Abstract
Background
In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a randomized, double-blind, practice-based, active-control, comparative effectiveness trial in high-risk hypertensive participants, risk of new-onset heart failure (HF) was higher in the amlodipine (2.5-10 mg/day) and lisinopril (10-40 mg/day) arms compared with the chlorthalidone (12.5-25 mg/day) arm . Similar to other studies, mortality rates following new-onset HF were very high (≥50% at 5 years), and were similar across randomized treatment arms. After the randomized phase of the trial ended in 2002, outcomes were determined from administrative databases.
Methods and Results
Using national databases, post-trial follow-up mortality through 2006 was obtained on participants who developed new-onset HF during the randomized (in-trial) phase of ALLHAT. Mean follow-up for the entire period was 8.9 years. Of 1761 participants with incident HF in-trial, 1348 died. Post-HF all-cause mortality was similar across treatment groups with adjusted hazard ratios (95% confidence intervals) of 0.95 (0.81-1.12) and 1.05 (0.89-1.25), respectively, for amlodipine and lisinopril compared with chlorthalidone, and 10-year adjusted rates of 86%, 87%, and 83%, respectively. All-cause mortality rates were also similar among those with reduced ejection fractions (84%) and preserved ejection fractions (81%) with no significant differences by randomized treatment arm.
Conclusions
Once HF develops, risk of death is high and consistent across randomized treatment groups. Measures to prevent the development of HF, especially blood pressure control, must be a priority if mortality associated with development of HF is to be addressed.
Keywords: heart failure, hypertension, diuretics, mortality, ejection fraction
INTRODUCTION
Heart failure (HF) is the most frequent reason for hospitalization among older Americans with annual dollar costs estimated at 37.2 billion. Its increasing prevalence in part reflects longer survival of patients with hypertension and coronary heart disease (CHD). Over 5.8 million Americans have HF; more than 670 000 new cases are diagnosed annually. The cost in lives lost remains high, with 1-year mortality exceeding 20% and 5-year mortality over 50%.1-4
Hypertension is a major risk factor for HF. Treatment of hypertension with long-acting thiazide-type diuretics has been shown to reduce its incidence by 49%-64%.5-7 Angiotensin converting enzyme inhibitors (ACEIs) have been shown to reduce the incidence of HF in patients at high risk for cardiovascular events by 20%-25%.8-10
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) was a multi-center, randomized, double-blind, active-controlled trial designed to determine if the primary outcome of fatal CHD or non-fatal myocardial infarction (MI) is lower for high-risk antihypertensive patients assigned to an ACEI (lisinopril), calcium channel-blocker (CCB; amlodipine), or α-blocker (doxazosin) compared with a thiazide-type diuretic (chlorthalidone). Several secondary endpoints were prespecified, including HF.11 After an average follow-up of 4.9 years (3.2 in the doxazosin/chlorthalidone comparison, which was terminated early), we reported that the diuretic-based treatment was superior to each of the comparator drugs in preventing new-onset HF (hazard ratio (HR)[95% confidence interval (CI)])=1.38[1.25-1.52]; 1.19[1.07-1.31]; 1.80[1.61-2.02], respectively, for the CCB, ACEI, and α-blocker compared with the diuretic).12, 13
The randomized active treatment phase of ALLHAT ended in March, 2002. Since then, per Protocol, ALLHAT participants were followed in administrative databases extending through 2006.14 The main results of the extended follow-up for the amlodipine and lisinopril comparisons with chlorthalidone have been presented.15 After an average 8.9 years of follow-up (4.9 years active treatment phase +4 years post-active follow-up), the risk of new-onset HF (hospitalization or death without hospitalization) remained elevated in the CCB group compared with the thiazide-type diuretic group (HR=1.12; 95% CI 1.02-1.22). The HF rate was not significantly different for lisinopril compared with chlorthalidone.16 The doxazosin/chlorthalidone comparison will be reported separately due to differential follow-up duration. In this manuscript, we evaluate mortality among participants who developed new-onset HF associated with hospitalization or death (with or without hospitalization) during the active treatment phase of ALLHAT, overall, by randomized treatment assignment, and by left ventricular ejection fraction (EF).
METHODS
Active Follow-up
Details of ALLHAT’s design have been published.11 Briefly, eligible participants were at least 55 years old with a systolic blood pressure (SBP) of 140 mmHg or higher, and/or a diastolic blood pressure (DBP) of 90 mmHg or higher, and/or were taking antihypertensive medication (<3 drugs) with a blood pressure of 160/100 mmHg or lower at randomization, and had at least 1 additional CHD risk factor (including preexisting cardiovascular and/or cerebrovascular disease). Individuals with a history of symptomatic HF or left ventricular EF<35% were excluded. Active follow-up of ALLHAT participants ended March 31, 2002.
Institutional Review Board Approval
It was recognized at the inception of ALLHAT that post-trial follow-up would be important in assessing long-term antihypertensive treatment effects. Informed consent from ALLHAT subjects was sought for participation in the study and for permission for post-study morbidity and mortality follow-up. The University of Texas Health Science Center Institutional Review Board (IRB) approved the long-term follow-up study.
Design
ALLHAT’s Extension Study protocol can be found at http://allhat.sph.uth.tmc.edu/.14 The prespecified primary outcome is cardiovascular disease (CVD) mortality (death due to CHD, stroke, HF, or other CVD). Secondary outcomes include total and cause-specific mortality, CVD (CVD mortality, hospitalized non-fatal MI, stroke, or HF), CHD (CHD mortality or hospitalized non-fatal MI), stroke (fatal or non-fatal hospitalized), HF (fatal or non-fatal hospitalized), and end-stage renal disease.14
Heart Failure Outcome
During active patient follow-up, HF events were assessed at follow-up visits and reported to the Clinical Trials Center (CTC). For all hospitalized events, copies of discharge summaries and/or death certificates accompanied investigator-submitted reports and were evaluated at the CTC for accuracy and completeness. The Endpoints Committee classified 10% of CHD and stroke events; no such central review was specified in the initial ALLHAT protocol for HF events.12 An extensive Heart Failure Validation Study undertaken at the end of active follow-up centrally adjudicated in a blinded manner all investigator-reported hospitalized HF events using established diagnostic criteria, including the prespecified ALLHAT algorithm (previously used in the SHEP trial).5 The ALLHAT Heart Failure Validation Study also collected EF information from HF hospitalizations.17, 18
During the extended follow-up, hospitalizations and deaths due to HF, and mortality in participants with previously diagnosed HF, were ascertained using administrative databases.14 Information on medications, BP, outpatient morbidity and treatment, and laboratory data was not collected.
Mortality Ascertainment
Cause of death was determined by the investigator during the trial. Mortality data were available for the entire cohort during in-trial and post-trial periods, except for Canadian participants, for whom database access was unavailable.
All-cause mortality was ascertained from the National Death Index (NDI) and the Social Security Administration (SSA). A death identified through passive (administrative database) surveillance was verified at the CTC after receipt from the appropriate administrative jurisdiction of a death certificate, used solely for verification of participant identity. Cause of death was then provided by the NDIPlus, and the International Classification of Diseases (ICD)-10 codes obtained from NDIPlus were collapsed into the 11 categories used in-trial.
Statistical Analysis
Statistical analyses were undertaken with the STATA software (version 11) (2009; Stata Corporation, College Station, TX, USA).To compare baseline characteristics of HF participants assigned to amlodipine or lisinopril versus chlorthalidone, and of those with versus without in-trial HF, contingency tables and t-tests were used. Analyses of the treatment effects on risk for primary and secondary outcomes were performed using Cox regression. The follow-up period includes both the randomized trial (mean follow-up duration, 4.9 years) and subsequent extension period follow-up (4 years). The estimated 10-year event rates for CVD mortality, total mortality, and hospitalized HF mortality in the chlorthalidone group were calculated using a Weibull survival model of observed results in the original study. Statistical power for each analysis was obtained using these rates and the sample sizes within the ALLHAT treatment groups and subgroups. For CVD mortality, the primary outcome, we estimated a 90% power with α=0.017 to detect an 11% risk reduction (HR=0.90) for the chlorthalidone group (10-year CVD mortality rate of 16%) compared to either the amlodipine or lisinopril group.14, 19 Post-HF mortality rates were calculated using the Kaplan-Meier method. Adjusted mortality rates and adjusted treatment HRs were calculated using Cox regression and baseline factors such as age, race, sex, and time to HF. The proportional hazards model assumption for treatment was checked by adding a time dependent covariate (treatment x log time) and by visual inspection of log-log survival plots for each of the Cox models. Time-dependent Cox regression was used to estimate the HRs associated with the treatment intervention separately for in-trial and post-trial periods. Treatment HRs for mortality were calculated using HF development as a time-dependent variable with tests for interactions to determine whether the effects of the treatment differed. Treatment changes, captured in the database, occurred frequently following incident HF hospitalizations during the active treatment phase of the trial. A shift in medication status within treatment groups was reported as percent of participants prior to a HF event whose medication changed, either going off or on a given medication, following the event. Given the many multivariate, subgroup, and interaction analyses performed, statistical significance at the 0.05 level should be interpreted cautiously.
RESULTS
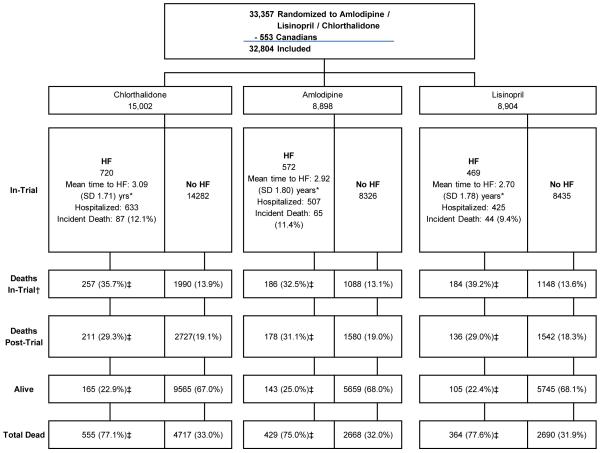
A total of 32 804 participants (plus 553 Canadian participants) were randomized to the ALLHAT chlorthalidone, amlodipine, and lisinopril arms. During the randomized treatment phase (through March, 2002), 720 of the 15 002 chlorthalidone participants (4.8%), 572 of the 8899 amlodipine participants (6.4%), and 469 of the 8904 lisinopril participants (5.3%) had hospitalized or fatal HF (total 1761 events). Of the participants with in-trial hospitalized or fatal HF events, 77%, or 1348 of the 1761, were dead at the end of the extended follow-up period (through December, 2006): 196 as incident in-trial HF events, 627 additional in-trial deaths, and 525 post-trial deaths (Figure 1 and Supplemental Table 1).
Figure 1. Consort diagram for heart failure extension.
*Wilcoxon test for equality of survival function p values are: A vs C: P=.12; L vs. C: P<.001
†Separate from/does not include incident HF deaths
‡Among In-Trial (Limited Access Data Set; LADS) Hospitalized HF Cases
Baseline characteristics for those with and without in-trial hospitalized or fatal HF are provided in Table 1. Significant differences between those who developed HF during the trial compared with those who did not were seen in most of the characteristics listed. Notably, those who developed HF were older, had higher SBP and DBP, were more likely to have a history of CVD, CHD, LVH, and type 2 diabetes, as well as lower estimated glomerular filtration rates. There were no differences in gender, race, or total cholesterol.
Table 1.
Baseline characteristics of ALLHAT participants (excluding Canadian participants) with vs. without in-trial hospitalized or fatal heart failure.
| Amlodipine/Lisinopril/Chlorthalidone Comparison | |||
|---|---|---|---|
| Baseline Characteristic | No In-Trial HF N=31 043 |
In-Trial Hospitalized or Fatal HF N=1761 |
P‡ |
| Age in years, mean(SD) | 66.7(7.6) | 70.3(8.2) | <0.001 |
| Age range in years, n(%) | |||
| 55-64 | 13 435(43.3) | 464(26.4) | <0.001 |
| ≥65 | 17 608(56.7) | 1297(73.6) | |
| Race, n(%) | |||
| Black | 11 146(35.9) | 626(35.6) | 0.76 |
| Non-Black | 19 897(64.1) | 1135(64.4) | |
| Women, n(%) | 14 597(47.0) | 796(45.2) | 0.14 |
| Education in years, mean(SD) | 11.0(4.0) | 10.7(3.9) | 0.01 |
| Medication use, n(%) | |||
| Antihypertensive treatment | 27 992(90.2) | 1641(93.2) | <0.001 |
| Aspirin | 10 934(35.2) | 759(43.1) | <0.001 |
| Blood Pressure in mm Hg, mean(SD) | |||
| SBP/DBP | 146(16)/84(10) | 148(16)/82(11) | <0.001/<0.001 |
| Treated SBP/DBP | 145(16)/84(10) | 147(16)/81(11) | <0.001/<0.001 |
| Pulse in beats/minute, mean(SD) | 74(11) | 75(11) | <0.001 |
| Eligibility risk factors* | |||
| Cigarette smoker | 6846(22.1) | 323(18.3) | <0.001 |
| ASCVD† | 15 728(50.7) | 1140(64.7) | <0.001 |
| History MI or stroke | 6949(22.4) | 635(36.1) | <0.001 |
| History coronary revascularization | 3843(12.4) | 381(21.6) | 0.001 |
| Other ASCVD | 7175(23.1) | 540(36.7) | <0.001 |
| Major ST depression or T-wave inversion | 3151(10.3) | 215(12.4) | 0.005 |
| Type 2 diabetes | 12 069(41.9) | 941(57.1) | <0.001 |
| HDL-C<0.9065 mmol/L | 3622(11.7) | 190(10.8) | 0.26 |
| LVH by echo or ECG | 6168(19.9) | 384(21.8) | 0.048 |
| History CHD | 7589(24.6) | 649(37.2) | <0.001 |
| LVH(Minnesota code) | 3415(12.9) | 261(17.3) | <0.001 |
| Body mass index, mean(SD) | 29.7(6.2) | 30.4(7.0) | <0.001 |
| Biochemical measures, mean(SD) | |||
| Total cholesterol(mmol/L) | 5.59(1.12) | 5.63(1.24) | 0.20 |
| Potassium(mmol/L) | 4.31(.51) | 4.36(.55) | <0.001 |
| Fasting glucose(mmol/L) | 6.79(3.14) | 7.74(3.83) | <0.001 |
| eGFR(ml/min/1.73m2) | 78.1(19.6) | 71.7(21.1) | <0.001 |
For trial eligibility, participants had to have at least 1 other risk factor in addition to hypertension. Thus the indicated risk factors are not mutually exclusive or exhaustive, and may not represent prevalence.
History of myocardial infarction or stroke; history of coronary revascularization; major ST segment depression on T wave inversion on any ECG in the past 2 years; other ASCVD (history of angina pectoris; history of intermittent claudication, gangrene, or ischemic ulcers; history of transient ischemic attack; coronary, peripheral vascular, or carotid stenosis 50% or more documented by angiography or Doppler studies; ischemic heart disease documented by reversible or fixed ischemia on stress thalium or dipyridamole thalium, ST depression ≥1 mm for ≥1 minute on exercise testing or Holter monitoring; reversible wall motion abnormality on stress echocardiogram; ankle-arm index <.9; abdominal aortic aneurysm detected by ultrasonography, CT scan, or X-ray; carotid or femoral bruits).
P value for contingency tables calculated using the Pearson chi-square statistic.
HF=heart failure; SBP/DBP=systolic blood pressure/diastolic blood pressure; ASCVD=atherosclerotic cardiovascular disease; HDLC=high density lipoprotein cholesterol; LVH-left ventricular hypertrophy; CHD=coronary heart disease; eGFR=estimated glomerular filtration rate (derived from application of the following simplified MDRD equation based on serum creatinine level, age, race, and sex; Estimated GFR=186.3×(Serum creatinine)−1.154×(Age, y)−0.203×1.212(if black)×0.742(if female)28; body mass index calculated by the weight in kilograms divided by the square of height in meters
Among those with in-trial hospitalized or fatal HF, the amlodipine group had significantly more, and the lisinopril group trended to more, black participants compared with the chlorthalidone group (P=0.04 and P=0.06, respectively; data not shown).
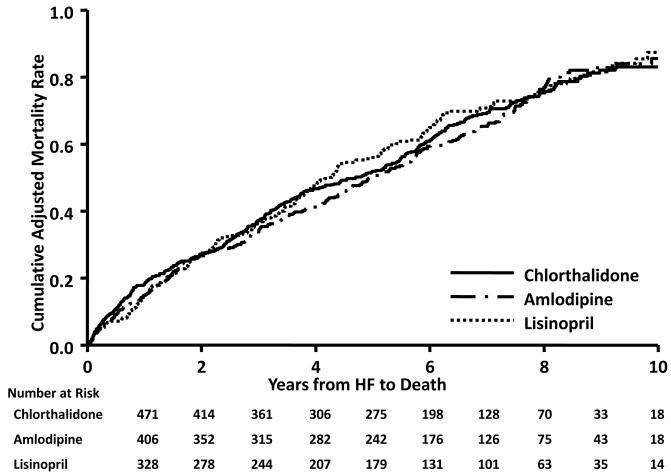
Mortality outcomes adjusted for characteristics associated with the initial in-trial hospitalization for HF, overall and by EF status (Preserved EF [PEF], EF≥50%; Reduced EF [REF], EF<50%) at the initial hospitalization for HF, are shown in Table 2.17 Mortality rates are similar across treatment comparisons, with adjusted 10-year all-cause mortality rates per 100 persons of 83 for chlorthalidone, 86 for amlodipine, and 87 for lisinopril. When divided into CVD and non-CVD mortality, 10-year rates are again similar across treatment groups, ranging from 57% in chlorthalidone to 66% in amlodipine for CVD mortality and from 55% in amlodipine to 62% in lisinopril for non-CVD mortality. There were no in-trial vs. post-trial treatment interactions affecting mortality outcomes for either treatment comparison (Supplemental Table 2). For all-cause, CVD, and non-CVD mortality, adjusted 10-year mortality rates are higher (though in some cases absolute numbers are quite small) for HF-REF compared to HF-PEF, with the exception of all-cause and non-CVD mortality in the lisinopril group. In both treatment comparisons and in both HF-REF and HF-PEF groups, mortality rates do not differ between treatment groups. Adjusted all-cause mortality curves by treatment group are shown in 17, 20Figure 2, and by EF status in Supplemental Figure 1. There were no statistically significant differences in risk for any of the mortality outcomes following HF-PEF or HF-REF for either treatment group comparison. The proportional hazard assumption for treatment for post-HF mortality was not violated. Further, there were no in-trial vs. post-trial treatment interactions affecting mortality outcomes for either treatment comparison, whether for HF-REF or HF-PEF (Supplemental Table 3).
Table 2.
Adjusted mortality rates and hazard ratios by treatment group for hospitalized heart failure (overall and by ejection fraction status) patients.
| Chlorthalidone | Amlodipine | Lisinopril | |||||
|---|---|---|---|---|---|---|---|
| n, 10-y rate/100 persons | n, 10-y rate/100 persons | HR* (95% CI) | P | n, 10-y rate/100 persons | HR*(95% CI) | P | |
| All-cause mortality † | |||||||
| Total | 555, 83.0 | 429, 85.6 | 0.95(0.81-1.12) | 0.555 | 364, 87.3 | 1.05(0.89-1.25) | 0.545 |
| PEF ‡ § | 79, 81.0 | 66, 71.3 | 0.82(0.58-1.16) | 0.263 | 68, 88.7 | 0.98 (0.70-1.38) | 0.885 |
| REF ‡ § | 127, 87.4 | 102, 83.2 | 0.96(0.72-1.28) | 0.786 | 70, 84.8 | 1.03(0.76-1.40) | 0.841 |
| CVD mortality † | |||||||
| Total | 374, 57.1 | 270, 65.9 | 0.92(0.75-1.13) | 0.435 | 225, 63.2 | 0.97(0.77-1.22) | 0.782 |
| PEF ‡ § | 44, 51.9 | 36, 49.2 | 0.85(0.53-1.36) | 0.502 | 31, 54.8 | 0.88(0.54-1.43) | 0.607 |
| REF ‡ § | 85, 69.8 | 64, 55.2 | 0.84(0.58-1.21) | 0.346 | 42, 60.5 | 0.90(0.61-1.34) | 0.606 |
| Heart failure † | |||||||
| Total | 141, 13.1 | 96, 11.3 | 0.67(0.41-1.10) | 0.111 | 78, 10.4 | 1.13(0.70-1.83) | 0.607 |
| PEF ‡ § | 10, 5.2 | 4, 11.1 | 0.51(0.13-2.07) | 0.333 | 5, 6.1 | 0.76(0.22-2.65) | 0.672 |
| REF ‡ § | 17, 10.1 | 17, 9.3 | 0.88(0.37-2.08) | 0.773 | 8, 9.5 | 1.02(0.40-2.60) | 0.960 |
| Other CVD † | |||||||
| Total | 233, 48.5 | 174,58.9 | 0.99(0.79-1.25) | 0.927 | 147, 55.4 | 0.92(0.71-1.19) | 0.509 |
| PEF ‡ § | 34, 49.6 | 32, 43.8 | 0.92(0.56-1.53) | 0.750 | 26, 49.6 | 0.90(0.53-1.53) | 0.706 |
| REF ‡ § | 68, 66.4 | 47, 49.6 | 0.84(0.56-1.25) | 0.384 | 34, 55.8 | 0.88(0.57-1.37) | 0.579 |
| Non-CVD mortality † | |||||||
| Total | 167, 58.1 | 152, 54.5 | 1.06(0.83-1.37) | 0.634 | 131, 61.5 | 1.23(0.94-1.62) | 0.125 |
| PEF ‡ § | 30, 53.6 | 30, 43.3 | 0.93(0.55-1.56) | 0.773 | 35, 76.3 | 1.20(0.73-1.98) | 0.478 |
| REF ‡ § | 39, 58.1 | 33, 63.9 | 1.14(0.70-1.85) | 0.605 | 25, 57.3 | 1.24(0.74-2.09) | 0.411 |
Comparisons are amlodipine to chlorthalidone and lisinopril to chlorthalidone.
Adjusted for baseline characteristics of age, race, gender, smoking, education, treatment of hypertension, SBP, DBP, pulse, type II diabetes, LVH by ECG, history of CHD, BMI, eGFR, and time to HF.
This represents the known ejection fraction status of those participants in the Heart Failure Validation Study (HFVS)17 and eligible for Extension.
Adjusted for baseline characteristics of age and time to HF.
EF=ejection fraction; HF=Heart failure; PEF=Preserved ejection fraction; REF=Reduced ejection fraction; CVD=cardiovascular disease; HR=hazard ratio; CI=confidence interval
Figure 2. Adjusted KM curves by treatment group for all-cause mortality of participants with hospitalized heart failure*.
*Adjusted for baseline characteristics of age, race, gender, smoking, education, treatment of hypertension, SBP, DBP, pulse, type II diabetes, LVH by ECG, history of CHD, BMI, eGFR, and time to HF.
Approximately 92% of participants with hospitalized (including hospitalized fatal) heart failure events during ALLHAT were part of the Heart Failure Validation Study (HFVS). This figure reflects only those participants with events meeting ALLHAT HF definition in the Validation Study17, 20 and eligible for Extension.
HF=heart failure
Table 3 presents the Cox multivariate regression analyses using HF as a time-varying covariate, i.e., does the occurrence of HF increase the subsequent risk of death? The HRs by mortality outcome and treatment group showed significantly increased risk of death due to all causes, CVD, and non-CVD. Results were the same when analyzed by EF group, with HRs slightly higher among those with HF-REF compared with HF-PEF, with the exception of non-CVD deaths for amlodipine and lisinopril. Overall, there were no statistically significant treatment-by-incident HF interactions in either of the analyses in Table 3.
Table 3.
Adjusted mortality hazard ratios for hospitalized heart failure (overall and by ejection fraction status) patients comparing those who developed heart failure vs those who did not develop heart failure (heart failure treated as a time-varying covariate)
| Total Mortality | CVD Death | HF Death | Other CVD | Non-CVD | |
|---|---|---|---|---|---|
| HR(95% CI), P | HR(95% CI), P | HR(95% CI), P | HR(95% CI), P | HR(95% CI), P | |
| Overall | |||||
| Total | 2.89(2.69-3.11), <0.001 | 3.84(3.49-4.23), <0.001 | 8.06(6.38-10.18), <0.001 | 3.40(3.05-3.78), <0.001 | 2.18(1.95-2.43), <0.001 |
| PEF * | 2.42(2.08-2.81), <0.001 | 2.73(2.20-3.38), <0.001 | 3.81(2.18- 6.67), <0.001 | 2.60(2.06-3.28), <0.001 | 2.15(1.73-2.67), <0.001 |
| REF * | 3.06(2.67-3.51), <0.001 | 4.27(3.58-5.09), <0.001 | 6.80(4.36-10.62), <0.001 | 3.99(3.29-4.83), <0.001 | 2.05(1.63-2.57), <0.001 |
| Chlorthalidone | |||||
| Total | 2.82(2.52-3.15), <0.001 | 3.81(3.29-4.41), <0.001 | 8.10(5.71-11.48), <0.001 | 3.33(2.82-3.93), <0.001 | 1.97(1.65-2.36), <0.001 |
| PEF * | 2.47(1.93-3.15), <0.001 | 2.91(2.08-4.08), <0.001 | 4.12(1.80- 9.42), 0.001 | 2.74(1.89-3.91), <0.001 | 1.89(1.29-2.77), 0.001 |
| REF * | 3.16(2.57-3.88), <0.001 | 4.48(3.45-5.82), <0.001 | 7.66(4.02-14.61), <0.001 | 4.15(3.11-5.52), <0.001 | 2.05(1.44-2.91), <0.001 |
| Amlodipine | |||||
| Total | 2.80(2.46-3.18), <0.001 | 3.80(3.19-4.51), <0.001 | 6.30(3.99-9.96), <0.001 | 3.54(2.93-4.27), <0.001 | 2.15(1.77-2.62), <0.001 |
| PEF * | 2.24(1.28-2.18), <0.001 | 2.83(1.95-4.12), <0.001 | 3.32(1.03-10.65), 0.044 | 2.81(1.89-4.17), <0.001 | 1.93(1.30-2.86), 0.001 |
| REF * | 2.33(1.88-2.89), <0.001 | 3.28(2.36-4.56), <0.001 | 5.30(2.27-12.36), <0.001 | 3.07(2.15-4.39), <0.001 | 1.78(1.20-2.66), 0.005 |
| Lisinopril | |||||
| Total | 3.19(2.78-3.67), <0.001 | 4.02(3.32-4.88), <0.001 | 10.92(7.03-16.94), <0.001 | 3.36(2.71-4.18), <0.001 | 2.59(2.10-3.19), <0.001 |
| PEF * | 2.64(2.01-3.46), <0.001 | 2.49(1.63-3.80), <0.001 | 4.12(1.50-11.36), <0.006 | 2.89(1.43-3.65), <0.001 | 2.78(1.93-4.00), <0.001 |
| REF * | 3.97(3.03-5.21), <0.001 | 5.77(4.07-8.19), <0.001 | 8.12(3.25-20.29), <0.001 | 5.45(3.72-7.97), <0.001 | 2.59(1.64-4.08), <0.001 |
Adjusted for HF (time-varying covariate); baseline risk factors of age, race (black/non-black), gender, smoking status, education, prior treatment for hypertension, SBP, DBP, heart rate, diabetes, LVH by ECG, history of CHD, body-mass index, and eGFR.
CI=confidence interval, CVD=cardiovascular disease, EF=ejection fraction, HF=heart failure, HR=hazard ratio, PEF=preserved ejection fraction, REF=reduced ejection fraction
For patients with medication data available before and after the HF event, there were significant medication shifts following hospitalization, including 39% of patients in each of the amlodipine and lisinopril groups having a diuretic added and 72% of chlorthalidone-assigned patients going off atenolol. (Supplemental Table 4).
DISCUSSION
Using national databases, we examined mortality in the 1761 participants who developed in-trial new-onset HF requiring hospitalization, and for whom mortality data were available (>99% of the cohort). Over three-quarters (77%) of the 1761 ALLHAT participants with initial HF events, fatal or requiring hospitalization, during the active, randomized phase of ALLHAT died over the extended follow-up period (mean follow-up time, 8.9 years). Mortality was high regardless of treatment group or EF category: all-cause 10-year mortality rates ranged from 83% in the chlorthalidone group to 87% in the lisinopril group. McCullough, et al., using administrative data sets exclusively, saw similar mortality rates among patients with heart failure in the community.21 In ALLHAT HF participants, mortality, whether attributed to any cause or limited to CVD or non-CVD causes, did not differ across randomized treatment comparisons; 10-year rates were 57%-66% for CVD and 55%-62% for non-CVD mortality. Among those with REF, 10-year mortality rates were similar across treatment groups (83%-87%); among those with PEF, mortality rates ranged from 81% in the chlorthalidone group to 89% in the lisinopril group.
The in-trial HF results of ALLHAT showed that the risk of HF defined as fatal, hospitalized or treated without hospitalization (ALLHAT prespecified endpoint) was higher in all comparator arms versus chlorthalidone (relative risk [RR]=1.38; 95% CI 1.25-1.52 and 1.19, 95% CI 1.07-1.31 for amlodipine and lisinopril, respectively, compared to chlorthalidone). For the amlodipine/chlorthalidone comparison for hospitalized or fatal HF, results were similar (RR=1.35; 95% CI 1.21-1.50); the lisinopril/chlorthalidone comparison showed no significant difference (RR=1.10, 95% CI 0.98-1.23).11, 12 These findings evoked questions as to diagnostic accuracy, relationship of HF to initial antihypertensive medications, and the eventual fate of the HF patients.22
Central adjudication validated the hospitalized HF diagnoses in the majority of patients (80% by Framingham criteria,23 71% by ALLHAT criteria, and 84% by reviewing cardiologists’ judgment), thus confirming results reported for the ALLHAT prespecified HF outcome (RR=1.46, 95% CI 1.27-1.68, P<0.0001; and 1.18 [CI 1.02-1.28], P=0.04, using ALLHAT diagnostic criteria for the amlodipine and lisinopril comparisons, respectively, with chlorthalidone).17
We have previously reported that the greatest benefit of thiazide-type diuretics in prevention of HF occurred during year 1 of follow-up.20 In seeking explanations for the early benefit, we explored whether discontinuation of medications at study entry could have contributed to the excess new-onset HF in the other drugs compared to chlorthalidone. A study of pre-ALLHAT medications taken by patients with incident in-trial HF showed no treatment group differences.24
We next sought to determine whether there were post-HF differences in survival (or, conversely, mortality) once patients were no longer subject to randomized medication assignments. The passive-follow-up of ALLHAT participants afforded us the opportunity to investigate long-term outcomes of the participants who developed their first hospitalized HF event during the active trial. It is clear from these data that, while randomized treatment groups did differ in HF incidence, mortality was similar and, as might be anticipated, high throughout.
New-onset HF is a common complication of hypertension and is associated with very high subsequent mortality, making prevention of HF an important goal of antihypertensive therapy.4, 23, 25In ALLHAT, 6-year incidence rates of adjudicated hospitalized HF were comparable in magnitude to those of stroke (5.6%) and were about half those of CHD (CHD death or non-fatal MI; 11.4%).17 These data reiterate that, in spite of current treatments for HF, once it develops, the outcome is poor.26, 27 ALLHAT demonstrated that blood pressure control is an achievable goal in high-risk patients treated in diverse medical settings,16, 28 and that a regimen that includes a thiazide-type diuretic remains unsurpassed in the treatment of hypertension, especially in the prevention of HF within the population.
This study has several limitations. The high-risk hypertensive population of ALLHAT may not represent the general community, and outcomes may not be generalizable to a lower-risk population. Nonetheless, similar mortality rates among HF patients in the community have been reported.20 Lacking post-trial medication data on the HF participants, we cannot assess the impact of antihypertensive medications prescribed during the follow-up period. We do know that the majority of participants across the treatment groups were prescribed diuretics following the HF event. It is likely that many of the participants’ medications were altered as new events arose or new medications became available. Further, other post-trial management strategies, including revascularization, implantable devices, and medical therapy as directed by professional treatment guidelines, could modify biologic effects of prior treatment. However, during the in-trial phase, which included the incident HF event, the randomization process assured that, except for the randomized treatment, all other factors, including medications, should not have differed across treatment groups. As noted, in-trial hospitalized HF events were adjudicated via outside reviewers according to strict criteria.17 We did not, however, classify events according to ischemic vs. nonischemic HF. Finally, although the ALLHAT CTC Endpoints Department reviewed all in-trial events (through discharge/death summaries and death certificates), no such review or adjudication was possible for post-trial deaths, for which cause of death was derived from NDI-provided ICD codes. Patient identities associated with these documents were, however, vigorously confirmed through a complex algorithm, and so only specific causes of death may have been inconsistently reported.
In a national and international setting of increasing HF incidence and consequent high costs in lives lost and dollars, addressing modifiable risk factors must be a priority. In ALLHAT, treatment group differences in the risk of developing HF are clear; yet, once the HF develops, neither the type of HF as denoted by EF category nor initial antihypertensive treatment significantly impacts the very high subsequent mortality rates. Prevention of HF is a public health imperative, and a diuretic-based antihypertensive regimen remains a fundamental component of HF prevention.
Supplementary Material
Acknowledgments
Funding Sources This study was supported by a contract with the National Heart, Lung, and Blood Institute. The ALLHAT investigators acknowledge study medications contributed by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Conflict of Interest Disclosures Dr. Cushman has consulted for Daiichi Sankyo, Novartis, Noven, Sanofi-Aventis, Takeda, and Theravance; has received honoraria from Bristol-Myers Squibb; and has received research grants from GlaxoSmithKline, Merck, and Novartis.
Dr. Davis has consulted for Amgen and Takeda.
Dr. Massie has consulted for Averion, Bristol-Myers Squibb, Gilead, Merck, Nile Therapeutics, Novartis, and Sanofi-Aventis; and has received payments for serving on the DSMB from Corthera and Takeda.
Dr. Oparil has consulted for Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Forest Pharmaceuticals, NicOx, and Novartis; and has received research grants from Amgen, Daiichi Sankyo, Gilead, Omron Healthcare, Pfizer, Schering Plough, and Takeda.
Dr. Probstfield has received research grants from Abbot Laboratories, Boehringer Ingelheim, GlaxcoSmithKline, and Sanofi-Aventis.
Dr.Thadani has consulted for Forest Laboratories, Gilead Colorado, Lilly/ICOS, and Merck; has received honoraria from Daiichi Sankyo and Eli Lilly; and has received research grants from Bristol-Myers Squibb, Eisai Medical Research, Eli Lilly, NIH, Novartis, Pfizer, and Schering-Plough.
Drs. Baraniuk, Cuyjet, Dart, Einhorn, Ford, Furberg, Graumlich, Jafri, Parish, Piller, Retta, Saklayen, and Simpson have no financial interests to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 3.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Kostis JB, Davis BR, Cutler J, Grimm RH, Jr, Berge KG, Cohen JD, Lacy CR, Perry HM, Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 6.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 7.Yip GW, Fung JW, Tan YT, Sanderson JE. Hypertension and heart failure: a dysfunction of systole, diastole or both? J Hum Hypertens. 2009;23:295–306. doi: 10.1038/jhh.2008.141. [DOI] [PubMed] [Google Scholar]
- 8.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 10.Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL, PEACE Trial Investigators Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM, for the ALLHAT Research Group Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 12.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 13.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed 4/7/2010];ALLHAT. 2010 Available at: http://allhat.sph.uth.tmc.edu/
- 15.Cushman WC, Davis BR, Pressel SL, Wright JT, Jr, Whelton PK, Cutler JA, Einhorn PT, Barzilay JI, Rahman M, Piller LB, Ford CE, Oparil S, Probstfield JL. Long-term follow-up in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). 2009 Late-Breaking Clinical Trial/Science Abstracts From the AHA Scientific Sessions 2009. Circulation. 2009;120:2160. [Google Scholar]
- 16.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM, for the ALLHAT Collaborative Research Group Success and predictors of blood pressure control in diverse North American settings: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 17.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR, ALLHAT Collaborative Research Group The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S, ALLHAT Collaborative Research Group Heart failure with preserved and reduced left ventricular ejection fraction in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunnett CW. A multiple comparisons procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 20.Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group Role of diuretics in the prevention of heart failure: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2006;113:2201–2210. doi: 10.1161/CIRCULATIONAHA.105.544031. [DOI] [PubMed] [Google Scholar]
- 21.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD, Resource Utilization Among Congestive Heart Failure (REACH) Study Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 22.Davis BR, Furberg CD, Wright JT, Jr, Cutler JA, Whelton P, ALLHAT Collaborative Research Group ALLHAT: setting the record straight. Arch Intern Med. 2004;141:39–46. doi: 10.7326/0003-4819-141-1-200407060-00013. [DOI] [PubMed] [Google Scholar]
- 23.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 24.Grimm RH, Davis BR, Piller LB, Cutler JA, Margolis KL, Barzilay J, Dart RA, Graumlich JF, Murden RA, Randall OS, ALLHAT Collaborative Research Group Heart failure in ALLHAT: did blood pressure medication at study entry influence outcome? J Clin Hypertens. 2009;11:466–474. doi: 10.1111/j.1751-7176.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338:1281–1285. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 26.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafazand M, Schaufelberger M, Lappas G, Swedberg K, Rosengren A. Survival trends in men and women with heart failure of ischaemic and non-ischaemic origin: data for the period 1987-2003 from the Swedish Hospital Discharge Registry. Eur Heart J. 2009;30:671–678. doi: 10.1093/eurheartj/ehn541. [DOI] [PubMed] [Google Scholar]
- 28.Margolis KL, Piller LB, Ford CE, Henriquez MA, Cushman WC, Einhorn PT, Colon PJ, Sr, Vidt DG, Christian R, Wong ND, Wright JT, Jr, Goff DC, Jr, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group Blood pressure control in Hispanics in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension. 2007;50:854–861. doi: 10.1161/HYPERTENSIONAHA.107.092650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.