are making, etc.) is certainly “fair game,” but conversation is welcome to diverge to other topics (e.g., hobbies, pets, school activities, video games, etc.). Recording equipment includes: recorder (tape or digital), AC adapter, tape or compact flash card (512 MB or more), back-up batteries.
Interactions Tips
-
Begin by informing the child of the purpose of the activity and introducing him/her to the audio equipment that is being used.
e.g., “I/We are interested in learning about how children talk. While we play with this Model Magic® and talk for the next 15 minutes, I am going to record our voices with this recorder. I want to record your voice so I can go back and listen to what you say. Have you ever used a recorder like this before?” As appropriate, explain how the recorder works, for example, “Our voices go into this microphone, through this wire, into this box, and onto the tape. After we are done, you can listen to us talking if you want.”
Tape the child’s first name, the date, etc. and play it back right away. This allows you to make sure the equipment is working and allows the child to see how it works. On digital, make sure to create a new file and repeat introduction.
Create an environment of trust and security in which the child feels you are truly interested in what he/she has to say (e.g., be responsive, comment about yourself, offer breaks as needed).
Specific suggestions for initiating and maintaining the interaction:
-
Set out the clay colors, select one, and Comment on your choice/activity.
For example, “I’m going to begin by making a castle. I wonder what color it should be.”
Limit requests, directions, and closed-questions, particularly at the beginning of the interaction. Instead use comments and open-ended questions (e.g., “I really like pepperoni on my pizza.” Or “I didn’t see the Spiderman movie. What was it like?”); The open phrase “Tell me about …” can be very helpful here (e.g., “Tell me about your favorite movie.” Or “Tell me what you do with your sister.”
Be enthusiastic (Use smiles, vocal inflection, and eye contact.)
Be patient and pause (Give the child plenty time and space to respond, up to 30 seconds of silence is fine)
Listen and follow the child’s interests with responses, comments, and questions (e.g., “Tell me more about that” “Pizza flavored ice cream? I’ve never heard of such a thing!”)
Use open-ended questions when appropriate (“What do you think of soccer?” rather than “Do you like soccer?”)
Try to introduce 3–4 different conversational topics that are not directly tied to the on-going clay activity (See suggested topics below).
Offer information of interest to the child (e.g., “In the movie Shrek, it was funny when the princess kicked the bad guys.”), including information about yourself (e.g., “I have two little sisters.” Or “When I was in second grade we had to do timed multiplication tables.”)
Limit repeating what the child says (i.e., glossing) unless you feel it will be necessary for the transcriber to understand what is being said. If you are going to repeat the child’s productions, be sure to repeat exactly what the child said (errors and all) and use a downward intonation.
Avoid judging or correcting the child.
Potential Conversational Topics
Popular characters/TV programs/Movies
e.g., “One of my favorite movies is—————. I like the part where—————. Tell me, about a movie or TV show that you like.”
School activities (e.g., activities, teacher, fieldtrip, gym, art, etc.)
e.g., “What was your school fieldtrip like?.”
Family/Siblings/Pets
e.g., “What do you and your brother do together?”
Relevant seasonal things (e.g., holidays, vacations, activities/sports)
e.g., “What did you do on the Fourth of July?”
Games/Sports/Hobbies
e.g., “I heard that you have a baseball game tonight. What will you be doing?”
Food
e.g., “I heard that you baked cookies last night. How did you make them?”
Footnotes
A source of potential confusion is the use of differing abbreviations in reference to genetic variance; for the current purpose Var (A) and h2 refer to the same thing (see Purcell, 2008 p, 378 for additional explanation).
Contributor Information
Laura Segebart DeThorne, Department of Speech and Hearing Science, University of Illinois, Urbana-Champaign;.
Sara Ann Hart, Department of Human Development and Family Science, The Ohio State University, Columbus.
References
- Anderson KE, Lytton H, Romney DM. Mothers’ interactions with normal and conduct-disordered boys: Who affects whom? Developmental Psychology. 1986;22:604–609. [Google Scholar]
- Beals DE, Tabors PO. Arboretum, bureaucratic and carbohydrates: Preschoolers’ exposure to rare vocabulary at home. First Language. 1995;15:57–76. [Google Scholar]
- Caspi A, Williams B, Kim-Cohen J, Craig IW, Milne BJ, Poulton R, Schalkwyk LC, Taylor A, Werts H, Moffitt TE. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proceedings of the National Academy of Sciences, Early Edition. 2008 doi: 10.1073/pnas.0704292104. Retrieved July 14, 2008 from http://www.pnas.org/content/early/recent. [DOI] [PMC free article] [PubMed]
- Conti-Ramsden G. Mothers in dialogue with language-impaired children. Topics in Language Disorders. 1985:58–68. [Google Scholar]
- Cramblit NS, Siegel GM. The verbal environment of a language-impaired child. Journal of Speech & Hearing Disorders. 1977:474–482. doi: 10.1044/jshd.4204.474. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Parenting and child behavioral adjustment in early childhood: A quantitative genetic approach to studying family processes. Child Development. 2000;71:468–484. doi: 10.1111/1467-8624.00158. [DOI] [PubMed] [Google Scholar]
- DeThorne LS, Channell R. Clinician-child interactions: Adjustments in linguistic complexity. American Journal of Speech-Language Pathology. 2007;16:119–127. doi: 10.1044/1058-0360(2007/016). [DOI] [PubMed] [Google Scholar]
- DeThorne LS, Coletto M, Wendorf L, Petrill SA, Johnson BW. Measuring lexical diversity in conversation: Can we separate the baby from the bathwater?. Poster session presented at the Symposium on Research in Child Language Disorders; Madison, WI. 2007. Jun, [Google Scholar]
- DeThorne LS, Petrill SA, Channell RW, Hart SA, Campbell RJ, Dealer-Deckard K, Thompson LA, Vandenbergh DJ. Genetic effects on children’s conversational language use. Journal of Speech-Language-Hearing Research. 2008;51:423–435. doi: 10.1044/1092-4388(2008/031). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Benjamin J, Belmaker RH. Behavioral genetics, genomics, and personality. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. Washington, DC: American Psychological Association; 2003. pp. 365–388. [Google Scholar]
- Evans DM, Martin NG. The validity of twin studies. GeneScreen. 2000;1:77–79. [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. Edinburgh: Oliver & Boyd; 1960. [Google Scholar]
- Field T, Dempsey J, Shuman H. Developmental follow-up of pre-term and post-term infants. In: Sigman M, Friedman S, editors. Preterm Birth and Psychological Development. New York: Academic Press; 1981. pp. 299–312. [Google Scholar]
- Fisher SE. Isolation of the genetic factors underlying speech and language disorders. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. Washington, DC: American Psychological Association; 2003. pp. 205–226. [Google Scholar]
- Gavin WJ, Giles L. Sample size effects on temporal reliability of language sample measures of preschool children. Journal of Speech and Hearing Research. 1996;39:1258–1262. doi: 10.1044/jshr.3906.1258. [DOI] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful Differences in the Everyday Experience of Young American Children. Baltimore: Paul H. Brookes; 1995. [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. New England Journal of Medicine. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. The American Journal of Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Bailey JM, Martin NG. Etiology of male sexual orientation in an Australian twin sample. Psychology, Evolution, & Gender. 2000;2.3:301–311. [Google Scholar]
- Leonard LB. Is specific language impairment a useful construct? In: Rosenberg S, editor. Advances in Applied Psycholinguistics: Vol. 1. Disorders of first-language development. New York: Cambridge University Press; 1987. pp. 1–39. [Google Scholar]
- Lidsky T, Schneider JS. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain. 2003;126(1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Loban W. Language development: Kindergarten through grade twelve. (Research Report No. 18) Urbana, IL: National Council of Teachers of English; 1976. [Google Scholar]
- Luke B, Keith LG. The contribution of singletons, twins, and triplets to low birth weight, infant mortality, and handicap in the United States. Journal of Reproductive Medicine. 1992;37:661–666. [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. 2. Mahwah, NJ: Lawrence Erlbaum; 2004. [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Merriman C. The intellectual resemblance of twins. Psychological Monographs. 1924;32(5):1–58. [Google Scholar]
- Miller J. Systematic Analysis of Language Transcripts (Research Version 8.0) [Computer software] Madison, WI: University of Wisconsin; 2004. [Google Scholar]
- Miller JF, Long S, McKinley N, Thormann S, Homes MA, Nockerts A. Language Sample Analysis II: The Wisconsin Guide. Madison, WI: Wisconsin Department of Public Education; 2005. [Google Scholar]
- Neale MC, Boker SM, Xie F, Maes HH. Mx: Statistical modeling. 7. Richmond, VA: Department of Psychiatry; 2006. [Google Scholar]
- Neale M, Miller MB. The use of likelihood-based confidence intervals in genetic methods. Behavior Genetics. 1997;27:113–120. doi: 10.1023/a:1025681223921. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Boston: Kluwer Academic; 1992. [Google Scholar]
- Neale MC, Miller MM. The use of likelihood-based confidence intervals in genetic models. Behavior Genetics. 1997;27:113–120. doi: 10.1023/a:1025681223921. [DOI] [PubMed] [Google Scholar]
- Nippold MA. Later Language Development: The School-Age and Adolescent Years. 2. Austin, TX: Pro-ed; 1998. [Google Scholar]
- Paul R, Elwood TJ. Maternal linguistic input to toddlers with slow expressive language development. Journal of Speech and Hearing Research. 1991;34:982–988. doi: 10.1044/jshr.3405.982. [DOI] [PubMed] [Google Scholar]
- Petrill SA, Deater-Deckard K, Thompson LA, DeThorne LS, Schatschneider C. Reading skills in early readers: Genetic and shared environmental effects. Journal of Learning Disabilities. 2006;39:48–55. doi: 10.1177/00222194060390010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behavior: A multivariate genetic analysis. Developmental Psychology. 1996;32:590–603. [Google Scholar]
- Plomin R, Bergeman CS. The nature of nurture: Genetic influence on “environmental” measures. Behavioral and Brain Sciences. 1991;14:373–427. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction arid correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 5. New York: Worth; 2008. pp. 305–333. [Google Scholar]
- Purcell S. Statistical methods in behavioral genetics. In: Plomin R, DeFries JC, McClearn GE, McGuffin P, editors. Behavioral Genetics. 5. New York: Worth; 2008. pp. 359–410. [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rowe DC. Assessing genotype-environment interactions and correlations in the postgenomic era. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral Genetics in the Postgenomic Era. Washington, DC: American Psychological Association; 2003. pp. 71–86. [Google Scholar]
- Rowe DC, Jacobson KC, Van den Oord EJCG. Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development. 1999;70:1151–1162. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- Rutter M, Thorpe K, Greenwood R, Northstone K, Golding J. Journal of Child Psychology and Psychiatry. 2003;44:326–341. doi: 10.1111/1469-7610.00125. [DOI] [PubMed] [Google Scholar]
- Sameroff A. Early influences on development: Fact or fancy? Merrill-Palmer Quarterly. 1975;21:263–294. [Google Scholar]
- Scarr S. Developmental theories for the 1990s: Development and individual differences. Child Development. 1992;63:1–19. [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Segal NL. Entwined lives: Twins and what they tell us about human behavior. New York: Dutton; 1999. [PubMed] [Google Scholar]
- Speltz ML, DeKlyen M, Calderon R, Greenberg MT, Fisher PA. Neuropsychological characteristics and test behavior in boys with early onset conduct problems. Journal of Abnormal Psychology. 1999;108:315–325. doi: 10.1037/0021-843X.108.2.315. [DOI] [PubMed] [Google Scholar]
- Stromswold K. Why aren’t identical twins linguistically identical? Genetic, prenatal and postnatal factors. Cognition. 2006;101:333–382. doi: 10.1016/j.cognition.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale IV: Guide for administering and scoring. Itasca: Riverside Publishing; 1986. [Google Scholar]
- U.S. Census Bureau. Census 2000 data for the state of Ohio. 2000 Retrieved October 20, 2008, from http://quickfacts.census.gov/qfd/states/39000.html.
- Vreugdenhil HJI, Lanting CI, Mulder PGH, Boersma ER, Weisglas-Kuperus N. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. Journal of Pediatrics. 2002;140(1):48–56. doi: 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- Whitehurst GJ, Fischel JE, Lonigan CJ, Valdez-Menchaca MC, DeBaryshe BD, Caulfield MB. Verbal interaction in families of normal and expressive-language-delayed children. Developmental Psychology. 1988;24(5):690–699. [Google Scholar]