Abstract
Mast cells (MCs) promote a wide range of localized and systemic inflammatory responses. Their involvement in immediate as well as chronic inflammatory reactions at both local and distal sites points to an extraordinarily powerful immunoregulatory capacity with spatial and temporal versatility. MCs are preferentially found in close proximity to both vascular and lymphatic vessels. On activation, they undergo a biphasic secretory response involving the rapid release of prestored vasoactive mediators followed by de novo synthesized products. Many actions of MCs are related to their capacity to regulate vascular flow and permeability and to the recruitment of various inflammatory cells from the vasculature into inflammatory sites. These mediators often work in an additive fashion and achieve their inflammatory effects locally by directly acting on the vascular and lymphatic endothelia, but they also can affect distal sites. Along these lines, the lymphatic and endothelial vasculatures of the host act as a conduit for the dissemination of MC signals during inflammation. The central role of the MC-endothelial cell axis to immune homeostasis is emphasized by the fact that some of the most effective current treatments for inflammatory disorders are directed at interfering with this interaction.
Introduction
Mast cells (MCs) are granulated hematopoietic cells that reside in nearly all tissues. They are characterized by their biphasic response to stimuli, which involves rapid degranulation and release of preformed inflammatory mediators followed by de novo synthesis and a slower but sustained secretion of a wide range of pharmacologically active mediators. Because of their preponderance at the host-environment interface and their large repertoire of cell surface receptors, such as the high-affinity IgE receptors, complement component receptors, and various TLRs, MCs are capable of responding to a wide variety of exogenous and endogenous stimuli, making them versatile detectors of allergens, tissue injury, and infection.1,2 Anaphylaxis, a rapid and whole body immune reaction, rheumatoid arthritis, a chronic inflammatory reaction localized to joints, and bacterial clearance at sites of infection are examples of 3 very distinct inflammatory responses that have all been linked to MCs and to their capacity to activate the host vasculature and to enhance large-scale trafficking of various immune cells.
The human or mammalian vascular system is composed of a network of vessels lined by endothelial cells (ECs). In addition to its critical role in gas exchange, blood components are distributed to tissues via this route, systemically and at high speed, including nutrients, hormones, growth factors, inflammatory mediators, and inflammatory cells. In the capillaries, part of the blood volume is filtered out of the vascular space by hydrostatic pressure. Some of this protein-depleted fluid reenters the blood on the venous side, and the remaining fluid is removed from the tissues by the lymphatic system. From the perspective of immune responses, this latter path through lymphatics (also formed of endothelial cell conduits) and lymph nodes (LNs) is critically important because it gives the host the ability to monitor these fluids for infection and to assess the inflammatory status of tissues. MCs also use this network to affect systemic immune responses in the host.
The close proximity of MCs to the host's vascular and lymphatic endothelia enables their products to act directly on ECs and also to enter the vasculature and spread to distal sites, promoting local and long-distance effects. Activation of vascular ECs is required for the timely recruitment of circulating leukocytes to a site of inflammation and for the regulation of vascular permeability and blood flow to the site. MCs produce many mediators that functionally overlap in promoting enhanced expression of adhesion molecules on ECs, vascular permeability, and blood flow. At rest, the blood endothelium is highly impermeable to molecules larger than ∼ 3 nm3; however, acute changes in vascular permeability result in loss of fluid and plasma proteins from the intravascular space into the interstitium near the affected blood vessel, leading to edema. This allows the delivery of large defensive humoral factors (such as immunoglobulin and complement) to the tissue and facilitates the extravasation of leukocytes. MCs have the capacity to both initiate and sustain cellular trafficking out of the vasculature because of the 2-phase release of vasoactive compounds (Table 1). By packaging some of their bioactive mediators within the matrix of their granules, structurally stable nano-sized particles, mediators appear to be slowly released, enhancing their activity.4 During inflammation, when these mediator-loaded particles are released in the vicinity of lymphatic vessels, some particles enter the lymphatics and traffic to the draining LNs.4 Many MC-derived or -associated products can also be readily detected in the blood after activation or during MC-promoted pathologic conditions,5,6 further showing their potential to generate systemic responses. Here, we describe the interactions between MC products and ECs during inflammation and their effect on the host.
Table 1.
Vasoactive MC mediators
| Vascular permeability | WPB exocytosis | Chemoattraction | Adhesion molecule up-regulation | Angiogenesis/lymphangiogenesis | |
|---|---|---|---|---|---|
| Preformed | |||||
| Histamine | + | + | * | + | |
| Tryptase | + | + | * | Δ | Δ |
| Chymase | * | Δ | |||
| Cathepsin G | + | + | * | ||
| Serglycin-heparin proteoglycan | Δ | Δ | |||
| TNF | + | Δ | + | + | |
| IL-8 | + | + | |||
| VEGF | + | + | Δ/* | + | |
| bFGF | Δ | Δ/* | + | ||
| TGFβ | Δ | + | |||
| Eicosanoids | |||||
| LTB4 | + | ||||
| LTC4 | + | + | |||
| LTD4 | + | + | |||
| LTE4 | + | + | |||
| PGD2 | Δ/* | * | |||
| PAF | + | + | + | + | |
| Selected de novo cytokines | |||||
| TNF | + | + | + | ||
| IL-1 | Δ/* | + | + | ||
| IL-6 | Δ | Δ | + | ||
| GM-CSF | * | ||||
| CCL1 | + | ||||
| MCP1 (CCL2) | + | Δ | /* | ||
| MIP1α (CCL3) | + | Δ/* | |||
| MIP1β (CCL4) | + | ||||
| MIP2 (CXCL2) | + | Δ |
+ indicates clear evidence for a direct role in this process; Δ, possible role/limited or conflicting evidence; and *, potentiates the effect of another mediator.
Proximity to the vasculature provides MCs broad spatial influence
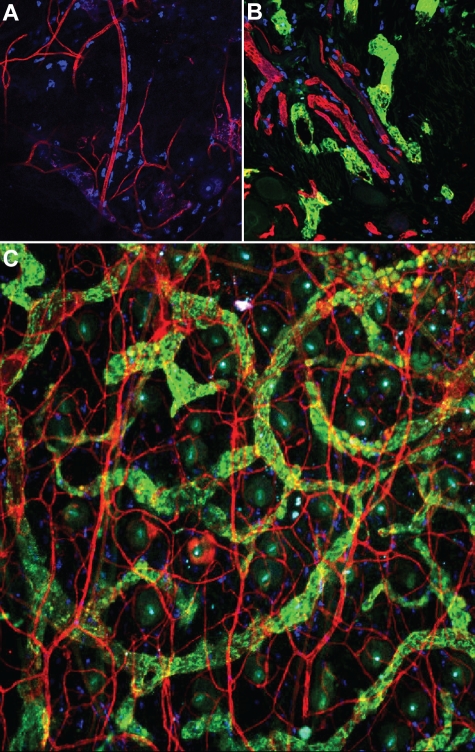
MCs are found in highest concentration immediately beneath the epithelial surfaces of the skin and the mucosae. Estimated concentrations of MCs range from 500 to 4000/mm3 in the lungs, 7000 to 12 000/mm3 in the skin, and 20 000/mm3 in the gastrointestinal tract.7 Their preferential location at the host-environment interface suggests a central role for these cells in immune surveillance that has been supported by numerous studies examining the responses of MCs to various pathogens. One of the earliest observations about MCs, made by their discoverer, Paul Ehrlich, is that they frequently adopt a perivascular localization within tissues8 (Figure 1A-C). MCs also exist in close proximity to lymphatic vessels in connective tissues (Figure 1B-C). The anatomy of peripheral tissues ensures that released MC mediators can affect both vasculatures soon after their elaboration, making the vascular networks distribution channels for MCs to quickly raise a systemic alarm response.
Figure 1.
MCs and vascular endothelium are intimately associated. (A) MCs (blue, stained with the heparin-binding probe, avidin) are visible lining blood vessels (CD31, red) in tissue from the mouse ear, which was stained in whole mount (20× mag. 20×/0.75). (B) Proximity of MCs (blue) to blood (red, CD31) and lymphatic (green, LYVE-1) vessels in the mouse skin (20× mag. 20×/0.75 objective). (C) Extensive association of MCs with both vasculatures is apparent after staining mouse ear tissue in whole mount as in other panels for vascular and lymphatic endothelia (10× magnification, 10×/0.25 NA objective). Images were acquired using a Nikon Eclipse TE200 microscope and EZ-C1 Version 3.6 software. Additional image processing was performed using Autoquant software and Adobe Photoshop (Version 9).
Activation of MCs can set off a cascade of events occurring directly at a site of insult, at distal sites of moderate distance from the site of MC activation (such as in draining LNs), or even systemically. These distal and systemic effects of MCs are achieved through distribution of MC products through blood or lymphatic vasculature. Yet MCs also facilitate the interaction of the central circulatory system with a local inflammatory site, in part by promoting the recruitment of cells into a site of inflammation, primarily from the bloodstream. For example, MCs can mediate early neutrophil recruitment, which is often the first line of defense against pathogens; however, in various models they have also been shown to promote the recruitment of a variety of other cell types into tissues, including eosinophils, dendritic cell (DC) precursors, T cells, and natural killer cells.1,9 Longer distance communication to distal sites, such as to the draining LN, potentiates immune responses. Along these lines, MCs are indispensable for the sequestration of lymphocytes within draining LNs, which promotes the presence of rare Ag-specific lymphocytes during the initiation of adaptive immune responses and the egress of multiple subtypes of DCs to draining LNs.1 These observations show one way that MCs coordinate host responses on a systemic level, by regulating the trafficking of immune cells from the blood to a site of localized inflammation, as well as to draining LNs.
Temporally defined action of MC products on vascular endothelium
The first phase of response, degranulation, can have functional effects within minutes of MC activation. This initial response can be further subdivided into the effects of the completely soluble mediators and those that remain associated with the structurally stable exocytosed granules. The effects of these MC-derived particles are potentially long lasting, because these particles can also remain extracellular for hours.4 MCs begin generating de novo mediators soon after activation, but the first to be elaborated are the arachidonic acid metabolites. With these come a second round of MC-derived vascular effects. The production of new protein mediators takes the longest and contributes to the so-called “late phase” of allergic inflammation. The following discussion of various MC mediators is structured around this progression.
Preformed mediators (first phase response)
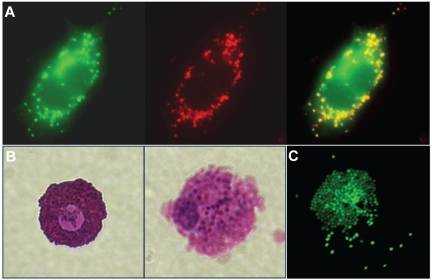
MC granules are built around a framework of proteoglycans composed of a serglycin core protein that is extensively decorated with heparin chains. The extremely high negative charge density of heparin, combined with the highly positively charged MC proteases, allows the formation of a polyelectrolyte complex. This charge-based binding is so stable that a granule retains its shape even after exocytosis and remains a discrete particle in the extracellular space.10 Granule-stored products that are not highly charged, including histamine, easily diffuse away from the matrix on exposure to the extracellular fluid. In contrast, other mediators such as certain proteases and TNF (Figure 2A) are retained in the granule matrix after exocytosis,4,11 which should affect their activity and may prolong their presence in the extracellular space near the releasing MCs. Activated MCs in the process of releasing granules can be viewed in Figure 2B-C. MC-derived particles have been visualized moving through tissues and lymphatics and have been shown to act as delivery vehicles for shuttling inflammatory mediators to distant sites, including the LNs draining a site of inflammation.4 As shown by TNF, it seems that when low abundance, short-lived signals are retained within the particle structure, protected from dilution, degradation, and free diffusion, their activity is enhanced in vivo.4 Another key unexplored potential is that, because the key granule component, heparin, binds to many extracellular signaling molecules, these particles may regulate and concentrate the activities of other granular mediators, as well as of mediators released from other nearby cells.
Figure 2.
Particulate release of MC Mediators. (A) Rat lymphoblastic leukemia-2H3 cells (a MC-like cell line), which express a TNF–green fluorescent protein fusion protein (green), incorporate TNF and other mediators, including serotonin (red), into their granules. Colocalization of these markers can be viewed in the merged image where the overlapping areas appear yellow. The construction of these cells is described in Kunder et al4 (60×/1.40 NA, oil objective). (B) A resting MC (left) and a compound 48/80-activated mast cell (right), both obtained from rat peritoneal lavage, and stained with toluidine blue (60×/1.40 NA oil objective). (C) An activated, rat peritoneal MC stained with avidin to show the granules, some of which have diffused away from the cell after release as discrete particles. Confocal images were acquired using a Nikon Eclipse TE200 microscope and EZ-4 Version 3.6 software. Bright field images were captured using a Nikon Eclipse TE200 microscope and a Nikon Coolpix 4500 camera. Additional image processing was performed using Adobe Photoshop Version 9.
Histamine is stored in high concentrations in MC granules,12 yet it is thought to be immediately solubilized during granule exocytosis to promote increases in vascular permeability. In support of this, antihistamines effectively reduce vascular permeability occurring with pharmacologic MC activation.13 The half-life of histamine is ∼ 1 minute.14 Thus, it exerts its effects quickly, and these effects also resolve quickly. This feature is critical for vascular homeostasis, because it ensures the retention of plasma proteins in the intravascular space. The mechanisms of histamine action on the vasculature during acute inflammatory responses are also well characterized and result from activation of the 2 primary receptors for histamine, H1 and H2.15 The H1 receptor is expressed most strongly on venular ECs and is responsible for most of histamine's acute vascular effects.16 Its activation greatly increases the permeability of the venular endothelium via the formation of gaps between ECs, resulting in extravasation of fluid as well as plasma proteins.16 H1 is a G-coupled protein receptor (GPCR) that uses the Gαq/11 family of proteins and, ultimately, promotes calcium flux within activated ECs. This calcium has several downstream targets that can influence vascular permeability, including endothelial nitric oxide synthase (eNOS),17 one function of which is to induce vascular smooth muscle relaxation.18 A component of the eNOS pathway, AKT1, in ECs was shown to be essential for histamine-induced vascular permeability.19 Histamine can also promote the influx of inflammatory cells into a tissue, which begins when circulating leukocytes encounter “activated endothelium.” The earliest event in this process involves the release of preformed products that microvascular ECs store within their own granules, Weibel-Palade bodies (WPBs), which contain mediators that include VWF, P-selectin, and (in previously stimulated cells) IL-8,20 and which can contribute to the rolling, activation, and diapedesis of leukocytes along postcapillary venules. Calcium-dependent exocytosis of WPBs can be induced by several agonists, including histamine.20 Endothelial presentation of P-selectin mediates early neutrophil rolling, but it is rapidly endocytosed within ∼ 20 minutes.21 The amounts of leukocyte-recruiting products stored within WPBs are not probable to support sustained influx into the tissue; however, it is probable that WPB exocytosis acts in concert with other early events as a critical bridge for cellular recruitment before the de novo products required for full-scale endothelial activation have been generated. Histamine also has synergistic effects in combination with other mediators, such as by enhancing the expression of E-selectin and ICAM1 on ECs responding to TNF.22 The most rapid actions of MCs on the vasculature appear to be histamine dependent.
Functional studies have begun to investigate the contributions of histamine to many pathologic or protective processes in vivo. Mice lacking histadine decarboxylase (HDC) are unable to convert histadine to histamine and have provided a tool to examine the physiologic relevance to histamine, although with the caveat that additional MC-related functions may be influenced because of altered MC numbers and granulation.23,24 One study has shown that histamine is unlikely to influence the drop in blood pressure associated with anaphylaxis,23 but others have implicated histamine in increased vascular permeability, such as at the blood-brain barrier.25 In the latter study, it was shown that mice lacking HDC were protected from severe malaria, which was attributed to reduced vascular leakage at the blood-brain barrier.25 However, overexpression of H1 on mouse ECs, unexpectedly, reduced blood-brain barrier permeability in a bacterially induced experimental encephalitis model.26 These contrasting findings show the need to further examine the effects of histamine and its many receptors on the vasculature. Interestingly, a study in zebrafish also implicated histamine in the branching of blood vessels.27 Because MCs are major histamine producers, they probably contribute to these physiologic processes in which histamine is consequential. However, further studies to define the specific contributions of MC-derived histamine are required, particularly in light of evidence that HDC-deficient mice have defects, such as in angiogenesis, that MC-deficient mice do not.28
Proteases, some of which are specific to MCs, are the most abundant proteins stored in MC granules and constitute a main structural component. These play a role in the storage of other granule-associated factors, because genetic deficiency of one protease can also affect the storage of other granule products.29 Use of mice deficient in unique MC proteases is beginning to define functional roles in EC processes for these granule components, despite the potential of altered granule composition in these animals. For example, in models of experimentally induced aneurysm, mice deficient in murine MC protease 6 (mMCP6)30 or mMCP431 had reduced pathology compared with controls. In a model of ischemia-reperfusion injury, mMCP5 (but not mMCP1)–deficient mice were protected.32 These reports show the unique contributions of individual MC proteases to vascular responses. MC tryptases are thought to promote neutrophil recruitment during inflammation because the injection of recombinant mMCP6 or human tryptase33 into mice was shown to promote rapid neutrophil influx and production of the neutrophil chemoattractant, IL-8.34 Another tryptase, mMCP7, is able to degrade fibrinogen and was implicated in regulation of coagulation due to its ability to degrade fibrinogen, which could have larger implications for limiting clot formation and promoting vascular leakage during inflammatory processes.35
The link between tryptase and endothelial activation has not been entirely elucidated, but it probably involves the activation of one of the family of protease-activated receptors, PAR2.36 These GPCRs on ECs are activated by tryptase through protease cleavage of part of the extracellular domain, allowing a tethered “ligand” to interact with the rest of the receptor, leading to signal transduction.37 PAR2 agonists can elicit leukocyte rolling and adhesion,38 and PAR2-deficient animals have defective early leukocyte rolling after surgical trauma.39 Like histamine, PAR2 activation stimulates an increase in cytoplasmic Ca++ that results in exocytosis of WPBs and, therefore, leukocyte recruitment.40,41 This effect was also seen when tryptase was used as the stimulus.42 Like histamine, tryptase can also stimulate platelet-activating factor (PAF) production by ECs.42 Likewise, it has been shown in vitro that tryptase can reduce the barrier function of ECs.43 PAR2 activators also up-regulate endothelial cyclooxygenase 2.44 In addition, endothelial IL-6 production in response to TNF is augmented by PAR2 activation,45 which provides further support of the notion that inflammatory mediators in various combinations can result in qualitatively or quantitatively different responses, including additive effects.
Chymases are also stored in MC granules and are thought to be regulators of vascular tone, although less is known about their role in inflammation. One study showed that intradermal injection of chymase greatly potentiated the size of histamine-induced wheals in allergic dogs, whereas it did not elicit wheals when injected alone.46 Chymase was first recognized for its role in angiotensin II generation; however, the ultimate contributions of MC chymase to angiotensin conversion in vivo are, as yet, uncertain.47 Chymase also promotes degradation of extracellular matrix, and its breakdown of fibronectin has been implicated in the apoptosis of smooth muscle cells.48 These functions are thought to have drastic effects on vascular homeostasis and may contribute to pathologic conditions, such as atherosclerosis. Indeed, MC chymase levels are correlated with atherosclerotic plaques in human patients and in animal models, highlighting the potential of chymase to affect vascular homeostasis with injury, disease, or age.47
Another abundant protease within MC granules is carboxypeptidase A. With reference to the vasculature, one report suggests it may participate (along with chymase) in angiotensin II generation, resulting in vasoconstriction in mice.49 MCs also store significant quantities of cathepsin G (primarily considered a neutrophil enzyme) in their granules.50 The physiologic function of MC-derived cathepsin G has not been determined, but some insight may be gleaned from the neutrophil literature. The signaling elicited by cathepsin G promotes intraendothelial gap formation,51 as well as PAF production.52 Like tryptase, it is capable of eliciting calcium flux in ECs.53
Heparin is a structural element of the MC granule, and MCs that cannot produce heparin also have significantly reduced levels of many other preformed mediators.54,55 It is also required to form complexes that remain insoluble in the extracellular space after exocytosis.11 A highly specific (ie, not simply charge-based) interaction of heparin is with antithrombin III, which is the basis for the use of heparin clinically as an anticoagulant. Although a similar role may be played in vivo inside the vasculature by heparan sulfate on the luminal surface of ECs, the interaction of antithrombin III with heparin may be important during inflammatory events to deter the activation of the coagulation cascade in the interstitial space. Heparin was also recently described as having another interaction with the coagulation cascade, through the activation of protease factor XII. The resulting production of bradykinin was shown, in vivo, to promote edema, adhesion of cells to the vascular endothelium, and blood vessel dilation resulting in lowered blood pressure.56 In vitro, heparin can also induce lacunae in endothelial monolayers and stimulate EC migration, events which might be relevant to the role of MCs in angiogenesis.57,58
A large number of biologic molecules, including growth factors and cytokines, bind to heparin because of its incredibly high negative charge density.59 Although with lower affinity than to heparin, these molecules also can bind to heparan sulfate, which is a less-sulfated constituent of the endothelial basement membrane.60 Heparin binding of growth factors and cytokines by such particles moving through edematous tissues might deliver signals to sites that would ordinarily be too distant to be influenced by small quantities of preformed mediators.
TNF is preformed in MCs61 and has rapid effects on ECs, increasing permeability by causing cytoskeletal rearrangements.62,63 We know these effects occur rapidly in vivo because infusion of recombinant TNF can cause hypotension and death within minutes.63 Although TNF also induces stress fiber formation,62 the mechanism of activation must be different from that of histamine, because TNF does not induce intracellular Ca++ accumulation. Instead, it stimulates the production of diacylglycerol (and consequent activation of protein kinase C) without inositol 1,4,5-triphosphate generation.64 Protein kinase C activation appears to be required for TNF-dependent endothelial contraction.65 TNF also induces transcriptional endothelial activation, including adhesion molecules and chemokines.66 For example, MC-derived TNF induces expression of E-selectin on inflamed vascular endothelium at sites of bacterial infection, as shown through the reconstitution of MC-deficient mice with BM-derived MCs from TNF-deficient animals.67 Finally, cyclooxygenase 2 is increased after TNF exposure, promoting the production of prostaglandins.66 These products fill the roles that P-selectin and possibly preformed IL-8 play in the earliest stage of the response once endothelial inflammatory transcription has commenced. Interestingly, the transcriptional program induced by TNF treatment is similar to that induced by bacterial cell wall component, lipopolysaccharide, which signals through TLR4 and ultimately also activates NFκB.68 Because TNF is prestored within MC granules, its potent effects on ECs can be exerted from the beginning of the response. The kinetics of TNF release also extend beyond the initial burst of quickly solubilized cytokine because TNF is at least partially retained within exocytosed particles.4 This could have implications not only for the temporal persistence of the cytokine's effects within the site of inflammation but also on the extent of the vascular network that can be influenced because of the flow of these particles in vivo, although within the constraints of anatomical barriers.
Newly synthesized mediators (second phase)
The most rapidly acting de novo–produced mediators are the eicosanoids, leukotrienes and prostaglandins. Meaningful quantities of these mediators can be released within minutes of stimulation, because no transcription is required, only enzymatic activity.69 As one indication of the diversity of potential responses mediated by MCs, not all degranulating stimuli result in the production of large amounts of eicosanoids.70 The association of these factors with anaphylaxis underscores the potential detrimental effects that MCs can have on a systemic level.
Leukotrienes are potent vasoactive inflammatory mediators, which were once collectively known as the slow-reacting substance of anaphylaxis (because it cannot be detected in unstimulated tissues and its release lags behind that of histamine and other preformed mediators). For the production of leukotrienes, arachidonic acid is first converted into leukotriene A4 (LTA4) by 5-lipoxygenase.71 The production of all 3 cysteinyl leukotrienes (LTC4, LTD4, and LTE4) depends on the initial conversion of LTA4 to LTC4 by LTC4 synthase (LTC4S).71 Activated MCs produce LTC4, which can be further converted to LTD4 and LTE4 in the extracellular environment.72 All 3 of these species produce potent, long-lasting wheal-and-flare responses on injection into human skin, suggesting they may enhance and prolong the immediate vascular changes evoked by histamine.73 In a hamster model, it was shown that LTC4 and LTD4 are 100 times more potent than histamine in eliciting increased vascular permeability.74 These mediators act primarily through the leukotriene receptor CysLT1, another GPCR expressed on endothelium.71 Like histamine or protease-activated receptors, leukotrienes elicit Ca++ mobilization in ECs, leading to secretion of WPBs and P-selectin externalization.75 This promotes neutrophil adhesion, which is probably enhanced by leukotriene-stimulated endothelial production of PAF.76 In LTC4S, CysLT1, and CysLT2 knockout animals, the increased vascular permeability seen during experimental allergic inflammation or zymosan peritonitis is greatly reduced.71 There is also evidence to suggest that MCs may emit smaller quantities of LTB4,77 which is a neutrophil and T-cell chemoattractant.78 In support of a role in neutrophil chemoattraction, animals deficient for LTA4 hydrolase (the enzyme that generates LTB4) have impaired early neutrophil recruitment in a zymosan peritonitis model,79 which is a process that is partially MC dependent.80 MC-derived leukotrienes also potentially contribute to vascular pathology, because it has been shown that in human tissues during aneurysm, leukotriene conversion is increased and that enhanced LTC4S expression colocalizes with MC markers.81
Prostaglandins constitute a second class of eicosanoids, of which prostaglandin D2 (PGD2) is the main form generated by MCs.82 PGD2 injection in human skin causes erythema without substantial edema (probably because of vasodilation) and inhibits platelet aggregation.83 It has also been shown to increase vascular permeability in rats.84 Intriguingly, although intranasal application of PGD2 did not cause any allergic-type symptoms in rats, when applied simultaneously with histamine, it increased the potency of histamine for causing these effects by 1000-fold.85 PGD2 was shown to enhance the ability of memory T cells to detect CCL21, promoting their transendothelial migration, by augmenting the function, but not expression, of CCR7 on those cells.86 PGD2 was also shown to inhibit LTC4 but not LTB4 production by BM-derived MCs,87 whereas LTE4 was shown to promote PGD2 production by human MCs,88 emphasizing the importance of further work to understand the temporal regulation of eicosanoid production.
PAF is also produced by activated MCs,89 as well as by endothelium in response to products produced by MCs, including histamine, proteases, leukotrienes, and cytokines, including TNF and IL-1.90 The PAF receptor is a GPCR whose activation results in cytoskeletal rearrangements within ECs.91 In addition to prompting increases in vascular permeability, PAF induces leukocyte recruitment,92 and, like histamine, this is mediated through gap formation at interendothelial junctions.93 PAF receptor–deficient animals are resistant to death from experimental passive systemic anaphylaxis, and they show no evidence of increased pulmonary vascular permeability, contrasting with wild-type animals.94 Similarly, intravenous PAF induces a rapid hypotensive anaphylactoid condition in mice, which is unrelated to platelet activation,95 and blockade of PAF can protect mice from anaphylaxis.96 In humans, levels of PAF also correlate to the severity of anaphylaxis.97 It is important to note, however, that basophils also can produce PAF and can promote anaphylaxis. In an IgG-mediated passive anaphylaxis mouse model (contrasting IgE-mediated), it was shown that basophils produced PAF with allergen challenge and that these, but not MCs, were required for shock.98 Because mouse MCs are not thought to express the high-affinity receptor for IgG, in contrast to human MCs,99 further studies are needed to determine the contributions of MCs and MC-derived PAF to IgG-mediated anaphylaxis in humans. In another example of interaction between multiple MC mediators, combined PAF and H1 inhibition almost completely abolished the hypotension seen in passive systemic anaphylaxis.100 Recent evidence that PAF also can induce MC degranulation has suggested that PAF may act in an autocrine fashion to augment local or systemic MC degranulation responses.101
MCs are an important source of cytokines during inflammatory responses. In various settings, they have the capacity to release a wide array of cytokines,102 with MC-derived TNF being one of the best characterized. In addition to releasing preformed TNF, activated MCs also actively produce and secrete it.61 In chronic inflammation, there are many cellular sources of TNF, so the specific contribution of MC-derived TNF remains unclear. Still, basic studies have shown accumulation of blood vessel–associated MCs at rheumatoid arthritic lesions103 and colocalization between MC markers and TNF.104 Another study hints at this association with chronic inflammation because airway hyperreactivity was reduced in a MC-dependent asthma model.105 IL-1 is produced by MCs and has similar effects to TNF, in that it enhances vascular permeability and up-regulates the expression of adhesion molecules, chemokines, and other effectors.106,107 It has also been shown to enhance TNF-dependent hyperpermeability.108 Another MC-produced cytokine that can affect endothelial barrier function is IL-6,109,110 which also may promote lymphocyte adhesion.111,112 Importantly, many of the cytokines that can be produced by MCs are known to modulate the functions of other hematopoietic cells involved in the inflammatory response and the generation of immunity. MC production of chemokines appears to promote cellular infiltration into sites of infection, as shown by the observed MC-dependent recruitment of NK cells to sites of viral challenge.9 Yet another critical factor to EC function that MCs can produce is VEGF, forms of which promote angiogenesis, as well as lymphangiogenesis. MC-derived VEGF is thought to mediate much of the influence of MCs on these 2 processes, although many other MC products, including TGFβ, fibroblast growth factor-2, TNF, and others, also have been ascribed angiogenic properties.113 With their extensive panel of de novo–generated and stimulus-specific inflammatory mediators, MCs may not only promote the recruitment of cells but also modulate their subsequent functions through specialized cytokine production and produce long-lasting vascular remodeling at a site of inflammation.
Action of MC products on lymphatic endothelium
In contrast to the vascular endothelium, less is known about the activities of the lymphatic endothelium during inflammation. Lymphatic vessels form the interstitium-lymph boundary and perform a barrier function in regulating the movement of substances into lymph. Unlike the vascular endothelium, the initial lymphatic endothelium is highly permeable to the flow of fluid.114 When the tissue becomes edematous, these junctions are pulled open by connections between the outer wall of the vessel and the surrounding connective tissue, and the diameter of such openings can reach several microns.115 This process has previously been assumed to be purely mechanical, but a few reports now hint that it is an active process. First, electron microscopy of initial lymphatics during inflammation (induced both by thermal injury and histamine injection) shows changes suggestive of cellular contraction, similar to what is seen in venular ECs in response to histamine.116,117 Such contraction would serve to pull open the junctions between lymphatic ECs, as it does in the blood vessel endothelium. Recently, histamine has been shown to directly act on isolated lymph vessels, promoting dose-dependent contraction, ex vivo, which can be blocked by antihistamines.118 In addition, a study showed that the permeability of lymphatic endothelial tubes in vitro is reduced by increased cAMP, and the same is known to be true for blood ECs.119 Another showed that tube permeability can be regulated by VEGF-C.120
Given the high level of similarity between blood and lymphatic ECs, it is reasonable to expect that many of the same rapid responses to MC-derived mediators occur in both. Both venous and lymphatic ECs contain WPBs,121 although their function has not been investigated in lymphatic endothelium. Lymphatic ECs also express eNOS, and its activity is induced by Ca++ ionophores, histamine, and TNF just as it is in the microvascular endothelium.122 Lymphatic ECs undergo significant transcriptional changes during inflammatory events, as well. For example, TNF induces the production of the adhesion molecules VCAM1 and ICAM1 in dermal lymphatic ECs, without which trafficking of DCs to the draining nodes is limited.123,124 These studies suggest that inflammatory adhesion molecules regulate cellular traffic into the lymphatic vasculature as well as out of the blood vasculature. Because Ag trafficking and presentation by DCs are crucial events in the development of adaptive immunity, this kind of regulation of the lymphatic endothelium probably influences long-term immunologic memory.
Distribution of MC signals via lymphatics
Lymphatic vessels connect peripheral sites, where pathogens first encounter host defenses, with secondary lymphoid tissues, where Ag presentation and the resulting specific immune responses originate. Peripheral MC-derived TNF promotes LN enlargement during immune responses by modulating B- and T-cell trafficking out of high endothelial venules.125 Yet how peripheral signals reach the LN, which is usually a considerable distance from the site of inflammation, has been unclear. One mechanism underlying this process is the packaging of signals within MC granules, which largely remain insoluble after release. Recent evidence shows that preformed MC-derived TNF remains associated with these particles after degranulation, enhancing the activity of this otherwise short-lived cytokine.4 In this way, lymphatics act as channels to target MC-derived signals to those cells participating in the initiation of adaptive responses.
Therapeutic and prophylactic implications
Because of the central role played by MCs in exacerbating inflammatory disorders involving EC activation and vascular leakage, many of the most effective therapies are directed at limiting MC-EC communication (Table 2). The most widely used of these therapies are directed at limiting the actions of prominent prestored or newly synthesized MC mediators such as histamine, leukotrienes, and TNF. Long used to treat pathologies associated with asthma, anaphylaxis, and allergy,126–128,130,131 MC “stabilizers” and other drugs that target MC products are now proposed as potential therapies for a wider range of diseases involving vasculopathy, from preventing metastases during cancer to limiting aneurysm formation.47,134
Table 2.
Clinical targeting of MCs or their products
| Target MC product | Example | Mechanism and indications | Reference(s) |
|---|---|---|---|
| Inhibition of MCs or their products | |||
| Antihistamines | First generation, for example, diphenhydramine | Mainstay of treatment for allergic disorders such as mastocytosis, chronic idiopathic urticaria, and acquired cold urticaria | 126 |
| Second generation, for example, loratadine, cetirizine, etc | Are nonsedating because they do not cross the blood-brain barrier, have proven especially effective in both perennial and seasonal allergic rhinitis | ||
| Antileukotrienes | Montelukast and the related agents Zafirlukast and Pranlukast | Inhibit the cysteinyl leukotriene receptor CysLT1 which is widely expressed, therefore effects of these drugs are not exclusive to the endothelium | 127 |
| Zileuton | Inhibits 5-lipoxygenase, the enzyme that converts arachidonic acid to LTA4 as the first step of leukotriene synthesis | ||
| Protease inhibition | Tryptase inhibition: developmental phase | Appears promising for allergic inflammation | 128 |
| Chymase inhibition: developmental phase | Animal models of aneurysm, atherosclerosis, myocardial infarction | 47,129 | |
| Anti-TNF agents | Etanercept | A soluble TNF receptor-Fc fusion protein indicated for rheumatoid arthritis, inflammatory bowel disease, and a variety of other inflammatory conditions | 130 |
| Infliximab | Anti-TNF monoclonal antibody indicated for rheumatoid arthritis, inflammatory bowel disease, and a variety of other inflammatory conditions | ||
| MC stabilizers | Cromolyn, Ketotifen and others prevent degranulation and limit MC activation | Asthma and allergic rhinitis | 131 |
| Kit receptor-targeting drugs | Imatinib and other tyrosine kinase inhibitors | Effective in some subsets of human patients with MC neoplastic disease. | 132 |
| Promoting MC function | |||
| MC-activating agents | Developmental phase | Small-molecule MC activators are being explored as adjuvants | 133 |
Concluding remarks
The vasculature plays a critical role in facilitating local and distal MC-mediated inflammation. One general principle that emerges is that MC-dependent inflammation is highly temporally organized. This organization results in a great deal of potential regulatory control at each step. The sequence of phases include immediately acting soluble-preformed mediators, less-soluble granule-associated preformed mediators, products of arachidonic acid metabolism, and finally the secretion of de novo proteins. It is probable that the actual sequence of events may vary, because transcriptional changes and enzymatic cascades can be programmed at different rates. Finally, the MC activation program will be influenced by autocrine and paracrine signaling from the surrounding environment. For each of these steps, most of the inflammatory sequelae that follow are mediated directly through or influenced by the vasculature. Many of these MC products are interpreted by GCPR on ECs, resulting in increased intracellular calcium as a second messenger. The orderly temporal progression of Gq-coupled MC mediators suggests that it is important to sustain this “alarm” signal in ECs from the beginning of the response. The centrality of Gq-mediated signaling in inflammation was shown in studies that revealed that mice with an endothelium-specific deficiency in Gq (and closely related G11) are protected against anaphylaxis-induced hypotension and vascular leakiness.135
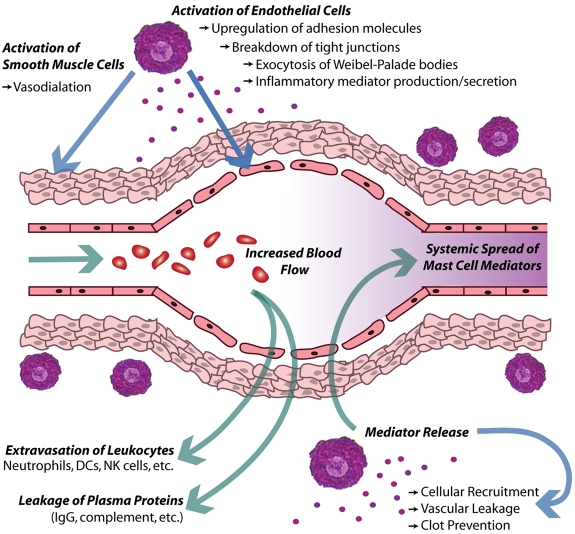
Inflamed blood vessels also support several phases of response. For a diagram that summarizes the acute effects of MC products on blood vessels during inflammation, see Figure 3. MC-derived mediators are a key trigger prompting WPB exocytosis from ECs, allowing the early inflammatory response and cellular recruitment to begin without the necessity of waiting for a transcriptional response. Finally, the long-term response of ECs involves the sustained production of new adhesion molecules and chemokines, supporting extensive recruitment of inflammatory cells. These MC-driven processes are crucial to host defense against viral, bacterial, and parasitic pathogens,1 but can also mediate tissue injury such as during asthma, ischemia reperfusion injury, autoimmune disease, and other pathologic conditions.2,102,136 Evidence that MC-dependent increases in permeability can occur at the blood-brain barrier, allowing access of parasites or metastases to this otherwise protected space,25 also show the tissue-specific consequences of MC responses. Furthermore, vascular remodeling is now recognized to be affected by MCs and their products, such as VEGF.113 This observation has implications for understanding the influence of MCs during varied processes such as tumor vascularization and wound healing.
Figure 3.
Local effect of MCs on the vasculature during acute inflammation. In this diagram, activated MCs release inflammatory mediators, which then induce changes along vascular endothelium. Some of these mediators directly act on ECs and smooth muscle cells to promote vasodilation and vascular leakiness. In addition, vascular ECs up-regulate many adhesion molecules and release WPBs to promote the rolling and extravasation of leukocytes into the inflamed tissue. Concurrently, increased vascular leakiness promotes the loss of fluid and blood proteins into the tissue, or edema. Additional MC-derived mediators can limit clotting and these responses cumulatively act to increase vascular flow through the site of inflammation. Presumably, the compromised barrier function of the vascular ECs would also facilitate the dissemination of MC products systemically.
It is also increasingly apparent that MC-derived inflammatory mediators interact with the lymphatic microvasculature and that initial lymphatic vessels do actively respond to inflammatory signals. This may regulate the local inflammatory environment, but other changes, such as the expression of adhesion molecules, seem directed at facilitating the development of adaptive immunity (eg, by enabling the entrance of DCs into the lymphatics). Lymphatic vesicles also appear to be conduits for the trafficking of discrete MC particles to the LN. Thus, regulation of the lymphatic endothelium by MCs is probably an important determinant of immune responses.
The large number of pharmacologic agents targeting MC products is testament to their importance, although most are aimed at preventing MC-dependent inflammation. Because the MC-EC axis is so central to inflammation, the events determined by it are probable to remain a fruitful source of new therapies.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01 AI50021, R21 DK077307, RO1 DK077159, U01AI082107, and R21 DA029731), Start-up Funding from Duke–National University of Singapore (S.N.A.), and Singapore National Medical Research Council (grant NIG/1053/2011, A.L.S.).
Authorship
Contribution: All authors contributed substantially to the writing of the paper and to the preparation of figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashley L. St John, Program in Emerging Infectious Diseases, Duke–National University of Singapore Graduate Medical School, Level 9, 8 College Road, Singapore; e-mail: ashley.st.john@duke-nus.edu.sg.
References
- 1.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siflinger-Birnboim A, Del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132(1):111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- 4.Kunder CA, St John AL, Li G, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206(11):2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ono E, Taniguchi M, Mita H, et al. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. 2009;39(1):72–80. doi: 10.1111/j.1365-2222.2008.03104.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26(3):451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman SI. Mast cell-mediated inflammation in asthma. Ann Allergy. 1989;63(6 Pt 2):546–550. [PubMed] [Google Scholar]
- 8.Ehrlich P. Beitraege zur Kenntniss der Anilinfaerbungen und ihrer Verwendung in der mikroskopischen Technik. Arch mikr anat u entwcklngsmech. 1877;13:263. [Google Scholar]
- 9.St John AL, Rathore AP, Yap H, et al. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A. 2011;108(22):9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohlich P, Anderson P, Uvnas B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz LB, Riedel C, Caulfield JP, Wasserman SI, Austen KF. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J Immunol. 1981;126(6):2071–2078. [PubMed] [Google Scholar]
- 12.Riley JF. Histamine in tissue mast cells. Science. 1953;118(3064):332. doi: 10.1126/science.118.3064.332. [DOI] [PubMed] [Google Scholar]
- 13.McLeod RL, Mingo GG, Kreutner W, Hey JA. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life Sci. 2005;76(16):1787–1794. doi: 10.1016/j.lfs.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Snyder SH, Axelrod J. Tissue metabolism of histamine-C14 in vivo. Fed Proc. 1965;24:774–776. [PubMed] [Google Scholar]
- 15.Ash AS, Schild HO. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heltianu C, Simionescu M, Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelm M, Feelisch M, Krebber T, Motz W, Strauer BE. Mechanisms of histamine-induced coronary vasodilatation: H1-receptor-mediated release of endothelium-derived nitric oxide. J Vasc Res. 1993;30(3):132–138. doi: 10.1159/000158987. [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39(4-5):173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 19.Di Lorenzo A, Fernandez-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009;106(34):14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117(2):113–122. doi: 10.1007/s00418-001-0368-9. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam M, Koedam JA, Wagner DD. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4(8):791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miki I, Kusano A, Ohta S, et al. Histamine enhanced the TNF-alpha-induced expression of E-selectin and ICAM-1 on vascular endothelial cells. Cell Immunol. 1996;171(2):285–288. doi: 10.1006/cimm.1996.0205. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsu H. Histamine synthesis and lessons learned from histidine decarboxylase deficient mice. Adv Exp Med Biol. 2011;709:21–31. doi: 10.1007/978-1-4419-8056-4_3. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsu H, Tanaka S, Terui T, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502(1-2):53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 25.Beghdadi W, Porcherie A, Schneider BS, et al. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J Exp Med. 2008;205(2):395–408. doi: 10.1084/jem.20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C, Diehl SA, Noubade R, et al. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;107(44):18967–18972. doi: 10.1073/pnas.1008816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CC, Huang CW, Cheng YS, Yu J. Histamine metabolism influences blood vessel branching in zebrafish reg6 mutants. BMC Dev Biol. 2008;8:31. doi: 10.1186/1471-213X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh AK, Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Defective angiogenesis in the inflammatory granulation tissue in histidine decarboxylase-deficient mice but not in mast cell-deficient mice. J Exp Med. 2002;195(8):973–982. doi: 10.1084/jem.20011782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feyerabend TB, Hausser H, Tietz A, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25(14):6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Sun J, Lindholt JS, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108(11):1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120(11):973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abonia JP, Friend DS, Austen WG, Jr, et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol. 2005;174(11):7285–7291. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161(4):1939–1946. [PubMed] [Google Scholar]
- 34.Huang C, Friend DS, Qiu WT, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160(4):1910–1919. [PubMed] [Google Scholar]
- 35.Huang C, Wong GW, Ghildyal N, et al. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J Biol Chem. 1997;272(50):31885–31893. doi: 10.1074/jbc.272.50.31885. [DOI] [PubMed] [Google Scholar]
- 36.Steinhoff M, Corvera CU, Thoma MS, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8(4):282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 37.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 38.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163(9):5064–5069. [PubMed] [Google Scholar]
- 39.Lindner JR, Kahn ML, Coughlin SR, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165(11):6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- 40.Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res. 2003;92(3):272–278. doi: 10.1161/01.res.0000057386.15390.a3. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Motley ED, Eguchi K, et al. Distinct roles of protease-activated receptors in signal transduction regulation of endothelial nitric oxide synthase. Hypertension. 2009;53(2):182–188. doi: 10.1161/HYPERTENSIONAHA.108.125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol Cell Physiol. 2005;289(6):C1485–C1491. doi: 10.1152/ajpcell.00215.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sendo T, Sumimura T, Itoh Y, et al. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signal. 2003;15(8):773–781. doi: 10.1016/s0898-6568(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 44.Houliston RA, Keogh RJ, Sugden D, Dudhia J, Carter TD, Wheeler-Jones CP. Protease-activated receptors upregulate cyclooxygenase-2 expression in human endothelial cells. Thromb Haemost. 2002;88(2):321–328. [PubMed] [Google Scholar]
- 45.Chi L, Li Y, Stehno-Bittel L, et al. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2001;21(4):231–240. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 46.Rubinstein I, Nadel JA, Graf PD, Caughey GH. Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J Clin Invest. 1990;86(2):555–559. doi: 10.1172/JCI114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doggrell SA, Wanstall JC. Vascular chymase: pathophysiological role and therapeutic potential of inhibition. Cardiovasc Res. 2004;61(4):653–662. doi: 10.1016/j.cardiores.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 48.Leskinen MJ, Lindstedt KA, Wang Y, Kovanen PT. Mast cell chymase induces smooth muscle cell apoptosis by a mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol. 2003;23(2):238–243. doi: 10.1161/01.atv.0000051405.68811.4d. [DOI] [PubMed] [Google Scholar]
- 49.Lundequist A, Tchougounova E, Abrink M, Pejler G. Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II. J Biol Chem. 2004;279(31):32339–32344. doi: 10.1074/jbc.M405576200. [DOI] [PubMed] [Google Scholar]
- 50.Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145(8):2652–2661. [PubMed] [Google Scholar]
- 51.Peterson MW. Neutrophil cathepsin G increases transendothelial albumin flux. J Lab Clin Med. 1989;113(3):297–308. [PubMed] [Google Scholar]
- 52.Camussi G, Tetta C, Bussolino F, Baglioni C. Synthesis and release of platelet-activating factor is inhibited by plasma alpha 1-proteinase inhibitor or alpha 1-antichymotrypsin and is stimulated by proteinases. J Exp Med. 1988;168(4):1293–1306. doi: 10.1084/jem.168.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson MW, Gruenhaupt D, Shasby DM. Neutrophil cathepsin G increases calcium flux and inositol polyphosphate production in cultured endothelial cells. J Immunol. 1989;143(2):609–616. [PubMed] [Google Scholar]
- 54.Forsberg E, Pejler G, Ringvall M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400(6746):773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 55.Humphries DE, Wong GW, Friend DS, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400(6746):769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 56.Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34(2):258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagunoff D, Rickard A. Mast cell granule heparin proteoglycan induces lacunae in confluent endothelial cell monolayers. Am J Pathol. 1999;154(5):1591–1600. doi: 10.1016/S0002-9440(10)65412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans–as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 60.Kanwar YS, Farquhar MG. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 62.Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato N, Goto T, Haranaka K, et al. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986;76(6):1113–1121. [PubMed] [Google Scholar]
- 64.Schutze S, Berkovic D, Tomsing O, Unger C, Kronke M. Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med. 1991;174(5):975–988. doi: 10.1084/jem.174.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferro T, Neumann P, Gertzberg N, Clements R, Johnson A. Protein kinase C-alpha mediates endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1107–L1117. doi: 10.1152/ajplung.2000.278.6.L1107. [DOI] [PubMed] [Google Scholar]
- 66.Viemann D, Goebeler M, Schmid S, et al. Transcriptional profiling of IKK2/NF-kappa B- and p38 MAP kinase-dependent gene expression in TNF-alpha-stimulated primary human endothelial cells. Blood. 2004;103(9):3365–3373. doi: 10.1182/blood-2003-09-3296. [DOI] [PubMed] [Google Scholar]
- 67.Shelburne CP, Nakano H, St John AL, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6(4):331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magder S, Neculcea J, Neculcea V, Sladek R. Lipopolysaccharide and TNF-alpha produce very similar changes in gene expression in human endothelial cells. J Vasc Res. 2006;43(5):447–461. doi: 10.1159/000095162. [DOI] [PubMed] [Google Scholar]
- 69.Levi-Schaffer F, Dayton ET, Austen KF, et al. Mouse bone marrow-derived mast cells cocultured with fibroblasts. Morphology and stimulation-induced release of histamine, leukotriene B4, leukotriene C4, and prostaglandin D2. J Immunol. 1987;139(10):3431–3441. [PubMed] [Google Scholar]
- 70.Benyon RC, Robinson C, Church MK. Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. Br J Pharmacol. 1989;97(3):898–904. doi: 10.1111/j.1476-5381.1989.tb12030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173(3):1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 72.Raulf M, Stuning M, Konig W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- 73.Soter NA, Lewis RA, Corey EJ, Austen KF. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 74.Dahlen SE, Bjork J, Hedqvist P, et al. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Datta YH, Romano M, Jacobson BC, Golan DE, Serhan CN, Ewenstein BM. Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-selectin surface expression in human umbilical vein endothelial cells. Circulation. 1995;92(11):3304–3311. doi: 10.1161/01.cir.92.11.3304. [DOI] [PubMed] [Google Scholar]
- 76.McIntyre TM, Zimmerman GA, Prescott SM. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A. 1986;83(7):2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mencia-Huerta JM, Razin E, Ringel EW, et al. Immunologic and ionophore-induced generation of leukotriene B4 from mouse bone marrow-derived mast cells. J Immunol. 1983;130(4):1885–1890. [PubMed] [Google Scholar]
- 78.Costa MF, de Souza-Martins R, de Souza MC, et al. Leukotriene B4 mediates gammadelta T lymphocyte migration in response to diverse stimuli. J Leukoc Biol. 2010;87(2):323–332. doi: 10.1189/jlb.0809563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163(12):6810–6819. [PubMed] [Google Scholar]
- 80.Kolaczkowska E, Seljelid R, Plytycz B. Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J Leukoc Biol. 2001;69(1):33–42. [PubMed] [Google Scholar]
- 81.Di Gennaro A, Wagsater D, Mayranpaa MI, et al. Increased expression of leukotriene C4 synthase and predominant formation of cysteinyl-leukotrienes in human abdominal aortic aneurysm. Proc Natl Acad Sci U S A. 2011;107(49):21093–21097. doi: 10.1073/pnas.1015166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 83.Mills DC, Macfarlane DE. Stimulation of human platelet adenylate cyclase by prostaglandin D2. Thromb Res. 1974;5(3):401–412. doi: 10.1016/0049-3848(74)90176-5. [DOI] [PubMed] [Google Scholar]
- 84.Flower RJ, Harvey EA, Kingston WP. Inflammatory effects of prostaglandin D2 in rat and human skin. Br J Pharmacol. 1976;56(2):229–233. doi: 10.1111/j.1476-5381.1976.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahman A, Inoue T, Ago J, Ishikawa T, Kamei C. Interactive effect of histamine and prostaglandin D2 on nasal allergic symptoms in rats. Eur J Pharmacol. 2007;554(2-3):229–234. doi: 10.1016/j.ejphar.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 86.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120(3):506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-507. [DOI] [PubMed] [Google Scholar]
- 87.He P, Laidlaw T, Maekawa A, Kanaoka Y, Xu K, Lam BK. Oxidative stress suppresses cysteinyl leukotriene generation by mouse bone marrow-derived mast cells. J Biol Chem. 2011;286(10):8277–8286. doi: 10.1074/jbc.M110.205567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paruchuri S, Jiang Y, Feng C, Francis SA, Plutzky J, Boyce JA. Leukotriene E4 activates peroxisome proliferator-activated receptor gamma and induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283(24):16477–16487. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mencia-Huerta JM, Lewis RA, Razin E, Austen KF. Antigen-initiated release of platelet-activating factor (PAF-acether) from mouse bone marrow-derived mast cells sensitized with monoclonal IgE. J Immunol. 1983;131(6):2958–2964. [PubMed] [Google Scholar]
- 90.Bussolino F, Camussi G. Platelet-activating factor produced by endothelial cells. A molecule with autocrine and paracrine properties. Eur J Biochem. 1995;229(2):327–337. [PubMed] [Google Scholar]
- 91.Bussolino F, Camussi G, Aglietta M, et al. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987;139(7):2439–2446. [PubMed] [Google Scholar]
- 92.Humphrey DM, McManus LM, Hanahan DJ, Pinckard RN. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984;50(1):16–25. [PubMed] [Google Scholar]
- 93.Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol. 2008;295(2):H898–H906. doi: 10.1152/ajpheart.00309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishii S, Kuwaki T, Nagase T, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187(11):1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Myers A, Ramey E, Ramwell P. Glucocorticoid protection against PAF-acether toxicity in mice. Br J Pharmacol. 1983;79(2):595–598. doi: 10.1111/j.1476-5381.1983.tb11034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arias K, Baig M, Colangelo M, et al. Concurrent blockade of platelet-activating factor and histamine prevents life-threatening peanut-induced anaphylactic reactions. J Allergy Clin Immunol. 2009;124(2):307–314. doi: 10.1016/j.jaci.2009.03.012. 314.e1-2. [DOI] [PubMed] [Google Scholar]
- 97.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 98.Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 100.Shibamoto T, Liu W, Cui S, Zhang W, Takano H, Kurata Y. PAF, rather than histamine, participates in mouse anaphylactic hypotension. Pharmacology. 2008;82(2):114–120. doi: 10.1159/000141516. [DOI] [PubMed] [Google Scholar]
- 101.Kajiwara N, Sasaki T, Bradding P, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125(5):1137–1145.e6. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 102.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9(11):1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Godfrey HP, Ilardi C, Engber W, Graziano FM. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- 104.Tetlow LC, Woolley DE. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995;54(11):896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakae S, Lunderius C, Ho LH, Schafer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy Clin Immunol. 2007;119(3):680–686. doi: 10.1016/j.jaci.2006.11.701. [DOI] [PubMed] [Google Scholar]
- 106.Watson ML, Lewis GP, Westwick J. Increased vascular permeability and polymorphonuclear leucocyte accumulation in vivo in response to recombinant cytokines and supernatant from cultures of human synovial cells treated with interleukin 1. Br J Exp Pathol. 1989;70(1):93–101. [PMC free article] [PubMed] [Google Scholar]
- 107.Williams MR, Kataoka N, Sakurai Y, Powers CM, Eskin SG, McIntire LV. Gene expression of endothelial cells due to interleukin-1 beta stimulation and neutrophil transmigration. Endothelium. 2008;15(1):73–165. doi: 10.1080/10623320802092443. [DOI] [PubMed] [Google Scholar]
- 108.Abe Y, Sekiya S, Yamasita T, Sendo F. Vascular hyperpermeability induced by tumor necrosis factor and its augmentation by IL-1 and IFN-gamma is inhibited by selective depletion of neutrophils with a monoclonal antibody. J Immunol. 1990;145(9):2902–2907. [PubMed] [Google Scholar]
- 109.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131(2):710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 110.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res. 2002;104(2):118–123. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 111.Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996;105(1):112–119. doi: 10.1046/j.1365-2249.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 113.Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett. 2008;269(1):1–6. doi: 10.1016/j.canlet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 114.Casley-Smith JR, Florey HW. The structure of normal small lymphatics. Q J Exp Physiol Cogn Med Sci. 1961;46:101–106. doi: 10.1113/expphysiol.1961.sp001502. [DOI] [PubMed] [Google Scholar]
- 115.Wenzel-Hora BI, Berens von Rautenfeld D, Majewski A, Lubach D, Partsch H. Scanning electron microscopy of the initial lymphatics of the skin after use of the indirect application technique with glutaraldehyde and MERCOX as compared to clinical findings. Lymphology. 1987;20(3):126–144. [PubMed] [Google Scholar]
- 116.Casley-Smith JR, Bolton T. Electron microscopy of the effects of histamine and thermal injury on the blood and lymphatic endothelium, and the mesothelium of the mouse's diaphragm, together with the influence of coumarin and rutin. Experientia. 1973;29(11):1386–1388. doi: 10.1007/BF01922834. [DOI] [PubMed] [Google Scholar]
- 117.Castenholz A. Structural and functional properties of initial lymphatics in the rat tongue: scanning electron microscopic findings. Lymphology. 1987;20(3):112–125. [PubMed] [Google Scholar]
- 118.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: endothelium-dependent responses. Dokl Biol Sci. 2010;432:176–180. doi: 10.1134/S0012496610030038. [DOI] [PubMed] [Google Scholar]
- 119.Price GM, Chrobak KM, Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res. 2008;76(1):46–51. doi: 10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breslin JW, Yuan SY, Wu MH. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res Biol. 2007;5(2):105–113. doi: 10.1089/lrb.2007.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harrison RL, Ewert A, Folse DS. Presence of Weibel-Palade bodies in lymphatic endothelium of the cat. Lymphology. 1986;19(4):170–171. [PubMed] [Google Scholar]
- 122.Leak LV, Cadet JL, Griffin CP, Richardson K. Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun. 1995;217(1):96–105. doi: 10.1006/bbrc.1995.2750. [DOI] [PubMed] [Google Scholar]
- 123.Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA. Human lymphatic endothelial cells express multiple functional TLRs. J Immunol. 2008;180(5):3399–3405. doi: 10.4049/jimmunol.180.5.3399. [DOI] [PubMed] [Google Scholar]
- 124.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203(12):2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McLachlan JB, Hart JP, Pizzo SV, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4(12):1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 126.Belsito DV. Second-generation antihistamines for the treatment of chronic idiopathic urticaria. J Drugs Dermatol. 2010;9(5):503–512. [PubMed] [Google Scholar]
- 127.Coreno A, Skowronski M, Kotaru C, McFadden ER., Jr Comparative effects of long-acting beta2-agonists, leukotriene receptor antagonists, and a 5-lipoxygenase inhibitor on exercise-induced asthma. J Allergy Clin Immunol. 2000;106(3):500–506. doi: 10.1067/mai.2000.109425. [DOI] [PubMed] [Google Scholar]
- 128.Erin EM, Leaker BR, Zacharasiewicz A, et al. Effects of a reversible beta-tryptase and trypsin inhibitor (RWJ-58643) on nasal allergic responses. Clin Exp Allergy. 2006;36(4):458–464. doi: 10.1111/j.1365-2222.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 129.Takai S, Jin D, Muramatsu M, Miyazaki M. Chymase as a novel target for the prevention of vascular diseases. Trends Pharmacol Sci. 2004;25(10):518–522. doi: 10.1016/j.tips.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 130.Wong M, Ziring D, Korin Y, et al. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126(2):121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Disodium cromoglycate. Lancet. 1972;2(7790):1299. [PubMed] [Google Scholar]
- 132.Ustun C, Deremer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35(9):1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 133.McLachlan JB, Shelburne CP, Hart JP, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 134.Theoharides TC, Rozniecki JJ, Sahagian G, et al. Impact of stress and mast cells on brain metastases. J Neuroimmunol. 2008;205(1-2):1–7. doi: 10.1016/j.jneuroim.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 135.Korhonen H, Fisslthaler B, Moers A, et al. Anaphylactic shock depends on endothelial Gq/G11. J Exp Med. 2009;206(2):411–420. doi: 10.1084/jem.20082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frangogiannis NG, Lindsey ML, Michael LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98(7):699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]