Abstract
Analysis of changes in gene expression is an important means to define molecular differences associated with the phenotypic changes observed in response to myocardial infarction (MI). Several studies in humans or animal models have reported differential miRNA expression in response to MI acutely (animal) or chronically (human). To determine the relative contribution of microRNA (miRNA) and mRNAs to acute and chronic temporal changes in response to MI, mRNA and miRNA expression profiles were performed in three time points post-MI. Changes in mRNA and miRNA expression was analyzed by arrays and confirmed by RT-PCR. Bioinformatic analysis demonstrated that several genes and miRNAs in various pathways are regulated in a temporal or phenotype-specific manner. Furthermore miRNA analyses indicated that miRNAs can target expression of several genes involved in multiple cardiomyopathy-related pathways. Our results suggest that: 1) Differentially regulated miRNAs are predicted to target expression of several genes in multiple biological processes involved in the response to MI; 2) antithetical and compensatory changes in miRNA expression are observed at later disease stages, including antithetical regulation of miR-29, which correlates with the expression of collagen genes, and upregulation of apoptosis-related miRNAs at early stages and antiapoptotic/growth promoting miRNAs at later stages; 3) temporally dependent changes in miRNA and mRNA expression post-MI are generally characterized by dramatic changes acutely postinjury and are normalized as disease progresses; 4) A combinatorial analysis of mRNA and miRNA expression may aid in determining factors involved in compensatory and decompensated responses to cardiac injury.
Keywords: microRNA, signal transduction, fetal gene program, gene expression
myocardial infarction (MI) is a major cause of morbidity and mortality worldwide. Partial or complete epicardial coronary artery occlusion impedes proper oxygen supply to the heart muscle, resulting in hypoxia and loss of viable cardiac tissue (26). The loss of cardiac muscle often results in decreased cardiac output, increasing stress on remaining viable myocardium, and consequently activation of multiple compensatory signaling pathways and changes in gene expression. These responses are initially compensatory but ultimately may result in cardiac decompensation, which, beyond simple loss of myocardium, can be the result of increased fibrosis, apoptosis, changes in myofilament protein phosphorylation (24), and CHANGES IN GENE EXpression that are associated with a fetal stage (8). Several of these changes are temporally related and can result in sequentially dependent or transient responses.
MicroRNAs (miRNA, miRs) are small noncoding ∼22 nucleotide (nt) RNAs capable of modulating the expression of many genes (1) with estimates suggesting that as much as 60% of the genome is subject to their regulation (7). miRs regulate gene expression by recognition of reverse complementary 6- to 8-nucleotide “seed” sequences, most frequently located within 3′-untranslated regions of target mRNAs. In eukaryotic systems, miRs act to cause translational repression or mRNA destabilization (4, 5). Several miRNAs have been implicated in regulating the expression of genes that are involved in multiple biological processes post-MI (for a recent review see Ref. 8), and temporal miRNA expression analysis has been undergone in mouse and rat MI models and include miRNA expression profile in rats and mice (3, 17, 23). These studies showed distinct differences in the identity and directionality of miRNA expression at different time points and infarcted areas of the myocardium. However, these animal model studies were limited to a time frame relatively early post-MI. In contrast, miRNA expression profiling in human heart failure secondary to an ischemic event has only been performed in patients with longstanding heart failure undergoing cardiac transplantation where changes in gene expression represent a chronic, end-stage phenotype. Thus, what happens to miR expression during the time period between acute MI and compensated heart failure remains relatively unexplored.
In the current study, we have performed a comprehensive array-based analysis of mRNA and miRNA expression in the noninfarcted left ventricle (LV) at three time points post-MI: 2 days, 2 wk, and 2 mo. Our results demonstrated a number of dramatic changes in gene expression at the early time point that were either normalized or reversed as ventricular remodeling progressed. Bioinformatic strategies were used to combine data from miRNA and mRNA expression profiles to identify potential targets for the regulated miRNAs. Our results suggest that time of intervention of miRNA therapeutics post-MI may result in distinct biological functions that could have relevance to disease programs and to treatment outcome.
METHODS
Surgical induction of MI.
Left coronary artery of 11 wk old C57Bl/6J male mice were permanently occluded as previously described (24). Control animals underwent an identical sham surgical procedure without coronary ligation. At the time of death, animals were anesthetized with an overdose of pentobarbital, the heart was rapidly excised, and the right ventricle was removed. Cardiac muscle was rapidly frozen in liquid nitrogen and stored at −70°C. In the current study, LV tissue was obtained from the same animals as described previously in Walker et al. (24). Tissue was taken from the free wall of the LV near the base of the heart (proximal to the ligation) and distal from the infarct zone. Infarction was confirmed at the time of the initial surgery by three-lead electrocardiography (EKG) and infarct size, at the time of death, was determined by wall motion score index (27) and by tracing the infarct during diastole in the B-mode long axis view. For miRNA and mRNA array experiments, two to four animals were used at each time point and compared with sham-operated age and sex-matched littermates. A total of 12 MI and 14 control animals were investigated. Protocol for animal work is in accordance with Public Health Service Animal Welfare Assurance, IDA3269-01 and was reviewed and approved by the Institutional Animal Care and Use Committee.
RNA extraction and RT-PCR.
Total RNA was extracted from tissue outside the infarcted area using the Mirvana kit (ABI) according to manufacturer's condition. For mRNA detection, 0.5 μg of RNA were reverse transcribed into cDNA using I-script (Bio-Rad), and RT-PCR was performed as previously described (19). Primers are described below, and target mRNA expression was normalized to the signal for 18S rRNA (19).
Reverse transcription of miRNAs was performed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's recommendations. In this case, 5 ng of miRNA was combined with dNTPs, MultiScribe reverse transcriptase, and the primer specific for the target miRNA. The resulting cDNA was diluted 15-fold and used in PCR reactions. PCR was performed according to manufacturer's recommendations (Applied Biosystems). In brief, cDNA was combined with the TaqMan assay specific for the target miRNA, and PCR reaction was done using the ABI7300 [α-myosin heavy chain (MyHC) forward (F): 5′ cccaggatctctggattggtct, αMyHC reverse (R): 5′ggcaggaagaggagtagcaga; βMyHC F: 5′ gagcattctcctgctgtttcctt, βMyHC R: 5′ ctcctctgctgaggcttccttt; sarco(endo)plasmic reticulum Ca2+-ATPase (Serca) F: 5′ gcattgcagtctggatcatcaaca, Serca R: 5′ gccaccatgaactgggtcatt; atrial natriuretic factor (ANF) F: 5′ gccggtagaagatgaggtcatg, ANF R: 5′ gcttcctcagtctgctcactca; B-type natriuretic peptide (BNP) F: 5′ cgctgggaggtcactcctat, BNP R: 5′ gctctggagactggctaggactt].
miRNA arrays.
miRNA expression analysis was performed by Dharmacon (Lafayette, CO) using arrays based on the Sanger miRBase 10.1 database, capable of detecting 567 miRNAs. Error-weighted ANOVA with Q-value multiple test correction was performed with log-transformed, interchip scaled data. In addition, Scheffé post hoc test was performed after each ANOVA. A P value of 0.05 was used as a cutoff for expressed miRNAs.
mRNA arrays.
mRNA expression analysis was performed by the University of Colorado Denver array core facility using Affymetrix Mouse GeneChip 1.0 ST version 1, capable of detecting 28,000 probes. Statistical analysis was done by ANOVA. A P value of 0.05 was used as an initial cutoff followed by a second cutoff that excluded fold differences <1.25-fold.
Statistical analyses.
For RT-PCR measurements all analyses were performed by ANOVA with Bonferroni post hoc test, with a P < 0.05 in a two-tailed distribution considered to be statistically significant. Statistical analyses for mRNA and miRNA arrays are described in the methodological section for each array.
RESULTS
Myocardial dysfunction in response to MI.
Immediately prior to death, EKG measurements were made as described in Walker et al. (24). There were no significant differences in infarct sizes in each group. A sharp decline in ejection fraction (EF%) was observed at 2 days post-MI (EF% = 43% in MI vs. 60% in sham) that was followed by a slower decline in EF% at the subsequent time points (39% at 2 wk and 30% at 2 mo) (24).
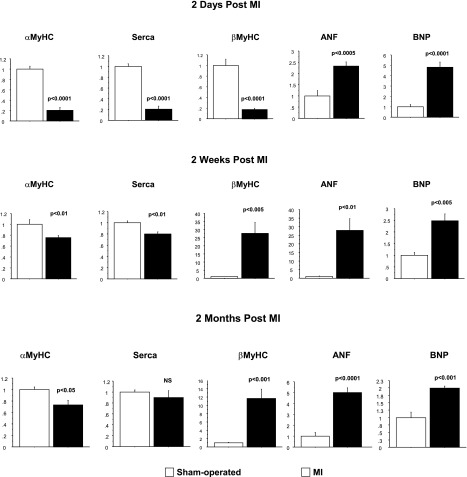
The time course of target gene expression changes to a pathological fetal program, characterized by a decrease in αMyHC/βMyHC ratio and SERCA 2a and an increase in ANF and BNP mRNA, is shown in Fig. 1. Interestingly, at 2 days post-MI, significant decreases in SERCA 2a, αMyHC, and βMyHC expression were observed; conversely, and as expected, there were marked increases in ANF and BNP expression. At 2 wk and 2 mo post-MI, βMyHC mRNA and protein (24) expression were significantly increased and the signature pattern of increased expression of the fetal gene program characteristic of pathologic hypertrophy and heart failure was observed.
Fig. 1.
Expression of the hypertrophic gene program is activated in response to ischemic injury. Total RNA was extracted from left ventricle (LV) of animals subjected to myocardial infarction and control animals and analyzed by RT-PCR. Gene expression in myocardial infarction (MI) groups was compared with sham-operated control groups.
Temporal identification of miRNA expression in MI samples.
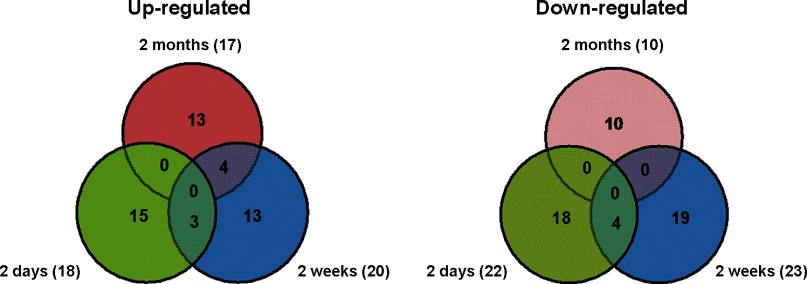
Temporal changes in miRNA expression were analyzed in mouse LV samples at all three time points post-MI and compared with sham-operated time-matched controls. As shown in Table 1 and Fig. 2, compared with sham-operated control, at 2 days post-MI, expression of 40 miRNAs was found to be changed significantly. At 2 wk post-MI, differential expression of 43 miRNAs was observed to undergo differential expression, whereas, at 2 mo post-MI, differential expression of 27 miRNAs was observed. Interestingly, the majority of differentially expressed miRNAs are uniquely regulated at each of the time points analyzed. Several of these, including the miR-15 family, miR-29, and miR-21 have been shown to undergo differential regulation in association with MI in previous studies. It is therefore likely that this subset of miRs are functionally important in post-MI pathological remodeling (8). All miRNAs that are significantly differentially expressed are reported.
Table 1.
miRNAs differentially regulated in 2 days, 2 wk and 2 mo post MI
| 2 days post-MI | 2 wk post-MI | 2 mo post-MI |
|---|---|---|
| Downregulated | ||
| mmu-miR-181b | mmu-miR-208a | mmu-miR-466c-5p |
| mmu-miR-678 | mmu-miR-138* | mmu-miR-30c-1* |
| mmu-miR-22 | mmu-miR-681 | mmu-miR-882 |
| mmu-miR-140* | mmu-miR-29c | mmu-miR-744 |
| mmu-miR-30a* | mmu-miR-27b* | mmu-miR-710 |
| mmu-let-7f | mmu-miR-323-5p | mmu-miR-760 |
| mmu-miR-299 | mmu-miR-696 | mmu-miR-615-5p |
| mmu-miR-421 | mmu-miR-295* | mmu-miR-294* |
| mmu-miR-27a; miR-27b | mmu-miR-188-5p | mmu-miR-329 |
| mmu-miR-181c | mmu-miR-770-3p | mmu-miR-150 |
| mmu-let-7 g | mmu-miR-130b | |
| mmu-miR-126-5p | mmu-miR-673-3p | |
| mmu-miR-876-3p | mmu-miR-486 | |
| mmu-miR-409-3p | mmu-miR-464 | |
| mmu-miR-10a | mmu-miR-345-5p | |
| mmu-miR-149 | mmu-miR-10a | |
| mmu-miR-208a | mmu-miR-378 | |
| mmu-miR-491 | mmu-miR-133a* | |
| mmu-miR-295* | mmu-miR-200c* | |
| vmmu-miR-30e* | mmu-miR-483 | |
| mmu-miR-376b | mmu-miR-149 | |
| mmu-miR-711 | ||
| mmu-miR-490 | ||
| Upregulated | ||
| mmu-miR-106b* | mmu-miR-181a-2* | mmu-miR-16 |
| mmu-miR-188-5p | mmu-miR-451 | mmu-miR-199a-3p; miR-199b |
| mmu-miR-106a | mmu-miR-16 | mmu-miR-195 |
| mmu-miR-485 | mmu-miR-195 | mmu-miR-29b |
| mmu-miR-296-5p | mmu-miR-678 | mmu-miR-29c |
| mmu-miR-466c-5p | mmu-miR-706 | mmu-miR-497 |
| mmu-miR-762 | mmu-miR-505 | mmu-miR-24-2* |
| mmu-miR-324-5p | mmu-miR-26b | mmu-miR-199a-5p |
| mmu-miR-21 | mmu-miR-714 | mmu-miR-199b* |
| mmu-miR-423-5p | mmu-miR-199a-3p; miR-199b | mmu-miR-675-5p |
| mmu-miR-551b | mmu-miR-126-3p | mmu-miR-26b |
| mmu-miR-181a-2* | mmu-miR-21 | mmu-miR-208a |
| mmu-miR-582-3p | mmu-miR-470 | mmu-miR-19b |
| mmu-miR-712 | mmu-miR-28 | mmu-miR-29a |
| mmu-miR-714 | mmu-miR-26a | mmu-miR-15a |
| mmu-miR-466f-5p | mmu-miR-212 | mmu-miR-350 |
| mmu-miR-711 | mmu-miR-484 | |
| mmu-miR-574-5p | mmu-miR-715 | |
| mmu-miR-497 | ||
All microRNAs (miRNAs) specifically regulated in each model are described. P value cutoff for described miRNAs (RT-PCR or arrays) was <0.05.
MI, myocardial infarction.
Fig. 2.
Distribution of microRNAs (miRNAs) differentially regulated in 2 days, 2 wk, and 2 mo post-MI. Numbers in each partition represent the number of commonly or uniquely regulated genes in each animal model. The size of each partition is not to scale. The total number of regulated genes is defined on the left and right side of the diagrams.
RT-PCR measurements of miRNA expression.
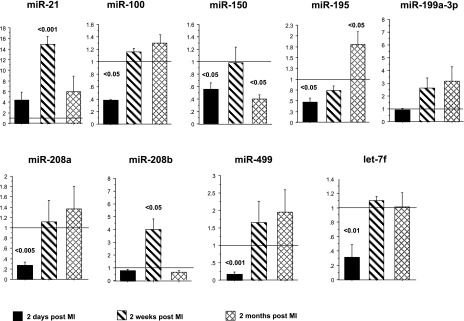
To validate miRNA array results, expression of a subset of nine miRNAs of interest was measured by RT-PCR. As shown in Fig. 3, directionality of expression was similar with both array and RT-PCR methodologies. However, the amplitude and significance of the measured differences were generally greater when detected by RT-PCR. Because expression of very few miRNAs was commonly regulated at different time points, RT-PCR allowed us to determine directionality of expression of a subset of miRNAs at different time points. Interestingly, with the exception of miR-21, initial (2 days post-MI) changes in miRNA expression tended to normalize by 2 wk post-MI, a finding consistent with previously reported proteomic changes (24). At later time points, expression of most miRNAs is either unchanged or is antithetical to 2 days post-MI, suggesting distinctly different and rapid compensatory mechanisms from those associated with the initial response to injury.
Fig. 3.
RT-PCR is a more sensitive method for miRNA expression analysis. Expression of a subset of miRNAs differentially regulated in the array analysis was analyzed by RT-PCR.
Expression of the so-called MyomiRs, miR-208a and 208b, has been correlated with the expression of their host genes, αMyHC and βMyHC (22). However, the current results suggest that expression of these miRNAs may be independent from the expression of their respective host genes. At 2 days post-MI, there is a dramatic downregulation of both αMyHC and βMyHC mRNA, and while expression of miR-208a is also decreased, miR-208b expression is unchanged. In contrast, at 2 wk post-MI, miR-208b is upregulated but expression is again normalized by 2 mo post-MI, whereas expression of βMyHC mRNA is increased at both time points. Expression of miR-208a is normalized (RT-PCR) or downregulated (array) at 2 wk and normalized at 2 mo post-MI, while expression of αMyHC is consistently downregulated. These results support the conclusion that expression of these miRNAs is independent of host gene expression. This conclusion is corroborated by a previous study showing that miR-208b is regulated by an independent promoter (14).
Temporal genome-wide expression profile.
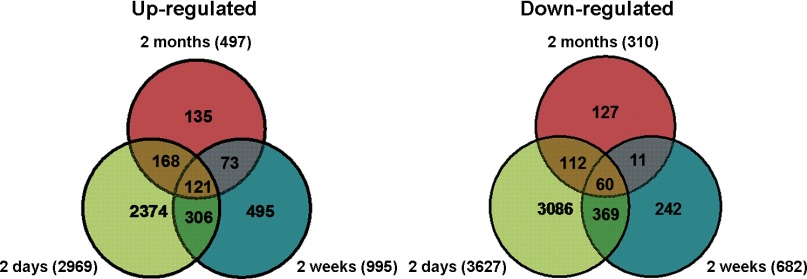
mRNA arrays were generated to compare changes in gene expression at each of the three post-MI time points. Although mRNA array data have been previously reported in MI mouse models, we performed these analyses because the use of different strains, different array platforms, and other factors can result in differences in gene expression that are model independent and might otherwise compromise a robust miRNA-mRNA comparison. Using a statistical cutoff of P < 0.05, as determined by ANOVA, with a subsequent fold-change cutoff of ≥1.25, inclusively, we found ∼9,000 genes to be up- or downregulated across all time points. As shown in Fig. 4, 2,969, 995, and 497 genes were significantly upregulated 2 days, 2 wk, and 2 mo post-MI, respectively. Of those, only 110 were commonly regulated at all time points (Supplemental Table S1).1 Similarly, 3,627, 682, and 310 genes were downregulated 2 days, 2 wk, and 2 mo post-MI, respectively. Of those, a small subset of 57 genes were regulated at all time points (Supplemental Table S2).
Fig. 4.
Distribution of genes differentially regulated 2 days, 2 wk, and 2 mo post-MI. Numbers in each partition represent the number of commonly or uniquely regulated genes in each animal model. The size of each partition is not to scale. The total number of regulated genes is defined on the left and right side of the diagrams.
Pathway analysis of mRNA changes.
To identify the representative biological/disease functions of genes differentially regulated in all time points post-MI, up- or downregulated genes were separately subjected to Ingenuity Pathway analysis (IPA) (http://www.ingenuity.com). As shown in Table 2, genes involved in cell death, growth and proliferation, metabolic pathways, and fibrosis are highly regulated post-MI at all time points.
Table 2.
Ingenuity Pathway analysis
| 2 days |
2 wk |
2 mo |
|||
|---|---|---|---|---|---|
| Down | Up | Down | Up | Down | Up |
| Heart Failure | Cell Death | Metabolic Disease | Cellular Growth and Proliferation | Cellular Development | Cell Death |
| Cardiac Fibrosis | Cellular Growth and Proliferation | Cardiovascular Disease | Cell Death | Cellular Growth | Cellular Growth and Proliferation |
| Cardiac Hypertrophy | Cell-to-Cell Signaling | Cardiac Congestive Heart Failure | Inflammatory Response | Cardiac Hypertrophy | Gene Expression |
| Metabolic Disease | Inflammatory Response | Heart Failure | Cardiac Fibrosis | Cardiac Infarction | Cardiac Necrosis/Cell Death |
| Cardiovascular Disease | Gene Expression | Inflammatory Disease | Cardiac Infarction | Cardiac Necrosis/Cell Death | Cardiac Hypertrophy |
| Skeletal and Muscular Disease | Cardiac Dysfunction | Cell Death | Coronary Artery Disease | Coronary Artery Disease | Cardiac Infarction |
| Coronary Artery Disease | Cardiac Necrosis/Cell Death | Coronary Artery Disease | Gene Expression | Cell Death | Cardiac Fibrosis |
Differentially regulated genes 2 days, 2 wk, and 2 mo post-MI were subjected to Ingenuity Pathway analysis; their biological functions are described.
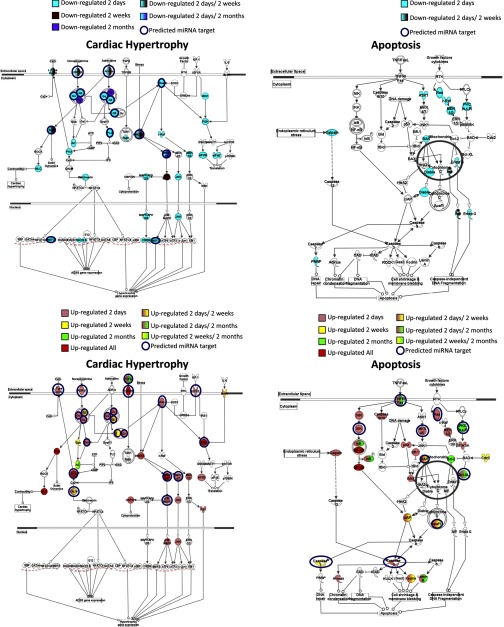
IPA was also performed to understand the cellular context of the differentially expressed genes. Apoptosis and cardiac hypertrophy pathways are shown in Fig. 5. Several genes implicated in cardiac hypertrophy are regulated at all time points, including genes involved in the inflammatory process, β-adrenergic receptors, PKA and MAPK pathways and transcription factors. In the apoptosis pathway, genes involved in promoting apoptosis are mostly upregulated 2 days post-MI, while genes linked to cell survival (Bcl2) or signaling (PKC) pathways are upregulated at later time points. This suggests an initial apoptotic response to injury that is followed by a hypertrophic growth response.
Fig. 5.
Canonical pathway analysis of differentially regulated genes. Genes up- or downregulated post-MI were subjected to Ingenuity Pathway Analysis. Cardiac hypertrophy and apoptosis pathways are shown. Genes are color coded according to regulation at different time points post-MI. miRNAs that can potentially regulate specific genes are annotated in the figure. A list of all mRNAs that are targets for the deregulated miRNAs can be found in Supplemental Table S5.
Differential expression of mRNAs targeted by regulated miRNAs post-MI.
To examine potential mRNA targets for regulated miRNAs, mRNA and miRNA array data sets were analyzed using the microRNA and mRNA integrated analysis (MMIA) software that integrates mRNA and miRNA expression data based on miRNA-predicted targets (13). mRNAs and miRNAs that were found to be differentially and antithetically regulated as determined by array analysis were input into the program. The MMIA report showed that of the downregulated genes, 254, 69, and 23 are predicted to be miRNA targets, at 2 days, 2 wk, and 2 mo post-MI, respectively. Similarly, 499, 363, and 55 upregulated genes are predicted to be miRNA targets at those same time points (Supplemental Tables S3 and S4).
mRNAs predicted to be regulated by miRNAs according to the MMIA analysis were further subjected to IPA and miRNA target prediction for various genes are annotated in the pathways in Fig. 5 and Supplemental Table S5. As detailed in this table, miRNAs known to be regulated in various models of cardiac hypertrophy and MI (miR-21, miR-208, miR-29, miR-27, let-7) are predicted to target expression of several genes in the cardiac hypertrophic and apoptosis pathways including the MAPK pathway, caspases, BCL, MEF2, and β-adrenergic receptor (25).
DISCUSSION
Temporal changes in miRNA expression.
This study focuses on temporal changes in miRNA and gene expression in response to MI. The temporal progression is of interest as it illustrates the engagement of different programmatic responses to injury as the heart transitions from the acute response to the loss of muscle mass to a more compensated, remodeled phenotype. Although temporal miRNA expression analysis have been performed previously in mouse and rat post-MI models and include miRNA expression profile 6 h post-MI in rats (3); 3 and 14 days post-MI in mice (23); and 2, 7, and 14 days post-MI in rats (17), these studies did not include later time points that correspond to a remodeled phenotype nor did they simultaneously examine coexpression of mRNAs. We show herein that there are dramatic changes in miRNA and mRNA expression acutely following MI (2 days) and that a number of these changes tend to normalize at later time points. Interestingly, there is little overlap of differentially expressed miRNAs between individual time points. This suggests that expression of a number of genes is differentially regulated at multiple stages post-MI.
We have previously demonstrated a similar pattern of response in a limited analysis of posttranslational sarcomeric protein modifications in this same model: phosphorylation of contractile proteins including the regulatory myosin light chain, myosin binding protein C, and troponin I is markedly decreased acutely and recovers to sham levels by 2 wk post-MI (24). The implications of these temporal changes in mRNA and miRNA expression are significant. Theoretically, miRNAs can regulate hundreds of target mRNAs simultaneously. Adding to the complexity, a single mRNA can be regulated simultaneously by several miRNAs. Therefore, subtle quantitative changes in miRNAs expressed at different time points have the potential to profoundly impact the pathways that are regulated in response to MI.
miRNA regulation of cardiac disease-related pathways.
Several miRNAs have been implicated in different biological functions in the response to MI. In particular, miR-29 has been demonstrated to have an important role in the regulation of collagen expression and in the development of fibrosis; specifically, downregulation of miR-29 results in upregulation of several collagen genes (23). Our results show that expression of miR-29 is decreased at 2 wk post-MI but upregulated at 2 mo post-MI, a finding that correlates temporally with the expression of collagen genes; whereas four and five genes are respectively upregulated in 2 days and 2 mo post-MI, and 14 are upregulated 2 wk post-MI.
miR-21, which in our study was markedly upregulated at all time points, can be downregulated or upregulated depending on disease setting (upregulated in hypertrophy, downregulated in ischemia) or proximity to area of infarct (decreased in the ischemic region, increased in peri-infarct zone) (14). Increased expression of miR-21 can have an antiapoptotic effect through inhibition of PTEN and the apoptotic proteins FasL and PDCD4 (3, 16) but can also result in the development of fibrosis through increased expression of matrix metalloproteinase (MMP) 2 in fibroblasts (15). Forced overexpression of miR-21 has been shown to reduce MI size and decrease LV dimensions (3). These results suggest that miR-21 overexpression may have a positive effect in the post-MI setting but that miR-21 therapy may also increase myocardial fibrosis.
In other studies, increased expression of miR-195 resulted in the development of cardiac hypertrophy and dilatation (21). The current results show an increase in miR-195 expression chronically post-MI, concordant with previous reported expression results in cardiac hypertrophy and heart failure (18). However, acutely post-MI, miR-195 expression is downregulated. The consequences of miR-195 downregulation have not been analyzed and may have a detrimental effect in the heart.
The temporal expression of miRNAs in response to injury may affect apoptosis. As seen in Fig. 5 and Supplemental Table S5, several proapoptotic mRNAs are upregulated 2 days post-MI that can be targeted by miRNAs (caspase 3 and 7). At 2 mo a different subset of mRNAs involved in cell survival and growth are upregulated, and their expression is also predicted to be regulated by antithetically expressing miRNAs (Bcl-2, PKC). These results support the notion that molecular genetic responses to an acute MI are complex and stage-specific suggesting that future post-MI miRNA-based therapeutics may vary based on time.
MyomiR expression in MI.
The so-called MyomiRs, miR-208a, miR-208b, and miR-499, are encoded from introns of αMyHC, βMyHC, and MyHC7b, respectively. miR-208a knockout animals lack expression of both miR-208b and miR-499 (20). Interestingly, studies of the role of MyomiRs have been somewhat inconsistent; one study showed little to no effect of overexpression or downregulation of miR-208a in the heart (20, 22), whereas another study showed an increase in LV diameter in diastole and systole, a decrease in fractional shortening, and an increase in βMyHC expression in response to miR-208a overexpression (2).
As stated above, the expression of MyomiRs is presumed to be directly correlated with expression of their host genes (20, 22). However, current results suggest that their expression can be independent of their host genes. In fact, in a recent study by Monteys et al. (12), it was noted that ∼35% of intronically encoded miRs have upstream promoter elements that function independently of their host genes; this includes miR-208b. The results presented herein show that downregulation of miR-208a is independent of the expression of miR-208b and miR-499, a result that differs from a previous study in which genetic knockout of miR-208a results in no detectable expression of miR-208b and miR-499. In fact, we observed downregulation of miR-208a acutely and at 4 mo post-MI (data not shown), while expression of miR-208b was increased at 2 wk and 4 mo post-MI, and expression of miR-499 was only changed acutely post-MI (Fig. 3). Since miR-208a and miR-208b have very similar seed sequences, they may have overlapping targets, and the effect of downregulation of miR-208a may abrogate the effect of upregulation of miR-208b. Because overexpression and knockout studies were done using genetically modified models (2, 20, 22), the global effect of modifying the expression of MyomiRs in a wild-type animal in response to injury is still not well defined.
mRNA regulation in cardiac disease-related pathways.
In contrast to miRNAs, a lot more is known about changes in mRNAs and their respective pathways in response to injury in the heart. As outlined above, fibrosis, contractile impairment, inflammation, and apoptosis are well-known processes important for disease progression after MI, and expression of several genes in these pathways change post-MI. As summarized in results, a small number of mRNAs is commonly regulated at all time points post-MI and most of these are involved in biological processes important in the post-MI response. Moreover, differentially regulated miRNAs are predicted to regulate genes involved in these process (Supplemental Table S3–S5), underlying the importance of miRNAs in regulating global responses to injury. Similar to miRNAs, mRNA expression is also temporally regulated, following an acute and compensatory responses pattern.
In addition to the differential expression of apoptosis-related genes described above, MMP genes also are upregulated 2 days post-MI, while their inhibitor, tissue inhibitor of metalloproteinases (TIMP1), is upregulated at 2 days and 2 mo post-MI. A likely explanation of this finding is the prevention of an overt degradation of extracellular matrix resulting in increased fibrosis.
Calcineurin plays an important role in the hypertrophic process (25). Importantly, the expression of calmodulin, which regulates calcineurin activity, is upregulated at 2 days and 2 wk post-MI. Conversely, its inhibitor RCAN2 is downregulated at 2 days; however, RCAN1 is upregulated at 2 mo post-MI. These findings suggest that an increase in calcineurin activity occurs at the early of stages of the post-MI response with a decrease in activity occurring at later time points. These results suggest the pathological acute response is followed by a countervailing chronic, compensatory response.
Of the genes commonly regulated at all time points, several have been shown to have important functions in ventricular response post-MI. For example, downregulation of PDE4 expression in heart failure has been implicated in hyperphosphorylation of the ryanodine receptor, resulting in a leaky receptor, vulnerability to arrhythmias, and impaired contractility (9); upregulation of the inflammatory response through increased expression of TGF-βR1 and Smad results in increased fibrotic tissue deposition (6); inhibition of protein tyrosine phosphatases and dual-specific phosphatase 6 decreases apoptosis and increases survival post-MI (11); hypoxia-inducible factor-1α is upregulated in hypoxic environments and is thought to be cardioprotective (10).
There are several important conclusions that can be derived from this study: 1) we show temporally dependent changes in miRNA and mRNA expression post-MI that in general are characterized by dramatic changes acutely postinjury and are normalized as disease progresses. We have recently completed a study of temporal changes in a mouse model of β-adrenergic receptor overexpression, and an opposite pattern of changes in gene expression was observed: few miRNAs and mRNAs changed at the early time points before the onset of overt cardiac pathology, and several miRNAs and mRNAs changed at later stages that correlated with cardiac dysfunction (Dockstader K; Nunley K, Karimpour-Fard A, Medway A, Nelson P, Port JD, Liggett SB, Bristow MR, Sucharov CC, unpublished observations). The differences in temporal expression of miRNAs in the overexpression and the MI model suggest that regulation of miRNA expression is highly dependent on changes in heart function (2). Differentially regulated miRNAs are predicted to target expression of several genes in multiple biological processes involved in the response to MI, including apoptosis, hypertrophic response, MAPK pathway, and Ca2+-signaling proteins, indicating that miRNAs are likely to have a global effect on biological processes in a disease setting (3). Antithetical and compensatory changes in miRNA expression are observed at later disease stages. This suggests that the function of miRNAs at different stages requires careful analysis: for example, miR-21 overexpression can be antiapoptotic but can result in fibrosis; downregulation of miR-29 can result in fibrosis, but the effect of its upregulation is not known; upregulation of miR-195 results in cardiac hypertrophy/heart failure, but the effect of its downregulation as observed acutely post-MI is not known. Since most miRNA studies have been done in genetically altered animals or in cell-based systems, the effect of changes in miRNA expression may differ depending on a pathologic context (4). A combinatorial analysis of mRNA and miRNA expression may aid in determining factors involved in compensatory and decompensated responses to cardiac injury.
Because miRNAs may ultimately be an important alternative to pharmacological treatment of heart failure, it will be essential to determine if an observed change in miRNA levels is the result of an injury or a compensatory response to acute changes to develop proper miRNA-based therapy.
GRANTS
This work was supported by the Temple Hoyne Buell Foundation (L. A. Walker, P. M. Buttrick) and National Heart, Lung, and Blood Institute Grants HL-051239, HL-101435 (J. D. Port), and R21 HL-097123, 1K01HL-088708-01 (C. C. Sucharov).
DISCLOSURES
J. D. Port: employee, shareholder, ARCA Biopharma; equity in miRagen Therapeutics, Inc. C. C. Sucharov: equity in miRagen Therapeutics, Inc.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ambros V. microRNAs: tiny regulators with great potential. Cell 107: 823–826, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772–2786, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem 284: 29514–29525, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol 15: 331–341, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13: 1877–1893, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res 3: 280–289, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123: 25–35, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ 15: 686–690, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem 283: 31246–31255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monteys AM, Spengler RM, Wan J, Tecedor L, Lennox KA, Xing Y, Davidson BL. Structure and activity of putative intronic miRNA promoters. RNA 16: 495–505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nam S, Li M, Choi K, Balch C, Kim S, Nephew KP. MicroRNA and mRNA integrated analysis (MMIA): a web tool for examining biological functions of microRNA expression. Nucleic Acids Res 37: W356–W362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Port JD, Sucharov C. Role of microRNAs in cardiovascular disease: therapeutic challenges and potentials. J Cardiovasc Pharmacol 56: 444–453, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82: 21–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 285: 20281–20290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi B, Guo Y, Wang J, Gao W. Altered expression of microRNAs in the myocardium of rats with acute myocardial infarction. BMC Cardiovasc Disord 10: 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell 19: 4141–4153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007. [DOI] [PubMed] [Google Scholar]
- 23. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027–13032, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol 48: 1180–1186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang WC, Juan AH, Panebra A, Liggett SB. MicroRNA let-7 establishes expression of β2-adrenergic receptors and dynamically down-regulates agonist-promoted down-regulation. Proc Natl Acad Sci USA 108: 6246–6251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White HD, Chew DP. Acute myocardial infarction. Lancet 372: 570–584, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Takagawa J, Sievers RE, Khan MF, Viswanathan MN, Springer ML, Foster E, Yeghiazarians Y. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol 292: H1187–H1192, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.