Abstract
Skeletal muscle atrophy can be a consequence of many diseases, environmental insults, inactivity, age, and injury. Atrophy is characterized by active degradation, removal of contractile proteins, and a reduction in muscle fiber size. Animal models have been extensively used to identify pathways that lead to atrophic conditions. We used genome-wide expression profiling analyses and quantitative PCR to identify the molecular changes that occur in two clinically relevant mouse models of muscle atrophy: hindlimb casting and Achilles tendon laceration (tenotomy). Gastrocnemius muscle samples were collected 2, 7, and 14 days after casting or injury. The total amount of muscle loss, as measured by wet weight and muscle fiber size, was equivalent between models on day 14, although tenotomy resulted in a more rapid induction of muscle atrophy. Furthermore, tenotomy resulted in the regulation of significantly more mRNA transcripts then did casting. Analysis of the regulated genes and pathways suggest that the mechanisms of atrophy are distinct between these models. The degradation following casting was ubiquitin-proteasome mediated, while degradation following tenotomy was lysosomal and matrix-metalloproteinase mediated, suggesting a possible role for autophagy. These data suggest that there are multiple mechanisms leading to muscle atrophy and that specific therapeutic agents may be necessary to combat atrophy resulting from different conditions.
Keywords: gene expression, proteasome, lysosome
skeletal muscle atrophy is induced in a variety of diseases (diabetes mellitus, cancer, acquired immunodeficiency syndrome, sepsis, and chronic obstructive pulmonary disease), following trauma (denervation and tendon injury), after prolonged immobility (casting and extended bed rest), after extended unloading (microgravity), or as a natural progression of aging. Four broad categories of muscle wasting diseases have been described, including denervation-induced atrophy, disuse atrophy as a result of immobilization, unloading-induced atrophy as a result of prolonged bed rest, and spaceflight and chronic disease-induced cachexia (23). In these diverse conditions, atrophy is characterized by the loss of muscle mass through increased activity of the various protein degradation pathways.
To study the molecular and biochemical changes that occur during muscle atrophy, animal models of muscle atrophy have been developed that mimic many characteristics of muscle loss in humans. Many models, both preclinical and clinical, have been characterized using microarray technologies to uncover the underlying molecular changes that accompany atrophy in skeletal muscles. Some of these studies identified a common transcriptional set of genes termed atrogenes that leads to increased rates of protein degradation during muscle atrophy (37). While most cellular protein degradation is mediated through four major pathways, including calcium-dependent cysteine proteases (calpains) (9), cysteine-aspartic acid proteases (caspases) (16, 50), lysosomal cysteine proteases (cathepsins) (3), and ubiquitin-mediated proteasome activity (30), many studies suggest a significant role for ubiquitin-mediated protein degradation in muscle atrophy. Two of the most highly regulated genes during muscle atrophy are the muscle-specific ubiquitin ligases Fbxo32 and Trim63, commonly referred to as atrogin-1/MAFbx and MuRF-1, respectively (5, 12, 49, 68). Loss of Fbox32/atrogin-1 or Trim63/MuRF-1 attenuate muscle loss induced by denervation (5, 24), and Trim63/MuRF-1 knockout mice are less susceptible to amino-acid deprivation-induced atrophy (34).
Two orthopedically relevant models of muscle atrophy, immobilization induced by casting and unloading induced by tenotomy, have been developed. These represent improved preclinical models that may more accurately reflect conditions observed in human populations. Unlike other models of muscle atrophy, these two models have not been extensively characterized by molecular profiling. Therefore, the primary goal of this study was to identify a common set of genes regulated during different models of muscle atrophy and determine if skeletal muscle atrophy induced by immobilization due to casting or by unloading due to tendon resection follow the same molecular mechanisms.
MATERIAL AND METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Wyeth Research, Cambridge, MA, and were conducted in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care. Muscle atrophy was induced through unloading by tendon laceration (tenotomy) and immobilization induced by hindlimb casting. For the tenotomy, 8-wk-old male C57Bl/6 mice were anesthetized by ether inhalation and subsequently intramuscularly injected with 0.1 ml/g ketamine-xylazine. The right Achilles tendon just proximal to the calcaneus was cut with a sterile scalpel. The mice were allowed to recover and move freely about their cage. An additional cohort of animals was sham-operated, which involved isolation and manipulation of the Achilles tendon without laceration. For immobilization, the entire right hindlimb of 8-wk-old male C57Bl/6 mice were casted using a layer of gauze padding wrapped in fiberglass tape. The foot was held in a neutral position to ensure the gastrocnemius, soleus, and plantaris muscles were not loaded during the period of casting. Muscle atrophy was also induced through administration of glucocorticoid or by blocking neuromuscular signaling through injection of botulinum toxin A (Botox). Glucocorticoid treatments were performed through subcutaneous implantation of a pellet containing 5 mg dexamethasone. Pellets were designed for a 21-day continuous release (Innovative Research of America, Sarasota, FL). Botox (20 pg; Sigma, St. Louis, MO) or saline was administered by intramuscular injection directly into the right quadriceps muscle. For all studies, animals were killed after 2, 7, or 14 days. The left and right gastrocnemius muscles were carefully removed, weighed, flash-frozen in liquid nitrogen, and stored at −80°C. Gastrocnemius wet weights were determined and paired t-tests between injured and contralateral controls (tenotomy and casted animals) or unpaired t-tests between treated and control (Botox- and glucocorticoid-treated animals) were performed.
RNA preparation and hybridization.
RNA extraction, isolation, and labeling were performed as previously described (29). cRNA (10 μg) was fragmented and hybridized to GeneChip® Mouse Genome 430 2.0 arrays according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). For each array, all probe sets were normalized to a mean signal intensity value of 100. The default GeneChip Operating Software statistical values were used for all analyses. Raw data can be found in the Gene Expression Omnibus repository under accession number GSE25908 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25908).
Identification of differentially expressed genes.
Expression values for all probe sets were subjected to locally weighted scatter-plot smoothing (LOWESS) transformation. Correlation analyses and hierarchical clustering verified that all replicas had similar expression patterns. Only transcripts that were expressed over 20 signal units in 80% of the samples in at least one group were used for further analyses. For each model, expression values were log2 transformed and a two-way analysis of variance (ANOVA) was performed. Transcripts were considered to be regulated if the P value based on two-way ANOVA analyses using time and treatment parameters and evaluating treatment was <0.01 and the fold change between any two groups was >1.5.
Identification of significantly regulated gene sets.
Significantly regulated biological pathways were identified using a modified version of the SigPathway algorithm (8) incorporating a modified normalization routine and using gene sets defined by the Molecular Signatures Database (60). A gene set was considered significant when q1 ≤ 0.05 and q2 ≤ 0.05 where q1 or q2 are the permutation-based false-discovery rates for the Q1 or Q2 hypotheses (see Ref. 62 for explanation of Q1 and Q2).
RT-PCR.
Candidate gene expression was confirmed by real-time quantitative RT-PCR using primer/probe sets from Applied Biosystems (Foster City, CA) and a custom-derived 384-well micro fluidic card (Applied Biosystems) containing assays for Ctsk (Mm00484039_m1), Fbxo32 (Mm00499523_m1), Mmp13 (Mm00439495_g1), Casp3 (Mm01195085_m1), Trim63 (Mm01188690_m1), Ctss (Mm00457902_m1), Mmp2 (Mm00439506_m1), and Capn2 (Mm00486669_m1), and normalized to four endogenous control genes Gapdh (Hs02758991_g1), Gusb (Mm03003537_s1), Polr2a (Rn01752026_m1), and 18s (Hs99999901_s1).
Histology.
Tissue samples were flash-frozen and sectioned for histochemical analysis. Muscle serial cross-sections (10 μm) were cut and stained with Alexa 555-conjugated wheat germ agglutinin (Invitrogen, Carlsbad, CA) to stain membrane-bound and extracellular sialic acid and N-acetylglucosaminyl residues. Stained sections were examined using an Eclipse E8000M light microscope (Nikon, Melville, NY) coupled to an Axiocam HRc ultra high-resolution color charge-coupled device camera (Carl Zeiss, Thornwood, NY). Muscle fiber types were identified by immunohistochemical analyses using antibodies for myosin heavy chain I and myosin heavy chain II purified from the mouse hybridoma cell lines BA-F8 and SC-75 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and Alexa 488-conjugated goat anti-mouse IgG or IgM (Invitrogen). Fiber area was determined from captured images using the Image-J software (National Institutes of Health, http://rsb.info.nih.gov/ij/). The total area of three distinct sections from three distinct animals were analyzed at ×4 magnification. Between 2,000 and 6,000 individual fibers were used in the calculations.
Proteasome proteolytic activity assay.
Skeletal muscle lysates for cathepsin and proteasome proteolytic activity assays were prepared using a motorized Dounce homogenizer in three volumes of ice-cold buffer (20 mM Tris, 1 mM EDTA, 1 mM EGTA, 1% glycerol, and 2 mM DTT). Homogenized samples were centrifuged at 13,000 g for 30 min at 4°C. Proteasome activity was measured in the isolated supernatants and cathepsin activity was measured in the pellets as described in the following section.
Cathepsin activity assay.
Pellets of homogenized skeletal muscle tissues were resuspended in 2 volumes of lysis buffer (50 mM sodium acetate, pH 5.0, 200 mM NaCl, and 0.1% Triton-X100), sonicated, and centrifuged at 13,000 g for 30 min at 4°C. Supernatants were stored at −80°C. Total protein content in the supernatants was determined using a DC protein assay (Bio-Rad, Hercules, CA). Total cathepsin activity was measured using 10 mM omni-cathepsin fluorogenic substrate (benzyloxycarbonyl-Phe-Arg-AMC; Enzo Life Sciences, New York, NY) and 50 μg of protein extract as previously described (53). Fluorescence was monitored at excitation and emission wavelengths of 380 nm and 460 nm, respectively, every 5 min for 15 min at 37°C. Negative controls were performed in the presence of 5 mM E64 (Sigma). Rates of cleavage were calculated based on the amount of AMC peptide cleavage, derived from a standard curve of free AMC, per mg of protein per hour.
Proteasome activity assay.
The chymotrypsin-like activity of the 20S proteosome was measured by cleavage of a fluorescent substrate, succinyl-Leu-Leu-Val-Tyr-AMC peptide (Millipore, Billerica, MA), using 1 μg total protein in the presence of 0.05% SDS as previously described (26, 63). Fluorescence was monitored at excitation and emission wavelengths of 380 nm and 460 nm, respectively, for 60 min at 37°C. Negative controls were performed in the presence of lactacystin. Activity was calculated based on the amount of peptide cleavage, derived from a standard curve, per mg of protein per minute.
Matrix metalloproteinase 2 activity assay.
Matrix metalloproteinase 2 (MMP2) activity was measured using the Biotrak MMP2 activity assay system (GE Healthcare, Piscataway, NJ). Flash-frozen skeletal muscle tissues were homogenized into powders with a dry, ice-cooled mortar and pestle. Five volumes of protein extraction buffer (50 mM HEPES, pH 7.5, 25 mM NaCl, 5 mM CaCl2, and 0.005% Brij-35) without EDTA or EGTA containing a protease inhibitor cocktail (Sigma) were added. Samples were rotated at 4°C for 30 min before centrifugation at 13,000 g for 30 min at 4°C. The supernatants were collected, aliquoted, and stored at −80°C. Protein concentrations were determined by bicinchoninic acid assays (Pierce, Rockford, IL). Active MMP2 and pro-MMP2 (inactive) levels were measured in tissue homogenates following the manufacturer's instructions. Reactions were incubated for 6 to 8 h before MMP2 levels were measured. Pro-MMP2 was activated using p-aminophenylmercuric acetate (APMA). Active MMP2 was measured in the absence of APMA. The concentrations of active MMP2 were interpolated from standard curves and were normalized to the protein concentration of each sample.
RESULTS
Animal models.
Gastrocnemius muscle weights decreased in two clinically relevant animal models of muscle disuse atrophy: hindlimb casting and tenotomy (Fig. 1). Two weeks after injury or immobilization, both tenotomy and casting resulted in decreased gastrocnemius muscle weights of ∼20% compared with their respective contralateral controls (27.2 ± 4.7%, P < 1.3 × 10−7 and 23.7 ± 8.7%, P < 0.00089 for tenotomy and casting, respectively) on day 14. While comparable muscle weight decreases were observed in both models on day 14, the rate of muscle weight loss differed. After 2 days, muscle weight from cast-immobilized animals decreased by 7.5 ± 6.9% (P < 0.003), while muscle weight from tenotomy-treated animals were not significantly different (Fig. 1). Our studies included 8-wk-old animals and the cohort of animals in the cast-immobilized group were still actively growing, while the cohort of animals in the tenotomy-treated animals were not. By day 14, contralateral control muscle weights from the cast-immobilized animals increased by 15.3 ± 8.3% (P < 0.001, compared with the same animal at day 0), while muscle weights from contralateral control muscles from tenotomy-treated animals did not change significantly. However, the differences in the contralateral control muscle weight is independent of treatment (cast-immobilization or tenotomy) as muscle weights from the contralateral control in either the cast-immobilized animals or the tenotomy-treated animals were not significantly different from their respective cage-control animals. To control for growth and other animal-to-animal variability, comparisons, when applicable, were performed with contralateral limbs for controls.
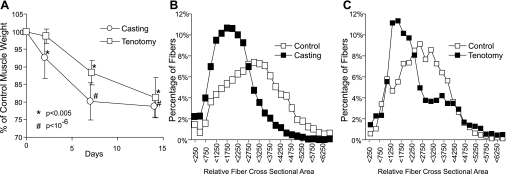
Fig. 1.
A: percent muscle loss of rat gastrocnemius muscles 2, 7, and 14 days after casting (circles, n = 8) or Achilles tendon resection (squares, n = 8) compared with contralateral controls. Losses were calculated by comparison to the respective contralateral control and were expressed as average losses. Error bars represent standard deviation. *P < 0.005 and #P < 1 × 10−6 compared with contralateral control limbs. B: frequency histogram of relative muscle fiber area 14 days after casting compared with contralateral control. Measurements were taken from 4,523 control and 5,448 cast-treated fibers from up to 5 different sections from 4 individual animals. C: frequency histogram of relative muscle fiber area after 14 days after Achilles tendon resection compared with contralateral control . Measurements were taken from 3,402 control and 2,019 tenotomy treated fibers up to 3 different sections from 2 individual animals.
Microscopic evaluation (Fig. 1) of the muscles indicated that the loss of muscle mass was associated with a decrease in the size of muscle fibers and increased interstitial space in both atrophy models. Histomorphometric analysis showed a decrease in muscle fiber size suggesting that the active atrophy, and not a lack of growth resulted in decreased muscle size. By day 14, the mean fiber size in gastrocnemius muscles decreased 58% (P < 0.00001) and 60% (P < 0.00001; Fig. 1, B and C) compared with the contralateral limb in the cast-immobilized and tenotomy-treated muscles, respectively.
Fiber type switching.
Microscopic evaluation of gastrocnemius muscles (n = 3) revealed that the muscles consisted primarily of type II fibers with very little, if any, type I fibers. After injury, the ratio of type II to type I fibers did not change (data not shown). However, atrophied muscles had lower mRNA expression levels of type I slow-twitch muscle markers. Most changes in muscle-marker gene expression in the cast-immobilized muscles were evident by day 7 with the largest differences seen by day 14. In contrast, the largest changes in muscle-marker expression in the tenotomy-treated muscles were seen by day 2 and returned to near control levels by day 14. Muscles from both cast-immobilized and tenotomy-treated limbs had reduced transcript levels for the slow-twitch muscle marker myosin heavy chain isoform I (Myh7; 0.6-fold by day 14, and 0.2-fold at day 2, respectively; Table 1). The transcript levels for a number of other slow-twitch muscle markers, including troponin I (Tnni1), troponin C (Tnnc), troponin T1 (Tnnt1), myosin light chain 2 (Myl2), and myosin light chain 3 (Myl3; Table 1), had reduced expression in both cast-immobilized and tenotomy-treated muscles. There was no change in expression of any of the fast-twitch myosin heavy chain genes (Myh1, Myh2, or Myh4), any type II troponin genes (Tnni2, Tnnc2, or Tnnt3), or the type II myosin light chain gene Myl1 (Table 1). These findings are likely due to high levels of expression that were above the dynamic range of this assay.
Table 1.
Top regulated genes involved in protein degradation and muscle function in casting and tenotomy-induced muscle atrophy
| Casting Fold Change |
Tenotomy Fold Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Gene Description | Casting P Value | Tenotomy P Value | 3 Days | 7 Days | 14 Days | 3 Days | 7 Days | 14 Days |
| Collagen Metabolism | |||||||||
| Mmp2 | matrix metalloproteinase 2 | 3.1E-06 | 9.0E-14 | 1.5 | 1.1 | 1.4 | 2.6 | 4.7 | 6.2 |
| Mmp14 | matrix metallopeptidase 14 | 9.3E-04 | 6.7E-13 | 1.2 | 1.0 | 1.6 | 3.1 | 7.3 | 7.9 |
| Mmp3 | matrix metalloproteinase 3 | 2.4E-02 | 1.4E-10 | 0.6 | 1.3 | 3.7 | 2.5 | 18.0 | 6.1 |
| Mmp11 | matrix metalloproteinase 11 | 3.0E-02 | 1.3E-06 | 2.2 | 1.3 | 1.1 | 2.8 | 2.0 | 2.1 |
| Adamts2 | a disintegrin-like and metalloprotease type 2 | 3.9E-02 | 2.5E-09 | 1.1 | 1.0 | 1.3 | 1.7 | 2.4 | 2.0 |
| Mmp13 | matrix metalloproteinase 13 | 4.4E-01 | 8.0E-14 | 0.6 | 1.8 | 2.5 | 8.4 | 1217 | 406.6 |
| Caspase Activity | |||||||||
| Casp8 | caspase 8 | 5.4E-02 | 6.3E-10 | 0.9 | 1.1 | 1.5 | 2.9 | 1.7 | 1.7 |
| Casp6 | caspase 6 | 2.3E-02 | 2.0E-05 | 1.0 | 1.0 | 1.4 | 2.6 | 1.8 | 1.4 |
| Casp3 | caspase 3 | 1.5E-02 | 3.0E-10 | 1.0 | 1.4 | 1.2 | 3.3 | 2.1 | 1.8 |
| Casp11 | caspase 11 | 6.3E-01 | 1.1E-08 | 0.6 | 1.2 | 1.5 | 2.6 | 2.3 | 1.9 |
| Casp1 | caspase 1 | 1.1E-01 | 1.4E-05 | 0.9 | 1.2 | 1.4 | 7.6 | 2.2 | 1.9 |
| Apaf1 | apoptotic protease activating factor 1 | 3.8E-01 | 1.7E-09 | 0.9 | 1.0 | 1.2 | 2.5 | 1.8 | 1.3 |
| Mitochodrial Function | |||||||||
| Abcb6 | ATP-binding cassette, sub-family B, 6 | 1.2E-01 | 2.8E-07 | 1.0 | 0.9 | 0.9 | 1.0 | 0.6 | 0.7 |
| Acaa2 | mitochondrial 3-oxoacyl-Coenzyme A thiolase | 8.4E-08 | 1.4E-06 | 1.0 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Acas2l | acetyl-Coenzyme A synthetase 2 like | 6.0E-07 | 1.7E-01 | 0.6 | 0.6 | 0.8 | 1.1 | 1.2 | 1.1 |
| Bckdhb | branched chain ketoacid dehydrogenase E1 | 6.6E-01 | 1.6E-06 | 1.1 | 1.2 | 0.8 | 0.6 | 0.9 | 0.8 |
| Cox6a1 | cytochrome c oxidase, subunit VI a1 | 1.6E-01 | 9.6E-10 | 0.8 | 1.0 | 1.1 | 2.1 | 1.5 | 1.6 |
| Dlat | dihydrolipoamide S-acetyltransferase | 4.9E-08 | 7.8E-12 | 1.0 | 0.7 | 0.8 | 0.5 | 0.6 | 0.6 |
| Mrpl17 | mitochondrial ribosomal L17 | 2.3E-09 | 2.6E-04 | 0.6 | 0.8 | 0.6 | 0.7 | 0.7 | 0.7 |
| Mrpl51 | mitochondrial ribosomal L51 | 1.9E-12 | 4.0E-07 | 0.6 | 0.7 | 0.7 | 0.5 | 0.6 | 0.8 |
| Ndufab1 | NADH dehydrogenase | 1.1E-05 | 3.2E-06 | 0.7 | 0.6 | 0.8 | 0.6 | 0.5 | 0.7 |
| Ndufs4 | NADH dehydrogenase 4 | 1.6E-01 | 5.5E-07 | 1.3 | 0.8 | 0.8 | 0.6 | 0.6 | 0.7 |
| Timm22 | translocase of inner mitochondrial membrane 2 | 2.3E-03 | 9.5E-05 | 0.9 | 0.8 | 0.7 | 0.6 | 0.6 | 0.8 |
| Timm8a | translocase of inner mitochondrial membrane 8 | 3.1E-10 | 2.2E-01 | 0.6 | 0.8 | 0.9 | 1.0 | 0.9 | 0.9 |
| Muscle Biogenesis | |||||||||
| Acta2 | actin, alpha 2 | 2.6E-05 | 6.6E-02 | 1.4 | 1.5 | 1.2 | 0.6 | 0.9 | 1.0 |
| Actc1 | actin, alpha, cardiac | 5.9E-07 | 1.8E-08 | 1.1 | 0.2 | 0.5 | 0.4 | 0.2 | 0.7 |
| Actg1 | actin, gamma, cytoplasmic 1 | 3.6E-05 | 1.0E-11 | 0.6 | 0.9 | 1.1 | 2.2 | 1.5 | 1.8 |
| Dmd | dystrophin, muscular dystrophy | 7.4E-05 | 6.7E-05 | 2.0 | 1.3 | 1.3 | 0.4 | 0.5 | 0.8 |
| Igf1 | Insulin-like growth factor 1 | 9.7E-01 | 7.8E-08 | 0.8 | 1.0 | 1.3 | 1.7 | 2.3 | 2.3 |
| Mef2c | myocyte enhancer factor 2C | 5.1E-05 | 6.3E-02 | 1.5 | 1.4 | 1.4 | 0.5 | 0.8 | 1.1 |
| Mybph | myosin binding protein H | 5.4E-06 | 1.7E-02 | 0.3 | 0.4 | 1.0 | 0.6 | 1.5 | 5.2 |
| Myh1 | myosin, heavy polypeptide 1 | 2.1E-01 | 4.2E-01 | 1.0 | 1.0 | 0.9 | 0.9 | 0.9 | 1.1 |
| Myh2 | myosin, heavy polypeptide 2 | 2.4E-02 | 1.2E-02 | 1.0 | 0.9 | 1.0 | 0.7 | 0.8 | 1.0 |
| Myh3 | myosin, heavy polypeptide 3 | 3.3E-07 | 4.2E-01 | 0.5 | 0.3 | 0.5 | 0.9 | 1.2 | 1.3 |
| Myh4 | myosin, heavy polypeptide 4 | 1.9E-01 | 2.6E-02 | 1.0 | 1.0 | 1.0 | 0.7 | 0.8 | 0.9 |
| Myh7 | myosin, heavy polypeptide 7 | 2.1E-03 | 1.1E-02 | 1.1 | 0.8 | 0.6 | 0.2 | 0.6 | 0.9 |
| Myl2 | myosin, light polypeptide 2, | 9.9E-12 | 4.0E-10 | 0.8 | 0.5 | 0.6 | 0.1 | 0.2 | 0.4 |
| Myl3 | myosin, light polypeptide 3 | 7.8E-06 | 1.3E-09 | 1.0 | 0.6 | 0.7 | 0.3 | 0.2 | 0.4 |
| Myog | myogenin | 4.7E-01 | 4.0E-11 | 0.9 | 1.0 | 1.4 | 3.6 | 1.7 | 1.8 |
| Myom2 | myomesin 2 | 3.1E-03 | 5.6E-05 | 1.2 | 1.0 | 1.0 | 0.6 | 0.7 | 1.1 |
| Tnnc1 | troponin C, cardiac/slow skeletal | 3.4E-03 | 2.3E-02 | 1.1 | 0.7 | 0.7 | 0.5 | 0.6 | 0.7 |
| Tnnc2 | troponin C2, fast | 2.3E-01 | 3.2E-01 | 0.9 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Tnni1 | troponin I, skeletal, slow 1 | 1.3E-08 | 3.8E-01 | 0.8 | 0.5 | 0.5 | 0.6 | 0.8 | 1.0 |
| Tnni2 | troponin I, skeletal, fast 2 | 3.6E-02 | 4.6E-05 | 1.1 | 0.9 | 0.6 | 0.5 | 0.6 | 0.4 |
| Tnnt1 | troponin T1, skeletal, slow | 1.4E-03 | 9.7E-01 | 1.1 | 0.7 | 0.6 | 0.6 | 1.1 | 1.6 |
| Tnnt3 | troponin T3, skeletal, fast | 3.1E-01 | 3.1E-01 | 1.1 | 1.1 | 0.9 | 1.0 | 1.0 | 0.9 |
| Tpm1 | tropomyosin 1, alpha | 1.6E-02 | 2.1E-06 | 0.7 | 0.9 | 1.1 | 1.4 | 1.5 | 1.7 |
| Tpm2 | tropomyosin 2, beta | 1.6E-08 | 1.5E-12 | 1.0 | 0.8 | 0.8 | 0.4 | 0.4 | 0.8 |
| Tpm3 | tropomyosin 3, gamma | 5.7E-01 | 2.8E-07 | 0.7 | 1.1 | 1.2 | 2.5 | 1.6 | 1.6 |
| Tpm4 | tropomyosin 4 | 3.5E-05 | 2.5E-09 | 0.5 | 0.8 | 1.2 | 2.8 | 2.3 | 1.9 |
| Lysosomal Function | |||||||||
| Apg4d | APG4 (ATG4) autophagy-related homolog D | 8.6E-10 | 9.5E-08 | 0.7 | 0.8 | 0.7 | 0.6 | 0.7 | 0.7 |
| Ctsc | cathepsin C | 2.5E-02 | 1.2E-14 | 1.0 | 1.1 | 1.5 | 4.2 | 2.0 | 1.4 |
| Lamp2 | lysosomal membrane glycoprotein 2 | 3.8E-02 | 5.8E-10 | 1.2 | 1.1 | 1.0 | 2.3 | 2.4 | 2.3 |
| Lip1 | lysosomal acid lipase 1 | 5.4E-01 | 1.7E-13 | 0.9 | 1.0 | 1.3 | 3.6 | 2.2 | 1.9 |
| Naglu | alpha-N-acetylglucosaminidase | 3.6E-06 | 1.2E-12 | 1.5 | 1.1 | 1.1 | 1.9 | 1.7 | 1.8 |
| Npc2 | Niemann Pick type C2 | 3.7E-02 | 4.9E-14 | 1.0 | 1.0 | 1.2 | 2.9 | 1.9 | 1.6 |
| Ctsb | cathepsin B | 7.1E-01 | 8.8E-16 | 0.9 | 1.0 | 1.1 | 3.3 | 1.7 | 1.4 |
| Ctsc | cathepsin C | 2.5E-02 | 1.2E-14 | 1.0 | 1.1 | 1.5 | 4.2 | 2.0 | 1.4 |
| Ctse | cathepsin E | 9.9E-01 | 1.7E-03 | 0.9 | 1.0 | 1.2 | 0.6 | 0.9 | 0.5 |
| Ctsh | cathepsin H | 7.2E-01 | 7.2E-14 | 0.7 | 1.0 | 1.4 | 6.6 | 3.0 | 2.2 |
| Ctsk | cathepsin K | 1.2E-04 | 1.9E-14 | 1.6 | 1.1 | 1.5 | 2.7 | 8.3 | 8.4 |
| Ctss | cathepsin S | 7.5E-03 | 1.0E-16 | 0.8 | 1.4 | 1.9 | 15.8 | 4.3 | 2.6 |
| Ctsz | cathepsin Z | 4.1E-01 | 3.8E-12 | 0.9 | 1.0 | 1.2 | 3.0 | 2.0 | 1.5 |
| Gla | galactosidase, alpha | 4.5E-01 | 1.2E-02 | 1.0 | 1.0 | 1.2 | 3.4 | 1.2 | 1.1 |
| Glb1 | galactosidase, beta 1 | 8.4E-03 | 3.1E-07 | 0.8 | 0.9 | 0.8 | 2.9 | 1.7 | 1.8 |
| Gusb | glucuronidase, beta | 7.6E-02 | 4.4E-12 | 0.7 | 0.9 | 1.1 | 3.7 | 1.6 | 1.4 |
| Hexa | hexosaminidase A | 8.1E-03 | 6.5E-15 | 0.8 | 1.0 | 1.0 | 2.5 | 1.8 | 1.5 |
| Hexb | hexosaminidase B | 4.4E-01 | 4.9E-12 | 1.1 | 1.1 | 1.0 | 7.0 | 2.9 | 2.5 |
| Lip1 | lysosomal acid lipase 1 | 5.4E-01 | 1.7E-13 | 0.9 | 1.0 | 1.3 | 3.6 | 2.2 | 1.9 |
| Man1a | mannosidase 1, alpha | 4.2E-01 | 4.0E-09 | 0.9 | 0.9 | 1.3 | 2.3 | 2.1 | 1.8 |
| Psap | prosaposin | 3.6E-01 | 1.0E-07 | 1.0 | 1.0 | 1.0 | 1.6 | 1.1 | 1.0 |
| Proteasome Function | |||||||||
| Psmc1 | protease 26S subunit, ATPase 1 | 1.7E-14 | 4.2E-01 | 1.6 | 1.3 | 1.1 | 1.1 | 1.0 | 1.0 |
| Psmc4 | proteasome 26S subunit, ATPase, 4 | 4.8E-09 | 4.7E-01 | 1.6 | 1.2 | 1.0 | 1.2 | 0.9 | 1.0 |
| Psmd2 | proteasome 26S subunit, non-ATPase, 2 | 2.5E-13 | 2.2E-02 | 1.3 | 1.6 | 1.1 | 1.3 | 1.0 | 1.0 |
| Ubc | ubiquitin C | 2.0E-06 | 5.9E-04 | 2.7 | 1.3 | 1.0 | 0.7 | 0.7 | 0.7 |
| Fbxo32 | F-box only protein 32 | 6.38E-09 | 0.0819765 | 2.7 | 1.9 | 1.3 | 1.5 | 1.3 | 0.8 |
| Trim63 | tripartite motif-containing 63 | 1.08E-05 | 0.1525747 | 1.9 | 1.1 | 1.0 | 1.6 | 1.0 | 0.9 |
Muscle regulatory factors.
Casting-induced atrophy did not regulate any of the known muscle regulatory factors, such as Myog, Myod1, Myf6, and Myf5. In contrast, tenotomy treatment increased Myog expression ∼3.5-fold by day 2 postinjury, which then returned to near control levels by day 7. Tenotomy also induced a small, but significant, increase in expression of Myf6 on day 2 and day 14 (Table 1).
Gene expression changes.
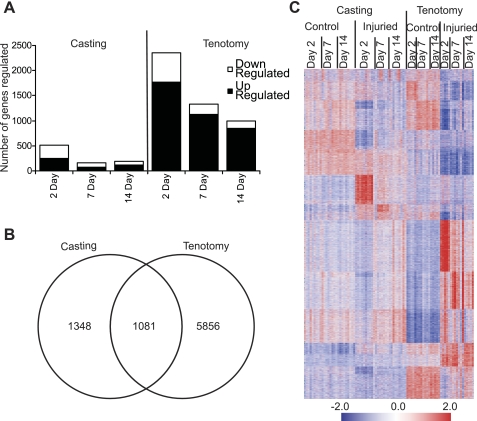
Genome-wide expression profiling identified 8,285 unique regulated transcripts between casted-immobilized and tenotomy treated limbs (ANOVA P < 0.01, fold change >1.5). Of these, 2,429 (1,301 up- and 1,128 downregulated) transcripts were regulated in the cast-immobilized muscles and 6,937 (3,543 up- and 3,394 downregulated) transcripts were regulated in the tenotomy-treated muscles (Tables 1 and 2, Fig. 2). The changes in gene expression in the tenotomy-treated animals were largely independent of the surgical procedure. Only 109 of the 6,937 (<1.6%) transcripts regulated in tenotomy-treated muscles were also regulated in sham-operated control muscles, and only 75 (<1.1%) were similarly induced or repressed in the treated and untreated animals. Casting and tenotomy induced significantly different qualitative and quantitative transcriptional responses and model-specific effects accounted for the majority of observed changes. Only 1,081 of the transcripts were regulated in both models (Fig. 2) with only 700 regulated in the same direction.
Table 2.
Top regulated genes in casting- and tenotomy-induced muscle atrophy
| Casting Fold Change |
Tenotomy Fold Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Gene Description | Casting P Value | Tenotomy P Value | 3 Days | 7 Days | 14 Days | 3 Days | 7 Days | 14 Days |
| Casting | |||||||||
| Bdh | 3-hydroxybutyrate dehydrogenase | 1.2E-13 | 1.7E-10 | 0.4 | 0.2 | 0.3 | 0.5 | 0.3 | 0.3 |
| Glcci1 | glucocorticoid-induced transcript 1 | 1.8E-12 | 2.2E-02 | 6.8 | 1.9 | 1.6 | 0.9 | 0.8 | 0.6 |
| Csrp3 | cysteine and glycine-rich protein 3 | 3.9E-10 | 2.0E-01 | 0.1 | 0.3 | 0.5 | 0.8 | 0.8 | 2.7 |
| Hspa1a | heat shock protein 1B | 5.6E-10 | 1.6E-05 | 0.2 | 0.4 | 0.6 | 2.6 | 1.2 | 2.8 |
| Hspa1b | heat shock protein 1B | 1.1E-09 | 1.9E-06 | 0.2 | 0.4 | 0.6 | 3.5 | 1.6 | 3.3 |
| Tfrc | transferrin receptor | 3.2E-09 | 9.4E-07 | 0.2 | 0.3 | 0.9 | 0.4 | 0.4 | 0.4 |
| P2ry1 | purinergic receptor P2Y | 2.7E-08 | 6.2E-02 | 0.1 | 0.4 | 0.4 | 0.4 | 0.4 | 1.0 |
| Actc1 | actin, alpha, cardiac | 5.9E-07 | 1.8E-08 | 1.1 | 0.2 | 0.5 | 0.4 | 0.2 | 0.7 |
| Nfe2l2 | nuclear factor, erythroid derived 2, like 2 | 1.8E-05 | 9.1E-01 | 4.5 | 2.0 | 1.8 | 1.1 | 1.1 | 0.9 |
| Stmn4 | stathmin-like 4 | 1.9E-05 | 7.3E-04 | 0.2 | 0.2 | 0.7 | 0.2 | 0.3 | 0.8 |
| Pnmt | phenylethanolamine-N-methyltransferase | 8.1E-05 | 4.3E-02 | 6.4 | 1.7 | 1.1 | 0.7 | 0.6 | 0.4 |
| Gm761 | gene model 761, (NCBI) | 3.7E-04 | 9.4E-01 | 4.9 | 2.2 | 1.4 | 0.9 | 2.9 | 0.4 |
| Serpina3n | serine (or cysteine) proteinase inhibitor | 3.8E-04 | 4.4E-14 | 0.9 | 2.4 | 6.3 | 15.1 | 81.6 | 18.0 |
| Clec4n | C-type lectin domain family 4, member n | 6.2E-04 | 4.5E-05 | 0.9 | 3.7 | 4.4 | 8.4 | 4.5 | 3.8 |
| Cd209e | Cd209e antigen | 1.7E-03 | 4.5E-01 | 1.2 | 1.1 | 6.8 | 1.7 | 1.2 | 1.0 |
| Srp54 | signal recognition particle 54 | 2.8E-03 | 9.5E-01 | 4.1 | 1.7 | 1.0 | 1.3 | 0.7 | 1.0 |
| St13 | suppression of tumorigenicity 13 | 3.5E-03 | 1.9E-02 | 0.7 | 0.2 | 0.7 | 3.6 | 2.7 | 0.7 |
| Acox2 | acyl-Coenzyme A oxidase 2 | 5.0E-03 | 2.9E-04 | 4.1 | 1.4 | 1.1 | 13.0 | 1.2 | 1.3 |
| Gsta3 | glutathione S-transferase, alpha 3 | 5.5E-03 | 3.7E-01 | 4.3 | 1.5 | 0.9 | 0.9 | 1.0 | 0.6 |
| Ctsg | cathepsin G | 7.8E-03 | 5.9E-01 | 1.2 | 4.3 | 1.8 | 1.4 | 1.0 | 1.3 |
| Tenotomy | |||||||||
| Gpnmb | glycoprotein (transmembrane) nmb | 1.1E-08 | 1.2E-17 | 1.9 | 1.6 | 1.5 | 23.0 | 3.9 | 3.9 |
| Lzp-s | P lysozyme structural | 8.3E-02 | 6.6E-16 | 0.8 | 1.3 | 1.7 | 22.7 | 3.9 | 2.5 |
| Postn | periostin, osteoblast specific factor | 4.7E-01 | 3.0E-15 | 0.6 | 0.6 | 1.8 | 19.9 | 19.1 | 14.2 |
| Sln | sarcolipin | 3.0E-01 | 2.8E-14 | 1.4 | 1.4 | 0.8 | 2.2 | 15.8 | 22.4 |
| Mmp13 | matrix metalloproteinase 13 | 4.4E-01 | 8.0E-14 | 0.6 | 1.8 | 2.5 | 8.4 | 1217 | 407 |
| Cthrc1 | collagen triple helix repeat containing 1 | 8.5E-01 | 1.4E-13 | 0.5 | 0.7 | 2.3 | 28.7 | 95.1 | 44.0 |
| Mpeg1 | macrophage expressed gene 1 | 5.2E-03 | 5.9E-13 | 1.0 | 1.6 | 1.9 | 55.2 | 8.4 | 4.5 |
| C1qtnf3 | C1q and tumor necrosis 3 | 5.0E-02 | 1.5E-12 | 0.7 | 0.4 | 1.0 | 9.3 | 40.4 | 25.0 |
| Spp1 | secreted phosphoprotein 1 | 5.8E-02 | 5.2E-12 | 0.6 | 1.9 | 1.9 | 24.0 | 11.4 | 4.2 |
| Tlr1 | toll-like receptor 1 | 3.5E-02 | 6.5E-12 | 1.2 | 1.2 | 3.1 | 23.7 | 9.2 | 7.8 |
| Ms4a7 | membrane-spanning 4-domains | 6.7E-02 | 1.1E-11 | 0.7 | 1.1 | 2.1 | 33.7 | 4.5 | 3.2 |
| Hcls1 | hematopoietic cell specific Lyn substrate 1 | 7.3E-01 | 3.6E-11 | 0.8 | 1.0 | 1.6 | 25.6 | 5.4 | 2.8 |
| Ccl12 | chemokine (C-C motif) ligand 12 | 3.5E-02 | 6.1E-11 | 0.4 | 3.8 | 6.4 | 22.4 | 20.0 | 9.2 |
| Cxcl16 | chemokine (C-X-C motif) ligand 16 | 3.4E-01 | 1.8E-10 | 1.3 | 0.8 | 1.9 | 37.1 | 8.8 | 4.3 |
| Lpxn | leupaxin | 5.0E-01 | 3.1E-10 | 0.6 | 1.3 | 1.8 | 20.9 | 4.4 | 4.4 |
| Birc5 | baculoviral IAP repeat-containing 5 | 1.9E-01 | 3.5E-10 | 0.9 | 1.7 | 2.2 | 22.0 | 17.9 | 5.3 |
| Trem2 | triggering receptor on myeloid cells 2 | 2.7E-02 | 1.9E-09 | 1.1 | 1.1 | 2.5 | 36.8 | 3.0 | 2.1 |
| Pscd4 | pleckstrin homology, Sec7 | 4.1E-01 | 1.9E-09 | 0.5 | 1.2 | 1.2 | 22.7 | 6.7 | 4.2 |
| Cdc2a | cell division cycle 2 homolog A | 5.2E-01 | 1.9E-09 | 0.4 | 1.7 | 2.8 | 28.6 | 17.0 | 6.8 |
| Myo1f | myosin IF | 5.6E-01 | 2.4E-09 | 0.5 | 1.2 | 1.3 | 20.2 | 4.8 | 9.4 |
| Il10ra | interleukin 10 receptor, alpha | 2.5E-01 | 2.4E-08 | 0.9 | 1.6 | 1.5 | 21.3 | 4.0 | 1.8 |
| Aif1 | allograft inflammatory factor 1 | 4.2E-02 | 3.3E-08 | 0.9 | 1.4 | 2.1 | 25.7 | 5.6 | 2.6 |
| Mfap4 | microfibrillar-associated protein 4 | 1.5E-03 | 4.7E-08 | 0.8 | 0.5 | 0.6 | 21.4 | 10.9 | 18.8 |
| Tcfec | transcription factor EC | 5.3E-01 | 1.0E-07 | 0.4 | 1.9 | 2.0 | 30.1 | 5.0 | 1.7 |
| Ms4a6d | membrane-spanning 4-domains | 2.0E-01 | 2.8E-07 | 0.5 | 1.4 | 2.9 | 22.5 | 7.5 | 2.4 |
| Ly9 | lymphocyte antigen 9 | 9.8E-01 | 4.3E-07 | 1.1 | 0.7 | 1.4 | 27.8 | 4.6 | 0.9 |
| Bub1 | budding uninhibited by benzimidazoles 1 | 4.7E-02 | 5.0E-07 | 1.2 | 2.0 | 2.3 | 46.9 | 5.0 | 1.5 |
| Ccr1 | chemokine (C-C motif) receptor 1 | 7.4E-02 | 5.8E-07 | 0.6 | 1.8 | 2.0 | 20.9 | 2.2 | 1.0 |
| C5r1 | complement component 5, receptor 1 | 8.5E-01 | 3.5E-06 | 0.6 | 1.3 | 1.5 | 19.1 | 1.7 | 1.5 |
| Prss35 | protease, serine, 35 | 7.5E-01 | 1.5E-05 | 1.1 | 1.0 | 1.1 | 1.1 | 5.5 | 36.8 |
| Uhrf1 | ubiquitin-like | 1.2E-01 | 2.1E-05 | 0.6 | 2.0 | 3.0 | 22.5 | 2.8 | 1.9 |
| Egr3 | early growth response 3 | 7.1E-01 | 3.9E-05 | 0.9 | 0.8 | 1.2 | 3.1 | 1.9 | 20.9 |
| Fosl1 | fos-like antigen 1 | 4.3E-02 | 4.3E-05 | 0.9 | 1.5 | 3.0 | 20.0 | 0.9 | 1.5 |
| Slc15a3 | solute carrier family 15, member 3 | 4.0E-01 | 1.4E-04 | 0.3 | 0.9 | 1.7 | 22.1 | 3.0 | 1.3 |
| Chi3l3 | chitinase 3-like 3 | 4.7E-02 | 4.7E-04 | 0.5 | 5.2 | 4.6 | 41.4 | 5.3 | 0.4 |
Fig. 2.
Expression of differentially expressed transcripts following induced muscle atrophy. A: the number of genes regulated during casting-induced muscle atrophy or tenotomy-induced muscle atrophy. B: the overlap of genes regulated between casting- and tenotomyinduced muscle atrophy. C: transcripts (8,285) with ≥ 1.5-fold changes (P < 0.01) in expression in either casting- and/or tenotomy-induced muscle atrophy models were hierarchically ordered and visualized in GeneData Expressionists. For each gene, relatively high expression levels are shown in red and relatively low expression levels are shown in blue.
Pathway analysis.
The 700 regulated transcripts that were common to both models were functionally annotated using the DAVID bioinformatics resources to identify enriched biological themes (13, 25). This analysis suggested that both cast-immobilization and tenotomy treatments resulted in decreased expression of myofibril genes, mitochondria-related genes, and genes involved in metabolic processes (glycolysis and amino acid metabolism), and increased expression of genes in the proteasomal protein degradation pathway. Using a more global pathway analysis tool, SigPathway (8), we identified 1,761 previously defined gene sets representing common biological pathways that were regulated in a least one time point in either model of muscle atrophy (Table 3). Consistent with other models of muscle atrophy, genes involved in energy production and mitochondrial functions were down regulated in both models. The major program for protein degradation, the proteasome, was regulated more significantly in the cast-immobilized muscles than in the tenotomy-treated muscles, while genes associated with lysosome functions were selectively regulated in the tenotomy-treated muscles. Furthermore, genes involved in inflammation and the extracellular matrix were selectively increased in the tenotomy-treated muscles.
Table 3.
Pathways regulated during muscle atrophy identified by Sigpathway analysis
| Casting |
Tenotomy |
|||||
|---|---|---|---|---|---|---|
| Gene Sets | 2 Days | 7 Days | 14 Days | 2 Days | 7 Days | 14 Days |
| CANCER MODULE_46 INFLAMMATION | −6.8 | 3.3 | 9.8 | 19.1 | 15.1 | 11.0 |
| EXTRACELLULAR_MATRIX | −6.2 | −5.5 | 2.4 | 7.4 | 12.0 | 14.4 |
| MITOCHONDRION | −0.2 | −8.2 | −11.2 | −11.7 | −11.9 | −12.4 |
| HSA03050_PROTEASOME | 10.3 | 11.9 | 3.9 | 4.9 | 0.5 | −0.6 |
| ELECTRON_TRANSPORT_CHAIN | −2.0 | −7.7 | −5.0 | −7.9 | −7.2 | −5.7 |
| HSA00190_OXIDATIVE_PHOSPHORYLATION | −0.9 | −7.1 | −5.9 | −6.8 | −5.5 | −4.5 |
| KREBS_TCA_CYCLE | 0.3 | −6.2 | −5.3 | −6.3 | −5.8 | −5.0 |
| GLYCOLYSIS_AND_GLUCONEOGENESIS | −1.5 | −5.8 | −3.5 | −3.7 | −3.5 | −2.8 |
| HSA00020_CITRATE_CYCLE | 0.6 | −5.2 | −5.7 | −5.4 | −4.6 | −4.2 |
| LYSOSOME | 1.3 | 0.7 | 0.9 | 5.1 | 3.2 | 2.8 |
Protein degradation.
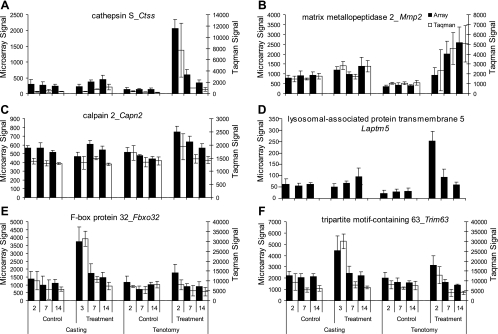
Casting- and tenotomy-induced muscle atrophy both regulated distinct sets of genes involved in multiple protein degradation pathways. Tenotomy induced expression of genes involved in calpain (Capn2), cathepsin (CtsK), caspase (Casp3), lysosome (Laptm5), and matrix-degradation (Mmp2) activities (Fig. 3). In contrast, the ubiquitin-mediated protein degradation pathway, as measured by Fbxo32/atrogin-1 and Trim63/MuRF1 expression, was regulated only in the casting model (Fig. 3). A custom TaqMan low density array was used to confirm the regulation of 44 individual genes by RT-PCR. These genes were selected because they were deemed to be biologically relevant and represent markers of distinct protein degradation pathways. All genes had comparable expression patterns in both RT-PCR and microarray analyses (Fig. 3).
Fig. 3.
Confirmation of microarray results by quantitative RT-PCR using select genes involved in protein degradation. mRNA expression levels of cathepsin K (Ctss, A), matrix metalloproteinase 2 (Mmp2, B), calpain 2 (Capn2, C), lysosomal-associated protein transmembrane 5 (Laptm5, D), F-box protein 32 (Fbxo32, E), and tripartite motif-containing 63 (Trim63, F) in the gastrocnemius muscle of mice whose muscles where immobilized by casting or tenotomy. Values represent the mean relative expression ± SD; n = 8 for microarray and n = 3 for qRT-PCR.
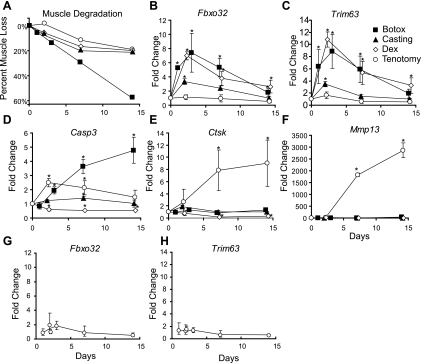
Since these two atrophy models regulated distinct sets of genes involved in various protein degradation pathways, we examined whether other models of muscle atrophy also differentially regulated these protein degradation pathways. Two additional muscle atrophy models, denervation induced by Botox injection and cachexia induced by glucocorticoid treatment, resulted in comparable loss of muscle mass as seen in the casted and tenotomy models (Fig. 4A). However, these models showed regulation of different genes involved in protein degradation. Botox injection and glucocorticoid treatment induced expression of Fbox32/atrogin-1 and Trim63/MuRF1 seven- to 10-fold compared with the three- to fourfold increase seen in the casting model and the relatively minor increase in expression following tendon laceration (Fig. 4, B and C). In contrast, the lysosomal marker cathepsin K and the matrix degradation marker Mmp13 were only significantly regulated following tendon laceration (Fig. 4, E and F). The apoptosis marker Casp3 was regulated only in the Botox- and tenotomy-treated animals (Fig. 4D).
Fig. 4.
Differential regulation of protein degradation genes between 4 models of skeletal muscle atrophy. A: percent muscle loss in 4 models of skeletal muscle atrophy. Losses were calculated by comparison to the respective contralateral control and were expressed as average losses. Fold changes in mRNA expression levels for Fbxo32 (B), Trim63 (C), Casp3 (D), cathepsin K (Ctsk, E), and Mmp13 (F) in gastrocnemius muscles during muscle atrophy induced by 4 different atrophy models as measured by qRT-PCR. Fold changes in messenger RNA expression levels of Fbxo32 (G) and Trimb3 (H) in the gastrocnemius muscle during tenotomy induced muscle atrophy as measured by qRT-PCR. Each point represents average fold changes compared with the respective contralateral control. Error bars represent SD; n = 3. *P < 0.05.
mRNA expression of Fbxo32/atrogin-1 and Trim63/Murf-1 is induced early during many models of muscle atrophy and returns to near baseline levels after 2 wk. Since high-level induction of Fbxo32/atrogin-1 and Trim63/Murf-1 was not seen 2 days after tenotomy, we explored earlier time points to determine if these genes were induced in the tenotomy model. Neither Fbxo32/atrogin-1 nor Trim63/Murf-1 expression was significantly regulated at 1, 2, or 3 days following tenotomy-induced muscle atrophy (Fig. 4, G and H).
Protease activity.
Gene expression profiling suggested a significant difference in proteolytic activity between the casting and tenotomy atrophy models. While differential gene expression is suggestive, protein activity does not always correlate with changes in mRNA expression. Therefore, it was important to confirm whether the gene expression changes correlated with changes in proteolytic activities.
Lysosomal activity.
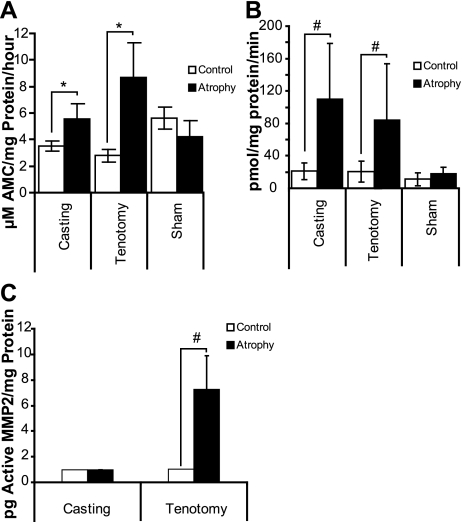
The role of the lysosome was evaluated by monitoring cathepsin activity using a pan-cathepsin peptide substrate cleavage assay. Activity increased more dramatically in gastrocnemius muscles from the tenotomy-treated animals than in the gastrocnemius muscles from the cast-immobilized animals. In the tenotomy-treated muscles, the rate of cleavage increased 3.2-fold (P < 3.2 × 10−8), while the rate of cleavage increased only 1.6-fold (P < 1.3 × 10−8) in the cast-immobilized muscles (Fig. 5A). Therefore, there was a twofold increase in lysosomal activity in tenotomy vs. cast-immobilized animals. Greater than 95% of the cathepsin activity was blocked by the Ca2+-dependent cathepsin inhibitor E64, indicating that the activity was specific (data not shown). Lysosome activity did not change significantly in the sham-operated animals.
Fig. 5.
Casting and tenotomy induced different rates of proteolytic cleavage. A: total cathepsin activity in tenotomy- and casting-induced atrophy was measured in total muscle extracts. Error bars represent SD; n = 8. B: proteasome activity in tenotomy- and casting-induced atrophy was measured in total muscle extracts. Error bars represent SD; n = 8. C: MMP2 activity in tenotomy- and casting-induced atrophy was measured in total muscle extracts. Error bars represent standard error; n = 10. *P < 1 × 10−7 and #P < 0.001 compared with the respective contralateral control limbs.
Proteasome activity.
Using the fluorogenic peptide substrate LLVY-AMC to measure 20S proteasome activity, we found that muscle extracts from cast-immobilized animals had a 5.2-fold increase (P < 0.008) in activity over extracts from the respective contralateral control muscles. Similarly, muscle extracts from tenotomy-treated muscles had a 4.1-fold increase (P < 0.01) in proteasome activity, while no significant increase in activity was observed from the sham-operated animals (Fig. 5B). All activity was blocked by the proteasome inhibitor lactacystin (data not shown).
MMP activity.
MMP2 activity increased only in muscles from tenotomy-treated animals. Tenotomy treatment increased the endogenous active MMP2 an average of 7.1-fold (P < 0.00098) over the limit of detection, while no endogenous active MMP2 was detected in muscles from cast-immobilized animals (Fig. 5C). In control muscles from both models, active MMP2 levels were close or below the level of detection for this assay, but total MMP2 levels (pro- and active MMP2) were similar. Control muscles from tenotomy-treated animals had an average of 241 ± 64.5 pg total MMP2 per mg of protein and control muscles from cast-immobilized animals had an average of 227 ± 134 pg total MMP2 per mg of protein.
DISCUSSION
In this study, we evaluated two relevant orthopedic models of skeletal muscle atrophy, casting and tenotomy, through the use of genome-wide expression profiling. While numerous studies have used expression profiling to identify molecular mechanisms of muscle atrophy (1, 2, 6, 7, 10, 21, 22, 27, 28, 31, 32, 37, 42–44, 49, 51, 55, 58, 65–67), this is one of the first studies to characterize the global transcriptional response of muscle atrophy induced by casting or by tenotomy.
Since muscle mass is a balance between anabolic protein synthesis and catabolic protein degradation, changes in mass can be caused by increases in catabolic signaling, decreases in anabolic signaling, or both. Like previous studies, we observed a significant decrease in expression of genes involved in energy production and carbohydrate metabolism concordant with an upregulation of genes involved in protein degradation and metabolism. However, the magnitude and the number of gene transcripts regulated between the cast-immobilization and tenotomy-treated animals suggest some distinct modes of action. Atrophy induced by tenotomy increased expression of MMP genes and genes involved in the lysosomal degradation pathway, while atrophy induced by casting up-regulated genes involved in the proteasome-mediated degradation pathway.
Pathological mechanism of action.
Casting and tenotomy resulted in distinct biophysical properties of the muscle. Tenotomy releases all load, results in muscle shortening and nerve damage, and prevents contraction of the muscle fibers through elimination of both passive and active stretch. Tendons begin to heal quickly and after 2 days have formed a repair site bridging the defect. The tendon repair tissue is of inferior structure and the muscle remained contracted during the time course of this study. As the tendon heals, the load increases, yet it remains significantly less than in the control animals. In contrast, casting does not affect muscle length and maintains passive tension and a constant load. These differences, which result in similar muscle loss, suggest that different mechanisms may be involved.
Characterization of other muscle atrophy models suggests a common transcriptional program during muscle atrophy (37), and therefore, we were surprised to see such marked differences in cast-immobilized and tenotomy-treated animals. The transcriptional differences may be related to differences in biophysical properties. Therefore, we examined protein degradation marker genes in two additional models of muscle atrophy, Botox- and glucocorticoid-induced muscle atrophy. Additionally, given that both the proteasome and lysosome pathways were distinctively regulated between cast-immobilized and tenotomy-treated animals, we evaluated the relative roles of each pathway in the other atrophy models. Glucocorticoid treatment stimulates protein breakdown and inhibits protein synthesis without affecting muscle load, while Botox treatment inhibits neuromuscular function by blocking presynaptic release of the neurotransmitter acetylcholine at the neuromuscular junction resulting in reduced muscle load. Although muscle loss was similar in all four models examined, the expression repertoire and magnitude of genes from different protein degradation pathways activated by each of the four models of muscle atrophy were distinct, suggesting that multiple mechanisms lead to muscle atrophy and that all four atrophy models induce different transcriptional responses.
While we examined transcriptional effects due to acute trauma, the mechanisms of muscle atrophy due to chronic disease are likely different. Autophagy-related genes are induced in various chronic models of muscle wasting, including starvation, cancer-induced cachexia, diabetes, and uremia (37, 45), but were not regulated in acute casting or tenotomy. Fbxo32/atrogin-1 and Trim63/Murf-1 are induced in many models of acute muscle atrophy, but are downregulated in aging-related muscle loss (17) and chronic spinal cord injury patients (40) and not regulated in sarcopenic human muscles (22). Fbxo32/atrogin-1 and Trim63/Murf-1 are induced early and transiently in animal models of muscle atrophy and, therefore, may have a role during initiation of chronic disease.
Ubiquitin-mediated proteasome activation.
The involvement of the ubiquitin-mediated proteasome pathway in muscle protein breakdown is well established and is initiated through two muscle-specific E3-ligases, Fbxo32/atrogin-1/MAFbx and Trim63/Murf-1, whose mRNA levels increase dramatically in multiple models of muscle atrophy (5, 12, 14, 24, 36, 38, 39, 41). In this study, Fbxo32/atrogin-1 and Trim63/Murf-1 were upregulated in the casting-, Botox-, and glucocorticoid-induced models of atrophy. However, there was little evidence of increased expression of either gene following tenotomy-induced atrophy. Expression of Fbxo32/atrogin-1 and Trim63/Murf-1 is transient and is induced early after induction of atrophy (54). While we cannot rule out that the time points chosen for this study were not optimized for detection of Fbxo32/atrogin-1 and Trim63/Murf-1, the results suggest that these genes were not significantly induced during tenotomy-induced atrophy. Furthermore, the levels of Fbxo32/atrogin-1 and Trim63/Murf-1 mRNA did not correlate with the level of muscle loss since losses were similar in all models examined. Another ubiquitin ligase, Cblb, is important for the activation of Fbxo32/atrogin-1 and can specifically degrade IRS1, a key regulator of skeletal muscle growth, which results in the inactivation of AKT1 and the up-regulation of Fbxo32/atrogin-1 (48). Consistent with the expression of Fbxo32/atrogin-1 and Trim63/Murf-1, casting, but not tenotomy, increased Cblb mRNA expression.
While casting dramatically increased expression of genes in the ubiquitin-mediated protein degradation pathway, tenotomy also increased expression of many genes involved in proteasome-mediated degradation, albeit at a much lower level. Furthermore, atrophied muscles from both models showed increased proteasome activity, suggesting that ubiquitin-mediated protein degradation is an important component in both models. However, activation of the proteasome pathway likely occurs through different mechanisms since tenotomy treatment increased proteasome activity in the absence of increased Fbxo32/atrogin-1 and Trim63/Murf-1 mRNA expression.
Lysosomal pathway activation.
While activation of ubiquitin-proteasome pathways plays a significant role in multiple types of muscle atrophy, the role of lysosomal activation is only beginning to be elucidated. Lysosome activation requires initiation of the autophagy program. Various myopathies have impaired autophagy-lysosomal pathways (3), and proper control of autophagy is important to maintain muscle mass (46). Regulation of genes involved in the autophagy-lysosomal pathway has been seen in some experimental models of muscle atrophy (15, 37), but these genes were not regulated in our casting model. In contrast, tenotomy-induced muscle atrophy induced expression of many lysosomal proteases, lysosomal glucosidases, and lysosomal lipases, while casting-induced atrophy did not regulate these genes (Table 1). However, genes involved in autophagy regulation (Atg8 and Bnip3) were not significantly regulated or were comparably increased (Atg4) in casting- and tenotomy-induced atrophy.
Lysosomal proteolysis and the induction of many autophagy-related genes are dependent on the FOXO3 transcription factor (45, 69). Muscle loss caused by denervation, starvation, or glucocorticoid treatment is also dependent on FOXO3 (55, 56). Additionally, the FOXO transcription factors can stimulate transcription of Fbxo32/atrogin-1 and Trim63/Murf-1 (56, 59). Since FOXO3 is involved in both lysosomal and proteasomal dependent pathways, FOXO3 may be a key checkpoint regulator of proteolysis. The differential activity of FOXO3 could lead to the activation of the different protein degradation pathways induced by casting and tenotomy.
Activation of additional degradation pathways.
While the proteasome and autophagy/lysosome pathways represent the major routes of proteolysis, we cannot rule out roles for other pathways, including the activity of calpains and caspases, both of which were regulated primarily during tenotomy-induced atrophy. Calpains remove myofilaments from the sarcomere through degradation of Z-band-associated proteins, which is important for muscle protein turnover since the proteasome does not degrade intact myofibrils (33). Additionally, calpain inhibitors prevent sepsis-induced muscle proteolysis independently of Fbxo32/atrogin-1 and Trim63/Murf-1 expression (19), suggesting that the calpain pathway is an important mediator of some muscle atrophy pathways.
Tenotomy-induced muscle atrophy induced the expression of a significant number of genes involved in inflammation. This induction is independent of the wound healing response because the tendon and the tendon repair site were removed from the muscle before analysis. Chronic inflammation may be a major contributor to age-related muscle wasting (11), and proinflammatory signaling is required for muscle homeostasis (47). Inhibition of inflammation through targeted ablation of Ikkb improves muscle physiology and protects against denervation-induced atrophy (47). Interestingly, very few inflammation-related genes were induced early in the casting model. The presence of inflammatory markers seen on day 14 in the casting model was likely due to irritation as the skin under the cast was inflamed and irritated.
Recent studies suggest that MMPs play an important role in response to muscle injury and repair (57) and rats under long-term hindlimb suspension have increased MMP2 activity (52). Our studies suggest that MMPs have differential activity in cast-immobilized and tenotomy-treated muscles. MMP2 is a zinc proteolytic enzyme that plays a role in the maintenance and structural integrity of the basal lamina (64). Given the role of MMPs in extracellular remodeling, our data suggest that the atrophy induced by tenotomy is characterized by extensive degradation and remodeling of the extracellular matrix providing access for remolding of muscle tissue.
Conclusions
We extensively characterized the molecular progression of muscle atrophy in two different animal models. Our studies were performed with young, actively growing animals, and therefore, we cannot determine the exact contribution of passive (lack of growth) vs. active muscle atrophy. The activation of many known atrophy-related genes, decreased muscle fiber size, and lack of significant physiological differences in muscles from cage controls vs. muscles from contralateral controls suggests that the major effect is an active degradation process. In addition, Velcade, a proteasome inhibitor, can partially rescue unilateral casting-induced muscle loss in actively growing animals (35). However, inhibition of growth may contribute to the overall changes in transcriptional profiles, and ongoing studies in older animals will help define the active degradation process.
Proteasome inhibition is effective at reducing muscle proteolysis (4, 18, 20, 61) and has been implicated as a possible therapeutic intervention for preventing muscle atrophy. However, our data suggests that inhibition of the proteasome may not prevent all types of muscle atrophy, and that alternative or multiple anticatabolic therapeutic interventions may be necessary for optimal treatment. While this study focused on understanding the mechanisms of catabolic activities on muscles, we have not yet addressed the role of anabolic responses in various models of atrophy. Therefore, we are currently exploring whether inducing an anabolic response would be beneficial in preventing muscle atrophy in multiple models.
DISCLOSURES
All authors were employed by Wyeth Research/Pfizer at the time of the research.
ACKNOWLEDGMENTS
The authors thank Christine Huard and Ying Zhang for microarray processing and Donna Gavin and Kathryn Wallace for histological processing.
Current address for J. Parkington: Novartis Institutes for BioMedical Research, Cambridge MA.
REFERENCES
- 1. Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG, Leinwand LL, Argraves WS, Bateman TA, Barth JL. Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol 106: 582–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batt J, Bain J, Goncalves J, Michalski B, Plant P, Fahnestock M, Woodgett J. Differential gene expression profiling of short and long term denervated muscle. FASEB J 20: 115–117, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37: 2098–2114, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Beehler BC, Sleph PG, Benmassaoud L, Grover GJ. Reduction of skeletal muscle atrophy by a proteasome inhibitor in a rat model of denervation. Exp Biol Med (Maywood) 231: 335–341, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics 31: 510–520, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Choi S, Liu X, Li P, Akimoto T, Lee SY, Zhang M, Yan Z. Transcriptional profiling in mouse skeletal muscle following a single bout of voluntary running: evidence of increased cell proliferation. J Appl Physiol 99: 2406–2415, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML, Wilson E, Saraf KA, Liu W, McCampbell AS, Dave B, Broaddus RR, Brown EL, Kao W, Skotnicki JS, Abou-Gharbia M, Winneker RC, Walker CL. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res 69: 6171–6178, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Dargelos E, Poussard S, Brule C, Daury L, Cottin P. Calcium-dependent proteolytic system and muscle dysfunctions: a possible role of calpains in sarcopenia. Biochimie 90: 359–368, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Debigare R, Maltais F, Cote CH, Michaud A, Caron MA, Mofarrahi M, Leblanc P, Hussain SN. Profiling of mRNA expression in quadriceps of patients with COPD and muscle wasting. COPD 5: 75–84, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. Int J Sports Med 27: 94–99, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dehoux MJ, van Beneden RP, Fernandez-Celemin L, Lause PL, Thissen JP. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett 544: 214–217, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 14. DeRuisseau KC, Kavazis AN, Deering MA, Falk DJ, Van Gammeren D, Yimlamai T, Ordway GA, Powers SK. Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J Appl Physiol 98: 1314–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J 360: 143–150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edström E, Altun M, Hägglund M, Ulfhake B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 663–674, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Fang CH, Wang JJ, Hobler S, Li BG, Fischer JE, Hasselgren PO. Proteasome blockers inhibit protein breakdown in skeletal muscle after burn injury in rats. Clin Sci (Lond) 95: 225–233, 1998 [PubMed] [Google Scholar]
- 19. Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Fischer D, Gang G, Pritts T, Hasselgren PO. Sepsis-induced muscle proteolysis is prevented by a proteasome inhibitor in vivo. Biochem Biophys Res Commun 270: 215–221, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Flück M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 289: R4–R14, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, Kandarian SC. Identification of a molecular signature of sarcopenia. Physiol Genomics 21: 253–263, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann NY Acad Sci 1211: 25–36 [DOI] [PubMed] [Google Scholar]
- 24. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Ikemoto M, Nikawa T, Takeda S, Watanabe C, Kitano T, Baldwin KM, Izumi R, Nonaka I, Towatari T, Teshima S, Rokutan K, Kishi K. Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB J 15: 1279–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Jagoe RT, Lecker SH, Gomes M, Goldberg AL. Patterns of gene expression in atrophying skeletal muscles: response to food deprivation. FASEB J 16: 1697–1712, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Jelier R, ‘t Hoen PA, Sterrenburg E, den Dunnen JT, van Ommen GJ, Kors JA, Mons B. Literature-aided meta-analysis of microarray data: a compendium study on muscle development and disease. BMC Bioinformatics 9: 291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res 28: 289–97, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Kandarian S. The molecular basis of skeletal muscle atrophy–parallels with osteoporotic signaling. J Musculoskelet Neuronal Interact 8: 340–341, 2008 [PubMed] [Google Scholar]
- 31. Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33: 155–165, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Komamura K, Shirotani-Ikejima H, Tatsumi R, Tsujita-Kuroda Y, Kitakaze M, Miyatake K, Sunagawa K, Miyata T. Differential gene expression in the rat skeletal and heart muscle in glucocorticoid-induced myopathy: analysis by microarray. Cardiovasc Drugs Ther 17: 303–310, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Koohmaraie M. Ovine skeletal muscle multicatalytic proteinase complex (proteasome): purification, characterization, and comparison of its effects on myofibrils with mu-calpains. J Anim Sci 70: 3697–3708, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376: 1224–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289: E969–E980, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15: 1537–1545, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leger B, Senese R, Al-Khodairy AW, Deriaz O, Gobelet C, Giacobino JP, Russell AP. Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40: 69–78, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806–C812, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Magnusson C, Svensson A, Christerson U, Tagerud S. Denervation-induced alterations in gene expression in mouse skeletal muscle. Eur J Neurosci 21: 577–580, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Mahoney DJ, Safdar A, Parise G, Melov S, Fu M, MacNeil L, Kaczor J, Payne ET, Tarnopolsky MA. Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am J Physiol Regul Integr Comp Physiol 294: R1901–R1910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Mourkioti F, Rosenthal N. NF-kappaB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med 86: 747–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, Ohno A, Okumura Y, Nonaka I, Yasutomo K, Baldwin KM, Kominami E, Higashibata A, Nagano K, Tanaka K, Yasui N, Mills EM, Takeda S, Nikawa T. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol 29: 4798–4811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, Kominami E, Nonaka I, Ogawa T, Adams GR, Baldwin KM, Yasui N, Kishi K, Takeda S. Skeletal muscle gene expression in space-flown rats. FASEB J 18: 522–524, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Plant PJ, Bain JR, Correa JE, Woo M, Batt J. Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J Appl Physiol 107: 224–234, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Raffaello A, Laveder P, Romualdi C, Bean C, Toniolo L, Germinario E, Megighian A, Danieli-Betto D, Reggiani C, Lanfranchi G. Denervation in murine fast-twitch muscle: short-term physiological changes and temporal expression profiling. Physiol Genomics 25: 60–74, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Reznick AZ, Menashe O, Bar-Shai M, Coleman R, Carmeli E. Expression of matrix metalloproteinases, inhibitor, and acid phosphatase in muscles of immobilized hindlimbs of rats. Muscle Nerve 27: 51–59, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Rossi S, Stoppani E, Martinet W, Bonetto A, Costelli P, Giuliani R, Colombo F, Preti A, Marchesini S, Fanzani A. The cytosolic sialidase Neu2 is degraded by autophagy during myoblast atrophy. Biochim Biophys Acta 1790: 817–828, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Skittone LK, Liu X, Tseng A, Kim HT. Matrix metalloproteinase-2 expression and promoter/enhancer activity in skeletal muscle atrophy. J Orthop Res 26: 357–363, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551: 33–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 23: 3251–3253, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest 100: 197–203, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tian L, Greenberg SA, Kong SW, Altschuler J, Kohane IS, Park PJ. Discovering statistically significant pathways in expression profiling studies. Proc Natl Acad Sci USA 102: 13544–13549, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ugai S, Tamura T, Tanahashi N, Takai S, Komi N, Chung CH, Tanaka K, Ichihara A. Purification and characterization of the 26S proteasome complex catalyzing ATP-dependent breakdown of ubiquitin-ligated proteins from rat liver. J Biochem (Tokyo) 113: 754–768, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Vu T, Werb Z. Gelatinase B, structure, regulation and function. In: Matrix Metalloproteinases, edited by Parks W, Mecham R. San Francisco: Morgan Kaufmann, 1998, p. 115–148 [Google Scholar]
- 65. Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol 582: 825–841, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Welle S, Brooks AI, Delehanty JM, Needler N, Bhatt K, Shah B, Thornton CA. Skeletal muscle gene expression profiles in 20–29 year old and 65–71 year old women. Exp Gerontol 39: 369–377, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149–159, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]