Abstract
The platelet P2Y12 purinoceptor (P2Y12R), which plays a crucial role in hemostasis, undergoes internalization and subsequent recycling to maintain receptor responsiveness, processes that are essential for normal platelet function. Here, we observe that P2Y12R function is compromised after deletion or mutation of the 4 amino acids at the extreme C-terminus of this receptor (ETPM), a putative postsynaptic density 95/disc large/zonula occludens-1 (PDZ)–binding motif. In cell line models, removal of this sequence or mutation of one of its core residues (P341A), attenuates receptor internalization and receptor recycling back to the membrane, thereby blocking receptor resensitization. The physiologic significance of these findings in the regulation of platelet function is shown by identification of a patient with a heterozygous mutation in the PDZ binding sequence of their P2Y12R (P341A) that is associated with reduced expression of the P2Y12R on the cell surface. Importantly, platelets from this subject showed significantly compromised P2Y12R recycling, emphasizing the importance of the extreme C-terminus of this receptor to ensure correct receptor traffic.
Introduction
ADP plays a central role in platelet activation by acting as a released autocrine mediator in platelet responses to other agonists. ADP activates 2 platelet G protein–coupled receptors (GPCRs), P2Y1 and P2Y12, which couple, respectively, to Gq and Gi-mediated pathways, and synergize to induce full platelet aggregation responses to ADP.1,2 Interaction of ADP with P2Y1 leads to mobilization of intracellular calcium and activation of Rho kinase, resulting in platelet shape change and an initial wave of rapidly reversible aggregation. In contrast, ADP stimulation of P2Y12 is associated with adenylyl cyclase inhibition and PI3K activation, resulting in sustained aggregation in synergy with the P2Y1 receptor (P2Y1R). Activation of both receptors is required for a full aggregation response to ADP.3
Recent work from our laboratory has shown that P2Y12 receptor (P2Y12R) responsiveness is rapidly and reversibly modulated in human platelets.4 On prolonged exposure to agonist, the responsiveness of P2Y12R, in human platelets, decreases because of receptor desensitization5 and subsequent internalization.6 In cell lines P2Y12Rs are regulated in a G protein–receptor coupled kinase (GRK)–dependent manner,5 leading to their rapid recruitment to clathrin-coated pits (CCPs) in an arrestin-dependent manner.7 The receptors are subsequently internalized and then recycled back to the cell surface after receptor dephosphorylation.4 This internalization and subsequent receptor recycling is required for the rapid resensitization of P2Y12R function in human platelets.4
Many GPCRs possess specific cytoplasmic sequences required for efficient trafficking of the receptor into either lysosomal or recycling pathways.8–10 In this study we have examined the importance of the C-terminus of the P2Y12R in regulating these dynamic regulatory and trafficking processes. Given the importance of this receptor for platelet activation in normal hemostasis and in the pathophysiology of disorders such as coronary heart disease,2 the understanding of how P2Y12R function is regulated is essential for the improvement of existing antiplatelet agents and the development of new therapeutic strategies.11
In this study, we demonstrate for the first time the critical importance of a putative postsynaptic density 95/disc large/zonula occludens-1 (PDZ)–binding motif at the extreme C-terminus of the P2Y12R (ETPM) for correct receptor traffic and function by expression in an immortalized cell line. Further, we report the identification of a subject with a P341A mutation within the PDZ-binding motif of their P2Y12R that both leads to reduced receptor expression on the platelet surface and compromises receptor traffic.
Methods
DMEM/F12 media, Lipofectamine 2000, and FBS, anti-hemagglutinin (HA) mAb (HA-11), and goat anti–mouse fluorescein-conjugated secondary Ab were purchased from Invitrogen. Complete protease inhibitor tablets and rhodamine-conjugated mouse monoclonal anti-HA Ab were from Roche. The anti-phosphothreonine Ab was from Cell Signaling Laboratories. The P2Y12R-specific Ab, directed to the extracellular surface N-terminus of the receptor, was from Imgenex. Radiochemicals were from Perkin Elmer Life Sciences. All other reagents were from Sigma-Aldrich.
Construction of P2Y12R constructs
HA-tagged P2Y12 constructs were generated as previously described.5 Three C terminal tail truncation mutants of the HA-tagged P2Y12R were engineered with the use of standard PCR techniques by introducing a stop codon after K303, E339, and T320. Point mutants were obtained by PCR amplification as for deletion mutants, with an antisense primer containing the desired mutation and a site for the restriction enzyme XbaI at the 5′ end, to facilitate subsequent cloning in HA-pcDNA3. The PCR products and pcNEO were digested with XhoI/XbaI, and, after purification, ligation was undertaken with T4 ligase overnight at 4°C. Subsequent products were transformed into DH5a cells, ampicillin-resistant colonies were amplified, and the correct sequence was confirmed by sequencing.
Cell culture and transfection
Chinese hamster ovary (CHO)–K1 cells were cultured in DMEM/F-12 (50:50) medium, 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Stably transfected cells were cultured in the above medium supplemented with 600 μg/mL geneticin as previously described.7 Surface receptor expression of wild-type and mutant receptor constructs was assessed by radioligand binding as previously described with the use of [3H]-2MeSADP (3 Ci [111 GBq]/mmol) and the P2Y12R antagonist AR-C69931MX to measure specific binding.6
In transient transfections, cells were grown to 80%-90% confluence and transfected with 0.5-10 μg of DNA with the use of Lipofectamine 2000 according to the manufacturer's instructions. Cell structure was assessed throughout to ensure cells were healthy, and cell shape was comparable with other studies that used these cells.12
Measurement of cAMP accumulation in CHO cells
Desensitization and signaling of P2Y12R responses in CHO cells were measured as previously described.4 In desensitization/resensitization experiments, cells were exposed to a desensitizing dose of ADP (10nM; 15 minutes) in the presence of the phosphodiesterase inhibitor Ro201724 (250μM). Apyrase (0.2 U/mL) was then added directly to each well to promote receptor resensitization and incubated at 37°C (30 minutes) to remove the desensitizing ADP. P2Y12R activity was assessed by adding forskolin (1μM) to cells in the absence or presence of ADP (0.01pM to 10μM), and plates were incubated at 37°C for 10 minutes. Cyclic AMP levels were subsequently assessed as previously described.4 Data are expressed as the percentage of inhibition of forskolin-stimulated adenylyl cyclase.
Measurement of receptor phosphorylation
Agonist-dependent P2Y12R phosphorylation was assessed as previously described.6 After treatment with ADP (10μM; 5 minutes) cells were placed on ice and washed twice with ice-cold PBS. All subsequent procedures were performed at 4°C unless otherwise stated. Cells were lysed and HA-tagged receptor immunoprecipitated with the use of HA-11. Immune complexes were then isolated and eluted from beads by the addition of 20 μL of electrophoresis sample buffer. After fractionation by SDS-PAGE and transfer to a nitrocellulose membrane, phospho-threonine levels were assessed with an anti–phospho-threonine Ab. Receptor immunoprecipitation was determined by reprobing membranes with a polyclonal anti-HA Ab/HRP-conjugated anti–rabbit IgG and visualization by ECL. P2Y12R expression was also confirmed with a P2Y12-specific Ab. The extent of receptor phosphorylation was quantified by densitometric analysis of resulting autoradiographs.
Internalization and immunofluorescence microscopy of HA-P2Y12 in CHO cells
HA-tagged surface receptor loss was assessed by ELISA as described previously.4,7 Cells were transiently transfected with pcDNA3 containing arrestin-2-dominant-negative mutant (arr-2-DNM; arrestin-2 [319-418]). Eps-15-dominant-negative mutant (Eps-15-DNM; EΔ95-295) or dynamin-dominant negative mutant (dyn-DNM; dynamin-K44A). After 24 hours of transfection, cells were split into 24-well tissue culture dishes. Twenty-four hours later, cells were incubated with DMEM/F12 containing apyrase (0.1 U/mL) for 1 hour at 37°C, washed, and then challenged with DMEM containing ADP (1nM to 1mM) for 0-60 minutes at 37°C. To induce receptor recycling apyrase was added (0.2 U/mL) to remove ADP. Changes in surface receptor expression were subsequently determined by an ELISA, taking advantage of the HA-epitope tag, and expressed as either the percentage of surface receptor or the percentage of the loss of surface receptor with the background signal from pcDNA3-transfected controls subtracted from all receptor-transfected values.
Cellular distribution of HA- or FLAG-tagged receptor in CHO cells was assessed by immunofluorescence microscopy.7 Briefly, cells were grown on poly-L-lysine–coated coverslips in 6-well plates. Twenty-four hours later, receptor distribution was assessed with a primary HA-11 (1:200) and goat anti–mouse fluorescein-conjugated secondary Ab (1:200) or a polyclonal anti-FLAG Ab and goat anti–rabbit rhodamine-conjugated secondary Ab (1:200). Coverslips were mounted with the use of Slow-Fade mounting medium and examined by microscopy on a Leica SP5-AOBS confocal laser scanning microscope attached to a Leica DM I6000 inverted epifluorescence microscope with phase-contrast and a Plan-Apo 63 × 1.40 NA oil immersion objective.
Arrestin-2–green fluorescent protein (arrestin-2–GFP) and arrestin-3–GFP redistribution was assessed as previously described.13 Briefly, arrestin-transfected cells were grown on poly-L-lysine–coated coverslips. Receptor distribution was assessed with a rhodamine-conjugated mouse monoclonal anti-HA Ab (1:100). Cells were then washed 3 times with PBS, and coverslips were mounted before imaging in a heated imaging chamber through which media and drugs could be added. Cells were examined by microscopy as described in the previous paragraph. All images were collected on Leica TCS-NT software and processed with Adobe Photoshop CS3.
Coimmunoprecipitation experiments
After drug treatment, cells were washed twice with ice-cold PBS and lysed, and coimmunoprecipitation experiments were performed as previously described.7 Arrestin-2–GFP was detected by immunoblotting with a monoclonal anti-GFP Ab. Proteins were detected by ECL.
P2RY12 sequencing
The P2RY12 coding sequence was PCR amplified from genomic DNA as 3 overlapping fragments with the use of oligonucleotide primers designed to incorporate universal M13-tails at the 5′ end (primer sequences indicated in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PCRs contained 30 ng of genomic DNA in a final volume of 15 μL of 1× ReddyMix Master Mix (ABgene Ltd) containing 3 pmoles of each primer and 1.5 or 3.0mM MgCl2, depending on the fragment being amplified. PCR products were purified with ExoSAP-IT for PCR Product Clean Up (GE Healthcare) and sequenced on an automated ABI 3730 DNA capillary sequencer. The P2RY12 1021C > G mutation was sought in other subjects by restriction analysis with the use of HinfI.
Platelet function studies
Whole blood, anticoagulated with sodium citrate (3.8%), was immediately centrifuged at 130g for 15 minutes to obtain platelet-rich plasma (PRP). A subsequent centrifugation at 1050g for 15 minutes was done to obtain platelet-poor plasma, which was used to set the 100% light transmission of the instrument. PRP (450 μL) was warmed at 37°C for 3 minutes, and 50 μL of Luciferine Luciferase was then added. After 30 seconds agonist was added. Platelet aggregation and secretion were performed on a Chrono-Log LumiAggregometer with ADP (1-100μM), collagen (3 μg/mL), and epinephrine (10μM). Platelet aggregation and ATP secretion were recorded for 3 minutes, and the maximum light transmission in this period was measured.14 Basal, prostaglandin E2 (PGE2; 10μM)– or forskolin (1μM)–stimulated cAMP levels were assessed in the absence and presence of ADP (1nM to 10μM) in platelets as previously described.6
Radioligand binding in human platelets
To induce receptor internalization platelets were stimulated with ADP (10μM; 15 minutes) or vehicle alone. ADP was then removed by the addition of 0.2 U/mL apyrase (15 minutes) to induce receptor recycling. P2Y1 and P2Y12 surface receptor expression was subsequently determined by ligand binding in fixed platelets as previously described.6
Experimental design and statistics
Data were analyzed by the iterative fitting program GraphPad Prism. Log concentration-effect curves were fitted to logistic expressions for single-site analysis, whereas t0.5 values for agonist-induced internalization were obtained by fitting data to single exponential curves. Where appropriate, statistical significance was assessed by Mann-Whitney U test or by 2-way ANOVA.
Results
Construction and stable expression of full-length and mutant P2Y12R constructs
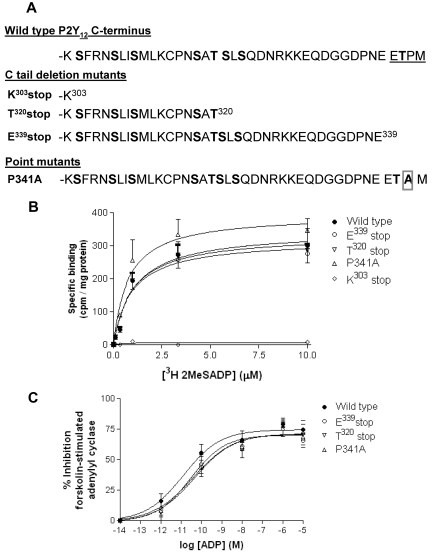
The goal of this study was to determine the functional significance of the C-terminus of the P2Y12 R. We constructed a series of C-tail deletion mutants of the P2Y12R (Figure 1A), removing the last 4 amino acids, a putative PDZ ligand (E339stop), a large proportion of the C-tail (T320stop), or the entire C-tail (K303stop), all tagged at their N-terminus with HA. In addition we made a receptor construct containing a point mutation in this putative PDZ ligand found at the extreme C-terminus of this receptor (P341A) to disrupt the integrity of this motif. Receptor constructs were stably expressed in P2Y12-null CHO cells. Ligand binding studies with the P2Y receptor ligand [3H]-2MeSADP (Figure 1B) showed that each of these receptor mutants was expressed at the cell surface at comparable levels to that of full-length receptor, with the exception of the K303 stop mutant that failed to be expressed at the cell surface. The lack of K303 stop surface expression was also confirmed by immunofluorescent microscopy (data not shown). Agonist-dependent activation of each of the other Gi-coupled P2Y12R mutants was comparable to that of the full-length receptor (Figure 1C) as indicated by inhibition of forskolin-stimulated adenylyl cyclase. Only the T320stop, E339stop, and P341A variant P2Y12Rs were studied further.
Figure 1.
Removal of the extreme C-terminus of the P2Y12 receptor does not affect surface expression or G protein coupling. (A) Sequence comparison of the COOH terminus domains of the human P2Y12 receptor constructs. Potential phosphorylation sites are in bold and the PDZ ligand found at the extreme C-terminus of the receptor is underlined. (B) Receptor levels were measured in CHO cells stably expressing wild-type or mutant receptor with the use of [3H]2MeSADP (0.1-10μM) in the presence of the P2Y12 receptor antagonist AR-C69931MX (1μM) to determine specific binding. Data are expressed as specific binding of [3H]2MeSADP (cpm) per milligram of protein and represent means ± SEMs of 5 independent experiments. (C) Agonist (ADP; 0.01pM to 10μM)–dependent inhibition of forskolin (1μM; 10 minutes)–stimulated adenylyl cyclase activity was assessed in CHO cells stably expressing wild-type and mutant receptor constructs. Data are expressed as percentage inhibition of forskolin-stimulated adenylyl cyclase and represent means ± SEMs of 5 independent experiments.
The extreme C-terminus of the P2Y12R regulates agonist-induced receptor desensitization
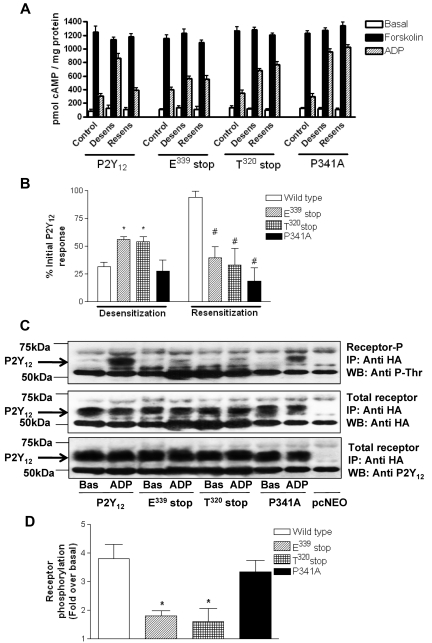
We have previously reported in both cell lines and platelets4 that pretreatment with ADP promotes desensitization of P2Y12, whereas removal of ADP with apyrase promotes a rapid resensitization of full-length P2Y12R, a result that was confirmed in this study in stably transfected CHO cells for inhibition of cAMP formation by forskolin (Figure 2A). Importantly, desensitization of both E339stop and T320stop was reduced by ∼ 50% (P < .05), indicating that the last 4 amino acids at the extreme C-terminus of this receptor are required for efficient P2Y12R desensitization (Figure 2B). Given that GRK-dependent phosphorylation has been shown to regulate P2Y12R desensitization,7 we examined the ability of these receptor constructs to be phosphorylated (Figure 2C). Agonist-induced phosphorylation of E339stop and T320stop was reduced by > 75% (P < .05) compared with the full-length receptor as shown in a representative experiment (Figure 2C) and confirmed by densitometric analysis of multiple blots (Figure 2D). Given that T340 is the only phospho-acceptor site in the last 4 amino acids, our results suggest that phosphorylation of this residue is probably required for agonist-induced P2Y12R desensitization, although the possibility that the extreme C-terminus of this receptor may interact with a regulatory kinase cannot be excluded. The desensitization and phosphorylation of the P341A construct meanwhile was not significantly attenuated, indicating that the integrity of the PDZ domain is unlikely to be required for receptor phosphorylation or desensitization. Importantly, however, as was the case for the E339stop and T320stop receptors, P341A did not resensitize after agonist removal (Figure 2A), indicating that the PDZ ligand is required for effective receptor resensitization.
Figure 2.
The extreme C-terminus of the P2Y12 receptor is required for normal agonist-induced regulation of receptor function. (A-B) P2Y12 desensitization and subsequent resensitization were assessed by comparing agonist (ADP; 10μM)–dependent inhibition of forskolin (1μM; 10 minutes)–stimulated adenylyl cyclase activity before (control) and after pretreatment with either ADP alone (10nM; 15 minutes; desens on graph) or after subsequent removal of desensitizing ADP with apyrase (0.2 U/mL; 15 minutes; resens on graph). Data represent means ± SEMs of 6 independent experiments. (A) Raw data are expressed as pmol cAMP/mg protein. (B) Data are expressed as percentage of initial P2Y12 response. *Statistical significance at P < .05 for data compared with respective densitized wild-type control (Mann-Whitney U test). #Statistical significance at P < .05 for resensitized compared with respective resensitized control (Mann-Whitney U test). (C-D) Phosphorylation of full-length and mutant HA-P2Y12 was assessed in CHO cells stably expressing receptor constructs. Cells were treated with ADP (10μM; 5 minutes) or vehicle alone, and HA-tagged receptors were immunoprecipitated from membrane lysates and run on SDS-PAGE before transfer to nitrocellulose membranes. Specific phosphorylated bands between 50 and 75 kDa not present in vector-alone pcNEO-transfected controls were subsequently identified with a phosphothreonine-specific Ab. Similar amounts of receptor immunoprecipitation were confirmed by reprobing membranes with a polyclonal anti-HA Ab/HRP-conjugated anti–rabbit IgG and visualization by ECL. Receptor immunoprecipitation was also confirmed by reprobing with a P2Y12-specific N-terminal Ab. Data are representative of 3 individual experiments. (C) Densitometric analysis of 3 experiments was performed, and data were expressed as fold increase over basal phosphorylation
The PDZ ligand of the P2Y12R is required for effective agonist-induced traffic
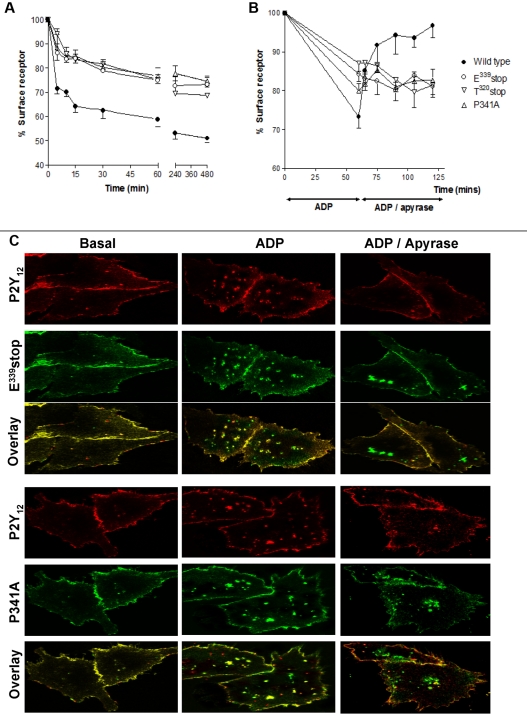
Receptor internalization and recycling are key regulators of P2Y12R resensitization in both cell lines and human platelets.4 Importantly, P2Y12R internalization does not appear to affect the degree of receptor desensitization. We therefore next sought to determine whether receptor traffic was altered for these mutant receptors. Making use of the N-terminal HA epitope–tagged versions of wild-type and variant receptor constructs stably expressed in CHO cells, we were able to quantify the agonist-induced surface receptor loss and subsequent recycling back to the cell surface by ELISA4,7,15 and immunofluorescence microscopy4 as previously described. Total surface receptor loss was attenuated for all mutant receptors compared with the full-length receptor with this attenuation of surface loss maintained after ≤ 8 hours of agonist exposure (Figure 3A). This result shows that an intact PDZ ligand is essential for normal internalization. Receptor recycling to the cell surface was completely blocked with the E339stop, T320stop, and P341A variant receptors, showing demonstrating a critical role for the PDZ-binding domain in the return to the membrane (Figure 3B).
Figure 3.
Agonist-induced internalization and traffic is blocked after removal or mutation of the PDZ ligand on the extreme C-terminus of the P2Y12 receptor. (A-B) CHO cells stably expressing either wild-type or mutant P2Y12 receptor were challenged with (A) ADP (10μM; 0-480 minutes) to induce receptor internalization or with (B) ADP (10μM; 60 minutes) to induce receptor internalization then apyrase (0.2 U/mL) to remove ADP and to promote subsequent receptor recycling. Surface receptor loss was subsequently assessed by ELISA as described in “Methods.” The data represent means ± SEMs of 5 independent experiments. (C) CHO cells stably expressing HA-tagged mutant receptor constructs were transiently transfected with FLAG-tagged wild-type P2Y12 receptor. Cells were preincubated with a monoclonal anti-HA or polyclonal anti-FLAG Ab at 4°C for 1 hour. Subsequently, cells were incubated at 37°C for 60 minutes in the absence or presence of agonist (ADP; 10μM). Cells were then incubated with apyrase for (0.2 U/mL) for 60 minutes. Receptor localization was determined by immunofluorescence in fixed cells and was visualized with a monoclonal fluorescein-conjugated or polyclonal rhodamine-conjugated secondary Ab. Groups of cells coexpressing wild-type receptor (red) with either E339stop (green) or P341A (green) are shown in the absence of agonist (Basal), after agonist treatment (ADP), or after agonist removal (ADP/apyrase). E339stop or P341A P2Y12 receptor (green) retained in the cells after agonist removal is clearly evident in the overlay. Data shown are representative of 3 independent experiments. Images were taken using a Leica SP5-AOBS confocal laser scanning microscope attached to a Leica DM I6000 inverted epifluorescence microscope with phase-contrast and a Plan-Apo 63×/1.40 NA oil immersion objective as stated in “Internalization and immunofluorescence microscopy.”
Further analysis by immunofluorescence microscopy confirmed that these variant receptors did not recycle efficiently to the cell surface after internalization and that they were retained in an intracellular sorting compartment (Figure 3C). In these experiments we transiently coexpressed FLAG-tagged wild-type receptor into cells stably expressing HA-tagged E339stop or P341A variant receptors. After agonist exposure the mutant receptors colocalized (shown in green) with wild-type receptor (shown in red; overlay in yellow Figure 3C; ADP). However, after agonist removal E339stop and P341A variant receptors remained retained in an intracellular sorting compartment, whereas wild-type receptor efficiently recycled back to the cell surface (Figure 3C; ADP/apyrase). Therefore, the PDZ ligand of the P2Y12R appears to play a critical role in regulating both receptor internalization and recycling to the surface, directing receptor sorting.
The PDZ ligand of the P2Y12R is required for arrestin-dependent internalization
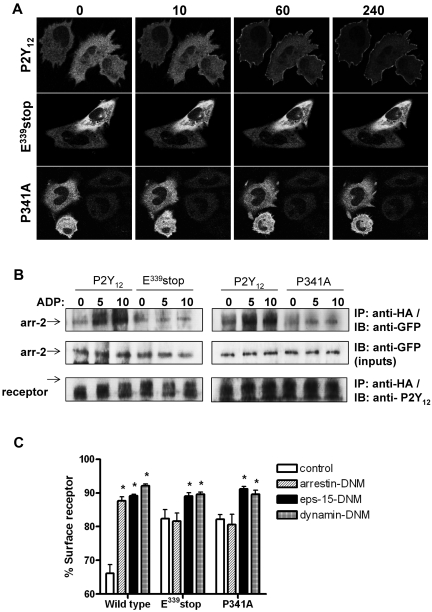
We next sought to determine why receptor internalization and traffic was attenuated in the variant P2Y12Rs lacking a functional PDZ ligand (P341A or E339stop). P2Y12R internalization is arrestin dependent with activation of P2Y12Rs leading to rapid recruitment of arrestin-2 and arrestin-3 from cytosol to membrane in transfected cell lines.7 Before agonist stimulation, arrestin-2–GFP displayed a diffuse cytoplasmic distribution in CHO cells expressing each of the receptor constructs (Figure 4A). After the addition of ADP (10μM) to cells expressing wild-type P2Y12R, rapid translocation of arrestin-2–GFP from cytosol to the membrane was observed. In contrast, arrestin-2–GFP did not translocate in cells expressing either P341A or E339stop. A similar series of observations were obtained in cells transfected with arrestin-3–GFP (data not shown). We next undertook a series of coimmunoprecipitation experiments to investigate possible arrestin-2/P2Y12R interaction (Figure 4B). With the use of the HA epitope to immunoprecipitate the receptor, we demonstrated coprecipitation of arrestin-2–GFP with the wild-type P2Y12R, but not with T320stop, E339stop, or the P341A mutant, after agonist addition (Figure 4B). Furthermore, overexpression (> 5-fold over endogenous levels) of a dominant-negative mutant form of arrestin-2 (319-418; arrestin-DNM) selectively attenuated agonist-induced wild-type but not mutant receptor internalization (Figure 4C). This arrestin mutant lacks the receptor-binding region found in the endogenous protein and competes with both wild-type arrestin-2 and arrestin-3 for clathrin binding.16 Therefore, an intact PDZ ligand appears to be required for arrestin/P2Y12R interaction and subsequent arrestin-dependent receptor internalization.
Figure 4.
An intact PDZ-binding ligand is required for arrestin- but not clathrin-dependent internalization of the P2Y12 receptor. (A) Cells stably expressing receptor constructs were transfected with arrestin-2–GFP. Before imaging, coverslips were mounted in an imaging chamber at 37°C. The initial diffuse cytoplasmic distribution of arrestin-2–GFP is shown before agonist stimulation (0 second). ADP (10μM) was added, and the redistribution of arrestin-2 was monitored in real time. The images shown were collected at 10, 60, and 240 seconds after agonist addition. Data shown are representative of 3 independent experiments. (B) Cells stably expressing receptor construct were transiently transfected with arrestin-2–GFP. Cells were stimulated with ADP (10μM; 5 and 10 minutes). Receptor was immunoprecipitated from cell lysates with the use of an anti-HA Ab (HA-11), and arrestin-2 association was assessed with an anti-GFP Ab. Equal loading of arrestin-2–GFP was confirmed in cell lysates taken before receptor immunoprecipitation. As shown arrestin-2/receptor association was only found in cells expressing the full-length P2Y12 purinergic receptor. Like E339stop and the P341A variant, T320stop did not associate with arrestin (data not shown). Data shown are representative of 3 independent experiments. (C) Receptor-expressing cells were transiently transfected with DNM forms of arrestin-2 (319-418; arrestin-DNM), eps-15 (E95-295; eps-15–DNM), dynamin (K44A; dynamin-DNM), or vector (pcDNA3) alone. Cells were subsequently challenged with ADP (10μM; 30 minutes), and surface receptor loss was assessed by ELISA. The data represent means ± SEMs of 7 independent experiments. *P < .05 compared with respective pcDNA3 vector transfected controls (Mann-Whitney U test).
The PDZ ligand of the P2Y12R is not required for clathrin-dependent internalization
Because we have previously demonstrated that wild-type P2Y12R internalizes through CCPs in a dynamin-dependent manner,7 we next examined the ability of the P341A or E339stop variants to undergo clathrin-dependent internalization. Eps-15–DNM blocks CCP formation by disrupting the interaction between eps-15 and the clathrin adaptor complex AP-2 essential for normal formation of CCPs.17 Dynamin-DNM is deficient in its ability to bind GTP and functions to inhibit dynamin-mediated scission of clathrin-coated vesicles from the plasma membrane.18 As shown in Figure 4C, expression of either eps-15–DNM or dynamin-DNM strongly inhibited ADP-induced (10μM; 30 minutes) internalization of wild-type and variant receptors. These results show that the variant receptors can, like the wild-type receptor, internalize in a clathrin- and dynamin-dependent manner.
The PDZ ligand of the P2Y12R is required for effective agonist-induced traffic in human platelets
The studies in CHO cells transfected with wild-type and variant constructs indicated the critical importance of the carboxyl-terminus and specifically the PDZ ligand in regulating receptor traffic and function. To corroborate this finding in platelets, and hypothesizing that a loss in function of the C-terminal tail would be associated with a mild bleeding tendency, we sought to identify patients with naturally occurring mutations in the carboxyl-terminus of the P2Y12R. In parallel to this work, we have been searching for mutations in the P2Y12R in patients enriched for bleeding.19 Given the similarities in bleeding symptoms between patients with type 1 VWD and platelet-based bleeding disorders and the growing evidence for locus heterogeneity in type 1 VWD, we focused our attention on a cohort of patients with bleeding symptoms and a diagnosis of type 1 VWD who had been recruited previously to the European Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) study.20 Phenotypic and genotypic characterizations of the MCMDM-1VWD cohort have confirmed the contribution of genetic loci outside of the VWF locus to the pathogenesis of type 1 VWD.20 Indeed, investigation of a subgroup of this cohort recently identified a novel P2RY12 gene defect in one of the index cases who was enrolled in the study with a diagnosis of type 1 VWD and a VWF defect.19 Sequencing of the P2Y12 gene (P2RY12) in the remaining index cases in this study identified a single patient who was heterozygous for a C > G transversion at nucleotide 1021 of the P2RY12 cDNA (+1 is the A of the initiator ATG codon), which was predictive of a P341A substitution within the P2Y12 PDZ ligand characterized above. The 1021C > G transversion was not listed in the dbSNP database, and did not occur among 100 healthy control subjects (200 alleles) recruited in the same center as the patient or in publically available databases, indicating that the alteration was unlikely to represent a novel polymorphism in the general population. The patient, who had a bleeding history consistent with the diagnosis of type 1 VWD (bleeding severity score = 1421) was not available for further investigation. However, the mother of the patient, who was reported to have normal VWF levels accompanied with mild bleeding tendency (bleeding severity score = 421) and was heterozygous for the P341A mutation, was available for investigation.
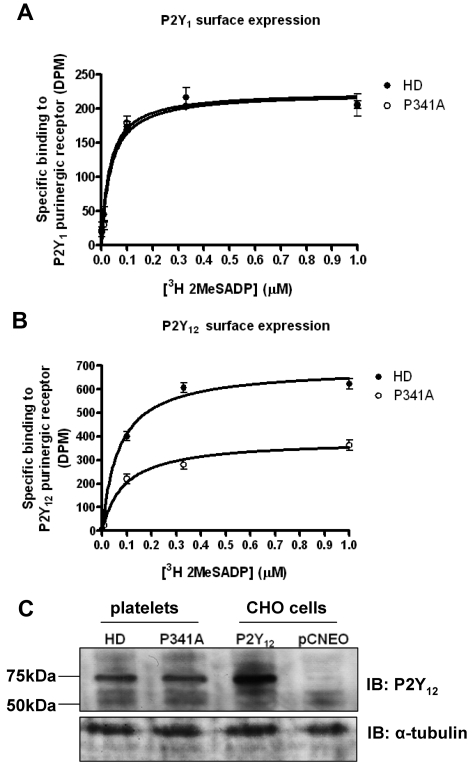
We initially examined surface expression of ADP receptors on platelets from the subject with the P341A variant by ligand binding with the use of [3H]-2MeSADP (100nM) in the presence of A3P5P (1mM; Figure 5A) or AR-C69931MX (1μM; Figure 5B) to distinguish either the P2Y1 or P2Y12 surface binding sites.4,7 Importantly, there was a significant reduction in P2Y12 surface binding sites in platelets (44.9% ± 5.5% decrease in Bmax) from the subject with the P2Y12-P341A variant versus a healthy donor control that was analyzed on the same day whose levels of P2Y12 surface expression were consistent with previous reports from our laboratory.6 In contrast P2Y1 surface receptor expression was unaltered. Western blotting of platelet cell lysates with a P2Y12-specific Ab (Figure 2B) showed no difference in total levels of P2Y12R in platelets from a healthy donor and the subject with the P2Y12-P341A variant (Figure 5C). The specificity of this Ab to assess P2Y12R expression was again confirmed in CHO cells stably expressing P2Y12R or vector alone controls. Therefore, only surface and not total P2Y12 expression appears to be altered in the subject with the P341A substitution compared with healthy donor controls, suggesting that the receptor has become trapped in an intracellular compartment.
Figure 5.
Expression of P2Y1 and P2Y12 receptors in platelets from a subject with a heterozygous P2Y12 P341A substitution. (A-B) P2Y1 and P2Y12 surface receptor levels were measured in fixed platelets from healthy donors (HDs) or from the subject with a heterozygous P2Y12 P341A mutation (P341A) by displacement of [3H]2MeSADP (1nM to 1μM) by receptor antagonists for P2Y1 (A3P5P; 1mM) and P2Y12 (AR-C69931MX; 1μM), respectively. Data are expressed as [3H]2MeSADP binding (DPM) and represent means ± SEMs. *Statistically significant reduction in P2Y12 binding levels at P < .05 for data compared with respective control data (Mann-Whitney U test). (C) Total P2Y12 receptor expression was assessed by immunoblotting with a P2Y12-specific Ab in cell lysates from HDs or P341A patient platelets and CHO cells stably expressing full-length P2Y12 or pcNEO control. Data shown are representative for 3 independent experiments. Densitometric analysis of 3 experiments was performed and, when data were expressed as fold increase over HD values of 1.1, 0.9 and 1.1 were obtained, indicating no change.
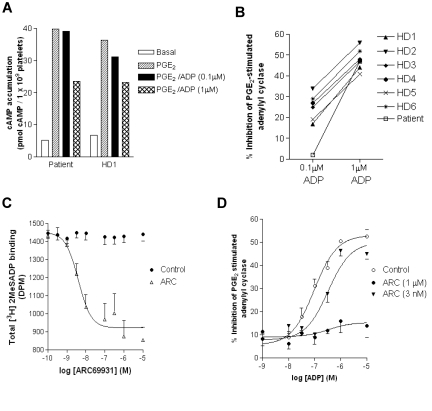
Interestingly, P2Y12R function appeared to be altered with submaximal concentrations of ADP (0.1μM), producing little inhibition of PGE2-stimulated adenylyl cyclase (Figure 6A-B), whereas healthy donor controls were able to produce a modest increase in P2Y12R activity (Figure 6A-B). At higher concentrations of ADP (1μM) P2Y12R activity was similar in both the patient and healthy donor controls (Figure 5A-B). Basal and PGE2-stimulated cAMP levels were also comparable between the patient and healthy donor controls (Figure 6A). Platelets from the subject showed sustained aggregation and dense granule secretion, which were similar to that of control platelets, in response to a range of ADP concentrations (1-100μM). Aggregation in response to collagen (3 μg/mL) and adrenaline (10μM) was also similar to that of control platelets, confirming the integrity of agonist-induced platelet activation in the subject.
Figure 6.
P2Y12 receptor signaling in platelets from a subject with a heterozygous P2Y12 P341A substitution. (A-B) P2Y12 receptor signaling was measured in platelets from healthy donors (HDs) or from the subject with a heterozygous P2Y12 P341A mutation (P341A). P2Y12 receptor signaling was assessed by comparing agonist (ADP; 0.1 and 1μM)–dependent inhibition of PGE2 (10μM; 5 minutes)–stimulated adenylyl cyclase activity. (A) The raw data from the patient and HD1 are expressed as pmol cAMP/1 × 109 platelets. (B) Data from the patient and a range of HDs are expressed as percentage of inhibition of PGE2-stimulated adenylyl cyclase. (C-D) The result of a partial reduction in P2Y12 receptor availability was further investigated. (C) Displacement of the P2Y-specific ligand [3H]-2MeSADP by ARC69931. Fixed platelets were incubated with [3H]2MeSADP (100nM), and bound ligand was displaced by increasing concentrations of the P2Y12 receptor antagonist AR-C69931MX (0.1nM to 10μM). Data are expressed as total 3H-2MeSADP binding (DPM) and represent means ± SEMs of 3 independent experiments. From this data 2 concentrations of AR-C69931MX were used: 3nM, which displaces ∼ 50% of specific ligand binding, and a maximal concentration of 1μM. (D) Inhibition of ADP-stimulated P2Y12 receptor activity by antagonism with different concentrations of AR-C69931 MX in human platelets. Agonist (ADP; 1nM to 10μM)–dependent inhibition of PGE2 (10μM; 5 minutes)–stimulated adenylyl cyclase activity was assessed in the absence and presence of AR-C69931 MX (3nM and 1μM). Data are expressed as the percentage of inhibition of PGE2-stimulated adenylyl cyclase and represent means ± SEMs of 3 independent experiments. Pretreatment with 3nM AR-C69931 MX shifts the EC50 for ADP from 95 ± 8nM in the absence and 380 ± 13nM in the presence of antagonist (P < .05, Mann-Whitney U test).
Because the patient was unavailable for further study and to determine whether the reduction in P2Y12R surface expression was responsible for the reduction in receptor signaling at low concentrations of ADP, we examined P2Y12R function in platelets where we pharmacologically reduced receptor availability. In these experiments we used 2 different concentrations of the P2Y12R antagonist AR-C69931MX, either at 3nM, which in our hands it displaces ∼ 50% of the P2Y radioligand [3H]-2MeSADP, or 1μM, which blocks all P2Y12 ligand binding (Figure 6C). The higher 1-μM concentration of AR-C69931MX as expected almost completely blocked ADP-stimulated P2Y12 inhibition of PGE2-stimulated adenylyl cyclase. Importantly, the lower concentration of AR-C69931MX (3nM), which produces a reduction in P2Y12 surface availability comparable to that seen in the patient, only inhibited ADP-stimulated P2Y12R activity at lower concentrations of ADP (ie, 0.1μM). At higher ADP concentrations (> 1μM) P2Y12R stimulation was maintained (Figure 6D). Therefore, similar to our patient studies partial reductions in P2Y12R surface availability only attenuated ADP stimulation at submaximal concentrations.
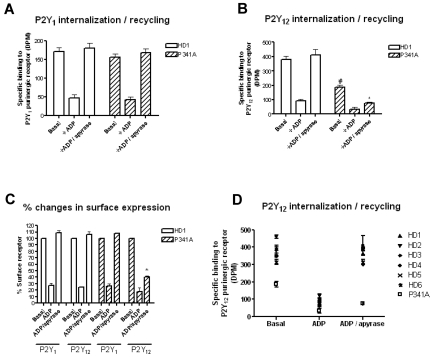
Because changes in levels of P2Y12 surface receptor expression were evident (Figure 5C), we next examined if receptor traffic was impaired in platelets from the subject with the P2Y12-P341A variant (Figure 7). Consistent with our previous studies,4 platelets from healthy donors showed a clear reduction in [3H]-2MeSADP binding to both P2Y1 (Figure 7A,C) and P2Y12 (Figure 7B-C) after pretreatment with ADP (10μM; 10 minutes). Agonist-induced loss of surface P2Y1 and P2Y12Rs were similar in platelets from the subject with the P341A variant of P2Y12 (ADP on Figure 7C). This was different to our findings in cell lines in which the P341A variant stably overexpressed in CHO cells showed attenuated receptor internalization. After lengthened periods of apyrase exposure for 30 minutes (ADP/apyrase on Figure 7B) both the P2Y1 and P2Y12 surface receptor levels returned to control levels as expected in platelets from healthy donor patients.4 In contrast, platelets from the subject with the P341A variant did not display rapid P2Y12R recycling. P2Y1R traffic meanwhile was similar to that in healthy donor platelets. Further studies with a larger group of healthy donors also showed consistent recycling of P2Y12R back to the cell surface after removal of ADP, whereas that of the patient was blocked (Figure 7D). Therefore, in agreement with our cell line studies, the P341A substitution within the PDZ ligand of the P2Y12R significantly impaired correct receptor recycling back to the membrane in human platelets.
Figure 7.
Reduced recycling of P2Y12 receptors in platelets from a subject with a heterozygous P2Y12 P341A mutation. Platelets from healthy donors (HDs) or from the subject with a heterozygous P2Y12 P341A mutation (P341A) were exposed to ADP (10μM; 10 minutes) to promote receptor internalization (ADP on graph) and then apyrase (0.2 U/mL; 30 minutes) to promote receptor recycling (ADP/apyrase on graph). P2Y1 and P2Y12 surface receptor levels were subsequently measured in fixed platelets with the use of [3H]-2MeSADP (100nM) in the presence of either the P2Y1 receptor antagonist A3P5P (1mM) or the P2Y12 receptor antagonist AR-C69931MX (1μM). (A-B) Data are expressed as specific [3H]-2MeSADP binding to the P2Y1 and P2Y12 receptors, respectively (DPM). (B) #Statistical significance at P < .05 for data compared with basal HD1 control. *Statistical significance at P < .05 for data compared with respective resensitized HD1 control (Mann-Whitney U test). (C) Data are expressed as the percentage of surface receptor and represent means ± SEMs. Statistical significance at P < .05 for data compared with respective resensitized HD1 control (Mann-Whitney U test). (D) Data are expressed as specific P2Y12 receptor binding (DPM) in human platelets and represent data from 6 healthy donors and the patient (P341A) in basal, ADP (10μM; 10 minutes) stimulated to promote receptor internalization, and then apyrase (0.2 U/mL; 30 minutes) to promote receptor recycling (ADP/apyrase on graph) conditions.
Discussion
Here, we report for the first time in tissue of a human origin that the integrity of a PDZ ligand is essential for normal protein function. This was shown through studies characterizing the molecular determinants regulating traffic of P2Y12 purinoceptors in human platelets. Through the course of our studies we showed that the presence of the PDZ ligand of the receptor regulated receptor traffic both in transfected cell lines and most importantly in human platelets. This receptor, which internalizes through clathrin-mediated endocytosis in a GRK- and arrestin-dependent manner,7 was mistrafficked after removal or disruption of its PDZ ligand leading to its retention in an endocytic-sorting compartment. Importantly, this mistraffic blocked the ability of the receptor to recycle back to the cell surface and hence blocked receptor resensitization, a process essential for normal platelet purinoceptor function.4 Critically, we have studied a human subject with a naturally occurring mutation predicted to disrupt the PDZ ligand in the P2Y12R. Platelets from this subject showed significantly compromised P2Y12R traffic. To our knowledge this is the first report of an identified mutation in the PDZ ligand of an endogenously expressed protein leading to an observable change in protein traffic. This provides valuable confirmation ex vivo of the physiologic significance of PDZ-mediated pathways.
P2Y12Rs play an essential role in ADP-induced platelet activation and are important pharmacologic targets in the treatment of arterial thrombotic disease.2,11 Their functional regulation is therefore critical for the control of hemostasis and thrombosis.2,4 Our studies have shown for the first time in human platelets that the PDZ motif of this receptor is critically required for efficient receptor recycling. We also show a reduction in surface versus total receptor in platelets from the subject with the P341A variant that we believe is because of the retention of receptor in intracellular sorting compartments after platelet exposure to low levels of ADP possibly released from damaged cells during their transit in the vasculature. It may also be the case that P2Y12-P341A variant expresses poorly at the cell surface in human platelets, although our cell line studies indicate that this receptor appears to be exported and inserted efficiently into the cell membrane.
Although the mild bleeding symptoms that have been associated with P2Y12 deficiency are usually recessively inherited, heterozygous defects have also been reported in patients with a hemorrhagic diathesis.22–24 Our recent study of a novel heterozygous P2Y12 defect and its association with bleeding in a family with type 1 VWD supports a contribution from other loci to the bleeding tendency in patients with type 1 VWD and provides further evidence for locus heterogeneity in this disorder.19 In this present study we have identified a patient with type 1 VWD and heterozygous for the P341A mutation. Although this patient (bleeding severity score = 1421) was not available for further investigation, the mother of the patient, who has normal VWF levels accompanied with mild bleeding tendency (bleeding severity score = 421), was studied.
Studies on this subject showed a significant decrease in surface P2Y12R coupled with a significant impairment of P2Y12R recycling. These changes are accompanied with a reduction in responsiveness to submaximal concentrations of ADP, although platelet aggregation and secretion after acute periods of maximal concentrations of ADP stimulation are not different from controls. We hypothesize that the failure of the P341A variant to recycle may lead to an impairment in ADP responsiveness after more prolonged periods of platelet activation, such as seen in the growing thrombus.25,26 Previously, we have shown that, in order for P2Y12Rs to resensitize after agonist-induced desensitization, they need to internalize and then subsequently recycle back to the cell surface.4 Any attenuation of receptor internalization could lead to an increased prevalence of desensitized receptors remaining on the cell surface, whereas the lack of recycling would prevent any resensitized receptor returning to the cell surface. Therefore, P2Y12R internalization and recycling are processes critical for maintenance of receptor responsiveness in platelets. Our cell line studies (Figure 2A) show that the responsiveness of the P2Y12-P341A mutant does not resensitize after agonist-induced desensitization. Given that continuous ADP signaling is required for thrombus stability,25,26 we therefore speculate that the effect on signaling produced by the blockade of P2Y12-P341A resensitization could affect on thrombus growth. Such a reduction in P2Y12R function may explain the mild bleeding symptoms seen in the heterozygous carrier of the P341A variant, although without further more detailed studies we cannot conclude that this mutation is causative. A more clear understanding of how the processes and timing of P2Y desensitization and subsequent resensitization link with thrombus formation, possibly provided by in vivo mouse thrombus formation models, is required to fully understand their effect. Interestingly, the P341A variant was associated with a reduction in responsiveness to submaximal concentrations of ADP. Our studies suggest that this reduction may reflect changes in surface receptor expression seen with the P341A variant because we can replicate our findings when we partially reduce surface receptor levels with the use of P2Y12R antagonists. Importantly in CHO cells transfected with the P341A variant agonist-dependent activation of the P341A receptor was similar to that of the wild-type receptor, indicating no obvious change in receptor/G protein coupling. Unfortunately, detailed further analysis examining the P341A variant in this patient and its relevance to whole platelet function, especially at these submaximal concentrations of ADP or after repeated ADP challenge, is not possible at this time.
Many GPCRs express C-terminal type I, type II, and type III PDZ ligands (see recent review including bioinformatic search10) with PDZ domain–containing proteins having established roles in receptor traffic, localization, and assembly of signaling complexes. For example, the β2AR has a C-terminal DSLL motif that conforms to a type I PDZ ligand, which controls receptor recycling.27 A type I PDZ ligand is also found in the β1AR and is capable of promoting PDZ-domain interactions and receptor recycling.28 In addition to C-terminal PDZ ligands, one study has indicated that an internal PDZ ligand present in the ETA endothelin receptor C-tail can regulate receptor recycling.29 Our study has also shown that the C-terminal ETPM motif of the P2Y12R, a type I PDZ motif, is required for receptor recycling. Deletion or mutation of this sequence leads to the accumulation of the receptor in an intracellular sorting compartment and blockade of receptor recycling. Interestingly, the PDZ-dependent recycling of the β2AR has recently been shown to be regulated by its interaction with sorting nexin 27.30 We have as of yet failed to identify a similar endocytic sorting protein binding partner that can regulate the PDZ-dependent recycling of the P2Y12R, although studies are ongoing. In addition to recycling, PDZ ligands have been implicated in regulating endocytosis of certain GPCRs.9,10 In our study, disruption or removal of the PDZ ligand slowed down the internalization of the receptor in CHO cells and blocked the ability of the receptor to interact with arrestin. Our previous studies have shown that receptor phosphorylation by GRKs regulates arrestin-dependent P2Y12R internalization.7 Interestingly, studies in arrestin-2−/− mice have suggested that arrestin-2 may play a role in thrombus formation in vivo,31 although arrestin-dependent P2Y12R internalization was not investigated in these mice. In order for arrestins to effectively interact with a GPCR they are believed to recognize 2 binding sites on receptors: one that is shown on agonist binding and a second that is shown on phosphorylation.32 The stability of arrestin/receptor association requires receptor phosphorylation, although initial recruitment appears to require an agonist-induced conformational change in receptor structure. In our studies receptor phosphorylation and desensitization were significantly attenuated after removal of the PDZ motif but were unaffected in the P341A variant. These data suggest that either an interaction of this PDZ motif with a regulatory kinase or phosphorylation of T340 found within this PDZ motif is required for receptor phosphorylation, thereby enhancing arrestin interaction. Interestingly, although the recombinant P341A variant was still phosphorylated, its ability to interact with arrestin was lost. Therefore, the integrity of this PDZ motif also appears to be required for arrestin interaction in CHO cells. In addition, the P341A variant still desensitized, in the absence of arrestin interaction, indicating that, although arrestin is required for effective rapid internalization, it is not required for the desensitization P341A variant.
Importantly, after mutation or removal of the PDZ ligand, the P2Y12R was still able to undergo clathrin-dependent internalization. A number of GPCRs, including the P2Y1R, have been shown to internalize via CCPs in an arrestin-independent manner.7,8 For example, arrestin-independent clathrin-mediated endocytosis of M3 muscarinic receptors requires βγ subunit-dependent recruitment of tubulin,33 and α1B-adrenoceptors are endocytosed through a clathrin-mediated pathway by direct interaction of the receptor with the AP2 complex machinery.34 This may in part explain why in our platelet studies the P2Y12-P341A still appeared to internalize. The internalization of P2Y12-P341A in human platelets may, as seen in cell line studies, also be slowed. Unfortunately, such detailed measurements of surface receptor loss require significant amounts of platelet material that are not available. The reduced surface expression in P2Y12-P341A platelets may also contribute to the perceived willingness of this receptor to internalize because any agonist-induced changes in surface receptor will be more apparent than that seen in cell lines that have a significant receptor reserve.
In conclusion we show for the first time for a protein natively expressed in human tissue that the integrity of a PDZ ligand is critical for protein function. Future screens of patients with mild bleeding disorders may show further important information about the critical importance of structural motifs for the maintenance of protein–protein interactions in the context of human disease.
Acknowledgments
The authors thank Mr John Anson for his contribution to the P2RY12 analyses. The contribution of the MCMDM-1VWD study partnership is gratefully acknowledged.
This work was supported by the British Heart Foundation (BHF; PG/06/038 and RG/09/007/27 917). S.J.M. is a BHF Research Fellow, and S.P.W. holds a BHF Chair.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.N. designed and performed research; M.E.D., A.A., and S.J.M. designed and performed research and wrote the paper; A.B.F. designed research and wrote the paper; A.D.M. wrote the paper; and S.P.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart J. Mundell, Department of Physiology and Pharmacology, School of Medical Sciences, University Walk, Bristol, BS8 1TD, United Kingdom; e-mail: s.j.mundell@bris.ac.uk.
References
- 1.Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM. ADP receptors–targets for developing antithrombotic agents. Curr Pharm Des. 2003;9(28):2303–2316. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- 2.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99(3):466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 3.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundell SJ, Barton JF, Mayo-Martin MB, Hardy AR, Poole AW. Rapid resensitization of purinergic receptor function in human platelets. J Thromb Haemost. 2008;6(8):1393–1404. doi: 10.1111/j.1538-7836.2008.03039.x. [DOI] [PubMed] [Google Scholar]
- 5.Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105(9):3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 6.Mundell SJ, Jones ML, Hardy AR, et al. Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol. 2006;70(3):1132–1142. doi: 10.1124/mol.106.023549. [DOI] [PubMed] [Google Scholar]
- 7.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic. 2006;7(10):1420–1431. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8(5):462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 10.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyth SS, Woulfe DS, Weitz JI, et al. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29(4):449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 12.Matharu AL, Mundell SJ, Benovic JL, Kelly E. Rapid agonist-induced desensitization and internalization of the A(2B) adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem. 2001;276(32):30199–30207. doi: 10.1074/jbc.M010650200. [DOI] [PubMed] [Google Scholar]
- 13.Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 14.Cattaneo M, Zighetti ML, Lombardi R, et al. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci U S A. 2003;100(4):1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51(5):711–720. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- 16.Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of b-arrestin dominat negative mutants. J Biol Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- 17.Benmerah A, Poupon V, Cerf-Bensussan N, Dautry-Varsat A. Mapping of Eps15 domains involved in its targeting to clathrin-coated pits. J Biol Chem. 2000;275(5):3288–3295. doi: 10.1074/jbc.275.5.3288. [DOI] [PubMed] [Google Scholar]
- 18.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly ME, Dawood BB, Lester WA, et al. Identification and characterization of a novel P2Y 12 variant in a patient diagnosed with type 1 von Willebrand disease in the European MCMDM-1VWD study. Blood. 2009;113(17):4110–4113. doi: 10.1182/blood-2008-11-190850. [DOI] [PubMed] [Google Scholar]
- 20.Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD). Blood. 2007;109(1):112–121. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 21.Kunicki TJ, Federici AB, Salomon DR, et al. An association of candidate gene haplotypes and bleeding severity in von Willebrand disease (VWD) type 1 pedigrees. Blood. 2004;104(8):2359–2367. doi: 10.1182/blood-2004-01-0349. [DOI] [PubMed] [Google Scholar]
- 22.Remijn JA, IJsselkijk MJ, Strunk AL, et al. Novel molecular defect in the platelet ADP receptor P2Y12 of a patient with haemorrhagic diathesis. Clin Chem Lab Med. 2007;45(2):187–189. doi: 10.1515/CCLM.2007.036. [DOI] [PubMed] [Google Scholar]
- 23.Mumford AD, Dawood BB, Daly ME, et al. A novel thromboxane A2 receptor D304N variant that abrogates ligand binding in a patient with a bleeding diathesis. Blood. 2010;115(2):363–369. doi: 10.1182/blood-2009-08-236976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson S, Daly M, Dawood B, et al. Phenotypic approaches to gene mapping in platelet function disorders–identification of new variant of P2Y12, TxA2 and GPVI receptors. Hamostaseologie. 2010;30(1):29–38. [PubMed] [Google Scholar]
- 25.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108(9):3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 26.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5′-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2006;47(1):155–162. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 27.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 28.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280(5):3305–3313. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- 29.Paasche JD, Attramadal T, Kristiansen K, et al. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol. 2005;67(5):1581–1590. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- 30.Lauffer BE, Melero C, Temkin P, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190(4):565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, D'Angelo L, Chavez M, Woulfe DS. Arrestin-2 differentially regulates PAR4 and ADP receptor signaling in platelets. J Biol Chem. 2011;286(5):3805–3814. doi: 10.1074/jbc.M110.118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23(4):621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Popova JS, Rasenick MM. Clathrin-mediated endocytosis of m3 muscarinic receptors. Roles for Gbetagamma and tubulin. J Biol Chem. 2004;279(29):30410–30418. doi: 10.1074/jbc.M402871200. [DOI] [PubMed] [Google Scholar]
- 34.Diviani D, Lattion AL, Abuin L, Staub O, Cotecchia S. The adaptor complex 2 directly interacts with the alpha 1b-adrenergic receptor and plays a role in receptor endocytosis. J Biol Chem. 2003;278(21):19331–19340. doi: 10.1074/jbc.M302110200. [DOI] [PubMed] [Google Scholar]