Abstract
Essential hypertension is highly prevalent in the elderly population, exceeding 70% in people older than 60 yr of age, and remains a leading risk factor for heart disease, stroke, and chronic renal disease. Elucidation of genetic determinants is critical but remains a challenge due to its complex, multifactorial pathogenesis. We investigated the role DEspR promoter variants, previously associated with male essential hypertension susceptibility, in blood pressure (BP) regulation. We detected a single nucleotide polymorphism within the DEspR 5′-regulatory region associated with increased BP in a male Sardinian cohort accounting for 11.0 mmHg of systolic BP (P < 10−15) and 9.3 mmHg of diastolic BP (P < 10−15). Sequence analysis of three normotensive subjects homozygous for the rs6535847 “normotension-associated T-allele” identified a canonical TATAAAA-box in contrast to a CATAAAA-motif in three hypertensive subjects homozygous for the rs6535847 “hypertension-associated C-allele.” In vitro analysis detected decreased transcription activity with the CATAAAA-motif promoter-construct compared with the canonical TATAAAA-box promoter-construct. Although BP did not differ between DEspR+/− knockout male mice and wild-type littermates at 6 mo of age, radiotelemetric BP measurements in 18 mo old inbred DEspR+/− knockout male mice known to have decreased DEspR RNA and protein detected higher systolic, mean, and diastolic BPs in DEspR+/− mice compared with littermate wild-type controls (P < 0.05). Our results demonstrate that promoter variants in DEspR associated with hypertension susceptibility and increased BP in Sardinian males affect transcription levels, which then affect BP in an age-dependent and male-specific manner. This finding is concordant with the late-onset and sex-specific characteristics of essential hypertension, thus reiterating the mandate for sex-specific analyses and treatment approaches for essential hypertension.
Keywords: genetics, blood pressure, sex-specific, promoter, mice
essential hypertension remains a major public health concern due to its high prevalence and key role as a risk factor for coronary artery disease, stroke, chronic renal disease, and peripheral vascular disease, leading causes of morbidity and mortality (3). Elucidation of genetic determinants is critical but remains a challenge due to its polygenic nature, influence of environmental factors on disease outcome, and the added confounding factor of inherent genetic heterogeneity of human populations (2, 4, 7). Hypertension is further compounded by phenotype variation due to its relatively late onset, variable disease course and target organ complications, sex-specific differences, and emerging impact of gestational environmental factors. This multifaceted complexity has made elucidation of hypertension susceptibility genes challenging, as evidenced by multicohort analysis of tens of thousands of patients leading to identification of loci associated with hypertension contributing only up to 0.5 mmHg despite association P values < 10−8 (14). Prospectively, the health value added by therapies targeting these “0.5 mmHg BP genes” would contrast the therapies targeting major hypertension susceptibility genes that account for 10-fold greater than observed 0.5 mmHg blood pressure (BP) effect (>5 mmHg) or significant increased risk for target organ complications. Unequivocally, identification of major hypertension susceptibility genes remains a clinical mandate. Lessons from large-cohort genome-wide association studies (GWAS) (14, 15, 21), which did not detect major hypertension susceptibility genes accounting for more than 5 mmHg BP, indicate that molecular genetic analysis of candidate hypertension susceptibility genes, defined through the identification of a functionally significant variant, genetic linkage, or association of said variant in both animal models and human case-control studies, and proof of concept in in vivo animal model experiments, remains a valid and still much-needed approach. Furthermore, deduced from the nondetection of major hypertension susceptibility genes in said large multiracial, multicohort studies (14, 15, 21) but detection in site-specific human cohort studies (5, 6) and animal model studies (8, 13), it becomes apparent that major hypertension genes are likely hypertension subtype-specific, sex-specific, and/or modified by environmental factors (diet, developmental programming) that are not accounted for.
Following this molecular genetic paradigm, we tested the hypothesis that the dual endothelin-1/vascular endothelial growth factor-signal peptide receptor or DEspR (GenBank gene ID Dear) is a major hypertension susceptibility gene in the Sardinian population based on its association with hypertension detected by gene-specific single nucleotide polymorphism (SNP) and haplotype analysis (6). DEspR, formerly Dear (6, 9, 20), was originally cloned from a Dahl salt-sensitive hypertensive rat brain cDNA library and was shown to be a single transmembrane receptor coupled to a Ca2+-mobilizing transduction pathway binding endothelin-1 (ET-1) and angiotensin II (ANG II) with equivalent affinities (20). Subsequent molecular studies elucidated that mouse and human DEspR do not bind ANG II but instead binds ET-1 and the vascular endothelial growth factor signal peptide with equal affinities (6, 9). Cumulative studies have reported that DEspR is involved in the modulation of BP in sex-specific ways as observed in animal model studies and in humans. Candidate gene analysis detected a DEspR S44P/M74T variant as genetically linked to hypertension susceptibility in salt-sensitive, hypertensive Dahl rat strain with significant linkage in females accounting for 14.5% of total trait variance (TTV) and suggestive linkage in males accounting for 6% of TTV (13). A total genome scan study conducted by us also detected DEspR as a candidate within a BP quantitative trait locus with significant linkage (logarithm of the odds 3.5) in female but not in male F2 [S × R] intercross rats (10). Concordantly, in humans, SNP and haplotype analyses detected strong association of a 5′-flanking region SNP and its corresponding haplotype marker set, h13, with hypertension susceptibility in a male Sardinian case-control cohort (6). We therefore conducted studies to identify and characterize putative functional polymorphisms in the DEspR 5′-flanking regulatory region that might contribute to allele-specific susceptibility or resistance to essential hypertension in the Sardinian population.
MATERIALS AND METHODS
Study population.
The study cohort from Sardinia has been previously described (5, 6). In brief, it consists of 712 subjects, 433 hypertensives and 279 normotensives; all enrolled at the Hypertension and Related Diseases Center of the Azienda Ospedaliero Universitaria-University of Sassari Medical School, Sassari, Sardinia, Italy. Studies were approved by the local ethics committee of Local Health Unit-University of Sassari Medical School. All subjects were white, unrelated, born in different domains of northern Sardinia, a geographical location with a high degree of genetic homogeneity (1, 17), and ascertained to be Sardinian for at least six generations. Hypertensive subjects with BP > 160/95 mmHg (n = 433, mean age = 51.0 ± 10.2 yr) with no secondary hypertension etiology were considered for the study. BP measurements were obtained prior to any medications. Since hypertension is a late-onset disease, normotensive controls (n = 279, mean age = 65.4 ± 10.6 yr) were not age-matched with hypertensive patients but instead limited to subjects older than 54 yr of age who had not been previously diagnosed or treated as hypertensive; had no family history of hypertension, cardiovascular, or cerebrovascular disease; and had BP < 138/85 mmHg on at least four occasions. This more accurately eliminates potential hypertensives who could have been included if normotensives represented the adult age group from 20–49 yr as has been done for other hypertensive study cohorts.
Cloning and sequencing of DEspR 5′-regulatory region.
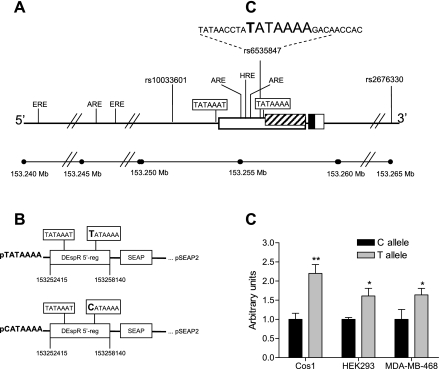
DEspR is located on chromosome 4 at coordinate 153258000. DEspR is nested within the FBXW7 locus, between FBXW7 exons 6 and 4, and it is transcribed in opposite direction respect to the FBXW7 transcription unit. We cloned and sequenced a 7,860 base pair (bp) fragment spanning the entire DEspR transcription unit (using as forward primer: 5′-TCCAGTTGTAAAAATACGCC-3′ and reverse primer: 5′-GCCTTCTTCAATGCCTTGTG-3′; chromosome 4 coordinates 153251394–153259254, Build 37.1; Fig. 2) from six patients, three carrying haplotypes associated with hypertension and three carrying haplotypes associated with normotension, respectively (Table 1). Each fragment was subcloned into the PT-vector system (Clontech, Palo Alto, CA) and then sequenced in its entirety in both directions to ascertain accuracy. Regulatory elements within DEspR 5′-regulatory region were investigated by using the DS Gene 1.5 software program.
Fig. 2.
Transcriptional activity of DEspR promoter variants. A: illustration of the DEspR 5′-regulatory region. Exons are represented as boxes; coding exon in black; untranslated regions in white and striped boxes. DEspR SNPs and their location are presented. Sequence and location of the DEspR rs6535847 C/T polymorphism are shown. The positions of the TATAAAT- and TATAAAA-boxes within DEspR 5′-regulatory region are shown. The location of potential estrogen response (ERE), androgen response (ARE), and hypoxia response elements (HRE) are indicated based upon 100% homology with corresponding consensus sequences. B: schematic of 2 DEspR (pTATAAAA, pCATAAAA) reporter gene constructs. C: relative transcriptional activity of pTATAAAA and pCATAAAA gene constructs in Cos1, HEK293, and MDA-MB-468 cells. *P < 0.05; **P < 0.002 (2-tailed student t-test).
Table 1.
Sequencing of DEspR 5′-regulatory regions of patients carrying haplotypes associated with hypertension and normotension
| Haplotype |
|||
|---|---|---|---|
| Patient | rs6535847 | rs2676330 | Association |
| 1 | CC | CC | HT |
| 2 | CC | CC | HT |
| 3 | CC | CC | HT |
| 4 | TT | TT | NT |
| 5 | TT | TT | NT |
| 6 | TT | TT | NT |
HT, hypertension; NT, normotension.
Reported associations are from Ref. 6.
Amplification of 5′ cDNA ends, 5′-rapid amplification of cDNA ends.
Amplification of 5′-cDNA ends was performed on total RNA from human kidney using the 5′ RACE System from Invitrogen (Carlsbad, CA). Initial cDNA was synthesized from human kidney total RNA (Invitrogen, Carlsbad, CA) using a primer GSP1 (5′-TGCTGGCTCATTCTAAAACTG-3′) specific for DEspR. Subsequent 5′-rapid amplification of cDNA ends (RACE) was carried out following manufacturer's instructions. For the primary PCR of the dC-tailed cDNA, we used primers GSP1 and AAP (5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG-3′) with the following PCR profile: 94°C for 2 min; 35 cycles of 94°C for 30 s, 55°C for 60 s, 72°C for 5 min; final extension at 72°C for 7 min. For the nested PCR that followed, we used a 1:10 dilution of the primary PCR as template and the following primer pairs: 1) GSP2 (5′-GTGTTCTTTTAAAGACTGCCTG-3′) and AUAP (5′-GGCCACGCGTCGACTAGTAC-3′), 2) GSP3 (5′-GTGTTTCTTATGCCTCAGCC-3′) and AUAP; with the PCR profile: 94°C for 2 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 1 min; final extension at 72°C for 7 min. PCR products obtained from this nested PCR were gel-purified and cloned into pCR II-TOPO (Invitrogen, Carlsbad, CA) for sequencing. We note that the sequence of promoter A region is significantly different from the one present in the public database (Build 37.1). The sequence presented was obtained from three independent pCR II-TOPO-150 bp clones that match the sequence present in all six patients' cloned 5′-regulatory regions.
Transcriptional activity of DEspR promoter regions.
To measure transcriptional activity, we used the Great EscAPe SEAP Reporter System 3 (Clontech Lab). This system uses SEAP, a secreted form of human placental alkaline phosphatase, as a reporter to monitor the activity of promoters and enhancers. Sequences were cloned into the pSEAP2-Basic Vector for transcriptional assay and a chemiluminescent substrate was used to monitor secreted phosphatase activity. We cloned the DEspR promoter regions onto pSEAP2 vector by PCR amplification using the following primers: forward: 5′-CAAACAGTCATCTGTACACCC-3′; reverse: 5′-TGCCCCTAGTTATATGTCAGC-3′ (chr4 coordinates 153252415–153258140, Build 37.1; 5725 bp fragment). Two constructs were generated, one carrying the rs6535847 T-polymorphism (pTATAAAA, Fig. 2B) and one carrying the rs6535847 C-polymorphism (pCATAAAA, Fig. 2B). We measured transcriptional activity in three cell lines, two of renal origin (HEK293, human embryonic kidney cell line; Cos1, monkey kidney cell line) and one human mammary tumor cell line (MDA-MB-468, ATCC) since DEspR is detected prominently in kidney (6) and mammary tumor cells (V. L. M. Herrera, N. Ruiz-Opazo, unpublished results). Transfections were performed essentially as described (11). Cells were maintained in appropriate growth media. Cells at 70% confluence (300,000 cells in P-35 dishes) were cotransfected with 3.0 μg of 5′-regulatory region-SEAP constructs plus 1.5 μg of pSV2-β-galactosidase (for internal control) using the DOTAP liposomal transfection reagent (Roche Molecular Biochemicals). After 6 h, cells were fed with fresh growth media. After 72 h, cell culture supernatants were collected and assayed for SEAP activity. Cells were harvested, and cell protein extracts were utilized for determination of β-galactosidase activity (Promega) to normalize SEAP activity. Each construct was tested in six replicates.
BP measurements in DEspR+/− and wild-type mice.
All animal procedures were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee. Eighteen-month-old DEspR+/− and wild-type DEspR+/+ littermate mice, ascertained to be >99.98% of C57BL/6 genetic background (BC10), were used for BP measurements by radiotelemetry. DEspR+/− haplo-deficient mice express ∼50% of DEspR protein (9) and 50% DEspR mRNA (12) when compared with age- and sex-matched wild-type controls in different tissues. Wild-type DEspR+/+ and DEspR+/− littermate mice were produced for the study from the same BC10 (+/+) × (+/−) intercross. BP was measured using intra-aortic abdominal radiotelemetric implants (PA-C10, Dataquest A.R.T. 4.2 system from Data Sciences International) obtaining BP measurements over 10 s every 5 min for 12–72 h. The average systolic (SBP), diastolic (DBP), and mean arterial pressures (MAP) were obtained, along with heart rate and activity.
Genotyping.
SNP genotyping was carried out by the Molecular Genetics Core Facility at the Boston University School of Medicine on an Applied Biosystems 7900 Real-Time PCR System. The rs6535847 SNP assay (TaqMan assay) was procured from Applied Biosystems. The genotyping completeness rate was 91%.
Statistical analysis.
Testing (Mann-Whitney Rank Sum Test, SigmaPlot 11.0) based on BP as quantitative trait was done by comparing BP in homozygous C/C carriers versus subjects carrying [C/T + T/T] genotypes.
RESULTS
Identification of two transcription start sites by 5′-RACE.
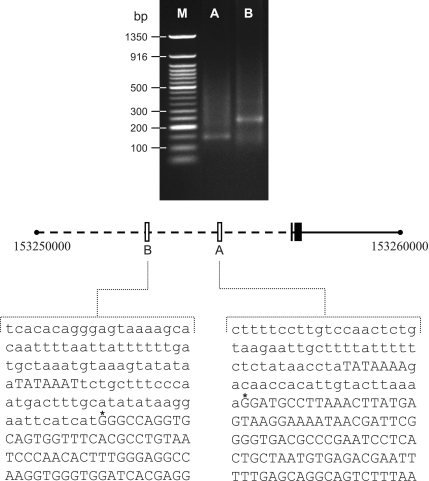
To identify putative transcription start sites within the DEspR transcription unit we performed 5′-RACE analysis using human total kidney RNA since DEspR is readily detected in kidney (6). A first PCR was performed with AAP primer and GSP1 primer, which hybridized within a sequence in closed proximity of DEspR initiation methionine. A nested PCR was then performed with AUAP plus GSP2 and GSP3 primers testing for two potential transcription start sites. As shown in Fig. 1, two distinct fragments of 150 and 250 bp were obtained after nested PCR corresponding to GSP2 (promoter A) and GSP3 (promoter B) respectively. The PCR products for GSP2 and GSP3 were subcloned and sequenced defining two promoter regions, A and B respectively, shown in Fig. 1. These results identify A as the promoter containing the T/CATAAAA-box polymorphism and more importantly it shows that promoter A is transcriptionally active in vivo.
Fig. 1.
Sequence of each promoter region as determined by 5′-rapid amplification of cDNA ends (RACE) analysis. Top: agarose gel (1.5%) analysis of 5′-RACE primer-specific nested PCR products. Lanes A and B represent amplifications with primer pairs AUAP/GSP2 (promoter A) and AUAP/GSP3 (promoter B), respectively. M, size markers (Invitrogen 1 Kb ladder). Bottom: the center line is the chromosome 4 map, with the open boxes representing the 2 promoters relative to DEspR coding exons (black boxes). Nucleotide sequence of promoters A and B are shown. *The 2 transcription start sites identified by 5′-RACE.
Scanning of DEspR 5′-regulatory region for candidate variants.
To investigate putative variants within the DEspR gene and 5′-regulatory region, we cloned and sequenced a 7860 bp fragment spanning the entire DEspR transcription unit (Fig. 2A) from three hypertensive patients homozygous for the “hypertensive C-allele” of SNPs rs6535847 and rs2676330 (Fig. 2A) comprising a hypertension-associated haplotype (previously haplotype h13) in Sardinian males (6), in contrast to three normotensive subjects homozygous for the “protective T-allele” of the normotension-associated haplotype (previously haplotype h14) in Sardinian males (6) (Table 1). We note that current SNP nomenclature and chromosome localization differ numerically from that previously used. Current SNPs rs6535847 and rs2676330 correspond to former SNP-naming hCV29317640l or Dear-SNP11 and hCV16274369 or Dear-SNP12, respectively (6). We note that the localization of DEspR by sequence in relation to these SNPs remains the same, although chromosomal distance is numerically different based on the “Build 37.1-Map” and that the protective T-allele (rs6535847-T) is the less frequent “minor allele” in the Sardinian cohort in contrast to the “hypertension susceptibility” C-allele (rs6535847-C), which is the more frequent or “major allele.”
Nucleotide sequence analysis in both directions elucidated that the normotension-associated DEspR 5′-regulatory region, hence containing the protective rs6535847-T allele has a canonical TATAAAA-box that corresponded to promoter A within the DEspR transcription unit (Fig. 1) located immediately downstream to a hypoxia response element (HRE) and two androgen response elements (AREs, Fig. 2A). In contrast, the hypertension-associated DEspR 5′-regulatory region, hence containing the hypertension susceptibility rs6535847-C allele has a CATAAAA motif instead (Fig. 2A). No other polymorphisms were detected in the 7680 bp long DNA fragment other than C/T at rs6535847.
Based on established functional characterization of TATAAAA-box containing promoters (16, 22), we hypothesized that the DEspR 5′-regulatory region containing the CATAAAA motif, hence only one (promoter B) noncanonical TATA box-containing promoter would be less “robust” than the DEspR 5′-regulatory region harboring an additional (promoter A) canonical TATAAAA box-containing promoter, thereby resulting in lower DEspR transcription levels, especially in response to modulation by regulatory elements in the DEspR 5′-regulatory region, such as HRE, AREs, and estrogen response elements (EREs, Fig. 2A). If hypo-modulation of DEspR transcription is functionally significant in BP regulation, then a priori, the C-allele should be associated with hypertension with increased BP as a quantitative trait, the DEspR 5′-regulatory region containing the CATAAAA motif should lead to lower transcription levels in in vitro assays and decreased DEspR levels in an animal model, such as seen in heterozygous DEspR+/− knockout mice (9, 12), should lead to increase BP.
Association of DEspR rs6535847 C/T polymorphism with BP as a quantitative trait.
We previously reported that DEspR rs6535847 C-allele (previously hCV29317640 or SNP11) (6) was associated with hypertension in our Sardinian case-control cohort when analyzed as an individual SNP (P < 0.0172) and as part of a haplotype, h13 (P = 0.0328) (6). To corroborate this association with hypertension as a dichotomous trait, we next analyzed the association of DEspR rs6535847 C/T polymorphism with BP as a quantitative trait. As shown in Table 2, male subjects homozygous for DEspR rs6535847 C-allele (C/C) had higher SBP and DBP than those heterozygous (C/T) or homozygous for the DEspR rs6535847 T-allele (ΔSBP = 11.0 mmHg, P < 10−15; ΔDBP = 9.3 mmHg, P < 10−15).
Table 2.
Analysis of DEspR (rs6535847) variant based on blood pressure as a quantitative trait
| Genotypes | n | Mean SBP ± SE, mmHg | Δ SBP | P | Mean DBP ± SE, mmHg | Δ DBP | P |
|---|---|---|---|---|---|---|---|
| Male + female cohort | |||||||
| C/C | 305 | 158.5 ± 2.2 | 101.2 ± 1.5 | ||||
| [C/T + T/T] | 337 | 154.8 ± 1.8 | 3.7 | 0.076 | 96.7 ± 1.2 | 4.5 | 0.021 |
| Female cohort | |||||||
| C/C | 145 | 154.1 ± 3.2 | 95.6 ± 1.9 | ||||
| [C/T + T/T] | 158 | 158.6 ± 2.8 | −4.5 | 0.324 | 96.3 ± 1.8 | −0.7 | 0.726 |
| Male cohort | |||||||
| C/C | 160 | 162.5 ± 2.9 | 106.3 ± 2.7 | ||||
| [C/T + T/T] | 179 | 151.5 ± 2.3 | 11.0 | <10−15 | 97.0 ± 2.2 | 9.3 | <10−15 |
Blood pressures were adjusted for age, body mass index, and case/control status.
n, Number of individuals; SBP, systolic blood pressure; DBP, diastolic blood pressure; Δ SBP, difference in systolic blood pressure; Δ DBP, difference in diastolic blood pressure; P, Mann-Whitney rank sum test P values.
DEspR 5′-regulatory region containing the CATAAAA motif exhibits decreased transcriptional activity.
To determine whether having a CATAAAA motif in DEspR 5′-regulatory region causes decrease transcriptional activity compared with a DEspR 5′-regulatory region having an additional canonical TATAAAA box-containing promoter, we tested transcriptional activity of DEspR promoter-constructs carrying said polymorphisms in vitro. We measured transcriptional activity using the Great EscAPe SEAP Reporter System 3, which uses SEAP as a reporter to monitor activity. Two constructs were tested, one carrying the rs6535847 T polymorphism (pTATAAAA, Fig. 2B) and the other carrying the rs6535847 C polymorphism (pCATAAAA, Fig. 2B) in three cell lines, two of renal origin (HEK293, human embryonic kidney cell line; Cos1, monkey kidney cell line) and one human mammary tumor cell line (MDA-MB-468) since DEspR is detected prominently in kidney (6) and mammary tumors (V. L. M. Herrera, N. Ruiz-Opazo, unpublished results). As shown in Fig. 2C, the DEspR pCATAAAA construct exhibited lower transcriptional activity than pTATAAAA construct in three cell lines tested (in Cos1, P < 0.002; in HEK293 and MDA-MB-468, P < 0.05). These observations demonstrate that the C/T polymorphism in DEspR promoter region is functionally significant.
Effect of decreased DEspR expression levels on BP.
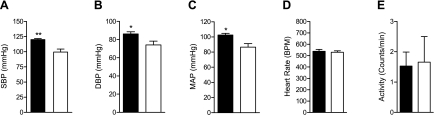
The DEspR−/− state was embryonic lethal (9), thus to determine whether decreased DEspR expression levels leads to increased BP in vivo, we analyzed BP in aging heterozygous DEspR+/− knockout male mice (18 mo of age), since hypertension is an aging-associated, late-onset disease in humans and since BP measurements obtained at an earlier time point (4–6 mo of age) did not detect differences in BP in male mice (9). We utilized highly inbred male DEspR+/− knockout mice (>99.98% of C57BL/6 genetic background) exhibiting 50% reduction in DEspR mRNA and protein (9, 12) levels. As shown in Fig. 3, male DEspR+/− mice had higher BP (SBP mean ± SE; male DEspR+/− at 120.1 ± 1.6 mmHg vs. male wild-type controls at 99.5 ± 5.1 mmHg, P < 0.009; DBP mean ± SE; male DEspR+/− at 86.3 ± 2.2 mmHg vs. male wild-type controls at 74.2 ± 4.1 mmHg, P < 0.05; MAP mean ± SE; male DEspR+/− at 102.5 ± 2.3 mmHg vs. male wild-type controls at 86.6 ± 4.7 mmHg, P < 0.05) than male wild-type controls, while heart rate and activity measures remain equivalent (Fig. 3), consistent with our prediction that lower DEspR expression will result in elevated BP.
Fig. 3.
Analysis of blood pressure, heart rate, and activity in heterozygous DEspR+/− and wild-type male mice at 18 mo of age. A: mean systolic blood pressure ± SE (SBP; mmHg). B: mean diastolic blood pressure ± SE (DBP; mmHg). C: mean mean arterial pressure ± SE (MAP; mmHg). D: mean heart rate ± SE (beats/min; BPM). E: mean activity ± SE (counts/min) in DEspR+/− (solid bars, n = 4) and wild-type (open bars, n = 4) male mice. *P < 0.05; **P < 0.009 (2-tailed student t-test).
DISCUSSION
DEspR as a modulator of hypertension susceptibility.
Elucidation of hypertension susceptibility genes in humans requires a multifaceted molecular genetic approach that substantiates the existence of a hypertension susceptibility gene through concordance of cumulative evidence for: 1) genetic association of the gene in question with essential hypertension in a human population, 2) elucidation and genetic association of specific functional variants that might contribute to susceptibility or resistance to high BP and subsequent, and 3) demonstration in a biological experimental system that the effect of specific functional variants on BP are consistent with the genetic association results. Accordingly, the concordance of our observations fulfills the step-wise candidate gene molecular genetic paradigm, thus providing compelling evidence that validates DEspR as a modulator of hypertension susceptibility in Sardinian males. DEspR rs6535847 C/T polymorphism was associated with an 11.0 mmHg SBP effect and a 9.3 mmHg DBP effect, which is significantly greater than the 1 mmHg BP effect of loci associated with hypertension identified in an extensive GWAS analysis (14). Notably, this rs6535847 C/T polymorphism caused a functionally significant sequence variance in the DEspR promoter region: CATAAAA box in the C-allele associated with hypertension compared with a canonical TATAAAA box as second promoter downstream to AREs, EREs, and one HRE in the protective T-allele. The decrease in DEspR transcription due to the absence of a second TATAAAA-box containing promoter resulted in decreased DEspR expression levels, which in aging, 18 mo old DEspR+/− male mice are observed to have increased BP as measured by radiotelemetry.
Aging and sex modulates DEspR effects on BP.
The role of DEspR in hypertension is quite complex since it is modulated by both sex and age as seen in this study. This observation projects that GWAS combining males and females and combining young adults and old adults will likely not be able to discern DEspR as a modulator of hypertension susceptibility. Observations of sex-specific and aging-specific effects of DEspR seen in the Sardinian cohort are corroborated by observations in studies of heterozygous DEspR+/− mice. While heterozygous DEspR+/− knockout female mice showed lower BP than wild-type female controls at 4–6 mo of age (9), heterozygous DEspR+/− knockout male mice did not exhibit any BP changes at 4–6 mo but exhibited higher BP than age-matched wild-type male controls at 18 mo. This is an interesting finding that mimics and/or correlates with the observation in humans in which hypertension is a late-onset disease that becomes more prevalent with aging, exceeding 70% in people older than 60 yr of age (18, 19).
We also note that the DEspR's sex-specific opposite effect on BP parallels observations in other traits affected by DEspR expression. We reported hippocampus-dependent cognitive deficits in heterozygous DEspR+/− knockout male mice but better hippocampus-dependent cognitive performance in DEspR+/− female mice when compared with age- and sex-matched control mice (12). Overall, DEspR effects on specific physiological functions are modulated by the sex hormone microenvironment. The precise molecular mechanisms involved in this sex-specific modulation of DEspR function remain to be elucidated, although canonical ERE and ARE are present in the 5′-regulatory region of DEspR and immediately upstream to the T/CATAAAA-box variant site.
Highlighting the complexity of DEspR roles, we do note, however, that the angiogenesis roles of DEspR affect developmental vascularization in both male and females (9) and that DEspR is also a genetic factor/modulator for hypertension in females, since DEspR exhibited genetic linkage to hypertension in an F2 (Dahl S × Dahl R) intercross rat population affecting females to a greater extent accounting for 14% of TTV compared with 6% of TTV in male F2 [S × R] intercross rats (10, 13). We do note, however, that this cohort was done at 5–6 mo of age (10) and that the rat DEspR variant is an structural polymorphism (S44P/M74T) (13) rather than a regulatory mutation as is found in human DEspR.
In conclusion, our results demonstrate DEspR as a hypertension susceptibility gene in a northern Sardinian sample in males. A functionally significant rs6535847 C/T polymorphism induces a promoter sequence change in DEspR 5′-regulatory region, which alters DEspR transcription and hence expression levels, which then modulate systolic, diastolic, and mean arterial pressures. Sex- and age-specific factors influence the effects of DEspR roles in hypertension or BP regulation, as observed in humans, as well as in rat and mouse model studies. The effects of age and sex are concordant with the late-onset characteristics of essential hypertension and predominance in males compared with premenopausal women. Although testing of DEspR as a hypertension susceptibility modulator needs to be done in other hypertensive cohorts, our findings reiterate the pathogenesis-based mandate for sex-specific studies with aging-associated considerations in humans and in animal model studies, as these could advance value-added insight into treatment approaches for essential hypertension.
GRANTS
Research described in this article was supported by National Heart, Lung, and Blood Institute Grant HL-98939, awarded to N. Ruiz-Opazo, and by Progetti Educazione Sanitaria Regione Sardegna awarded to N. Glorioso.
DISCLOSURES
Boston University has filed a patent (application no. PCT/US2005/041594)for DEspR in which V. Herrera and N. Ruiz-Opazo are inventors.
AUTHOR CONTRIBUTIONS
Author contributions: N. G. and N. R. -O. conception and design of research; N. G., V. L. M. H., G. A., C. T., P. B., E. B., and N. R. -O. analyzed data; N. G., V. L. M. H., and N. R. -O. edited and revised manuscript; N. G. and N. R. -O. approved final version of manuscript; V. L. M. H., T. D., G. A., C. T., P. B., and E. B. performed experiments; V. L. M. H. and N. R. -O. interpreted results of experiments; V. L. M. H. and T. D. prepared figures; N. R. -O. drafted manuscript.
REFERENCES
- 1. Cappello N, Rendine S, Griffo R, Mameli GE, Succa V, Vona G, Piazza A. Genetic analysis of Sardinia, I: data on 12 polymorphisms in 21 linguistic domains. Ann Hum Genet 60: 125–141, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Charchar FJ, Zimmerli LU, Tomaszewski M. The pressure of finding human hypertension genes: new tools, old dilemmas. J Hum Hypertens 22: 821–828, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Cowley AW. The genetic dissection of essential hypertension. Nat Rev Genetics 7: 829–840, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Ehret GB, Morrison AC, O'Connor AA, Grove ML, Baird L, Schwander K, Weder A, Cooper RS, Rao DC, Hunt SC, Boerwinkle E, Chakravarti A. Replication of the Wellcome Trust genome-wide association study of essential hypertension: the Family Blood Pressure Program. Eur J Hum Genet 16: 1507–1511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glorioso N, Filigheddu F, Troffa C, Dettori F, Soro A, PinnaParpaglia P, Tsikoudakis A, Myers RH, Herrera VL, Ruiz-Opazo N. Interaction of α1Na,K-ATPase and Na,K,2Cl-cotransporter genes in human essential hypertension. Hypertension 38: 204–209, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Glorioso N, Herrera VL, Bagamasbad P, Filigheddu F, Troffa C, Argiolas G, Bulla E, Decano JL, Ruiz-Opazo N. Association of ATP1A1 and Dear SNP-haplotypes with essential hypertension: sex-specific and haplotype-specific effects. Circ Res 100: 1522–1529, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Gong M, Hubner N. Molecular genetics of human hypertension. Clin Sci 110: 315–326, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Herrera VL, Xie HX, Lopez LV, Schork NJ, Ruiz-Opazo N. The alpha1 Na,K-ATPase gene is a susceptibility hypertension gene in the Dahl salt-sensitive HSD rat. J Clin Invest 102: 1102–1111, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrera VL, Ponce LRB, Bagamasbad PD, VanPelt BD, Didishvili T, Ruiz-Opazo N. Embryonic lethality in Dear gene-deficient mice: new player in angiogenesis. Physiol Genomics 23: 257–268, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Herrera VL, Tsikoudakis A, Ponce LRB, Matsubara Y, Ruiz-Opazo N. Sex-specific QTLs and interacting-loci underlie salt-sensitive hypertension and target-organ complications in Dahl S/jrHS hypertensive rats. Physiol Genomics 26: 172–179, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Herrera VL, Bagamasbad P, Didishvili T, Decano JL, Ruiz-Opazo N. Overlapping genes in Nalp6/PYPAF5 locus encodes two V2-type vasopressin isoreceptors: angiotensin-vasopressin receptor (AVR) and non-AVR (NAVR). Physiol Genomics 34: 65–77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrera VL, Decano JL, Bagamasbad P, Kufahl T, Steffen M, Ruiz-Opazo N. Sex-specific hippocampal-dependent cognitive deficits and increased neuronal autophagy in DEspR haploinsufficiency in mice. Physiol Genomics 35: 316–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneko Y, Herrera VL, Didishvili T, Ruiz-Opazo N. Sex-specific effects of dual ET-1/AngII receptor (Dear) variants in Dahl salt-sensitive/resistant hypertension rat model. Physiol Genomics 20: 157–164, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Wellcome Trust Case Control Consortium. Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41: 666–676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nussinov R, Owens J, Maizel JV. Sequence signals in eukaryotic upstream regions. Biochim Biophys Acta 866: 109–119, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Piazza A. Who are the Europeans? Science 260: 9–11, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Plouin PF, Rossignol P, Bobrie G. Hypertension in the elderly. Bull Acad Natl Med 190: 793–805, 2006 [PubMed] [Google Scholar]
- 19. Redon J, Cea-Calvo L, Lozano JV, Marti-Canales JC, Llisterri JL, Aznar J, Gonzalez-Esteban J. Blood pressure and estimated risk of stroke in the elderly population of Spain: the PREV-ICTUS study. Stroke 38: 1167–1173, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VL. Molecular characterization of a dual endothelin-1/angiotensin II receptor. Mol Med 4: 96–108, 1998 [PMC free article] [PubMed] [Google Scholar]
- 21. The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wefald FC, Devlin BH, Williams RS. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature 344: 260–262, 1990 [DOI] [PubMed] [Google Scholar]