Abstract
Cryptococcus adeliensis is a recently described new fungal species which has been isolated from decaying algae in Terre Adelie, Antarctica. We report the first known case of meningitis caused by C. adeliensis in a patient with acute myeloid leukemia undergoing allogeneic peripheral blood stem cell transplantation.
CASE REPORT
A 40-year-old female was diagnosed with acute myeloid leukemia (AML) in November 2000. She received two courses of chemotherapy in November and December 2000 with partial response. In March 2001, the first relapse of AML occurred, and she was treated with gemtuzumab ozogamicin (monoclonal antibody against the cellular surface antigen CD33 conjugated with the cytotoxic antibiotic calicheamicin). Beginning 15 March 2001, she complained about an increasingly disturbed gait due to a weakness in the legs. Magnetic resonance imaging (MRI) of the brain was performed on 11 April 2001, and the results were normal.
The patient was readmitted to the hospital on 7 May 2001 for allogeneic peripheral blood stem cell transplantation (PBSCT) from a nonrelated donor. On admission she suffered from a progressive spastic paraparesis with brisk tendon reflexes, leading to an increasingly disturbed gait. The next day, the patient received a central venous catheter, and conditioning for allogeneic PBSCT was performed with total-body irradiation and chemotherapy with cyclophosphamid, fludarabine, and antithymocyte globulin. The patient received antibiotic and antimycotic prophylaxis with ciprofloxacin and oral itraconazole (400 mg/day, liquid formulation). On 15 May 2001, allogeneic PBSCT was performed. On the same day, the patient developed fever and signs of septicemia. Blood cultures yielded Staphylococcus epidermidis and Pseudomonas fluorescens, and antibiotic therapy with ceftazidime, tobramycin, and teicoplanin was initiated. The fever was resolved, but the paraparesis progressed. In addition, the patient developed a diffuse headache, which was controlled by morphine derivatives. Lumbar puncture and a second MRI of the brain were performed 2 days after PBSCT.
This second MRI showed a bifrontal augmentation of the outer cerebrospinal fluid space and a prominent bifrontal atrophy with otherwise normal parenchyma.
The lumbar puncture yielded clear cerebrospinal fluid (CSF) with 59 cells/μl (83% lymphocytes, 12% monocytes, 5% neutrophils). CSF glucose was normal, with 3.7 mmol/liter; protein and lactate were slightly elevated, with 0.482 g/liter and 3.6 mmol/liter, respectively. Gram staining of the centrifuged CSF did not show any microorganisms. An India ink smear revealed very few nonencapsulated round yeast cells. The Cryptococcus species latex agglutination test (IMMY, Norman, Okla.) for the detection of cryptococcal glucuronoxylomannan capsule antigen was negative. Bacterial cultures performed on sheep blood agar and chocolate agar at 37°C yielded no growth after incubation for 48 h. After 3 days incubation on Sabouraud-glucose (2%) agar, growth of tiny colonies with a yeast-like appearance was visible at 30°C but not at 37°C. On Guizotia abyssinica agar at 30°C, the colonies were smooth and cream colored and there was no blackening of the agar. After being incubated for 14 days at 30°C on Sabouraud agar, the colonies developed a light orange tinge. They became pasty with lobate borders. Initial identification of the fungal isolate was done by micromorphological and biochemical means.
On rice agar, spherical yeast cells with unilateral budding were visible. No pseudohyphae were produced. The blastospores were noncapsulated, as revealed by India ink smears. The urease hydrolyzation on Christensen's urea medium was positive. The strain failed to grow on Sabouraud agar at 37°C, grew slowly at 30 and 4°C, and grew best at 25°C.
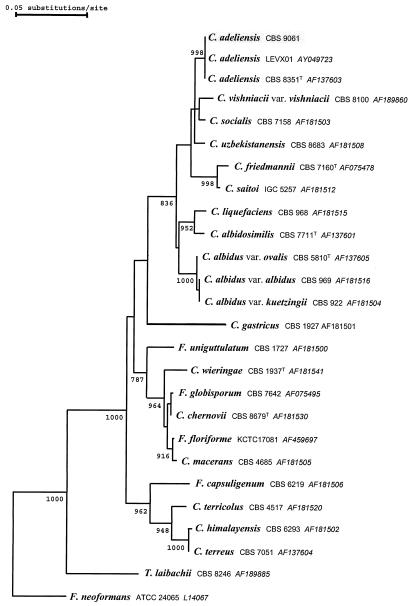
Carbohydrate assimilation tests were performed using the ID 32C yeast identification system (bioMérieux, Marcy l'Etoile, France). The assimilation tests were positive with sucrose, l-arabinose, cellobiose, raffinose, maltose, trehalose, 2-keto-gluconate, sorbitol, d-xylose, rhamnose, glucuronate, melezitose, mannitol, inositol, and glucose. The profile after 7 days of incubation at 25°C (biocode 4473324151, esculin positive) matched excellently with the profile for Cryptococcus albidus in the APILAB database (ID, 99.9%; T 0.85). Due to the recently described species diversity within the C. albidus species cluster (14), the sequences of the internal transcribed spacer (ITS 1 and ITS 2) and the D1/D2 region of the 26S rDNA were determined. Both were found to be 100% identical (Fig. 1) to the corresponding sequences of Cryptococcus adeliensis (accession numbers AF145328 and AF137603, respectively) as well as to that of an isolate derived from sheep feces in Spain (accession number AY049723).
FIG. 1.
Rooted phylogenetic tree inferred by analysis of 26S rDNA sequences using the neighbor-joining method based on a dissimilarity matrix corrected for multiple mutations per site according to Jukes and Cantor. Confidence values for individual branches were determined by bootstrap analysis based on 1,000 bootstrap trees that were generated from resampled characters. All calculations were performed with the software package TREECON, version 1.3b. Bootstrap values of ≥750 are displayed on the respective nodes. F., Filobasidiella; T., Trichosporon.
The MICs of antimycotic agents were determined by microdilution according to National Committee for Clinical Laboratory Standards guideline M27-A (9) and by Etest on RPMI 1640 agar (AB Biodisk, Solna, Sweden). After incubation at 25°C for 48 h, the following MICs based on the National Committee for Clinical Laboratory Standards guideline and Etest, respectively, were determined: amphotericin B, 0.125 and 0.094 mg/liter; flucytosine, >64 and >32 mg/liter; fluconazole, 32 and >256 mg/liter; itraconazole, 0.25 and 2 mg/liter; and voriconazole, 0.25 and 0.125 mg/liter.
A total of four blood cultures drawn between days 3 and 9 after PBSCT remained sterile despite prolonged incubation at a decreased temperature (21 days at 30°C).
The patient received 5 mg of liposomal amphotericin (AmBisome)/kg of bodyweight from 19 May. After 2 days, 120 mg of flucytosine/kg was added (12), and after 3 days, additional amphotericin B at a dose of 0.25 mg was applied intrathecally every third day. CSF samples drawn on days 2, 4, 6, and 9 after the beginning of the antifungal therapy remained sterile.
Despite antifungal therapy, the patient's neurologic state steadily deteriorated; i.e., the patient became somnolent and suffered from focal epileptic seizures. She developed fever and acute respiratory distress syndrome and required mechanical ventilation. The patient was transferred to the intensive care unit, where she died 14 days later due to multiorgan failure despite broad-spectrum antibiotic and antimycotic therapy. Autopsy, including histopathological examinations by Grocott stain, did not reveal any fungal elements in the leptomeninx or brain at the time of death.
Most cryptococcal infections are caused by Cryptococcus neoformans. Cryptococci other than C. neoformans have rarely been reported to cause meningitis, cryptococcemia, or pulmonary disease in immunocompromised patients, with C. albidus being the predominant species reported (6-8). The C. albidus group shows an intraspecies diversity; i.e., several strains have recently been reclassified upon ribosomal DNA sequence analysis (4, 14). This is the first report of a case of meningitis due to C. adeliensis. C. adeliensis has recently been described as a new species (13). The description was based on phenotypic characteristics and on the comparative sequence analysis of the D1/D2 large subunit of the 26S rDNA gene and ITS 1 and ITS 2 of the ribosomal DNA. The type strain has been isolated from Terre Adelie, Antarctica, from decayed algae. It belongs to the C. albidus group and is characterized by the absence of growth on lactate and quinate, tolerance of 0.01% cycloheximide, and weak growth at 30°C. It produces a cold-adapted xylanase (10), a trait also revealed in our isolate when growing at 15°C on yeast-nitrogen base medium with birch xylan as the sole carbohydrate source (2).
The majority of patients with cryptococcosis suffer from substantial T-cell dysfunction, like patients with AIDS or lymphoma. Immunologic defects that are typically associated with cryptococcosis are lymphopenia and immune dysfunction due to prior steroid or fludarabine use (5). Our patient was lymphopenic for several weeks due to the underlying disease (AML) and the chemotherapy courses she received. She was treated with fludarabine only 1 week before PBSCT. The clinical symptoms of cryptococcosis developed gradually over 2 months. They were nonspecific and not severe at the beginning, which led to a delay in diagnosis. Only after allogeneic PBSCT with severe immunosuppression did the paraparesis increase and a diffuse headache and focal seizures occur. Otherwise unexplained neurologic symptoms in hematologic patients—even if mild in the beginning—require laboratory examination for cryptococcosis (5). Differential diagnoses include metastatic brain tumors, leptomeningeal disease, and bacterial meningitis.
The laboratory diagnosis of cryptococcosis caused by non-C. neoformans species may be difficult for the following reasons. (i) Microscopic and chemical analyses of the CSF may reveal only slightly abnormal values for cell counts, protein, and lactate concentrations. (ii) The cryptococcal antigen test may be negative, as strains may be nonencapsulated. (iii) Cryptococci other than C. neoformans, including C. adeliensis, may fail to grow in vitro at 37°C. Therefore, it is important to incubate an additional fungal medium inoculated with the CSF specimens at a decreased temperature if cryptococcosis is suspected. (iv) Due to the heterogeneity of the non-C. neoformans group, reliable species identification requires sequence analysis of ribosomal gene fragments.
There are no standard recommendations for antimycotic therapy for cryptococcosis other than that caused by C. neoformans. Susceptibility testing of the patient's isolate of C. adeliensis indicated susceptibility to amphotericin B, resistance to flucytosine and fluconazole, and reduced susceptibility to itraconazole and voriconazole compared to that for C. neoformans reference strains. C. albidus strains with this resistance pattern have been described previously (14). Data from several studies suggest a correlation of the MIC of fluconazole with the clinical outcome in cryptococcosis (11). Both microdilution and Etest seem to be suitable methods for in vitro susceptibility testing, as their results correlate well (1). It is important to identify strains for which MICs of azoles are high, as fluconazole monotherapy is recommended for consolidation therapy (12) and has even been used as a single drug for the treatment of cryptococcal meningitis (15).
The antimycotic therapy of our patient was performed according to standard recommendations for C. neoformans meningitis (12). Liposomal amphotericin B instead of amphotericin B was used because of development of a renal dysfunction. Furthermore, it was shown that liposomal amphotericin led to more rapid sterilization of CSF than did amphotericin B (3).
Due to the treatment with high doses of intravenous liposomal amphotericin supplemented by intrathecal conventional amphotericin B, the CSF of our patient was sterilized and the cryptococcal meningoencephalitis was resolved, as proven by autopsy. Thus, the contribution of the cryptococcal infection to the symptoms of acute respiratory distress syndrome and multiorgan failure remains unsolved. The blood cultures constantly devoid of any cryptococci in combination with the results of the postmortem histology indicated a lethal role of nonfungal effectors, such as the kind of medication and an infection with agents other than Cryptococcus species.
In conclusion, meningitis due to cryptococci other than C. neoformans may occur in immunocompromised patients. The neurologic symptoms may be noncharacteristic and may not be severe at the beginning. Cryptococci other than C. neoformans may be unable to grow in vitro at 37°C, and they may be resistant to antifungal agents, including azoles.
Nucleotide sequence accession number. Our isolate (M3287) was deposited at the culture collection of the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands) under accession number CBS 9061.
Acknowledgments
We thank Ines Völz for excellent technical assistance and Reinhard Kappe for critical reading of the manuscript.
REFERENCES
- 1.Aller, A. I., E. Martin-Mazuelos, M. J. Gutierrez, S. Bernal, M. Chavez, and F. J. Recio. 2000. Comparison of the Etest and microdilution method for antifungal susceptibility testing of Cryptococcus neoformans to four antifungal agents. J. Antimicrob. Chemother. 46:997-1000. [DOI] [PubMed] [Google Scholar]
- 2.Biely, P., Z. Kratky, M. Vranska, and D. Urmanicova. 1980. Induction and inducers of endo-1,4-beta-xylanase in the yeast Cryptococcus albidus. Eur. J. Biochem. 108:323-329. [DOI] [PubMed] [Google Scholar]
- 3.Chen, S. C. 2002. Cryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin B. J. Antimicrob. Chemother. 49(Suppl. 1):57-61. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca, A., G. Scorzetti, and J. W. Fell. 2000. Diversity in the yeast Cryptococcus albidus and related species as revealed by ribosomal DNA sequence analysis. Can. J. Microbiol. 46:7-27. [PubMed] [Google Scholar]
- 5.Kontoyiannis, D. P., W. K. Peitsch, B. T. Reddy, E. E. Whimbey, X. Y. Han, G. P. Bodey, and K. V. I. Rolston. 2001. Cryptococcosis in patients with cancer. Clin. Infect. Dis. 32:E145-E150. [DOI] [PubMed] [Google Scholar]
- 6.Kordossis, T., A. Avlami, A. Velegraki, I. Stefanou, G. Georgakopoulos, C. Papalambrou, and N. J. Legakis. 1998. First report of Cryptococcus laurentii meningitis and a fatal case of Cryptococcus albidus cryptococcaemia in AIDS patients. Med. Mycol. 36:335-339. [PubMed] [Google Scholar]
- 7.Krumholz, R. A. 1972. Pulmonary cryptococcosis. A case due to Cryptococcus albidus. Am. Rev. Respir. Dis. 105:421-424. [DOI] [PubMed] [Google Scholar]
- 8.Melo, J. C., S. Srinivasan, M. L. Scott, and M. J. Raff. 1980. Cryptococcus albidus meningitis. J. Infect. 2:79-82. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Petrescu, I., J. Lamotte-Brasseur, J. P. Chessa, P. Ntarima, M. Claeyssens, B. Devreese, G. Marino, and C. Gerday. 2000. Xylanase from the psychrophilic yeast Cryptococcus adeliae. Extremophiles 4:137-144. [DOI] [PubMed] [Google Scholar]
- 11.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 13.Scorzetti, G., I. Petrescu, D. Yarrow, and J. W. Fell. 2000. Cryptococcus adeliensis sp. nov., a xylanase producing basidiomycetous yeast from Antarctica. Antonie Leeuwenhoek 77:153-157. [DOI] [PubMed] [Google Scholar]
- 14.Sugita, T., M. Takashima, R. Ikeda, T. Nakase, and T. Shinoda. 2001. Intraspecies diversity of Cryptococcus albidus isolated from humans as revealed by sequences of the internal transcribed spacer regions. Microbiol. Immunol. 45:291-297. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi, H., H. Ikemoto, K. Watanabe, A. Ito, K. Hara, and S. Kohno. 1996. Fluconazole monotherapy for cryptococcosis in non-AIDS patients. Eur. J. Clin. Microbiol. Infect. Dis. 15:787-792. [DOI] [PubMed] [Google Scholar]