Abstract
We have analyzed the prevalence of Shiga toxin-producing Escherichia coli (STEC) in stool specimens of patients with diarrhea or other gastrointestinal alterations from the Xeral-Calde Hospital of Lugo City (Spain). STEC strains were detected in 126 (2.5%) of 5,054 cases investigated, with a progressive increase in the incidence from 0% in 1992 to 4.4% in 1999. STEC O157:H7 was isolated in 24 cases (0.5%), whereas non-O157 STEC strains were isolated from 87 patients (1.7%). STEC strains were (after Salmonella and Campylobacter strains) the third most frequently recovered enteropathogenic bacteria. A total of 126 human STEC isolates were characterized in this study. PCR showed that 43 (34%) isolates carried stx1 genes, 45 (36%) possessed stx2 genes and 38 (30%) carried both stx1 and stx2. A total of 88 (70%) isolates carried an ehxA enterohemolysin gene, and 70 (56%) isolates possessed an eae intimin gene (27 isolates with type γ1, 20 with type β1, 8 with type ζ, 5 with type γ2, and 3 with type ɛ). STEC isolates belonged to 41 O serogroups and 66 O:H serotypes, including 21 serotypes associated with hemolytic uremic syndrome and 30 new serotypes not previously reported among human STEC strains in other studies. Although the 126 STEC isolates belonged to 81 different seropathotypes (associations between serotypes and virulence genes), only four accounted for 31% of isolates. Seropathotype O157:H7 stx1 stx2 eae-γ1 ehxA was the most common (13 isolates) followed by O157:H7 stx2 eae-γ1 ehxA (11 isolates), O26:H11 stx1 eae-β1 ehxA (11 isolates), and O111:H- stx1 stx2 eae-γ2 ehxA (4 isolates). Our results suggest that STEC strains are a significant cause of human infections in Spain and confirm that in continental Europe, infections caused by STEC non-O157 strains are more common than those caused by O157:H7 isolates. The high prevalence of STEC strains (both O157:H7 and non-O157 strains) in human patients, and their association with serious complications, strongly supports the utilization of protocols for detection of all serotypes of STEC in Spanish clinical microbiology laboratories.
Shiga toxin-producing Escherichia coli (STEC) strains, also called verotoxin-producing E. coli (VTEC) strains, represent the most important recently emerged group of food-borne pathogens (25, 37). Members of this group are a major cause of gastroenteritis that may be complicated by hemorrhagic colitis (HC) or the hemolytic uremic syndrome (HUS) which is the main cause of acute renal failure in children (2, 46, 49). Since its identification as a pathogen in 1982, STEC O157:H7 has been the cause of a series of outbreaks, especially in Canada, Japan, the United Kingdom, and the United States (25, 48, 50). Domestic ruminants, mainly cattle, sheep, and goats, have been implicated as the principal reservoir (5, 7, 8). Transmission occurs through consumption of undercooked meat, unpasteurized dairy products and vegetables, or water contaminated by feces of carriers because STEC strains are found as part of the normal intestinal floras of the animals. Person-to-person transmission has also been documented (25, 37).
STEC strains that cause human infections belong to a large number of O:H serotypes. (A total of 472 serotypes are listed in the authors' website [http://www.lugo.usc/ecoli]). A world-wide review of literature realized by K.A. Bettelheim, recording well over 1,000 reports of isolations of non-O157 STEC strains, is also available (http://www.sciencenet.com.au/vtectable.htm) [5, 9, 15, 20, 25, 33, 39, 42, 43; L. Beutin, G. Krause, S. Zimmermann, K. Gleier, S. Kaulfuss, and H. Schmidt, Abstr. 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Edinburgh, United Kingdom, abstr. P192, p. 175, 2003; A. Kai, N. Konishi, H. Obata, K. Hatakeyama, C. Monma, Y. Shimojima, H. Suzuki, T. Akiba, S. Morozumi, T. Itoh, and Y. Kudoh, Abstr. 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Edinburgh, United Kingdom, abstr. P242, p. 202, 2003; and F. Scheutz, B. Olesen, and P. Gerner-Smidt, Abstr. 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Kyoto, Japan, abstr. 207, p. 98, 2000]. Most outbreaks and sporadic cases of HC and HUS have been attributed to strains of the enterohemorrhagic serotype O157:H7 (2, 27, 46). Unlike other E. coli strains, STEC O157:H7 does not ferment sorbitol and is β-glucuronidase negative. These differences make it easy to identify O157:H7 strains in clinical samples and food products (6, 13, 26, 34). Human infections caused by STEC O157:H7 are under nationwide surveillance in many countries. However, the detection of other non-O157 STEC infections is often limited to a small number of specialized laboratories because STEC O157 colonies are more easily detectable on some culture media than non-O157 STEC types, which are thus often missed in laboratory diagnosis using stool and food specimens. However, as non-O157 STEC strains are more prevalent in animals and as contaminants in foods, humans are probably more exposed to these strains. Infections with some non-O157 STEC types (such as O26:H11 or O26:H-, O91:H21 or O91:H-, O103:H2, O111:H-, O113:H21, O117:H7, O118:H16, O121:H19, O128:H2 or O128:H-, O145:H28 or O145:H-, and O146:H21) are frequently associated with severe illness in humans, but the role of other STEC non-O157 types in human disease needs further examination [5, 15, 22, 26, 34; Beutin et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P192, and I. S. T. Fisher, W. J. Reilly, O. N. Gill, S. J. O′Brien, and H. R. Smith, Abstr. 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Edinburgh, United Kingdom, abstr. P214, p. 187, 2003].
STEC strains produce two potent phage-encoded cytotoxins called Shiga toxins (Stx1 and Stx2) or verotoxins (VT1 and VT2) (25, 37). In addition to toxin production, another virulence-associated factor expressed by STEC is a protein called intimin which is responsible for intimate attachment of STEC to the intestinal epithelial cells, causing attaching and effacing lesions in the intestinal mucosas. Intimin is encoded by the chromosomal gene eae, which is part of a pathogenicity island termed the locus for enterocyte effacement (23). Severe diarrhea (especially HC) and HUS were closely associated with STEC types carrying the eae gene for intimin (5, 15, 32, 39). Differentiation of intimin alleles represents an important tool for STEC typing in routine diagnostics as well as in epidemiological and clonal studies. The C-terminal end of intimin is responsible for receptor binding, and it has been suggested that different intimins are responsible for different host tissue cell tropisms. Intimin type-specific PCR assays identified 14 variants of the eae gene that encode 14 different intimin types and subtypes (α1, α2, β1, β2, γ1, γ2/θ, δ/κ, ɛ, ζ, η, ι, λ, μ, and ν) (1, 8, 32, 47, 51; M. Blanco, J. E. Blanco, G. Dahbi, and J. Blanco, submitted for publication). A factor that may also affect virulence of STEC is the enterohemolysin Ehly, also called enterohemorrhagic E. coli hemolysin (EHEC-HlyA), which is encoded by an ehxA gene (4, 45).
The objectives of this study were to determine the prevalence of STEC O157:H7 and non-O157 in the stool samples submitted for routine pathogen identification to the clinical microbiology laboratory of the Hospital Xeral-Calde of our city (Lugo, Spain) and also to establish the serotypes, virulence genes, and intimin types of STEC strains isolated between 1992 and 1999.
{Data from this study have been partly presented as a poster communication at the 5th International Symposium on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections, Edinburgh, United Kingdom, 8 to 11 June 2003 [J. E. Blanco, M. Blanco, A. Mora, G. Dhabi, M. P. Alonso, M. A. Coira, and J. Blanco, Abstr. 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Edinburgh, United Kingdom, abstr. P194, p. 176, 2003]}.
MATERIALS AND METHODS
Clinical specimens, culture, and STEC screening.
Over more than 6 years (from October 1992 to August 1999), 5,054 unduplicated fecal samples (only one per patient) from inpatients and outpatients of all ages that were submitted to the clinical microbiology laboratory for routine pathogen identification were screened for STEC. All stool specimens were grown in cultures by standard methods for bacterial enteric pathogen identification of strains of such genera as Salmonella, Shigella, Yersinia, Campylobacter, and Aeromonas (29).
During the first period of the study (1992 to 1994), fecal samples were plated onto MacConkey agar (MAC) and onto sorbitol MacConkey (SMAC) medium, and from each sample 10 E. coli-like colonies (lactose positive, lactose negative, or sorbitol negative) were chosen and examined for Shiga toxin (verotoxin) production on Vero and HeLa cells.
During the second period of the study (1995 to 1999), the stools were placed in 5 ml of buffered peptone water supplemented with vancomycin (8 mg liter−1), cefixime (0.05 mg liter−1), and cefsulodin (10 mg liter−1) (BPWvcc) and incubated for 6 h at 37°C. A total of 1 ml of this enrichment culture was added to 20 μl of magnetic beads coated with O157 antibodies (anti-E. coli O157 Dynabeads; Dynal, Oslo, Norway), and immunomagnetic separation was performed according to the manufacturer's instructions. The concentrated target cells were plated onto SMAC agar and on cefixime tellurite sorbitol MacConkey (CTSMAC) medium (13). All stools were additionally plated directly onto MAC and CTSMAC media. STEC strains were detected by PCR using specific primers for amplification of stx1, stx2, and eae-γ1 genes. For PCR, a loopful of bacterial growth taken from the first streaking area of the fecal culture plates was suspended in 0.5 ml of sterile distilled water and boiled for 5 min to release the DNA. From each PCR-positive fecal culture, 10 E. coli-like colonies obtained from MAC, SMAC, and/or CTSMAC plates were analyzed by PCR to obtain the STEC isolates for further characterization. When no positive-testing single colony was found among the first 10, at least 40 more colonies were tested. When none of the assayed coliform colonies still tested positive in the PCR, the sample was reported as PCR positive without STEC isolation. Production of Shiga toxins (verotoxins) by PCR-positive isolates was confirmed by cytotoxicity tests on Vero and HeLa cells.
All STEC isolates were subsequently characterized biochemically with the API 20E system (Biomerieux, Marcy l'Etoile, France) and serotyped. For patients from whom all isolates were identical with respect to the profiles of virulence genes and O:H serotypes, only one colony was selected. When one patient yielded colonies with different virulence genes or O:H serotypes, one of each was selected for further characterization.
Production and detection of Shiga toxins (verotoxins) in Vero and HeLa cells.
For production of Shiga toxins, one loopful of each isolated colony was inoculated into 50-ml Erlenmeyer flasks containing 5 ml of tryptone soy broth (pH 7.5) with mitomycin C and incubated for 20 h at 37°C (shaken at 200 rpm) and then centrifuged (6,000 × g) for 30 min at 4°C. The Vero and HeLa cell culture assays were performed using nearly confluent cell monolayers grown in plates with 24 wells. At the time of assay, the growth medium (RPMI with polymyxin sulfate) was changed (0.5 ml per well) and 75 μl of undiluted culture supernatant was added. Cells were incubated at 37°C in a 5% CO2 atmosphere, and the morphological changes in cells were observed using a phase-contrast inverted microscope after 24 and 48 h of incubation (7).
Detection of virulence genes by PCR.
From the PCR-positive primary fecal culture, E. coli-like colonies were grown in subcultures on tryptone soy agar. The bacterial cells were suspended in 250 μl of sterile water, boiled at 100°C for 5 min to release the DNA, and centrifuged. The supernatant was used in the PCR as described below. Base sequences and predicted sizes of amplified products for the specific oligonucleotide primers used in this study are shown in Table 1. The majority of the oligonucleotide primers were designed by us according to the nucleotide sequences of the virulence genes. Multiplex PCR was used only for detection of stx1 and stx2 genes. Isolates positive for the eae gene as determined with universal EAE-1 and EAE-2 primers were afterwards analyzed with all different variant primers. Amplification of bacterial DNA was performed using 30-μl volumes containing 7 μl of the prepared sample supernatant; 150 ng of the oligonucleotide primers; 0.2 mM (each) dATP, dGTP, dCTP, and dTTP; 10 mM Tris-HCl (pH 8.8); 1.5 mM MgCl2; 50 mM KCl; and 1 U of Biotaq DNA polymerase (Bioline, London, United Kingdom). The conditions for the PCR were 94°C for 2 min (for initial denaturation of DNA within the sample) followed by 35 cycles of 94°C for 1 min (denaturation), 55 to 64°C (see Table 1) for 1 min (primer annealing), and 72°C for 1 min (DNA synthesis) performed with a thermal cycler (model PCR express; Hybaid, Southhampton, United Kingdom). The amplified products were visualized by standard submarine gel electrophoresis using 10 μl of the final reaction mixture on a 2% agarose gel in TBE buffer (89 mM Tris, 89 mM boric acid, 2.5 mM EDTA). The samples were electrophoresed for 20 to 40 min at 130 V. Amplified DNA fragments of specific sizes were located by UV fluorescence after staining with ethidium bromide. Molecular size markers (HaeIII digest of φx174DNA) (Promega, Madison, Wis.) were included in each gel (8).
TABLE 1.
PCR primers and conditions for amplification of STEC virulence genes
| Gene | Primer | Oligonucleotide sequence (5′-3′)b | Fragment size (bp) | Annealing temperature (°C) | Primer coordinates | GenBank accession no. |
|---|---|---|---|---|---|---|
| stx1 | VT1-A | CGCTGAATGTCATTCGCTCTGC | 302 | 55 | 113-134 | M17358 |
| VT1-B | CGTGGTATAGCTACTGTCACC | 394-414 | ||||
| stx2 | VT2-A | CTTCGGTATCCTATTCCCGG | 516 | 55 | 50-69 | M59432 |
| VT2-B | CTGCTGTGACAGTGACAAAACGC | 543-565 | ||||
| ehxA | HlyA1 | GGTGCAGCAGAAAAAGTTGTAG | 1551 | 60 | 238-259 | X79839 |
| HlyA4 | TCTCGCCTGATAGTGTTTGGTA | 1767-1788 | ||||
| eae | EAE-1 | GGAACGGCAGAGGTTAATCTGCAG | 775 | 55 | 1441-1460 | AF022236 |
| EAE-2a | GGCGCTCATCATAGTCTTTC | 2193-2215 | ||||
| eae-α1 | EAE-FB | AAAACCGCGGAGATGACTTC | 820 | 60 | 1909-1928 | AF022236 |
| EAE-A | CACTCTTCGCATCTTGAGCT | 2709-2728 | ||||
| eae-α2 | IH2498aF | AGACCTTAGGTACATTAAGTAAGC | 517 | 60 | 2099-2122 | AF530555 |
| IH2498aR | TCCTGAGAAGAGGGTAATC | 2597-2615 | ||||
| eae-β | EAE-FB | AAAACCGCGGAGATGACTTC | 830 | 64 | 1909-1928 | AF453441 |
| EAE-B | CTTGATACACTTGATGACTGT | 2718-2738 | ||||
| eae-β1 | EA-B1-F | CGCCACTTAATGCCAGCG | 811 | 60 | 1928-1945 | AF453441 |
| EAE-B | CTTGATACACCTGATGACTGT | 2718-2738 | ||||
| eae-β2 | EA-B2-F | CCCGCCACTTAATCGCACGT | 807 | 60 | 1929-1948 | AF043226 |
| EAE-B | CTTGATACACCTGATGACTGT | 2715-2735 | ||||
| eae-γ1 | EAE-FB | AAAACCGCGGAGATGACTTC | 804 | 60 | 1909-1928 | AF071034 |
| EAE-C1 | AGAACGCTGCTCACTAGATGTC | 2691-2712 | ||||
| eae-γ2/θ | EAE-FB | AAAACCGCGGAGATGACTTC | 808 | 58 | 1909-1928 | AF025311 |
| EAE-C2 | CTGATATTTTATCAGCTTCA | 2697-2716 | ||||
| eae-δ/κ | EAE-FB | AAAACCGCGGAGATGACTTC | 833 | 60 | 1909-1928 | U66102 |
| EAE-D | CTTGATACACCCGATGGTAAC | 2721-2741 | ||||
| eae-ɛ | EAE-FB | AAAACCGCGGAGATGACTTC | 722 | 66 | 1909-1928 | AF116899 |
| LP5 | AGCTCACTCGTAGATGACGGCAAGCG | 2605-2630 | ||||
| eae-ζ | Z1 | GGTAAGCCGTTATCTGCC | 206 | 62 | 2062-2079 | AF449417 |
| Z2 | ATAGCAAGTGGGGTGAAG | 2250-2267 | ||||
| eae-η | EAE-FB | AAAACCGCGGAGATGACTTC | 712 | 62 | 1899-1918 | AJ308550 |
| LP8 | TAGATGACGGTAAGCGAC | 2593-2610 | ||||
| eae-ι | EAE-FB | AAAACCGCGGAGATGACTTC | 807 | 66 | 1909-1928 | AJ308551 |
| LP7 | TTTATCCTGCTCCGTTTGCT | 2695-2715 | ||||
| eae-λ | 68.4F | CGGTCAGCCTGTGAAGGGC | 466 | 64 | 2061-2079 | AF530557 |
| 68.4R | ATAGATGCCTCTTCCGGTATT | 2506-2526 | ||||
| eae-μ | FV373F | CAACGGTAAGTCTCAGACAC | 443 | 64 | 114-133 | AJ579305 |
| FV373R | CATAATAAGCTTTTTGGCCTACC | 534-556 | ||||
| eae-ν | IH1229aF | CACAGCTTACAATTGATAACA | 311 | 60 | 269-289 | AJ579306 |
| IH1229aR | CTCACTATAAGTCATACGACT | 559-579 |
Universal (detects all types of eae variants) oligonucleotide primer pair EAE-1 and EAE-2 with homology to the 5′ conserved region of eae gene.
The HlyA1 and Hly4 primer pair was designed by Schmidt et al. (45). The remaining primer pairs were designed by us according to the nucleotide sequences of the genes (H88) (Blanco et al., submitted).
Serotyping.
The determination of O and H antigens was carried out by the method described by Guinée et al. (19) employing all available O (O1 to O181) and H (H1 to H56) antisera. All antisera were obtained and absorbed with the corresponding cross-reacting antigens to remove the nonspecific agglutinins. The O antisera were produced in the Laboratorio de Referencia de E. coli (Lugo, Spain; http://www.lugo.usc.es/ecoli), and the H antisera were obtained from the Statens Serum Institut (Copenhagen, Denmark).
E. coli control strains.
The E. coli strains used as controls were as follows: EPEC-2348 (human, O127:H6, eae-α1), AEEC-IH2498a (human, O125:H6, eae-α2), REPEC-RDEC-1 (rabbit, O15:H-, eae-β1), EPEC-359 (human, O119:H6, eae-β2), STEC-EDL933 (human, O157:H7, stx1, stx2, eae-γ1, ehxA), STEC-VTB308 (bovine, O111:H-, stx1, eae-γ2), STEC-TW07926 (human, O111:H8, stx1, stx2, eae-θ), EPEC-BP12665 (human, O86:H34, eae-δ), AEEC-6044/95 (human, O118:H5, eae-κ), STEC-VTB-286 (bovine, O103:H2, stx1, eae-ɛ), STEC-VTO-50 (ovine, O156:H-, stx1, eae-ζ), AEEC-CF11201 (human, O125:H-, eae-η), AEEC-7476/96 (human, O145:H4, eae-ι), AEEC-68-4 (human, O34:H-, eae-λ), EPEC-373 (human, O55:H51, eae-μ), AEEC-IH1229a (human, O10:H-, eae-ν), and K12-185 (negative for stx1, stx2, eae, and ehxA). Strains were stored at room temperature in nutrient broth with 0.75% agar.
Clinical data and statistical test.
Clinical data were collected by retrospective analysis of medical records. Statistical analysis was performed by using a Yates-corrected chi-square test. P values of <0.05 indicated statistical significance.
RESULTS
Prevalence of STEC in human stool samples.
We have analyzed the presence of STEC in stool specimens of patients with diarrhea or other gastrointestinal alterations from the Hospital Xeral-Calde of Lugo. STEC strains were detected in 126 (2.5%) of 5,054 cases investigated, with a progressive increase in the incidence from 0% in 1992 to 4.4% in 1999. STEC O157:H7 was isolated in 24 cases (0.5%), whereas non-O157 strains were isolated from 87 patients (1.7%) (Table 2). Two patients simultaneously excreted STEC O157:H7 and non-O157 types. Thus, in 109 (87%) of the 126 STEC-positive stool specimens, STEC isolates were recovered. All STEC isolates gave positive results in the Vero and HeLa cell cytotoxicity test.
TABLE 2.
STEC prevalence in human clinical stool samples
| Year | Methodology used for detection and isolation of STECb | No. of stool samples (coprocultives)
|
|||
|---|---|---|---|---|---|
| Total no. analyzed | No. (%) STEC positiveb with:
|
||||
| STEC detected | O157:H7 isolated | Non-O157 isolated | |||
| 1992 | For O157:H7, SMAC + Vero/HeLa cells; | 186 | 0 (0) | 0 (0) | 0 (0) |
| 1993 | for non-O157, MAC + Vero/HeLa cells | 439 | 4 (0.9) | 0 (0) | 4 (0.9) |
| 1994 | 510 | 7 (1.4) | 0 (0) | 7 (1.4) | |
| 1995 | For O157:H7, | 707 | 10 (1.4) | 3 (0.4) | 7 (1.0) |
| 1996 | BPWvcc+IMS+CTSMAC+ | 684 | 9 (1.3) | 1 (0.1) | 8 (1.2) |
| 1997 | SMAC+PCR-stx1-stx2-eae-γ1; | 850 | 26 (3.0) | 7 (0.8) | 17 (2.0) |
| 1998 | for non-O157, | 1,048 | 42 (4.0) | 4 (0.4) | 27 (2.6) |
| 1999 | MAC+CTSMAC+PCR-stx1-stx2 | 630 | 28 (4.4) | 9 (1.4) | 17 (2.7) |
| Total | 5,054 | 126 (2.5) | 24 (0.5) | 87 (1.7) | |
IMS, immunomagnetic separation; PCR-stx1-stx2-eae-γ1, PCR with primers specific for stx1, stx2, and eae-γ1; PCR-stx1-stx2, PCR with primers specific for stx1 and stx2.
STEC O157:H7 and STEC non-O157 strains were isolated in two samples.
STEC strains were the third most frequently recovered enteropathogenic bacteria strains, after Salmonella and Campylobacter strains. In the study population, Salmonella spp. were isolated from 8.0% of patients, Campylobacter spp. were isolated from 4.9%, Aeromonas spp. were isolated from 0.4%, Yersinia spp. were isolated from 0.3%, and Shigella spp. were isolated from 0.1%. In the stool specimens of 2 patients with STEC O157:H7 and 11 patients with STEC non-O157, other enteropathogens were isolated (6 Salmonella spp. isolates and 7 Campylobacter jejuni isolates).
STEC O157:H7 was more frequently isolated during the summer months. Of 24 specimens that yielded STEC O157:H7, 15 (63%) were collected from June through August. STEC non-O157 strains were isolated throughout the year with only a moderate seasonal variation, 69% of the cases being detected from April to September.
The largest number of STEC O157:H7 isolates was obtained from children 1 to 10 years of age (12 cases) and adults 61 to 100 years of age (9 cases). Non-O157 STEC infections were distributed without an obvious variation among the different age groups. The isolation proportions for STEC were not significantly different for male and female patients.
When clinical data from patients with STEC O157:H7 isolation were compared to those from patients with non-STEC O157 isolates, important statistical differences were found. Thus, patients infected with STEC O157:H7 more often presented with bloody diarrhea and/or HC (46 versus 21%; P < 0.05) and acute renal failure and/or HUS (17 versus 5%; P < 0.05) than those infected with non-O157 (Table 3). Furthermore, three people (78, 83, and 87 years old, respectively) died with STEC O157:H7 infection. STEC strains were isolated in two cases of HUS: STEC O157:H7 from one girl (2 years old) and STEC O20:H19 from one adult (73 years old).
TABLE 3.
Clinical features of patients positive for STEC isolation
| Clinical features | No. (%) positive for STEC O157:H7 (n = 24) | No. (%) positive for STEC non-O157a (n = 85) | No. (%) positive for STEC O26:H11 (n = 12) |
|---|---|---|---|
| Diarrhea | 21 (88) | 59 (69) | 7 (58) |
| Bloody diarrhea or HC | 11 (46) | 18 (21) | 2 (17) |
| Abdominal cramps | 12 (50) | 33 (39) | 5 (42) |
| Vomiting | 10 (42) | 34 (40) | 5 (42) |
| Fever | 5 (21) | 21 (25) | 1 (8) |
| HUS | 1 (4) | 1 (1) | 0 (0) |
| Acute renal failure (ARF) | 3 (13) | 3 (4) | 0 (0) |
| HC, HUS, and/or ARF | 12 (50) | 18 (21) | 2 (17) |
| Death | 3 (13) | 0 (0) | 0 (0) |
| Hospitalization | 17 (71) | 64 (75) | 10 (83) |
Includes STEC O26:H11 isolates.
All STEC infections detected in this study were sporadic, and the sources of infection were not identified in most cases. However, outbreak investigations were not routinely performed, and a number of these infections could be part of unidentified outbreaks. In 12 cases (8 O157:H7 and 4 non-O157), stool specimens from parents who had been in contact with STEC-positive patients were examined and asymptomatic carriers were detected among the relatives of six patients with STEC O157:H7 infection and of three patients with non-O157 STEC infection. One of the most curious cases concerned a positive-testing stool sample from an asymptomatic mother of a girl with HUS; the sample showed plentiful pure growth of STEC O157:H7 on CTSMAC agar. Another prominent case was that of a patient (87 years old) who died after HC and acute renal failure, while his nephew (63 years old) was found to be an asymptomatic carrier of the same type of STEC O157:H7. Besides, in the case of a patient 73 years of age with HUS caused by STEC O20:H19 vt1 vt2, strains with the same seropathotype were recovered from an asymptomatic relative and from two of four cows of the family farm. We isolated STEC O113:H21 vt2 from another patient 52 years of age with diarrhea and abdominal pain and also from the stools of his wife and a son and from 4 of 13 cows of the family farm.
Detection of STEC in SMAC and CTSMAC media and biochemical characteristics.
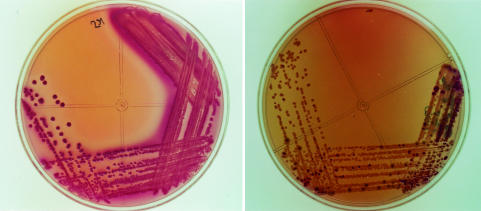
During this study, 5 of 24 STEC O157:H7-positive specimens did not exhibit sorbitol-negative colonies on SMAC agar but the isolation of STEC O157:H7 colonies by culture on CTSMAC medium was possible (Fig. 1). The CTSMAC medium was also a very useful tool for selective isolation of non-O157 STEC eae-positive strains. None of the 24 STEC O157:H7 strains isolated in this study fermented sorbitol after overnight incubation and all were β-glucuronidase negative. All non-O157 STEC isolates fermented sorbitol, except for three: one O75:H8 isolate, one O172:H- isolate, and one OX177:H- isolate (the latter did not ferment lactose either). Two STEC O150:H- isolates were lactose negative, β-glucuronidase negative, and urease positive. One STEC O2:H27 isolate was β-glucuronidase negative.
FIG. 1.
Culture on SMAC (left) and CTSMAC (right) media of a STEC O157:H7-positive stool specimen from a patient with HUS. The presence of sorbitol-negative colonies on the SMAC plate (left) was obscured by the overwhelming number of sorbitol fermenters.
Virulence genes.
A total of 126 human STEC isolates were characterized in this study. PCR showed that 43 (34%) isolates carried stx1 genes, 45 (36%) possessed stx2 genes, and 38 (30%) carried both stx1 and stx2. Enterohemolysin (ehxA) and intimin (eae) virulence genes were detected in 88 (70%) and in 70 (56%) of the isolates, respectively.
Serotypes and seropathotypes.
STEC isolates belonged to 41 O serogroups and 66 O:H serotypes, including 21 serotypes associated with HUS in previous studies (O1:H7, O1:H-, O20:H19, O26:H11, O26:H-, O26:H?, O46:H31, O91:H21, O91:H-, O98:H-, O103:H2, O111:H-, O113:H21, O118:H16, O128:H2, O145:H-, O157:H7, O172:H-, O174:H-, ONT:H4, and ONT:H-) and 30 new serotypes not previously reported among human STEC isolates in other studies. Although the 126 STEC isolates belonged to 81 different seropathotypes (association between serotype and virulence genes), only four accounted for 31% of the isolates. Seropathotype O157:H7 stx1 stx2 eae-γ1 ehxA (13 isolates) was the most common followed by O157:H7 stx2 eae-γ1 ehxA (11 isolates), O26:H11 stx1 eae-β1 ehxA (11 isolates), and O111:H- stx1 stx2 eae-γ2 ehxA (4 isolates) (Table 4).
TABLE 4.
Seropathotypes (serotypes and virulence genes) of human STEC isolates (n = 126)
| Serotypea | Total no. of isolates | stx1 | stx2 | eae | ehxA |
|---|---|---|---|---|---|
| O1:H7 | 1 | − | + | − | − |
| O1:H7 | 1 | + | + | − | + |
| O1:H- | 1 | + | + | + (−)b | + |
| O2:H11 | 1 | − | + | − | − |
| O2:H27 | 1 | − | + | − | − |
| O2:H- | 1 | + | − | − | − |
| O6:H49 | 1 | − | + | − | + |
| O8:H11 | 1 | + | − | − | − |
| O8:H19 | 1 | − | + | − | + |
| O9:H21 | 2 | − | + | − | − |
| O20:H19 | 1 | + | + | − | − |
| O26:H11 | 11 | + | − | + (β1) | + |
| O26:H11 | 1 | + | − | + (β1) | − |
| O26:H11 | 1 | − | + | + (β1) | + |
| O26:H? | 1 | + | − | + (β1) | + |
| O26:H- | 1 | + | − | + (β1) | + |
| O41:H2 | 1 | − | + | + (−) | + |
| O46:H31 | 1 | − | + | − | − |
| O64:H25 | 1 | + | − | + (NR)c | + |
| O69:H11 | 1 | + | − | + (β1) | + |
| O75:H8 | 1 | + | + | − | + |
| O76:H19 | 1 | + | − | − | + |
| O77:H41 | 1 | + | + | + (−) | + |
| O77:H41 | 1 | − | + | − | + |
| O79:H14 | 1 | + | − | − | − |
| O81:H? | 1 | + | − | − | + |
| O83:H- | 1 | − | + | − | − |
| O84:H? | 1 | + | − | + (ζ) | + |
| O84:H2 | 1 | + | − | + (ζ) | + |
| O91:H21 | 1 | + | + | − | + |
| O91:H- | 2 | + | − | − | + |
| O91:H- | 1 | + | + | − | + |
| O98:H- | 2 | + | − | + (ζ) | + |
| O103:H2 | 2 | + | − | + (ɛ) | + |
| O103:H21 | 1 | − | + | − | − |
| O110:H28 | 1 | + | + | − | − |
| O111:H- | 1 | + | − | + (−) | − |
| O111:H- | 4 | + | + | + (γ2) | + |
| O113:H4 | 1 | − | + | − | + |
| O113:H4 | 1 | − | + | − | − |
| O113:H21 | 1 | − | + | − | + |
| O113:H21 | 2 | − | + | − | − |
| O116:H- | 1 | − | + | − | − |
| O116:H4 | 1 | + | + | − | − |
| O117:H28 | 1 | + | + | − | − |
| O117:H- | 1 | + | + | − | − |
| O118:H16 | 2 | + | − | + (β1) | + |
| O123:H19 | 1 | + | − | − | − |
| O128:H2 | 1 | + | + | + (−) | + |
| O141:H2 | 1 | + | + | − | + |
| O145:H8 | 1 | − | + | + (γ1) | + |
| O145:H- | 1 | − | + | + (γ1) | + |
| O145:H- | 1 | + | − | + (γ1) | + |
| O146:H21 | 1 | − | + | − | − |
| O146:H21 | 2 | + | + | − | + |
| O150:H- | 2 | + | − | + (ζ) | + |
| O153:H21 | 1 | + | − | − | − |
| O153:H33 | 1 | + | − | − | − |
| O153:H- | 1 | + | − | − | − |
| O156:H4 | 1 | − | + | − | − |
| O156:H- | 1 | + | − | + (ζ) | + |
| O157:H7 | 11 | − | + | + (γ1) | + |
| O157:H7 | 13 | + | + | + (γ1) | + |
| O162:H4 | 1 | − | + | + (−) | + |
| O166:H28 | 2 | − | + | − | − |
| O172:H- | 1 | − | + | + (ɛ) | + |
| O174:H- | 2 | − | + | − | − |
| OX177:H- | 1 | − | + | + (β1) | + |
| ONT:H4 | 2 | + | + | − | − |
| ONT:H8 | 1 | + | − | − | + |
| ONT:H8 | 1 | − | + | − | − |
| ONT:H10 | 1 | + | + | − | − |
| ONT:H19 | 1 | − | + | − | + |
| ONT:H21 | 1 | + | − | + (ζ) | − |
| ONT:H28 | 1 | − | + | − | − |
| ONT:H41 | 1 | − | + | − | + |
| ONT:H? | 1 | + | − | + (β1) | + |
| ONT:H- | 2 | + | + | − | + |
| ONT:H- | 1 | + | + | + (γ2) | + |
Names of serotypes previously associated with human STEC causing HUS are in boldface characters; names of new serotypes not previously found among human STEC isolates are underlined.
Isolates that were negative for the eae gene [(−)].
Typing of eae (intimin) genes was realized years after the isolation of the strains. Some of them had lost the viability, so eae typing could not be realized [(NR)].
In six stool specimens and in two samples, STEC strains belonging to two and three different seropathotypes, respectively, were isolated.
Typing of eae (intimin) genes.
A total of 27 isolates of serotypes O157:H7 (24 strains), O145:H8 (1 strain), and O145:H- (2 strains) possessed intimin type γ1, 20 isolates of serotypes O26:H11 (13 strains), O26:H? (1 strain), O26:H- (1 strain), O69:H11 (1 strain), O118:H16 (2 strains), OX177:H- (1 strain), and ONT:H? (1 strain) showed intimin type β1, 8 isolates of serotypes O84:H? (1 strain), O84:H- (1 strain), O98:H- (2 strains), O150:H- (2 strains), O156:H- (1 strain), and ONT:H21 (1 strain) showed intimin type ζ, 5 isolates of serotypes O111:H- (4 strains) and ONT:H- (1 strain) showed intimin type γ2, and 3 isolates of serotypes O103:H2 (2 strains) and O172:H- (1 strain) possessed intimin type ɛ (Table 4).
DISCUSSION
Our findings suggest that STEC strains are a significant cause of human infections in Spain and confirm that in continental Europe, infections by non-O157 STEC strains are more common than those caused by O157:H7 strains [12; Fisher et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P214]. Non-O157 STEC strains were isolated in Germany with higher frequency than O157:H7 strains, according to the findings of Gunzer et al. (20) (7% versus 3%), Karch et al. (24) (1% versus 0.4%) and Pulz (42) (2% versus 0.4%). Similar results were found by Pradel et al. (40) (3% versus 0%) in France, Burnens et al. (11) (1% versus 0%) in Switzerland, and Piérard et al. (39) (0.7% versus 0.2%) in Belgium. In Denmark, non-O157 STEC strains were found three times more often than O157:H7 strains [Scheutz et al., 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. 207]. In Italy between 1988 and 2000, 98 cases of STEC O157:H7 infection versus 147 cases of non-O157 infection were identified (A. Caprioli, S. Morabito, F. Minelli, M. L. Marziano, S. Gorietti, T. Pichiorri, and A. E. Tozzi, Notiziario dell'Istituto Superiore di Sanitá. V. 14:S1, 2001). Non-O157 STEC may also play a more important role in disease than STEC O157:H7 in Argentina, Australia, Chile, and South Africa (14, 26). In Canada, the United States, Japan, England, and Scotland, in contrast, the prevalence of non-O157 is very low (much lower than that of STEC O157:H7) [28, 33, 48, 50; Fisher et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P214, and J. Yatsuyanagi, S. Saito, I. Itoh, H. Sato, and Y. Miyajima, Abstr. 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Kyoto, Japan, abstr. 233, p. 111, 2000]. We do not know whether the low prevalence of non-O157 in these countries is due to a lack of consistent testing. However, recent reports indicate that the prevalence of STEC strains in the United States is 1.3 to 4.0%, with non-O157 strains accounting for 25 to 63% of human isolates [10, 17, 21, 31; D. Acheson, T. Ngo, and R. Chitrakar, Abstr. 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. 225, p. 107, Kyoto, Japan, 2000, and E. J. Klein, J. R. Stapp, D. R. Boster, C. R. Clausen, X. Quin, J. Wells, D. L. Swerdlow, and P. I. Tarr, Abstr. 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Kyoto, Japan, abstr. 257, p. 123, 2000]. Thus, the prevalence of non-O157 STEC in the United States is much greater than was suspected. Interestingly, in January 2002, the American Society for Microbiology Public and Scientific Affairs Board Committee on Agriculture and Food Microbiology submitted a recommendation to the U.S. Department of Agriculture Food Safety and Inspection Service (FSIS) to include testing for non-O157 STEC (34).
Serotypes found in Spain were similar to those found in several other countries. Thus, serotype O26:H11 was the most common type found among human non-O157 STEC strains isolated in Spain. This serotype was the first or second (after O103:H2) most common non-O157 serotype detected in Germany [3, 24; Beutin et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P192], Belgium (39), Denmark [Scheutz et al., 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. 207], Finland (15), Canada (28, 33), the United States [17, 34; Acheson et al., 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. 225], and Japan [Kai et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P242, and Yatsuyanagi et al., 4th Int. Symp. Workshop Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. 233]. The present study revealed a wide variety of non-O157 STEC serotypes not previously isolated in cases of human infection. Thus, our results contribute to an increase in knowledge in the epidemiological research involving STEC in Spain and worldwide and to more efficient detection of these infections.
An important change in STEC detection and isolation procedures occurred after 1994, when more sensitive methods such as enrichment culture in BPWvcc, immunomagnetic separation, culture on CTSMAC, and detection of virulence genes by PCR were introduced. This increase in sensitivity was confirmed with an increase in detection (1.0% in 1992 to 1994 versus 2.9% in 1995 to 1999) of STEC. Furthermore, all 24 STEC O157:H7 strains isolated in this study were recovered after 1994.
As in other studies in which the PCR was not applied on pure bacteria, we failed to isolate colonies of STEC in some of the PCR-positive samples (13%). Piérard et al. (39), using a protocol similar to ours, failed to isolate an STEC colony in 19% of PCR-positive stools. The probable explanation for this phenomenon is the high sensitivity of the PCR technique, which returns a positive result when a STEC colony is mixed with commensal E. coli in a proportion even as low as 10−4 to 10−8 (39).
SMAC, containing 1% sorbitol instead of 1% lactose, uses the inability of STEC O157:H7 to ferment sorbitol to improve detection. Generally, the use of SMAC is perceived as the “gold standard” for the isolation of STEC O157:H7, although our results as well as other findings prove otherwise (13, 34). Visible sorbitol-negative colonies do not always prevail over the sorbitol-positive enteric floras (Fig. 1). The presence of only a few colonies is often obscured by the overwhelming number of sorbitol fermenters. During this study, 5 of 24 STEC O157:H7-positive specimens did not exhibit sorbitol-negative colonies on SMAC agar; however, the isolation of STEC O157:H7 colonies by culture on CTSMAC medium was possible. Thus, our results confirm that the recovery of STEC O157:H7 can be improved by the addition of cefixime and tellurite to SMAC agar (13). However, the growth of some STEC O157:H7 strains on CTSMAC agar is inhibited by tellurite sensitivity. Therefore, the use of a second isolation medium such as SMAC agar is recommended. All 24 STEC O157:H7 strains isolated in this study were negative for sorbitol fermentation and were well recovered on CTSMAC medium. Like Fukushima et al. (18), we found that CTSMAC medium is also very useful for selective isolation of non-O157 STEC eae-positive strains.
In routine diagnosis, there is no definitive biochemical characteristic, such as sorbitol fermentation, which would distinguish non-O157 STEC isolates from commensal E. coli members of the floras. However, all O157:H7 strains and a significant proportion of non-O157 strains have been observed to produce enterohemolysin (Ehly), a putative virulence factor that facilitates the detection of STEC isolates through the use of enterohemolysin agar (4). In our study, 70% of STEC isolates were positive for an ehxA gene that encodes production of Ehly. The ehxA gene also seems to be associated with carriage of the eae gene (15, 37); isolates possessing the eae gene were statistically more likely to be positive for the ehxA gene than those which did not carry this gene (92 and 35%, respectively, were positive for the ehxA gene; P < 0.05).
The eae gene, which has been shown to be necessary for attaching and effacing activity, encodes a 94- to 97-kDa outer-membrane protein termed intimin (23). Numerous investigators have underlined the strong association between carriage of the eae gene and the capacity of STEC isolates to cause severe human disease, especially HUS [15, 35; Beutin et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P192]. This important virulence gene was detected in 100% of the STEC O157:H7 and in 45% of the non-O157 human isolates assayed in the present study. Nevertheless, production of intimin is not essential for pathogenesis, as a number of sporadic cases of HUS have been caused by eae-negative non-O157 STEC isolates. Thus, STEC O104:H21 and O113:H21 isolates lacking the eae gene were responsible for an outbreak and a cluster of three HUS cases in the United States and Australia, respectively (35, 37). In the present study, an STEC O20:H19 eae-negative strain was isolated from a patient with HUS. Furthermore, Paton and Paton (36) recently described a novel megaplasmid-encoded adhesin (Saa) which we have detected in some human STEC isolates lacking the eae gene (data not shown). This adhesin might be an important virulence factor of eae-negative STEC isolates capable of causing severe diseases in humans.
Analysis of the nucleotide sequences of the intimin genes from different STEC and enteropathogenic E. coli isolates has shown a high degree of homology in the 5′ two-thirds of the genes and a significant degree of heterogeneity in the 3′ one-third of the genes. A total of 14 variants of the eae gene were identified by intimin type-specific PCR assays that used oligonucleotide primers complementary to the 3′ end of the specific intimin genes that encode the intimin types α1, α2, β1, β2, γ1, γ2/θ, δ/κ, ɛ, ζ, η, ι, λ, μ, and ν (1, 8, 32, 47, 51; Blanco et al., submitted). Like other authors [1, 32; Beutin et al., 5th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., abstr. P192], we found that the intimin types β1 and γ1 are the most frequently detected types in human STEC isolates. The intimin β1 was mainly found among strains belonging to serotype O26:H11, whereas intimin type γ1 was detected in all 24 STEC O157:H7 isolates assayed. The recently described intimin types η, ι, λ, μ, and ν were not detected in human STEC isolated in Spain.
In Spain, as in many other countries, STEC strains have been frequently isolated from cattle (7), sheep (8), and food (6), and they represent a significant cause of sporadic cases of human infection. STEC isolates have caused eight outbreaks in Spain: six caused by serotype O157:H7 (mainly of phage type 2), one by serotype O26:H11, and one by serotype O111:H− (38, 41) (http://www.lugo.usc.es/ecoli/SEROTIPOSOUTBREAKS.htm).The high level of prevalence of STEC (both O157:H7 and non-O157 strains) in human patients in Spain and its association with serious complications, combined with the high level of prevalence in animals and meat products, strongly supports the utilization of protocols for detection of all serotypes of STEC as a routine procedure in Spanish clinical microbiology laboratories. Until now, the detection of non-O157 isolates has not been possible for most clinical laboratories. Nowadays, however, different PCR protocols for the detection of STEC have been published, making diagnosis of non-O157 infections possible (15, 30, 36, 39, 44). In addition, kits designed for Shiga toxin detection by immunological methods (Premier EHEC immunoassay [Meridian Bioscience, Cincinnati, Ohio], ProSpecT Shiga Toxin E. coli Microplate assay [Alexon-Trend, Ramsey, Minn.], VTEC-Screen [Denka Seiken Co., Tokyo, Japan], and VTEC-RPLA [Oxoid Ltd., Basingstoke, Hampshire, United Kingdom]) (4, 16, 26, 44) are now commercially available. Using these PCR or immunologic techniques, it will be possible to better define the role of non-O157 STEC strains as agents of diarrhea, HC, and HUS.
Acknowledgments
We thank Monserrat Lamela for skillful technical assistance.
This work was supported by grants from the Fondo de Investigación Sanitaria (grants FIS G03-025-COLIRED-O157 and FIS 98/1158), the Xunta de Galicia (grant PGIDIT02BTF26101PR), the Comisión Interministerial de Ciencia y Tecnología (grants CICYT-ALI98-0616 and CICYT-FEDER-1FD1997-2181-C02-01), and the European Commission (FAIR programme grants CT98-4093 and CT98-3935). A. Mora and G. Dahbi acknowledge the Xunta de Galicia and the Agencia Española de Cooperación Internacional (AECI) for research fellowships.
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banatvala, N., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, J. G. Wells, and the Hemolytic Uremic Syndrome Study Collaborators. 2001. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J. Infect. Dis. 183:1063-1070. [DOI] [PubMed] [Google Scholar]
- 3.Beutin, L., S. Aleksic, S. Zimmermann, and K. Gleier. 1994. Virulence factors and phenotypical traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med. Microbiol. Immunol. 183:13-21. [DOI] [PubMed] [Google Scholar]
- 4.Beutin, L., S. Zimmermann, and K. Gleier. 1996. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J. Clin. Microbiol. 34:2812-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco, J., M. Blanco, J. E. Blanco, A. Mora, M. P. Alonso, E. A. González, and M. I. Bernárdez. 2001. Epidemiology of verocytotoxigenic Escherichia coli (VTEC) in ruminants, p. 113-148. In G. Duffy, P. Garvey, and D. McDowell (ed.), Verocytotoxigenic Escherichia coli. Food and Nutrition Press Inc., Trumbull, Conn.
- 6.Blanco, J. E., M. Blanco, A. Gutiérrez, C. Prado, M. Rio, L. Fernández, M. J. Fernández, V. Saínz, and J. Blanco. 1996. Detection of enterohaemorrhagic Escherichia coli O157:H7 in ground beef using immunomagnetic separation. Microbiología SEM 12:385-394. [PubMed] [Google Scholar]
- 7.Blanco, M., J. E. Blanco, J. Blanco, E. A. González, A. Mora, C. Prado, L. Fernández, M. Rio, J. Ramos, and M. P. Alonso. 1996. Prevalence and characteristics of Escherichia coli serotype O157:H7 and other verotoxin-producing E. coli in healthy cattle. Epidemiol. Infect. 117:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco, M., J. E. Blanco, A. Mora, J. Rey, J. M. Alonso, M. Hermoso, J. Hermoso, M. P. Alonso, G. Dhabi, E. A. González, M. I. Bernárdez, and J. Blanco. 2003. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from healthy sheep in Spain. J. Clin. Microbiol. 41:1351-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bockemühl, J., S. Aleksic, and H. Karch. 1992. Serological and biochemical properties of Shiga-like toxin (verocytotoxin)-producing strains of Escherichia coli, other than O-group O157, from patients in Germany. Zentralbl. Bakt. 276:189-195. [DOI] [PubMed] [Google Scholar]
- 10.Bokete, T. N., C. M. O′Callahan, C. R. Clausen, N. M. Tang, N. Tran, S. L. Moseley, R. T. Fritsche, and P. I. Tarr. 1993. Shiga-like toxin-producing Escherichia coli in Seattle children: a prospective study. Gastroenterology 105:1724-1731. [DOI] [PubMed] [Google Scholar]
- 11.Burnens, A. P., P. Boss, F. Orskov, I. Orskow, U. B. Schaad, F. Müller, R. Heinzle, and J. Nicolet. 1992. Occurrence and phenotypic properties of verotoxin-producing Escherichia coli in sporadic cases of gastroenteritis. Eur. J. Clin. Microbiol. Infect. Dis. 11:631-634. [DOI] [PubMed] [Google Scholar]
- 12.Caprioli, A., and A. E. Tozzi. 1998. Epidemiology of Shiga toxin-producing Escherichia coli infections in continental Europe, p. 38-48. In J. B. Kaper and A. D. O′Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 13.Chapman, P. A., and A. Siddons. 1996. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from cases of bloody diarrhoea, non-bloody diarrhoea and asymptomatic contacts. J. Med. Microbiol. 44:267-271. [DOI] [PubMed] [Google Scholar]
- 14.Desmarchelier, P. M. 1997. Enterohemorrhagic Escherichia coli—the Australian perspective. J. Food Prot. 60:1447-1450. [DOI] [PubMed] [Google Scholar]
- 15.Eklund, M., F. Scheutz, and A. Siitonen. 2001. Clinical isolates of non-O157 Shiga toxin-producing Escherichia coli: serotypes, virulence characteristics, and molecular profiles of strains of the same serotype. J. Clin. Microbiol. 39:2829-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evangelista, A. T., A. L. Truant, and P. B. Bourbeau. 2002. Rapid systems and instruments for the identification of bacteria. In A. L. Truant (ed.), Manual of commercial methods in clinical microbiology. ASM Press, Washington, D.C.
- 17.Fey, P. D., R. S. Wickert, M. E. Rupp, T. J. Safranek, and S. H. Hinrichs. 2000. Prevalence of non-O157:H7 Shiga toxin-producing Escherichia coli in diarrheal stool samples from Nebraska. Emerg. Infect. Dis. 6:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima, H., K. Hoshina, and M. Gomyoda. 2000. Selective isolation of eae-positive strains of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 38:1684-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinée, P. A. M., W. H. Jansen, T. Wadström, and R. Sellwood. 1981. Escherichia coli associated with neonatal diarrhoea in piglets and calves. Curr. Top. Vet. Anim. Sci. 13:126-162. [Google Scholar]
- 20.Gunzer, F., H. Böhm, H. Rüssmann, M. Bitzan, S. Aleksic, and H. Karch. 1992. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J. Clin. Microbiol. 30:1807-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelacic, J. K., T. Damrow, G. S. Chen, S. Jelacic, M. Bielaszewska, M. Ciol, H. M. Carvalho, A. R. Melton-Celsa, A. D. O′Brien, and P. I. Tarr. 2003. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J. Infect. Dis. 188:719-727. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, R. P., R. C. Clarke, J. B. Wilson, S. Read, K. Rahn, S. A. Renwick, K. A. Sandhu, D. Alves, M. A. Karmali, H. Lior, S. A. Mcewen, J. S. Spika, and C. L. Gyles. 1996. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food Prot. 59:1112-1122. [DOI] [PubMed] [Google Scholar]
- 23.Kaper, J. B., S. Elliott, V. Sperandio, N. T. Perna, G. F. Mayhew, and F. R. Blattner. 1998. Attaching and effacing intestinal histopathology and the locus of enterocyte effacement, p. 163-182. In J. B. Kaper and A. D. O′Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 24.Karch, H., H. Huppertz, J. Bockemühl, H. Schmidt, A. Schwarzkopf, and R. Lissner. 1997. Shiga toxin-producing Escherichia coli infections in Germany. J. Food Prot. 60:1454-1457. [DOI] [PubMed] [Google Scholar]
- 25.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehl, S. C. 2002. Role of the laboratory in the diagnosis of enterohemorrhagic Escherichia coli infections. J. Clin. Microbiol. 40:2711-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keskimäki, M., M. Saari, T. Heiskanen, and A. Siitonen. 1998. Shiga toxin-producing Escherichia coli in Finland from 1990 through 1997: prevalence and characteristics of isolates. J. Clin. Microbiol. 36:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie, A. M. R., P. Lebel, E. Orrbine, P. C. Rowe, L. Hyde, F. Chan, W. Hohnson, P. N. McLaine, and the Synsorb Pk Study Investigators. 1998. Sensitivities and specificities of Premier E. coli O157 and Premier EHEC enzyme immunoassays for diagnosis of infection with verotoxin (Shiga-like toxin)-producing Escherichia coli. J. Clin. Microbiol. 36:1608-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 30.Nielsen, E. M., and M. T. Andersen. 2003. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J. Clin. Microbiol. 41:2884-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novicki, T. J., J. A. Daly, S. L. Mottice, and K. C. Carroll. 2000. Comparison of sorbitol MacConkey agar and a two-step method which utilizes enzyme-linked immunosorbent assay toxin testing and a chromogenic agar to detect and isolate enterohemorrhagic Escherichia coli. J. Clin. Microbiol. 38:547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pai, C. H., N. Ahmed, H. Lior, M. Johnson, H. V. Sims, and D. E. Woods. 1988. Epidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective study. J. Infect. Dis. 157:1054-1057. [DOI] [PubMed] [Google Scholar]
- 34.Parck, C. H., H. J. Kim, and D. L. Hixon. 2002. Importance of testing stool specimens for Shiga toxins. J. Clin. Microbiol. 40:3542-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton, A. W., and J. C. Paton. 2002. Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J. Clin. Microbiol. 40:271-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pebody, R. G., C. Furtado, A. Rojas, N. McCarthy, G. Nylen, P. Ruutu, T. Leino, R. Chalmers, B. de Jong, M. Donnelly, I. Fisher, C. Gilham, L. Graverson, T. Cheasty, G. Willshaw, M. Navarro, R. Salmon, P. Leinikki, P. Wall, and C. Bartlett. 1999. An international outbreak of Vero cytotoxin-producing Escherichia coli O157 infection among tourists; a challenge for the European infectious disease surveillance network. Epidemiol. Infect. 123:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piérard, D., D. Stevens, L. Moriau, H. Lior, and S. Lauwers. 1997. Isolation and virulence factors of Vero cytotoxin-producing Escherichia coli in human stool samples. Clin. Microbiol. Infect. 3:531-540. [DOI] [PubMed] [Google Scholar]
- 40.Pradel, N., V. Livrelli, C. De Champs, J. B. Palcox, A. Reynaud, F. Scheutz, J. Sirot, B. Joly, and C. Forestier. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prats, G., C. Frías, N. Margall, T. Llovet, L. Gaztelurrutia, R. Elcuaz, A. Canut, R. M. Bartolomé, L. Torroba, Y. Dorronsoro, J. Blanco, M. Blanco, N. Rabella, P. Coll, and B. Mirellis. 1996. Colitis hemorrágica por Escherichia coli verotoxigénica. Presentación de 9 casos. Enferm. Infecc. Microbiol. Clin. 14:7-15. [PubMed] [Google Scholar]
- 42.Pulz, M. 1997. Epidemiology and current importance of enterohaemorrhagic Escherichia coli (EHEC) in North Bavaria (1996). Gesundheitswesen 59:656-660. [PubMed] [Google Scholar]
- 43.Ramotar, K., E. Henderson, R. Szumski, and T. J. Louie. 1995. Impact of free verotoxin testing on epidemiology of diarrhea caused by verotoxin-producing Escherichia coli. J. Clin. Microbiol. 33:1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheutz, F., L. Beutin, and H. R. Smith. 2001. Clinical detection of verocytotoxin-producing E. coli (VTEC), p. 25-56. In G. Duffy, P. Garvey, and D. McDowell (ed.), Verocytotoxigenic Escherichia coli. Food and Nutrition Press Inc., Trumbull, Conn.
- 45.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 47.Tarr, C. L., and S. Whittam. 2002. Molecular evolution of the intimin gene in O111 clones of pathogenic Escherichia coli. J. Bacteriol. 184:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarr, P. I., T. E. Besser, D. D. Hancock, W. E. Keene, and M. Goldoft. 1997. Verotoxigenic Escherichia coli infection: United States overview. J. Food Prot. 60:1466-1471. [DOI] [PubMed] [Google Scholar]
- 49.Todd, W. T. A., and S. Dundas. 2001. The management of VTEC O157 infection. Int. J. Food Microbiol. 66:103-110. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, J. B., R. P. Johnson, R. C. Clarke, K. Rahn, S. A. Renwick, D. Alves, M. A. Karmali, P. Michel, E. Orrbine, and J. S. Spika. 1997. Canadian perspectives on verocytotoxin-producing Escherichia coli infection. J. Food Prot. 60:1451-1453. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, W. L., B. Köhler, E. Oswald, L. Beutin, H. Karch, S. Morabito, A. Caprioli, S. Suerbaum, and H. Schmidt. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J. Clin. Microbiol. 40:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]