Abstract
Beauveriolides I and III, isolated from the culture broth of fungal Beauveria sp. FO-6979, showed potent inhibitory activity of lipid droplet accumulation in primary mouse peritoneal macrophages. The cellular molecular target of this inhibitory activity was studied in macrophages. Beauveriolides I and III strongly inhibited the cholesteryl ester (CE) synthesis with IC50 values of 0.78 and 0.41 μM, respectively, without showing significant effects on the triacylglycerol and phospholipid synthesis. Furthermore, lysosomal cholesterol metabolism to CE in macrophages was inhibited by the compounds, indicating that the inhibition site lies within steps between cholesterol departure from the lysosome and CE synthesis in the endoplasmic reticulum. Therefore, acyl-CoA:cholesterol acyltransferase (ACAT) activity in the membrane fractions prepared from mouse macrophages was studied, resulting in a dose-dependent inhibition by beauveriolides I and III with IC50 values of 6.0 and 5.5 μM, respectively. Thus, we showed that the beauveriolides inhibit macrophage ACAT activity specifically, resulting in blockage of the CE synthesis, leading to a reduction of lipid droplets in macrophages. ACAT activity in the membrane fractions prepared from mouse liver and Caco-2 cells was also inhibited, indicating that the beauveriolides block both ACAT-1 and -2. Moreover, beauveriolides I and III exert antiatherogenic activity in both low-density lipoprotein receptor- and apolipoprotein E-knockout mice without any side effects such as diarrhea or cytotoxicity to adrenal tissues as observed for many synthetic ACAT inhibitors. Beauveriolides I and III are the first microbial cyclodepsipeptides having an in vivo antiatherosclerotic effect and show promise as potential lead compounds for antiatherosclerotic agents.
Hypercholesterolemia involves heterogeneous disorders of lipid metabolism characterized by elevated levels of plasma total cholesterol and low-density lipoprotein (LDL)-derived cholesterol. It is definitively linked to increased morbidity and mortality due to myocardial infraction. 3-Hydroxy-3-methylglutaryl-CoA reductase, one of the rate-limiting enzymes in the cholesterol biosynthetic pathway, proved to be an effective target of inhibition for the treatment of hypercholesterolemia, and derivatives of fungal compactin (ML236B) and mevinolin (monacolin K), inhibitors of this enzyme, have been used clinically as cholesterol-lowering or antiatherosclerotic agents (1–5).
On the other hand, these achievements have stimulated the search for new cholesterol-lowering agents with distinct mechanisms of action. Accordingly, a variety of inhibitors of microbial origin (6) have been reported that include, hymeglusin (an 3-hydroxy-3-methylglutaryl-CoA synthase inhibitor) (7, 8), zaragozic acids or squalestatins (squalene synthase inhibitors) (9, 10), pyripyropenes [inhibitors of acyl-CoA:cholesterol acyltransferase (ACAT)] (11) and ferroverdins (inhibitors of cholesteryl ester transfer protein) (12).
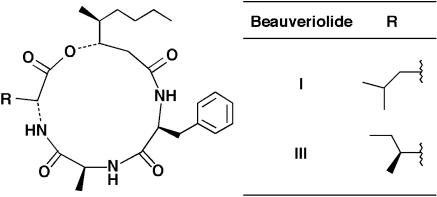
In the early stage of atherosclerogenesis, macrophages penetrate into the intima, efficiently take up modified LDL, store cholesterol and fatty acids as a form of neutral lipids in the cytosolic lipid droplets, and are converted into foam cells, leading to the development of atherosclerosis in the arterial wall (13–15). Recently, we established a cell-based assay system of lipid droplet synthesis using mouse macrophages as a model of macrophage-derived foam-cell formation (16). Screening for inhibitors with this system led to the discovery of fungal beauveriolides I and III (Fig. 1), which are members of the cyclodepsipeptide family (17, 18). These compounds could cause a reduction in the size and number of the cytosolic lipid droplets in macrophages without the cytotoxicity; however, the target site of this inhibition was unclear.
Fig. 1.
Structures of beauveriolides.
In this article, we show that beauveriolides I and III are the first microbial products orally active in mouse models of atherosclerogenesis by inhibiting ACAT activity.
Materials and Methods
Materials. Beauveriolides I and III were purified from a culture broth of Beauveria sp. FO-6979 as reported (18). [1-14C]Oleic acid (50 mCi/mmol) and [1-14C]cholesterol (54mCi/mmol) were purchased from DuPont/NEN, and [1-14C]oleoyl-CoA (oleoyl-CoA) (54 mCi/mmol) was from Amersham Pharmacia Biosciences. DMEM and Hanks' balanced salt solution were purchased from Nissui Seiyaku (Tokyo), GIT medium was from Nippon Seiyaku (Tokyo), and penicillin (10,000 units/ml), streptomycin (10,000 μg/ml), and glutamine (200 mM) solutions were from GIBCO. Phosphatidylcholine, phosphatidylserine, dicetylphosphate, cholesterol, 3β-hydroxy-5-pregnen-20-one (pregnenolone), oil red O, and fatty acid-free BSA were all purchased from Sigma–Aldrich. CL-283,546, an ACAT inhibitor,¶ was a generous gift from J. Hess, Pfizer Diagnostics. Plastic microplates (48-well) were purchased from Corning.
Animals. Female ICR mice (25–30 g) were obtained from the Japan SLC, Hamamatsu, Japan. Low-density lipoprotein receptor (LDL-R)-knockout mice and apolipoprotein E (apoE)-knockout mice on a C57BL/6 background were purchased from The Jackson Laboratory.
Mouse Peritoneal Macrophages. Mouse peritoneal macrophages from female ICR mice were prepared as described (19). Peritoneal cells were harvested from unstimulated mice by using Hank's balanced salt solution and then suspended at 2 × 106 cells per ml in GIT medium. Aliquots (0.25 ml) were dispensed into a 48-well plastic microplate or a tissue culture chamber (LAB-TEK 8-chamber, Nalge Nunc) and incubated in a humidified CO2 (5% vol/vol) atmosphere at 37°C for 2 h, after which each plate was washed three times with 0.25 ml of Hank's balanced salt solution to remove the unattached cells. The medium was then replaced immediately with 0.25 ml of DMEM containing 8% (vol/vol) lipoprotein-deficient serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) (hereafter referred to as medium A).
Assay for Cell Viability. Macrophage viability was measured in the presence of the inhibitors by using alamar Blue (Iwaki Glass, Tokyo).
Preparation of Liposomes. Multilamellar liposomes were prepared as described (19). In brief, a lipid mixture of phosphatidylcholine (1 μmol), phosphatidylserine (1 μmol), dicetylphosphate (0.2 μmol), and cholesterol (1.5 μmol) in chloroform were dried and then suspended in 1 ml of 0.3 M glucose. To prepare [14C]cholesterol-supplemented liposomes, [1-14C]cholesterol (0.37 μmol, 20 μCi) was added to the lipid mixture.
Assay for 14C-Labeled Neutral Lipid Synthesis by Macrophages. Assay for [14C]cholesteryl ester ([14C]CE) and [14C]triacylglycerol ([14C]TG) syntheses from [14C]oleic acid in macrophages was carried out by the method described (16). In brief, macrophages (5 × 105 cells per 0.25 ml of medium A) were cultured in a 48-well plastic microplate, and then 2.5 μl of a sample (methanol solution) and 10 μl of liposomes together with 5 μl of [14C]oleic acid (1 nmol, 0.05 μCi, 10% ethanol/PBS solution) were added to each culture. After a 14-h incubation, the medium was removed, and the cells in each well were washed three times with PBS. The cells were lysed by adding 0.25 ml of PBS containing 0.1% (wt/vol) SDS, and the cellular lipids were extracted by the method of Bligh and Dyer (20). The organic solvent was reduced by centrifugation under vacuum, the total lipids were separated on a TLC plate (silica gel F254, 0.5 mm thick, Merck) and analyzed with a bio-imaging analyzer (BAS 2000, Fuji) as described (16).
Cellular Neutral Lipid Staining. Macrophages were cultured in a tissue culture chamber with liposomes and inhibitors as described above. After the 14-h incubation, the cells were washed three times with PBS and fixed by soaking in 10% formalin. Nuclei and intracellular neutral lipid droplets were then stained with hematoxylin and oil red O, respectively, and the stained cells were examined by light microscopy (Vanox-S model, Olympus, Tokyo).
Assay for the Metabolism of Lysosomal [14C]Cholesterol by Macrophages. The metabolism of lysosomal [14C]cholesterol in mouse macrophages was measured as described (21). In brief, macrophages (5 × 105 cells) were inoculated for 2 h in a 48-well plastic microplate, washed with Hank's balanced salt solution, and placed in 0.25 ml of medium A containing 10 μl of liposomes supplemented with [14C]cholesterol (3.7 nmol, 200 nCi) and pregnenolone (added as a 2.5-μl methanol solution to make a final concentration of 10 μM). After incubation for 12 h, the medium was removed, and the cells were washed twice with buffer B (150 mM NaCl and 50 mM Tris·HCl, pH 7.4) containing BSA (2 mg/ml), then with buffer B without BSA, and subsequently incubated in 0.25 ml of medium A containing inhibitors (added as a 2.5-μl methanol solution) for 5 h. The cells were washed three times with PBS, and the cellular lipids were extracted twice with 1 ml of hexane-2-propanol (3:2). After reducing the organic solvent by evaporation, the total lipids were separated on a TLC plate and the radioactivity of [14C]CE was measured according to the same method described above.
Preparation of Microsomes from Mouse Livers and the Membrane Fraction from Macrophages. Mouse livers (0.5 g) or mouse peritoneal macrophages (2 × 108 cells) were homogenized in 3.0 ml of cold buffered sucrose solution (pH 7.2) containing 100 mM sucrose, 50 mM KCl, 40 mM KH2PO4, and 30 mM EDTA (hereafter referred to as buffer A) in a Teflon homogenizer. The microsomal fraction or the membrane fraction was pelleted by centrifugation at 100, 000 × g for 1 h at 4°C, resuspended in the buffer at a concentration of 5 mg of protein per ml and stored at –80°C until use.
Assay for ACAT Activity. ACAT activity was determined by using the isotope method described (22) with minor modifications. In brief, an assay mixture containing 2.5 mg/ml BSA in buffer A and [14C]oleoyl-CoA (20 μM, 0.5 μCi), together with a test sample (added as a 10-μl methanol solution to make a final concentration of 0–100 μM), and the microsomal or membrane fractions (100 μg of protein) in a total volume of 200 μl were incubated at 37°C for 5 min. The reaction was stopped by adding 1.2 ml of CHCl3/MeOH (2:1), and the product cholesteryl [14C]oleate was extracted by the method of Bligh and Dyer (20). After removing the organic solvent by evaporation, the residue was separated on a TLC plate and the radioactivity of cholesteryl [14C]oleate was measured as described above.
In Vivo Antiatherosclerotic Activity. LDL-R-knockout mice and apoE-knockout mice were housed in a pathogen-free barrier facility (12-h/12-h light/dark cycle) and were fed a normal rodent chow diet (CLEA Japan, Osaka) for 8 weeks after weaning. At this time the diets were changed to 0.15% cholesterol-supplemented diets (CLEA Japan), and beauveriolide III (50 and 25 mg/kg of body weight) suspended in 0.05% sodium CM-cellulose or only 0.05% sodium CM-cellulose (control) was administered orally every day for 2 months. Eighteen male mice (9 as controls and 9 for the beauveriolide III experiments) were used for this in vivo evaluation. Blood was collected from the retroorbital venous plexus at 0, 1, and 2 months. Blood glucose levels were measured immediately after bleeding with an Advantage II (Roche Diagnostics).
Colorimetric assays were used to measure plasma levels of total cholesterol (Determiner TC555 kit, Kyowa Medex, Tokyo), triacylglycerol (Triglyceride G-test kit, Wako Pure Chemicals, Osaka), and free fatty acid (NEFA C-test kit, Wako Pure Chemicals). For atherosclerotic lesion analyses, mice were killed by cervical dislocation after blood collection. Whole aortas were collected and stained with Sudan IV, and cross sections of proximal aorta were prepared and stained with oil red O as described (23). The luminal side of the stained aortas was photographed. Image capture and analysis was performed by using photoshop 6.0 (Adobe Systems, Tokyo). The extent of atherosclerosis was expressed as the percent of the lesion area of the entire aorta. The hearts were perfused with PBS containing 3% (wt/vol) formalin, were embedded in OCT compound (Sakura Tissue-Tek, Tokyo), and 6-μm-thick serial sections were cut by using a Cryostat (Leica). Four sections, each separated by 60 μm, were used to evaluate the lesions: two at the end of the aortic sinus and two at the junctional site of the aortic sinus and ascending aorta. The sections were counterstained with oil red O and hematoxylin. Images of the sections were captured with a digital camera (CoolSnap Photometrics, Olympus) mounted on a light microscope (Vanox-S model, Olympus) and analyzed with photoshop 6.0 (Adobe Systems).
Other Analytical Methods. Protein concentrations were determined according to the method of Bradford (24) by using the Bio-Rad Protein Assay Kit.
Statistics. All experimental data are expressed as mean ± SD. The significance of the difference of the means was determined by using Student's t test. A P value of <0.05 was taken as significant. Regression analyses were performed with statview (SAS Institute, Tokyo).
Results
Inhibition of Lipid Droplet Accumulation in Macrophages by Beauveriolides. In this macrophage assay, massive amounts of lipid droplets were accumulated in cytosols when the macrophages were incubated with the liposome (16). Under these conditions, a dose-dependent inhibition (3–10 μM) of lipid droplet accumulation was caused by beauveriolides I and III, and no morphological changes or cytotoxic effects were observed on macrophages for concentrations of up to 20 μM (18). By contrast, structurally related cyclodepsipeptides like enniatins showed none or only slight inhibition of lipid droplet accumulation even at 20 μM, and although beauvericin (10 μM) showed inhibition of lipid droplet accumulation, it was accompanied with severe morphological changes and cytotoxicity (≈50% cell viability by the alamar Blue assay) in macrophages (data not shown). Thus, among the cyclodepsipeptides tested, beauveriolides I and III were very specific inhibitors of cytosolic lipid droplet accumulation in mouse peritoneal macrophages.
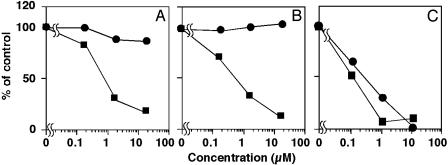
Selective Inhibition of CE Synthesis in Macrophages by Beauveriolides. Under the conditions of lipid droplet accumulation in mouse macrophages, about 40% of exogenously added [14C]oleic acid was incorporated into the neutral lipids, cholesteryl [14C]oleate (CE) and [14C]TG, which are the main constituents of lipid droplets in the cytosols (16). As shown in Fig. 2, beauveriolides I and III strongly inhibited [14C]CE synthesis in a dose-dependent fashion with IC50 values of 0.78 and 0.41 μM, respectively, but showed almost no inhibition of [14C]TG synthesis even at the highest dose, indicating that beauveriolides inhibit CE synthesis selectively in macrophages. Beauvericin (Fig. 2), enniatins, and bassianolides showed no selective inhibition; not only [14C]CE and [14C]TG syntheses, but also 14C-phospholipid synthesis were inhibited with similar IC50 values, indicating that it was due to their cytotoxic effect on macrophages (data not shown). Thus, among the cyclodepsipeptides beauveriolides I and III are the only compounds inhibiting CE synthesis selectively. These data support the results of the lipid droplet accumulation in macrophages as described above.
Fig. 2.
Effect of beauveriolides I and III and beauvericin on 14C-labeled neutral lipid synthesis from [14C]oleic acid by macrophages. Macrophage monolayers obtained from 5 × 105 cells per well in a microplate were incubated in 0.25 ml of medium A with a phospholipid/cholesterol liposome composed of phosphatidylcholine, phosphatidylserine, dicetylphosphate, and cholesterol at a molar ratio 10:10:2:15 and [14C]oleic acid in the absence or presence of indicated amounts of beauveriolide I (A) or III (B) or beauvericin (C). After a 14-h incubation, cholesteryl [14C]oleate (▪) and [14C]TG (•) were separated on TLC, determined with a radioscanner as described in Materials and Methods, and plotted as percent of control (without a drug).
Inhibition of Postlysosomal Cholesterol Metabolism in Macrophages by Beauveriolides. To gain insight regarding the target molecule of beauveriolides in inhibition of lipid droplet accumulation in macrophages, the effect of beauveriolides on postlysosomal process of cholesterol metabolism was studied. When macrophages were incubated with [14C]cholesterol-supplemented liposomes in the presence of 10 μM pregnenolone, [14C]CE formation was almost completely suppressed, and unesterified [14C]cholesterol accumulated in lysosomes of macrophages (21). After removal of pregnenolone by washing the cells with buffer, lysosomal [14C]cholesterol metabolism to [14C]CE was restarted in the presence or absence of beauveriolides. As shown in Fig. 3, beauveriolides I and III inhibited [14C]CE synthesis in a dose-dependent fashion with IC50 values of 0.37 and 0.21 μM, respectively, which are very similar to those for [14C]CE synthesis from [14C]oleic acid (Fig. 2 A and B). Under the same condition, cytochalasin D, an inhibitor of actin polymerization involved in endocytosis of the liposomes (21), produced no effect on [14C]CE synthesis from lysosomal [14C]cholesterol even at 20 μM (Fig. 3), whereas the compound inhibited [14C]CE synthesis from [14C]oleic acid with an IC50 value of 2.4 μM (Table 1). Also, CL-283,546, a synthetic ACAT inhibitor, inhibited both [14C]CE synthesis from lysosomal [14C]cholesterol and [14C]CE synthesis from [14C]oleic acid with almost the same IC50 values (Table 1 and Fig. 3). These data demonstrated that the beauveriolides block the postlysosomal process of cholesterol metabolism resulting in inhibition of CE synthesis in macrophages.
Fig. 3.
Effect of beauveriolides I and III on the metabolism of lysosomal [14C]cholesterol. Macrophages (5 × 105 cells in 0.25 ml of medium) grown in a 48-well plastic microplate were incubated with 10 μl of [14C]cholesterol (3.7 nmol, 200 nCi)-supplemented liposomes for 12 h in the presence of 10 μM pregnenolone. After incubation, the medium was removed, and the cells in each well were washed twice with buffer B containing BSA (2 mg/ml) and once with buffer B; they were then incubated in 0.25 ml of medium A containing beauveriolide I (▪), III (•), CL-283,546 (□), or cytochalasin D (♦). After a 5-h incubation, [14C]CE was separated by TLC, and radioactivity was measured with a radioscanner as described in Materials and Methods. The results were plotted as percent of control (without a drug).
Table 1. Effects of beauveriolides and other compounds on CE synthesis and ACAT activity.
| ACAT
|
|||||
|---|---|---|---|---|---|
| Compound | CE synthesis in macrophage | Postlysosomal cholesterol metabolism | Mouse macrophage | Mouse liver | Caco-2 |
| Beauveriolide I | 0.78 | 0.37 | 6.0 | 1.5 | 2.5 |
| Beauveriolide III | 0.41 | 0.21 | 5.5 | 1.5 | 1.0 |
| Beauvericin | 0.13 | 0.01 | 1.0 | 8.0 | NT |
| Cytochalasin D | 2.4 | >20 | NT | >100 | NT |
| CL-283,546 | 0.035 | 0.04 | 0.001 | 0.005 | 0.055 |
IC50 values stated are micromolar. NT, not tested.
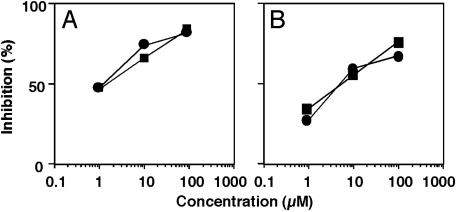
Inhibition of Mouse Macrophage ACAT Activity by Beauveriolides. The experiment using pregnenolone indicated that the inhibition site of beauveriolides is between the point of cholesterol departure from lysosomes and the point of cholesterol esterification in the ER. Therefore, their effect on ACAT activity was evaluated because other cyclodepsipeptides like beauvericin and enniatins with larger cyclic skeletons are known to inhibit ACAT activity (25). For this, microsomes prepared from mouse peritoneal macrophages, mouse livers, and human Caco-2 cells were used as an enzyme source. Beauveriolides I and III were found to inhibit the ACAT activity of mouse macrophage microsomes (expressed as ACAT-1) (26) in a dose-dependent manner with IC50 values of 6.0 and 5.5 μM, respectively, and the ACAT activity of mouse liver microsomes (expressed as ACAT-2) (27) in a dose-dependent manner with IC50 values of 1.5 μM for both compounds (Fig. 4). They also inhibited the ACAT activity in microsomes of human Caco-2 cells (expressed as ACAT-2) (28) with similar IC50 values (Table 1). Under the same conditions, beauvericin showed the most potent inhibition of ACAT activity in microsomes of all of the sources tested. These data revealed that the beauveriolides moderately inhibit ACAT-1 and -2 with similar potency.
Fig. 4.
Inhibition of ACAT activity in the mouse macrophage membrane fraction and mouse liver microsomes by beauveriolides I and III. Mouse livers (0.5 g) or mouse peritoneal macrophages (2 × 108 cells) were suspended in 3 ml of cold buffered sucrose solution (pH 7.2) containing 100 mM sucrose, 50 mM KCl, 40 mM KH2PO4, and 30 mM EDTA (hereafter referred to as buffer A) in a Teflon homogenizer. The liver microsomal fraction and the membrane fraction of macrophages, prepared as described in Materials and Methods, were used as the enzyme source. ACAT activity was assayed in an assay mixture containing 2.5 mg/ml BSA in buffer A and 20 μM [14C]oleic acid (0.5 μCi), together with beauveriolide I (▪) or III (•) (0–100 μM) and the microsomal fraction or the membrane fraction (100 μg of protein). After a 5-min incubation at 37°C, [14C]CE was separated by TLC, and radioactivity was measured with a radioscanner as described in Materials and Methods.
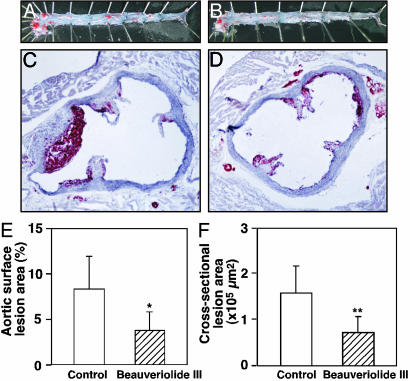
Inhibition of Atherosclerogenesis in ApoE-Knockout Mice by Beauveriolide. After 2-month oral administration of beauveriolide III (25 mg·kg–1·day–1) to apoE-knockout mice, atherosclerotic lesion area in the arch region of the whole aorta was decreased by 55% compared with the control group (3.8% vs. 8.4%, P < 0.01) (Fig. 5 A, B, and E). Reduction of atherosclerotic lesions was also shown in all regions of the aorta, with the most striking difference found in the proximal portion of the aortas. The cross-sectional lesion areas of hearts of the beauveriolide III-treated group were significantly smaller (by 52%) than those of the control group (78,005 vs. 162,801 μm2, P < 0.05) (Fig. 5 C, D, and F). No significant differences occurred in the body weight, blood glucose, plasma total cholesterol, plasma triglycerides, and plasmafree fatty acids between the two groups (data not shown). Similarly, atherosclerotic lesions of whole aortas and hearts of LDL-R-knockout mice treated with beauveriolide III (50 mg·kg–1·day–1) were also reduced by 40% and 60%, respectively (data not shown). Furthermore, beauveriolide-treated mice showed no side effects, such as diarrhea or cytotoxicity to adrenal tissues, during the experiments as observed for many synthetic ACAT inhibitors (29).
Fig. 5.
Effect of beauveriolide III on aortic atherosclerosis in apoE–/– mice. ApoE–/– mice were fed 0.15% cholesterol-supplemented diets with or without beauveriolide III (25 mg·kg–1·day–1) for 2 months (n = 9, each group). (A and B) Pinned-out aortas showing sudan-IV-stained lesions from apoE–/– mice that received 0.05% sodium CM-cellulose containing beauveriolide III (25 mg·kg–1·day–1 orally for 2 months) (B) and only 0.05% sodium CM-cellulose (control) (A). (C and D) Cross sections of proximal aortic roots of hearts showing oil-red-O-staining lesions in apoE–/– mice treated with beauveriolide III (D) and control (C). Comparison of the size of whole aorta surface (E) for A and B, and cross-sectional (F) lesions for C and D between the control and beauveriolide III-treated groups. ApoE–/– mice were fed 0.15% cholesterol-supplemented diets with or without beauveriolide III (25 mg·kg–1·day–1) for 2 months (n = 9). Bar indicates mean and error bars represent ± SD; *, P < 0.01; **, P < 0.05.
Discussion
A number of beauveriolide-related compounds, having the 13-membered cyclic skeleton composed of two l-amino acids, one d-amino acid, and one 3-hydroxylic fatty acid, have been reported (D. Matsuda, I.N., H.T., S. Kobayashi, R. Zocher, H. Kleinhauf, and S.Ō., unpublished work; refs. 17, 18, and 31), but their biological activities have not been clearly defined. Initially, we found that beauveriolides I and III show very potent inhibitory activity of lipid droplet accumulation in macrophages. Furthermore, our recent study on the structure–activity relationship (data not shown) showed that this activity is limited to the two compounds and, moreover, that d-allo-Ile/d-Leu and l-Phe in the molecules are important for the inhibitory activity. These findings prompted us to study the molecular target of the beauveriolides in macrophages. Several types of inhibitors of lipid droplet accumulation in macrophages have been reported (31–34). Sterol derivatives such as U18666A (31), progesterone, and pregnenolone (32) inhibit the movement of cholesterol out of the lysosome or inhibit the activity of multidrug-resistant P-glycoproteins in the plasma membrane (33), and a large number of ACAT inhibitors block cholesterol esterification in the endoplasmic reticulum (ER). These compounds are known to specifically inhibit CE synthesis in macrophages (34). On the other hand, triacsins, inhibitors of acyl-CoA synthetase, also block lipid droplet accumulation, but the compounds inhibit both CE and TG syntheses by depletion of acyl-CoA (16). Beauveriolides inhibit CE synthesis specifically (Fig. 2), and the inhibition site lies within some steps after cholesterol leaves lysosomes (Fig. 3). Therefore, ACAT, an ER enzyme, was tested as a target of beauveriolide. Finally, we demonstrated that beauveriolides I and III inhibit ACAT activity in microsomes prepared from mouse macrophages with IC50 values of 6.0 and 5.5 μM, respectively, and also mouse livers with IC50 values of 1.5 μM for both compounds. Recent molecular biological studies revealed the presence of two isozymes ACAT-1 and ACAT-2 (27, 28, 35). ACAT-1 is ubiquitously expressed, and high-level expression is observed in sebaceous glands, steroidogenic tissues, and macrophages. In rodents, ACAT-2 is expressed predominantly in the liver and intestine and in humans it is in the intestine. Therefore, it was strongly suggested that beauveriolides I and III inhibit both ACAT-1 and ACAT-2 to similar extents or inhibit ACAT-2 somewhat more strongly than ACAT-1 (Fig. 4). From the structure–activity relationship (data not shown), the results of ACAT inhibition by beauveriolide analogs are essentially similar to the results of the inhibition of lipid droplet accumulation in macrophages. In mouse macrophage microsomes, beauvericin that has a larger cyclic skeleton inhibited ACAT activity more strongly than beauveriolides (Table 1), but the compound did not show specific inhibition of CE synthesis (Fig. 2C) and had cytotoxic effect on macrophages. Thus, among several 13- and 18-membered cyclodepsipeptides tested, beauveriolides I and III are the only compounds that inhibit both ACAT activity and CE synthesis in macrophages, leading to a decrease in lipid droplet accumulation. Accordingly, in vivo anti-atherogenic activity of beauveriolides was studied in two mouse models.
Beauveriolide III proved orally active in both apoE-knockout mice (Fig. 5) and LDL-R-knockout mice (data not shown). After oral administration of at least 25 mg·kg–1·day–1 for 2 months to apoE-knockout mice, the atherosclerotic lesions of aorta and heart were reduced 54% and 52%, respectively. Beauveriolide III showed no side effects such as diarrhea or cytotoxicity to adrenal tissues during the experiments even at 100 mg·kg–1·day–1. Most synthetic ACAT inhibitors like CL-283,546 showed toxic effects on the adrenal gland (29). No data have been conclusive as to whether the toxic effects on the adrenal gland are inherent in the mechanism of action of these drugs. However, certain synthetic inhibitors like avasimibe proved effective in vivo but had no effect on the adrenal gland (36). Presently, the involvement of ACAT-1 and ACAT-2 as a target of antiatherosclerogenic drugs is a matter of controversy. Some ACAT inhibitors could reduce the development of atherosclerotic lesions independently of an effect on plasma cholesterol levels in cholesterol-fed rabbits and hamsters; however, with other inhibitors, the reduction of cholesterol levels depended on their effect on plasma cholesterol levels (37, 38). In pharmacological and genetic studies in animals, it was shown that specific inhibition of ACAT-1 may increase lesion size because of accumulation of free cholesterol in the lesions (39). Therefore, selective ACAT-1 inhibition should be approached cautiously in humans (40). ACAT-2-deficient transgenic mice have a reduction in CE synthesis in the small intestine and liver, which in turn produces protection against diet-induced hypercholesterolemia and gallstone formation (41). Furthermore, ACAT-2- and apoE-deficient mice have triglyceriderich apoB-containing lipoproteins and no atherogenic lesions (42). A selective inhibitor of ACAT-2 may be useful for preventing diet-induced hypercholesterolemia (41), but the development of such drugs has not been successful. Very recently, fungal pyripyropene A, discovered by our group, was found to be a very specific ACAT-2 inhibitor (L. L. Rudel, personal communication). Avasimibe, which inhibits both ACAT-1 and ACAT-2 activities, reduces atherosclerosis in several animal models and is being evaluated in clinical trials (37, 40). Our results show that beauveriolides, which also inhibit both ACAT-1 and ACAT-2, have antiatherogenic activity in both LDL-R and apoE-knockout mice without any side effects such as diarrhea or cytotoxicity to adrenal tissues. Beauveriolides I and III, microbial cyclodepsipeptides previously unreported to have in vivo antiatherosclerotic effect, show promise as potential lead compounds for antiatherosclerotic agents.
Acknowledgments
We thank Miss Makiko Masuda and Mr. Daisuke Matsuda for excellent assistance throughout this work. This work was supported by the “Research for the Future” Program of the Japan Society for the Promotion of Science (Grant JSPS-RFTF96I00304), the “21st Century COE Program” of the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and Uehara Memorial Foundation.
Abbreviations: CE, cholesteryl ester; TG, triacylglycerol; LDL, low-density lipoprotein; LDL-R, LDL receptor; ACAT, acyl-CoA:cholesterol acyltransferase; apoE, apolipoprotein E.
Footnotes
Katocs, A. S. J., Wang, C.-H. & Largis, E. E. (1988) FASEB J. 2, A1219 (abstr.).
References
- 1.Endo, A. (1992) J. Lipid Res. 33, 1569–1582. [PubMed] [Google Scholar]
- 2.Alberts, A. W., Chen, J., Kuron, G., Hunt, V., Huff, J., Hoffman, C., Rothrock, J., Lopez, M., Joshua, H., Harris, E., et al. (1980) Proc. Natl. Acad. Sci. USA 77, 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd, J., Cobbe, S. M., Ford, I., Isles, C. G., Lorimer, A. R., MacFarlane, P. W., McKillop, J. H. & Packard, C. J. (1995) N. Engl. J. Med. 333, 1301–1307. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen, T. R., Kjekshus, A. G., Berg, K., Haghfelt, T., Faergeman, O., Thorgeirsson, G., Pyorala, K., Miettinen, T., Olsson, A. G., Wedel, H., et al. (1994) Lancet 344, 1383–1389.7968073 [Google Scholar]
- 5.McKenney, J. M., McCormick, L. S., Schaefer, E. J., Black, D. M. & Watkins, M. L. (2001) Am. J. Cardiol. 88, 270–274. [DOI] [PubMed] [Google Scholar]
- 6.Tomoda, H., Namatame, I. & Ōmura, S. (2002) Proc. Jpn. Acad. 78, 217–240. [Google Scholar]
- 7.Tomoda, H., Kumagai, H., Takahashi, Y., Tanaka, Y., Iwai, Y. & Ōmura, S. (1988) J. Antibiot. 41, 247–249. [DOI] [PubMed] [Google Scholar]
- 8.Ōmura, S., Tomoda, H., Kumagai, H., Greenspan, M. D., Yodkovitz, J. B., Chen, J. S., Alberts, A. W., Martin, I., Mochales, S. & Monaghan, R. L. (1987) J. Antibiot. 40, 1356–1357. [DOI] [PubMed] [Google Scholar]
- 9.Baxter, A., Fitzgerald, B. J., Hutson, J. L., McCarthy, A. D., Motteram, J. M., Ross, B. C., Sapra, M., Snowden, M. A., Watson, N. S. & Williams, R. J. (1992) J. Biol. Chem. 267, 11705–11708. [PubMed] [Google Scholar]
- 10.Dufresne, C., Wilson, K. E., Singh, S. B., Zink, D. L., Bergstrom, J. D., Rew, D., Polishook, J. D., Meinz, M., Huang, L. & Silverman, K. C. (1993) J. Nat. Prod. 56, 1923–1929. [DOI] [PubMed] [Google Scholar]
- 11.Ōmura, S., Tomoda, H., Kim, Y. K. & Nishida, H. (1993) J. Antibiot. 46, 1168–1169. [DOI] [PubMed] [Google Scholar]
- 12.Tomoda, H., Tabata, N., Shinose, M., Takahashi, Y., Woodruff, H. B. & Ōmura, S. (1999) J. Antibiot. 52, 1101–1107. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein, J. L., Ho, Y. K., Basu, S. K. & Brown, M. S. (1979) Proc. Natl. Acad. Sci. USA 76, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, M. S., Goldstein, J. L., Krieger, M., Ho, Y. K. & Anderson, R. G. (1979) J. Cell Biol. 82, 597–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrity, R. G. (1981) Am. J. Pathol. 103, 181–190. [PMC free article] [PubMed] [Google Scholar]
- 16.Namatame, I., Tomoda, H., Arai, H., Inoue, K. & Ōmura, S. (1999) J. Biochem. 125, 319–327. [DOI] [PubMed] [Google Scholar]
- 17.Namatame, I., Tomoda, H., Tabata, N., Si, S. & Ōmura, S. (1999) J. Antibiot. 52, 7–12. [DOI] [PubMed] [Google Scholar]
- 18.Namatame, I., Tomoda, H., Si, S., Yamaguchi, Y., Masuma, R. & Ōmura, S. (1999) J. Antibiot. 52, 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa, K., Arai, H. & Inoue, K. (1990) J. Biol. Chem. 265, 5226–5231. [PubMed] [Google Scholar]
- 20.Bligh, E. G. & Dyer, W. (1959) Can. J. Biochem. Physiol. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Furuchi, T., Aikawa, K., Arai, H. & Inoue, K. (1993) J. Biol. Chem. 268, 27345–27348. [PubMed] [Google Scholar]
- 22.Field, F. J., Cooper, A. D. & Erickson, S. K. (1982) Gastroenterology 83, 873–880. [PubMed] [Google Scholar]
- 23.Yagyu, H., Ishibashi, S., Chen, Z., Osuga, J., Okazaki, M., Perrey, S., Kitamine, T., Shimada, M., Ohashi, K., Harada, K., et al. (1999) J. Lipid Res. 40, 1677–1685. [PubMed] [Google Scholar]
- 24.Bradford, M. M. (1976) Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 25.Tomoda, H., Huang, X. H., Cao, J., Nishida, H., Nagao, R., Okuda, S., Tanaka, H., Ōmura, S., Arai, H. & Inoue, K. (1992) J. Antibiot. 45, 1626–1632. [DOI] [PubMed] [Google Scholar]
- 26.Uelmen, P. J., Oka, K., Sullivan, M., Chang, C. C., Chang, T. Y. & Chan, L. (1995) J. Biol. Chem. 270, 26192–261201. [DOI] [PubMed] [Google Scholar]
- 27.Cases, S., Novak, S., Zheng, Y. W., Myers, H. M., Lear, S. R., Sande, E., Welch, C. B., Lusis, A. J., Spencer, T. A., Krause, B. R., et al. (1998) J. Biol. Chem. 273, 26755–26764. [DOI] [PubMed] [Google Scholar]
- 28.Oelkers, P., Behari, A., Cromley, D., Billheimer, J. T. & Sturley, S. L. (1998) J. Biol. Chem. 273, 26765–26771. [DOI] [PubMed] [Google Scholar]
- 29.Reindel, J. F., Dominick, M. A., Bocan, T. M., Gough, A. W. & McGuire, E. J. (1994) Toxicol. Pathol. 22, 510–518. [DOI] [PubMed] [Google Scholar]
- 30.Kuzma, M., Jegorov, A., Kacer, P. & Havlicek, V. (2001) J. Mass Spectrom. 36, 1108–1115. [DOI] [PubMed] [Google Scholar]
- 31.Liscum, L., Ruggiero, R. M. & Faust, J. R. (1989) J. Cell. Biol. 108, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aikawa, K., Furuchi, T., Fujimoto, Y., Arai, H. & Inoue, K. (1994) Biochim. Biophys. Acta 1213, 127–134. [PubMed] [Google Scholar]
- 33.Debry, P., Nash, E. A., Neklason, D. W. & Metherall, J. E. (1997) J. Biol. Chem. 272, 1026–1031. [DOI] [PubMed] [Google Scholar]
- 34.Sliskovic, D. R. & Trivedi, B. K. (1994) Curr. Med. Chem. 1, 204–255. [Google Scholar]
- 35.Farese, R. V., Jr. (1998) Curr. Opin. Lipidol. 9, 119–123. [DOI] [PubMed] [Google Scholar]
- 36.Sliskovic, D. R., Picard, J. A., O'Brien, P. M., Liao, P., Roark, W. H., Roth, B. D., Anderson, M. A., Mueller, S. B., Bocan, T. M., Bousley, R. F., et al. (1998) J. Med. Chem. 41, 682–690. [DOI] [PubMed] [Google Scholar]
- 37.Delsing, D. J., Offerman, E. H., van Duyvenvoorde, W., van Der Boom, H., de Wit, E. C., Gijbels, M. J., van Der Laarse, A., Jukema, J. W., Havekes, L. M. & Princen, H. M. (2001) Circulation 103, 1778–1786. [DOI] [PubMed] [Google Scholar]
- 38.Nicolosi, R. J., Wilson, T. A. & Krause, B. R. (1998) Atherosclerosis 137, 77–85. [DOI] [PubMed] [Google Scholar]
- 39.Accad, M., Smith, S. J., Newland, D. L., Sanan, D. A., King, L. E., Jr., Linton, M. F., Fazio, S. & Farese, R. V., Jr. (2000) J. Clin. Invest. 105, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewer, H. B., Jr. (2000) J. Clin. Invest. 105, 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buhman, K. K., Accad, M., Novak, S., Choi, R. S., Wong, J. S., Hamilton, R. L., Turley, S. & Farese, R. V., Jr. (2000) Nat. Med. 6, 1341–1347. [DOI] [PubMed] [Google Scholar]
- 42.Willner, E. L., Tow, B., Buhman, K. K., Wilson, M., Sanan, D. A., Rudel, L. L. & Farese, R. V., Jr. (2003) Proc. Natl. Acad. Sci. USA 100, 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]