Abstract
Purpose
To examine the association between exposure to antidepressants and emergency department or inpatient admission for sudden cardiac death and ventricular arrhythmia (SD/VA), and to examine the impact of dose and cytochrome P-450 inhibition
Methods
A cohort study was conducted within 1999–2003 Medicaid claims data from beneficiaries of five large states, supplemented with Medicare claims for dually-eligible individuals. Exposures were prescription claims for antidepressants of interest or a reference antidepressant. Outcomes were incident first-listed emergency department or principal inpatient diagnoses indicative of SD/VA originating in the outpatient setting, an outcome previously found to have a positive predictive value of 85%.
Results
In 1.3 million person-years of antidepressant exposure, we identified 4,222 SD/VA outcomes for a rate of 3.3/1,000 person-years (95% CI, 3.2 – 3.4). Compared to paroxetine (a referent with a putatively favorable cardiovascular risk profile), adjusted hazard ratios (HRs) were 0.80 (0.67 – 0.95) for bupropion, 1.24 (0.93 – 1.65) for doxepin, 0.79 (0.55 – 1.15) for lithium, and 1.26 (1.11 – 1.42) for mirtazapine. HRs for amitriptyline, citalopram, fluoxetine, nefazodone, nortriptyline, sertraline, trazodone, and venlafaxine were near unity. For antidepressants having non-null risks (bupropion and mirtazapine), we observed no relationship with antidepressant dose and some relationships with concomitant cytochrome P-450 inhibition.
Conclusions
Of antidepressants studied, only mirtazapine had a statistically significantly greater SD/VA risk versus paroxetine. However, baseline differences between these users suggest that this finding may be attributable to residual confounding. Eleven other antidepressants had SD/VA risks no greater than that of paroxetine, thereby providing reassurance regarding the comparative cardiovascular safety of antidepressants.
Keywords: Antidepressive Agents; Death, Sudden, Cardiac; Arrhythmias, Cardiac; Medicaid; Medicare; Centers for Medicare and Medicaid Services; Cohort Studies; Pharmacoepidemiology
INTRODUCTION
Drug-induced sudden cardiac death (SD) and ventricular arrhythmia (VA) have arisen as major public health concerns in the past fifteen years, resulting in the removal of more drugs from the market than any other adverse drug effect. The University of Arizona’s Center for Education and Research on Therapeutics (CERT) names more than 90 currently available, potentially arrhythmogenic drugs based on evidence or suspicion that each drug prolongs the electrocardiographic QT interval and/or causes torsade de pointes (TdP).1 However, putative markers of risk, such as a drug’s ability to prolong the QT interval or inhibit cardiac ion channel currents in laboratory settings, are not reliable predictors of its arrhythmogenicity in clinical use.2 Therefore, pharmacoepidemiologic studies assessing arrhythmogenicity in clinical populations are critical to informing clinical and public health decisions regarding specific drugs, and to clarifying our understanding of the etiology of drug-induced arrhythmias.
While no antidepressants have been withdrawn from the United States (U.S.) market for arrhythmogenicity,3 fourteen agents have been listed by the Arizona CERT as having TdP risk.1 While case reports and studies of intermediate markers abound,2;4–8 only two prior large epidemiologic studies evaluated arrhythmogenic outcomes in antidepressant users. The first, a retrospective cohort study utilizing Tennessee Medicaid claims from 1988–1993, quantified the risk of SD in tricyclic antidepressant (TCA) and selective serotonin reuptake inhibitor (SSRI) users compared to nonusers.9 The adjusted incidence rate ratios (IRRs) for TCAs and SSRIs were 1.12 (95% confidence interval (CI), 0.89 – 1.40) and 0.95 (0.42 – 2.15), respectively. While this study was large by conventional standards (~65,000 exposed person-years), it may have still been too small to identify an increase over the low baseline incidence of SD/VA, which is about 0.5 – 2 events per thousand person-years.10–14 Furthermore, this study was limited because it did not report agent-specific risks, did not examine more recently approved antidepressants, and may have been subject to confounding by indication because of comparisons to nonusers.
A second prior epidemiologic study, a nested case-control study utilizing 1995–2005 data from a United Kingdom medical records database, quantified the risk of SD or near death among users of venlafaxine compared to fluoxetine-, citalopram-, and dosulepin-user referents.15 The adjusted odds ratios for venlafaxine compared to these agents were 0.66 (0.38 – 1.14), 0.89 (0.50 – 1.60), and 0.83 (0.46 – 1.52), respectively. These effect estimates were based on a limited number of events among venlafaxine users (N = 18). Furthermore, this study examined only four antidepressants, only three of which are available in the U.S.
Therefore, we sought to examine potential associations between 21, U.S.-available antidepressants and a composite outcome of SD/VA among administrative claims data of over 30 million enrollees. In addition to examining associations between each antidepressant and SD/VA, we sought to characterize dose-response relationships and potential associations with drugs that inhibit the metabolism of antidepressants.
METHODS
Overview and Study Population
We performed a cohort study of the comparative safety of antidepressants with regard to serious arrhythmia within a large population of Medicaid enrollees. The cohort consisted exclusively of person-time exposed to amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine, doxepin, fluoxetine, fluvoxamine, imipramine, lithium, maprotiline, mirtazapine, nefazodone, nortriptyline, paroxetine, protriptyline, sertraline, trazodone, trimipramine, or venlafaxine. To limit the potential for confounding by indication, no antidepressant-unexposed group was included. Rather, paroxetine served as the referent exposure because of its limited effect on the QT interval,16–20 even in overdose,21 its overall very favorable cardiovascular profile,17;22 and its extensive use in the general population. The methodologic decision to not include an antidepressant-unexposed group was further made to highlight the question of comparative safety – as clinicians already perceiving a need to treat their patients with an antidepressant require data to inform their selection of an agent, rather than whether or not to treat.
The data for this study included that of the Medicaid programs of California, Florida, New York, Ohio, and Pennsylvania from 1999–2003, which we obtained from the Centers for Medicare and Medicaid Services (CMS).23 These states comprise about 13 million Medicaid enrollees at any one time (~35% of the Medicaid population), and 32 million enrollees cumulatively, who contribute nearly 72 million person-years of observation. The data consist of final-action claims that have undergone quality assurance review and editing by CMS. Because 15–17% of Medicaid beneficiaries are co-enrolled in Medicare,24 our data include Medicare claims for all dually-eligible persons in these states to ensure a more complete picture of their medical care. We previously performed quality assurance analyses of the linked Medicaid and Medicare data, the results of which suggested that the data are of high quality.25
This study was approved by the University of Pennsylvania’s Committee on Studies Involving Human Beings, which granted waivers of informed consent and of Health Insurance Portability and Accountability Act authorization.
Eligible Person-time
We included all person-time beginning with a prescription for a study drug and ending with the earliest of the prescription claim’s days supply field, 30 days, or filling a subsequent prescription for a study drug, provided that no diagnosis code for the outcome of interest (listed below) had yet been observed in any claim type for that enrollee. We assumed that each prescription lasted for a maximum of 30 days because Medicaid prescriptions for these drugs in these states tend to be dispensed in 30-day increments, as we confirmed by examining frequency distributions of the days supply and the number of days between subsequent prescriptions for the same enrollee. We excluded person-time defined by prescriptions for multiple study drugs filled on the same date.
Beneficiaries were eligible to contribute antidepressant prescriptions to one or more of the 21 study drug groups named above, provided that the beneficiary was an incident user of the respective antidepressant. An incident user was defined as a beneficiary without a prescription claim for the given antidepressant in the six months prior to the prescription claim of interest.
We performed secondary analyses excluding person-time contributed by a) second and later prescriptions for a given agent, to elucidate the effect in the initial prescription, b) enrollees in managed care plans, because data for these persons may be incomplete, and c) enrollees with cancer, as such persons may be different from the general population in ways not reflected in claims data.26
Ascertainment of Exposure, Dose, and Covariates
Eligible study time was considered exposed to one of the 21 antidepressants named above. The exposure variable was therefore determined by the identity of the drug for the prescription that contributed the relevant person-time. This was done by mapping the National Drug Code (NDC) of each prescription claim of interest to a commercially-available NDC look-up database (Lexicon; Cerner Multum, Inc.: Kansas City, Missouri)
Daily dose was calculated assuming that the prescription was consumed over the days supply, and categorized separately for each antidepressant into quartiles, or tertiles when necessary because of a low number of events.
As the concomitant administration of antidepressants and metabolic clearance inhibitors may lead to functional high dose exposures of antidepressants, and the risk of QT interval prolongation or TdP may be more likely in this setting,8 we examined the association between each antidepressant and SD/VA in persons receiving cytochrome P-450 (CYP) 1A2, 2B6, 2C19, 2C9, 2D6, and 3A4 inhibitors. Each of these isozymes affects the metabolism of at least one of the antidepressants of interest (see appendix Table A1). As lithium is not metabolized by the liver, we examined agents that would be expected to modify the renal clearance of this agent. To isolate an effect of CYP inhibition (or renal clearance inhibition) on the antidepressant’s pharmacokinetics rather than a direct arrhythmogenic effect the CYP inhibitor itself, we considered CYP inhibitors with known or suspected arrhythmogenic effects separately from those having no such effects.
We defined three types of potential confounding variables (based on chronic illnesses, posited risk factors for SD/VA, association with indications for antidepressant therapy, etc.): a) chronic diseases, defined as a diagnosis ever before the current study prescription; b) drug markers of chronic disease, defined as a prescription ever before the current study prescription; and c) current drugs, defined as a prescription in the 28 days prior through 10 days after the current study prescription. Codes are available from the authors.
Ascertainment of Outcome
As our goal was to study the effects of antidepressants in an ambulatory population, our outcome of interest was an outpatient-originating SD/VA event precipitating hospital presentation. Incident outcomes were identified in ED and inpatient Medicaid and Medicare claims having at least one of the following discharge International Classification of Diseases 9th Revision (ICD-9) diagnoses in a first-listed or principal position: paroxysmal ventricular tachycardia (427.1), ventricular fibrillation and flutter (427.4), ventricular fibrillation (427.41), ventricular flutter (427.42), cardiac arrest (427.5), sudden death (798), instantaneous death (798.1), or death occurring in less than 24 hours from onset of symptoms, not otherwise explained (798.2). We previously validated this outcome by reviewing primary medical records in a random sample of 116 CMS beneficiaries with such diagnoses. The positive predictive value for identifying outpatient-originating SD/VA not due to extrinsic causes was 85% (95% CI, 78% – 91%).27
Statistical Analysis
We first calculated unadjusted incidence rates (with 95% CIs) for each antidepressant. For drugs with at least 30 SD/VA events, we then used proportional hazards regression models to calculate adjusted hazard ratios (HRs) for the associations between antidepressants of interest and the outcome, using paroxetine as the referent. Potential confounding factors were evaluated using a change-in-estimate approach, and were included in the adjusted model if the covariate’s introduction changed the HR by ≥ 10%. To assess for covariates that in-and-of themselves had little effect on the HR, but when combined with other factors had a considerable influence on the point estimate, we utilized a forward-stepwise confounder selection approach to confirm the results of the change-in-estimate model. Analyses were conducted using SAS v9.1 (SAS Institute, Inc.: Cary, North Carolina) and Stata v9.2 (StataCorp LP: College Station, Texas).
Role of the Funding Source
This study was supported by R01HL076697 and KL2RR024132 from the National Institutes of Health. Other than suggestions made by peer reviewers during the grant review process, the funding sources had no role in the study’s design, conduct, or interpretation.
RESULTS
We identified 3,397,470 antidepressant users contributing 1,287,446 person-years of observation during which 4,222 incident SD/VAs occurred. Table 1 shows the unadjusted incidence rates of SD/VA among users of each antidepressant. The incidence rate among antidepressant-exposed beneficiaries was 3.28 (95% CI, 3.18 – 3.38) per 1,000 person-years, ranging from 0 per 1,000 for protriptyline and maprotiline to 12.64 (0.32 – 70.42) per 1,000 for trimipramine. Unadjusted IRRs, using paroxetine as the referent, are also presented in Table 1. As eight of the 21 antidepressants had < 30 events, they were excluded from further, multivariable analyses.
TABLE 1.
Unadjusted incidence rate of sudden death and ventricular arrhythmia (as a first-listed emergency department or principal inpatient diagnosis) in beneficiaries exposed to antidepressants, by pharmacologic class
| Drug | Users | Person-years | SD/VA events |
Crude incidence rate per 1,000 person- years (95% CI) |
Unadjusted incidence rate ratio versus paroxetine (95% CI) |
|---|---|---|---|---|---|
| TRICYCLIC (2° and 3° AMINES) | |||||
| amitriptyline | 354,298 | 106,373 | 322 | 3.03 (2.71–3.38) | 0.96 (0.84–1.10) |
| amoxapine | 676 | * | * | 9.20 (1.11–33.23) | 2.93 (0.35–10.60) |
| clomipramine | 6,815 | * | * | 2.20 (0.81–4.79) | 0.70 (0.26–1.53) |
| desipramine | 13,232 | 3,499 | 16 | 4.57 (2.61–7.43) | 1.45 (0.83–2.37) |
| doxepin | 52,638 | 13,843 | 54 | 3.90 (2.93–5.09) | 1.24 (0.92–1.63) |
| imipramine | 58,027 | 18,375 | 24 | 1.31 (0.84–1.94) | 0.41 (0.26–0.62) |
| nortriptyline | 91,071 | 25,229 | 95 | 3.77 (3.05–4.60) | 1.20 (0.96–1.48) |
| protriptyline | 1,592 | 375 | 0 | - | - |
| trimipramine | 287 | * | * | 12.64 (0.32–70.42) | 4.02 (0.10–22.45) |
| TETRACYCLIC | |||||
| maprotiline | 307 | 94 | 0 | - | - |
| mirtazapine | 214,072 | 84,230 | 546 | 6.48 (5.95–7.05) | 2.06 (1.84–2.30) |
| TRIAZOLOPYRIDINE | |||||
| trazodone | 282,980 | 81,776 | 282 | 3.45 (3.06–3.88) | 1.09 (0.95–1.26) |
| AMINOKETONE | |||||
| bupropion | 340,163 | 99,205 | 165 | 1.66 (1.42–1.94) | 0.53 (0.44–0.63) |
| PHENETHYLAMINE | |||||
| venlafaxine | 225,379 | 89,218 | 289 | 3.24 (2.88–3.64) | 1.03 (0.90–1.18) |
| PHENYLPIPERAZINE | |||||
| nefazodone | 70,794 | 26,517 | 60 | 2.26 (1.73–2.91) | 0.72 (0.54–0.93) |
| SELECTIVE SEROTONIN REUPTAKE INHIBITOR | |||||
| citalopram | 294,434 | 134,210 | 484 | 3.61 (3.29–3.94) | 1.14 (1.02–1.28) |
| fluoxetine | 301,585 | 130,795 | 367 | 2.81 (2.53–3.11) | 0.89 (0.78–1.01) |
| fluvoxamine | 23,842 | 12,249 | 27 | 2.20 (1.45–3.21) | 0.70 (0.46–1.03) |
| paroxetine | 560,822 | 247,595 | 780 | 3.15 (2.93–3.38) | reference |
| sertraline | 452,216 | 189,394 | 669 | 3.53 (3.27–3.81) | 1.12 (1.01–1.24) |
| ANTI-BIPOLAR | |||||
| lithium | 52,240 | 21,444 | 33 | 1.54 (1.06–2.16) | 0.49 (0.33–0.69) |
omitted per Centers for Medicare and Medicaid Services privacy requirement
Table 2 shows characteristics of users of the remaining 13 antidepressants. Users were predominantly female and non-elders, and included considerable numbers of minorities, as is representative of the Medicaid population under study. Compared to the other antidepressants, a larger proportion of mirtazapine users were elderly (35.9%). The proportion of antidepressant prescriptions dispensed while a beneficiary was a nursing home resident was generally low across different users (2.9% to 15.6%) except for mirtazapine, in which 31.6% of its prescriptions were in such beneficiaries, consistent with its more frequent use in elders. Chronic disease diagnoses were also generally consistent across users, except for some diagnoses capturing specific indications of a particular antidepressant. For instance, 66.1% of lithium’s prescriptions were accompanied by a prior diagnosis of bipolar disorder, as lithium is principally used for this condition. In contrast, no more than 20% of any other antidepressant’s prescriptions were accompanied by such a diagnosis. Furthermore, given the age distribution of its users, mirtazapine prescriptions had the highest proportions of the following prior chronic disease diagnoses versus other antidepressants: anemia, arrhythmia/conduction disorder, cancer, cerebrovascular disease, coronary artery disease, enuresis, heart failure/cardiomyopathy, hypertension, hypothyroidism, kidney disease, organic psychosis, pulmonary circulation disease, and valvular heart disease. Drug markers of chronic disease exhibited a similar pattern. Current use of drugs was generally consistent across users.
TABLE 2.
Characteristics of beneficiaries filling prescriptions for antidepressants with sufficient numbers of events to permit their study
| amitriptyline | bupropion | citalopram | doxepin | fluoxetine | lithium | mirtazapine | nefazodone | nortriptyline | paroxetine | sertraline | trazodone | venlafaxine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| User-level variables | |||||||||||||
| users | 354,298 | 340,163 | 294,434 | 52,638 | 301,585 | 52,240 | 214,072 | 70,794 | 91,071 | 560,822 | 452,216 | 282,980 | 225,379 |
| female sex | 71.9% | 63.3% | 71.9% | 66.6% | 71.8% | 55.8% | 65.0% | 68.5% | 74.2% | 71.4% | 71.4% | 66.2% | 73.2% |
| age in years < 35 35 to 44 45 to 54 55 to 64 65 to 74 ≥ 75 |
20.5% 21.2% 19.7% 14.6% 13.7% 10.3% |
38.6% 23.1% 18.2% 10.9% 5.8% 3.4% |
29.4% 19.3% 16.4% 11.0% 9.8% 14.1% |
18.9% 23.7% 20.6% 13.0% 11.9% 11.9% |
36.9% 19.7% 15.5% 9.8% 8.5% 9.7% |
50.8% 24.2% 15.3% 5.8% 2.4% 1.5% |
21.7% 16.6% 15.4% 10.5% 10.1% 25.8% |
28.2% 26.3% 21.3% 11.3% 6.4% 6.5% |
20.2% 19.5% 18.7% 14.2% 14.3% 13.0% |
32.0% 18.7% 15.3% 10.8% 11.0% 12.2% |
36.3% 17.3% 14.3% 10.2% 9.5% 12.4% |
25.6% 23.1% 19.5% 11.5% 8.9% 11.3% |
31.6% 23.0% 18.6% 10.7% 7.5% 8.6% |
| race white non-white |
42.8% 57.2% |
63.4% 36.7% |
60.2% 39.8% |
53.2% 46.9% |
55.9% 44.1% |
64.5% 35.6% |
62.5% 37.5% |
57.1% 42.9% |
46.7% 53.3% |
52.4% 47.6% |
55.6% 44.4% |
56.0% 44.0% |
64.0% 36.0% |
| Prescription-level variables | |||||||||||||
| prescriptions | 1,459,479 | 1,484,516 | 1,929,663 | 208,576 | 1,823,463 | 348,998 | 1,307,780 | 382,916 | 355,657 | 3,385,018 | 2,708,288 | 1,298,668 | 1,352,882 |
| nursing home residence | 2.9% | 4.3% | 15.6% | 6.2% | 9.5% | 6.9% | 31.6% | 8.0% | 9.3% | 9.3% | 12.9% | 14.2% | 11.3% |
| Diagnoses ever in past | |||||||||||||
| ADHD | 1.0% | 13.4% | 3.2% | 1.8% | 4.1% | 16.6% | 5.8% | 3.6% | 2.4% | 3.3% | 5.2% | 5.0% | 3.6% |
| anxiety/panic disorder/OCD | 28.2% | 36.4% | 43.0% | 38.0% | 36.4% | 33.6% | 40.5% | 43.6% | 29.7% | 42.8% | 40.0% | 37.3% | 48.1% |
| alcohol abuse | 8.5% | 11.6% | 10.3% | 13.5% | 9.0% | 14.9% | 11.5% | 11.9% | 6.3% | 8.5% | 8.7% | 14.9% | 11.1% |
| anemia | 30.8% | 23.2% | 36.2% | 32.8% | 29.7% | 24.3% | 43.6% | 26.4% | 32.0% | 32.7% | 33.3% | 32.6% | 32.0% |
| arrhythmia/conduction disorder | 17.5% | 13.6% | 24.0% | 19.6% | 17.1% | 12.6% | 29.0% | 15.2% | 19.4% | 20.7% | 21.5% | 20.1% | 19.7% |
| asthma/COPD | 34.7% | 35.9% | 36.2% | 39.7% | 32.3% | 29.3% | 39.0% | 33.7% | 32.5% | 34.6% | 34.4% | 36.7% | 36.4% |
| bulimia nervosa | 0.2% | 0.4% | 0.4% | 0.2% | 0.5% | 0.4% | 0.3% | 0.4% | 0.2% | 0.3% | 0.3% | 0.4% | 0.6% |
| bipolar disorder | 5.1% | 19.2% | 14.6% | 12.2% | 12.1% | 66.1% | 14.1% | 16.9% | 6.3% | 10.0% | 11.0% | 17.0% | 17.9% |
| cancer | 20.7% | 17.8% | 24.3% | 22.6% | 18.6% | 14.2% | 26.3% | 16.8% | 22.2% | 20.6% | 22.6% | 21.3% | 23.7% |
| cerebrovascular disease | 17.2% | 10.9% | 23.9% | 17.4% | 16.7% | 7.1% | 31.0% | 14.1% | 21.8% | 20.1% | 21.7% | 19.3% | 18.6% |
| coronary artery disease | 26.7% | 18.8% | 31.0% | 26.8% | 22.6% | 10.6% | 35.5% | 20.5% | 28.3% | 28.0% | 28.2% | 25.3% | 25.3% |
| depression | 37.7% | 61.9% | 70.8% | 52.4% | 63.4% | 60.9% | 70.4% | 72.5% | 45.6% | 60.8% | 64.8% | 58.6% | 75.3% |
| diabetes mellitus | 35.9% | 21.7% | 31.3% | 28.7% | 25.7% | 16.9% | 30.8% | 23.1% | 32.6% | 28.7% | 29.1% | 27.7% | 28.2% |
| enuresis | 8.5% | 8.5% | 13.2% | 9.5% | 10.5% | 9.5% | 14.6% | 8.5% | 10.7% | 11.2% | 11.2% | 11.8% | 11.9% |
| headache | 37.0% | 26.9% | 30.5% | 32.7% | 28.1% | 23.5% | 27.5% | 29.8% | 43.5% | 29.7% | 28.6% | 30.0% | 34.0% |
| HIV/AIDS | 6.9% | 5.9% | 5.3% | 7.2% | 4.2% | 3.9% | 4.6% | 5.2% | 4.4% | 4.4% | 4.5% | 5.8% | 4.2% |
| HF/cardiomyopathy | 17.0% | 11.3% | 22.1% | 17.1% | 15.3% | 5.8% | 27.7% | 12.2% | 18.8% | 18.5% | 20.0% | 17.9% | 16.9% |
| hypercholesterolemia | 38.9% | 31.1% | 38.1% | 37.1% | 32.2% | 24.4% | 32.2% | 31.8% | 36.9% | 36.9% | 35.0% | 32.8% | 36.6% |
| hypertension | 54.9% | 37.7% | 53.8% | 53.0% | 44.2% | 28.1% | 57.6% | 43.3% | 54.6% | 50.5% | 50.0% | 49.2% | 48.2% |
| hypothyroidism | 16.4% | 15.7% | 22.7% | 18.7% | 18.3% | 21.5% | 23.9% | 16.5% | 17.9% | 19.2% | 19.3% | 18.9% | 21.9% |
| insomnia | 12.1% | 7.3% | 8.7% | 13.9% | 7.8% | 6.3% | 11.3% | 9.4% | 10.6% | 9.2% | 8.2% | 15.6% | 10.6% |
| irritable bowel syndrome | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | - | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| kidney disease | 19.7% | 13.2% | 20.5% | 19.8% | 16.0% | 8.7% | 23.5% | 13.3% | 19.8% | 17.8% | 19.2% | 17.9% | 17.9% |
| liver disease | 19.1% | 16.8% | 18.7% | 22.1% | 16.7% | 18.6% | 18.1% | 16.9% | 16.7% | 17.3% | 16.9% | 20.1% | 18.2% |
| neuropathic pain | 37.0% | 20.2% | 25.6% | 30.1% | 20.9% | 11.6% | 23.3% | 20.6% | 37.3% | 23.3% | 22.8% | 23.4% | 26.9% |
| neutropenia | 1.5% | 1.2% | 1.8% | 2.0% | 1.3% | 2.4% | 1.8% | 1.2% | 1.3% | 1.5% | 1.5% | 1.7% | 1.5% |
| obesity | 11.8% | 14.3% | 14.0% | 11.4% | 13.0% | 13.7% | 8.0% | 11.5% | 10.6% | 11.6% | 12.5% | 12.2% | 15.8% |
| organic psychosis | 6.4% | 8.1% | 20.8% | 10.4% | 12.8% | 12.3% | 35.6% | 11.8% | 12.1% | 13.4% | 16.8% | 19.3% | 15.8% |
| premenstrual tension syndrome | 0.3% | 0.5% | 0.5% | 0.4% | 1.1% | 0.6% | 0.2% | 0.5% | 0.4% | 0.4% | 0.5% | 0.4% | 0.7% |
| PTSD | 1.6% | 4.5% | 3.5% | 2.8% | 3.0% | 6.8% | 3.4% | 5.2% | 2.1% | 2.6% | 3.6% | 3.7% | 4.7% |
| pulmonary circulation disease | 3.4% | 3.0% | 4.5% | 3.9% | 3.0% | 1.5% | 4.7% | 2.4% | 3.3% | 3.5% | 4.0% | 3.6% | 3.8% |
| Raynaud’s syndrome | 0.5% | 0.4% | 0.4% | 0.4% | 0.3% | 0.2% | 0.4% | 0.2% | 0.5% | 0.4% | 0.4% | 0.4% | 0.4% |
| rheumatoid arthritis | 25.5% | 14.8% | 21.2% | 23.4% | 17.8% | 9.4% | 22.0% | 17.7% | 24.8% | 20.6% | 19.4% | 19.6% | 20.9% |
| symptomatic menopausal state | 8.8% | 6.9% | 8.1% | 8.7% | 7.2% | 4.6% | 5.9% | 7.6% | 8.7% | 7.6% | 7.0% | 7.1% | 9.5% |
| schizophrenic disorders | 5.6% | 17.4% | 17.6% | 14.7% | 16.5% | 45.5% | 17.1% | 20.3% | 6.5% | 14.0% | 13.6% | 21.4% | 17.1% |
| smoking | 11.5% | 17.6% | 12.1% | 15.4% | 10.4% | 14.5% | 12.6% | 11.1% | 8.9% | 10.4% | 10.6% | 15.0% | 14.5% |
| substance abuse | 19.5% | 27.7% | 19.7% | 27.2% | 18.3% | 28.5% | 20.3% | 22.4% | 13.5% | 17.2% | 17.3% | 26.8% | 23.1% |
| Tourette’s disorder | 0.1% | 0.3% | 0.2% | 0.1% | 0.2% | 0.5% | 0.1% | 0.2% | 0.1% | 0.2% | 0.2% | 0.2% | 0.2% |
| valvular heart disease | 12.8% | 9.3% | 16.0% | 13.9% | 11.6% | 6.0% | 16.8% | 9.9% | 13.0% | 14.5% | 14.5% | 12.4% | 13.1% |
| Drugs used ever in past | |||||||||||||
| ACEI/ATIIRB | 34.9% | 21.2% | 31.2% | 29.5% | 25.1% | 10.0% | 30.6% | 22.1% | 32.7% | 29.7% | 29.4% | 26.6% | 26.7% |
| adrenergic bronchodilator (inhaled and oral) | 34.0% | 36.2% | 34.1% | 36.5% | 31.1% | 26.1% | 34.3% | 32.4% | 31.6% | 31.9% | 33.1% | 34.8% | 35.8% |
| anorexiant/anti-obesity agent | 0.8% | 8.0% | 2.2% | 1.4% | 2.7% | 10.1% | 4.5% | 2.4% | 1.8% | 2.0% | 3.3% | 3.6% | 2.8% |
| antiadrenergic, centrally-and peripherally-acting, non-α1 selective antagonists | 7.2% | 9.3% | 7.2% | 7.7% | 6.5% | 12.6% | 9.8% | 6.4% | 7.0% | 6.7% | 7.7% | 8.9% | 6.8% |
| antiarrhythmic, class I (oral; excluding phenytoin) | 0.5% | 0.3% | 0.6% | 0.8% | 0.4% | 0.1% | 0.7% | 0.4% | 0.6% | 0.6% | 0.6% | 0.5% | 0.5% |
| antiarrhythmic, class III (oral) | 0.7% | 0.4% | 1.1% | 0.7% | 0.7% | 0.1% | 1.2% | 0.4% | 0.7% | 0.9% | 0.9% | 0.7% | 0.7% |
| antidiabetic agent | 27.0% | 13.8% | 19.4% | 17.8% | 16.5% | 8.3% | 17.7% | 14.3% | 22.6% | 17.8% | 18.5% | 17.3% | 17.6% |
| β-blocker, systemic | 24.7% | 16.6% | 24.3% | 23.5% | 19.2% | 14.1% | 23.3% | 19.1% | 27.0% | 22.8% | 22.4% | 21.8% | 21.6% |
| CCB, non-verapamil | 24.4% | 14.2% | 22.6% | 21.7% | 17.7% | 6.7% | 23.1% | 16.5% | 24.7% | 22.0% | 21.4% | 19.2% | 18.1% |
| CCB, verapamil | 4.6% | 3.0% | 4.0% | 4.6% | 3.3% | 1.7% | 3.9% | 3.5% | 5.2% | 3.9% | 3.7% | 3.7% | 4.0% |
| corticosteroid (inhaled) | 15.5% | 16.6% | 14.6% | 17.2% | 13.1% | 9.9% | 12.6% | 14.4% | 14.5% | 14.0% | 13.9% | 14.9% | 15.9% |
| corticosteroid (oral) | 24.2% | 21.4% | 21.6% | 29.0% | 18.7% | 13.3% | 20.0% | 18.0% | 23.1% | 19.7% | 20.8% | 21.0% | 24.0% |
| digoxin | 4.6% | 2.7% | 6.6% | 4.8% | 4.6% | 0.8% | 9.9% | 3.6% | 4.8% | 5.6% | 6.1% | 5.1% | 4.7% |
| diuretic, loop | 19.0% | 12.5% | 21.8% | 19.6% | 15.9% | 5.7% | 25.4% | 13.2%) | 19.0% | 17.4% | 19.4% | 18.6% | 18.4% |
| diuretic, thiazide | 22.8% | 14.7% | 20.6% | 21.4% | 16.6% | 6.9% | 18.3% | 15.9% | 22.5% | 19.5% | 19.2% | 18.1% | 18.8% |
| immunosuppressants used for organ transplantation | 1.0% | 0.6% | 0.9% | 1.1% | 0.6% | 0.1% | 0.5% | 0.4% | 1.0% | 0.6% | 0.8% | 0.6% | 0.8% |
| lipid-lowering agent | 29.4% | 20.5% | 25.5% | 24.8% | 21.3% | 11.4% | 20.4% | 21.1% | 27.4% | 25.0% | 23.9% | 21.3% | 24.2% |
| nitrate | 15.2% | 9.8% | 16.4% | 14.2% | 11.9% | 3.0% | 18.2% | 11.0% | 16.2% | 14.8% | 15.0% | 13.2% | 13.0% |
| thyroid hormone | 9.4% | 9.5% | 13.1% | 11.0% | 10.8% | 15.0% | 13.9% | 9.8% | 10.5% | 10.6% | 11.1% | 11.3% | 13.4% |
| vasodilator, non-nitrate | 1.0% | 0.4% | 1.0% | 0.9% | 0.7% | 0.1% | 1.0% | 0.4% | 0.9% | 0.8% | 1.0% | 0.78% | 0.6% |
| warfarin | 4.8% | 3.2% | 6.7% | 4.8% | 4.4% | 1.2% | 8.0% | 3.1% | 5.3% | 4.9% | 5.9% | 5.0% | 5.4% |
| xanthine derivative | 4.4% | 3.9% | 3.9% | 5.1% | 3.6% | 1.7% | 3.9% | 4.2% | 3.9% | 4.2% | 3.9% | 4.1% | 4.0% |
| Drugs used currently | |||||||||||||
| adrenergic bronchodilator (inhaled and oral)* | 11.8% | 13.0% | 11.0% | 12.6% | 9.9% | 6.5% | 10.9% | 10.6% | 10.3% | 10.6% | 10.9% | 11.6% | 11.2% |
| amantadine/foscarnet | 0.2% | 0.3% | 0.5% | 0.3% | 0.4% | 0.8% | 0.5% | 0.5% | 0.4% | 0.3% | 0.4% | 0.6% | 0.4% |
| antiarrhythmic, class Ia | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| antiarrhythmic, classes Ib/c* | 0.1% | <0.1% | 0.1% | 0.1% | <0.1% | <0.1% | 0.1% | <0.1% | 0.1% | 0.1% | <0.1% | 0.1% | 0.1% |
| antiarrhythmic, class III* | 0.3% | 0.2% | 0.6% | 0.4% | 0.3% | <0.1% | 0.7% | 0.2% | 0.4% | 0.5% | 0.5% | 0.4% | 0.4% |
| antiemetic 5-hydroxytryptamine3 receptor antagonist | 0.2% | 0.1% | 0.2% | 0.2% | 0.1% | 0.1% | 0.2% | 0.2% | 0.2% | 0.1% | 0.2% | 0.2% | 0.2% |
| antipsychotic | 11.7% | 31.0% | 32.0% | 21.5% | 28.6% | 70.7% | 34.2% | 37.2% | 13.2% | 25.0% | 26.3% | 34.9% | 32.7% |
| arsenic trioxide | - | - | - | - | - | - | - | - | - | - | <0.01% | - | - |
| aspirin | 6.8% | 4.0% | 5.7% | 4.5% | 4.3% | 1.6% | 4.7% | 3.9% | 6.8% | 5.4% | 5.7% | 4.9% | 4.4% |
| azole antifungal | 2.2% | 1.9% | 1.8% | 2.6% | 1.7% | 1.0% | 1.5% | 1.7% | 1.7% | 1.5% | 1.6% | 1.8% | 2.0% |
| β-blocker, systemic | 13.6% | 8.9% | 13.7% | 12.0% | 10.3% | 6.8% | 13.3% | 9.6% | 14.9% | 12.4% | 12.8% | 11.8% | 11.5% |
| CCB* | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| CCB, non-verapamil | 13.8% | 7.9% | 12.9% | 11.4% | 9.9% | 3.3% | 13.3% | 9.0% | 14.3% | 12.5% | 12.4% | 10.8% | 10.0% |
| CCB, verapamil | 2.0% | 1.3% | 1.7% | 1.8% | 1.4% | 0.6% | 1.7% | 1.5% | 2.3% | 1.6% | 1.6% | 1.6% | 1.8% |
| chloral hydrate | 0.1% | 0.1% | 0.1% | 0.2% | 0.1% | 0.2% | 0.1% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| cisapride | 0.2% | 0.1% | 0.1% | 0.2% | 0.1% | <0.1% | 0.1% | 0.1% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% |
| clindamycin | 0.5% | 0.4% | 0.5% | 0.5% | 0.4% | 0.4% | 0.4% | 0.4% | 0.5% | 0.4% | 0.4% | 0.5% | 0.5% |
| cyclooxygenase-2 inhibitor | 17.1% | 10.2% | 13.3% | 13.7% | 10.9% | 3.8% | 12.7% | 12.2% | 16.9% | 12.3% | 12.5% | 11.8% | 13.5% |
| diuretic, loop | 9.3% | 6.1% | 12.1% | 9.6% | 8.2% | 2.2% | 14.9% | 6.6% | 9.8% | 8.8% | 10.8% | 9.9% | 9.4% |
| diuretic, potassium-sparing | 3.7% | 2.4% | 3.3% | 3.8% | 2.8% | 1.0% | 3.4% | 2.5% | 3.4% | 2.9% | 3.1% | 3.3% | 3.2% |
| diuretic, thiazide | 10.3% | 6.4% | 8.9% | 8.5% | 7.1% | 2.3% | 7.4% | 6.6% | 10.0% | 8.3% | 8.4% | 7.4% | 7.9% |
| droperidol | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| ephedrine/PPA/PSE | 3.9% | 3.6% | 3.1% | 3.6% | 3.1% | 2.3% | 2.5% | 4.1% | 3.9% | 3.0% | 3.1% | 3.5% | 3.4% |
| epinephrine | 0.1% | 0.1% | 0.1% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| famotidine | 3.1% | 2.2% | 3.2% | 3.3% | 2.9% | 1.7% | 4.2% | 3.0% | 3.2% | 3.3% | 3.0% | 3.2% | 2.8% |
| felbamate/fosphenytoin | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| galantamine | 0.1% | 0.1% | 0.4% | 0.1% | 0.2% | 0.1% | 0.7% | 0.1% | 0.1% | 0.2% | 0.3% | 0.2% | 0.3% |
| (hydroxy)chloroquine/mefloquine | 0.8% | 0.3% | 0.4% | 0.6% | 0.3% | 0.1% | 0.3% | 0.3% | 0.6% | 0.3% | 0.3% | 0.4% | 0.5% |
| hydroxyzine | 3.1% | (3.4% | 3.5% | 8.9% | 3.1% | 2.9% | 3.6% | 4.5% | 3.0% | 3.1% | 3.1% | 4.2% | 3.8% |
| macrolide antibiotic | 4.6% | 4.5% | 4.2% | 5.2% | 3.9% | 3.0% | 3.7% | 4.0% | 3.8% | 4.0% | 4.2% | 4.3% | 4.5% |
| magnesium supplement | 0.4% | 0.2% | 0.4% | 0.4% | 0.3% | 0.1% | 0.5% | 0.2% | 0.4% | 0.2% | 0.4% | 0.4% | 0.4% |
| meperidine | 0.2% | 0.1% | 0.1% | 0.2% | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| methadone | 0.8% | 0.4% | 0.4% | 1.0% | 0.3% | 0.2% | 0.5% | 0.4% | 0.8% | 0.3% | 0.3% | 0.6% | 0.6% |
| NSAID | 18.6% | 11.2% | 9.7% | 13.3% | 11.3% | 6.8% | 7.7% | 12.4% | 16.3% | 11.2% | 10.0% | 12.2% | 11.6% |
| octreotide | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| pentamidine | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| phentermine/sibutramine | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% | <0.1% |
| potassium supplement | 5.4% | 3.9% | 7.7% | 6.0% | 5.3% | 1.7% | 10.2% | 4.6% | 6.1% | 5.6% | 7.0% | 6.4% | 6.5% |
| quinine | 1.7% | 0.6% | 0.9% | 1.5% | 0.8% | 0.1% | 0.9% | 0.7% | 1.3% | 0.9% | 0.8% | 1.0% | 0.8% |
| quinolone antibiotic | 5.0% | 4.1% | 5.5% | 5.5% | 4.5% | 2.3% | 6.9% | 4.3% | 4.9% | 4.8% | 5.1% | 5.1% | 5.4% |
| sildenafil | 1.5% | 1.4% | 0.8% | 1.5% | 0.8% | 0.4% | 0.7% | 1.1% | 0.8% | 0.9% | 0.9% | 0.9% | 0.7% |
| tacrolimus | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | <0.1% | 0.1% | <0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| tamoxifen | 0.3% | 0.3% | 0.4% | 0.4% | 0.3% | 0.1% | 0.5% | 0.3% | 0.4% | 0.4% | 0.5% | 0.3% | 0.6% |
| tizanidine | 1.1% | 0.7% | 0.7% | 1.2% | 0.5% | 0.3% | 0.8% | 0.6 | 1.3% | 0.5% | 0.7% | 0.8% | 1.1% |
| trimethoprim-sulfamethoxazole | 3.5% | 2.2% | 2.6% | 3.3% | 2.3% | 1.5% | 3.0% | 2.4% | 2.7% | 2.3% | 2.4% | 2.9% | 2.4% |
limited to agents posited to prolong the QT interval
ADHD = attention deficit hyperactivity disorder; OCD = obsessive-compulsive disorder; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; AIDS = acquired immune deficiency syndrome; HF = heart failure; PTSD = post-traumatic stress disorder; ACEI = angiotensin-converting enzyme inhibitor; ATIIRB = angiotensin-II receptor blocker; CCB = calcium channel blocker; PPA = phenylpropanolamine; PSE = pseudoephedrine; NSAID = nonsteroidal anti-inflammatory drug
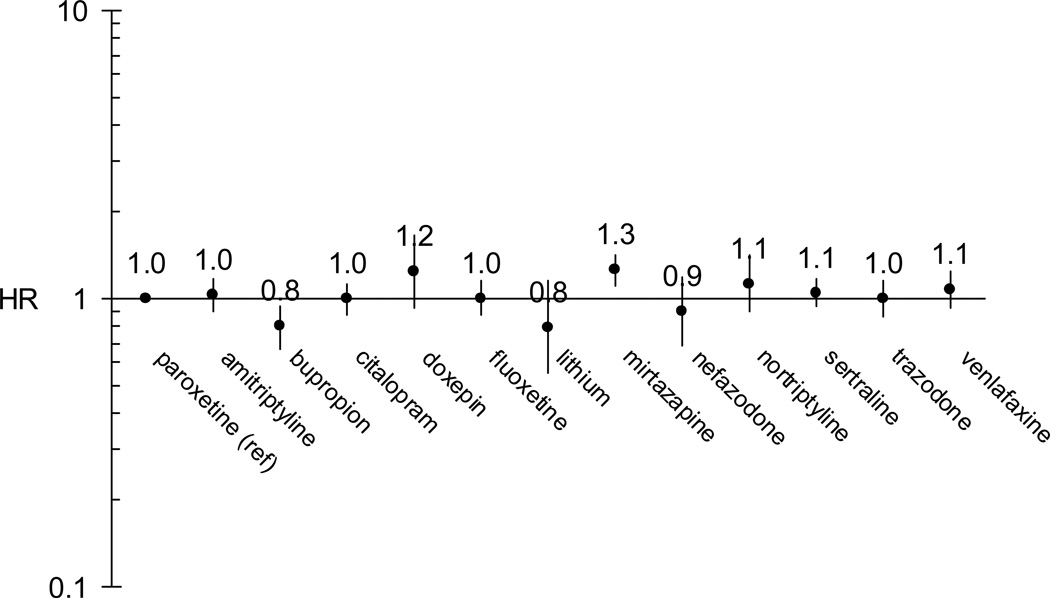
Figure 1 shows HRs for each antidepressant in a model adjusted for age, sex, race, state, nursing home residence, an ever-past diagnosis of bipolar disorder, and ever-past use of an angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker. The former five covariates were forced into each model while the latter two were identified as confounders via the change-in-estimate approach. Compared to paroxetine, mirtazapine had an elevated HR of 1.26 (1.11 – 1.42) and bupropion had a reduced HR of 0.80 (0.67 – 0.95). HRs for the remaining antidepressants were not statistically significantly different from that of paroxetine, although doxepin’s point estimate (HR = 1.24, 0.93 – 1.65) was nearly identical to that of mirtazapine. The forward-stepwise confounder selection model results were very similar to the change-in-estimate confounder selection results above, and are therefore not presented.
FIGURE 1.
Adjusted* hazard ratios for SD/VA associated with antidepressant exposure, using paroxetine as the reference
*adjusted for age, sex, race, state, nursing home residence, an ever-past bipolar disorder diagnosis, and an ever-past angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker prescription
A secondary analysis including person-time of only the first prescription for a given agent found no elevated risks with mirtazapine (HR = 1.01, 0.77 – 1.33) or doxepin (HR = 0.80, 0.43 – 1.47), in a model adjusted for the same covariates listed above. HRs for other antidepressants were slightly attenuated, but similar to the primary analysis. Other secondary analyses, excluding enrollees in managed care plans and excluding those with cancer, yielded point estimates generally similar to those of the primary analysis. In particular, the HRs for mirtazapine remained elevated at 1.35 (1.07 – 1.71) and 1.27 (1.09 – 1.48), respectively. The HRs for doxepin were 0.91 (0.48 – 1.72) and 1.24 (0.86 – 1.78), respectively. The reduced hazard associated with bupropion was no longer statistically significant, with HRs of 0.73 (0.52 – 1.02) and 0.88 (0.71 – 1.09), respectively.
As dose and clearance inhibitor analyses were intended to confirm statistically elevated or protective risks demonstrated in the primary analysis, we report only the effect estimates for mirtazapine and bupropion. In a model adjusted for age, sex, race, state, nursing home residence, number of prior antidepressant prescriptions, and an ever-past substance abuse diagnosis, HRs for the second through fourth mirtazapine dose quartiles (i.e., > 15 and ≤ 22.5 mg; > 22.5 and ≤ 30 mg; > 30 mg) were 0.81 (0.49 – 1.35), 1.05 (0.86 – 1.28), and 0.95 (0.70 – 1.29) respectively, compared to the lowest dose quartile (i.e., ≤ 15 mg). In a model adjusted for the same covariates, HRs for the second and third bupropion dose tertiles (i.e., 300 mg; > 300 mg) were 0.67 (0.48 – 0.94) and 0.84 (0.45 – 1.58) respectively, compared to the lowest dose tertile (i.e., < 300 mg).
In a model adjusted for age, sex, race, state, and nursing home residence, HRs for mirtazapine plus a known or suspected arrhythmogenic 1A2, 2D6, and 3A4 (isozymes responsible for mirtazapine’s metabolism) inhibitor were 1.85 (1.47 – 2.33), 2.13 (1.50 – 3.03), and 1.58 (1.06 – 2.35) respectively, compared to mirtazapine in the absence of such an inhibitor. The HRs for mirtazapine plus a non-arrhythmogenic 1A2, 2D6, and 3A4 inhibitor were 1.49 (0.70 – 3.15), 1.25 (1.03 – 1.53), and 1.61 (1.22 – 2.11) respectively, compared to mirtazapine in the absence of such an inhibitor. In a model adjusted for the same covariates, the HR for bupropion plus a known or suspected arrhythmogenic 2C19 (an isozyme responsible for bupropion’s metabolism) inhibitor was 1.64 (0.60 – 4.44). The HR for bupropion plus a non-arrhythmogenic 2C19 inhibitor was 1.25 (0.86 – 1.80). There was no concomitant prescribing of bupropion and a 2B6 inhibitor, another isozyme responsible for the drug’s metabolism. In these clearance inhibitor models, we were unable to assess for confounding by covariates other than those mentioned above because of too few events occurring during concomitant prescribing of the antidepressants and inhibitors.
DISCUSSION
These results suggest that TCAs (amitriptyline, doxepin, nortriptyline), SSRIs (citalopram, fluoxetine, sertraline), a triazolopyridine (trazodone), an aminoketone (bupropion), a phenethylamine (venlafaxine), a phenylpiperazine (nefazodone), and an anti-bipolar agent (lithium) are associated with risks of SD/VA that are no greater than that of paroxetine, an SSRI considered to have a very favorable cardiovascular profile17;22 including no QT interval prolongation.16–20 Mirtazapine, a tetracyclic, was associated with a risk of SD/VA greater than that of paroxetine, yet the HR of 1.26 (1.11 – 1.42) is a modest elevation and could be the result of multiple comparisons, or residual or unmeasured confounding. Mirtazapine clearly was used in an older, more diseased, and less ambulatory population than the other antidepressants. Thus, while demographics and numerous comorbidities (and drug markers of comorbidities) were considered as potential model covariates, it is possible that some confounding remained and may have created a spurious association with our outcome.
Given the lack of a dose-response relationship with mirtazapine, our inability to assess potential confounders beyond those considered baseline (age, sex, race, state, nursing home residence) to confirm the positive associations in our analysis of concomitantly-prescribed, non-arrhythmogenic clearance inhibitors, and our a priori anticipation that mirtazapine would not have an elevated risk, we hesitate to interpret this association as causal.
Similarly, the negative association with bupropion could be the result of multiple comparisons, residual confounding, or unmeasured confounding, as this agent was utilized in a younger, less diseased, and more ambulatory population. On the other hand, we recognize that bupropion has been putatively associated with QT interval shortening,28;29 although this property is not unique to this aminoketone antidepressant.4
This study has limitations. First, an important limitation of any non-randomized pharmacoepidemiologic study is the potential for confounding by indication. We attempted to limit this potential by studying only person-time with exposure to an antidepressant and by measuring and adjusting for a number of potential confounding factors. Nevertheless, we cannot rule out the possibility that unmeasured factors may have contributed to the observed associations.
Second is the potential for incomplete ascertainment of outcomes. For example, because this study relied upon ED and inpatient diagnoses, it would have missed fatal events that did not result in presentation to the hospital. However, studies suggest that 69–80% of persons experiencing an out-of-hospital cardiac arrest30;31 and up to 88% of persons experiencing a witnessed ventricular tachycardia survive to hospital admission,32 although literature estimates do vary by year and subject age.33 Furthermore, trends suggest that survival until hospital admission among such persons is increasing over time.34 An approach by which out-of-hospital events could be captured, via use of death certificate diagnoses alone, has been shown to have a poor positive predictive value for identifying SD/VA.35–38
Third, because of a low number of events, we were unable to estimate HRs for imipramine and maprotiline, two of the three antidepressants most commonly implicated as causing TdP.6 Maprotiline had no events in little observed person-time.
Fourth, the ascertainment of certain covariates within administrative billing claims (such as Medicaid and Medicare) may be incomplete. In particular, determining a beneficiary’s smoking status and alcohol/substance use or abuse is notoriously difficult, and was principally limited to related or surrogate diagnoses appearing on encounter claims (e.g., alcoholic cirrhosis as a surrogate for an alcohol user). Given our reliance on proxies for these difficult-to-measure covariates, occurrences of such conditions were likely under-ascertained.23
Finally, generalizability may be limited to the Medicaid population. However, as such persons may be at higher baseline risk for SD/VA than the general U.S. population, the lack of apparent associations with numerous antidepressants seems reassuring.
Overall, these results provide reassurance regarding the comparative cardiac safety of antidepressants, suggesting that there are not large differences in arrhythmogenic risk among them.
Take Home Messages / Key Points.
A number of antidepressants are thought to prolong the electrocardiographic QT interval, a putative marker of arrhythmogenicity.
Few epidemiologic studies though have examined drug-specific risks of sudden cardiac death and ventricular arrhythmia (SD/VA), a clinically-meaningful outcome.
Eleven antidepressants under study had hazards of SD/VA no greater than paroxetine, an antidepressant thought to have a favorable cardiovascular profile.
These findings provide reassurance regarding the comparative cardiovascular safety of antidepressants.
Supplementary Material
Acknowledgements
This study was supported by grants R01HL076697 and KL2RR024132 from the National Institutes of Health. Other than suggestions made by peer reviewers during the grant review process, the funding sources had no role in the study’s design, conduct, or interpretation.
Dr. Bilker has consulted for Johnson & Johnson, Wyeth, AstraZeneca, and Hisamitsu, all unrelated to this topic. Dr. Kimmel has received research funding from Pfizer and consulted for Novartis, Pfizer, and Centocor, all unrelated to this topic. Dr. Hennessy has received research funding from AstraZeneca and Bristol-Myers Squibb, and consulted for AstraZeneca, Teva, Wyeth, and a law firm representing Pfizer, all unrelated to this topic.
We thank Cristin P. Freeman, MPH (University of Pennsylvania) and Gerri Barosso RD, MPH, MS (Research Data Assistance Center, University of Minnesota) for their expertise in the use of Centers for Medicare and Medicaid Services data. We thank Liu Qing, BS (University of Pennsylvania) and Hopiy Kim, BS (University of Pennsylvania) for their statistical programming.
Footnotes
Presented In Abstract Form At:
25th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (Providence, Rhode Island), August 19, 2009.
Potential Conflicts of Interest:
Dr. Leonard and Mr. Newcomb have no conflicts of interest to report.
References
- 1.Arizona Center for Education and Research on Therapeutics and The Critical Path Institute. QT drug lists by risk group. [Accessed 05/19/2010]; http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm.
- 2.Sala M, Lazzaretti M, DeVidovich G, Caverzasi E, Barale F, d'Allio G, et al. Electrophysiological changes of cardiac function during antidepressant treatment. Ther Adv Cardiovasc Dis. 2009;3(1):29–43. doi: 10.1177/1753944708096282. [PMID: 19124389] [DOI] [PubMed] [Google Scholar]
- 3.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165:1363–1369. doi: 10.1001/archinte.165.12.1363. [PMID: 15983284] [DOI] [PubMed] [Google Scholar]
- 4.Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479–498. doi: 10.1517/14656566.3.5.479. [PMID: 11996627] [DOI] [PubMed] [Google Scholar]
- 5.Sala M, Coppa F, Cappucciati C, Brambilla P, d'Allio G, Caverzasi E, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs. 2006;7(3):256–263. [PMID: 16555686] [PubMed] [Google Scholar]
- 6.Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert Opin Drug Saf. 2008;7(2):181–194. doi: 10.1517/14740338.7.2.181. [PMID: 18324881] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Noord C, Straus SM, Sturkenboom MC, Hofman A, Aarnoudse AJ, Bagnardi V, et al. Psychotropic drugs associated with corrected QT interval prolongation. J Clin Psychopharmacol. 2009;29(1):9–15. doi: 10.1097/JCP.0b013e318191c6a8. [PMID: 19142100] [DOI] [PubMed] [Google Scholar]
- 8.Zemrak WR, Kenna GA. Association of antipsychotic and antidepressant drugs with Q-T interval prolongation. Am J Health Syst Pharm. 2008;65:1029–1038. doi: 10.2146/ajhp070279. [PMID: 18499875] [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75:234–241. doi: 10.1016/j.clpt.2003.09.019. [PMID: 1500197] [DOI] [PubMed] [Google Scholar]
- 10.Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, Reynolds RF, et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325(7372):1070. doi: 10.1136/bmj.325.7372.1070. [PMID: 12424166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt CM, Hertz RP, Ellis BE, Crowell SP, Louv W, Moyé L. Risk of developing life-threatening ventricular arrhythmia associated with tefenadine in comparison with over-the-counter antihistamines, ibuprofen and clemastine. Am J Cardiol. 1994;73:346–352. doi: 10.1016/0002-9149(94)90006-x. [PMID: 8109548] [DOI] [PubMed] [Google Scholar]
- 12.Enger C, Cali C, Walker AM. Serious ventricular arrhythmias among users of cisapride and other QT-prolonging agents in the United States. Pharmacoepidemiol Drug Saf. 2002;11(6):477–486. doi: 10.1002/pds.725. [PMID: 12426932] [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. State-specific mortality from sudden cardiac death--United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51(6):123–126. [PMID: 11898927] [PubMed] [Google Scholar]
- 14.Walker AM, Szneke P, Weatherby LB, Dicker LW, Lanza LL, Loughlin JE, et al. The risk of serious cardiac arrhythmias among cisapride users in the United Kingdom and Canada. Am J Med. 1999;107(4):356–362. doi: 10.1016/s0002-9343(99)00241-7. [PMID: 10527038] [DOI] [PubMed] [Google Scholar]
- 15.Martinez C, Assimes TL, Mines D, Dell'aniello S, Suissa S. Use of venlafaxine compared with other antidepressants and the risk of sudden cardiac death or near death: a nested case-control study. BMJ. 2010;340:c249. doi: 10.1136/bmj.c249. [PMID: 20139216] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer WF, Blumhardt CL. The safety profile of paroxetine. J Clin Psychiatry. 1992;53 Suppl:61–66. [PMID: 1531828] [PubMed] [Google Scholar]
- 17.Edwards JG, Goldie A, Papayanni-Papasthatis S. Effect of paroxetine on the electrocardiogram. Psychopharmacology (Berl) 1989;97(1):96–98. doi: 10.1007/BF00443420. [PMID: 2523548] [DOI] [PubMed] [Google Scholar]
- 18.Kuhs H, Rudolf GA. Cardiovascular effects of paroxetine. Psychopharmacology (Berl) 1990;102(3):379–382. doi: 10.1007/BF02244107. [PMID: 2147517] [DOI] [PubMed] [Google Scholar]
- 19.Martin DE, Zussman BD, Everitt DE, Benincosa LJ, Etheredge RC, Jorkasky DK. Paroxetine does not affect the cardiac safety and pharmacokinetics of terfenadine in healthy adult men. J Clin Psychopharmacol. 1997;17(6):451–459. doi: 10.1097/00004714-199712000-00003. [PMID: 9408807] [DOI] [PubMed] [Google Scholar]
- 20.Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Jr, Pollock BG, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279(4):287–291. doi: 10.1001/jama.279.4.287. [PMID: 9450712] [DOI] [PubMed] [Google Scholar]
- 21.Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277–285. doi: 10.1081/clt-120037428. [PMID: 15362595] [DOI] [PubMed] [Google Scholar]
- 22.Yeragani VK, Pohl R, Jampala VC, Balon R, Ramesh C, Srinivasan K. Effects of nortriptyline and paroxetine on QT variability in patients with panic disorder. Depress Anxiety. 2000;11(3):126–130. [PMID: 10875054] [PubMed] [Google Scholar]
- 23.Hennessy S, Carson JL, Ray WA, Strom BL. Medicaid Databases. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. Sussex: John Wiley; 2005. [Google Scholar]
- 24.Medicare Payment Advisory Commission. Washington, DC: Medicare Payment Advisory Commission; 2004. Jun, Report to the Congress: New Approaches in Medicare. [Google Scholar]
- 25.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through CMS. Med Care. 2007;45(12):1216–1220. doi: 10.1097/MLR.0b013e318148435a. [PMID: 18007173] [DOI] [PubMed] [Google Scholar]
- 26.Ramsey SD, Zeliadt SB, Richardson LC, Pollack L, Linden H, Blough DK, et al. Disenrollment from Medicaid after recent cancer diagnosis. Med Care. 2008;46(1):49–57. doi: 10.1097/MLR.0b013e318158ec7f. [PMID: 18162855] [DOI] [PubMed] [Google Scholar]
- 27.Hennessy S, Leonard CE, Freeman CP, Deo R, Newcomb C, Kimmel SE, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf. 2010;19(6):555–562. doi: 10.1002/pds.1869. [PMID: 19844945] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiev A, Masco HL, Wenger TL, Johnston JA, Batey SR, Holloman LC. The cardiovascular effects of bupropion and nortriptyline in depressed outpatients. Ann Clin Psychiatry. 1994;6(2):107–115. doi: 10.3109/10401239409148989. [PMID: 7804386] [DOI] [PubMed] [Google Scholar]
- 29.Wenger TL, Cohn JB, Bustrack J. Comparison of the effects of bupropion and amitriptyline on cardiac conduction in depressed patients. J Clin Psychiatry. 1983;44(5 Pt 2):174–175. [PMID: 6406452] [PubMed] [Google Scholar]
- 30.Bunch TJ, White RD, Bruce GK, Hammill SC, Gersh BJ, Shen WK, et al. Prediction of short-and long-term outcomes by electrocardiography in survivors of out-of-hospital cardiac arrest. Resuscitation. 2004;63(2):137–143. doi: 10.1016/j.resuscitation.2004.05.008. [PMID: 15531064] [DOI] [PubMed] [Google Scholar]
- 31.Manfredini R, Portaluppi F, Grandi E, Fersini C, Gallerani M. Out-of-hospital sudden death referring to an emergency department. J Clin Epidemiol. 1996;49(8):865–868. doi: 10.1016/0895-4356(96)00114-x. [PMID: 8699205] [DOI] [PubMed] [Google Scholar]
- 32.Myerburg RJ, Kessler KM, Zaman L, Conde CA, Castellanos A. Survivors of prehospital cardiac arrest. JAMA. 1982;247(10):1485–1490. [PMID: 7035698] [PubMed] [Google Scholar]
- 33.Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104(22):2699–2703. doi: 10.1161/hc4701.099784. [PMID: 11723022] [DOI] [PubMed] [Google Scholar]
- 34.Bunch TJ, Hammill SC, White RD. Outcomes after ventricular fibrillation out-of-hospital cardiac arrest: expanding the chain of survival. Mayo Clin Proc. 2005;80(6):774–782. doi: 10.1016/S0025-6196(11)61532-2. [PMID: 15945529] [DOI] [PubMed] [Google Scholar]
- 35.Arking DE, Chugh SS, Chakravarti A, Spooner PM. Genomics in sudden cardiac death. Circ Res. 2004;94(6):712–723. doi: 10.1161/01.RES.0000123861.16082.95. [DOI] [PubMed] [Google Scholar]
- 36.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44(6):1268–1275. doi: 10.1016/j.jacc.2004.06.029. [PMID: 15364331] [DOI] [PubMed] [Google Scholar]
- 37.Fox CS, Evans JC, Larson MG, Lloyd-Jones DM, O'Donnell CJ, Sorlie PD, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am J Cardiol. 2005;95(7):856–859. doi: 10.1016/j.amjcard.2004.12.011. [PMID: 15781015] [DOI] [PubMed] [Google Scholar]
- 38.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Jr, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82(1):50–53. doi: 10.1016/s0002-9149(98)00240-9. [PMID: 9671008] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.