Abstract
The curative potential of MHC-matched allogeneic bone marrow transplantation (BMT) is in part because of immunologic graft-versus-tumor (GvT) reactions mediated by donor T cells that recognize host minor histocompatibility antigens. Immunization with leukemia-associated antigens, such as Wilms Tumor 1 (WT1) peptides, induces a T-cell population that is tumor antigen specific. We determined whether allogeneic BMT combined with immunotherapy using WT1 peptide vaccination of donors induced more potent antitumor activity than either therapy alone. WT1 peptide vaccinations of healthy donor mice induced CD8+ T cells that were specifically reactive to WT1-expressing FBL3 leukemia cells. We found that peptide immunization was effective as a prophylactic vaccination before tumor challenge, yet was ineffective as a therapeutic vaccination in tumor-bearing mice. BMT from vaccinated healthy MHC-matched donors, but not syngeneic donors, into recipient tumor-bearing mice was effective as a therapeutic maneuver and resulted in eradication of FBL3 leukemia. The transfer of total CD8+ T cells from immunized donors was more effective than the transfer of WT1-tetramer+CD8+ T cells and both required CD4+ T-cell help for maximal antitumor activity. These findings show that WT1 peptide vaccination of donor mice can dramatically enhance GvT activity after MHC-matched allogeneic BMT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) can be curative for patients with high risk leukemia and other hemato-lymphoid malignancies.1 The curative potential is in part because of immunologic graft-versus-tumor (GvT) reactions mediated by T cells contained in the donor graft.2,3 Several lines of clinical evidence have validated the importance of GvT reactions. There were significantly higher relapse rates in acute and chronic myeloid leukemia patients who received syngeneic (identical twin) or T-cell depleted (TCD) grafts compared with recipients who received T-cell replete allografts from human leukocyte antigen (HLA)–matched donors.4 In transplant recipients who had leukemia relapse, the infusion of donor lymphocytes induced sustained complete remissions, including molecular remissions in some patients.5,6 The effector T-cell populations that mediate GvT reactions and their target antigens remain relatively poorly defined. After HLA-matched allogeneic HCT, GvT reactions are predominantly mediated by the donor T cells that recognize host minor histocompatibility antigens (mHAgs).7–9 Donor CD8+ and CD4+ T-cell clones that are cytotoxic for target cells expressing recipient mHAgs presented by major histocompatibility complex (MHC) class I and class II molecules, respectively, can be isolated from recipients of T-cell replete grafts.10 Despite the potential for donor T-cell mediated GvT reactions, the main reason for an unsuccessful outcome after allogeneic HCT remains disease relapse.
One obvious strategy to enhance GvT reactions would be to generate cytotoxic T lymphocytes (CTLs) against tumor antigens by immunizing the donor before the graft harvest. The use of recipient derived “whole” tumor cell vaccines produced curative GvT reactions in several different strain combinations of mouse models of bone marrow transplantation (BMT), yet it also resulted in unacceptable acute graft-versus-host disease (GVHD).11 The increased GVHD was attributed to the presence of immunodominant mHAgs on the “whole” tumor cell vaccine.11,12 In these studies, however, some donor T-cell clones that mediated GvT activity were identified as tumor specific and distinct from those that mediated lethal GVHD.8,11–13 Thus, in theory, if donors could be immunized against a tumor-associated antigen (TAA) without simultaneously being immunized against mHAgs, it is conceivable that such a vaccine could potentiate the GvT reactions without aggravating GVHD.14
The Wilms tumor gene, WT1, encodes a TAA expressed at high levels in most human myeloid malignancies and in some murine leukemia cell lines.15–17 There is conclusive evidence that “processed” peptides derived from endogenous WT1 protein are presented in the context of MHC class I epitopes on leukemic blasts, and are immunogenic.18 Three 9-mer WT1 peptides (WT126, WT221, and WT235), which contain H-2Db binding anchor motifs were tested in mice (C57BL/6, H-2Db) for in vivo induction of CTLs directed against these WT1 peptides.19 Only one peptide, WT126, with the highest binding affinity for H-2Db molecules induced vigorous CTL responses. The CTLs specifically lysed not only WT126-pulsed target cells dependent on WT126 concentration, but also WT1-expressing FBL3 tumor cells in an H-2Db-restricted manner.19 Recent clinical studies showed that vaccination of patients with leukemia with the HLA-A*0201-restricted WT126 epitope induced leukemia-reactive CD8+ T cells that can contribute to leukemia control.20
In the current study, we characterized the T-cell immune response after WT126 peptide immunization of normal C57BL/6 (H-2b) mice to determine whether vaccination was protective against syngeneic FBL3 leukemia tumor challenge and was therapeutic in FBL3 leukemia-bearing C57BL/6 mice. We confirmed that peptide immunization was effective as a prophylactic vaccination before tumor challenge, yet was ineffective as a therapeutic vaccine in tumor-bearing mice. In other experiments, we used LP/J (H-2b) and C3H.SW (H-2b) donors of bone marrow transplants (BMT) with C57BL/6 recipients to determine whether the GvT reactions after MHC-matched transplantation were enhanced by the addition of WT126 peptide donor immunization before graft harvest. The transfer of splenic lymphocytes from peptide vaccinated donors into tumor-bearing recipient mice resulted in eradication of tumor in the majority of mice. The transfer of total CD8+ T cells from immunized donors required CD4+ T-cell help for maximal antitumor activity.
Methods
Animals
Albino Thy 1.2 C57BL/6 (H-2b), wild-type C3H.SW (H-2b), C57BL/6 (H-2b), and LP/J (H-2b) male mice, 6 to 10 weeks old were purchased from the breeding facility of the Department of Comparative Medicine, Stanford University or The Jackson Laboratory. All mice were housed in the Stanford University Medical Center Animal Facility. All experiments were approved by the Stanford Administrative Panel on Laboratory Animal Care and conducted in accordance with Stanford University Animal Facility and National Institutes of Health guidelines.
Cell lines
FBL3 is a Friend leukemia virus-induced erythroleukemia cell line of C57BL/6 (H-2Db) origin and was provided by Dr B. Chesebro (National Institute of Allergy and Infectious Diseases). Luciferase labeled FBL-3 cells were transduced with the GFP-firefly luciferase fusion (GLF) gene that was subcloned from pJW.GFP-yLuc (kindly provided by Dr M. H. Bachmann) into pHR2 to generate pHR2-GLF. Lentiviral particles expressing GLF were prepared as previously described.21 FBL3 is a WT1-naturally expressing cell line that is highly metastatic, and intravenous injection of at least 1 × 105 FBL3 cells into unirradiated mice is uniformly lethal within 4 weeks. H11, a C57BL/6 origin, pre-B cell lymphoma used as a control tumor was generated by infection of primary bone marrow cells with the retrovirus vector MSCV-neo/p190Bcr-Abl22 (provided by Dr M. Cleary, Stanford School of Medicine). Both FBL3 and H11 express MHC class I but not MHC class II receptors.
Antibodies for flow cytometry
Unconjugated anti-CD16/32 (2.4G2), anti-CD8 phycoerythrin (PE;53-6.7), anti-TCRβ allophycocyanin (APC;H57-597), anti-CD62L fluorescein isothiocyanate (FITC;Mel-14), anti-CD44 PE (IM7), anti-CD44 FITC (IM7), anti-Ly 9.1 APC (30C7), anti-IFNγ PE (XMG1.2), and anti-B220 Pacific Blue (RA36B2) monoclonal antibodies (mAbs) were purchased from BD Biosciences. Anti-CD8 Alexa 700 (53-6.7) was obtained from Biolegend. WT1-tetramer (RMFPNAPYL-H-2Db) PE and WT1-tetramer (RMFPNAPYL-H-2Db) APC were obtained from the National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA) as well as Dr M. Davis (Stanford School of Medicine). Staining, flow cytometric analysis, sorting, and analysis using Cytobank (http://www.cytobank.org) have been previously described in detail.23
Cell preparations
The preparation of TCD bone marrow (BM) cells has been previously reported.24 T-cell contamination of TCD-BM was < 0.1%. Single spleen cell suspensions were enriched for CD4, CD8, or total T cells with magnetic microbead-based isolation kits using the AUTOMACS system (Miltenyi Biotech). Enriched cell subsets were ≥ 95% pure as judged by re-analysis of sorted cells. To monitor percent WT1-tetramer+ cells and donor chimerism, peripheral blood lymphocytes (PBL) were stained and analyzed by flow cytometry. To determine intracellular IFN-γ production, PBLs were incubated for 24 hours with 5 × 105 irradiated FBL3 or H11 tumor cells at a 1:1 cell ratio and monensin (BD Biosciences) was added for the last 8 hours. The percentage of gated CD8+ T-cell staining positively for CD44 and IFN-γ was determined using the BD CytoFix/CytoPerm Plus Kit (BD Biosciences), and analyzed by flow cytometry.
WT1 peptide vaccine
The previously described WT1-126 peptide (amino acids 126-134 RMFPNAPYL derived from WT1 protein), is an MHC class I (H-2Db)-binding peptide.25 WT1-126 was synthesized and HPLC purified by the Stanford Protein and Nucleic Acid Core Facility. WT1-126 peptide (100 μg) was dissolved in 100uL PBS and aliquots were stored frozen at 20°C. The WT1 peptide vaccine consisted of emulsions of 100ug of WT1-126 peptide in PBS combined with 100uL of Incomplete Freund's Adjuvant (IFA, F5506, Sigma-Aldrich) at a 1:1 volume ratio and was prepared by 15-minute vortex before subcutaneous (s.c.) abdominal injection. The control vaccine consisted of 100uL PBS without peptide combined with 100uL of IFA at a 1:1 volume, prepared by 15-minute vortex before s.c. injection. Animals received 1-4 weekly s.c. abdominal injections of the WT1 peptide vaccine or control vaccine.
FBL3 tumor inoculation and bone marrow transplant model
FBL3 cells (1 × 105) were injected intravenously by retro-orbital route into 6–10 week old, C57BL/6, LP/J, or C3H.SW mice to assess immune responses and protection against tumor growth. For the BMT experiments, 6–10 week old recipient C57BL/6 mice received lethal irradiation with 950 cGy of total body irradiation (TBI) using a Philips x-ray unit (250 kV, 15 mA) divided in two fractions, separated by 6 hours. Irradiated mice were injected intravenously with 5 × 102 FBL3 cells 6 hours after the second dose of irradiation. On the day of BMT (24 hours after FBL3 tumor inoculation) 5 × 106 TCD BM cells from IFA vaccinated donor mice and 2 × 107 splenocytes from WT1 vaccinated or IFA control vaccinated donor mice were injected IV together into the tail vein. In some experiments recipient mice received TCD BM with 2 × 106 CD8+ T cells with or without 6 × 106 CD4+ T cells. GVHD was documented as previously described.24 The MHC minor-mismatch strain combinations with vaccinated and unvaccinated donors used in the BMT experiments result in GVHD characterized by weight loss, skin changes, and mortality developing beyond 60 days after transplantation (unvaccinated donors: supplemental Figure 1A-B, vaccinated donors: supplemental Figure 1C-D, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).26–29 Although vaccinated versus unvaccinated donors were not compared in the same experiment, the weight loss and mortality observed were similar. Accordingly, analysis of tumor growth was limited to the first 50 days after transplantation. For these experiments the two minor-mismatch strain combinations, LP/J→C57BL/6 and C3H.SW→C57BL/6, were selected because GVHD in the former is CD4-dependent and CD8-dependent in the latter.26–29
Assessment of graft-versus leukemia and in vivo bioluminescence imaging
In vivo bioluminescence imaging of surviving mice at each time point was performed according to Edinger et al.21 Briefly, mice received an intraperitoneal injection with luciferin (375mg/kg body weight).30 Ten minutes later, mice were imaged using the Xenogen In Vivo Imaging System (IVIS) 200 (Caliper LifeSciences). Luciferase image analysis was performed using Living Image 3.0 (Caliper LifeSciences). Luciferase light units were quantified in average radiance per region of interest (photons emitted/whole mouse/second).
Statistical analysis
Overall survival curves according to the Kaplan-Meier method were constructed, and the log-rank test was used to determine statistical differences in animal survival. Prism software was used to analyze luciferase light units, and determine statistical significance of differences between groups by applying an unpaired Student t test. Differences in mean IFN-γ cytokine production of replicate in vitro assays were analyzed using the 2-tailed Student t test. For all tests, P values < .05 were considered significant.
Results
WT1 peptide immunization before but not after FBL3 tumor inoculation protects against tumor growth
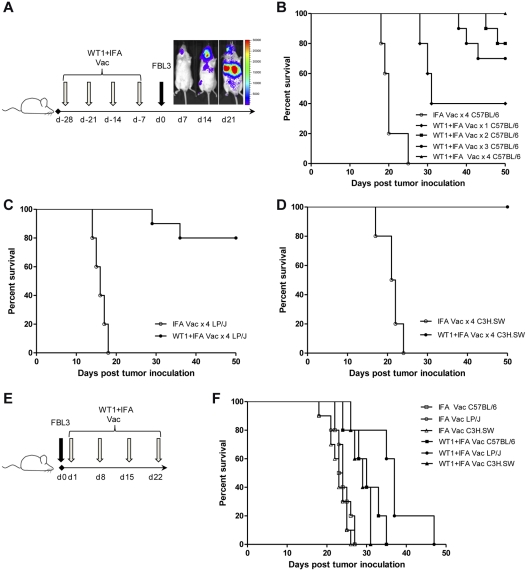
We first investigated if WT1 peptide vaccination administered before FBL3 tumor inoculation could protect against the growth of leukemia in normal C57BL/6, C3H.SW, and LP/J mice (Figure 1A). Figure 1B shows that all C57BL/6 mice that received control immunizations containing IFA without WT1 peptide, died from tumor progression by day 25 after FBL3 challenge, and BLI confirmed this was associated with the presence of disseminated tumor. In contrast, survival of prophylactically vaccinated, FBL3-challenged mice was significantly improved after 1 to 4 weekly WT1 peptide vaccinations before FBL3 tumor inoculation; all mice in the group that received 4 weekly WT1 peptide vaccinations before FBL3 tumor challenge survived beyond 50 days (P < .001 vs IFA injected group), between 75% and 80% of mice survived if 2 or 3 WT1 vaccinations preceded FBL3 tumor challenge (P < .001), and 40% of mice survived if one WT1 vaccination preceded FBL3 tumor challenge (P = .002; Figure 1B). The evaluation of 2 other murine strains with the same MHC class I type, (H-2Db), LP/J, and C3H.SW, confirmed protection of all prophylactically vaccinated FBL3-challenged mice. Survival was significantly improved after 4 weekly prophylactic WT1 peptide vaccinations before FBL3 tumor challenge compared with mice that received control IFA vaccination (P < .001; Figure 1C-D).
Figure 1.
WT1 peptide vaccination is protective but not therapeutic against tumor challenge. (A) Prophylactic vaccination scheme. One to 4 subcutaneous vaccinations of 100 ug of WT1 peptide and 100 uL of Incomplete Freund's Adjuvant (IFA) or 100 uL of IFA alone were administered weekly before intravenous tumor challenge with 1 × 105 FBL3 cells on day 0. Representative bioluminescence demonstrates disseminated tumor by 14 days after tumor inoculation in unvaccinated mice. (B) Survival of C57BL/6 mice after tumor challenge and 4 weekly vaccinations with IFA alone (○) compared with 1 (♦, P = .002), 2 (■, P < .001), 3 (●, P < .001), or 4 vaccinations (▴, P < .001) with WT1 peptide and IFA (n = 10 mice per group). (C-D) Survival of LP/J and C3H.SW mice after 4 weekly vaccinations with IFA alone (○), or WT1 peptide and IFA (●) before tumor challenge (C, LP/J P < .001; D, C3H.SW P < .001). (E) Therapeutic vaccination scheme. Subcutaneous vaccinations of 100 ug of WT1 peptide and 100 uL of IFA or 100 uL of IFA alone were administered weekly starting 24 hours after intravenous inoculation of 1 × 105 FBL3 cells on day 0. (F) Survival of C57BL/6, C3H.SW, or LP/J mice after intravenous inoculation with 1 × 105 FBL3 cells on day 0 and vaccination on day 1, 8, 15, and 22 with WT1 peptide and IFA (closed symbols) or with IFA alone (open symbols; C57BL/6, ■ vs □, P < .001; C3H.SW, ▴ vs Δ, P < .001; LP/J, ● vs ○, P < .001). There were 10 mice per group in all experiments with survival monitored at least every other day.
We next determined if 4 therapeutic WT1 peptide vaccinations beginning 24 hours after FBL3 tumor inoculation would also inhibit tumor growth (Figure 1E). Figure 1F shows that among the 3 murine strains, 4 weekly WT1 vaccinations administered after FBL3 tumor inoculation prolonged median survival by only 1 to 2 weeks compared with mice that received control vaccination with IFA alone, and all therapeutically vaccinated mice died by day 50.
WT1 peptide vaccination generates memory CD44hiCD8+ T cells that are WT1-tetramer+ and can produce IFN-γ after tumor cell stimulation
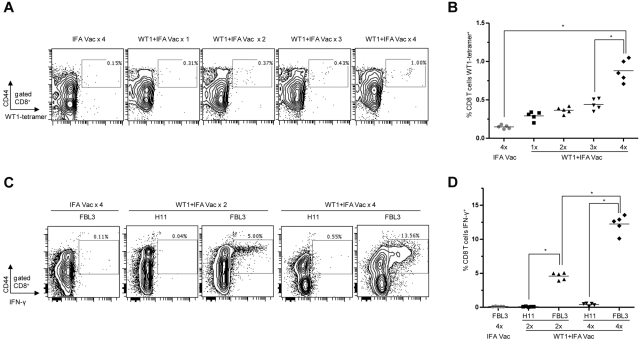
We assessed whether WT1 peptide vaccination induced a WT1 antigen specific CD8+ T-cell response. Figure 2A shows flow cytometry plots staining for CD44 and WT1-tetramer among gated CD8+ T cells obtained from PBLs from C57BL/6 mice after 1-4 weekly WT1 vaccinations compared with 4 weekly control IFA vaccinations. In all cases, PBLs were collected 28 days after the first vaccination. Increased percentages of WT1-tetramer+CD44hi cells were observed among gated CD8+ T cells with each additional WT1 peptide vaccination. After 4 weekly WT1 peptide vaccinations the WT1-tetramer+CD44hi cells rose to a mean of 0.75% among the gated CD8+ T cells in the blood which was significantly increased compared with mice that received fewer peptide vaccinations (P < .01), and compared with vaccination with IFA alone (0.15%; P < .01; Figure 2B).
Figure 2.
WT1 peptide vaccination induces memory CD8+ T cells which are functional and WT1-specific. (A-D) Peripheral blood lymphocytes (PBLs) from C57BL/6 mice were isolated 28 days after the first vaccination. (A) Representative flow cytometry plots show percent of gated CD8 T cells staining positively for CD44 and WT1-tetramer (box) after 1 to 4 vaccinations. Plots are representative of 5 mice per group. (B) Mean percentage of CD8+ WT1-tetramer+ cells among gated CD8+ T cells (*P < .001). (C) Representative flow cytometry plots show percent of gated CD8+ T cells stained positively for CD44 and intracellular IFN-γ (box) after 24-hour coculture with irradiated FBL3 tumor cells or H11 tumor cells (negative control). Plots are representative of 5 mice per group. (D) Mean percentages of CD8+IFN-γ+ T cells among gated CD8+ T cells (*P < .001).
We next determined if the CD8+CD44hi T-cell population was specifically reactive to WT1 expressing tumor cells by stimulating PBLs obtained from WT1 or IFA control vaccinated mice with an equal number of irradiated FBL3 or H11 tumor cells for 24 hours. Figure 2C shows a marked increase from 0.1 to 5 and 13.5% in cells staining positively for CD44 and IFN-γ among gated CD8+ T cells in mice that received 2 and 4 WT1 peptide vaccinations, respectively, after coculture with FBL3 tumor cells compared with mice injected with IFA (P < .01). In contrast, PBLs obtained from similar groups of WT1 vaccinated mice revealed minimal IFN-γ production after incubation with irradiated WT1 nonexpressing, H11 tumor cells (Figure 2C-D). The mean percentages of IFN-γ producing cells were significantly different when FBL3 and H11 stimulations were compared (P < .001; Figure 2D).
Donor WT1 peptide vaccination combined with allogeneic BMT enhances antileukemia activity against the FBL3 tumor
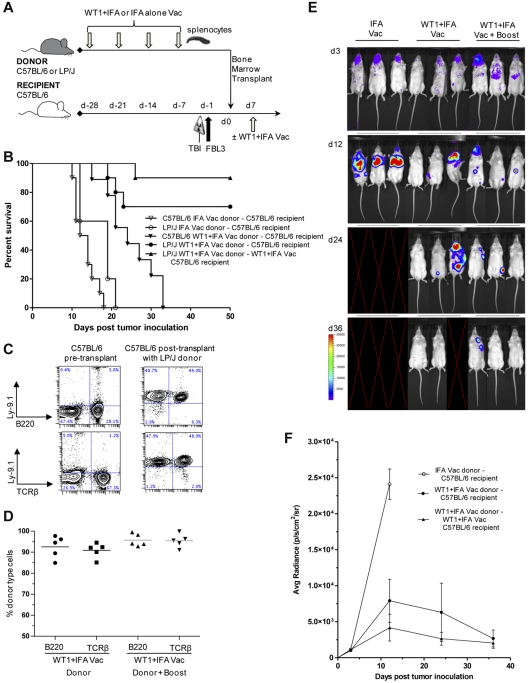
We next assessed whether the T-cell immune response induced by WT1 vaccination could be efficiently transferred into irradiated FBL3 tumor-inoculated recipients and inhibit tumor growth in murine BMT models. For these experiments, 4 weekly WT1 vaccinations were administered to LP/J (minor mismatch) or C57BL/6 (syngeneic) donor mice before the transfer of 5 × 106 TCD BM cells and 2 × 107 splenocytes into lethally irradiated C57BL/6 recipients (Figure 3A). Recipient mice received 5 × 102 FBL3 tumor cells 6 hours after TBI and 24 hours before the IV infusion of the donor inoculum.
Figure 3.
Donor WT1 peptide vaccination combined with allogeneic BMT enhances antileukemic activity against FBL3. (A) Vaccination and bone marrow transplant scheme. 4 subcutaneous vaccinations of 100ug of WT1 peptide and 100uL of IFA or 100uL of IFA alone were administered weekly to C57BL/6 or LP/J donors before isolation and transfer of 2 × 107 splenocytes into lethally irradiated C57BL/6 recipients. All recipients were given also 5 × 106 T-cell depleted bone marrow cells from IFA vaccinated donors. Recipients received 5 × 102 FBL3 tumor cells intravenously 6 hours after lethal radiation and 24 hours before intravenous transfer of donor cells. Seven days after cell transfer one group of recipients received a vaccine “boost” with WT1 peptide and IFA. (B) Survival after vaccination with WT1 peptide and IFA (closed symbols) compared with IFA alone (open symbols) after syngeneic, concomitant observed controls (C57BL/6→C57BL/6, ▾ vs ▿, P < .001) or allogeneic (LP/J→C57BL/6, ● vs ○, P < .001; ▾ vs ●, P < .001) bone marrow and splenocyte transplantation (n = 10 mice per group). Some recipients were given a vaccine boost (LP/J→C57BL/6, ▴ vs ●, P = .239). (C) PBLs were isolated from C57BL/6 controls or 28 days after transplantation of C57BL/6 recipients with LP/J donor cells and assayed for staining for donor marker Ly9.1 versus TCRβ or versus B220. Left panels show representative normal control C57BL/6 mice and right panels show C57BL/6 recipients of LP/J donor transplants. (D) Plots are mean percentages of donor type cells among T cells of 5 mice per group. (E-F) Representative images of 3 mice per group and bioluminescence intensity (mean ± SE for groups starting with 10 mice per group) of albino C57BL/6 recipients at 3, 12, 24, and 36 days after bone marrow and splenocyte transplantation from IFA alone vaccinated (○) or WT1 peptide + IFA vaccinated LP/J donors with (▴) or without vaccine “boost” (●; ○ vs ● at day 12, P < .001). Blank images represent death of recipients.
Syngeneic BMT model.
Figure 3B shows that after transplantation of TCD BM and splenocytes from IFA vaccinated C57BL/6 donors into irradiated FBL3 tumor-inoculated C57BL/6 recipients, all recipient mice died from tumor progression within 3 weeks of transplantation. A survival improvement was observed when cells from WT1 vaccinated C57BL/6 donors were transferred into irradiated FBL3 tumor-inoculated C57BL/6 hosts (P < .001), yet all mice still died from tumor progression by 5 weeks after transplant.
Allogeneic MHC minor-mismatch BMT model.
In contrast to the syngeneic model, the majority (70%) of FBL3 tumor-inoculated C57BL/6 recipient mice survived beyond 50 days after the infusion of TCD BM and splenocytes from WT1 peptide vaccinated LP/J donors (WT1 vs IFA vaccinated, P < .001; Figure 3B). The addition of a single WT1 “booster” vaccination administered to recipients on day 7 after transplantation resulted in 50-day survival rates of 90% (Figure 3B). After transplantation of TCD BM and splenocytes from IFA vaccinated LP/J donors into FBL3 tumor-inoculated C57BL/6 recipients, all mice died from tumor progression within 21 days of transplantation.
Chimerism assessment.
We assessed donor cell chimerism among T and B-cells in the peripheral blood of the surviving C57BL/6 mice (Figure 3C-D) at 4 weeks after allogeneic transplantation. All recipients were predominantly (> 84%) donor type (Ly9.1+) among B220 B-cells and TCRβ+ T cells. The addition of a single recipient “booster” vaccine on day +7 resulted in a nonsignificant trend toward higher levels of B and T-cell donor chimerism.
In vivo tumor BLI.
To account for the differences in animal survival after BMT, we investigated the extent and rapidity of luciferase-labeled FBL3 tumor cell expansion in irradiated recipients (Figure 3E-F). Representative examples of groups are shown in Figure 3E BLI patterns. Irradiated C57BL/6 mice inoculated with luciferase-labeled FBL3 tumor cells and infused 24 hours later with TCD BM and splenocytes from IFA vaccinated allogeneic LP/J donors showed marked tumor expansion by day +12 after transplantation. These recipients died before additional BLI on day +24. In contrast, surviving C57BL/6 mice that received TCD BM and splenocytes from WT1 vaccinated LP/J donors showed significantly less luciferase-labeled FBL3 tumor expansion on day +12 (P < .001; Figure 3E-F). Two of 3 mice showed no tumor signal at day +36 and 1 died before day +36 in examples in Figure 3E. There was a nonsignificant trend toward improved FBL3 tumor cell clearance in mice that received the day 7 after transplantation WT1 “booster” vaccine compared with mice that did not, albeit by day 36 after transplantation both groups showed minimal detection of luciferase-labeled FBL3 tumor cells. Figure 3F compares the mean photon emission from the 3 groups of mice starting with 10 mice per group.
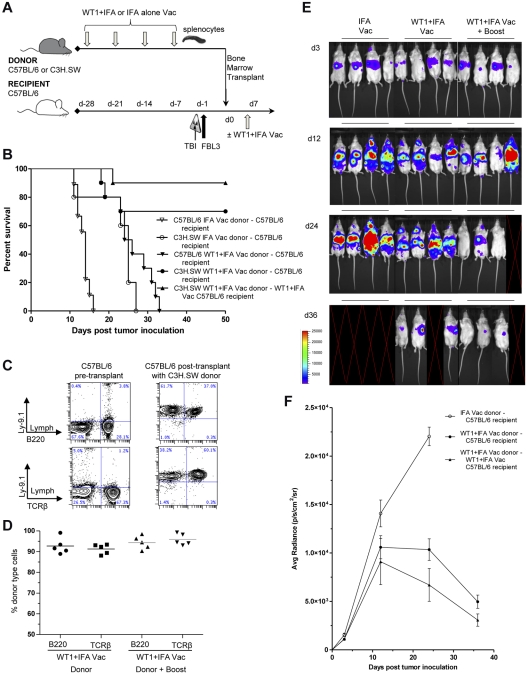
Donor WT1 vaccination protects against FBL3 tumor growth using C3H.SW donors
To test whether strain differences might influence the WT1 peptide-induced enhancement of GvT activity after BMT, another MHC minor-mismatch strain combination was evaluated. In these experiments, the BMT scheme outlined in Figure 3A was followed except C3H.SW instead of LP/J mice in addition to C57BL/6 were used as donors, and recipients were syngeneic C57BL/6 or allogeneic C3H.SW mice (Figure 4A). Similar to the previous BMT strain combinations, tumor-challenged C57BL/6 recipients all died within ∼ 2 weeks of transplantation of TCD BM and splenocytes from IFA vaccinated syngeneic donors (Figure 4B). WT1 vaccination of donors improved survival in the syngeneic C57BL/6 BMT model, yet was not completely protective as all mice in this group died from tumor progression before 5 weeks. However, survival beyond 50 days improved to ∼ 65% of mice when TCD BM and splenocytes from WT1 vaccinated C3H.SW donors were transplanted into irradiated allogeneic FBL3 tumor-inoculated C57BL/6 recipients (P < .001; Figure 4B). There was a trend toward further improved survival after a single WT1 “booster” vaccination, 7 days after transplantation, and this group had survival rates of 90% (Figure 4B). Donor cell chimerism among T and B-cells in the peripheral blood was assessed 28 days after transplantation in the 2 groups of surviving allogeneic C57BL/6 recipients (Figure 4C-D). All recipients were predominantly (> 92%) donor type (Ly9.1+) among B220 B-cells and TCRβ+ T cells.
Figure 4.
Donor WT1 peptide vaccination enhances graft-versus-tumor activity of the minor-mismatch C3H.SW→C57BL/6 bone marrow transplant model. (A) Vaccination and bone marrow transplant scheme was the same as in Figure 3A except that C3H.SW donors were used instead of LP/J donors. (B) Survival after vaccination with WT1 peptide and IFA (closed symbols) compared with IFA alone (open symbols) after syngeneic, concomitant observed controls (C57BL/6→C57BL/6, ▾ vs ▿, P < .001) or allogeneic (C3H.SW→C57BL/6, ● vs ○, P < .001; ▾ vs ●, P < .001) bone marrow and splenocyte transplantation (n = 10 mice per group). Some recipients were given a vaccine boost (C3H.SW→C57BL/6, ▴ vs ●, P = .265). (C) PBLs were isolated from C57BL/6 controls or 28 days after transplantation of C57BL/6 recipients with C3H.SW donor cells and assayed for staining for donor marker Ly9.1 versus TCRβ or versus B220. Left panels show representative normal control C57BL/6 mice and right panels show C57BL/6 recipients of C3H.SW donor transplants. (D) Plots are mean percentages of donor type cells among T cells of 5 mice per group. (E-F) Representative images of 4 mice per group and bioluminescence intensity (mean ± SE for groups starting with 10 mice per group) of albino C57BL/6 recipients at 3, 12, 24, and 36 days after bone marrow and splenocyte transplantation from IFA alone vaccinated (○) or WT1 peptide + IFA vaccinated C3H.SW donors with (▴) or without vaccine “boost” (●; ○ vs ● at day 24, P < .001). Blank images represent death of recipients.
Bioluminescence imaging confirmed that recipients of adjuvant only, or WT1 vaccinated syngeneic donor TCD BM and splenocytes died as a result of progressive leukemia tumor burden (Figure 4E-F). Representative examples of BLI patterns are shown in Figure 4E. Irradiated C57BL/6 mice inoculated with luciferase-labeled FBL3 tumor cells and infused 24 hours later with allogeneic TCD BM and splenocytes from IFA vaccinated C3H.SW donors also showed significant tumor expansion by day +24 after transplantation (Figure 4E-F). In contrast, the 2 groups of surviving C57BL/6 mice that received TCD BM and splenocytes from WT1 vaccinated allogeneic C3H.SW donors showed significantly (P < .001) less luciferase-labeled FBL3 tumor expansion on day +24 (Figure 4E-F). There was a nonsignificant trend toward improved FBL3 tumor cell clearance in mice that received the WT1 “booster” vaccine 7 days after transplantation compared with recipients that did not. By day 36 after transplantation both groups showed minimal detectable luciferase-labeled FBL3 tumor cells.
Optimum antileukemia activity induced by WT1 peptide vaccination requires CD8+ T cells from WT1 peptide vaccinated donors combined with CD4+ T cells
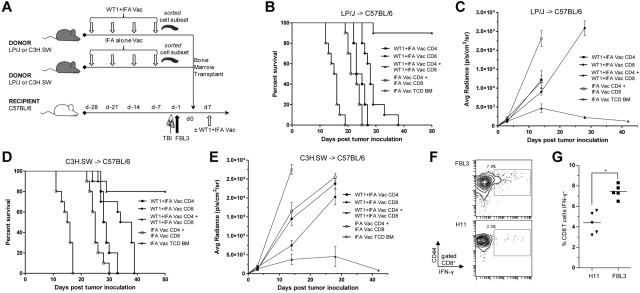
We next investigated the T-cell donor population that transferred antileukemic activity against FBL3 tumor cell growth. Vaccination and BMT scheme was the same as in Figures 3A and 4A. However, on the day of transplant C57BL/6 recipients received 5 × 106 TCD BM cells from IFA adjuvant only or WT1 peptide vaccinated allogeneic donors along with 6 × 106 CD4+ T cells, 2 × 106 CD8+ T cells, or both (Figure 5A). All recipients received a WT1 “booster” vaccination 7 days after BMT.
Figure 5.
Enhanced antileukemia activity induced by WT1 peptide vaccination requires CD8+ T cells from WT1 peptide vaccinated donors combined with CD4+ T cells. (A) Vaccination and bone marrow transplant scheme was the same as in Figures 3A and 4A. (B and D) Survival after allogeneic LP/J→C57BL/6 (B) or C3H.SW→C57BL/6 (D) transplantation (n = 10 mice per group) with 5 × 106 T-cell depleted bone marrow cells (○) from IFA vaccinated donors or with T-cell depleted bone marrow from IFA alone vaccinated donors and combination of 6 × 106 CD4 T cells and 2 × 106 CD8 T cells from IFA alone vaccinated donors (□), 6 × 106 CD4 T cells from WT1 peptide and IFA vaccinated donors (■), 2 × 106 CD8 T cells from WT1 peptide and IFA vaccinated donors (●), or combination of 6 × 106 CD4 T cells and 2 × 106 CD8 T cells from WT1 peptide and IFA vaccinated donors (▴; ● vs ▴, B, P < .001; D, P = .002). (C-E) Bioluminescence intensity (mean ± SE) of only surviving mice at each imaging time point in B and D (● vs ▴, C, P < .001; E, P < .001 at day 28). (F) Representative flow cytometry plots show percent of gated CD8+ T cells stained positively for CD44 and intracellular IFN-γ (box) after 24-hour coculture with irradiated FBL3 tumor cells or H11 tumor cells (negative control). (G) Means are of 5 mice per group (*P < .001). PBLs from recipient C57BL/6 mice in F and G were isolated 28 days after cell transfer of 6 × 106 CD4 T cells and 2 × 106 CD8 T cells from WT1 peptide and IFA-vaccinated donors (group ▴ in Figure 5D).
Figure 5B shows that transplantation of TCD BM combined with either CD8+ T cells or CD4+ T cells from WT1 vaccinated LP/J donors failed to protect allogeneic recipients against FBL3 tumor cell growth, and all recipients died within 38 days of BMT. Recipients given TCD BM alone all died by day +19. Monitoring of FBL3 leukemia tumor burden by BLI confirmed increasing photoemission intensity by day +30 after transplantation indicating leukemia progression was the cause of death for these groups of mice (Figure 5C). In contrast, the transfer of both CD8+ T cells and CD4+ T cells from WT1 vaccinated LP/J donors resulted in 50-day survival rates of ∼ 90% (Figure 5B; CD4+ or CD8+ vs CD4+ and CD8+ P < .001) and was associated with significantly reduced FBL3 tumor burden 28 days after transplantation as monitored by BLI (Figure 5C; P < .001).
To confirm the antitumor activity of the donor inoculum required both CD8+ T cells and CD4+ T cells from WT1 vaccinated donors we validated the experiment using another strain combination. Figure 5D shows that the infusion of CD8+ T cells or CD4+T cells from WT1 vaccinated C3H.SW donors failed to improve survival compared with recipients of IFA vaccinated grafts, and all C57BL/6 recipient mice in these groups died within 38 days of transplantation. Recipients given TCD-BM alone all died by day +18. Bioluminescence imaging confirmed that these groups of mice had significantly (P < .001) increased FBL3 tumor cell expansion 28 days after transplantation (Figure 5E). The infusion of CD8+ T cells with CD4+ T cells from WT1 peptide-vaccinated C3H.SW donors into irradiated C57BL/6 recipients resulted in 80% of mice surviving beyond 50 days (Figure 5D) and BLI confirmed that the surviving mice had minimal bioluminescent evidence of leukemia after approximately 40 days (Figure 5E).
To assess the reactivity and specificity of the chimeric donor T cells toward FBL3 tumor cells, we obtained PBLs from recipient C57BL/6 mice 28 days after allogeneic transplantation of TCD BM combined with 6 × 106 CD4+ T cells and 2 × 106 CD8+ T cells from WT1 vaccinated LP/J donors. Figure 5F and G show the percentage of gated CD8+ T cells stained positively for CD44 and intracellular IFN-γ after 24-hour incubation with irradiated FBL3 stimulators was significantly increased compared with the percentage positive after incubation with irradiated H11 stimulators (P < .001).
WT1-tetramer+ CD8+ T cells from WT1-vaccinated donors provide attenuated antileukemia activity against FBL3, and require CD4+ T cells for activity
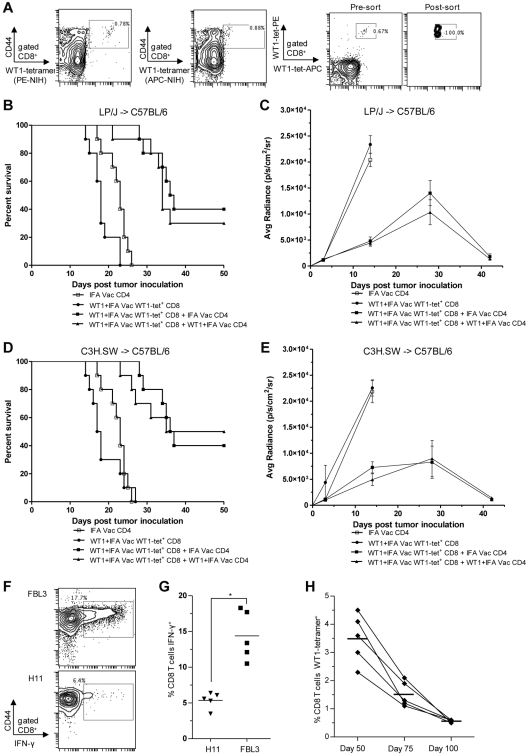
In further experiments, we determined if the sorted WT1-tetramer+CD44hiCD8+ T cells were as effective as total CD8+ T cells at inhibiting FBL3 tumor progression in the BMT models. We sorted 1 × 105 WT1-tetramer+CD44hi CD8+ T cells from WT1-vaccinated LP/J and C3H.SW donors and transferred them into irradiated C57BL/6 mice with 5 × 106 donor TCD BM cells with or without 6 × 106 CD4+ T cells from WT1-immunized and nonimmunized donors. Figure 6A shows the sorting thresholds used to purify WT1-tetramer+ T cells among gated CD8+CD44hi cells. Two tetramers were used for staining, and only cells staining positively for both were included for sorting. Purity of WT1-tetramer+ T cells was > 90%. Figure 6B through D show that in the absence of CD4+ T-cell help, the transfer of the WT1-tetramer+ sorted cell population obtained from WT1-vaccinated donors did not inhibit FBL3 tumor progression in irradiated C57BL/6 recipients as all mice died before day 28, and BLI confirmed abundant tumor growth (Figure 6C-E). The addition of CD4+ T cells from either WT1 peptide or adjuvant alone vaccinated donors to the WT1-tetramer+ sorted cells significantly improved survival (P < .001), and ∼ 30%-50% of recipient mice survived beyond 50 days (Figure 6B-D). Bioluminescence imaging among surviving mice showed clearing of FBL3 tumor among survivors (Figure 6C-E). There were no significant differences in survival when CD4+ T cells were obtained from WT1-vaccinated versus IFA alone vaccinated donor (P > .05).
Figure 6.
WT1-tetramer+ CD8+ T cells from WT1-vaccinated donors provide attenuated antileukemia activity against FBL3, and require CD4+ T cells for potent activity. Donor vaccination scheme was the same as in Figure 5A. (A) Representative analysis of WT-1–specific CD8 T cells from vaccinated donors that were identified and sorted based on 2-color positive staining for 2 WT1-tetramers and for CD44 expression. CD44hiWT1-tetramer+ CD8 T cells were injected with or without CD4 T cells into irradiated C57BL/6 recipients. (B-D) Survival after allogeneic (B) LP/J→C57BL/6 and (D) C3H.SW→C57BL/6 transplantation (n = 10 mice per group) with 6 × 106 CD4 T cells from IFA alone vaccinated donors (□), 1 × 105 WT1-tetramer+ CD8+ T cells from WT1 peptide and IFA vaccinated donors (●), combination of 1 × 105 WT1-tetramer+ CD8 T cells and 6 × 106 CD4 T cells both from WT1 peptide and IFA vaccinated donors (▴; ▴ vs ●, B, P < .001, D, P < .001) and combination of 1 × 105 WT1-tetramer+ CD8 T cells from WT1 peptide and IFA vaccinated donors and 6 × 106 CD4 T cells from IFA alone vaccinated donors (■; ■ vs ●, B, P < .001; D, P < .001). (C-E) Bioluminescence intensity(mean ± SE) of only surviving mice at each imaging time point in panels B and D (■ vs ●, C, P < .001; E, P < .001 at day 14). (F) PBLs from recipient C57BL/6 mice were isolated 28 days after cell transfer of 1 × 105 WT1-tetramer+ CD8+ T cells from WT1 peptide and IFA vaccinated donors and 6 × 106 CD4 T cells from IFA alone vaccinated donors (group ■ in panel D). Representative flow cytometry plots show percent of gated CD8+ T cells staining positively for CD44 and intracellular IFN-γ (box) after 24-hour coculture with irradiated FBL3 tumor cells or H11 tumor cells (negative control). (G) Means are of 5 mice per group (*P < .001). (H) PBLs from 5 recipient C57BL/6 mice from pilot and validation experiments, were isolated 50, 75, and 100 days after cell transfer of 1 × 105 WT1-tetramer+ CD8+ T cells from WT1 peptide and IFA vaccinated donors and 6 × 106 CD4 T cells from IFA alone vaccinated donors (group ■ in Figure 6D), and assayed for percent WT1-tetramer+ CD8+ T cells (means denoted by bars at day 50, 75, and 100 = 3.5%, 1.5%, and 0.56%, respectively).
The fate of the infused donor T-cell inoculum was determined by evaluating PBLs from chimeric C57BL/6 recipients on days 28, 50, 75, and 100 days after transfer of WT1-tetramer+ T cells from vaccinated C3H.SW donors and CD4+ T cells from IFA-vaccinated C3H.SW donors. The chimeric CD8+CD44hi T cells collected 28 days after allogeneic transplantation had significantly increased IFN-γ production (P < .001) in vitro after incubation with irradiated FBL3 versus H11, leukemia stimulators (Figure 6F-G). The percentage WT1-tetramer+ T cells among gated CD8+ T cells in the peripheral blood decreased gradually between days 50, 75 and 100 from a mean percent of 3.5% to 1.5%, and 0.56%, respectively (Figure 6H).
Discussion
Allogeneic HCT from an HLA-matched donor is the treatment of choice for selected patients with high risk or chemotherapy refractory acute myelogenous leukemia because it offers a high potential for cure. A significant contribution to the curative potential is derived from alloreactive donor immune cells in the graft directed against host mHAgs. The goal of the current study was to determine whether allogeneic transplantation combined with immunotherapy using a WT1 peptide vaccination of donors induced more potent antitumor activity than either therapy alone and we tested this concept using several murine models.
In the immunotherapy experiments, nontumor-bearing mice that received 4 weekly prophylactic immunizations with an MHC class-I restricted WT1 peptide resisted challenge with WT1-expressing leukemia cells whereas weekly therapeutic vaccinations administered to mice starting 24 hours after tumor inoculation was ineffective at tumor growth inhibition. The efficacy of the prophylactic immunizations was associated with an immune response recognized by the expansion of a WT1 antigen specific CD44hiCD8+ memory T-cell population that was reactive to FBL3 WT1-expressing tumor cells as judged by IFN-γ secretion. Our findings are consistent with others who showed that prophylactic immunizations with a variety of WT1 vaccination systems induced the expansion of WT1 specific CTLs that were effective at protecting against tumor growth, but were minimally effective as a therapeutic treatment in tumor-bearing animals.19,31 Thus, immunotherapy alone with WT1 peptide vaccination was ineffective in tumor-bearing mice. In trials with patients with leukemia, therapeutic vaccination with tumor-associated antigens, notably WT1 and PR1 peptides, failed to induce significant tumor regression, and fewer than one-half of those with active leukemia at the time of immunization developed an immune response as determined by a doubling of the percentage of tetramer positive T cells in the blood.20,32,33 In contrast, peptide vaccinations of patients with leukemia in complete remission led to the emergence of PR1 or WT1+CD8+ T cells that was associated with a transient reduction in WT1 gene expression, a molecular measure of tumor burden.33 These results suggest that active disease suppresses the response to tumor antigens.
The alloreactivity in the MHC minor-mismatch strain combinations used in the current BMT experiments without WT1 peptide vaccination provided modest antitumor activity, and FBL3 tumor-bearing animals survived on average 1-3 weeks longer than did syngeneic recipients. There were no cures in either allogeneic or syngeneic recipients in the absence of donor vaccination and all animals died from progressive leukemia. The observation that syngeneic BMT provides less GvT activity than allogeneic transplantation was also observed in the clinical setting.4,34 When allogeneic, but not syngeneic donor mouse BMT was combined with immunotherapy using splenocytes from WT1-immunized donors, dramatic antitumor efficacy was observed, and the majority of transplant recipients appeared cured and free of leukemia as judged by BLI. The results indicate that the donor immune response to alloantigens combined with the tumor-specific antigen response provides a synergistic therapy. Another murine model has demonstrated improved alloreactive GvT reactions when immunotherapy directed against a potent transfected immunogen was combined with allogeneic BMT.35,36 Donor immunization with influenza nucleoprotein (NP) provided improved therapeutic activity (20% survival at day 60 after transplantation) against 205-NP, a fibrosarcoma cell line engineered to express influenza NP antigen. In another study,35 immunoglobulin idiotype protein vaccination of donor BALB/c (H-2d) mice to induce antibody responses resulted in a modest effect against the subcutaneous 38C13 lymphoma in C3H (H-2k) BMT recipients, and tumor cures were observed in a minority.35 Idiotype protein vaccination of donor DBA/2 (H-2d) mice, similarly, induced GvT against the HOPC myeloma after BMT using BALB/c (H-2d) recipients, but it is not clear whether GvT was mediated by T cells and/or antibody in this model.37
In the current study, the GvT reactions that resulted in cures required the adoptive transfer of donor CD4+ T cells combined with CD8+ T cells. Transplantation of the CD4+ or CD8+ T cells from WT1-vaccinated donors failed to protect against FBL3 leukemia progression. Whereas it was clearly expected that CD8+ T cells from WT1-immunized donors would be required for GvT reactions, because the peptide binds to MHC class I, it was somewhat unexpected that cooperation with CD4 T-cell help would be a requirement for antitumor reactions. Others have shown that memory CD8+ T cells generated under CD4+ help-dependent or independent conditions required CD4 T-cell help for secondary expansion and persistence after antigen re-exposure.38 CD4+ T cells needed to be included in the transplant to achieve cures and the efficacy of these T cells did not depend on WT1 donor vaccination. This suggests that the CD4+ T cells may have been stimulated by the minor histocompatibility antigens of the host and in turn enhanced the immune potency of the CD8+ T cells. CD4+ T-cell help may contribute to the observed expansion and persistence of the WT1-tetramer+ CD8+ T cells at days 50 through 100.
The infusion of sorted WT1-tetramer+CD44hi CD8+ T cells was less effective than total CD8+ T cells in preventing FBL3 tumor progression in the BMT models, and required CD4+ T-cell help from vaccinated or unvaccinated donors. Although the WT1-tetramer+CD44hi CD8+ T cells may be functionally impaired by the TCR stimulation transiently induced during tetramer staining and sorting, the difference in the GvT activity of WT1-tetramer+CD44hi CD8+ T cells is more likely the result of T-cell reactivity to tumor antigen without the additional alloreactivity contained in total CD8+ T cells. Thus, WT1-tetramer+CD44hi CD8+ T cells would not have been expected to have potent GvT activity without the additional synergistic benefit of total CD8+ T-cell alloreactivity.
GVHD leading to death did occur in both minor-mismatch strain combinations, but did not interfere with the ability to monitor GvT activity within the first 50 days after transplantation. Nonablative conditioning with total lymphoid irradiation and anti-thymocyte serum has been shown to protect against GVHD without interfering with GvT in a major MHC-mismatch model.39 Allogeneic transplantation combined with immunotherapy using a WT1 peptide vaccine will be tested for GvT without GVHD using the latter conditioning regimen in future studies.
In conclusion, tumor peptide immunization without BMT was not effective against established leukemia, and allogeneic BMT without immunotherapy provided only modest GvT activity in the MHC-matched strain combinations that were studied. However, immunization of immunocompetent healthy donor mice provided transferable immunity, which was significantly enhanced by allogeneic, but not syngeneic BMT, and resulted in eradication of tumor in transplant recipients. The results demonstrate a powerful synergy between donor immunization and MHC-matched, BMT. This approach may have value for clinical translation which is currently in development with early phase trials for acute myeloid leukemia and multiple myeloma.40,41
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-58 250, HL-57 743 and CA-49 605) and the Leukemia & Lymphoma Society. M.J.G. is supported by the Howard Hughes Medical Institute Research Training Fellowship. H.E.K. is supported by the American Society of Hematology Research Training Award for Fellows, American Society of Hematology Scholar Award, American Society of Clinical Oncology Young Investigator Award, and Leukemia & Lymphoma Society Special Clinical Fellow Award.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.E.K., A.M.M., M.J.G. and S.D. designed and performed experiments, analyzed data, and wrote the paper; E.N., J.B., and D.C. designed and performed experiments; and S.S. and R.L. designed experiments, reviewed data, and were cosenior authors of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Samuel Strober, MD, Stanford University School of Medicine, CCSR Bldg, Rm 2215-C, 300 Pasteur Dr, Stanford, CA 94305-5166; e-mail: sstrober@stanford.edu.
References
- 1.Lowsky R, Negrin R. Hematopoietic Cell Transplantation. In: Kaushansky K, Lichtman MA, Beutler E, Kipp TJ, Seligsohn U, Prchal JF, editors. William's Hematology. 8th ed. New York, NY: McGraw Hill; 2010. pp. 313–342. [Google Scholar]
- 2.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 3.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 5.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. [PubMed] [Google Scholar]
- 6.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994;330(2):100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 7.Rotzschke O, Falk K, Wallny HJ, Faath S, Rammensee HG. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990;249(4966):283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- 8.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93(7):2336–2341. [PubMed] [Google Scholar]
- 9.Wallny HJ, Rammensee HG. Identification of classical minor histocompatibility antigen as cell-derived peptide. Nature. 1990;343(6255):275–278. doi: 10.1038/343275a0. [DOI] [PubMed] [Google Scholar]
- 10.Warren EH, Greenberg PD, Riddell SR. Cytotoxic T-lymphocyte-defined human minor histocompatibility antigens with a restricted tissue distribution. Blood. 1998;91(6):2197–2207. [PubMed] [Google Scholar]
- 11.Anderson LD, Jr, Petropoulos D, Everse LA, Mullen CA. Enhancement of graft-versus-tumor activity and graft-versus-host disease by pretransplant immunization of allogeneic bone marrow donors with a recipient-derived tumor cell vaccine. Cancer Res. 1999;59(7):1525–1530. [PubMed] [Google Scholar]
- 12.Anderson LD, Jr, Savary CA, Mullen CA. Immunization of allogeneic bone marrow transplant recipients with tumor cell vaccines enhances graft-versus-tumor activity without exacerbating graft-versus-host disease. Blood. 2000;95(7):2426–2433. [PubMed] [Google Scholar]
- 13.Dickinson AM, Wang XN, Sviland L, et al. In situ dissection of the graft-versus-host activities of cytotoxic T cells specific for minor histocompatibility antigens. Nat Med. 2002;8(4):410–414. doi: 10.1038/nm0402-410. [DOI] [PubMed] [Google Scholar]
- 14.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167–177. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84(9):3071–3079. [PubMed] [Google Scholar]
- 16.Miwa H, Beran M, Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992;6(5):405–409. [PubMed] [Google Scholar]
- 17.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96(4):1480–1489. [PubMed] [Google Scholar]
- 18.Oka Y, Tsuboi A, Kawakami M, et al. Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancers. Curr Med Chem. 2006;13(20):2345–2352. doi: 10.2174/092986706777935104. [DOI] [PubMed] [Google Scholar]
- 19.Oka Y, Udaka K, Tsuboi A, et al. Cancer immunotherapy targeting Wilms' tumor gene WT1 product. J Immunol. 2000;164(4):1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 20.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101(2):640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 22.Smith KS, Rhee JW, Cleary ML. Transformation of bone marrow B-cell progenitors by E2a-Hlf requires coexpression of Bcl-2. Mol Cell Biol. 2002;22(21):7678–7687. doi: 10.1128/MCB.22.21.7678-7687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotecha N, Flores NJ, Irish JM, et al. Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell. 2008;14(4):335–343. doi: 10.1016/j.ccr.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179(10):6547–6554. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 25.Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms' tumor gene (WT1) product. Immunogenetics. 2000;51(2):99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 26.Schultz KR, Bader S, Paquet J, Li W. Chloroquine treatment affects T-cell priming to minor histocompatibility antigens and graft-versus-host disease. Blood. 1995;86(11):4344–4352. [PubMed] [Google Scholar]
- 27.Mauch P, Lipton JM, Hamilton BL, Obbagy J, Nathan D, Hellman S. Reduction of lethal graft-versus-host disease: transplantation of cultured murine bone marrow across minor histocompatibility differences. Blood. 1985;66(3):542–547. [PubMed] [Google Scholar]
- 28.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14(suppl 1):129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy GF, Whitaker D, Sprent J, Korngold R. Characterization of target injury of murine acute graft-versus-host disease directed to multiple minor histocompatibility antigens elicited by either CD4+ or CD8+ effector cells. Am J Pathol. 1991;138(4):983–990. [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4(4):297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima H, Kawasaki K, Oka Y, et al. WT1 peptide vaccination combined with BCG-CWS is more efficient for tumor eradication than WT1 peptide vaccination alone. Cancer Immunol Immunother. 2004;53(7):617–624. doi: 10.1007/s00262-003-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qazilbash MH, Wieder ED, Thall PF, et al. PR1 Peptide Vaccine-Induced Immune Response Is Associated with Better Event-Free Survival in Patients with Myeloid Leukemia [abstract]. ASH Annual Meeting Abstracts. 2007;110(11):283. [Google Scholar]
- 33.Rezvani K, Price DA, Brenchley JM, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9(3):245–251. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 34.Fefer A, Sullivan KM, Weiden P, et al. Graft versus leukemia effect in man: the relapse rate of acute leukemia is lower after allogeneic than after syngeneic marrow transplantation. Prog Clin Biol Res. 1987;244:401–408. [PubMed] [Google Scholar]
- 35.Kwak LW, Pennington R, Longo DL. Active immunization of murine allogeneic bone marrow transplant donors with B-cell tumor-derived idiotype: a strategy for enhancing the specific antitumor effect of marrow grafts. Blood. 1996;87(7):3053–3060. [PubMed] [Google Scholar]
- 36.Anderson LD, Jr, Mori S, Mann S, Savary CA, Mullen CA. Pretransplant tumor antigen-specific immunization of allogeneic bone marrow transplant donors enhances graft-versus-tumor activity without exacerbation of graft-versus-host disease. Cancer Res. 2000;60(20):5797–5802. [PubMed] [Google Scholar]
- 37.Zeis M, Steinmann J, Petrela E, Hartung G, Schmitz N, Uharek L. Transfer of idiotypic protein primed allogeneic marrow grafts elicits potent graft-versus-myeloma effects in mice. Bone Marrow Transplant. 2001;27(3):279–285. doi: 10.1038/sj.bmt.1702785. [DOI] [PubMed] [Google Scholar]
- 38.Ryu SJ, Jung KM, Yoo HS, et al. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009;113(18):4273–4280. doi: 10.1182/blood-2008-09-181263. [DOI] [PubMed] [Google Scholar]
- 39.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: The role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9(6):355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 40.Neelapu SS, Munshi NC, Jagannath S, et al. Tumor antigen immunization of sibling stem cell transplant donors in multiple myeloma. Bone Marrow Transplant. 2005;36(4):315–323. doi: 10.1038/sj.bmt.1705057. [DOI] [PubMed] [Google Scholar]
- 41.Bishop MR, Kwak LW, Fowler DH, et al. Sibling donor immunization with patient-derived Id-KLH vaccine before reduced-intensity allogeneic hematopoietic cell transplantation for multiple myeloma [abstract]. J Clin Oncol (Meeting Abstracts) 2009;27(15S):7024. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.